Abstract
INTRODUCTION
Interleukin -1 was the first cytokine identified and is a powerful inducer of fever and inflammation. The biologically active receptor for IL-1, shares signaling pathways with some pathogen recognition receptors, the toll like receptors (TLRs) which early on suggested an important role in innate immune function.
DISCUSSION
The discovery that some intracellular “danger receptors”, the NOD like receptors (NLRs) can assemble to form multimolecular platforms, the inflammasomes, that not only sense intracellular danger but also activate IL-1β, has provided the molecular basis for the integration of IL-1 as an early response mediator in danger recognition. The critical role of balancing IL-1 production and signaling in human disease has recently been demonstrated in rare human monogenic diseases with mutations that affect the meticulous control of IL-1 production, release and signaling by leading to decreased or increased TLR/IL-1 signaling. In diseases of decreased TLR/IL-1 signaling (IRAK-4 and MyD88 deficiencies) patients are at risk for infections with gram positive organisms; and in diseases of increased signaling, patients develop systemic autoinflammatory diseases (Cryopyrin associated periodic syndromes (CAPS), and deficiency of the IL-1 receptor antagonist (DIRA)).
CONCLUSION
Monogenic defects in a number of rare diseases that affect the balance of TLR/IL-1 signaling have provided us with opportunities to study the systemic effects of IL-1 in human diseases. The molecular defects in CAPS and DIRA provided a therapeutic rationale for targeting IL-1 and the impressive clinical results from IL-1 blocking therapies have undoubtedly confirmed the pivotal role of IL-1 in human disease and spurred the exploration of modifying IL-1 signaling in a number of genetically complex common human diseases.
Keywords: IL-1, autoinflammatory diseases, NOMID/CINCA, DIRA, NLRP3, IL1RN, IL-1Ra, NOD like receptors, anakinra, neonatal disorder, genetic disease
Introduction to the Integration of Danger Recognition and IL-1 Regulation
The Role of TLRs in Danger Recognition and Shared Signaling Pathways with the IL-1 Receptor Type I
Our immune system has evolved to protect the integrity of our body and its functions against external “danger” such as infections, and endogenous “danger” such as cellular waste and injury. While inflammation as a response to danger is a well studied phenomenon, the understanding of inflammation as a physiologic response necessary to maintain tissue homeostasis is evolving [1] and emphasizes the importance of careful regulation and balancing of an “appropriate” inflammatory response.
Exogenous and endogenous danger signals can be sensed by the innate immune system through the recognition of patterns common to the danger stimuli. In the case of extracellular pathogens, these are highly conserved signals termed pathogen-associated molecular patterns (PAMPs) [2]. PAMPs interact with extracellular leucine rich residues (LRR) on transmembrane Toll-like receptors (TLRs) and the intracellular region of the TLR activates a downstream signaling cascade. In 1998, TLRs and IL-1 receptors were grouped in the interleukin-1 receptor/Toll-like receptor superfamily as the members have a conserved intracellular signaling domain, the Toll-IL-1 receptor (TIR) domain [3].
The recognition of endogenous danger signals by a recently discovered new type of receptors, the intracellular nucleotide- binding oligomerization domain (NOD) like receptors (NLRs), led to the understanding that some of these molecules can form multimolecular complexes that not only sense endogenous danger but also activate proteolytic caspases that can cleave and activate and secrete cytokines important in an early immune response [4]. The NLR necessary for activation of caspase-1 and resultant pro-IL-1 processing is NLRP3 also known as NALP3, cryopyrin, CIAS1, or PYPAF1. These NLR complexes are called inflammasomes and will be discussed in more detail below.
The IL-1 Family and its Receptors and the Regulation of IL-1
Of the 11 IL-1 family members, IL-1α and IL-1β are the best studied and most relevant to the currently known human diseases described below. IL-1α is active in its precursor form [5] whereas IL-1β requires proteolytic cleavage for activation as described above. Both cytokines bind to two different IL-1 receptors, the functional IL-1 receptor type I which contains the intracellular (TIR) signaling domain and the “decoy” IL-1 receptor type II which is lacking this domain rendering it inactive. When IL-1α or IL-1β bind to the IL-1R Type 1, an active receptor signaling complex is formed by association with the IL-1 receptor accessory protein (IL-1RAcP) and approximating the two intracellular TIR signaling domains allowing recruitment of MyD88 and other downstream mediators, Figure 1a. Binding of biologically active IL-1α and β to the receptor is also regulated by the IL-1receptor antagonist (IL-1Ra), that was isolated in 1986 as a soluble factor that blocked the binding of IL-1 to its receptor [6] [7]. The gene structure of IL-1Ra is homologous to that of IL-1α and IL-1β like most other members of the IL-1 family, and its gene location is in a cluster on the human chromosome 2q14 3 [8]. IL-1Ra competes with IL-1α and IL-1β for binding to the Type I IL-1R, but in contrast to the IL-1 cytokines prevents the simultaneous engagement of the IL-1RAcP and therefore the formation of active IL-1R1/IL-1RAcP signaling complexes, Figure 1b. The medication anakinra, the recombinant form of IL-1Ra, was approved by the Food and Drug Administration for the treatment of rheumatoid arthritis [9].
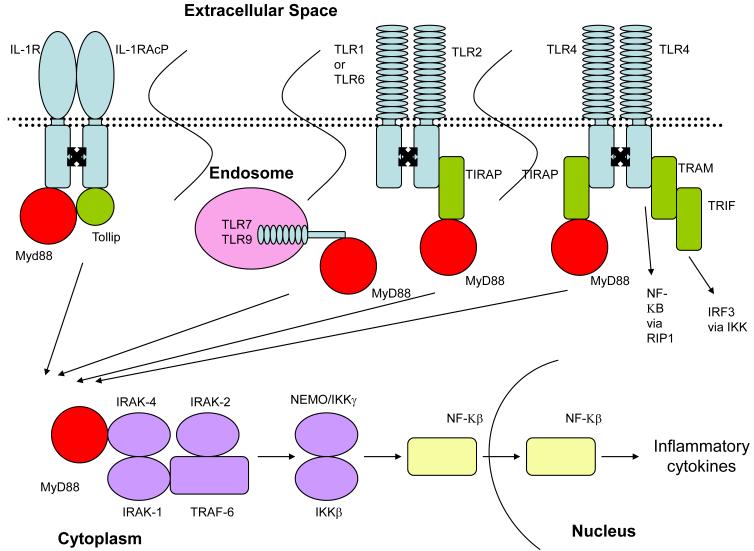
Figure 1a.
Convergence of Pathways in TLR and IL-1R Signaling
Upon activation, the IL-1 receptor dimerized with the IL-1 receptor accessory protein. The two cytoplasmic TIR domains approximate and recruit MyD88 and Tollip. TLRs also dimerizes upon activation. This dimerization results in the recruitment of TIRAP and MyD88. TLR4 can also recruit TRAM and TRIF resulting in NF-KB and IRF3 production. TLR7 and TLR9 are activated in endosomes and also recruit MyD88. Regardless of the pathway responsible for MyD88 production, it then interacts with IRAK-4 recruiting IRAK-1, IRAK-2, and TRAF-6. This ultimately results in NF-KB production and translocation to the nucleus to produce inflammatory cytokines.
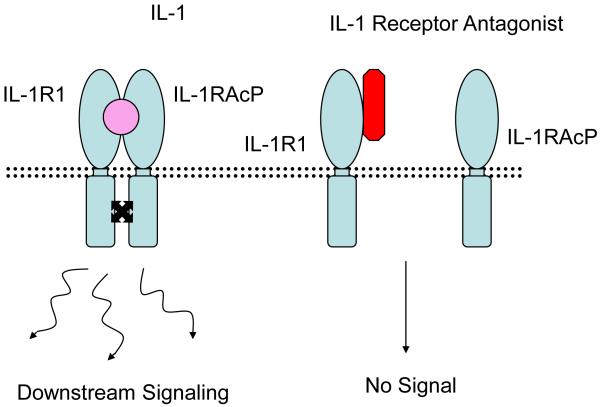
Figure 1b.
Blockade of IL-1 Signaling by IL-1 Receptor Antagonist
IL-1 receptor antagonist competes with IL-1 for binding to the IL-1 receptor preventing recruitment of IL-1 accessory protein and downstream signaling.
The major sources of IL-1β are blood monocytes, tissue macrophages, and dendritic cells. The early induction of IL-1 to exogenous and endogenous danger and its ability to induce gene expression and synthesis of potent inflammatory mediators make IL-1 a pivotal “alarm” cytokine. Among the inflammatory mediators are cyclooxygenase type 2 (COX-2), type 2 phospholipase A, and inducible nitric oxide synthase (iNOS) which accounts for prostaglandin-E2 (PGE2), platelet activating factor, and nitric oxide (NO) production. IL-1 also increases the expression of adhesion molecules such as intercellular adhesion molecule-1 on mesenchymal cells and vascular cell adhesion molecule–1 on endothelial cells [5] and has angiogenic properties [10]. In contrast to IL-1β, the biologic activity of IL-1α is not dependent on the inflammasome, and biologically active IL-1α precursor is present intracellularly (and may be released with cell death) and on the cell membrane [8]. IL-1α requires secretion to the extracellular space for activation which can also be mediated by caspase-1 but likely also other mechanisms [11].
Transcriptional and Translational Regulation of IL-1
IL-1β as a prototypic early response cytokine is tightly regulated on a transcriptional and translational level [5]. At the level of transcription, non-TLR stimuli such as C5a can induce high levels of IL-1β mRNA but stimulation through a TLR seems necessary to stabilize and transcribe the message into the inactive IL-1β precursor, pro-IL-1β [12]. In addition to the stable mRNA induction by nearly all TLRs, IL-1 can induce itself both in vivo and in monocytes in vitro [13, 14] Interestingly, IL-1 induced mRNA is even more stable than microbial stimulant induced IL-1β message [15]. This IL-1 induced positive feed back loop at a transcriptional and translational level may play an important role in perpetuating and amplifying IL-1 stimulation in the IL-1 mediated diseases that are discussed below.
Activation and Secretion of IL-1
Some Inflammasomes are potent IL-1 activators. However, in macrophages two signals for IL-1 production and secretion are required. TLR signaling is an obligate first step to induce stable mRNA transcription and translation leading to pro-IL-1β accumulation in the cytosol. The second step is the activation of the inflammasome and thus the activation of caspase-1 which is necessary to process and secrete cleaved and activated IL-1β. Caspase-1 activation is triggered by ATP which opens the ligand-gated ion channel, P2X7, receptor to potassium (K+) ion efflux, and causes a fall in intracellular K+ concentration [16]. Activation of the NLRP3 inflammasome can be inhibited by addition of potassium to cell lysis extracts [17]. As activated caspase-1 is required for IL-1 processing and secretion, inflammasome activation becomes the rate limiting step in IL-1 activation. It is interesting to speculate that tissue damage which leads to the release of K+, ATP, and uric acid may accelerate inflammasome activation in the context of pathogenic infections, but not if TLR stimulation occurs without a second stimulus derived from tissue stress. This would allow for NLRs to modify and differentially respond to pathogenic and non pathogenic TLR stimuli. Figure 2.
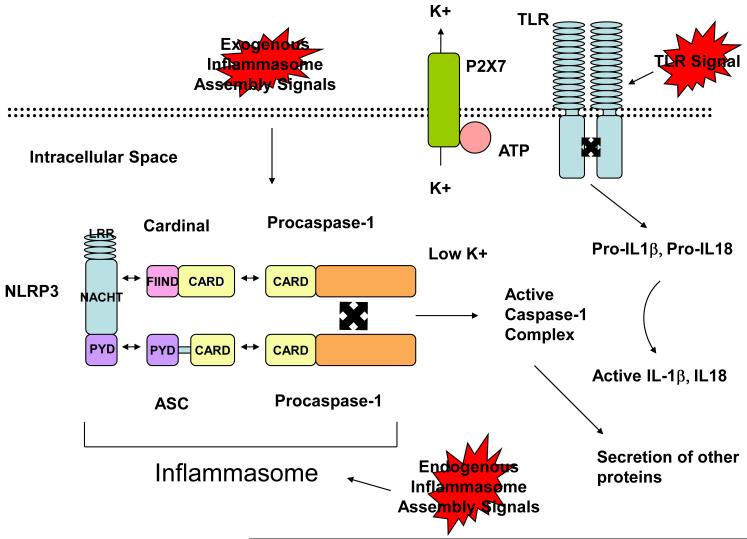
Figure 2.
Inflammasome Activation and Assembly
Exogenous and endogenous danger signals responsible for inflammasome assembly are largely not well understood. LRR on NLRP3 recognize danger signals resulting in the binding of FIIND in Cardinal to the NACHT domain of NLRP3 and the binding of the pyrin domain of NLRP3 to the pyrin domain of ASC. This results in the interaction of the respective CARD domains of Cardinal and ASC with a CARD domain of procaspase-1. The two procaspase-1 molecules autocatalyze to form an active caspase-1 complex. This requires a low potassium environment which depends on ATP binding to the P2X7 receptor on potassium channels. Pro-IL-1b, Pro-IL-18, and Pro-IL-33 are produced following TLR signaling. These proteins can then be cleaved to active IL-1β, and active IL-18, and active IL-33 (in vitro). The generation of active caspase-1 can also result in the secretion of other proteins not necessarily requiring active cleavage.
Downstream Signaling of IL-1
Upon stimulation, the two cytoplasmic TIR domains, one on each receptor chain approximate and recruit the adaptor protein MyD88 and Tollip. MyD88 recruitment triggers the phosphorylation of the IL-1 receptor–associated kinases IRAK-4, IRAK-2, and IRAK-1, and TRAF-6 is recruited which leads to NF-kB activation. The discovery that the cytoplasmic domain of the IL-1 receptor type I is highly homologous to that of TLRs suggested their link in participating in host defense against infection. However, there are differences in the signaling of TLRs and the IL-1 receptor. For example the TLR4 receptor can recruit two different TIR domain adapters, the translocating chain-associating membrane protein (TRAM) and TIR-domain-containing adapter-inducing interferon-β (TRIF). Signaling through the TRAM is MyD88 dependent as seen in signaling through the IL-1 receptor, and TRIF recruitment leads to the activation of interferon regulatory factor 3 (IRF3), the transcription factor required for induction of type 1 interferons. [18]. Figure 1a. The extracellular structures of the TLR and the IL-1R differ in that the IL-1R subfamily has immunoglobulin (Ig) domains and the TLR subfamily members have a leucine rich repeat domain (LRR), a motif that can directly bind PAMPs.
NOD like Receptors (NLRs) as Part of Inflammasomes, Sensing Danger and the Pivotal Role of IL-1 as Coordinator of an Innate Immune Response
In contrast to the transmembrane TLRs which are mostly located on the cell surface (except for TLR3, TLR7, TLR8, and TLR9 which are endosomal), NLRs are cytoplasmic and largely responsible for the recognition of intracellular danger and damage. NLRs also contain a leucin rich repeat (LRR) domain, a transmembrane NACHT domain, and an N-terminal effector domain. Analogous to the LRR domain of TLRs, the LRR of NLRs is likely responsible for the recognition of danger signals however, the mechanisms by which the NLR LRR domains sense danger is largely unknown. Direct binding to danger signals has not been demonstrated, and it has been hypothesized that activation of NLRs occurs through more general mechanisms downstream of an insult.
The human NLR family has 23 members [4]. Four NLR family members, NLRP3, NLRP1, NLRC4 and NAIP5 participate in the formation of caspase-1 activating platforms which are called “inflammasomes” [19]. Although the NLRP1 (also called CARD7, DEFCAP) inflammasome was the first described [19], the NLRP3 inflammasome is the best studied and has recently been implicated in a number of human diseases. NLRP3 forms a multiprotein complex, with the adaptor protein apoptosis-associated speck-like protein containing a caspase activating and recruitment domain (ASC) and Cardinal [20]. The association of NLRP3 with ASC and with Cardinal is required for recruitment of two procaspase-1 molecules via homotypic interaction of the caspase activating and recruitment domains (CARD) of ASC and Cardinal [21]. The approximation of two pro-caspase molecules leads to autocatalysis into active caspase-1 [22] which cleaves pro-IL-1 and pro-IL18 into their active forms. Recently inflammasome activated caspase-1 has been linked to unconventional secretion of a variety of leaderless proteins such as proIL-1α and FGF-2 [11]. Figure 1a.
The NLRP3 inflammasome is activated by microbial (exogenous) stimuli including LPS [20, 23-25]; nucleic acids [23, 24, 26, 27]; muramyl dipeptide (MDP) [28]; several toxins including the Streptomyces hygroscopicus derived nigericin and the marine dinoflagellate derived maitotoxin [16]; environmental, large inorganic crystallinic structures, such as asbestos and silica [29-31]; and recently adjuvants including aluminum hydroxide (alum) used to boost vaccine responses were found to stimulate the inflammasome [30, 32-34]. The NLRP3 inflammasome also senses cellular (endogenous) danger signals such as ATP [16], uric acid crystals [25], hyaluronan and heparan sulfate [35] and amyloid-β fibrils [36]. The fact that the inflammasome is activated by endogenous, non-infectious stimuli which are released from stressed or dying cells and that it can coordinate not only an inflammatory response but a response involved in cytoprotection and tissue repair [11] suggests that NLRs may have a role in tissue homeostasis.
The Concept of Autoinflammation as Excessive inflammation and Immunodeficiencies as Insufficient Inflammation in Response to Danger Sensing
Historically, the family of autoinflammatory diseases initially comprised a small group of hereditary fever syndromes that presented with fever and systemic and organ specific inflammation without obvious inflammatory triggers [37]. The first characterized Mendelian autoinflammatory diseases were familial Mediterranean fever (FMF) caused by autosomal recessive mutations in MEFV1 encoding pyrin [38, 39] and the TNF receptor associated periodic syndrome (TRAPS) caused by mutations in the p55 TNF receptor, TNFRSF1A [40]. The genetic defect in both diseases pointed to dysregulated innate immune responses. In order to distinguish these disorders from the “autoimmune” disorders that are caused by dysregulated adaptive immune responses marked by antigen specific T-cells and/ or the development of pathogenic autoantibodies, Dan Kastner coined the term “autoinflammatory” conditions. Patients with autoinflammatory diseases also lack evidence of infections, allergic diseases or immunodeficiencies. Since 1997 the group of monogenic autoinflammatory diseases has grown to include hyperimmunoglobulin D with periodic fever syndrome (HIDS) due to mutations in mevalonate kinase, MVK [41, 42]; Blau syndrome and childhood sarcoidosis due to mutations in NOD2 [43, 44]; and pyogenic arthritis, pyoderma gangrenosum, and acne (PAPA) due to mutations in PSTPIP1 [45]. The cryopyrin associated periodic syndrome (CAPS) family of mutations are due to mutations in CIAS1/NLRP3 encoding cryopyrin [46-48] while deficiency of IL-1 receptor antagonist (DIRA) is caused by deleting mutations in the IL-1 receptor antagonist, IL1RN [49, 50]. The molecular genetic defects of two disorders in particular, CAPS and DIRA, and their impressive response to IL-1 blockade have helped delineate the role of IL-1 in human disease.
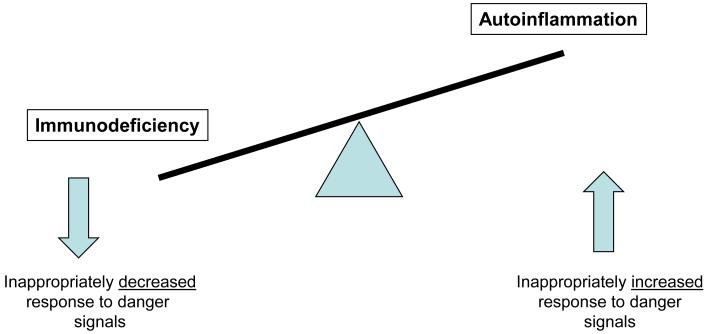
Genetic modifications that lead to stimulation of proinflammatory pathways by either constitutive over-action of proinflammatory effector pathways or lack of antiinflammatory effector pathways lead to autoinflammation. In contrast, genetic modifications resulting in the inability to recognize and coordinate an inflammatory response to eliminate infectious organisms lead to an immunodeficiency. In this sense immunodeficiencies and autoinflammatory diseases are immune dysregulatory conditions where the balance to maintain an adequate inflammatory response to danger signals has been tipped towards inappropriately low and inappropriately excessive responses. This imbalance will be demonstrated in disorders affecting TLR/IL-1R signaling which is the main focus of this review Figure 3.
Figure 3.
Dysregulated Balance of Immune Response to Danger Signals
Monogenic Diseases of Decreased TLR/IL-1R Signaling
MyD88 and IRAK-4 Deficiencies
As a functional IL-1 response is critical in the recognition of some danger signals, it follows that an insufficient ability to activate IL-1 would result in an impaired ability to respond to innate immune threats. MyD88 recruits IRAK-4 following TLR and IL-1 receptor activation and intact function of both adaptors is required for the proper function of IL-1 cytokines [51, 52]. Figure 1a. Knockout mice deficient in MyD88 and IRAK-4 are vulnerable to a broad range of pathogens including most bacteria, viruses, fungi, and parasites [53, 54]. IRAK-4 and MyD88 deficiencies have been discovered as inherited autosomal recessive disorders in humans. Similar to the mouse models blood and fibroblast cells do not activate downstream MAPK and NFκB mediated secretion of TNF and IL-6 in response to known IL-1 receptor ligands [53, 55]. In contrast to the knockout mouse models, children with MyD88 and IRAK-4 deficiencies have a less severe phenotype. Clinically, the defect in children with MyD88 and IRAK-4 deficiencies is limited to an increased susceptibility to Streptococcus pneumoniae, Staphylococcus aureus, and Pseudomonas aeruginosa infections, however patients may also have an increased susceptibility to other gram-negative bacteria [53, 55-57]. The bacterial range of infections is relatively limited compared to mice with no described atypical or intracellular infections and intact resistance to most ubiquitous microorganisms, including viruses, fungi, and parasites, as well as a number of bacteria [58]. This implies that in humans, there may be redundant immunologic danger signal recognition for the majority of pathogens. With the exception of recurrent infections, patients are otherwise healthy and appear to have a diminished risk for serious infection once adolescence is reached.
Monogenic and Genetically Complex Diseases of Increased TLR/IL-1 Signaling
Cryopyrin-Associated Periodic Syndrome (CAPS)
A major breakthrough in understanding the role of IL-1 in human disease came from Hal Hoffman’s discovery in 2001 that the autosomal dominantly inherited syndromes Familial Cold Autoinflammatory Syndrome (FCAS) and Muckle-Wells Syndrome (MWS) result from mutations in the CIAS1 gene (later also called NALP3, NLRP3, PYPAF1) encoding cryopyrin [46]. The spontaneously occurring not inherited disease, chronic infantile neurologic, cutaneous and arthritis syndrome (CINCA), called Neonatal Onset Multisystem Inflammatory Disease (NOMID) in the US, was later found to be caused by spontaneous de novo mutations in the same gene [47, 48] linking these previously thought to be unrelated syndromes to a single disease spectrum with FCAS on the mild and NOMID on the most severe end of the spectrum. The disease spectrum is referred to as cryopyrin associated periodic syndromes (CAPS). Mutations in CIAS1/NLRP3 are autosomal dominant gain of function mutations that result in an overactive inflammasome and increased conversion of pro-caspase-1 to active caspase-1 with resultant increased IL-1β and IL-18, levels [16, 59].
Patients with FCAS present with episodes of inflammation including fevers, an urticarial-like rash, joint pain, conjunctivitis, and headaches after exposure to cold temperatures or even air drafts. Typical episodes last for 12 to 48 hours and then resolve. The long term outcome is good, end organ damage or amyloidosis is uncommon. Patients with MWS have similar symptoms of fevers, urticarial-like rash, joint pain, conjunctivitis, and headaches however in contrast to patients with FCAS, these symptoms can be continuous and are not typically precipitated by cold exposure. Patients may also develop episcleritis and optic disc edema. Unlike FCAS, sensorineural hearing loss is common in patients with MWS presenting in the second to third decade of life and amyloidosis can occur in up to 25% of patients [60].
The most severe of the cryopyrin associated syndromes is NOMID. Patients with NOMID typically present within the first days after birth with systemic inflammation, fevers, urticarial-like rash, malaise. Joint swelling and conjunctivitis can develop at birth but usually within the first months of life. CNS inflammation with leptomeningitis and neutrophilic pleocytosis in the cerebrospinal fluid are common. Severely affected children develop hydrocephalus, brain atrophy, and cognitive impairment. Patients typically develop sensorineural hearing loss within the first year of life and on magnetic resonance imaging (MRI) cochlear enhancement is seen and suggests cochleitis as the cause of hearing loss. Conjunctivitis, anterior and posterior uveitis, and subcorneal infiltrates can lead to retinal scarring, corneal clouding, and blindness. Arthralgia is common, true arthritis in most cases is very mild however between 50 to 70% of patients with NOMID develop characteristic bony overgrowth of the epiphyses of the long bones. Most commonly involved is the epiphyses of the distal femur and proximal femur of the knee, which frequently leads to joint deformities and contractures, Figure 4. Other features include elevated inflammatory markers, short stature, and leucocytosis. With appropriate early intervention, inflammatory changes are largely reversible. Bony overgrowth changes typically do not respond to therapy and once organ damage such as significant hearing loss or visions loss or hydrocephalus with cerebral atrophy have developed these changes are not reversible, making early treatment imperative. About 50% of NOMID patients do not have mutations in CIAS1 but are clinically indistinguishable from the CIAS1 mutation positive patients suggesting the involvement of other genes in the disease pathogenesis [47]. Interestingly, in mutation positive patients, stimulation of peripheral blood mononuclear cells with LPS leads to activation of caspase-1 and secretion of IL-1β without the presence of a second ATP signal, thus suggesting a constitutive overactivation of the NLRP3 inflammasome and an exaggerated response to LPS stimulation [61].
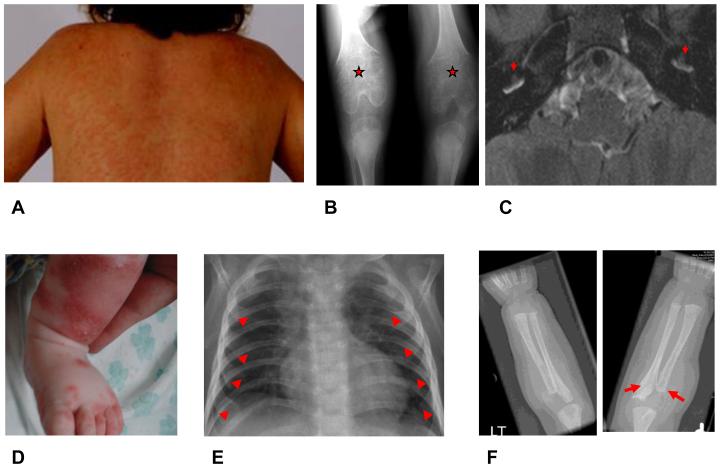
Figure 4.
Clinical Manifestations of NOMID and DIRA
Images A through C – Clinical manifestations of NOMID. A) Neutrophilic urticarial like rash B) Bony proliferation at the growth end plate C) Inner ear cochlear enhancement on MRI. Images D through F – Clinical manifestations of DIRA. D) Neutrophilic pustular rash E) Widening of anterior ribs F) Periostitis with osteolysis.
Despite clinical heterogeneity, patients with FCAS, MWS, and NOMID respond dramatically and invariably to IL-1 blockade. Studies have shown efficacy of anakinra (Kineret®, recombinant IL-1 receptor antagonist) and the long acting drugs rilonacept (Arcalyst®, IL-1 Trap, a fusion protein of the IL-1 receptor and the Fc portion of IgG) and canakinumab (Ilaris®, IL-1β blocking antibody) for the treatment of CAPS. All patients show rapid improvement in clinical and laboratory parameters with treatment [62-67]. The drugs are generally well tolerated and these medications have now become the standard of care in the treatment of these conditions. Although anakinra has been used longer in the treatment of CAPS, rilonacept and canakinumab have undergone a development program for orphan diseases and are now FDA approved for the treatment of FCAS / MWS [68]. Consistent with the clinical observations of the varied disease severity in FCAS, MWS and NOMID/CINCA, increasing doses of IL-1 blocking agents such as anakinra are necessary to control more severe disease. Typically subcutaneous anakinra 0.5-1.5mg/kg/day is sufficient to suppress disease activity in FCAS patients however, in NOMID patients doses up to 6mg/kg/day are required to control disease. The resolution of symptoms and the achievement of inflammatory remission not only in the blood but also in the specific organs with treatment by IL-1 inhibitors is an important proof of concept that the systemic and organ specific inflammation seen in these disorders is dependent on IL-1β. The success of the treatment of FCAS with IL-1 blockade serves as an example of how understanding the pathophysiologic processes responsible for inflammatory diseases can lead to rational targeting of affected pathways. Notably, only bony overgrowth changes progress on treatment which suggests that continuation of growth is likely independent of IL-1 [69].
Deficiency of the IL-1 Receptor Antagonist (DIRA)
DIRA is a newly described autosomal recessive disease due to mutations of IL1RN that lead to non-expression of the encoded protein, IL-1 Ra [49, 50]. Three homozygous mutations are point mutations in the IL1RN that lead to a stop codon and no transcription and no expression of the protein are detected [49]; another mutation constitutes a 175kb genomic deletion that includes the IL1RN locus but also 5 adjacent genes located in the same gene cluster on chromosome 2q [49, 50]. The IL1RN mutations are present in founder populations in Newfoundland, the Netherlands, Puerto Rico, and possibly Lebanon and further founder mutations have since been found in 2 other populations (personal communications). Non-expression of IL-1Ra leads to unopposed IL-1 receptor activation and an increased response to IL-1α and IL-1β stimulation [49]. Affected patients present with a neonatal inflammatory syndrome that similar to NOMID has a high mortality.
Perinatally, patients develop a pustular rash, joint swelling, painful osteolytic lesions and periosteitis typically affecting the distal ribs and the long bones and characteristic heterotopic bone formation presenting as cloaking around the proximal femur have been seen in all children described so far. Interestingly, in contrast to NOMID, fevers are rare. Although NOMID and DIRA patients present with a neutrophilic dermatitis, the rash is urticarial-like in NOMID and CAPS but pustular in DIRA. Meningitis, cochlear inflammation and progressive hearing loss, as well as signs of increased intracranial pressure and eye inflammation, all features of NOMID, are not seen in DIRA patients. CNS vasculitis and interstitial lung disease are rare manifestations of DIRA and not seen in CAPS, Table 1. Patients only respond incompletely to high doses of prednisone, however a rapid and dramatic response to anakinra has been seen in all children. The response to treatment may not be complete in patients with the genomic deletion, suggesting a possible contribution of the other deleted genes to the inflammatory phenotype.
Table 1.
Differences Between IL-1 Mediated Monogenic Autoinflammatory Diseases
| FCAS | MWS | NOMID | DIRA | |
|---|---|---|---|---|
| Genetic defect |
CIAS1 autosomal dominant mutation |
CIAS1 autosomal dominant mutation |
CIAS1 autosomal dominant mutation |
IL1RN autosomal recessive deletion |
| Functional consequences |
Overactive cryopyrin leading to increased caspase-1 conversion and IL-1β, and possibly IL-18, IL-33 production |
Overactive cryopyrin leading to increased caspase-1 conversion and IL-1β, and possibly IL-18, IL-33 production |
Overactive cryopyrin leading to increased caspase-1 conversion and IL-1β, and possibly IL-18, IL-33 production |
Lack of IL-1 receptor antagonist leading to unopposed IL-1α and IL-1β signaling |
| Systemic manifestations |
Cold induced fevers, elevated inflammatory markers, joint pain |
Fevers, elevated inflammatory markers, joint pain |
Fevers, elevated inflammatory markers, joint pain |
Elevated inflammatory markers |
| Audiology manifestations |
None | Cochleitis and hearing loss | Cochleitis and hearing loss | None |
| Ophthalmologic manifestations |
Conjunctivitis | Conjunctivitis | Conjunctivitis, uveitis, papilledema, optic nerve atrophy, vision loss |
None |
| CNS manifestations | None | Mild intermittent meningitis | Elevated intracranial pressure with hydrocephalus, leptomeningitis, cognitive impairment |
CNS vasculitis (rare) |
| Bone manifestations | None | None | Bony overgrowth with deformity | Widening of anterior rib ends, periosteal elevation, multifocal osteolytic lesions |
| Skin manifestations | Cold induced neutrophilic urticarial like rash |
Neutrophilic urticarial like rash |
Neutrophilic urticarial like rash | Neutrophilic pustular rash |
| Other | None | None | Growth retardation | Growth retardation, interstitial lung disease |
The Role of IL-1 in Other Monogenic Autoinflammatory Diseases
A common theme among the monogenic autoinflammatory diseases is the dysregulation of innate immune pathways many of which involve the aberrant regulation of IL-1, Table 2. Monogenic autoinflammatory disorders with clinical and molecular evidence of IL-1 involvement include FMF, PAPA, and HIDS/MKD.
Table 2.
“Monogenic” Autoinflammatory Syndromes
| Inflammatory Pathogenesis |
Disease | Gene | Protein | Inheritance pattern |
Disease onset | Flare/fever pattern |
Specific organ inflammation |
Treatment |
|---|---|---|---|---|---|---|---|---|
| IL-1 Mediated | CAPS: | |||||||
| FCAS |
CIAS1 (1q44) |
Cryopyrin | Autosomal dominant |
First 6 months of life, cold induced |
<24 h | Skin, Eyes, Joints | IL-1 blockade | |
| MWS |
CIAS1 (1q44) |
Cryopyrin | Autosomal dominant |
Infancy to adolescence |
24-48 h | Skin, Eyes, Joints, Inner ears, Meninges (mild) |
IL-1 blockade | |
| NOMID |
CIAS1 (1q44) |
Cryopyrin | Autosomal dominant/ de novo |
Neonatal or early infancy |
Continuous with flares |
Skin, Eyes, Joints, Inner ears, Meninges, Bony epiphyseal hyperplasia |
IL-1 blockade | |
| DIRA |
IL1RN (2q14) |
IL-1 receptor antagonist |
Autosomal Recessive |
Neonatal or early infancy |
Continuous with flares |
Skin, bones, lungs (rare), vasculitis (rare) |
Anakinra | |
| Partially IL-1 Mediated |
FMF |
MEFV (16p13) |
pyrin | Autosomal recessive |
80% of the cases occur before the age of 20 |
1-3 d | Skin, Joints, Peritoneum, Pleura |
Colchicine, rarely IL-1 and TNF blockade or thalidomide if colchicine resistant |
| HIDS |
MVK (12q24) |
Mevalonate kinase |
Autosomal recessive |
Median age at onset 6 months |
3-7 d | Skin, Eyes, Joints, Prominent lymph nodes |
NSAIDS, corticosteroids, TNF and IL-1 blockade |
|
| PAPA |
CD2BP1 (15q24) |
PSTPIP1 | Autosomal dominant |
Early childhood | Common | Skin, Joints | Local and systemic corticosteroids, TNF or IL-1 blockade |
|
| Other Pathways |
TRAPS |
TNFRSF1A (12p13) |
TNF receptor 1 |
Autosomal dominant |
Median age at onset 3 yrs |
1-4 wk | Skin, Eyes, Joints, Peritoneum, Pleura |
TNF blockade, steroids, IL-1 blockade, colchicine is ineffective |
| PGA¶ |
NOD2 (16q12) |
Nod2 | Autosomal Dominant/ de novo |
Early childhood | Uncommon | Skin, Eyes, Joints | NSAIDS, Corticosteroids, methotrexate, cyclospo- rine, TNF or IL-1blockade |
|
| Majeed’s syndrome |
LPIN2 (18p11) |
Lipin2 | Autosomal recessive |
Early infancy (1- 19 months) |
Weeks- months |
Bones, Periosteum, Anemia |
NSAIDS, corticosteroids, interferon-α |
|
| Cherubism |
SH3BP2 (4p16) |
SH3BP2 | Autosomal dominant |
Childhood, spontaneous remission by 3rd decade |
Uncommon | Jaws, Eyes (rare) | NSAIDS, TNF inhibition, interferon-α, azithromycin, bisphosphonates |
|
| IL-10 receptor deficiency |
IL10RA (11q23) IL10RB (21q22) |
IL-10 receptor, IL10RB also forms IL-22, -26, -28, -29 receptors |
Autosomal Recessive |
Neonatal or early infancy |
Continuous with flares |
Colitis with fistula formation, folliculitis in patients with IL10RB mutations |
Bone marrow transplantation |
Familial Mediterranean fever, FMF, is the most common monogenic periodic fever syndrome and is caused by mutations in the MEFV gene on chromosome 16p13, which encodes the protein pyrin, also known as marenostrin. As indicated by the name of the diseases, the mutations are most common in ethnic groups around the Mediterranean Sea [38, 39]. Patients present with 1- to 3-day episodes of fever, serositis, and arthralgia or arthritis. Although patients are typically asymptomatic between attacks, inflammatory markers such as erythrocyte sedimentation rate, C-reactive protein, and serum amyloid A levels can remain elevated even during inactive disease states and can lead to the development of amyloidosis and kidney failure. Although FMF is considered an autosomal recessive disease, clinical manifestations of FMF can be present in patients with only a single MEFV mutation and carriers for single MEFV mutations may have subclinical evidence of inflammation [70, 71]. The role of pyrin in causing disease remains unresolved and experimental models lend data suggesting an anti-inflammatory or a proinflammatory role of pyrin depending on experimental conditions. Pyrin interacts with tubulin and colocalizes with microtubules. The anti-inflammatory actions have been demonstrated in models where wild type pyrin can bind to ASC, a component of several inflammasomes and sequester ASC making it unavailable to form an inflammasome. Wildtype pyrin also binds to caspase-1 and inhibits the production of mature IL-1β [72, 73]; its proinflammatory properties were demonstrated in an overexpression model where pyrin can form a caspase activating inflammasome that secretes IL-1 [74]. In both scenarios, mutations in pyrin would lead to more inflammation. FMF is effectively treated and attacks can be prevented in most patients with colchicine, which inhibits microtubule formation. Anecdotal reports suggest that IL-1 inhibition may work in some colchicine resistant patients [72, 75-79]. The fact that no treatment modality is completely satisfactory in controlling the disease in all patients suggests the involvement of pyrin in more complex effector mechanisms than seen in patients with CAPS.
Pyogenic arthritis with pyoderma gangrenosum and acne (PAPA) syndrome is a rare autosomal dominant disorder only described in a few families. It presents with recurrent arthritis, sterile purulent synovial fluid, and neutrophilic skin lesions ranging from cystic acne to pyoderma gangrenosum. The CD2-binding protein 1 (CD2BP1) is mutated and the protein encoded is PSTPIP1, proline serine threonine phosphatase-interacting protein 1 [45]. Interaction with the protein tyrosine phosphatase PEST causes phosphorylation of PSTPIP1which prevents its binding to pyrin. Mutations in PSTPIP1 prevent its phosphorylation and lead to prolonged binding to pyrin. Mutated PSTPIP1 is proinflammatory but it is unclear whether the inhibitory function is mediated through prevention of the inhibitory function of pyrin on the NLRP3 inflammasome because of the increased binding of pyrin to mutated PSTPIP1 [80] or through its binding to pyrin causing a conformational change which would enable pyrin to oligomerize with adaptor proteins and form a pyrin inflammasome more readily as suggested in another model [81]. In both scenarios the proposed net effect is increased production of IL1β. Treatment with steroids, anti-TNF, and IL-1 inhibition lead to clinical benefit. However, even patients on high doses of IL-1 blocking agents can develop disease flares suggesting a more complex role of PSTPIP1 in the disease pathogenesis.
Hyperimmunoglobulinemia D with periodic fever syndrome (HIDS) / mevalonate kinase deficiency (MKD), is an autosomal recessive disease spectrum. Mutations in the MVK at sites that encode parts of the enzyme that are associated with the catalytic activity of mevalonate kinase lead to a complete loss of the enzymatic activity and result in a very severe presentation with recurrent fever, mental retardation, developmental abnormalities and often early death. This severe condition is historically referred to as MKD. A residual enzymatic activity of about 5% causes a milder episodic disease with attacks lasting 1-3 days that present with recurrent fever, lymphadenopathy, abdominal pain, and rash. The disease can often be triggered by immunizations. Mevalonate kinase converts mevalonic acid to 5-phospho-mevalonic acid, an early step in the pathway of cholesterol synthesis. As part of this pathway, anti-inflammatory isoprenoids are synthesized. Initial confusion whether the disease is caused by access of mevalonate, the substrate accumulating upstream of the dysfunctional enzyme, or due to the inability to produce isoprenoids which require precursors that are not produced due to the lack of MK activity, has recently been clarified by providing evidence favoring the latter hypothesis. In vitro data from simvastatin treated cells mimicking MVK deficiency suggests that dysregulated isoprenoid biosynthesis activates the small GTPase Rac1. This was required for both protein kinase B (PKB) activation and increased caspase-1 activity leading to active IL-1β secretion. Importantly, inhibition of Rac1 in peripheral blood mononuclear cells isolated from MKD patients resulted in a reduction in IL-1β release [82]. IL-1 blocking therapy with anakinra has resulted in disease improvement in some patients which clinically confirms a partial role of IL-1 in HIDS [83, 84].
The Role of IL-1 in Genetically Complex Autoinflammatory Diseases
The clinical effect of IL-1 blockade or clinical similarities of the IL-1 associated monogenic diseases with a number of genetically complex diseases has recently suggested a role for IL-1 in these disorders. IL-1 blockade has been efficacious in the treatment of gout [85-87], diabetes [88] and a case of Behcet’s disease [89]. In an animal model of Alzheimer’s disease, amyloid β fibrils were able to stimulate IL-1β release in an inflammasome dependent fashion [90] and in a model of asbestos induced lung fibrosis [29] inflammasome dependent IL-1 release was also observed suggesting a possible role for IL-1 in these conditions. The clinical manifestations of DIRA are similar to the bone lesions seen in another inflammatory disease, chronic recurrent multifocal osteomyelitis (CRMO) and an animal model of CRMO, the cmo mouse; the skin lesions are similar to those seen in pustular psoriasis. In the cmo mouse model high levels of IL-1 have been found in the bone lesions [91, 92]. IL-1 blocking therapy has also become the treatment of choice in patients with systemic onset Still’s disease [93, 94] and may be important in the pathogenesis of conditions that have not been traditionally considered inflammatory such as atherosclerosis [95].
The prominence of excessive IL-1 signaling as demonstrated in the diseases above is by no means the only effector pathway in autoinflammatory diseases. As dysregulated modular effector pathways affecting the balance of an inflammatory response to danger signals become evident, novel genes regulating such pathways become candidates for dysregulation in yet unknown inflammatory conditions. As an example, the recent description of homozygous mutations in the IL-10 receptor that lead to the loss of IL-10 signaling, pointed to the important role of the anti-inflammatory cytokine IL-10 in controlling an inflammatory response to commensal bacteria in the gut. The lack of IL-10 signaling leads to the development of inflammatory colitis starting during the first year of life [96]. Substantiating the role of IL-10 in complex disorders associated with gut inflammation are SNPs associated with decreased IL-10 production that were identified in genome wide association studies in inflammatory bowel disease [97, 98] as well as an association with IL-10 promotor polymorphisms producing low IL-10 levels in Behcet’s disease [99].
Genetic modifications of other effector pathways have been associated with an increased risk of infections. For example mutations in genes leading to decreased IL-12 signaling (IFGNR1, IFGNR2, STAT-1, NEMO, IL12B, IL12RB1) [100] predispose to mycobacterial infections. Dectin-1 [101] and CARD9 mutations [102] lead to decreased STAT3 and IL-17 signaling [103] in response to fungal infections, causing increased susceptibility to mucocutaneous fungal infections including vulvovaginal candidiasis and onchomycosis.
Conclusion
All autoinflammatory diseases share an inappropriately “increased” level of inflammation which contrasts with the immunodeficiencies that result in infections due to an inappropriately decreased inflammatory response to infectious and other danger stimuli. The concept and scope of auto-inflammatory diseases is evolving, and the recent discovery of innate immune dyregulation in common polygenic diseases such as gout, diabetes, silicosis, asbestosis, and possibly Alzheimer’s disease has provided important clues to disease pathogenesis that is opening new avenues for therapeutic interventions. The role of the innate immune system in diseases traditionally considered to be “autoimmune” or caused by adaptive immune dysregulation, such as lupus erythematosus and rheumatoid arthritis, is becoming increasingly appreciated. This indicates that innate and adaptive immune dysreguation are likely linked and may provide a rationale for using innate immune modifying therapies in these diseases.
The molecular discovery of the genetic basis in a number of monogenic autoinflammatory diseases continues to illustrate the inflammatory phenotype in human diseases and thus continues to reveal key inflammatory pathways regulating organ specific inflammation. Diseases such as CAPS, DIRA, IRAK-4 deficiency, and MyD88 deficiency illustrate the effects of a dysregulated IL-1 pathway and have spurred interest in the exploration of novel therapeutic targets that may include suppression of IL-1β production and release, neutralization of secreted IL-1, inhibition of IL-1R signal transduction [4], and modification of inflammasome activation. As these drugs become available, exciting new opportunities to explore the role of the key inflammatory pathways in clinical trials in more common complex diseases become possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–35. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R, Janeway CA., Jr. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91(3):295–8. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 3.O’Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: 10 years of progress. Immunol Rev. 2008;226:10–8. doi: 10.1111/j.1600-065X.2008.00701.x. [DOI] [PubMed] [Google Scholar]
- 4.Jha S, Ting JP. Inflammasome-associated nucleotide-binding domain, leucine-rich repeat proteins and inflammatory diseases. J Immunol. 2009;183(12):7623–9. doi: 10.4049/jimmunol.0902425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–50. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 6.Balavoine JF, et al. Prostaglandin E2 and collagenase production by fibroblasts and synovial cells is regulated by urine-derived human interleukin 1 and inhibitor(s) J Clin Invest. 1986;78(4):1120–4. doi: 10.1172/JCI112669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hannum CH, et al. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature. 1990;343(6256):336–40. doi: 10.1038/343336a0. [DOI] [PubMed] [Google Scholar]
- 8.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87(6):2095–147. [PubMed] [Google Scholar]
- 9. http://www.accessdata.fda.gov/drugsatfda_docs/label/2003/anakamg062703LB.pdf.
- 10.Voronov E, et al. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci U S A. 2003;100(5):2645–50. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keller M, et al. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132(5):818–31. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 12.Kaspar RL, Gehrke L. Peripheral blood mononuclear cells stimulated with C5a or lipopolysaccharide to synthesize equivalent levels of IL-1 beta mRNA show unequal IL-1 beta protein accumulation but similar polyribosome profiles. J Immunol. 1994;153(1):277–86. [PubMed] [Google Scholar]
- 13.Dinarello CA, et al. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J Immunol. 1987;139(6):1902–10. [PubMed] [Google Scholar]
- 14.Greten FR, et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 2007;130(5):918–31. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schindler R, Ghezzi P, Dinarello CA. IL-1 induces IL-1. IV. IFN-gamma suppresses IL-1 but not lipopolysaccharide-induced transcription of IL-1. J Immunol. 1990;144(6):2216–22. [PubMed] [Google Scholar]
- 16.Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440(7081):228–32. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 17.Kahlenberg JM, Dubyak GR. Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am J Physiol Cell Physiol. 2004;286(5):C1100–8. doi: 10.1152/ajpcell.00494.2003. [DOI] [PubMed] [Google Scholar]
- 18.Kagan JC, et al. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9(4):361–8. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–26. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 20.Sutterwala FS, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24(3):317–27. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Mariathasan S, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430(6996):213–8. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 22.Li P, et al. Characterization of mice deficient in interleukin-1 beta converting enzyme. J Cell Biochem. 1997;64(1):27–32. doi: 10.1002/(sici)1097-4644(199701)64:1<27::aid-jcb5>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 23.Kanneganti TD, et al. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem. 2006;281(48):36560–8. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- 24.Kanneganti TD, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440(7081):233–6. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 25.Martinon F, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–41. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 26.Di Paolo NC, et al. Virus binding to a plasma membrane receptor triggers interleukin-1 alpha-mediated proinflammatory macrophage response in vivo. Immunity. 2009;31(1):110–21. doi: 10.1016/j.immuni.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muruve DA, et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452(7183):103–7. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 28.Marina-Garcia N, et al. Pannexin-1-mediated intracellular delivery of muramyl dipeptide induces caspase-1 activation via cryopyrin/NLRP3 independently of Nod2. J Immunol. 2008;180(6):4050–7. doi: 10.4049/jimmunol.180.6.4050. [DOI] [PubMed] [Google Scholar]
- 29.Dostert C, et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320(5876):674–7. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hornung V, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9(8):847–56. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cassel SL, et al. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci U S A. 2008;105(26):9035–40. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H, et al. Cutting edge: inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J Immunol. 2008;181(1):17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eisenbarth SC, et al. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453(7198):1122–6. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kool M, et al. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008;181(6):3755–9. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 35.Yamasaki K, et al. NLRP3/cryopyrin is necessary for interleukin-1beta (IL-1beta) release in response to hyaluronan, an endogenous trigger of inflammation in response to injury. J Biol Chem. 2009;284(19):12762–71. doi: 10.1074/jbc.M806084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halle A, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9(8):857–65. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masters SL, et al. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease (*) Annu Rev Immunol. 2009;27:621–68. doi: 10.1146/annurev.immunol.25.022106.141627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.The International FMF Consortium Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. Cell. 1997;90(4):797–807. doi: 10.1016/s0092-8674(00)80539-5. [DOI] [PubMed] [Google Scholar]
- 39.A candidate gene for familial Mediterranean fever. Nat Genet. 1997;17(1):25–31. doi: 10.1038/ng0997-25. [DOI] [PubMed] [Google Scholar]
- 40.McDermott MF, et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999;97(1):133–44. doi: 10.1016/s0092-8674(00)80721-7. [DOI] [PubMed] [Google Scholar]
- 41.Drenth JP, et al. International Hyper-IgD Study Group Mutations in the gene encoding mevalonate kinase cause hyper-IgD and periodic fever syndrome. Nat Genet. 1999;22(2):178–81. doi: 10.1038/9696. [DOI] [PubMed] [Google Scholar]
- 42.Houten SM, et al. Mutations in MVK, encoding mevalonate kinase, cause hyperimmunoglobulinaemia D and periodic fever syndrome. Nat Genet. 1999;22(2):175–7. doi: 10.1038/9691. [DOI] [PubMed] [Google Scholar]
- 43.Kanazawa N, et al. Early-onset sarcoidosis and CARD15 mutations with constitutive nuclear factor-kappaB activation: common genetic etiology with Blau syndrome. Blood. 2005;105(3):1195–7. doi: 10.1182/blood-2004-07-2972. [DOI] [PubMed] [Google Scholar]
- 44.Miceli-Richard C, et al. CARD15 mutations in Blau syndrome. Nat Genet. 2001;29(1):19–20. doi: 10.1038/ng720. [DOI] [PubMed] [Google Scholar]
- 45.Wise CA, et al. Mutations in CD2BP1 disrupt binding to PTP PEST and are responsible for PAPA syndrome, an autoinflammatory disorder. Hum Mol Genet. 2002;11(8):961–9. doi: 10.1093/hmg/11.8.961. [DOI] [PubMed] [Google Scholar]
- 46.Hoffman HM, et al. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29(3):301–5. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aksentijevich I, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 2002;46(12):3340–8. doi: 10.1002/art.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feldmann J, et al. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am J Hum Genet. 2002;71(1):198–203. doi: 10.1086/341357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aksentijevich I, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med. 2009;360(23):2426–37. doi: 10.1056/NEJMoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reddy S, et al. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. N Engl J Med. 2009;360(23):2438–44. doi: 10.1056/NEJMoa0809568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koziczak-Holbro M, et al. IRAK-4 kinase activity is required for interleukin-1 (IL-1) receptor- and toll-like receptor 7-mediated signaling and gene expression. J Biol Chem. 2007;282(18):13552–60. doi: 10.1074/jbc.M700548200. [DOI] [PubMed] [Google Scholar]
- 52.Muzio M, et al. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science. 1997;278(5343):1612–5. doi: 10.1126/science.278.5343.1612. [DOI] [PubMed] [Google Scholar]
- 53.von Bernuth H, et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321(5889):691–6. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 55.Picard C, et al. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003;299(5615):2076–9. doi: 10.1126/science.1081902. [DOI] [PubMed] [Google Scholar]
- 56.Enders A, et al. Two siblings with lethal pneumococcal meningitis in a family with a mutation in Interleukin-1 receptor-associated kinase 4. J Pediatr. 2004;145(5):698–700. doi: 10.1016/j.jpeds.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 57.Ku CL, et al. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. J Exp Med. 2007;204(10):2407–22. doi: 10.1084/jem.20070628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ku CL, et al. Inherited disorders of human Toll-like receptor signaling: immunological implications. Immunol Rev. 2005;203:10–20. doi: 10.1111/j.0105-2896.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- 59.Janssen R, et al. Enhanced interleukin-1beta and interleukin-18 release in a patient with chronic infantile neurologic, cutaneous, articular syndrome. Arthritis Rheum. 2004;50(10):3329–33. doi: 10.1002/art.20494. [DOI] [PubMed] [Google Scholar]
- 60.Muckle TJ. The ‘Muckle-Wells’ syndrome. Br J Dermatol. 1979;100(1):87–92. doi: 10.1111/j.1365-2133.1979.tb03572.x. [DOI] [PubMed] [Google Scholar]
- 61.Gattorno M, et al. Persistent efficacy of anakinra in patients with tumor necrosis factor receptor-associated periodic syndrome. Arthritis Rheum. 2008;58(5):1516–20. doi: 10.1002/art.23475. [DOI] [PubMed] [Google Scholar]
- 62.Goldbach-Mansky R, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med. 2006;355(6):581–92. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hawkins PN, et al. Spectrum of clinical features in Muckle-Wells syndrome and response to anakinra. Arthritis Rheum. 2004;50(2):607–12. doi: 10.1002/art.20033. [DOI] [PubMed] [Google Scholar]
- 64.Lovell DJ, Bowyer SL, Solinger AM. Interleukin-1 blockade by anakinra improves clinical symptoms in patients with neonatal-onset multisystem inflammatory disease. Arthritis Rheum. 2005;52(4):1283–6. doi: 10.1002/art.20953. [DOI] [PubMed] [Google Scholar]
- 65.Hoffman HM, et al. Efficacy and safety of rilonacept (interleukin-1 Trap) in patients with cryopyrin-associated periodic syndromes: results from two sequential placebo-controlled studies. Arthritis Rheum. 2008;58(8):2443–52. doi: 10.1002/art.23687. [DOI] [PubMed] [Google Scholar]
- 66.Maksimovic L, et al. New CIAS1 mutation and anakinra efficacy in overlapping of Muckle-Wells and familial cold autoinflammatory syndromes. Rheumatology (Oxford) 2008;47(3):309–10. doi: 10.1093/rheumatology/kem318. [DOI] [PubMed] [Google Scholar]
- 67.Lachmann HJ, et al. Use of canakinumab in the cryopyrin-associated periodic syndrome. N Engl J Med. 2009;360(23):2416–25. doi: 10.1056/NEJMoa0810787. [DOI] [PubMed] [Google Scholar]
- 68.IL-1beta-targeted antibody approved for rare autoinflammatory disorders. Nat Rev Drug Discov. 2009;8(8):605. doi: 10.1038/nrd2953. [DOI] [PubMed] [Google Scholar]
- 69.Hill SC, et al. Arthropathy of neonatal onset multisystem inflammatory disease (NOMID/CINCA) Pediatr Radiol. 2007;37(2):145–52. doi: 10.1007/s00247-006-0358-0. [DOI] [PubMed] [Google Scholar]
- 70.Kalyoncu M, et al. Are carriers for MEFV mutations “healthy”? Clin Exp Rheumatol. 2006;24(5 Suppl 42):S120–2. [PubMed] [Google Scholar]
- 71.Lachmann HJ, et al. Clinical and subclinical inflammation in patients with familial Mediterranean fever and in heterozygous carriers of MEFV mutations. Rheumatology (Oxford) 2006;45(6):746–50. doi: 10.1093/rheumatology/kei279. [DOI] [PubMed] [Google Scholar]
- 72.Chae JJ, et al. The B30.2 domain of pyrin, the familial Mediterranean fever protein, interacts directly with caspase-1 to modulate IL-1beta production. Proc Natl Acad Sci U S A. 2006;103(26):9982–7. doi: 10.1073/pnas.0602081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Papin S, et al. The SPRY domain of Pyrin, mutated in familial Mediterranean fever patients, interacts with inflammasome components and inhibits proIL-1beta processing. Cell Death Differ. 2007;14(8):1457–66. doi: 10.1038/sj.cdd.4402142. [DOI] [PubMed] [Google Scholar]
- 74.Yu JW, et al. Cryopyrin and pyrin activate caspase-1, but not NF-kappaB, via ASC oligomerization. Cell Death Differ. 2006;13(2):236–49. doi: 10.1038/sj.cdd.4401734. [DOI] [PubMed] [Google Scholar]
- 75.Belkhir R, et al. Treatment of familial Mediterranean fever with anakinra. Ann Intern Med. 2007;146(11):825–6. doi: 10.7326/0003-4819-146-11-200706050-00023. [DOI] [PubMed] [Google Scholar]
- 76.Calligaris L, et al. The efficacy of anakinra in an adolescent with colchicine-resistant familial Mediterranean fever. Eur J Pediatr. 2008;167(6):695–6. doi: 10.1007/s00431-007-0547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gattringer R, et al. Anakinra in two adolescent female patients suffering from colchicine-resistant familial Mediterranean fever: effective but risky. Eur J Clin Invest. 2007;37(11):912–4. doi: 10.1111/j.1365-2362.2007.01868.x. [DOI] [PubMed] [Google Scholar]
- 78.Kuijk LM, et al. Effective treatment of a colchicine-resistant familial Mediterranean fever patient with anakinra. Ann Rheum Dis. 2007;66(11):1545–6. doi: 10.1136/ard.2007.071498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roldan R, et al. Anakinra: new therapeutic approach in children with Familial Mediterranean Fever resistant to colchicine. Joint Bone Spine. 2008;75(4):504–5. doi: 10.1016/j.jbspin.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 80.Shoham NG, et al. Pyrin binds the PSTPIP1/CD2BP1 protein, defining familial Mediterranean fever and PAPA syndrome as disorders in the same pathway. Proc Natl Acad Sci U S A. 2003;100(23):13501–6. doi: 10.1073/pnas.2135380100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu JW, et al. Pyrin activates the ASC pyroptosome in response to engagement by autoinflammatory PSTPIP1 mutants. Mol Cell. 2007;28(2):214–27. doi: 10.1016/j.molcel.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kuijk LM, et al. HMG-CoA reductase inhibition induces IL-1beta release through Rac1/PI3K/PKB-dependent caspase-1 activation. Blood. 2008;112(9):3563–73. doi: 10.1182/blood-2008-03-144667. [DOI] [PubMed] [Google Scholar]
- 83.Bodar EJ, et al. Effect of etanercept and anakinra on inflammatory attacks in the hyper-IgD syndrome: introducing a vaccination provocation model. Neth J Med. 2005;63(7):260–4. [PubMed] [Google Scholar]
- 84.Cailliez M, et al. Anakinra is safe and effective in controlling hyperimmunoglobulinaemia D syndrome-associated febrile crisis. J Inherit Metab Dis. 2006;29(6):763. doi: 10.1007/s10545-006-0408-7. [DOI] [PubMed] [Google Scholar]
- 85.McGonagle D, et al. Management of treatment resistant inflammation of acute on chronic tophaceous gout with anakinra. Ann Rheum Dis. 2007;66(12):1683–4. doi: 10.1136/ard.2007.073759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.So A, et al. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther. 2007;9(2):R28. doi: 10.1186/ar2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Terkeltaub R, et al. The interleukin 1 inhibitor rilonacept in treatment of chronic gouty arthritis: results of a placebo-controlled, monosequence crossover, non-randomised, single-blind pilot study. Ann Rheum Dis. 2009;68(10):1613–7. doi: 10.1136/ard.2009.108936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Larsen CM, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356(15):1517–26. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 89.Botsios C, et al. Resistant Behcet disease responsive to anakinra. Ann Intern Med. 2008;149(4):284–6. doi: 10.7326/0003-4819-149-4-200808190-00018. [DOI] [PubMed] [Google Scholar]
- 90.Sanz JM, et al. Activation of microglia by amyloid {beta} requires P2X7 receptor expression. J Immunol. 2009;182(7):4378–85. doi: 10.4049/jimmunol.0803612. [DOI] [PubMed] [Google Scholar]
- 91.Ferguson PJ, et al. A missense mutation in pstpip2 is associated with the murine autoinflammatory disorder chronic multifocal osteomyelitis. Bone. 2006;38(1):41–7. doi: 10.1016/j.bone.2005.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grosse J, et al. Mutation of mouse Mayp/Pstpip2 causes a macrophage autoinflammatory disease. Blood. 2006;107(8):3350–8. doi: 10.1182/blood-2005-09-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lequerre T, et al. Interleukin-1 receptor antagonist (anakinra) treatment in patients with systemic-onset juvenile idiopathic arthritis or adult onset Still disease: preliminary experience in France. Ann Rheum Dis. 2008;67(3):302–8. doi: 10.1136/ard.2007.076034. [DOI] [PubMed] [Google Scholar]
- 94.Goldbach-Mansky R, Kastner DL. Autoinflammation: the prominent role of IL-1 in monogenic autoinflammatory diseases and implications for common illnesses. J Allergy Clin Immunol. 2009;124(6):1141–9. doi: 10.1016/j.jaci.2009.11.016. quiz 1150-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Crossman DC, et al. Investigation of the effect of Interleukin-1 receptor antagonist (IL-1ra) on markers of inflammation in non-ST elevation acute coronary syndromes (The MRC-ILA-HEART Study) Trials. 2008;9:8. doi: 10.1186/1745-6215-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Glocker EO, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361(21):2033–45. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Franke A, et al. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat Genet. 2008;40(11):1319–23. doi: 10.1038/ng.221. [DOI] [PubMed] [Google Scholar]
- 98.Imielinski M, et al. Common variants at five new loci associated with early-onset inflammatory bowel disease. Nat Genet. 2009;41(12):1335–40. doi: 10.1038/ng.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wallace GR, et al. IL-10 genotype analysis in patients with Behcet’s disease. Hum Immunol. 2007;68(2):122–7. doi: 10.1016/j.humimm.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 100.Filipe-Santos O, et al. Inborn errors of IL-12/23- and IFN-gamma-mediated immunity: molecular, cellular, and clinical features. Semin Immunol. 2006;18(6):347–61. doi: 10.1016/j.smim.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 101.Ferwerda B, et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med. 2009;361(18):1760–7. doi: 10.1056/NEJMoa0901053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Glocker EO, et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med. 2009;361(18):1727–35. doi: 10.1056/NEJMoa0810719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Holland SM, Vinh DC. Yeast infections--human genetics on the rise. N Engl J Med. 2009;361(18):1798–801. doi: 10.1056/NEJMe0907186. [DOI] [PubMed] [Google Scholar]