Abstract
The neurotransmitter dopamine (DA) is an important molecule bridging the nervous and immune systems. DA through autocrine/paracrine manner modulates the functions of immune effector cells by acting through its receptors present in these cells. DA also has unique and opposite effects on T cell functions. Although DA activates naïve or resting T cells, but it inhibits activated T cells. In addition, changes in the expression of DA receptors and their signaling pathways especially in T cells are associated with altered immune functions in disorders like schizophrenia and Parkinson’s disease. These results suggest an immunoregulatory role of DA. Therefore targeting DA receptors and their signaling pathways in these cells by using DA receptor agonists and antagonists may be useful for the treatment of diseases where DA induced altered immunity play a pathogenic role.
Keywords: dopamine, immunity, T cells, dendritic cells, macrophages, NK cells, B cells, microglia
1. Introduction
Besides conventional roles of neurotransmitters in neural communication, a large amount of evidence indicates that neurotransmitters mediate cross talk between the nervous and immune systems (Eskandari and Sternberg, 2002). Among these neurotransmitters, the role of DA is particularly interesting because in addition to regulating behavior, movement, endocrine, cardiovascular, renal and gastrointestinal functions (Basu et al., 1995; Chakroborty et al., 2008; Mezey et al., 1999; Missale et al., 1998), DA can also modulate immune functions (Basu and Dasgupta, 2000). DA is synthesized by different immune effector cells and its receptors are present in these cells (Basu et al., 1993; Basu and Dasgupta, 2000; Eldrup et al., 1989; Ferrari et al., 2004; Kirillova et al., 2008; Le Fur et al., 1980; McKenna et al., 2002; Nakano et al., 2008, 2009). Furthermore the sympathetic innervation of lymphoid tissues can also be dopaminergic in nature, particularly during stress (Bencsics et al., 1997; Mignini et al., 2003). As the majority of recent reports indicate unique interactions between dopamine and T cells, the main focus of this mini-review is on DA mediated regulation of T cell function.
2. DA modulate the functions of immune effector cells by acting through its receptors present in these cells
DA is a monoamine catecholamine neurotransmitter, which acts through its D1 and D2 classes of receptors present in the target cells (Missale et al., 1998). The D1 class includes the D1 and D5 subtypes, which on activation increase intracellular cAMP (Missale et al., 1998). In contrast, the D2 class of receptors, which includes D2, D3, and D4 subtypes, inhibits intracellular cAMP on stimulation (Missale et al., 1998).
Several studies now indicate the presence of DA D1 D2, D3, D4 and D5 receptors in normal human leukocytes (Ferrari et al., 2004; Kirillova et al., 2008; McKenna et al., 2002; Nakano et al., 2008, 2009). Among the leukocyte subpopulations, T lymphocytes, monocytes have low, neutrophils, eosinophils have moderate and B, NK cells have high and more consistent expression of DA receptors (McKenna et al., 2002). In addition, DA D1 receptors are present in human dendritic cells (Nakano et al., 2008). Recently DA uptake system has been identified in the lymphocytes (Amenta et al., 2001; Caronti et al., 2001; Mill et al., 2002).
Furthermore, dopaminergic innervation of lymphoid tissues through sympathetic nerves has also been described, thereby suggesting direct DA-mediated neural regulation of immune effector cells (Bencsics et al., 1997; Mignini et al., 2003). Although these cells primarily come in contact with DA in lymph nodes, spleen, bone marrow and circulation (Basu and Dasgupta, 2000), but DA synthesized and released by T and dendritic cells can also act on DA receptors present in the T cells through an autocrine/paracrine loop (Bergquist, et al., 1994; Cosentino, et al., 2007; Nakano et al., 2009). DA regulates several important functions of these cells (Besser et al., 2005; Ghosh et al., 2003; Levite et al., 2001; Saha et al., 2001a, 2001b; Sarkar et al., 2006; Watanabe et al., 2006). Thus understanding the changes in the immune disorders associated with abnormal dopaminergic activities will help to elucidate the immunomodulatory role of this important neurotransmitter (Basu and Dasgupta, 2000).
3. Altered immunity is seen in diseases with abnormal dopamine function
Altered immune functions have been observed in diseases like schizophrenia and Parkinson’s disease with abnormal dopaminergic systems (Ilani et al., 2001; Nagai et al., 1996; Wandinger et al., 1999). A significantly higher expression of DA D3 receptors and increased IFN-γ synthesis by T cells are reported in untreated schizophrenic patients (Boneberg et al., 2006; Ilani et al., 2001). On the contrary, decreased expression of DA D3 receptors and IFNγ synthesis by peripheral lymphocytes are seen in Parkinson’s disease (Nagai et al., 1996; Wandinger et al., 1999). Because DA D3 receptor mediated increase in IFNγ synthesis by T cells has been demonstrated (Ilani et al., 2004), therefore these immune abnormalities are probably due to the changes in expression of DA D3 receptors and its signaling pathways in the T cells of patients with schizophrenia and Parkinson’s disease (Ilani et al., 2004).
Furthermore as dysfunction of the central dopaminergic system is associated with Parkinson’s disease and schizophrenia, it will be therefore important to mention here that animal studies have indicated brain DA mediated regulation of peripheral immune functions (Basu and Dasgupta, 2000). It has also been recently shown that central dopaminergic hypoactivity increases the risk of inflammation during infection or tissue injury (Engler et al. 2009).
4. DA regulates the functions of immune effector cells through autocrine/paracrine loop
CD4+CD25+ regulatory T lymphocytes (Tregs) are specialized T cells, which play a key role in the control of immune homeostasis (Cosentino et al., 2007). Recently, it has been demonstrated that Tregs contain substantial amounts of dopamine (Cosentino et al., 2007), which after being released acts on the DA D1 receptors present in these cells and subsequently suppress IL-10 and TGFβ synthesis by these cells (Cosentino et al., 2007). In addition, the released DA by acting on DA D1 receptors down-regulates Treg-dependent inhibition of effector T-lymphocyte proliferation and this occurs without affecting the production of TNFα or IFNγ (Cosentino et al., 2007).
Similarly, a paracrine regulatory loop of DA has been shown in the interface of dendritic and T cells (Nakano et al., 2009). DA stored in human monocytic-dendritic cells following its release acts on the D1 receptors present in the naïve T cells, increase cyclic AMP and cause differentiation of these cells into Th2 lineage in response to anti-CD3 plus anti-CD28 mAb (Nakano et al., 2009). However, in absence of dopamine release, T cell differentiation shifts towards Th1 lineage (Nakano et al., 2009). DA is released from these cells following antigen-specific dendritic-T cell interaction (Nakano et al., 2009). Furthermore as stimulation of cAMP increase DA concentration in dendritic cells and because DA by acting through its D1 receptors can increase cAMP concentration, therefore it is possible that the released DA auto-regulates its synthesis in these cells via acting through DA D1 receptors present in these cells (Nakano et al., 2009). These findings indicate that endogenous DA subserves an autocrine/paracrine regulatory loop in the cells of the immune system (Cosentino et al., 2007; Nakano et al., 2009).
5. DA activates resting T cells in absence of any additional stimulating agent
Stimulation of DA D2 and D3 receptors in normal resting peripheral human T lymphocytes, activate α4β1 and α5β1 integrins in these cells, thereby promoting adhesion of these cells to the extracellular matrix component, fibronection (Levite et al., 2001). This action of DA may be critical for trafficking and extravasation of T cells across the blood vessels and tissue barriers (Levite et al., 2001). This study is supported by Watanabe et al., 2006 who have shown that DA stimulates adhesion of CD8+ T cells to fibronectin and ICAM through integrins. These authors have further demonstrated that DA induced chemotactic migration of naïve CD8+Tcells is synergistic with chemokines like CCL19, CCL21 and CXCL12 (Watanabe et al., 2006). This action of DA is reported to be mediated through its D3 receptors present in these cells (Watanabe et al., 2006). DA is also reported to induce cytokine secretion by resting T cells (Besser et al., 2005). It has been shown that stimulation of DA D3 and D1/D5 receptors increase the secretion of TNFα and stimulation of DA D2 receptors induce IL-10 secretion without affecting the secretion of IFNγ and IL-4 (Besser et al., 2005) (Fig. 1A).
Fig. 1.
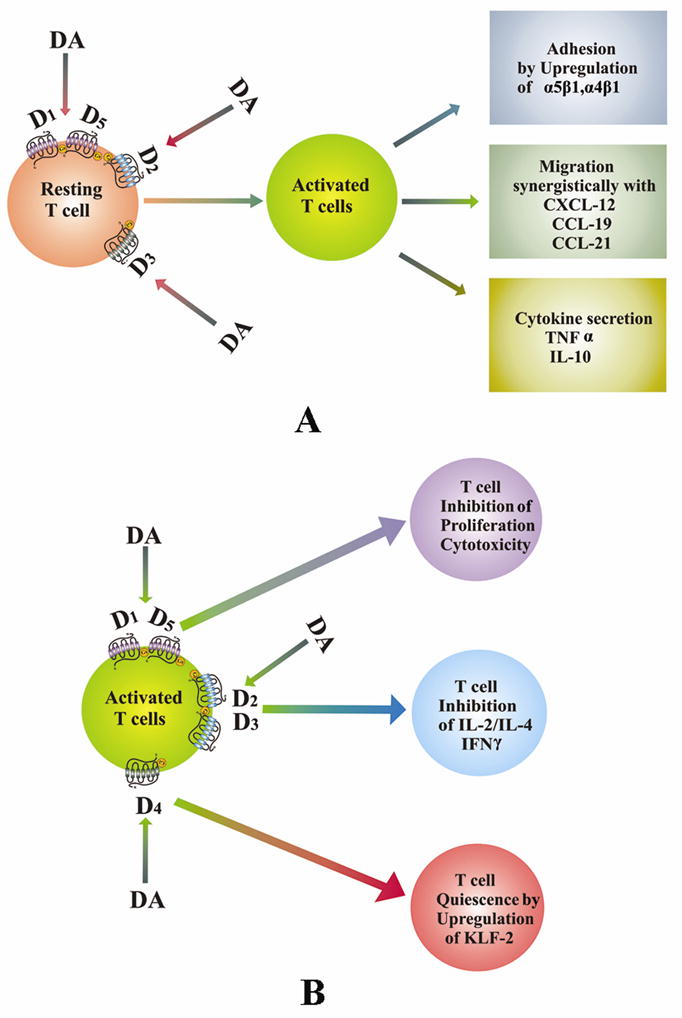
Role of dopamine in T cell functions. (A). DA activates naïve or resting T cells. DA by acting through its receptors stimulates adhesion, migration and cytokine secretion by these cells. (B). DA inhibits activated T cells. DA inhibits the activation of T cells when present during stimulation of T cell receptors and thus inhibits proliferation, cytokine secretion and induces T cell quiescence in these cells by acting through DA receptors. DA, dopamine; D1–D5, dopamine D1–D5 receptors.
6. DA inhibits activation of stimulated T cells
Although DA activated resting T cells, but anti CD3 and IL-2 induced proliferation and cytotoxicity of CD4+ and CD8+ T cells collected from normal human subjects are significantly inhibited when these cells are treated in vitro with high DA concentration observed in the plasma (48.6 pg/ml) of lung cancer patients suffering from uncoping stress (Saha et al., 2001a, 2001b). The molecular mechanism of this action is attributed to DA D1 receptor induced increase in the intracellular cAMP (Saha et al., 2001a, 2001b). Similarly, stimulation of DA D2 and D3 receptors in T cells has been shown to inhibit activated T cell receptor induced cell proliferation, and secretion of IL-2, IFNγ and IL-4 by down regulating the expressions of non receptor tyrosine kinase lck and fyn (Ghosh et al., 2003) (Fig. 1B).
We have also recently shown that stimulation of DA D4 receptors in human T cells during T cell receptor activation is associated with its quiescence (Sarkar et al., 2006). DA induces quiescence of these cells by upregulating the transcription factor, KLF2 via inhibition of ERK1/ERK2 in these cells (Sarkar et al., 2006) (Fig. 1B).
7. DA modulates the functions of NK cells, Splenic cells, Macrophages, B cells and Microglial cells
There are reports which indicate that DA can also modulate the functions of other cells in the immune system (Basu and Dasgupta, 2000). Although Reduced NK cell activities and ovalbumine induced delayed type hypersensitivity responses are reported in animals with hyperdopaminergic systems (Teunis et al., 2004; Kavelaars et al., 2005), but increased LPS-induced cytokine production by macrophages and ovalbumine induced humoral responses have been observed in these animals (Kavelaars et al., 2005).
Moreover, DA treatment has shown to stimulate the proliferation of murine splenocytes by acting through its D2 receptors present in these cells (Carr et al., 2003). A recent report indicates DA mediated inhibition of proliferation in both resting and malignant B lymphocytes (Meredith et al., 2006). Also, DA promotes apoptosis in cycling B cells through oxidative stress. However this action of DA is not demonstrated in resting lymphocytes (Meredith et al., 2006).
Microglial cells are the important immune effector cells in the brain (Chang and Liu, 2000; Färber et al., 2005; Orr et al., 2005; Theodore et al., 2008). After CNS infection, exposure to inflammatory stimuli, or interaction with blood-derived cells, these cells become activated to perform several innate immune functions, including induction of inflammation, cytotoxicity, and regulation of T-cell responses through presentation of antigen. Recent studies have demonstrated the presence of both DA D1 and D2 classes of receptors in the microglial cells (Chang and Liu, 2000; Färber et al., 2005). DA by acting via its D1 receptors regulates the synthesis of microglial nitric oxide, an immune mediator with high antiviral activity (Chang and Liu, 2000; Färber et al., 2005). It has been also shown that DA regulates the migration of these cells in vitro by acting through its receptors present in these cells (Färber et al., 2005).
8. Summary and conclusions
Taken together, the studies outlined above indicate that there is a well defined dopaminergic system in immunity (Basu and Dasgupta, 2000), DA is an important regulator of normal immunity (Basu and Dasgupta, 2000) and changes in the status of DA concentrations and/or receptors, especially in the T cells are responsible for abnormal immune functions seen in patients with schizophrenia and Parkinson’s disease (Ilani et al., 2001, 2004; Nagai et al., 1996; Wandinger et al., 1999). It will be therefore interesting to study if this DA mediated changes in the immune system is also linked to the etiology of these diseases (Ilani et al., 2001, 2004; Nagai et al., 1996; Wandinger et al., 1999).
Because a recent report indicates functional dopaminergic system in the thymus of rats (Mignini et al., 2009), therefore DA may have a role in the maturation and selection of lymphocytes (Mignini et al., 2009). It will therefore be prudent to investigate whether DA has any role in the formation of memory T cells since DA is not only synthesized in T cells, but there is also a functional dopaminergic autocrine regulatory loop in these cells (Bergquist, et al., 1994; Cosentino, et al., 2007). Therefore, elucidation of the detailed mechanisms by which DA activates resting T cells and inhibits stimulated T cells will be necessary to design new and effective therapies in future to modulate the functions of T cells in both health and diseases.
Finally, and most importantly, DA and its agonists or antagonists are being used in the clinics at present for the treatment of other diseases (Katzung, 2004); therefore rapid clinical trials may be undertaken using these inexpensive drugs for the treatment of immune disorders. However these drugs should be used with caution in patients with microbial sepsis as it may suppress the immune functions in these patients (Oberbeck, et al., 2006).
Acknowledgments
This work was supported in parts by DRDO (LSRB/24/EPB/2001) Government of India Grant (P.S.D.); Council of Scientific and Industrial Research Government of India Fellowship 9/30(43)/2005-EMR-1 to B.B., National Institutes of Health, USA Grants CA118265 (S. B.), CA 124763 (S. B.), and Department of Defense Grant, USA grant W81XWH-07-1-0051 (S. B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amenta F, Bronzetti E, Cantalamessa F, El-Assouad D, Felici L, Ricci A, Tayebati SK. Identification of dopamine plasma membrane and vesicular transporters in human peripheral blood lymphocytes. J Neuroimmunol. 2001;117:133–142. doi: 10.1016/s0165-5728(01)00317-4. [DOI] [PubMed] [Google Scholar]
- Basu S, Dasgupta PS, Chowdhury Roy J. Altered plasma level and uptake of dopamine by platelets in some human malignant tumors. Biog Amines. 1995;11:31–38. [Google Scholar]
- Basu S, Dasgupta PS. Dopamine, a neurotransmitter, influences the immune system. J Neuroimmunol. 2000;102:113–124. doi: 10.1016/s0165-5728(99)00176-9. [DOI] [PubMed] [Google Scholar]
- Basu S, Dasgupta PS, Lahiri T, Chowdhury JR. Uptake and biodistribution of dopamine in bone marrow, spleen and lymph nodes of normal and tumor bearing mice. Life Sci. 1993;53:415–424. doi: 10.1016/0024-3205(93)90645-j. [DOI] [PubMed] [Google Scholar]
- Bencsics A, Sershen H, Baranyi M, Hashim A, Lajtha A, Vizi ES. Dopamine, as well as norepinephrine, is a link between noradrenergic nerve terminals and splenocytes. Brain Res. 1997;761:236–243. doi: 10.1016/s0006-8993(97)00313-2. [DOI] [PubMed] [Google Scholar]
- Bergquist J, Tarkowski A, Ekman R, Ewing A. Discovery of endogenous catecholamines in lymphocytes and evidence for catecholamine regulation of lymphocyte function via an autocrine loop. Proc Natl Acad Sci U S A. 1994;91:12912–12916. doi: 10.1073/pnas.91.26.12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besser MJ, Ganor Y, Levite M. Dopamine by itself activates either D2, D3 or D1/D5 dopaminergic receptors in normal human T-cells and triggers the selective secretion of either IL-10, TNF alpha or both. J Neuroimmunol. 2005;169:161–171. doi: 10.1016/j.jneuroim.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Boneberg EM, von Seydlitz E, Pröpster K, Watzl H, Rockstroh B, Illges H. D3 dopamine receptor mRNA is elevated in T cells of schizophrenic patients whereas D4 dopamine receptor mRNA is reduced in CD4+-T cells. J Neuroimmunol. 2006;173:180–187. doi: 10.1016/j.jneuroim.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Caronti B, Antonini G, Calderaro C, Ruggieri S, Palladini G, Pontieri FE, Colosimo C. Dopamine transporter immunoreactivity in peripheral blood lymphocytes in Parkinson’s disease. J Neural Transm. 2001;108:803–807. doi: 10.1007/s007020170030. [DOI] [PubMed] [Google Scholar]
- Carr L, Tucker A, Fernandez-Botran R. In vivo administration of L-dopa or dopamine decreases the number of splenic IFN gamma-producing cells. J Neuroimmunol. 2003;137:87–93. doi: 10.1016/s0165-5728(03)00047-x. [DOI] [PubMed] [Google Scholar]
- Chang JY, Liu LZ. Catecholamines inhibit microglial nitric oxide production. Brain Res Bull. 2000;52:525–530. doi: 10.1016/s0361-9230(00)00291-4. [DOI] [PubMed] [Google Scholar]
- Chakroborty D, Chowdhury UR, Sarkar C, Baral R, Dasgupta PS, Basu S. Dopamine regulates endothelial progenitor cell mobilization from mouse bone marrow in tumor vascularization. J Clin Invest. 2008;118:1380–1389. doi: 10.1172/JCI33125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino M, Fietta AM, Ferrari M, Rasini E, Bombelli R, Carcano E, Saporiti F, Meloni F, Marino F, Lecchini S. Human CD4+CD25+regulatory T cells selectively express tyrosine hydroxylase and contain endogenous catecholamines subserving an autocrine/paracrine inhibitory functional loop. Blood. 2007;109:632–642. doi: 10.1182/blood-2006-01-028423. [DOI] [PubMed] [Google Scholar]
- Eldrup E, Richter EA, Christensen NJ. DOPA, norepinephrine, and dopamine in rat tissues: no effect of sympathectomy on muscle DOPA. Am J Physiol. 1989;256:E284–287. doi: 10.1152/ajpendo.1989.256.2.E284. [DOI] [PubMed] [Google Scholar]
- Engler H, Doenlen R, Riether C, Engler A, Niemi MB, Besedovsky HO, Rey AD, Pacheco-López G, Feldon J, Schedlowski M. Time-dependent alterations of peripheral immune parameters after nigrostriatal dopamine depletion in a rat model of Parkinson’s disease. Brain Behav Immun. 2009 Feb 6; doi: 10.1016/j.bbi.2009.01.018. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Eskandari F, Sternberg EM. Neural-immune interactions in health and disease. Ann N Y Acad Sci. 2002;966:20–27. doi: 10.1111/j.1749-6632.2002.tb04198.x. [DOI] [PubMed] [Google Scholar]
- Färber K, Pannasch U, Kettenmann H. Dopamine and noradrenaline control distinct functions in rodent microglial cells. Mol Cell Neurosci. 2005;29:128–138. doi: 10.1016/j.mcn.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Ferrari M, Cosentino M, Marino F, Bombelli R, Rasini E, Lecchini S, Frigo G. Dopaminergic D1-like receptor-dependent inhibition of tyrosine hydroxylase mRNA expression and catecholamine production in human lymphocytes. Biochem Pharmacol. 2004;67:865–873. doi: 10.1016/j.bcp.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Ghosh MC, Mondal AC, Basu S, Banerjee S, Majumder J, Bhattacharya D, Dasgupta PS. Dopamine inhibits cytokine release and expression of tyrosine kinases, Lck and Fyn in activated T cells. Int Immunopharmacol. 2003;3:1019–1026. doi: 10.1016/S1567-5769(03)00100-0. [DOI] [PubMed] [Google Scholar]
- Ilani T, Ben-Shachar D, Strous RD, Mazor M, Sheinkman A, Kotler M, Fuchs S. A peripheral marker for schizophrenia: Increased levels of D3 dopamine receptor mRNA in blood lymphocytes. Proc Natl Acad Sci U S A. 2001;98:625–628. doi: 10.1073/pnas.021535398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilani T, Strous RD, Fuchs S. Dopaminergic regulation of immune cells via D3 dopamine receptor: a pathway mediated by activated T cells. FASEB J. 2004;18:1600–1602. doi: 10.1096/fj.04-1652fje. [DOI] [PubMed] [Google Scholar]
- Katzung BG. Basic and clinical pharmacology. Appleton and Lange; Stamford, CT: 2004. [Google Scholar]
- Kavelaars A, Cobelens PM, Teunis MA, Heijnen CJ. Changes in innate and acquired immune responses in mice with targeted deletion of dopamine transporter gene. J Neuroimmunol. 2005;161:162–168. doi: 10.1016/j.jneuroim.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Kirillova GP, Hrutkay RJ, Shurin MR, Shurin GV, Tourkova IL, Vanyukov MM. Dopamine receptors in human lymphocytes: radioligand binding and quantitative RT PCR assays. J Neurosci Methods. 2008;174:272–280. doi: 10.1016/j.jneumeth.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Fur G, Phan T, Uzan A. Identification of stereospecific [3H]spiroperidol binding sites in mammalian lymphocytes. Life Sci. 1980;27:1587–1591. doi: 10.1016/0024-3205(80)90653-0. [DOI] [PubMed] [Google Scholar]
- Levite M, Chowers Y, Ganor Y, Besser M, Hershkovits R, Cahalon L. Dopamine interacts directly with its D3 and D2 receptors on normal human T cells, and activates beta1 integrin function. Eur J Immunol. 2001;31:3504–3512. doi: 10.1002/1521-4141(200112)31:12<3504::aid-immu3504>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- McKenna F, McLaughlin PJ, Lewis BJ, Sibbring GC, Cummerson JA, Bowen-Jones D, Moots RJ. Dopamine receptor expression on human T-and B-lymphocytes, monocytes, neutrophils, eosinophils and NK cells: a flow cytometric study. J Neuroimmunol. 2002;132:34–40. doi: 10.1016/s0165-5728(02)00280-1. [DOI] [PubMed] [Google Scholar]
- Meredith EJ, Holder MJ, Rosén A, Lee AD, Dyer MJ, Barnes NM, Gordon J. Dopamine targets cycling B cells independent of receptor/transporter for oxidative attack: Implications for non-Hodgkin’s lymphoma. Proc Natl Acad Sci USA. 2006;103:13485–13490. doi: 10.1073/pnas.0605993103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezey E, Eisenhofer G, Hansson S, Harta G, Hoffman BJ, Gallatz K, Palkovits M, Hunyady B. Non-neuronal dopamine in gastrointestinal system. Clin Exp Pharmacol Physiol Suppl. 1999;26:S14–S22. [PubMed] [Google Scholar]
- Mignini F, Streccioni V, Amenta F. Autonomic innervation of immune organs and neuroimmune modulation. Auton Autacoid Pharmacol. 2003;23:1–25. doi: 10.1046/j.1474-8673.2003.00280.x. [DOI] [PubMed] [Google Scholar]
- Mignini F, Tomassoni D, Traini E, Amenta F. Dopamine, vesicular transporters and dopamine receptor expression and localization in rat thymus and spleen. J Neuroimmunol. 2009;206:5–13. doi: 10.1016/j.jneuroim.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Mill J, Asherson P, Browes C, D’Souza U, Craig I. Expression of the dopamine transporter gene is regulated by the 3′ UTR VNTR: Evidence from brain and lymphocytes using quantitative RT-PCR. Am J Med Genet. 2002;114:975–979. doi: 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Ueno S, Saeki Y, Soga F, Hirano M, Yanagihara T. Decrease of the D3 dopamine receptor mRNA expression in lymphocytes from patients with Parkinson’s disease. Neurology. 1996;46:791–795. doi: 10.1212/wnl.46.3.791. [DOI] [PubMed] [Google Scholar]
- Nakano K, Higashi T, Hashimoto K, Takagi R, Tanaka Y, Matsushita S. Antagonizing dopamine D1-like receptor inhibits Th17 cell differentiation: preventive and therapeutic effects on experimental autoimmune encephalomyelitis. Biochem Biophys Res Commun. 2008;373:286–291. doi: 10.1016/j.bbrc.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Nakano K, Higashi T, Takagi R, Hashimoto K, Tanaka Y, Matsushita S. Dopamine released by dendritic cells polarizes Th2 differentiation. Int Immunol. 2009;21:645–654. doi: 10.1093/intimm/dxp033. [DOI] [PubMed] [Google Scholar]
- Oberbeck R, Schmitz D, Wilsenack K, Schüler M, Husain B, Schedlowski M, Exton MS. Dopamine affects cellular immune functions during polymicrobial sepsis. Intensive Care Med. 2006;32:731–739. doi: 10.1007/s00134-006-0084-y. [DOI] [PubMed] [Google Scholar]
- Orr CF, Rowe DB, Mizuno Y, Mori H, Halliday GM. A possible role for humoral immunity in the pathogenesis of Parkinson’s disease. Brain. 2005;128:2665–2674. doi: 10.1093/brain/awh625. [DOI] [PubMed] [Google Scholar]
- Saha B, Mondal AC, Basu S, Dasgupta PS. Circulating dopamine level, in lung carcinoma patients, inhibits proliferation and cytotoxicity of CD4+ and CD8+ T cells by D1 dopamine receptors: an in vitro analysis. Int Immunopharmacol 1. 2001a;1363:1374. doi: 10.1016/s1567-5769(01)00068-6. [DOI] [PubMed] [Google Scholar]
- Saha B, Mondal AC, Majumder J, Basu S, Dasgupta PS. Physiological concentrations of dopamine inhibit the proliferation and cytotoxicity of human CD4+CD8+T cells in vitro: a receptor-mediated mechanism. Neuroimmunomodulation. 2001b;9:23–33. doi: 10.1159/000049004. [DOI] [PubMed] [Google Scholar]
- Sarkar C, Das S, Chakroborty D, Chowdhury UR, Basu B, Dasgupta PS, Basu S. Cutting edge: Stimulation of dopamine D4 receptors induce T cell quiescence by up-regulating Kruppel-like factor-2 expression through inhibition of ERK1/ERK2 phosphorylation. J Immunol. 2006;177:7525–7529. doi: 10.4049/jimmunol.177.11.7525. [DOI] [PubMed] [Google Scholar]
- Teunis MA, Heijnen CJ, Cools AR, Kavelaars A. Reduced splenic natural killer cell activity in rats with hyperreactive dopaminergic system. Psychoneuroendocrinology. 2004;29:1058–1064. doi: 10.1016/j.psyneuen.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Theodore S, Cao S, McLean PJ, Standaert DG. Targeted overexpression of human alpha-synuclein triggers microglial activation and an adaptive immune response in a mouse model of Parkinson disease. J Neuropathol Exp Neurol. 2008;67:1149–1158. doi: 10.1097/NEN.0b013e31818e5e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandinger KP, Hagenah JM, Klüter H, Rothermundt M, Peters M, Vieregge P. Effects of amantadine treatment on in vitro production of interleukin-2 in de-novo patients with idiopathic Parkinson’s disease. J Neuroimmunol. 1999;98:214–220. doi: 10.1016/s0165-5728(99)00093-4. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Nakayama T, Nagakubo D, Hieshima K, Jin Z, Katou F, Hashimoto K, Yoshie O. Dopamine selectively induces migration and homing of naive CD8+ T cells via dopamine receptor D3. J Immunol. 2006;176:848–856. doi: 10.4049/jimmunol.176.2.848. [DOI] [PubMed] [Google Scholar]


