Abstract
Objective:
We evaluated the associations of teenage insulin and adolescent diet with 10-year weight gain in an analysis sample of black and white girls matched for pubertal stage, BMI (or fat mass), and insulin at ages 9-10. We hypothesized that pre-teen insulin and insulin resistance (IR) would interact with dietary factors to positively predict increases in BMI. Further, we hypothesized that increased insulin and IR, interacting with higher caloric intake during adolescence, would lead to greater increments in BMI in black girls than in white girls.
Patients and Methods:
Prospective 10-yr follow-up of 215 pairs of black and white schoolgirls matched at baseline by BMI (or fat mass), insulin, and pubertal stage, with repeated measures of body habitus, insulin, and dietary intake.
Results:
When matched for BMI, black girls had higher fat free mass and white girls had higher fat mass at ages 9-10 years. Black-white differences in caloric intake were not significant at ages 9-10, but black girls consumed more calories at age 19. Black girls consumed a greater percent of calories from fat throughout. At age 19, black girls had higher BMI, fat mass index, and insulin. When matched at ages 9-10 for fat mass, black girls were heavier, had higher BMI, and greater fat free mass. By ages 18-19, black girls continued to have higher BMI, but had accrued higher fat mass and a higher percentage of body fat. By stepwise multiple regression, 10-year increases in BMI were predicted by age 9-10 BMI, 10 year change in insulin, and a 3-way interaction between age 9-10 insulin, adolescent caloric intake, and race (higher in black girls), all p <.0001.
Conclusions:
Insulin at ages 9-10 interacts with caloric intake to increase BMI by age 19. There appear to be intrinsic black-white metabolic differences that lead to greater gains in fat during adolescence in black girls. Evaluating BMI and insulin at ages 9-10 could identify girls (particularly black) who would optimally benefit from dietary and exercise interventions to avoid obesity.
Keywords: Insulin, insulin resistance, caloric intake, obesity, adolescent girls, race, pre-teen predictors of adolescent weight gain
INTRODUCTION
Black-white differences in the prevalence of obesity in women are thought to contribute significantly to their differences in cardiovascular disease (CVD) morbidity and mortality. 1 Obesity differences begin during the peri-pubertal and adolescent periods. 2 Evaluating 15-year secular trends in BMI and CVD risk factors in Princeton School District middle-school children (1975-1990), Morrison et al reported that BMI had increased in all sex-race groups, with the largest increase in black females. Concomitantly, the largest increases in total cholesterol, blood pressure, and heart rate were in black females. 3 In the NHLBI Growth and Health Study (NGHS), increased BMI (>85th percentile) and central adiposity in 9-and 10-year old black and white girls were associated with increased lipids and blood pressure and with increased clustering of CVD risk factors. 4 Therefore, identifying pre-teen predictors of adolescent weight gain and central adiposity could provide focused avenues for prevention.
The NGHS was designed to identify factors contributing to black-white differences in weight. 5 The NGHS analyses have been complicated by the fact that black girls were taller and heavier, with greater BMI at enrollment, ages 9-10. These early differences were due in part to the earlier onset of puberty in black girls, 5 since onset of puberty increases accretion of height, weight, fat-free mass, and fat mass. At the same time, however, increased prepubertal body mass is associated with early onset of puberty. The onset of puberty increases systemic insulin resistance 6 which, in turn, may contribute to increased body weight in childhood and adulthood. 7, 8
Two clinical centers of NGHS collected fasting blood for the measurement of insulin in Years 1 (age 9-10), 7 (age 15-16) and 10 (age 18-19), in addition to annual measurement of body habitus, pubertal status, and diet. 5, 9 In the current report, we evaluate the role of pre-teen insulin and adolescent diet in 10-year weight gain in two analysis samples of paired black and white girls. Since BMI, fat mass, and insulin all change with pubertal development,6 9 we first matched for pubertal stage, BMI, and insulin at age 9-10, and second, matched for pubertal stage, fat mass, and insulin at age 9-10. Thus the black and white female cohorts started the analysis period with similar measures, by matching. We hypothesized that pre-teen insulin and insulin resistance (IR) would interact with dietary factors to positively predict increases in BMI. Further, we hypothesized that increased insulin and IR, interacting with higher caloric intake during adolescence, would lead to greater increments in BMI in black girls than in white girls.
Patients and Methods
The NGHS was a 10-year, multi-center study of the development of obesity and its effects on CVD risk factors in black and white girls, 5, 9 enrolling 9- and 10-year-old girls. Race was self-declared and as per the NGHS protocol, enrollment was restricted to racially concordant households. The Cincinnati OH clinic recruited girls from public and parochial schools in the inner city, within-city residential neighborhoods, and suburban areas; the Washington, DC clinic recruited girls from a health maintenance organization. Procedures followed were in accordance with the Institutional Review Boards of the two Centers, which approved the study. Signed informed consent was obtained from the girls' parents or guardians and assent from the girls.
Clinical Measures
Obesity was assessed annually according to a standard protocol 5 using the body mass index (BMI = kg/m2), 10-12 Table 1. Beginning in Year 2, waist circumference was measured at the minimum waist as an indicator of fat patterning, Table 1. In addition, bioelectrical impedance was measured using a BIA 101 body composition analyzer (RJL Systems, Detroit, MI). Resistance (R) and reactance (Xc) were measured to the nearest ohm on the right side of the body using a tetrapolar placement of electrodes. 13 Measures of R and (FFMI = FFM/m2), fat mass (FM), fat mass index (FMI = FM/m2), and percent body fat. 14
Table 1.
Median Anthropometric and Clinical Values for 215 Black-White Girls, Pair-matched at Enrollment by Pubertal Stage, BMI and Insulin, at Ages 9-10 (Visit 1), and Ages 18-19 (Visit 10)
| At Ages 9-10 (Visit 1) | At Ages 18-19 (Visit 10) | Changes from Visit 1 to Visit 10 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| White Girls | Black Girls | White Girls | Black Girls | White Girls | Black Girls | |||||
| n | Medi an |
n | Median | n | Media n |
n | Median | Median % change |
Median % change |
|
| Age | 215 | 10.0 | 215 | 10.1 | 215 | 19.0 | 215 | 19.1 | +90% | +90% |
| % Pubertal | 58.6% | Post pubertal | ||||||||
| Height (cm) | 215 | 140 | 215 | 142† | 214 | 165 | 213 | 165 | +17.5% | +16.1%## |
| Weight (kg) | 215 | 34.9 | 215 | 35.5† | 214 | 62.5 | 215 | 61.7 | +82.5% | +82.8% |
| BMI (kg/m2) | 215 | 17.2 | 215 | 17.2 | 214 | 22.7 | 213 | 22.9§ | +32.7% | +37.2%† |
| Waist (cm) | 209 | 62.0 | 214 | 62.0 | 211 | 71.8 | 214 | 71.4 | +18.6% | +17.1% |
| Fat Free Mass (kg) | 214 | 25.6 | 211 | 27.5## | 210 | 44.2 | 208 | 44.1 | +73.8% | +63.8%## |
| Fat Mass (kg) | 214 | 8.8 | 211 | 7.0## | 210 | 18.1 | 208 | 18.8 | +115% | +172%## |
| % Body Fat | 214 | 26.1 | 211 | 20.3## | 210 | 29.9 | 208 | 30.2 | +15.4% | +46.2%## |
| Glucose (mg/dl) | 187 | 93 | 208 | 92† | 157 | 86 | 159 | 87 | −7.1% | −5.2% |
| Insulin (μU/ml) | 215 | 9.3 | 215 | 9.4 | 165 | 7.0 | 161 | 9.0§ | −24.3% | −7.6%§ |
| HOMA IR | 187 | 1.10 | 208 | 1.05 | 155 | 0.80 | 157 | 1.00# | −25.0% | −7.1%† |
p<.05
p<.025
p<.01
p<.001
p<.0001, white girls versus black girls compared by Wilcoxon test
Self-reported parental BMI data was obtained, along with the schoolgirls' semi-quantitative identification of parental obesity by selecting one of 9 outline figure rating drawings15 ranging from very thin to very fat which best represented their parents' physiognomy. The parental figure ratings score15 provided by the schoolgirls correlated with both paternal and maternal self reported BMI (r= .77, p<.0001, r=.73, p<.0001).
Pubertal maturation was visually assessed by a modification of Tanner staging to include areolar development instead of breast development 16 by trained, certified staff.
Serum insulin, without stabilizing polyethylene glycol treatment,17 measured by competitive protein-binding radioimmunoassay, and glucose levels were measured after an overnight fast (≥ 8 hr) using the Michigan Diabetes Research and Training Center (Ann Arbor) in Year 1 (age 9-10) and the Endocrine Lab at the University of Cincinnati/Children's Medical Center in Year 10 (age 18-19), Table 1. Glucose was measured at Year 1 using a hexokinase reagent (Boehringer-Mannheim, Inc. FRG) and at Year 10 using the glucose oxidase method with the Hitachi 704 Chemistry Analyzer (Roche Diagnostics, Indianapolis, IN). Coefficients of variation ranged from 5 to 11% for insulin and 2 to 7% for glucose in Year 1 and were 9% and 4%, respectively in Year 10.
Frozen serum from age 9-10, although stored at −80 degrees C, was not kept for re-measurement at ages 18-19, 9 years later, due to concerns about stability18 over 9 years storage. Feldman and Chapman18 reported decrements in serum insulin of 74% after storage at −20 degrees C for 28 months. Both laboratories used the same competitive protein-binding radioimmunoassay. In statistical analyses where change in insulin was an explanatory variable, we used race-specific Z scores in place of raw insulin levels, to diminish possible laboratory differences in measurements of insulin (Tables 6-8).
Table 6.
Spearman correlations between changes (?) of BMI, waist circumference, fat free mass, fat mass, % of body weight as fat, insulin Z score, glucose and HOMA IR from visit 1 to visit 10
| ?BMI | ?Waist | ?FFM | ?FatM | ?%Fat | ?Insulin Z | ?Glucose | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Black | White | Black | White | Black | White | Black | White | Black | White | Black | White | Black | White | |
| ?Waist | .85# | .81# | ||||||||||||
| ?FFM | .73# | .77# | .67# | .67# | ||||||||||
| ?FatM | .86# | .94# | .75# | .78# | .52# | .76# | ||||||||
| ?%Fat | .58# | .56# | .51# | .48# | .11 | .18† | .74# | .58# | ||||||
| ?Insulin Z | .28§ | .27† | .30# | .27† | .29§ | .31# | .17* | .23† | .18* | .26§ | ||||
| ?Glucose | .25§ | .17* | .22† | .26† | .23† | .22† | .26§ | .15 | .15 | .04 | .25† | .30§ | ||
| ?IR | .31# | .33# | .32# | .33# | .29§ | .33# | .18* | .26† | .20* | .29§ | .996# | .996# | .26† | .32§ |
p<.05
p<.01
p<.001
p<.0001
Table 8.
Significant Predictors for 10-year Change in Percent Body Fat Separately in White and Black Girls Between Ages 9-10 (Visit 1) and Ages 18-19 (Visit 10).
| Response variables Significant explanatory variables |
? | SE(ß) | Partial R-square |
P |
|---|---|---|---|---|
| Percent of body fat increase for White girls (n=162) | ||||
| Baseline BMI | −0.016 | 0.0034 | 13.5% | <0.0001 |
| Change in insulin Z score* % of total calories as protein |
+0.0016 | 0.00063 | 4.5% | 0.012 |
| % of calories as saturated fat | +0.016 | 0.0061 | 3.5% | 0.0094 |
| Father's obesity level | +0.016 | 0.0079 | 2.1% | 0.038 |
| Percent of body fat increase for Black girls (n=148) | ||||
| Change in insulin Z score* % of total calories as protein |
+.0024 | .0011 | 3.6% | .022 |
Regression model by stepwise selection from explanatory variables: age, BMI, Insulin and maturation stage at baseline, parents' obesity level, change in Insulin Z score over 10 year follow-up, percent of calories from protein, from fat, from carbohydrate, from saturated fat (mean of interviews) during 10 year follow-up, and interaction terms (nutrients* baseline Insulin, nutrients* change in Insulin Z score).
We used fasting insulin as the indicator of insulin resistance based on reports by Huang et al 19 and Schwartz et al. 20 Huang et al 19 studied HOMA IR in white and African-American children and concluded that the utility of the HOMA equation in predicting insulin sensitivity was similar to fasting insulin alone. Recently, Schwartz et al.20 compared indices of insulin resistance by the euglycemic–hyperinsulinemic clamp and alternative methods, reporting, “surrogate measures are only modestly correlated with the clamp measurements of insulin sensitivity and do not offer any advantage over fasting insulin.”
Dietary Data
In 8 of the 10 yearly follow-up visits (Years 1, 2, 3, 4, 5, 7, 8, and 10), a three-day dietary diary was completed by the girls and retrieved by Registered Dietitians. The data were entered and summarized for analyses using the most current version of the Nutrition Data System for Research software developed for the Nutrition Coordinating Center (NCC), University of Minnesota, Minneapolis, MN, for calculation of total calories, and calories from protein, fat, saturated fat, and carbohydrate, Table 2, Figure 1. The mean (± SD) number of 3-day diet diary records available in the analysis sample was 6.9 ±1.3.
Table 2.
Median Anthropometric and Clinical Values for 172 Black-White Girls, Pair-matched at Enrollment by Pubertal Stage, fat mass and Insulin, at Ages 9-10 (Visit 1), and Ages 18-19 (Visit 10)
| At Ages 9-10 (Visit 1) | At Ages 18-19 (Visit 10) | Changes from Visit 1 to Visit 10 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| White Girls | Black Girls | White Girls | Black Girls | White Girls | Black Girls | |||||
| n | Medi an |
n | Median | n | Media n |
n | Median | Median % change |
Median % change |
|
| Weight (kg) | 172 | 32.6 | 172 | 36.3# | 172 | 59.8 | 172 | 64.9§ | +84.7% | +79.7% |
| BMI (kg/m2) | 172 | 16.5 | 171 | 17.8# | 172 | 22.3 | 170 | 24.1# | +33.2% | +35.3%* |
| Waist (cm) | 167 | 60.5 | 214 | 62.3# | 170 | 70.2 | 171 | 73.2§ | +18.0% | +16.9% |
| Fat Free Mass (kg) | 172 | 24.7 | 172 | 27.7## | 172 | 43.0 | 172 | 44.9 | +77.2% | +63.1%§ |
| Fat Mass (kg) | 172 | 7.7 | 172 | 7.7 | 172 | 16.6 | 172 | 20.7§ | +119% | +151%## |
| % Body Fat | 172 | 23.9 | 172 | 22.6§ | 172 | 28.9 | 172 | 31.8## | +18.1% | +36.2%## |
| Glucose (mg/dl) | 143 | 93 | 168 | 92 | 127 | 86 | 139 | 86 | −6.3% | −5.9% |
| Insulin (μU/ml) | 172 | 9.4 | 172 | 9.2 | 126 | 7.0 | 139 | 9.0§ | −26.8% | +1.0%* |
| HOMA IR | 143 | 1.00 | 168 | 1.00 | 121 | 0.80 | 136 | 1.05§ | −27.3% | 0.0% |
p<.05
p<.025
p<.01
p<.001
p<.0001, white girls versus black girls compared by Wilcoxon test
Figure 1.
Median and interquartile range for daily caloric intake in black and white girls from age 10 through age 19; p values from Wilcoxon tests.
Statistical Methods
All analyses were performed using SAS Version 9.1.
Because the radioimmunoassays for insulin for Years 1 and 10 were performed in different laboratories, to avoid possible analytical bias, we carried out analyses involving changes in insulin, using the changes in race specific Z- scores for these values. Z scores were calculated separately for each race, at enrollment and at year 10, using race-visit-specific means and standard deviations (SD), as follows: Z-score=[insulin-mean insulin]/SD.
Studies were done after matching by age 9-10 pubertal stage, BMI, and insulin (215 pairs, Tables 1,3-8) and matching by age 9-10 pubertal stage, insulin, and fat mass (172 pairs, Table 2).
Table 3.
Median Total Caloric Intake and Percentage of Calories from Protein, Fat, Carbohydrate, and Saturate Fat In White and Black Girls, Pair-matched at Enrollment by Pubertal Stage, BMI and Insulin, at Ages 9, 10 (Visit 1), and Ages 18, 19 (Visit 10 )
| At Ages 9-10 (Visit 1) | At Ages 18-19 (Visit 10) | Changes from Visit 1 to Visit 10 | ||||
|---|---|---|---|---|---|---|
| White Girls (n=209) |
Black Girls (n=178) |
White Girls (n=152) |
Black Girls (n=178) |
White Girls (n=149) |
Black Girls (n=148) |
|
| Median | Median | Median | Median | Median % change |
Median % change |
|
| Total Cal | 1777 | 1783 | 1742 | 1941# | −3% | +7%§ |
| % Protein | 14% | 14% | 14% | 14% | −2% | −5% |
| % Fat | 34% | 36%* | 29% | 35%## | −18% | −4%## |
| % Carb | 53% | 52% | 58% | 52%## | +10% | +4%# |
| % Sat Fat | 13% | 13% | 10% | 12%## | −28% | −9%## |
p<.05
p<.025
p<.01
p<.001
p<.0001, white girls versus black girls compared by Wilcoxon test
Wilcoxon non-parametric tests were used to compare differences in study variables between races at baseline (ages 9-10) and at follow-up (ages 18-19), and percent of changes, Tables 1-3. Change in nutrient intake, weight, BMI, waist, fat free mass, fat mass, % body fat, glucose, insulin, and HOMA IR were calculated as values at ages 18-19 minus values at ages 9-10 without consideration of fluctuations in the intervening 10 years (Tables 1-3).
Wilcoxon non-parametric tests were used to compare black-white differences in total caloric intake/day throughout the study (Figure 1).
Separately by race, Spearman correlations between glucose, insulin or HOMA IR with measures of BMI, waist circumference, fat free mass, fat mass, and percent of body weight as fat were calculated for visit 1 (ages 9-10), and visit 10 (ages 18-19), Table 4. None of the correlations between insulin or IR with BMI were curvilinear. Spearman correlations were also calculated between BMI, fat mass, insulin, HOMA IR and glucose at ages 9-10 with the same variables at ages 18-19 (Table 5). Spearman correlations were calculated between changes in body mass, insulin, and glucose from ages 9-10 to 18-19 (Table 6). Spearman correlations were calculated between parental BMI and parental obesity as judged by their children using figure rating drawings.15
Table 4.
Spearman correlations at ages 9-10 (Visit 1) and at ages 18-19 (Visit 10) between glucose, insulin and HOMA IR with measures of BMI, waist circumference, fat free mass, fat mass, and % of body weight as fat
| BMI | Waist | Fat free mass | Fat mass | % body weight as fat | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Black | White | Black | White | Black | White | Black | White | Black | White | ||
| Glucose | Visit 1 | .21† | .19* | .22§ | .26§ | .16* | .11 | .16* | .16* | .10 | .16* |
| Visit 10 | .38# | .14 | .38# | .18* | .33# | .16* | .36# | .12 | .33# | .07 | |
| Insulin | Visit 1 | .45# | .47# | .38# | .43# | .41# | .37# | .33# | .43# | .23§ | .37# |
| Visit 10 | .45# | .46# | .47# | .48# | .36# | .43# | .43# | .44# | .38# | .41# | |
| HOMA IR | Visit 1 | .47# | .49# | .40# | .46# | .43# | .41# | .34# | .45# | .24§ | .39# |
| Visit 10 | .45# | .45# | .47# | .48# | .35# | .42# | .43# | .43# | .39# | .40# | |
p<.05
p<.01
p<.001
p<.0001
Table 5.
Spearman correlations between age 9-10 BMI, fat mass, insulin, HOMA IR and glucose with age 18-19 BMI, fat mass, insulin, HOMA IR and glucose
| Age 18-19 BMI | Fat Mass | Insulin | HOMA IR | Glucose | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Black | White | Black | White | Black | White | Black | White | Black | White | ||
| Age 9-10 | |||||||||||
| BMI | .71# | .72# | .62# | .64# | .27§ | .29§ | .27§ | .28§ | .26§ | .09 | |
| Fat mass | .57# | .64# | .62# | .68# | .24† | .27§ | .24† | .24† | .20† | .07 | |
| Insulin | .24§ | .32# | .19† | .25§ | .15 | .25§ | .16† | .24† | .18* | .004 | |
| HOMA IR | .25§ | .31# | .21† | .24§ | .15 | .23† | .15 | .21* | .18* | −.01 | |
| Glucose | .24§ | .06 | .23† | −.0007 | .09 | .05 | .10 | .07 | .32# | .16* | |
p<.05
p<.01
p<.001
p<.0001
To explain changes in BMI, after variance-stabilizing transformation (arc tangent transformation), regression analyses were carried out for 215 pairs using a stepwise selection procedure, Table 7. Explanatory variables included race (white=1, black=2), age, BMI, insulin, maturation stage at age 9-10, and parents' obesity level. Additional explanatory variables included the 10-year change in insulin, mean total calorie intake over 10-year follow-up, and product terms of age 9-10 insulin with mean total calorie intake, 10-year change in insulin with mean total calorie intake, and race interaction with these two terms (Table 7). After arc tangent transformation, similar regressions were done for 10- year change in waist circumference (using waist circumference at age 10-11, the earliest waist measure available, and the last available waist measure), Table 7. Results were similar whether insulin or HOMA IR was used in the analyses, so only results based on insulin are presented.
Table 7.
Significant Predictors for 10-year Change in BMI and Waist Circumference in Black and White Girls from Ages 9-10 (Visit 1) to Ages18-19 (Visit 10).
| Response variables Significant explanatory variables |
ß | SE(ß) | Partial R-square |
P |
|---|---|---|---|---|
| BMI increase (n=325) | ||||
| Baseline BMI | +0.042 | 0.0084 | 8.2% | <0.0001 |
| Change in Insulin Z score | +0.19 | 0.022 | 11.1% | <0.0001 |
| Baseline Insulin * total calories * race | +0.0000055 | 0.0000011 | 5.8% | <0.0001 |
| Waist increase (n=320) | ||||
| Change in Insulin Z score | +0.27 | 0.034 | 8.5% | <0.0001 |
| Baseline Insulin | +0.032 | 0.0057 | 8.1% | <0.0001 |
Regression model by stepwise selection from explanatory variables: race (white=1, black=2), age, BMI, Insulin, and maturation stage at baseline, parents' obesity level, change in Insulin Z score over 10 year follow-up, total calorie intake (mean of interviews) during 10 year follow-up and interaction terms (total calorie intake * baseline Insulin, total calorie intake * change in Insulin Z score, total calorie intake * baseline Insulin* race, total calorie intake * change in Insulin Z score * race).
After arc tangent transformation, regression models were also run with the increase in percent body fat as the dependent variable and age, BMI, insulin, and maturation stage at baseline, parents' obesity level, change in insulin over 10-year follow-up, percentage of calories from protein, from fat, from carbohydrate, from saturated fat (mean data over 10-year follow-up), and interaction terms (nutrients*baseline insulin, nutrients*change in insulin) as explanatory variables, Table 8. These models were run separately for race (Table 8), after the initial pooled-race model indicated that race was the only significant explanatory variable for increase in percent of body fat.
RESULTS
The 10-year follow-up of NGHS girls included 80% of eligible girls. The 80% of former schoolchildren studied 10 years after their initial assessment did not differ (p >0.05) from the 20% without follow-up by age, BMI, glucose, or waist circumference. After covariance adjusting for age and race, at ages 9-10, participants did not differ (p>0.05) from non-participants by fasting serum insulin. Hence, the NGHS cohort did not reflect a selection bias at the time of follow-up. At ages 9-10 (Year 1), 518 white and 554 black girls enrolled in the Cincinnati and Washington DC clinics had fasting blood drawn for measurement of insulin and glucose concentrations. Although there were more black than white pubertal girls at ages 9-10 (408 of 544 black girls vs 193 of 507 white girls), all black-white comparisons were made after matching by maturation stage, BMI (or fat mass), and insulin at ages 9-10 (Tables 1,2).
Insulin values did not follow normal distributions in either race group.
Analyses Based on BMI Matching
The analyses based on matching by age 9-10 BMI, maturation stage, and insulin were based on 215 matched white-black pairs (Table 1). Of these participants, 165 white girls and 161 black girls had insulin measured at visit 10, ages 18-19 (Table 1). As determined by the matching procedure, distributions of BMI did not differ at ages 9-10, Table 1. Nevertheless, white girls had higher fat mass (8.8 vs 7.0 kg, p<.0001) and percent body fat (26.1% vs 20.3%, p<.0001), while black girls had higher median fat-free mass (27.5 vs 25.6 kg, p<.0001). At ages 18-19, black girls had higher BMI (22.9 vs 22.7, p=.007) and marginally higher fat mass (18.8 vs 18.1 kg, p= .059).
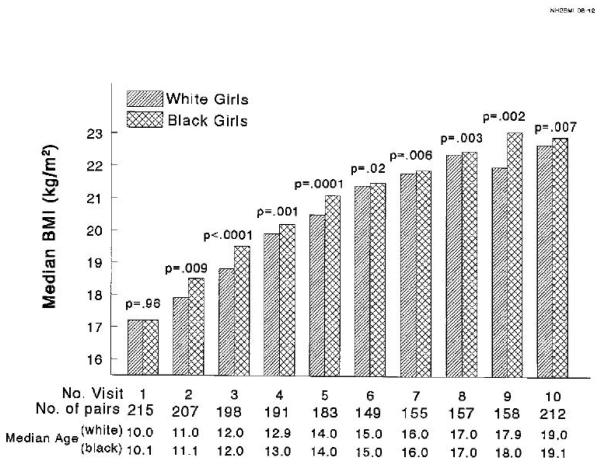
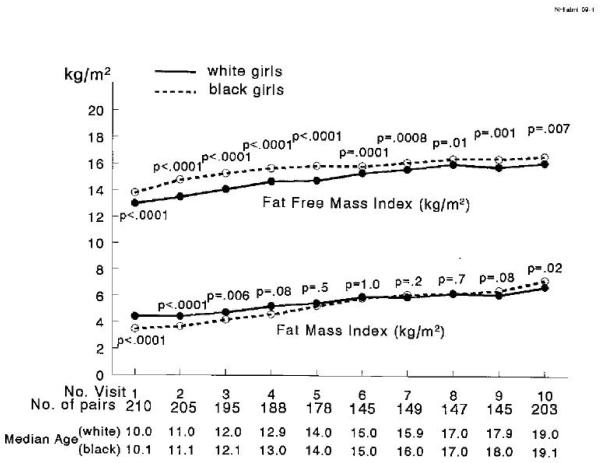
From median age 11 to 19, black girls had higher BMI, Figure 2. Black girls also had a higher fat-free mass index (kg/m2) from age 10 through 19, Figure 3. The fat mass index (fat mass in kg/m2) was higher in white girls from age 10 through 12, not significantly different from 13 to 18, and higher in black girls at age 19 (Figure 3). Percent body fat was higher in white girls from age 10 to 14 and not significantly different from age 15 to 19 (Figure 4). Summarizing the changes in body composition from age 10 to 19, black girls had a greater median percentage increase in BMI (37.2% vs 32.7%, p =.01), in fat mass (172% vs 115%, p<.0001), and in percent body fat (46.2% vs 15.4%, p<.0001) than white girls, Table 1.
Figure 2.
Median BMI (kg/m2) at each visit in black versus white girls, ages 10 to 19, with black and white girls matched at age 10 by pubertal status, BMI, and insulin; p values from paired Wilcoxon tests.
Figure 3.
Median fat free mass index (kg/m2) and fat mass index (kg/m2) at each visit in black versus white girls, ages 10 to 19, with black and white girls matched at age 10 by pubertal status, BMI, and insulin; p values from paired Wilcoxon tests.
Figure 4.
Median percent body fat at each visit in black versus white girls, ages 10 to 19, with black and white girls matched at age 10 by pubertal status, BMI, and insulin; p values from paired Wilcoxon tests.
By matching, black and white girls in these analyses did not differ for fasting serum insulin at ages 9-10, but black girls had higher median insulin at ages 15-16 (11.3 vs 9.7 μU/ml, p = 0.001) and at ages 18-19 (9.0 vs 7.0 μU/ml, p = 0.002), Table 1. The decrease in insulin accompanying the completion of puberty was less in black than white girls ( −7.6 % vs −24.3%, p=.0017) (Table 1).
Black and white girls consumed a similar number of calories per day (1783 vs 1777 kcal) at ages 9-10, but black girls consumed more calories at ages 18-19 (1941 vs 1742 kcal, p =.0005), Table 3. Thus, caloric intake decreased 3 percent in white girls, but increased 7 percent in black girls, Table 3. In both black and white girls, caloric intake rose with age through age 12, Figure 1. The divergence in caloric intake first occurred at age 13 and was present at every dietary evaluation thereafter with white girls ingesting fewer calories over time and black girls ingesting more calories over time, Figure 1.
Black girls consumed a larger percentage of calories as fat at both ages 9-10 (36 vs 34%, p =.027) and ages 18-19 (35 vs 29%, p <0.0001), Table 3. During adolescence, fat consumption decreased in both groups, but decreased more in white than black girls (18 vs 4%, p <.0001), Table 3. Consumption of saturated fats was less in white girls at ages 18-19 (10 vs 12 %, p < 0.0001). White girls had marginally higher consumption of carbohydrates at baseline (53 vs 52% p = 0.061) but higher consumption at ages 18-19 (58 vs 52%, p <0.0001), Table 3. Thus, the changes in caloric intake and in the pattern of fat, saturated fat and carbohydrate consumption as a percent of calories during adolescence differed significantly between black and white girls, Table 3.
At ages 9-10 and 18-19, glucose, and insulin were, for the most part, all significantly correlated with BMI, waist circumference, fat free mass, fat mass, and percent body fat (Table 4). The relationships between insulin and BMI and between insulin and other measures of body mass in Table 4 were linear, not curvilinear.
Analyses Based on Fat Mass Matching
After matching black and white girls by fat mass, maturation stage, and insulin at visit 1 (ages 9-10) we studied 172 white-black pairs of girls at ages 9-10 and 18-19, Table 2. Of these 172 pairs, 126 white and 139 black girls had insulin measured at visit 10 (Table 2). After matching for fat mass, pubertal stage, and insulin, at age 9-10 black girls were heavier, had higher BMI, and greater fat free mass, Table 2. By ages 18-19, black girls continued to have higher weight, BMI, had accrued higher fat mass, and had a higher percent body fat (Table 2).
Longitudinal BMI-Insulin Associations
As summarized in Table 5, BMI at ages 9-10 was closely correlated with BMI and fat mass at ages 18-19, and was correlated with insulin, HOMA IR, and glucose (Table 5). Age 9-10 insulin was positively correlated with BMI and fat mass at ages 18-19 (Table 5).
Ten-year changes in insulin Z scores were significantly correlated with 10-year changes in BMI, waist, fat free mass, fat mass, and percent body weight as fat (Table 6).
Ten year change in BMI was significantly associated with age 9-10 BMI, with the 10-year change in insulin Z score, and with a three- way interaction term of age 9-10 insulin, average 10 year caloric intake, and race (Table 7). Twenty-five percent of the variance in BMI increase during the 10 year follow-up could be accounted for by change in insulin Z score, baseline BMI, and the 3 way interaction of age 10 insulin, total calories over 10 years, and race (Table 7). The interaction of caloric intake during adolescence and age 9-10 insulin had a greater effect on the increase in BMI in black girls than white girls (Table 7), superimposed on the significantly greater intake of calories by black girls (Table 3, Figure 1). However there was no significant threshold effect for BMI; girls with top quintile BMI at age 9-10 did not have larger increases in BMI over the 10 years of follow up than the girls in the lower quintiles (data not shown)
The 10-year increase in waist circumference was associated with the 10 year change in insulin Z score (partial R2 = 8.5%, p <.0001) and with age 9-10 insulin (partial R2 = 8.1%), Table 7. The regression model explained 16.6% of the variance in waist circumference changes over the 10-year follow-up (Table 7).
In analyzing factors explaining the increase in percent body fat, in white girls, the change in percent body fat was associated with age 9-10 BMI, with the interaction of the change in insulin Z score with protein as a percent of calories, with the percent of calories as saturated fat, and with paternal anthropometric obesity score,15 Table 8. These explanatory variables accounted for 23.6% of the variance in the increase in percent body fat in white girls, Table 8. To explain the negative coefficient associated with age 9-10 BMI, we examined the mean BMI at Years 1 and 10 in girls who were pubertal versus pre-pubertal at baseline, noting the mean change in BMI in the subgroups. At age 9-10, pubertal girls had higher BMI (18.4 vs 16.2 kg/m2, p < 0.0001) and higher percent body fat (26.6 v 24.2%, p = 0.003), but at Year 10 (ages 18-19) there was no difference in percent body fat (30.3 vs 28.6 %, p = 0.14). Thus, the girls who were pubertal at enrollment had higher baseline BMI and a smaller increase in percent body fat, while girls who were pre-pubertal at enrollment had more growth yet to come.
In black girls, similar to white girls, the change in percent body fat was associated with the interaction of the change in insulin Z score with protein as a percent of calories, Table 8.
DISCUSSION
The central, novel findings in our 10-year prospective, longitudinal study of the development of obesity in black and white girls, matched by BMI (or fat mass), maturation status, and fasting serum insulin at ages 9-10, were as follows:
1). Higher age 9-10 insulin interacted with higher caloric intake to produce a greater increase in BMI, and this effect was greater in black than white girls. We speculate that this insulin*caloric intake*race interaction is central to an intrinsic black-white metabolic difference from ages 9-10 to 18-19 in which black girls gain more fat than white girls through adolescence.
2). From the BMI matching data, despite equal BMI at baseline, and a lower fat mass at baseline, black girls had a higher BMI at age 18-19 than white girls. From the fat mass matching data, despite equal fat mass at baseline and a lower percent body fat, black girls had higher fat mass and higher percent body fat at age 18-19. This implies that when matched “metabolically” at baseline, black girls gain more fat mass than white girls through adolescence. Along with the BMI matching data, this finding suggests that there is an intrinsic black-white metabolic difference from ages 9-10 to 18-19 in which black girls gain more fat than white girls through adolescence. Accretion of more fat mass and higher BMI over a 10-year period is consistent with higher caloric intake at age from age 13 to 19 in black girls
3) At ages 9-10, black and white girls had different body composition despite their similar (matched) BMI, with black girls taller and heaver, with greater fat free mass, and white girls with greater fat mass and percent body fat.
4) Black girls had greater 10-year increases in BMI and smaller post pubertal decrements in insulin, and so had significantly greater BMI and insulin at age 18-19.
5) Ten-year change in insulin Z score was a positive predictor of increases in BMI and waist circumference.
These results suggest that reducing insulin levels and moderating caloric intake from ages 9-10 to 18-19 should be beneficial for overweight girls with particular relevance in black girls. Since age 9-10 BMI was a significant predictor of 10- year increments in BMI, increased BMI at ages 9-10 should alert the clinician to check insulin (and IR) levels and discuss optimal diets and exercise programs, particularly in black girls.
The finding of a positive role for hyperinsulinemia interacting with 10-year caloric intake to increase weight gain is similar to reports by Odeleye 7 in Pima Indian children and Mosca et al 8 in adults, but conflicts with reports by Travers et al, 21 Maffeis et al. 22 and Hoffman et al 23 who found that insulin resistance leads to less weight gain and less body fatness. More research is needed to determine why insulin resistance appears to have such different effects in these different study populations.
In our current report, congruent with results from previous tracking studies, 24-26 pre-teen BMI was a major, independent predictor of change in BMI at age 18-19 in girls.
Our study has the following limitations. First, participants were not a random selection of the US, as in NHANES, 27 but came from a biracial school population and an HMO program. Thus, the data, while suggestive, need to be confirmed and cannot be extrapolated to all adolescent girls. Second, MRI 28 or CT visceral fat measurements to estimate intra- and extra- visceral fat measurements were not done. Third, three-day dietary diary data may less optimally reflect actual dietary intake than a 7-day record. 29 Fourth, the insulin levels at ages 9-10 and at ages 18-19 were performed in two different laboratories and though each laboratory used competitive protein-binding radioimmunoassays, this could represent a potential problem because the same insulin assay in different laboratories may give different results. 30 However, in the regression models that included change in insulin, Z-score transformation of insulin or IR provided nearly identical results as the untransformed data. This suggests that the observations of the effects of changes in insulin were independent of putative assay differences. Fifth, We used fasting insulin and glucose values to estimate insulin resistance, rather than the more accurate euglycemic clamp. 31
If elevated insulin is documented at age 9-10, accompanied by obesity, and if it increases during adolescence, then steps to restrict diet, increase physical activity, and decrease insulin and IR, may, speculatively, be warranted. An intensive nutrition program combined with strength training failed to produce changes in insulin sensitivity or body composition in obese Latino adolescents.32 In obese adolescents in Tennessee, whereas lifestyle changes did not produce significant weight loss, metformin plus lifestyle intervention resulted in significant weight loss.33 In a randomized, double-blind, placebo controlled trial in adolescents with insulin resistance, diet-exercise modification did not lead to weight loss, but addition of metformin increased weight loss in girls.34 In Australian obese children, in a randomized, double-blind, cross-over trial, metformin therapy resulted in significant improvement in body composition and fasting insulin.35 In normoglycemic morbidly obese adolescents, a randomized double blind, placebo-controlled trial revealed that combined metformin treatment and low-calorie diet had a significant anti-obesity effect in hyperinsulinemic obese adolescents compared to low calorie diet alone. 36 Freemark and Bursey 37 treated 29 obese black and white 12-19 year old adolescents with diet and metformin, successfully reducing both insulin and weight. Glueck et al 38, 39 have reported that the insulin sensitizer, metformin, when combined with diet, reduces insulin resistance and weight in obese, hyperinsulinemic adolescent girls with polycystic ovary syndrome (PCOS). Arslanian et al 40 reported that 1700 mg/day metformin treatment in obese adolescents with PCOS and impaired glucose tolerance was beneficial in improving glucose tolerance and insulin sensitivity, in lowering hyperinsulinemia, and in reducing elevated androgen levels.
We speculate that diet, exercise, and, if needed, metformin, have promise in primary prevention of metabolic syndrome if initiated in hyperinsulinemic obese adolescents. We speculate that initiating these interventions in a conservative, stepped fashion might reduce the development of obesity and development of impaired fasting glucose and type 2 diabetes, leading to an ultimate goal of primary prevention of both progressive obesity and type 2 diabetes. In our current study, the significant association between the interaction of age 9-10 elevated insulin and caloric intake for increases in BMI highlights insulin and diet as major modifiable targets in the prevention of or reduction of obesity, particularly in black girls.
Acknowledgments
Research was supported in part by NIH-NHLBI Contract HC-55023-26 and HL 48941 and by the Lipoprotein Research Fund of the Jewish Hospital of Cincinnati
Abbreviations
- BMI
body mass index
- NGHS
National Heart, Lung, and Blood Institute Growth and Health Study
- FFM
fat free mass
- FFMI
fat free mass index
- FM
fat mass
- FMI
fat mass index
- CVD
cardiovascular disease
- R
resistance
- Xc
reactance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interest for any authors. No honorarium, grant, or other form of payment was given to anyone to produce the manuscript.
REFERENCES
- 1.Williamson DF, Kahn HS, Byers T. The 10-y incidence of obesity and major weight gain in black and white US women aged 30-55 y. Am J Clin Nutr. 1991;53:1515S–8S. doi: 10.1093/ajcn/53.6.1515S. [DOI] [PubMed] [Google Scholar]
- 2.Troiano RP, Flegal KM, Kuczmarski RJ, Campbell SM, Johnson CL. Overweight prevalence and trends for children and adolescents. The National Health and Nutrition Examination Surveys, 1963 to 1991. Arch Pediatr Adolesc Med. 1995;149:1085–91. doi: 10.1001/archpedi.1995.02170230039005. [DOI] [PubMed] [Google Scholar]
- 3.Morrison JA, James FW, Sprecher DL, Khoury PR, Daniels SR. Sex and race differences in cardiovascular disease risk factor changes in schoolchildren, 1975-1990: the Princeton School Study. Am J Public Health. 1999;89:1708–14. doi: 10.2105/ajph.89.11.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrison JA, Sprecher DL, Barton BA, Waclawiw MA, Daniels SR. Overweight, fat patterning, and cardiovascular disease risk factors in black and white girls: The National Heart, Lung, and Blood Institute Growth and Health Study. J Pediatr. 1999;135:458–64. doi: 10.1016/s0022-3476(99)70168-x. [DOI] [PubMed] [Google Scholar]
- 5.Obesity and cardiovascular disease risk factors in black and white girls: the NHLBI Growth and Health Study. Am J Public Health. 1992;82:1613–20. doi: 10.2105/ajph.82.12.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Svec F, Nastasi K, Hilton C, Bao W, Srinivasan SR, Berenson GS. Black-white contrasts in insulin levels during pubertal development. The Bogalusa Heart Study. Diabetes. 1992;41:313–7. doi: 10.2337/diab.41.3.313. [DOI] [PubMed] [Google Scholar]
- 7.Odeleye OE, de Courten M, Pettitt DJ, Ravussin E. Fasting hyperinsulinemia is a predictor of increased body weight gain and obesity in Pima Indian children. Diabetes. 1997;46:1341–5. doi: 10.2337/diab.46.8.1341. [DOI] [PubMed] [Google Scholar]
- 8.Mosca CL, Marshall JA, Grunwald GK, Cornier MA, Baxter J. Insulin resistance as a modifier of the relationship between dietary fat intake and weight gain. Int J Obes Relat Metab Disord. 2004;28:803–12. doi: 10.1038/sj.ijo.0802621. [DOI] [PubMed] [Google Scholar]
- 9.Klein DJ, Aronson Friedman L, Harlan WR, et al. Obesity and the development of insulin resistance and impaired fasting glucose in black and white adolescent girls: a longitudinal study. Diabetes Care. 2004;27:378–83. doi: 10.2337/diacare.27.2.378. [DOI] [PubMed] [Google Scholar]
- 10.Cole T. Weight-stature indices to measure underweight, overweight, and obesity. In: Hines JH, editor. Anthropometric assessment of nutritional status. Wiley-Liss; New York: 1991. pp. 83–111. [Google Scholar]
- 11.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002:1–190. [PubMed] [Google Scholar]
- 12.Barlow SE, Dietz WH. Obesity evaluation and treatment: Expert Committee recommendations. The Maternal and Child Health Bureau, Health Resources and Services Administration and the Department of Health and Human Services. Pediatrics. 1998;102:E29. doi: 10.1542/peds.102.3.e29. [DOI] [PubMed] [Google Scholar]
- 13.Lukaski HC. Methods for the assessment of human body composition: traditional and new. Am J Clin Nutr. 1987;46:537–56. doi: 10.1093/ajcn/46.4.537. [DOI] [PubMed] [Google Scholar]
- 14.Guo SS, Khoury PR, Sprecker B, Heubi J, Chumlea WC, Siervogel RM, Morrison JA. Prediction of fat free mass in black and white pre-adolescent and adolescent girls from anthropometry and impedance. Am J of Human Biology. 1993;5:735–45. doi: 10.1002/ajhb.1310050617. [DOI] [PubMed] [Google Scholar]
- 15.Sorensen TI, Stunkard AJ, Teasdale TW, Higgins MW. The accuracy of reports of weight: children's recall of their parents' weights 15 years earlier. Int J Obes. 1983;7:115–22. [PubMed] [Google Scholar]
- 16.Tanner JM. Normal growth and techniques of growth assessment. Clin Endocrinol Metab. 1986;15:411–51. doi: 10.1016/s0300-595x(86)80005-6. [DOI] [PubMed] [Google Scholar]
- 17.Arnqvist H, Olsson PO, von Schenck H. Free and total insulin as determined after precipitation with polyethylene glycol: analytical characteristics and effects of sample handling and storage. Clin Chem. 1987;33:93–6. [PubMed] [Google Scholar]
- 18.Feldman JM, Chapman BA. Radioimmunoassay of insulin in serum and plasma. Clin Chem. 1973;19:1250–4. [PubMed] [Google Scholar]
- 19.Huang TT, Johnson MS, Goran MI. Development of a prediction equation for insulin sensitivity from anthropometry and fasting insulin in prepubertal and early pubertal children. Diabetes Care. 2002;25:1203–10. doi: 10.2337/diacare.25.7.1203. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz B, Jacobs DR, Jr., Moran A, Steinberger J, Hong CP, Sinaiko AR. Measurement of insulin sensitivity in children: comparison between the euglycemic-hyperinsulinemic clamp and surrogate measures. Diabetes Care. 2008;31:783–8. doi: 10.2337/dc07-1376. [DOI] [PubMed] [Google Scholar]
- 21.Travers SH, Jeffers BW, Eckel RH. Insulin resistance during puberty and future fat accumulation. J Clin Endocrinol Metab. 2002;87:3814–8. doi: 10.1210/jcem.87.8.8765. [DOI] [PubMed] [Google Scholar]
- 22.Maffeis C, Moghetti P, Grezzani A, Clementi M, Gaudino R, Tato L. Insulin resistance and the persistence of obesity from childhood into adulthood. J Clin Endocrinol Metab. 2002;87:71–6. doi: 10.1210/jcem.87.1.8130. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman RP, Stumbo PJ, Janz KF, Nielsen DH. Altered insulin resistance is associated with increased dietary weight loss in obese children. Horm Res. 1995;44:17–22. doi: 10.1159/000184584. [DOI] [PubMed] [Google Scholar]
- 24.Freedman DS, Khan LK, Serdula MK, Ogden CL, Dietz WH. Racial and ethnic differences in secular trends for childhood BMI, weight, and height. Obesity (Silver Spring) 2006;14:301–8. doi: 10.1038/oby.2006.39. [DOI] [PubMed] [Google Scholar]
- 25.Deshmukh-Taskar P, Nicklas TA, Morales M, Yang SJ, Zakeri I, Berenson GS. Tracking of overweight status from childhood to young adulthood: the Bogalusa Heart Study. Eur J Clin Nutr. 2006;60:48–57. doi: 10.1038/sj.ejcn.1602266. [DOI] [PubMed] [Google Scholar]
- 26.Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. Racial differences in the tracking of childhood BMI to adulthood. Obes Res. 2005;13:928–35. doi: 10.1038/oby.2005.107. [DOI] [PubMed] [Google Scholar]
- 27.Eddy D, Schlessinger L, Kahn R, Peskin B, Schiebinger R. The Relationship between Insulin Resistance and Related Metabolic Variables to Coronary Artery Disease: A Mathematical Analysis. Diabetes Care. 2008 doi: 10.2337/dc08-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegel MJ, Hildebolt CF, Bae KT, Hong C, White NH. Total and intraabdominal fat distribution in preadolescents and adolescents: measurement with MR imaging. Radiology. 2007;242:846–56. doi: 10.1148/radiol.2423060111. [DOI] [PubMed] [Google Scholar]
- 29.Bingham SA, Gill C, Welch A, et al. Validation of dietary assessment methods in the UK arm of EPIC using weighed records, and 24-hour urinary nitrogen and potassium and serum vitamin C and carotenoids as biomarkers. Int J Epidemiol. 1997;26(Suppl 1):S137–51. doi: 10.1093/ije/26.suppl_1.s137. [DOI] [PubMed] [Google Scholar]
- 30.Robbins DC, Andersen L, Bowsher R, et al. Report of the American Diabetes Association's Task Force on standardization of the insulin assay. Diabetes. 1996;45:242–56. doi: 10.2337/diab.45.2.242. [DOI] [PubMed] [Google Scholar]
- 31.Ferrannini E, Mari A. How to measure insulin sensitivity. J Hypertens. 1998;16:895–906. doi: 10.1097/00004872-199816070-00001. [DOI] [PubMed] [Google Scholar]
- 32.Davis JN, Kelly LA, Lane CJ, et al. Randomized Control Trial to Improve Adiposity and Insulin Resistance in Overweight Latino Adolescents. Obesity (Silver Spring) 2009 doi: 10.1038/oby.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harden KA, Cowan PA, Velasquez-Mieyer P, Patton SB. Effects of lifestyle intervention and metformin on weight management and markers of metabolic syndrome in obese adolescents. J Am Acad Nurse Pract. 2007;19:368–77. doi: 10.1111/j.1745-7599.2007.00236.x. [DOI] [PubMed] [Google Scholar]
- 34.Love-Osborne K, Sheeder J, Zeitler P. Addition of metformin to a lifestyle modification program in adolescents with insulin resistance. J Pediatr. 2008;152:817–22. doi: 10.1016/j.jpeds.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srinivasan S, Ambler GR, Baur LA, et al. Randomized, controlled trial of metformin for obesity and insulin resistance in children and adolescents: improvement in body composition and fasting insulin. J Clin Endocrinol Metab. 2006;91:2074–80. doi: 10.1210/jc.2006-0241. [DOI] [PubMed] [Google Scholar]
- 36.Kay JP, Alemzadeh R, Langley G, D'Angelo L, Smith P, Holshouser S. Beneficial effects of metformin in normoglycemic morbidly obese adolescents. Metabolism. 2001;50:1457–61. doi: 10.1053/meta.2001.28078. [DOI] [PubMed] [Google Scholar]
- 37.Freemark M, Bursey D. The effects of metformin on body mass index and glucose tolerance in obese adolescents with fasting hyperinsulinemia and a family history of type 2 diabetes. Pediatrics. 2001;107:E55. doi: 10.1542/peds.107.4.e55. [DOI] [PubMed] [Google Scholar]
- 38.Glueck CJ, Wang P, Fontaine R, Tracy T, Sieve-Smith L. Metformin to restore normal menses in oligo-amenorrheic teenage girls with polycystic ovary syndrome (PCOS) J Adolesc Health. 2001;29:160–9. doi: 10.1016/s1054-139x(01)00202-6. [DOI] [PubMed] [Google Scholar]
- 39.Glueck CJ, Aregawi D, Winiarska M, et al. Metformin-diet ameliorates coronary heart disease risk factors and facilitates resumption of regular menses in adolescents with polycystic ovary syndrome. J Pediatr Endocrinol Metab. 2006;19:831–42. doi: 10.1515/jpem.2006.19.6.831. [DOI] [PubMed] [Google Scholar]
- 40.Arslanian SA, Lewy V, Danadian K, Saad R. Metformin therapy in obese adolescents with polycystic ovary syndrome and impaired glucose tolerance: amelioration of exaggerated adrenal response to adrenocorticotropin with reduction of insulinemia/insulin resistance. J Clin Endocrinol Metab. 2002;87:1555–9. doi: 10.1210/jcem.87.4.8398. [DOI] [PubMed] [Google Scholar]