Abstract
A link between celiac disease and schizophrenia has been postulated for several years, based primarily on reports of elevated levels of antibody to gliadin in patients. We sought to examine the proposed connection between schizophrenia and celiac disease by characterizing the molecular specificity and mechanism of the anti-gliadin immune response in a subset of individuals with schizophrenia. Blood samples from individuals with schizophrenia and elevated anti-gliadin antibody titer were examined for celiac disease-associated biomarkers, including antibodies to transglutaminase 2 (TG2) enzyme and deamidated gliadin peptides, as well as the HLA-DQ2 and -DQ8 MHC genes. The anti-gliadin antibody response was further characterized through examination of reactivity towards chromatographically separated gluten proteins. Target proteins of interest were identified by peptide mass mapping. In contrast to celiac disease patients, an association between the anti-gliadin immune response and anti-TG2 antibody or HLA-DQ2 and -DQ8 markers was not found in individuals with schizophrenia. In addition, the majority of individuals with schizophrenia and anti-gliadin antiboy did not exhibit antibody reactivity to deamidated gliadin peptides. Further characterization of the antibody specificity revealed preferential reactivity towards different gluten proteins in the schizophrenia and celiac disease groups. These findings indicate that the anti-gliadin immune response in schizophrenia has a different antigenic specificity from that in celiac disease and is independent of the action of transglutaminase enzyme and HLA-DQ2/DQ8. Meanwhile, the presence of elevated levels of antibodies to specific gluten proteins points to shared immunologic abnormalities in a subset of schizophrenia patients. Further characterization and understanding of the immune response to gluten in schizophrenia may provide novel insights into the etiopathogenesis of specific disease phenotypes.
Keywords: Gluten, gliadin, schizophrenia, gluten sensitivity, celiac disease, antibody
1. Introduction
There is increasing evidence for the involvement of immunologic factors in schizophrenia (Patterson, 2008; Torrey and Yolken, 2001). Among the various reported immunologic abnormalities, increased immune sensitivity to gluten and association with celiac disease have been described in several reports (Bender, 1953; Cascella et al., 2009; Dohan et al., 1972; Graff and Handford, 1961; Jin et al., 2008; Reichelt and Landmark, 1995). Glutens, made up of two main fractions, gliadins and glutenins, are the main storage proteins of wheat and are comprised of about 100 different proteins in a given wheat cultivar (variety). When the various wheat cultivars are considered, the number of different gluten proteins is even greater, although almost all cultivars are made up of the same main types, α-type (also known as α/β type), γ-type, ω-type, low-molecular-weight glutenin subunits, and high-molecular-weight glutenin subunits (Jabri et al., 2005). Gluten sensitivity may be defined as a state of heightened immune response to ingested gluten, the most common clinical manifestation of which is celiac disease, an inflammatory enteropathy that is characterized by villous atrophy and lymphocytic infiltration in the small intestine in genetically predisposed individuals (Alaedini and Green, 2005). Genetic susceptibility for celiac disease is associated with close genetic linkage to class II human leukocyte antigens (HLA) DQ2 (DQA1 *05/DQB1 02) and DQ8 (DQA1 *0301/DQB1 *0302) that are involved in the presentation of gluten peptides to T cells (Louka and Sollid, 2003). Presence of antibodies to gluten and to the enzyme transglutaminase 2 (TG2) is a hallmark of celiac disease. The TG2 protein is an important element of the disease, not only as a target autoantigen, but also as an enzyme that enhances the immunostimulatory effect of gluten peptides through the deamidation and conversion of glutamines to glutamate residues (Briani et al., 2008). In fact, antibodies to selectively deamidated gluten are now known to be more specific for celiac disease than antibodies against the native species (Mothes, 2007; Naiyer et al., 2008).
The clinical presentation of celiac disease is highly variable, ranging from asymptomatic to severe malnutrition. In the now commonly diagnosed atypical form, gastrointestinal symptoms may be absent or less pronounced, while patients present with extra-intestinal features such as anemia, osteoporosis, short stature, and infertility (Alaedini and Green, 2005). Neurologic and psychiatric abnormalities have also been reported to be associated with celiac disease in various studies (Bushara, 2005). In addition, elevated levels of anti-gliadin antibody have been associated with some of these deficits, even in the apparent absence of the characteristic mucosal pathology (Burk et al., 2001; Bushara et al., 2001; Hadjivassiliou et al., 1998). The current literature indicates shared immunologic abnormalities and genetic associations between schizophrenia and celiac disease and is suggestive of an elevated relative risk of non-affective psychosis in celiac patients (Jungerius et al., 2008; Kalaydjian et al., 2006; Wei and Hemmings, 2005). A number of case studies and small trials indicate significant positive response to gluten-free diet in some individuals with schizophrenia (Kalaydjian et al., 2006). However, methodological shortcomings in some of these studies and contradictory findings by other groups have led to a lack of consensus on the relevance of gluten in schizophrenia (Kalaydjian et al., 2006). Specifically, the prevalence of true celiac disease versus anti-gliadin antibodies in patients with schizophrenia is a subject of contention. In addition, little is known about the specificity and mechanism of the immune response to gluten in these patients, or its role in disease pathogenesis. The aim of this study was to investigate the link between gluten sensitivity and schizophrenia by characterizing the immune response in patients and control subjects. Our results reveal an immunologic response to gluten in individuals with schizophrenia that is clearly different from that in celiac disease.
2. Materials and methods
2.1. Subjects
Serum samples from 17 individuals with schizophrenia (9 female, 8 male; mean age 37.6 ± 3.0 y), selected because of previously documented elevated IgG and IgA antibodies to gliadin (hereon referred to as gluten-sensitive individuals with schizophrenia), were studied. The initial screening of patients for antibodies to gliadin had been done using a commercially available ELISA kit (IBL International). The characteristics of the population from which these individuals was selected have been described previously (Dickerson et al., 2003). In addition, serum specimens from 25 biopsy-proven celiac disease patients with elevated IgG and IgA antibody to gliadin and 20 healthy subjects without elevated levels of anti-gliadin antibody were included in the study as controls. Diagnosis of celiac disease was made according to previously described criteria (Alaedini and Green, 2005).
2.2. Animal immunization
New Zealand White rabbits (n=2) were immunized using a commercial gliadin extract (Sigma-Aldrich). Immunizations were carried out with injection of 200 μg of gliadin protein in Complete Freund’s Adjuvant on day 0, and 100 μg on days 14, 28, and 42. Serum was collected on day 77.
2.3. Anti-gliadin antibody detection
All serum samples were tested for anti-gliadin antibody by ELISA as previously described (Alaedini et al., 2007). Briefly, 96-well round-bottom polystyrene plates (BD Biosciences) were coated with 50 μL/well of a 0.01 mg/mL solution of gliadin (Sigma-Aldrich) in 0.1 M carbonate buffer (pH 9.6). Control wells were coated only with buffer. After overnight incubation at 4°C, all wells were washed and blocked by incubation with 1% BSA in PBS-T for 1.5 h at room temperature. Serum samples were diluted at 1:200 for IgA measurement and 1:800 for IgG measurement and added at 50 μL/well in duplicates. After washing the wells, they were incubated with peroxidase-conjugated goat anti-human IgG (Amersham Biosciences) or IgA (MP Biomedicals) secondary antibody for 1 h. After washing the wells, developing solution, comprising 27 mM citric acid, 50 mM Na2HPO4, 5.5 mM o-phenylenediamine, and 0.01% H2O2 (pH 5), was added. Absorbance was measured at 450 nm after incubating the plates for 30 min. Absorbance values were corrected for non-specific binding by subtraction of the absorbance of the corresponding non-coated wells. Cutoff values were assigned as two standard deviations above the mean for the healthy control group results.
2.4. Anti-TG2 antibody detection
IgA antibodies to recombinant human TG2 were measured in all serum samples that were positive for IgG and/or IgA anti-gliadin antibodies using an ELISA kit, according to the manufacturer’s protocol (Euroimmun AG).
2.5. HLA typing
The HLA-DQ2 and -DQ8 haplotypes were measured by real time polymerase chain reaction as previously described in 13 of the individuals with schizophrenia for whom whole blood was available (Reinton et al., 2006).
2.6. Anti-deamidated gliadin antibody detection
Serum samples were tested for reactivity to a previously described glutamine-glutamate substituted trimer of a fusion peptide containing the sequences PLQPEQPFP and PEQLPQFEE (Schwertz et al., 2004). Antibody measurement was done by ELISA, according to the manufacturer’s protocol (Euroimmun AG).
2.7. Extraction and fractionation of gluten proteins
Twenty grams of flour (endosperm) from the wheat cultivar Cheyenne were sifted into 200 mL of 0.01 M acetic acid and mixed for 30 min at 35 °C. The dissolved fraction was separated by centrifugation and precipitated by the addition of 600 mL of 1.5% NaCl (room temperature, 15 min). The precipitate was collected by centrifugation and dissolved in 0.01 M acetic acid. The resulting solution (155 mL) was dialyzed (cutoff 6,000–8,000 Da) once against 0.01 M acetic acid overnight at 4 °C and twice more under the same conditions against de-ionized water. The solution remaining in the dialysis tubing was lyophilized to collect 1 g of gluten protein. Gel electrophoresis of the resulting mixture indicated the clear presence of all main types of gluten proteins. Extracted proteins (220 mg) were fractionated by size exclusion chromatography on a BioGel P-100 column (2 × 100 cm) (Bio-Rad) equilibrated with 0.1 M acetic acid and the elution pattern was followed at 280 nm (Figure 2). The eluate was divided into 6 fractions, which were lyophilized. Fraction 1 contained high and low molecular weight glutenins, fraction 2 had ω-gliadins and low molecular weight glutenins, and fractions 3 through 6 were comprised primarily of α/β- and γ-gliadins.
Figure 2.
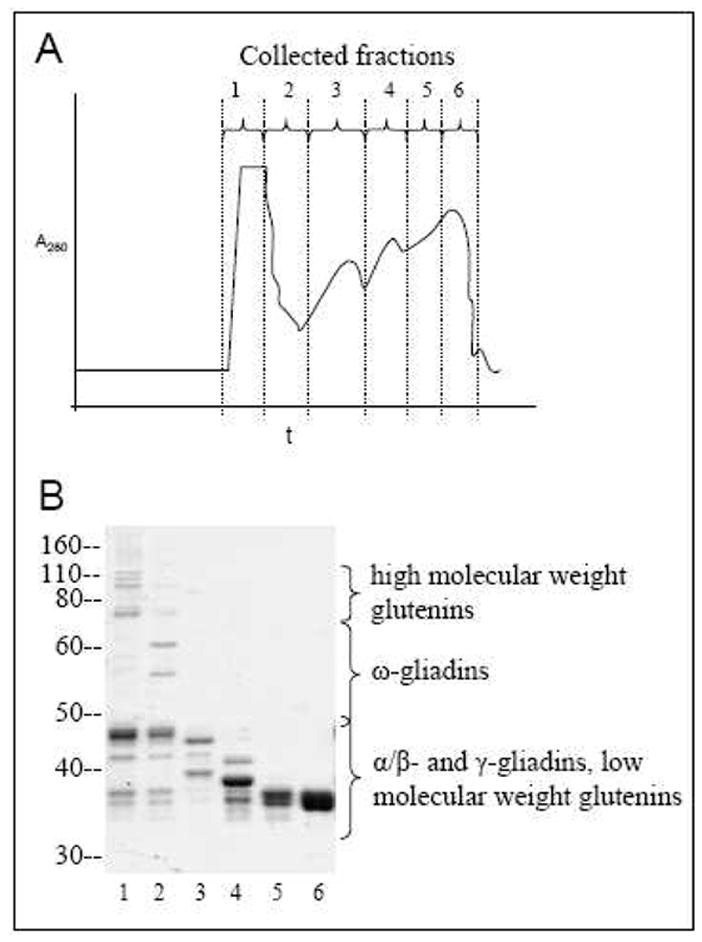
A) Chromatogram of gluten proteins eluted from the BioGel P-100 size exclusion column, following acetic acid extraction. B) SDS-PAGE profile (Coomassie-stained) of fractions 1–6 of gluten proteins collected from the column.
2.8. Immunoblotting
Western blot was carried out using the six separated gluten fractions from above. Protein samples (0.66 μg protein/lane) were dissolved in sample buffer, heated for 10 min at 75°C, and separated by SDS-PAGE using NuPAGE 10% bis-tris gels (Invitrogen). Transfer onto nitrocellulose membrane (Pall Corporation) using tris-glycine buffer containing 20% methanol was done overnight. The membrane was incubated for 2 h in blocking solution (5% milk + 0.5% BSA in TBS-T). Incubation with patient and control serum specimens (1:2,000 – 1:6,000) or gliadin-immunized rabbit serum (1:10,000) in dilution buffer (10% blocking solution + 10% fetal bovine serum in TBS-T) was done for 1 h. The secondary antibody used was HRP-conjugated anti-human or anti-rabbit IgG (Amersham Biosciences). Detection of bound antibodies was by the ECL system (Millipore) and autoradiography film (Fuji or Kodak).
Reactivity of antibodies from several patients with a protein doublet at ~33 kDa was further characterized as follows. Individual bands of the doublet were excised from the gel and destained with 0.05M Tris (pH 8.5)/30% acetonitrile for 30 min at 37 °C. Gel pieces were washed once with 0.05M Tris (pH 8.5)/30% acetonitrile and 3 times with water. Gel bands were crushed and incubated in 0.1 M NaOH for 20 min at 37 °C. Sample buffer was added directly to the crushed gel pieces, and SDS-PAGE, transfer, and immunoblotting were carried out as before.
2.9. Mass spectrometry-assisted identification of proteins
Individual protein bands of interest in the reactive doublet of fraction 5 from above (~33 kDa) were excised from the gel, washed, and destained. Each band was reduced by adding 100 μL of 0.01 M DTT in 0.1 M Tris (pH 8.5) and heating (55°C, 1–2 h). After cooling, the liquid was removed and replaced with 100 μL of 0.015 M iodoacetamide in 0.1 M Tris (pH 8.5). The reaction was allowed to proceed for 30 min in the dark, after which the liquid was removed. The gel bands were prepared for digestion by washing once with 200 μL of 0.05 M Tris (pH 8.5)/25% acetonitrile and twice with 200 μL of 0.05 M Tris (pH 8.5)/50% acetonitrile for 20 min with shaking. After removing the washes, the gel was dried for 30 min in a Speed-Vac concentrator. The gel bands were digested by adding 0.1 μg of subtilisin (Sigma) in 10 μL of 0.025 M Tris (pH 8.5) and an additional 10–15 μL of buffer, followed by incubation in a heating block (37°C, 2 h). Peptides were extracted twice with 50% acetonitrile/2% trifluoroacetic acid and the combined extracts were reduced in volume and analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) using a Micromass Q-TOF hybrid quadrupole/time-of-flight mass spectrometer with a nanoelectrospray source (Waters). Capillary voltage was set at 1.8 kV and cone voltage at 32V; collision energy was set according to mass and charge of the ion from 14 eV to 50 eV. Chromatography was performed on an LC Packings HPLC system (Dionex) with a C18 PepMap column (Dionex) using a linear acetonitrile gradient at 200 nL/min. Raw data files were processed using the MassLynx ProteinLynx software (Waters), and .pkl files were subjected to a search, using the Mascot program at www.matrixscience.com.
2.10. Data analysis
Group differences were analyzed by the Mann-Whitney U test or Welch’s t-test (continuous data), and Fisher’s exact test (nominal data). Differences with P values of <0.05 were considered to be significant. Continuous data are expressed as mean ± standard error of mean (SEM).
3. Results
3.1. Anti-gliadin antibody
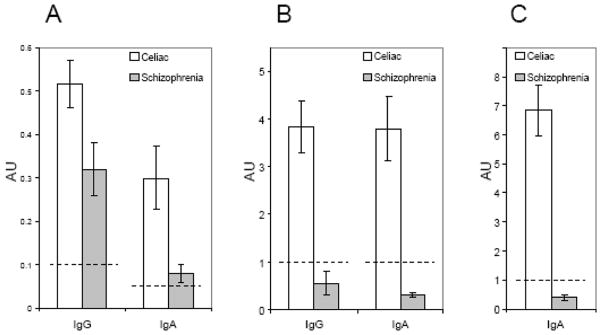
The levels of IgG and IgA anti-gliadin antibodies were compared in schizophrenia and celiac disease subjects who were previously found to be positive. As depicted in figure 1, the levels of IgG and IgA anti-gliadin antibodies were lower in the schizophrenia group as compared to the celiac disease group (P<0.05 for IgG, P<0.005 for IgA).
Figure 1.
Comparison of mean antibody levels in celiac disease and gluten-sensitive schizophrenia patients. A) Antibodies to native gliadin. Individuals with schizophrenia that were positive for anti-gliadin antibodies had lower levels of IgG (P<0.05) and IgA (P<0.005) antibodies to gliadin than celiac disease patients. B) Antibodies to a deamidated (glutamine-glutamate substituted) trimer of a fusion gliadin peptide containing PLQPEQPFP and PEQLPQFEE sequences in the same patients as in panel A. Individuals with schizophrenia who were positive for anti-gliadin antibodies had significantly lower mean IgG and IgA antibody reactivity to deamidated gliadin than celiac disease patients (P<0.001). C) IgA antibodies to recombinant human TG2. Levels were significantly lower in the schizophrenia group than the celiac disease group (P<0.001). Dotted lines indicate established cutoff values for positivity. Error bars represent the standard error of the mean.
3.2. Anti-TG2 antibody
IgA antibodies against human TG2, which have a high sensitivity and specificity for celiac disease, were measured in all gluten-sensitive patients and controls. While 23 of 25 (92 %) celiac disease patients had IgA anti-TG2 antibody reactivity, only 1 of the 17 (6 %) gluten-sensitive schizophrenia patients was positive (P<0.001). Comparison of normalized mean antibody reactivity for the two groups also revealed significantly lower levels of anti-TG2 IgA antibodies in schizophrenia patients (P<0.001) (Figure 1C). None of the healthy control serum samples were positive.
3.3. HLA typing
There was not a significantly increased correlation between gluten sensitivity and HLA-DQ2/DQ8 haplotypes in patients with schizophrenia as compared with the general population. 5/13 (38.5 %) of the gluten-sensitive individuals with schizophrenia displayed HLA-DQ2 and/or DQ8 (1 DQ2, 4 DQ8) haplotypes. In most populations, 90% or more of celiac disease patients carry the DQ2 haplotype and the rest have DQ8, compared to 20%–40% in healthy individuals having either (Alaedini and Green, 2005; Sollid, 2000).
3.4. Anti-deamidated gliadin antibody
IgG and IgA antibodies to a deamidated (glutamine-glutamate substituted) fusion gliadin peptide trimer were measured in all subjects. While 22 of 25 (88 %) celiac disease patients exhibited IgG antibody reactivity to deamidated gliadin, only 1 of the 17 (6 %) gluten-sensitive individuals with schizophrenia was positive (P<0.001). Similarly, 23 of 25 (92 %) celiac disease patients and none of the 17 gluten-sensitive individuals with schizophrenia were positive for the IgA antibodies to deamidated gliadin (P<0.001). Comparison of normalized mean antibody reactivity for the two groups also indicated significantly lower levels of IgG and IgA antibodies to the modified peptide in schizophrenia patients (P<0.001) (Figure 1B). None of the healthy control serum samples were positive for antibodies to deamidated gliadin.
3.5. Immunoblotting
Following the measurement of antibody levels by ELISA, serum specimens from representative subjects were further characterized with regard to reactivity towards fractionated gluten proteins. The gluten proteins used in this study were purified by extraction with acetic acid and size exclusion chromatography. The eluted six fractions were further separated by SDS-PAGE (Figure 2) and transferred to nitrocellulose membrane for immunoblotting. The observed antibody reactivity to the gluten proteins was heterogeneous in immunized rabbits, celiac disease patients, and schizophrenia patients. However, while antibodies from celiac patients and immunized rabbits bound to many gliadins and/or glutenins, antibodies from the individuals with schizophrenia bound to a relatively smaller set of gluten molecules (Figure 3). Serum samples from a few of these patients displayed an antibody specificity profile that was similar to celiac disease (Figure 3, patient S4), but antibodies from most sera bound to a limited repertoire of proteins in the glutenin and gliadin fractions (Figure 3, patients S1–S3). Antibodies from 6 individuals with schizophrenia reacted with a protein band of ~33 kDa in gluten fraction 5, either exclusively, or in conjunction with weaker binding to glutenin proteins in fractions 1 and 2. None of the celiac disease sera exhibited exclusive or prominent reactivity to this protein band. The band appeared as a doublet in Coomassie-stained gels. In order to determine which member of the protein doublet was targeted by the anti-gluten antibodies of patients with schizophrenia, protein bands were individually excised from Coomassie-stained gel, extracted, and re-analyzed. Patient antibody reactivity was found to be directed primarily at the upper band, with only residual binding to the lower band (Figure 4).
Figure 3.
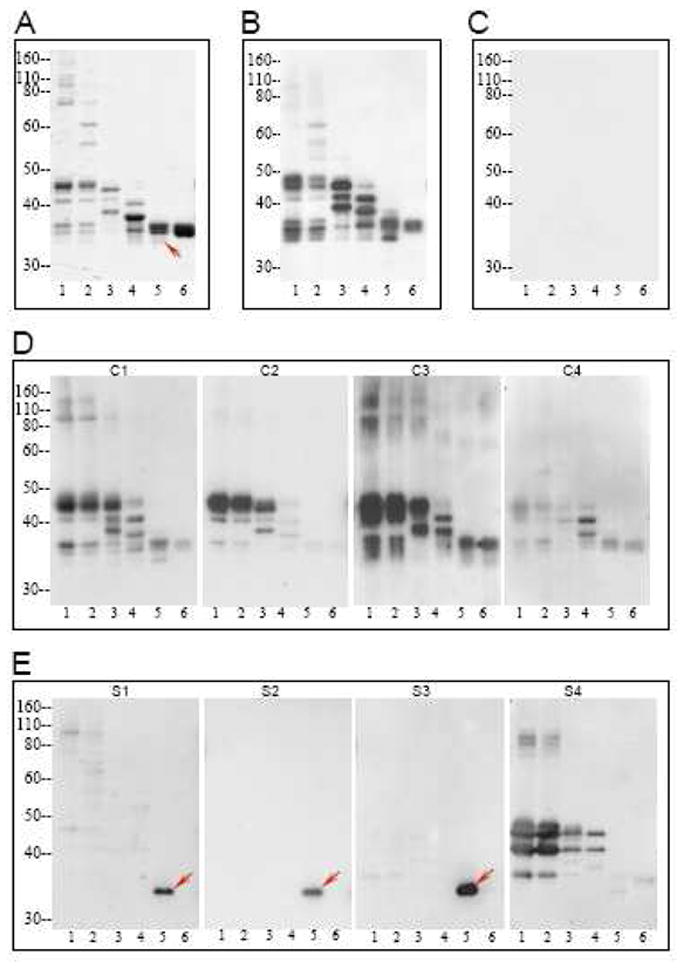
Pattern of antibody reactivity to gluten proteins of the Cheyenne cultivar in individuals with schizophrenia and controls. A) SDS-PAGE profile of fractions 1–6 of gluten proteins. B–E) Reactivity of serum antibodies from gliadin-immunized rabbits (B), a representative healthy control (C), representative patients 1–4 with celiac disease (D), and representative patients 1–4 with schizophrenia and elevated anti-gliadin antibody (E) towards transferred gluten proteins from panel A. Detection of bound antibodies was by the ECL system and autoradiography film. Arrows in panels A and E point to the ~33 kDa doublet to which antibody reactivity was found in schizophrenia patients.
Figure 4.
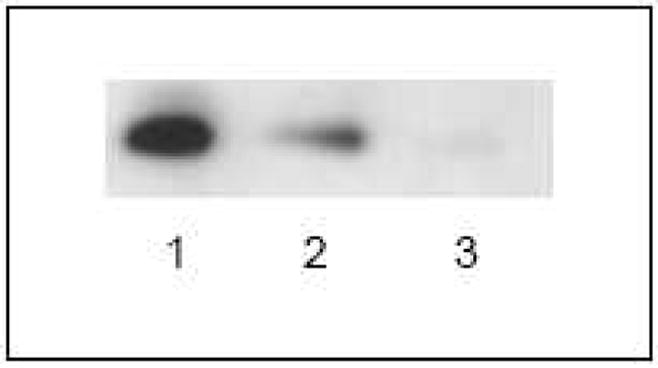
Characterization of the specificity of serum antibody to the ~33 kDa doublet. Protein bands were individually excised, extracted, and re-analyzed by immunoblotting with the serum of representative individuals with schizophrenia in order to identify the target protein. Lane 1) Protein doublet, containing upper and lower bands; lane 2) upper protein band; lane 3) lower protein band. Patient immune reactivity was found to be directed primarily at the upper band.
3.6. Identification of ~33 kDa target proteins
The protein bands of the ~33 kDa doublet were individually excised, digested with subtilisin after chemical modification, and analyzed by LC/MS-MS. The detected peptide masses were searched against the NCBI protein sequence database. Results of the database search revealed two γ-gliadins (accession numbers ABO37962 and AAQ63857) as the top two significant candidate proteins in the immunoreactive upper band, with 15 and 11 peptides matched and 42% and 32% sequence coverage, respectively. Similarly, the database search revealed two nearly identical γ-gliadins (accession numbers ABO37962 and AAK84776) as the top two significant candidate proteins in the lower band of the doublet, with 34 peptides matched (both proteins) and 58% and 57% sequence coverage, respectively.
4. Discussion
Schizophrenia is a complex disorder affecting approximately 1% of the population and is caused by a combination of genetic and environmental factors. The immune system is believed to play a considerable role in the disease process. Recently-reported single nucleotide polymorphism (SNP)-based genome-wide studies in schizophrenia populations show significant association with several markers spanning the major histocompatibility complex (MHC) region on chromosome 6 (Purcell et al., 2009; Shi et al., 2009; Stefansson et al., 2009). Dating back to the 1950s, schizophrenia has been linked to anti-gliadin antibodies and celiac disease in several studies (Bender, 1953; Cascella et al., 2009; Dohan et al., 1972; Graff and Handford, 1961; Jin et al., 2008; Reichelt and Landmark, 1995). A number of case reports and small trials also indicate significant positive response to gluten-free diet in some individuals with schizophrenia (Kalaydjian et al., 2006). Considering the limited response to traditional therapies by many individuals with schizophrenia and the side effects of current treatments, identification of a subset of patients who might benefit from an effective dietary approach without significant side effects would represent an important step forward in the understanding and treatment of the disease. However, the currently limited knowledge of the significance of anti-gliadin antibodies in the context of schizophrenia in general, combined with the results of a number of studies that do not support a link with celiac disease, have cast doubt on the relevance of gluten and gluten sensitivity to schizophrenia (Lambert et al., 1989; Ludvigsson et al., 2007).
In this study, our aim was to address these issues by assessing the prevalence of celiac disease-associated biomarkers and through the characterization of the anti-gluten immune response at the molecular level in individuals with schizophrenia and elevated anti-gliadin antibodies and in control subjects. We found no correlation between elevated anti-gliadin antibodies and the presence of increased anti-TG2 antibody levels or celiac-associated HLA genes in individuals with schizophrenia. Considering the high negative predictive value of the anti-TG2 antibody and HLA markers for celiac disease, it can be concluded with high certainty that the majority of these patients do not have celiac disease. We have also shown that the anti-gliadin antibody responses in celiac disease and gluten-sensitive individuals with schizophrenia are not necessarily directed at the same molecules. Equally interesting is the finding that the antibodies in schizophrenia do not appear to show significant affinity for deamidated gliadin peptides, again implying the absence of celiac disease in the majority of gluten-sensitive individuals with schizophrenia. As such, the anti-gluten immune response in schizophrenia patients is likely to be independent of the TG2 enzyme and to not require HLA-DQ2 and/or -DQ8 molecules for driving the process of antigen presentation and antibody production.
It is important to note that the sample size in this study is not large enough to exclude the possibility of celiac-associated markers (HLA-DQ2/DQ8 genes, anti-TG2 antibodies, anti-deamidated gliadin antibodies), and thereby celiac disease, being more prevalent in schizophrenia patients than in normal healthy individuals. However, it is adequately large to determine with high certainty that unlike in celiac disease, the anti-gluten immune response in schizophrenia is not associated with the above-mentioned biomarkers and therefore does not imply celiac disease in the overwhelming majority of individuals. This agrees with the results of a recently published large study, which found that most schizophrenia patients with elevated anti-gliadin antibody titers do not have anti-TG2 and anti-endomysial antibodies (Cascella et al., 2009). Nevertheless, anti-TG2 antibodies were reported to be found in 5.4% of schizophrenia patients versus 0.80% of the control group, implying increased prevalence of celiac disease among schizophrenia patients. However, this finding contradicts the absence of any difference in the incidence of anti-endomysial antibody between the two groups in the same study. Antibodies to endomysial tissue primarily target the TG2 enzyme and a very high correlation between anti-TG2 and anti-endomysial antibodies has been established previously (Rostom et al., 2006).
The observation that some schizophrenia patients have elevated anti-gliadin antibodies is intriguing. While the majority of these individuals do not appear to have celiac disease, the presence of antibodies to gluten points to the existence of shared mechanisms with celiac disease. Increased intestinal permeability, a presumed essential event in the triggering of celiac disease, may be one such factor. In fact, abnormal intestinal permeability in the absence of established bowel disease has been described in some individuals with chronic psychiatric disorders (Wood et al., 1987). More recent studies indicate that common variants of the gene myosin IXB (MYO9B), which encodes a myosin molecule involved in actin remodeling of epithelial enterocytes, might be associated with an increased risk of developing celiac disease and schizophrenia (Jungerius et al., 2008; Monsuur et al., 2005). If confirmed by other investigators, the reported shared genetic susceptibility would shed light on the mechanism of impairment of the epithelial barrier in at least a subset of celiac and schizophrenia patients and partly explain the prevalence of the immune response to gluten peptides, albeit being targeted at different protein species and epitopes, in both diseases.
Can the observed anti-gliadin antibodies have a role in the etiopathogenesis of schizophrenia in some patients? While antibodies to gluten have not been shown to have a pathogenic role in any disease, some possibilities may be considered. Antibodies to foreign proteins have been shown to cross-react with certain self antigens and elicit disease (Raveche et al., 2005; Yuki et al., 2004). As such, they can act as autoantibodies and have the potential to exert a pathogenic effect through various mechanisms, including direct interaction with their target, activation of complement, or binding to Fc receptors on macrophages, neutrophils, and other immune cells (Alaedini and Green, 2008; Dalakas, 2008). Antibodies can also be involved in disease pathogenesis through the activation of toll-like receptor pathways and secretion of various inflammatory molecules that affect the function of other cells, some of which could very well have a role in cognitive and neurodevelopmental defects (Crow, 2007; Nawa and Takei, 2006). The reported association of elevated titers of antibodies against different pathogens with impaired cognitive function in schizophrenia might be influenced by such a mechanism (Shirts et al., 2008). Alternatively, the antibody response might not play any role itself, instead being only indicative of the presence of immunogenic gluten peptides in the circulation. Increased urinary excretion of gluten peptides in patients with schizophrenia has been observed previously (Reichelt and Landmark, 1995). Referred to as gluten exorphins, these peptides have been shown to have potent opioid-like properties and to affect hormonal balance, behavior, and learning in animal models (Fanciulli et al., 2005; Fanciulli et al., 2002; Takahashi et al., 2000). Therefore, the reported effect of gluten-free diet in some patients might be explained by the direct role some gluten peptides can exert on the nervous system.
Increased levels of anti-gliadin antibodies in schizophrenia point to a mechanism involving the disruption of intestinal permeability and immunologic abnormalities in some individuals with schizophrenia. However, the mechanism of the observed antibody reactivity to gluten in schizophrenia appears to be fundamentally different from celiac disease. As such, the heightened immune response to gluten deserves further attention and research in determining its importance and relevance to the pathogenesis of schizophrenia, independent of concomitant celiac disease. Further detailed characterization of the antibody response to gluten and other dietary proteins of concern, including casein, is likely to enhance our understanding of the significance of the immune system and its response to foreign antigens in schizophrenia and other neuropsychiatric abnormalities. The elucidation of this relationship may lead to new methods for the diagnosis, prevention, and treatment of schizophrenia and related disorders.
Acknowledgments
This work was supported by The Stanley Medical Research Institute Grant 08R-2061 and NIH-National Institute of Neurological Disorders and Stroke Grant 5R21NS055323-02. We thank Dr. Mary Ann Gawinowicz at the Protein Core Facility of Columbia University for mass spectrometric analyses and insightful discussions. We would also like to thank Ms. Maria Minaya for help in collecting and organizing the celiac disease specimens, and Ms. Nancy Laird for technical assistance in the preparation of gluten samples. We are grateful to all the participants involved in this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alaedini A, Green PH. Narrative review: celiac disease: understanding a complex autoimmune disorder. Ann Intern Med. 2005;142:289–298. doi: 10.7326/0003-4819-142-4-200502150-00011. [DOI] [PubMed] [Google Scholar]
- Alaedini A, Green PH. Autoantibodies in celiac disease. Autoimmunity. 2008;41:19–26. doi: 10.1080/08916930701619219. [DOI] [PubMed] [Google Scholar]
- Alaedini A, Okamoto H, Briani C, et al. Immune cross-reactivity in celiac disease: anti-gliadin antibodies bind to neuronal synapsin I. J Immunol. 2007;178:6590–6595. doi: 10.4049/jimmunol.178.10.6590. [DOI] [PubMed] [Google Scholar]
- Bender L. Childhood schizophrenia. Psychiatr Q. 1953;27:663–681. doi: 10.1007/BF01562517. [DOI] [PubMed] [Google Scholar]
- Briani C, Samaroo D, Alaedini A. Celiac disease: from gluten to autoimmunity. Autoimmun Rev. 2008;7:644–650. doi: 10.1016/j.autrev.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Burk K, Bosch S, Muller CA, et al. Sporadic cerebellar ataxia associated with gluten sensitivity. Brain. 2001;124:1013–1019. doi: 10.1093/brain/124.5.1013. [DOI] [PubMed] [Google Scholar]
- Bushara KO. Neurologic presentation of celiac disease. Gastroenterology. 2005;128:S92–97. doi: 10.1053/j.gastro.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bushara KO, Goebel SU, Shill H, Goldfarb LG, Hallett M. Gluten sensitivity in sporadic and hereditary cerebellar ataxia. Ann Neurol. 2001;49:540–543. [PubMed] [Google Scholar]
- Cascella NG, Kryszak D, Bhatti B, et al. Prevalence of Celiac Disease and Gluten Sensitivity in the United States Clinical Antipsychotic Trials of Intervention Effectiveness Study Population. Schizophr Bull. 2009 doi: 10.1093/schbul/sbp055. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow MK. Type I interferon in systemic lupus erythematosus. In: Pitha PM, editor. CTMI. Vol. 316. Springer: Berlin Heidelberg; 2007. pp. 359–386. [DOI] [PubMed] [Google Scholar]
- Dalakas MC. B cells as therapeutic targets in autoimmune neurological disorders. Nat Clin Pract Neurol. 2008;4:557–567. doi: 10.1038/ncpneuro0901. [DOI] [PubMed] [Google Scholar]
- Dickerson FB, Boronow JJ, Stallings C, Origoni AE, Ruslanova I, Yolken RH. Association of serum antibodies to herpes simplex virus 1 with cognitive deficits in individuals with schizophrenia. Arch Gen Psychiatry. 2003;60:466–472. doi: 10.1001/archpsyc.60.5.466. [DOI] [PubMed] [Google Scholar]
- Dohan FC, Martin L, Grasberger JC, Boehme D, Cottrell JC. Antibodies to wheat gliadin in blood of psychiatric patients: possible role of emotional factors. Biol Psychiatry. 1972;5:127–137. [PubMed] [Google Scholar]
- Fanciulli G, Dettori A, Demontis MP, Tomasi PA, Anania V, Delitala G. Gluten exorphin B5 stimulates prolactin secretion through opioid receptors located outside the blood-brain barrier. Life Sci. 2005;76:1713–1719. doi: 10.1016/j.lfs.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Fanciulli G, Dettori A, Tomasi PA, et al. Prolactin and growth hormone response to intracerebroventricular administration of the food opioid peptide gluten exorphin B5 in rats. Life Sci. 2002;71:2383–2390. doi: 10.1016/s0024-3205(02)02036-2. [DOI] [PubMed] [Google Scholar]
- Graff H, Handford A. Celiac syndrome in the case histories of five schizophrenics. Psychiatr Q. 1961;35:306–313. doi: 10.1007/BF01566581. [DOI] [PubMed] [Google Scholar]
- Hadjivassiliou M, Grunewald RA, Chattopadhyay AK, et al. Clinical, radiological, neurophysiological, and neuropathological characteristics of gluten ataxia. Lancet. 1998;352:1582–1585. doi: 10.1016/s0140-6736(98)05342-2. [DOI] [PubMed] [Google Scholar]
- Jabri B, Kasarda DD, Green PH. Innate and adaptive immunity: the yin and yang of celiac disease. Immunol Rev. 2005;206:219–231. doi: 10.1111/j.0105-2896.2005.00294.x. [DOI] [PubMed] [Google Scholar]
- Jin SZ, Xu Q, Wu N, et al. A study of gluten antibody levels in serum among patients with schizophrenia. Schizophr Res. 2008;98:S145. [Google Scholar]
- Jungerius BJ, Bakker SC, Monsuur AJ, Sinke RJ, Kahn RS, Wijmenga C. Is MYO9B the missing link between schizophrenia and celiac disease? Am J Med Genet B Neuropsychiatr Genet. 2008;147:351–355. doi: 10.1002/ajmg.b.30605. [DOI] [PubMed] [Google Scholar]
- Kalaydjian AE, Eaton W, Cascella N, Fasano A. The gluten connection: the association between schizophrenia and celiac disease. Acta Psychiatr Scand. 2006;113:82–90. doi: 10.1111/j.1600-0447.2005.00687.x. [DOI] [PubMed] [Google Scholar]
- Lambert MT, Bjarnason I, Connelly J, et al. Small intestine permeability in schizophrenia. Br J Psychiatry. 1989;155:619–622. doi: 10.1192/s0007125000018092. [DOI] [PubMed] [Google Scholar]
- Louka AS, Sollid LM. HLA in coeliac disease: unravelling the complex genetics of a complex disorder. Tissue Antigens. 2003;61:105–117. doi: 10.1034/j.1399-0039.2003.00017.x. [DOI] [PubMed] [Google Scholar]
- Ludvigsson JF, Osby U, Ekbom A, Montgomery SM. Coeliac disease and risk of schizophrenia and other psychosis: a general population cohort study. Scand J Gastroenterol. 2007;42:179–185. doi: 10.1080/00365520600863472. [DOI] [PubMed] [Google Scholar]
- Monsuur AJ, de Bakker PI, Alizadeh BZ, et al. Myosin IXB variant increases the risk of celiac disease and points toward a primary intestinal barrier defect. Nat Genet. 2005;37:1341–1344. doi: 10.1038/ng1680. [DOI] [PubMed] [Google Scholar]
- Mothes T. Deamidated gliadin peptides as targets for celiac disease-specific antibodies. Adv Clin Chem. 2007;44:35–63. doi: 10.1016/s0065-2423(07)44002-1. [DOI] [PubMed] [Google Scholar]
- Naiyer AJ, Hernandez L, Ciaccio EJ, et al. Comparison of commercially available serologic kits for the detection of celiac disease. J Clin Gastroenterol. 2009;43:225–232. doi: 10.1097/MCG.0b013e31816200e5. [DOI] [PubMed] [Google Scholar]
- Nawa H, Takei N. Recent progress in animal modeling of immune inflammatory processes in schizophrenia: implication of specific cytokines. Neurosci Res. 2006;56:2–13. doi: 10.1016/j.neures.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: Etiology, pathology and animal models. Behav Brain Res. 2008 doi: 10.1016/j.bbr.2008.12.016. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Purcell SM, Wray NR, Stone JL, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009 doi: 10.1038/nature08185. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raveche ES, Schutzer SE, Fernandes H, et al. Evidence of Borrelia autoimmunity-induced component of Lyme carditis and arthritis. J Clin Microbiol. 2005;43:850–856. doi: 10.1128/JCM.43.2.850-856.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt KL, Landmark J. Specific IgA antibody increases in schizophrenia. Biol Psychiatry. 1995;37:410–413. doi: 10.1016/0006-3223(94)00176-4. [DOI] [PubMed] [Google Scholar]
- Reinton N, Helgheim A, Shegarfi H, Moghaddam A. A one-step real-time PCR assay for detection of DQA1*05, DQB1*02 and DQB1*0302 to aid diagnosis of celiac disease. J Immunol Methods. 2006;316:125–132. doi: 10.1016/j.jim.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Rostom A, Murray JA, Kagnoff MF. American Gastroenterological Association (AGA) Institute technical review on the diagnosis and management of celiac disease. Gastroenterology. 2006;131:1981–2002. doi: 10.1053/j.gastro.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Schwertz E, Kahlenberg F, Sack U, et al. Serologic assay based on gliadin-related nonapeptides as a highly sensitive and specific diagnostic aid in celiac disease. Clin Chem. 2004;50:2370–2375. doi: 10.1373/clinchem.2004.036111. [DOI] [PubMed] [Google Scholar]
- Shi J, Levinson DF, Duan J, et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009 doi: 10.1038/nature08192. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirts BH, Prasad KM, Pogue-Geile MF, Dickerson F, Yolken RH, Nimgaonkar VL. Antibodies to cytomegalovirus and Herpes Simplex Virus 1 associated with cognitive function in schizophrenia. Schizophr Res. 2008;106:268–274. doi: 10.1016/j.schres.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollid LM. Molecular basis of celiac disease. Annu Rev Immunol. 2000;18:53–81. doi: 10.1146/annurev.immunol.18.1.53. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, Steinberg S, et al. Common variants conferring risk of schizophrenia. Nature. 2009 doi: 10.1038/nature08186. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Fukunaga H, Kaneto H, Fukudome S, Yoshikawa M. Behavioral and pharmacological studies on gluten exorphin A5, a newly isolated bioactive food protein fragment, in mice. Jpn J Pharmacol. 2000;84:259–265. doi: 10.1254/jjp.84.259. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Yolken RH. The schizophrenia-rheumatoid arthritis connection: infectious, immune, or both? Brain Behav Immun. 2001;15:401–410. doi: 10.1006/brbi.2001.0649. [DOI] [PubMed] [Google Scholar]
- Wei J, Hemmings GP. Gene, gut and schizophrenia: the meeting point for the gene-environment interaction in developing schizophrenia. Med Hypotheses. 2005;64:547–552. doi: 10.1016/j.mehy.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Wood NC, Hamilton I, Axon AT, et al. Abnormal intestinal permeability. An aetiological factor in chronic psychiatric disorders? Br J Psychiatry. 1987;150:853–856. doi: 10.1192/bjp.150.6.853. [DOI] [PubMed] [Google Scholar]
- Yuki N, Susuki K, Koga M, et al. Carbohydrate mimicry between human ganglioside GM1 and Campylobacter jejuni lipooligosaccharide causes Guillain-Barre syndrome. Proc Natl Acad Sci U S A. 2004;101:11404–11409. doi: 10.1073/pnas.0402391101. [DOI] [PMC free article] [PubMed] [Google Scholar]