Abstract
Spaceflight represents a unique physiological challenge to humans, altering hormonal profiles and tissue insulin sensitivity. Among these hormonal alterations, hypercortisolemia and insulin insensitivity are thought to negatively affect muscle mass and function with spaceflight. As insulin sensitivity influences the accumulation of muscle triglycerides, we examined this relationship during hypercortisolemia and inactivity. Six young healthy volunteers were confined to bed rest for 28 days. To mimic the stress response observed during spaceflight, hypercortisolemia (20–24mg/dL) was induced and maintained by oral ingestion of hydrocortisone. On days 1 and 28 of bed rest, insulin sensitivity across the leg was assessed with a local (femoral arterial insulin infusion) 2 stage hyperinsulinemic-euglycemic clamp (stage 1: 35 µU/min/ml leg; stage 2: 70 µU/min/ml leg). Intramuscular lipid was measured with magnetic resonance spectroscopy. Following bed rest, there was a decrease in insulin sensitivity, as assessed by glucose uptake during hyperinsulinemia (from 9.1±1.3 (mean ± SEM) mg/kg.leg/min to 5.2±0.7 mg/kg.leg/min (P=0.015)). Intramuscular triglyceride increased from 0.077±0.011 to 0.136±0.018 (signal area of fat/signal area of standard; P=0.009). Intramuscular lipid content correlated with the glucose uptake at day 28, (R= −0.85; P=0.035). These data demonstrate that muscular inactivity and hypercortisolemia are associated with an increase in intramuscular triglyceride and skeletal muscle insulin resistance in previously healthy subjects.
Introduction
Spaceflight results in a hormonal imbalance that predisposes astronauts to the loss of muscle mass and function. The existing evidence indicates that hormones important for muscle anabolism are either altered in production/concentration, such as testosterone (42), or by tissue action, as is the case with insulin (25, 41). To further exacerbate the potential for muscle catabolism, spaceflight results in an increased production/excretion of cortisol (40). Thus, the prevailing hormonal influence favors muscle protein loss. This alteration in hormonal production and activity is particularly deleterious when combined with muscular inactivity. We have previously demonstrated that muscular inactivity predisposes the muscle to the catabolic effects of hypercortisolemia (17). Studies indicate that even short-term inactivity contributes to insulin resistance (26, 39, 43). Decreased insulin stimulated glucose uptake can be seen within 7 days of bed rest in healthy individuals, and within 24 hours when combined with fasting (4, 26, 44). In a patient population, hyperglycemia due to insulin resistance is directly linked to increased morbidity and mortality (48). During spaceflight, insulin resistance is most likely an important factor contributing to the loss of muscle mass and function.
The stress response, whether induced by illness or spaceflight, entails the increase in the counter-regulatory hormone cortisol (18). Cortisol release in healthy adults follows a diurnal pattern; however, in the stress state the diurnal pattern of cortisol release is lost, and concentrations remain elevated throughout the day (18, 54). Cortisol has also been demonstrated to have detrimental effects on glucose tolerance (23, 27, 37). Whereas several studies have looked at the effect of bed rest/muscular inactivity alone on insulin sensitivity, none have examined the combination of inactivity and hypercortisolemia that is noted during spaceflight, as well as during hospitalization.
Further, little is known about the interaction of fat metabolism and insulin sensitivity during bed rest or hypercortisolemia. Multiple studies have found an interaction between altered fat metabolism and glucose uptake in patients with type 2 diabetes mellitus (T2DM) . This has been reviewed extensively, and the prevailing thought is that intracellular triglyceride products such as diacylglycerol (DAG) or ceramides directly effect the insulin signaling pathway, and that concentrations of these compounds are elevated secondary to increased concentrations of intramyocellular triglycerides (IMTG) and increased delivery of FFA to tissues (35). Although it has been determined that short term administration of cortisol increases plasma FFA (27) and regional and systemic lipolysis (14), an investigation of the effects of long-term hypercortisolemia on insulin sensitivity has not been accomplished.
We studied the effects of prolonged inactivity and hypercortisolemia on insulin resistance and intramuscular lipid in healthy volunteers. We hypothesized that the combination of inactivity and hypercortisolemia would decrease insulin sensitivity and that it would further be related to increased deposition of muscle triglycerides. To discern this interaction, we measured glucose uptake across the leg, plasma fat metabolism, and skeletal muscle triglyceride content and metabolites.
Methods
Subjects
Six healthy male (age 27±2 years) volunteers participated in this project . All volunteers provided informed, written consent and the project was approved by the institutional review board at the University of Texas Medical Branch (UTMB). Subject eligibility was assessed using multiple medical screening tests as previously described, and all lipid and hepatic tests were within normal ranges (31). Additionally, a standard 2 hour oral glucose tolerance test was performed and all subjects had a normal glucose response to the challenge.
Admission and Pre- and Post-testing
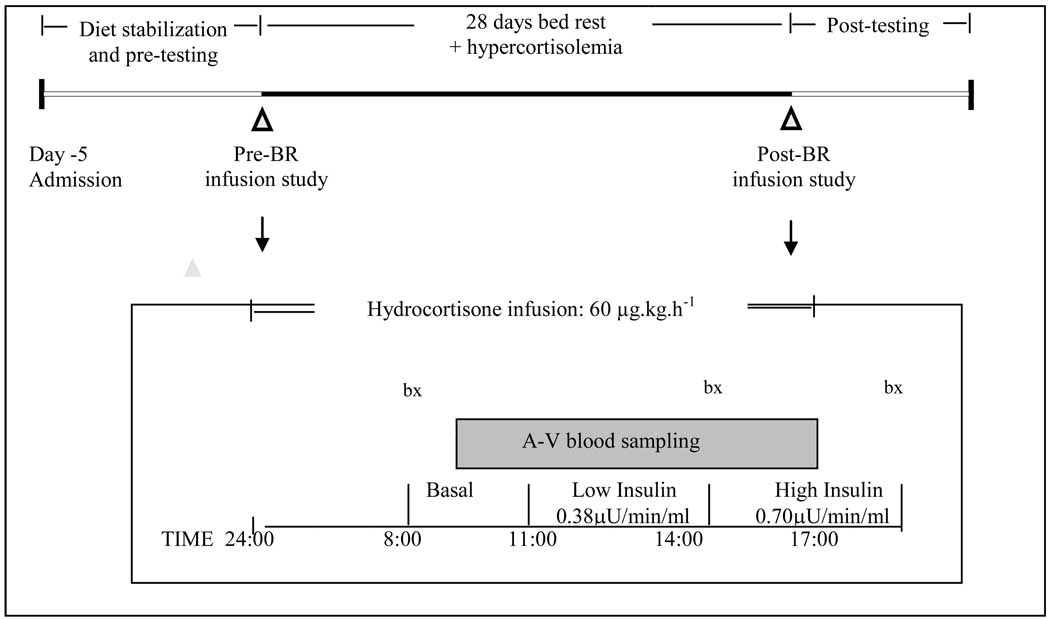
The experimental protocol is depicted in Figure 1. Subjects were admitted and housed in the General Clinical Research Center (GCRC) at UTMB for pre-testing and five days of dietary stabilization prior to the start of bed rest. During this period, subjects were sedentary but remained ambulatory. The Harris-Benedict equation was used to estimate daily energy requirements as previously described (31, 32). Subjects were placed on a three day rotating diet with daily nutrient intake evenly distributed between three meals (0830, 1300, 1830). Carbohydrate, fat and protein intake represented 59%, 27% and 14% of the daily energy intake respectively (16, 31, 32). Water was provided ad libitum. MRS spectroscopy was performed prior to bed rest after 2–3 days of diet stabilization and post-bed rest (day 29), immediately prior to the resumption of weight bearing.
Figure 1.
Experimental and pre- and post- bed rest infusion protocol (expanded).
Experimental Paradigm
Glucose clamp studies were performed on days 1 and 28 of bed rest. Subjects received 10–15 mg of oral hydrocortisone sodium succinate 5 times every day during bed rest (Days 2–27: 0800, 1200, 1600, 2000, 2400). This administration regimen resulted in circulating cortisol of approximately 20 µg/dl, while simultaneously removing the diurnal variation of cortisol secretion.
Infusion Protocol
At 2400 on both day 1 and 28, a constant peripheral venous infusion (18-guage polyethylene catheter: Insyte-W, Becton-Dickinson, Sandy, UT) of hydrocortisone sodium succinate (Pharmacia, Corp., MI), (60 µg/kg/h) was initiated to reduce fluctuations in plasma cortisol concentrations during measurement of insulin sensitivity. Peripheral blood samples for determination of plasma concentrations of cortisol, glucose and insulin were drawn through a contralateral 18-guage polyethylene peripheral venous catheter.
At 0700, 8 cm polyurethane catheters (Cook catheters, Bloomington, IN) were inserted into the femoral vein and artery of one leg under local anesthesia and maintained patent by normal saline. Femoral arterial and venous blood samples were obtained during the post-absorptive period between 0800 and 1100. Leg plasma flow was calculated from steady-state indocyanine green (ICG) concentrations and converted to leg blood flow using hematocrit as previously described (3, 22).
Following the 3 hour basal period, the first step of a 2 step across the leg insulin clamp was initiated with a low level of insulin (0.38 µU/min/ml of leg volume) for 3 hours. Insulin was infused directly into the femoral artery to minimize its systemic effects. Systemic glucose was clamped between 85–95 mg/dL, checked every 10 min and maintained via peripheral infusion of 25% dextrose when appropriate. After 3 hours, the infusion rate of insulin was increased to 0.70 µU/min/ml of leg volume, while the whole blood glucose concentration was maintained. The calculation of leg glucose uptake was performed with glucose values from the last hour of each of the clamps by dividing the amount of glucose infused per min by 25% of the total body weight, to approximate the contribution of total mass by the leg.
At 0800, 1400 and 1700, muscle biopsy samples (approx. 50 mg) were taken from the lateral portion of the vastus lateralis using a 5 mm Bergstrom biopsy needle and local anesthesia (1% lidocaine) as previously described (2, 30).
Bed rest
Subjects maintained strict bed rest throughout the study and were continually monitored by the scientific and GCRC nursing staffs. Subjects were encouraged to change position periodically to alleviate positional discomfort and to eat. Bathing and hygiene activities and urine collection were performed during bed rest. Subjects were permitted to use a bedside commode for bowel movements, but the time out of bed was non-weight bearing and limited to approximately 5 minutes.
Sample Analysis
IMTG quantification
Samples were analyzed as previously described (9, 10). Briefly, IMTG was measured from the soleus in 4 consistent voxles with a 1H knee coil on a GE Advantage 1.5 Tesla whole body imager (General Electric, Milwaukee, WI). A tube of 20 % Intralipid© (IV high fat total parenteral feeding solution – Baxter Healthcare Deerfield Park, IL) was taped to the leg and placed inside the knee coil to obtain a standard external reference to normalize IMTG concentrations (33). Peak positions were determined by time domain fitting using jMRUi (24, 49–51). The prior knowledge information used for the AMARES fits have been previously published by Rico-Sanz and our previous work (9–11, 36). This process was repeated for the intralipid phantom. The TG levels were computed as a ratio relative to the Intralipid© standard using the following formula:
where PM is the tissue lipid methylene peak area, VM is the total measured tissue voxel volume, PI is the Intralipid peak area, and VI is the Intralipid voxel volume. The results of this calculation are a ratio and expressed in arbitrary units.
Muscle DAG analysis
Muscle DAG palmitate concentrations were measured as previously described from frozen muscle tissue samples (46).
Glucose analysis
Plasma glucose was measured with an YSI 2300 Stat glucose/lactate analyzer (YSI, Inc. Yellow Springs, OH).
Insulin Analysis
Plasma was separated and stored at −80°C until analysis. After thawing, insulin levels were measured using RIA per the manufactures instructions (Diagnostic Laboratories, Los Angeles, CA). The coefficient of variation for these measurements in our lab is <10%.
Plasma Free Fatty Acid Analysis
Total free fatty acids were measured using a chromatographic kit per the manufactures instructions (Wako Diagnostics, Osaka, Japan).
Plasma Glycerol
Total free glycerol was measured using the free glycerol determination kit from Sigma (St. Loius, Mo), Catalog Number FG0100 per the manufactures instructions.
Statistics
Data reported are means ± Standard Error of the Mean. For IMTG and glucose uptake, a paired T-test was used to compare pre and post bed rest values in individuals pre and post-bedrest. DAG, plasma NEFA and plasma glycerol were analyzed using ANOVA with Tukey’s post-hoc analysis. Pearson correlations with linear regression analysis were performed with IMTG and insulin resistance vs. bedrest. All statistics were performed on Sigma Stat (Version 2.01).
Results
Subject body composition
As has already been published, subjects lost an average of −2.8 ± 0.6 kg with more 1 kg of lean body mass loss in the lower extremities (29). There was a minimal decrease in body fat mass (−95.6 ± 288.1 g).
Glucose
Fasting glucose concentrations were 90±7 mg/dl and 95±5 mg/dl on study days 1 and 28, respectively (p>0.05).
Clamp insulin concentrations
Basal insulin concentrations obtained from the femoral vein were similar pre and post bed rest (11.4±2.1 µIU/mL Pre and 14.2±2.9 Post; p=0.175). Insulin concentrations (femoral vien) during the low level of the clamp were 37.5±5.3 µIU/mL pre and 38.9±5.5 µIU/mL post and during the high level of clamp were 70.6±8.3 µIU/mL pre and 71.0±6.2 µIU/mL post.
Leg glucose uptake
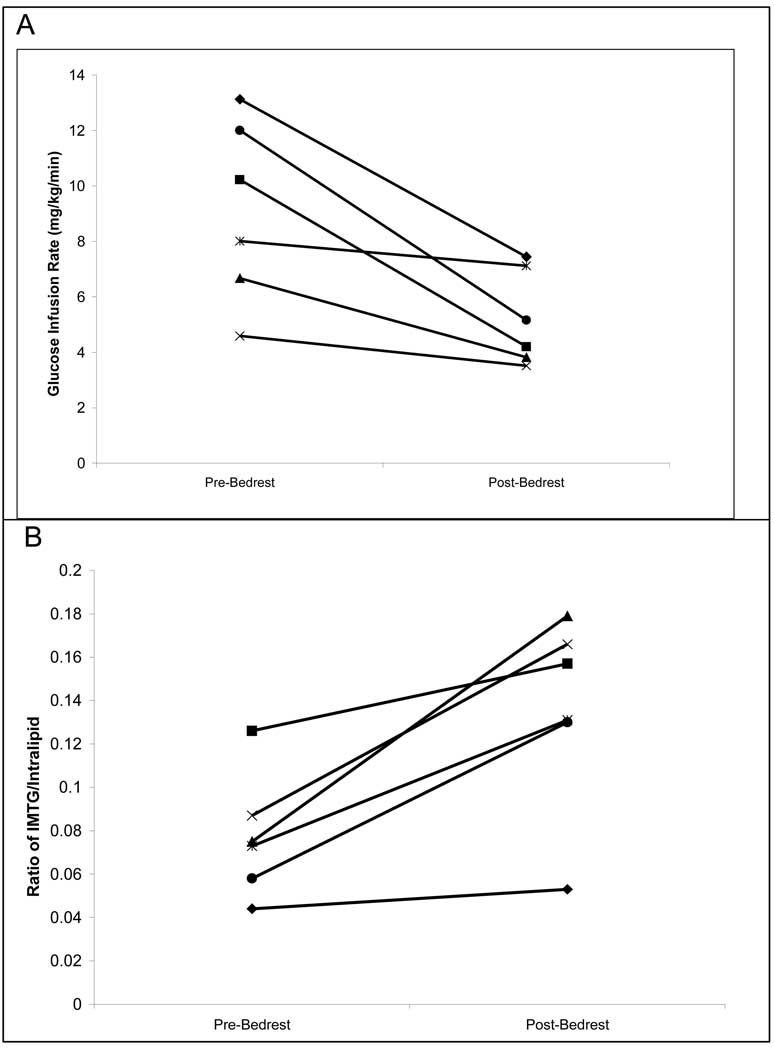
During the Stage 1 clamps on day 1 and day 28, there was no change in insulin stimulated glucose uptake (2.97±0.37 mg/kg leg/min vs 3.13±0.35; Pre vs. Post; P>0.1). There was a significant decrease in post-bed rest insulin stimulated glucose uptake during the stage 2 clamp on day 28 (Figure 2A; 9.1±1.3 mg/kg leg/min vs. 5.2±0.98; Pre vs. Post; P=0.015).
Figure 2.
Individual subject values of leg glucose uptake (A) and intramuscular triglycerides (IMTG; B). A) leg glucose uptake (as assessed by glucose infusion rate to maintain euglycemia) before and after bed rest in each individual patient. Uptake decreases significantly following bed rest; (P=0.015). B) IMTG in each individual patient before and after bed rest. IMTG increased in each subject and was significant for the group; P=0.009
IMTG
The ratio of intracellular fat to external standard for pre-bed rest IMTG was 0.077 ± 0.016 arbitrary units (AU). Following bed rest, it significantly increased (P=0.009) to 0.136± 0.026 AU. All subjects had at least a 20% increase in IMTG following bed rest, and two individuals had more than a two-fold increase (Figure 2B).
Correlations
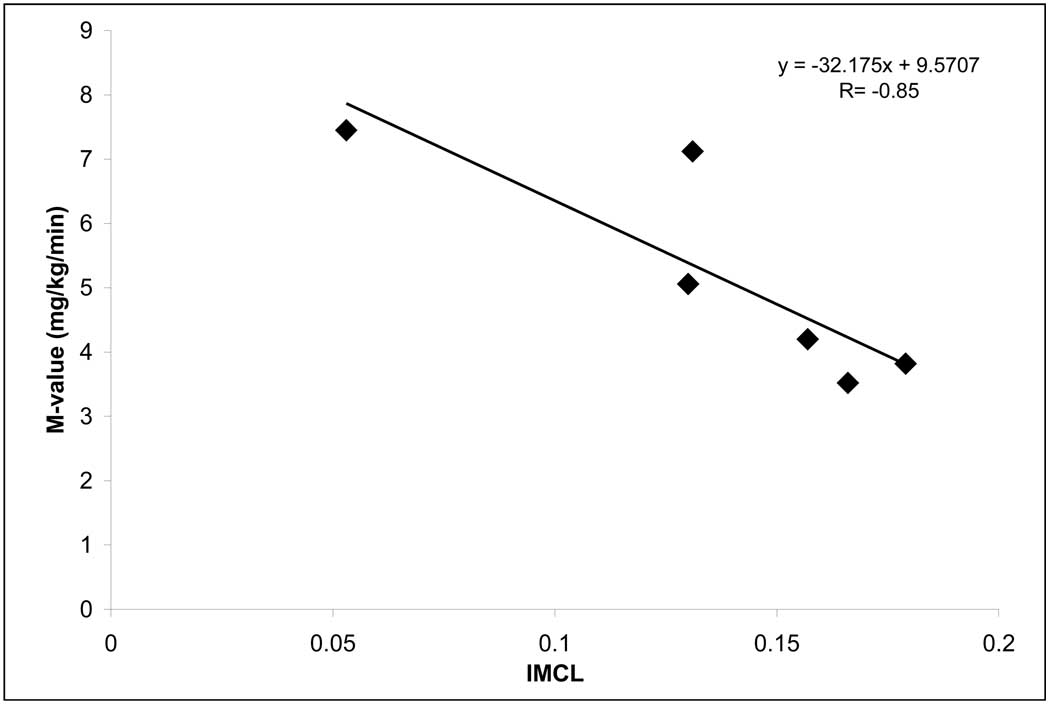
After bed rest, insulin sensitivity and IMTG were strongly correlated (R= −0.85, P=0.035, Figure 3).
Figure 3.
IMTG and glucose clamp correlations post bed rest. Leg glucose uptake (mg/kg/min) and IMTG (AU; pt value compared to standard) were significantly related following 28 days of hypercortisolemic bed rest with a R= −0.85; (P=0.035).
Plasma NEFA
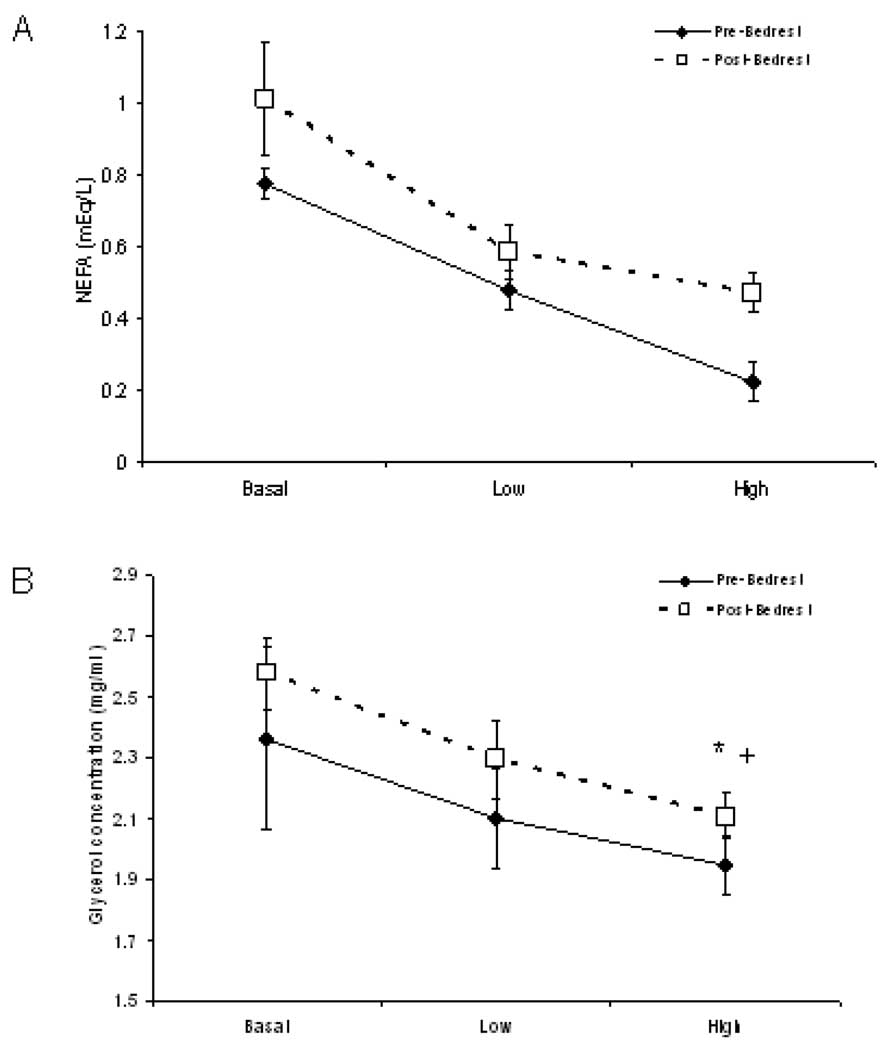
Plasma NEFA concentrations were unchanged before and after bed rest, although there was a tendency to increase at the high insulin concentration (Figure 4A). NEFA levels decreased in response to insulin equally before and after bed rest.
Figure 4.
Femoral vein plasma non-esterified free fatty acid (NEFA) and glycerol concentrations in response to 2 levels of intra-arterial insulin infusion. A) Plasma NEFA concentrations decreased in response to insulin equally before and after bed rest. B) Plasma glycerol decreased in response to insulin equally before and after bed rest. +; p =0.003 pre vs post at the high insulin level. *; p=0.01 high insulin vs basal, pre and post bed rest.
Plasma Glycerol
Plasma glycerol concentrations were unchanged in the basal and low insulin period of the clamp before and after bed rest (Figure 4B). The concentration of plasma glycerol was significantly greater following bed rest during the high insulin level phase of the clamp. Glycerol levels decreased in response to insulin similarly before and after bed rest.
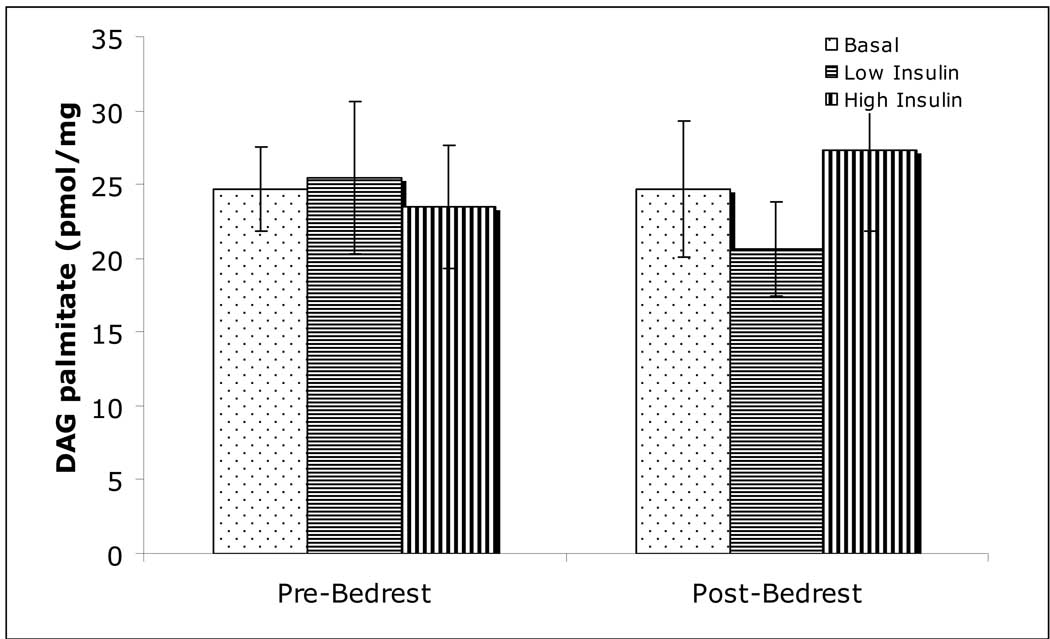
Muscle DAG
The concentration of DAG palmitate did not change with increasing concentrations of insulin, and did not change with bed rest (Figure 5).
Figure 5.
Intramuscular diacylglycerol palmitoyl concentrations. Concentrations did not change with exposure to insulin, or following bed rest.
Discussion
Muscular inactivity and concomitant hypercortisolemia are commonly experienced by astronauts, as well as medical and surgical patients. We found that the combination of these two catabolic stressors induced peripheral insulin resistance and increased IMTG accumulation in previously healthy volunteers. Peripheral insulin resistance and hyperglycemia are of serious concern to both astronauts and patient populations, and may be related to altered fat metabolism. Our results indicate that impairment of muscle glucose uptake was significantly correlated with the increase in IMTG. However, the mechanism by which intracellular lipids contribute to insulin resistance is not clear, as we did not find increases in intramuscular DAG, or in fasted plasma NEFA or glycerol concentrations.
The individuals enrolled in this study were healthy, with normal pre-study blood test and screening results. Specifically, pre bed rest IMTG values (0.08±0.01AU) were identical to a previously studied cohort of 18–32 year old healthy volunteers (10). Plasma NEFA and glycerol concentrations were within normal range, as were pre bed rest measurements for insulin sensitivity. Thus, the demonstrated changes were attributable to the combination of bed rest and hypercortisolemia.
Our results suggest a relationship between the accumulation of IMTG and peripheral insulin sensitivity. Following bed rest the IMTG content increased in every subject by at least 20% and there was a strong correlation between the two following bed rest. We have recently shown that IMTG is elevated in insulin resistant burn trauma patients (9). Studies in burned animals have also shown that IMTG accumulates rapidly in animals with high cortisol concentrations (1). Thus, in multiple models, there is strong relationship between hypercortisolemia, insulin resistance, and IMTG.
IMTG is likely elevated as a result of both a decrease in metabolic rate, secondary to inactivity, and an increase in NEFA delivery due to the prevailing hypercortisolemia (14). Increased NEFA concentrations have been linked to insulin resistance in many models (5). Although the fasted values of NEFA were not different before and after bed rest, plasma NEFA and glycerol were higher during hyperinsulinemia, indicating that lipolysis was not suppressed to the same extent following bed rest and hypercortisolemia, and that adipose tissue as well as muscle tissue was experiencing insulin resistance. An overall decreased metabolic rate has been demonstrated following bed rest and lipid oxidation rates have also been shown to decrease by up to 90% following 7 days of bed rest (4). In our previous study of insulin resistant burn patients, decreased mitochondrial function and a decreased rate of lipid oxidation was linked to insulin resistance. This mechanism may have been present, in part, in our volunteers (8, 13, 34). It maybe that in ambulatory, healthy adults, several factors such as nutrition, activity and genetics influence IMTG pool size, but in illness, inactivity or stress, the pool size become more directly related to insulin sensitivity, since IMTG and glucose uptake were not linearly associated prior to bed-rest, but were strongly associated afterwards.
Whereas IMTG is often elevated in insulin resistance, several studies have indicated that increases in DAG are also associated with insulin resistance. DAG is thought to activate protein kinase C (PKC), which in turn inactivates the insulin receptor substrate-1 (IRS-1) by serine phosphorylation(21, 35). However, despite parallel increases in insulin resistance and IMTG, we did not find any changes in DAG. These findings are similar to those in burn patients, where DAG concentrations were comparable to healthy controls (12). Further evidence is provided in a recent study by Dubé et al. where exercise training in obese older subjects resulted in an increase in IMTG and a decrease in DAG and ceramide (15). These findings point to the complexity of insulin resistance and the idea that DAG may not be a primary causal/controlling factor.
While bed rest alone can mimic the physical inactivity associated with trauma or illness and is often utilized as a ground-based model for spaceflight, it cannot duplicate the complex hormonal and metabolic disruptions of the stress state (52, 55). The chronic administration of exogenous cortisol in this study was designed to elevate plasma cortisol concentrations to the upper physiological range; consistent with values observed following moderate trauma (53, 56), and certain aspects of spaceflight (57). Other investigators noted that insulin secretion in response to a glucose load increased after 20 days of bed rest, reflecting peripheral insulin resistance (26). Similar results were found after 7 days of bed rest (4) and 20 days of bed rest (58). These results in healthy volunteers are consistent with data from patients restricted to bed rest following elective surgery (28). Unfortunately, the across the leg clamp, while better for direct assessment of muscle insulin sensitivity, prevents a direct comparison of insulin resistance with previous whole-body determinations in studies investigating bed rest alone (29).
Excess cortisol secretion has been linked to insulin resistance in both observational and experimental studies. Increased cortisol concentrations have also been demonstrated in patients with type 2 diabetes mellitus (T2DM) (26, 39). Studies in healthy volunteers indicate that cortisol increases plasma glucose concentrations via action on adipose, muscle and hepatic tissue, and may act by activating inflammatory pathways (6, 7, 20, 23, 27, 37, 45). As already published from this patient population, cytokines such as TNF-B and Il-6 did not increase, although we did not measure IL-1 TNF-alpha (47). It is unlikely that inflammation per see played a large role in the cortisol mediated insulin resistance in this patient model. While a shortcoming of this study was the absence of a bed rest alone group, it is clear that the combination of cortisol and bed rest is a model most relevant to patient and astronaut populations, and that an understanding of this combination has greater practical application.
We have previously shown that the combination of inactivity and stress facilitate a 3-fold greater loss of lean muscle mass than bed rest alone (7, 37, 38, 45). It is likely that some of the mechanisms tied to disuse atrophy are also related to the decreased peripheral glucose uptake. The insulin like growth factor-1 pathway, with downstream signals PI3K, Akt and protein S6 kinase (ps6k) has been shown to be important to muscle hypertrophy (29). Decreased levels of Akt protein have been found with disuse (19). PI3K and Akt are also critical in the insulin signaling pathway to increase glucose uptake in muscle (21). This pathway is also affected by hypercortisolemia, and this combination may be responsible for the greater muscle loss in these subjects compared to those in bed rest alone (7, 29, 32, 37). The loss of muscle protein may further affect insulin signaling by reducing the amount of metabolically-active tissue.
In conclusion, prolonged inactivity and hypercortisolemia induces insulin resistance in skeletal muscle which is associated with an increase in IMTG, with IMTG concentrations directly corresponding to leg glucose uptake. The increase in IMTG may be indicative of this combined insult and may not be related to DAG. Further exploration of the combined effect of inactivity and stress may lead to a better understanding of how to ameliorate these associated conditions.
Acknowledgements
We gratefully acknowledge the assistance of Melissa Bailey, Stephaine Blasé, Christopher Danesi, Dennis Dornfest, Cathy Bryant and Dave Crocker for their invaluable assistance with MRS sample and data processing.
This project was supported by NSBRI grant NPFR00205, NASA grant NAG9-1155, NIH 5 RO1 GM 57295 and Shriners Hospital grant 8490. Studies were conducted on the General Clinical Research Center (GCRC) at The University of Texas Medical Branch at Galveston and funded by grant M01 RR 00073 from the National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No author claims a conflict of interest
References
- 1.Astrakas LG, Goljer I, Yasuhara S, Padfield KE, Zhang Q, Gopalan S, Mindrinos MN, Dai G, Yu YM, Martyn JA, Tompkins RG, Rahme LG, Tzika AA. Proton NMR spectroscopy shows lipids accumulate in skeletal muscle in response to burn trauma-induced apoptosis. Faseb J. 2005;19:1431–1440. doi: 10.1096/fj.04-2005com. [DOI] [PubMed] [Google Scholar]
- 2.Bergstrom J, Furst P, Noree LO, Vinnars E. Intracellular free amino acid concentration in human muscle tissue. J App Physiol. 1974;36:693–697. doi: 10.1152/jappl.1974.36.6.693. [DOI] [PubMed] [Google Scholar]
- 3.Biolo G, Fleming DY, Maggi S, Wolfe RR. Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle. Am J Physiol (Endocrinol Metab) 1995;268:E75–E84. doi: 10.1152/ajpendo.1995.268.1.E75. [DOI] [PubMed] [Google Scholar]
- 4.Blanc S, Normand S, Pachiaudi C, Fortrat JO, Laville M, Gharib C. Fuel homeostasis during physical inactivity induced by bed rest. J Clin Endocrinol Metab. 2000;85:2223–2233. doi: 10.1210/jcem.85.6.6617. [DOI] [PubMed] [Google Scholar]
- 5.Boden G, Chen X, Ruiz J, White JV, Rossetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest. 1994;93:2438–2446. doi: 10.1172/JCI117252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter-Su C, Okamoto K. Effect of insulin and glucocorticoids on glucose transporters in rat adipocytes. Am J Physiol. 1987;252:E441–E453. doi: 10.1152/ajpendo.1987.252.4.E441. [DOI] [PubMed] [Google Scholar]
- 7.Coderre L, Vallega GA, Pilch PF, Chipkin SR. In vivo effects of dexamethasone and sucrose on glucose transport (GLUT-4) protein tissue distribution. Am J Physiol. 1996;271:E643–E648. doi: 10.1152/ajpendo.1996.271.4.E643. [DOI] [PubMed] [Google Scholar]
- 8.Cree MG, Aarsland A, Herndon DN, Wolfe RR. Role of fat metabolism in burn trauma-induced skeletal muscle insulin resistance. Crit Care Med. 2007;35:S476–S483. doi: 10.1097/01.CCM.0000278066.05354.53. [DOI] [PubMed] [Google Scholar]
- 9.Cree MG, Newcomer BR, Herndon DN, Qian T, Sun D, Morio B, Zwetsloot JJ, Dohm GL, Fram RY, Mlcak RP, Aarsland A, Wolfe RR. PPAR-alpha agonism improves whole body and muscle mitochondrial fat oxidation, but does not alter intracellular fat concentrations in burn trauma children in a randomized controlled trial. Nutr Metab (Lond) 2007;4:9. doi: 10.1186/1743-7075-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cree MG, Newcomer BR, Katsanos CS, Sheffield-Moore M, Chinkes D, Aarsland A, Urban R, Wolfe RR. Intramuscular and liver triglycerides are increased in the elderly. J Clin Endocrinol Metab. 2004;89:3864–3871. doi: 10.1210/jc.2003-031986. [DOI] [PubMed] [Google Scholar]
- 11.Cree MG, Newcomer BR, Read LK, Sheffield-Moore M, Paddon-Jones D, Chinkes D, Aarsland A, Wolfe RR. Plasma triglycerides are not related to tissue lipids and insulin sensitivity in elderly following PPAR-alpha agonist treatment. Mech Ageing Dev. 2007;128:558–565. doi: 10.1016/j.mad.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cree MG, Zwetsloot JJ, Herndon DN, Newcomer BR, Fram RY, Angel C, Green JM, Dohm GL, Sun D, Aarsland A, Wolfe RR. Insulin sensitivity is related to fat oxidation and protein kinase C activity in children with acute burn injury. J Burn Care Res. 2008;29:585–594. doi: 10.1097/BCR.0b013e31817db88f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cree MG, Zwetsloot JJ, Herndon DN, Qian T, Morio B, Fram R, Sanford AP, Aarsland A, Wolfe RR. Insulin sensitivity and mitochondrial function are improved in children with burn injury during a randomized controlled trial of fenofibrate. Ann Surg. 2007;245:214–221. doi: 10.1097/01.sla.0000250409.51289.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Djurhuus CB, Gravholt CH, Nielsen S, Pedersen SB, Moller N, Schmitz O. Additive effects of cortisol and growth hormone on regional and systemic lipolysis in humans. American journal of physiology. 2004;286:E488–E494. doi: 10.1152/ajpendo.00199.2003. [DOI] [PubMed] [Google Scholar]
- 15.Dube JJ, Amati F, Stefanovic-Racic M, Toledo FG, Sauers SE, Goodpaster BH. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete's paradox revisited. American journal of physiology. 2008;294:E882–E888. doi: 10.1152/ajpendo.00769.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrando AA, Lane HW, Stuart CA, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole-body protein synthesis. Am J Physiol (Endocrinol Metab) 1996;270:E627–E633. doi: 10.1152/ajpendo.1996.270.4.E627. [DOI] [PubMed] [Google Scholar]
- 17.Ferrando AA, Stuart CS, Sheffield-Moore M, Wolfe RR. Inactivity amplifies the catabolic response of skeletal muscle to cortisol. J Clin Endocrinol Metab. 1999;84:3515–3521. doi: 10.1210/jcem.84.10.6046. [DOI] [PubMed] [Google Scholar]
- 18.Gelfand RA, Matthews DE, Bier DM, Sherwin RS. Role of counterregulatory hormones in the catabolic response to stress. J Clin Invest. 1984;74:2238–2248. doi: 10.1172/JCI111650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glass DJ. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol. 2003;5:87–90. doi: 10.1038/ncb0203-87. [DOI] [PubMed] [Google Scholar]
- 20.Holt HB, Wild SH, Postle AD, Zhang J, Koster G, Umpleby M, Shojaee-Moradie F, Dewbury K, Wood PJ, Phillips DI, Byrne CD. Cortisol clearance and associations with insulin sensitivity, body fat and fatty liver in middle-aged men. Diabetologia. 2007;50:1024–1032. doi: 10.1007/s00125-007-0629-9. [DOI] [PubMed] [Google Scholar]
- 21.Hulver MW, Dohm GL. The molecular mechanism linking muscle fat accumulation to insulin resistance. Proc Nutr Soc. 2004;63:375–380. doi: 10.1079/pns2004351. [DOI] [PubMed] [Google Scholar]
- 22.Jorfeld L, Warhen J. Leg blood flow during exercise in man. Clin Sci. 1971;41:459–473. doi: 10.1042/cs0410459. [DOI] [PubMed] [Google Scholar]
- 23.Khani S, Tayek JA. Cortisol increases gluconeogenesis in humans: its role in the metabolic syndrome. Clin Sci (Lond) 2001;101:739–747. doi: 10.1042/cs1010739. [DOI] [PubMed] [Google Scholar]
- 24.Klose U. In vivo proton spectroscopy in presence of eddy currents. Magnetic Resonance in Medicine. 1990;14:26–30. doi: 10.1002/mrm.1910140104. [DOI] [PubMed] [Google Scholar]
- 25.Macho L, Koska J, Ksinantova L, Pacak K, Hoff T, Noskov VB, Grigoriev AI, Vigas M, Kvetnansky R. The response of endocrine system to stress loads during space flight in human subject. Adv Space Res. 2003;31:1605–1610. doi: 10.1016/s0273-1177(03)00097-8. [DOI] [PubMed] [Google Scholar]
- 26.Mikines KJ, Richter EA, Dela F, Galbo H. Seven days of bed rest decrease insulin action on glucose uptake in leg and whole body. J Appl Physiol. 1991;70:1245–1254. doi: 10.1152/jappl.1991.70.3.1245. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen MF, Caumo A, Chandramouli V, Schumann WC, Cobelli C, Landau BR, Vilstrup H, Rizza RA, Schmitz O. Impaired basal glucose effectiveness but unaltered fasting glucose release and gluconeogenesis during short-term hypercortisolemia in healthy subjects. Am J Physiol Endocrinol Metab. 2004;286:E102–E110. doi: 10.1152/ajpendo.00566.2002. [DOI] [PubMed] [Google Scholar]
- 28.Nygren J, Thorell A, Efendic S, Nair KS, Ljungqvist O. Site of insulin resistance after surgery: the contribution of hypocaloric nutrition and bed rest. Clin Sci (Lond) 1997;93:137–146. doi: 10.1042/cs0930137. [DOI] [PubMed] [Google Scholar]
- 29.Paddon-Jones D, Sheffield-Moore M, Cree MG, Hewlings SJ, Aarsland A, Wolfe RR, Ferrando AA. Atrophy and impaired muscle protein synthesis during prolonged inactivity and stress. J Clin Endocrinol Metab. 2006;91:4836–4841. doi: 10.1210/jc.2006-0651. [DOI] [PubMed] [Google Scholar]
- 30.Paddon-Jones D, Sheffield-Moore M, Creson DL, Sanford AP, Wolf SE, Wolfe RR, Ferrando AA. Hypercortisolemia alters muscle protein anabolism following ingestion of essential amino acids. Am J Physiol Endocrinol Metab. 2003;284:E946–E953. doi: 10.1152/ajpendo.00397.2002. [DOI] [PubMed] [Google Scholar]
- 31.Paddon-Jones D, Sheffield-Moore M, Urban RJ, Aarsland A, Wolfe RR, Ferrando AA. The catabolic effects of prolonged inactivity and acute hypercortisolemia are offset by dietary supplementation. J Clin Endocrinol Metab. 2005;90:1453–1459. doi: 10.1210/jc.2004-1702. [DOI] [PubMed] [Google Scholar]
- 32.Paddon-Jones D, Sheffield-Moore M, Urban RJ, Sanford AP, Aarsland A, Wolfe RR, Ferrando AA. Essential amino acid and carbohydrate supplementation ameliorates muscle protein loss in humans during 28 days bedrest. J Clin Endocrinol Metab. 2004;89:4351–4358. doi: 10.1210/jc.2003-032159. [DOI] [PubMed] [Google Scholar]
- 33.Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, Vanzulli A, Testolin G, Pozza G, Del Maschio A, Luzi L. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes. 1999;48:1600–1606. doi: 10.2337/diabetes.48.8.1600. [DOI] [PubMed] [Google Scholar]
- 34.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med. 2006;119:S10–S16. doi: 10.1016/j.amjmed.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rico-Sanz J, Thomas EL, Jenkinson G, Mierisova S, Iles R, Bell JD. Diversity in levels of intracellular total creatine and triglycerides in human skeletal muscles observed by 1HMRS. J Appl Physiol. 1999;87:2068–2072. doi: 10.1152/jappl.1999.87.6.2068. [DOI] [PubMed] [Google Scholar]
- 37.Rizza RA, Mandarino LJ, Gerich JE. Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor detect of insulin action. J Clin Endocrinol Metab. 1982;54:131–138. doi: 10.1210/jcem-54-1-131. [DOI] [PubMed] [Google Scholar]
- 38.Ruzzin J, Wagman AS, Jensen J. Glucocorticoid-induced insulin resistance in skeletal muscles: defects in insulin signalling and the effects of a selective glycogen synthase kinase-3 inhibitor. Diabetologia. 2005;48:2119–2130. doi: 10.1007/s00125-005-1886-0. [DOI] [PubMed] [Google Scholar]
- 39.Shangraw RE, Stuart CA, Prince MJ, Peters EJ, Wolfe RR. Insulin responsiveness of protein metabolism in vivo following bedrest in humans. Am J Physiol. 1988;255:E548–E558. doi: 10.1152/ajpendo.1988.255.4.E548. [DOI] [PubMed] [Google Scholar]
- 40.Stein TP, Schluter MD. Excretion of IL-6 by astronauts during spaceflight. The American journal of physiology. 1994;266:E448–E452. doi: 10.1152/ajpendo.1994.266.3.E448. [DOI] [PubMed] [Google Scholar]
- 41.Stein TP, Schulter MD, Boden G. Development of insulin resistance by astronauts during spaceflight. Aviation, space, and environmental medicine. 1994;65:1091–1096. [PubMed] [Google Scholar]
- 42.Strollo F, Riondino G, Harris B, Strollo G, Casarosa E, Mangrossa N, Ferretti C, Luisi M. The effect of microgravity on testicular androgen secretion. Aviation, space, and environmental medicine. 1998;69:133–136. [PubMed] [Google Scholar]
- 43.Stuart CA, Shangraw RE, Prince MJ, Peters EJ, Wolfe RR. Bed-rest-induced insulin resistance occurs primarily in muscle. Metabolism. 1988;37:802–806. doi: 10.1016/0026-0495(88)90018-2. [DOI] [PubMed] [Google Scholar]
- 44.Svanfeldt M, Thorell A, Brismar K, Nygren J, Ljungqvist O. Effects of 3 days of "postoperative" low caloric feeding with or without bed rest on insulin sensitivity in healthy subjects. Clin Nutr. 2003;22:31–38. doi: 10.1054/clnu.2002.0589. [DOI] [PubMed] [Google Scholar]
- 45.Tappy L, Randin D, Vollenweider P, Vollenweider L, Paquot N, Scherrer U, Schneiter P, Nicod P, Jequier E. Mechanisms of dexamethasone-induced insulin resistance in healthy humans. J Clin Endocrinol Metab. 1994;79:1063–1069. doi: 10.1210/jcem.79.4.7962275. [DOI] [PubMed] [Google Scholar]
- 46.Thyfault JP, Cree MG, Zheng D, Zwetsloot JJ, Tapscott EB, Koves TR, Ilkayeva O, Wolfe RR, Muoio DM, Dohm GL. Contraction of insulin resistant muscle normalizes insulin action in association with increased mitochondrial activity and fatty acid catabolism. Am J Physiol Cell Physiol. 2006 doi: 10.1152/ajpcell.00311.2006. [DOI] [PubMed] [Google Scholar]
- 47.Uchakin PNSR, Paddon-Jones D, Tobin BW, Ferrando AA, Wolfe RR. Cytokine secretion and latent herpes virus reactivation with 28 days of horizontal hypokinesia. Aviat Space Environ Med. 2007;78:608–612. [PubMed] [Google Scholar]
- 48.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 49.van den Boogaart A. MRUI MANUAL V. 96.3. A user's guide to the Magnetic Resonance User Interface Software Package. Delft: Delft Technical University Press; 1997. [Google Scholar]
- 50.van den Boogaart A, Van Hecke A, Van Huffel P, Graveron-Demilly S, van Ormondt D, de Beer R. MRUI: a graphical user interface for accurate routine MRS data analysis. Proceedings of the ESMRMB 13th Annual Meeting; Prague. 1996. p. 318. [Google Scholar]
- 51.Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. Journal of Magnetic Resonance. 1997;129:35–43. doi: 10.1006/jmre.1997.1244. [DOI] [PubMed] [Google Scholar]
- 52.Wolfe RR. Regulation of skeletal muscle protein metabolism in catabolic states. J Clin Nutr Metab. 2005;8:61–65. doi: 10.1097/00075197-200501000-00009. [DOI] [PubMed] [Google Scholar]
- 53.Wolfe RR. Regulation of skeletal muscle protein metabolism in catabolic states. Ann Surg. 2005;8:61–65. doi: 10.1097/00075197-200501000-00009. [DOI] [PubMed] [Google Scholar]
- 54.Woolf PD. Hormonal responses to trauma. Crit Care Med. 1992;20:216–226. doi: 10.1097/00003246-199202000-00011. [DOI] [PubMed] [Google Scholar]
- 55.Woolf PD. Hormonal responses to trauma. J Clin Invest. 1984;74:216–226. doi: 10.1097/00003246-199202000-00011. [DOI] [PubMed] [Google Scholar]
- 56.Woolf PD. Hormonal responses to trauma. Crit Care Med. 1992;20:216–226. doi: 10.1097/00003246-199202000-00011. [DOI] [PubMed] [Google Scholar]
- 57.Woolf PD. Hormonal responses to trauma. Am J Physiol. 1996;20:216–226. doi: 10.1097/00003246-199202000-00011. [DOI] [PubMed] [Google Scholar]
- 58.Yanagibori R, Suzuki Y, Kawakubo K, Makita Y, Gunji A. Carbohydrate and lipid metabolism after 20 days of bed rest. Acta Physiol Scand Suppl. 1994;616:51–57. [PubMed] [Google Scholar]