Abstract
Background
To minimize participants’ burden and the need for disrobing, a 7-lead electrocardiogram (ECG) recording using a single mid-sternal chest lead was recorded at the initial stages of The REasons for Geographic And Racial Differences in Stroke (REGARDS) Study. ECG-detected left ventricular hypertrophy (ECG-LVH) by Cornell voltage (RaVL +SV3) cannot be assessed from this method because of the absence of V3. We examined the possibility that the S wave amplitude in the mid-sternal lead (SV) could be used as a surrogate for SV3.
Methods
The REGARDS study is a US national study where 7-lead ECGs were performed in 8,330 (29%) participants and standard 12-lead EGCs were performed in 20,811 (71%). Cornell voltage was calculated as the sum of aVL amplitude + SV (in the 7-lead group) or SV3 (in the 12-lead group). Logistic regression analysis was used to examine and compare the magnitude of the association between the LVH risk factors with ECG-LVH in both groups, and Cox Proportional Hazards analysis was used to examine and compare the hazard ratios of overall mortality and cardiovascular mortality associated with ECG-LVH in both groups.
Results
Regardless of the Cornell voltage calculation method, ECG-LVH was significantly associated with LVH risk factors, and with the exception of sex there was no evidence of a difference in the magnitude of the association. ECG-LVH from both approaches were significantly and similarly associated with both all cause and cardiovascular mortality.
Conclusion
ECG-LVH by Cornell voltage calculated from a 7-lead ECG (using SV in the formula) has demographic and clinical associations that are similar to that calculated from a standard 12-lead ECG (using SV3). In epidemiologic studies recording 7-lead ECG, SV could be used as an alternative to SV3 in the Cornell voltage formula.
INTRODUCTION
Electrocardiogram (ECG) recording using a single mid-sternal lead instead of the standard 6 chest leads has been suggested to minimize participants’ burden and the need for disrobing in The REasons for Geographic And Racial Differences in Stroke (REGARDS) Study, a US national study with more than 30,000 participants (1). Using a mid-sternal electrode plus the standard 4 limb electrodes provides only 7-lead ECG, from which standard approaches for calculation of the Cornell voltage criteria of left ventricular hypertrophy (LVH) cannot be performed, as it requires measurement of S wave amplitude in V3 (SV3) (2). As the REGARDS study progressed and experience with the cohort was gained, concerns decreased regarding the additional participant burden and barriers from more complete disrobing required by a standard 12-lead ECG. Furthermore, 12-lead ECG will provide more information regarding anterior or anterolateral myocardial infarction/ischemia as well as more options to detect ECG-LVH, a major risk for stroke and a component of the Framingham stroke risk score (3). Therefore, after recording 7-lead ECGs (using the mid-sternal electrode) in almost one third of the study population, the possibility of collecting more information from the standard chest leads including calculation of Cornell voltage led to recording standard 12-lead ECGs in the rest of the study participants. In an effort to detect ECG-LVH by Cornell voltage in the REGARDS participants with 7-lead ECG, we conducted this analysis. We hypothesized that S wave amplitude in the mid-sternal lead (SV) could be used as an alternative to SV3 in the Cornell voltage formula. Our hypothesis is based on the assumption that there is redundancy in the 12-lead ECG which stems from the fact that the 6 precordial sites are close to each other (4–8). This in turn infers that a mid sternal lead, which is anatomically close to V3, may contain similar information to V3. If this hypothesis is true, calculating Cornell voltage using SV should detect ECG-LVH that has demographic and clinical associations that are not different from the ECG-LVH detected by the conventional Cornell voltage using the standard SV3. This analysis has the potential to enable similar epidemiologic studies which used 7-lead ECGs to detect ECG-LVH by Cornell voltage criteria in their participants.
METHODS
Study population
The REasons for Geographic And Racial Differences in Stroke (REGARDS) Study is a national longitudinal study initiated in January 2003 to elucidate the causes of geographic and racial disparities in stroke mortality (1). REGARDS was designed to recruit 30,000 community-dwelling black and white participants (50% from each group) from the continental US with oversampling from the stroke belt, an area of excess stroke mortality. Participants were recruited from those randomly selected from a commercially available nationwide list purchased from Genesys. The study originally recruited individuals 55 years and older; however, because the larger black/white disparities in mortality at younger ages the age eligibility was broadened in May 2004 to include those aged 45 to 54. Participants were contacted by mail, then telephone. Participants completed a standardized telephone interview including demographic information and medical history. An in-home examination was performed subsequently to obtain physical measures (blood pressure, height, and weight), ECG, medication inventory, and fasting blood and urine samples. Participants are followed by telephone twice per year for stroke and myocardial infarction events. Efforts are currently underway to retrieve medical records and death certificates for all death events to allow adjudication of the cause of death; however for this analysis, CVD mortality was defined as death from coronary or stroke causes as reported by family members. Written informed consent was obtained using methods approved by the institutional review boards of all participating institutions. Race was defined by self-report requesting participants to select their race from a list. Age, sex, education level, annual family income and use of antihypertensive therapy were based on self-report; history of stroke, TIA, and heart disease (myocardial infarction or heart attack, coronary artery bypass surgery, coronary angioplasty, or stenting) were defined based on self-report of a physician diagnosis. Blood pressure was the average of two measurements taken by a trained technician after the participant was seated for 5 minutes, measured using a standard protocol and regularly tested aneroid sphygmomanometer. Diabetes was defined as fasting glucose greater than 6.99mmol/L (126mg/dl), non-fasting glucose greater than 11.1mmol/L (200mg/dl), or self-reported medication use for diabetes. Both the phone interview and in-home examination were completed for 30,239 participants. Participants with missing data and those with QRS duration >120 ms in their ECGs were excluded from these analyses. Excluded participants did not differ in sociodemographic characteristics from included participants.
ECG recording in REGARDS
The ECG recording in REGARDS initially aimed to only diagnose atrial fibrillation (AF), being one of the major risk factors for stroke. Hence, the first 8,330 REGARDS participants underwent 7-lead ECG recording acquired by applying the standard 4 limb electrodes and a mid-sternal electrode. However, during the course of the study, it was felt that 12-lead ECG should (and could) be used. Therefore, the rest of REGARDS participants (N= 20,811) underwent a standard 12-lead-ECG recording. The change in the ECG protocol and the broadening of the age eligibility to include those aged 45 to 54 occurred at approximately the same time, and as such there are relatively few participants aged 45 to 54 evaluated by the 7-lead protocol. All ECGs were read centrally at the EPICARE center located at Wake Forest University School of Medicine. Cornell voltage was calculated as the sum of the SV+ aVL and SV3+aVL in participants with 7-lead ECGs and 12-lead respectively. LVH was diagnosed if Cornell voltage ≥ 2200 ms in women or ≥2800 ms in men (2).
Statistical analysis
Frequency distributions of all variables were first inspected to identify anomalies and outliers. Series of logistic regression models were developed to examine associations between demographic and LVH risk factors (exposure variables) with ECG-LVH by 7-lead ECG and 12-lead ECG, separately (outcome variable). A first model assessed associations with age, sex and race. To examine LVH associations with demographic factors, adjusted body mass index (BMI), hypertension, and diabetes, each of those variables were introduced individually into a second set of demographic adjusted models. The final set of models included all the variables in the first and second set of models (age, sex, race, BMI, hypertension and diabetes). In all cases, models included separate estimates for those with 7-lead and for those with 12-lead data of the association of each predictive factors with the prevalent ECG-LVH (for example, the same model was used to estimate the regression coefficient for age was estimated for those with 7-lead data, and the separate regression coefficient for age was estimated for those with 12-lead data). The difference in the magnitude of the association between those with 7-lead and 12-lead studies was assessed using a linear contrast of the estimated coefficients from this model.
Cox proportional hazards analysis was used to examine the hazard ratios (and 95% CI) for all cause mortality and reported cardiovascular (CVD) mortality, separately, associated with LVH by 7-lead ECG and 12-lead ECG, separately. The models were first adjusted for age, sex and race. Hypertension, diabetes, BMI, history of heart disease, and history of previous stroke or transient ischemic attacks (TIA) were then included in a second set of models. The final models included all the variables in the second set of models in addition to socio-economic status (SES). SES was defined by education level defined at four levels: less than high school graduate, high school graduate, some college, or a college graduate; and annual family income also defined at four levels: < $20K, $20k to $35K, $35K to $75K, or over $75K. In all cases, a separate term assessing the hazard ratio for LVH assessed by 7-lead and 12-lead protocols was estimated, and the difference between these hazard ratios assessed by a linear contrast.
RESULTS
Of the 30,239 participants in REGARDS, 29,566 (97.8%) participants had complete ECG data. An additional 29 (<0.1%) participants were deleted because of missing data regarding the ECG date, and a further 396 (1%) were deleted because the time between telephone interview and in-home visit (including the ECG) was greater than 183 days, resulting in an analysis cohort of 29,141 participants. Of these, 8,330 (29%) were studied with a 7-lead ECG and 20,811 (71%) with a standard 12-lead ECG. There was no overlap in the 12-lead and 7-lead groups.
Table 1 shows the characteristics of the study population stratified by ECG recording method. Compared to the 12-lead group, participants who underwent 7-lead ECG were older (67.0 ± 8.2 vs. 64.0 ± 9.7) with fewer females (35.8% vs. 62.8%) but more African Americans (42.2% vs. 40.9%). Differences in age and sex were anticipated as a product of the eligibility criteria and protocol adjustments for selecting individual participants. Except the BMI, which was almost equal between groups, the 7- lead group had more CVD risk factors (hypertension and diabetes) and more history of heart disease and stroke, reflecting the larger proportion of males and older people assessed early in the study.
Table 1.
Characteristics of the study population stratified by ECG recording method
| 7-Lead ECG N= 8,330 |
12-Lead ECG N=20,811 |
|
|---|---|---|
| Age (years) | 67.0 + 8.2 | 64.0 + 9.7 |
| Black (%) | 42.2 | 40.9 |
| Female (%) | 35.8 | 62.8 |
| Body Mass Index (Kg/m2) | 29.01 ± 5.76 | 29.43 ± 6.37 |
| Hypertension (%) | 61.9 | 58.2 |
| Systolic Blood Pressure (mmHg) | 130.3 ± 17.0 | 126.5 ± 16.6 |
| Diastolic Blood Pressure (mmHg) | 77.3 + 10.4 | 76.2 + 9.8 |
| Diabetes (%) | 24.0 | 21.1 |
| History of Heart Disease (%) | 24.2 | 22.1 |
| History of Stroke or TIA (%) | 11.3 | 9.5 |
Continuous variables are shown as mean± SD
Given the significant differences in the demographic and clinical characteristics between the 7-lead group and 12-lead group, with the earlier having more LVH risk factors, it was not surprising that the crude unadjusted age-, sex-, and race-specific prevalence of LVH was higher in the 7-lead group (Table 2). However, as shown in Table 3, after adjusting for the confounding effect of age, sex and race, there were no statistically significant differences between the associations of ECG-LVH and the demographic characteristics or LVH risk factors by each method. The odds ratios associated with ECG-LVH in the 7-lead group were not statistically different from those in the 12-lead groups (P values for odds comparison >0.05). Although male sex was significantly associated with LVH detected by both the modified Cornell voltage (7-lead group) and the conventional Cornell voltage (standard 12-lead group), the earlier group showed higher odds ratio [(OR (95% CI) in the full model: 3.86 (3.20 – 4.65) vs. 2.27 (1.94 – 2.65) respectively; P-value for OR comparison <0.0001)].
Table 2.
Unadjusted “crude” age-, sex, race-specific prevalence of ECG-LVH stratified by the ECG recording method
| Strata | 7-Lead ECG N (%) |
12-Lead ECG N (%) |
|
|---|---|---|---|
| Age (Years) | 45–54 | 59 (6.8%) | 3606 (3.0%) |
| 55–64 | 3550 (6.4%) | 7557 (3.9%) | |
| 64–74 | 3029 (7.4%) | 6342 (5.7%) | |
| 75+ | 1692 (10.7%) | 3297 (8.2%) | |
| Sex | Female | 2985 (14.7%) | 13059 (6.3%) |
| Male | 5345 (3.6%) | 7752 (2.7%) | |
| Race | African American | 3513 (12.5%) | 8516 (7.6%) |
| White | 4817 (4.0%) | 12295 (3.2%) | |
Table 3.
Odds ratios and 95% Confidence interval (OR: 95% CI) of ECG-LVH in the 7-Lead and 12-Lead ECG groups in a multivariable logistic regression analysis
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Joint Age-Race-Sex Model | Impact of Age-Race-Sex adjusted Individual Risk Factors |
Full Multivariable Model | |||||||
| (Age+ race+ sex+ BMI+ Hypertension +diabetes) | |||||||||
| (age+ race + sex) + BMI or Hypertension or Diabetes |
|||||||||
| 7-Lead ECG | 12-Lead ECG | P* | 7-Lead ECG | 12-Lead ECG | P* | 7-Lead ECG | 12-Lead ECG | P* | |
| Age (10- years) |
1.50 (1.42 – 1.59) |
1.48 (1.40 – 1.57) |
0.46 | N/A | N/A | N/A | 1.37 (1.27 – 1.47) |
1.38 (1.30 – 1.47) |
0.83 |
| African American |
2.84 (2.37 – 3.94) |
2.49 (2.19 – 2.83) |
0.24 | N/A | N/A | N/A | 2.42 (2.00 – 2.93) |
2.02 (1.76 – 2.32) |
0.13 |
| Male Sex | 3.88 (3.25 – 4.65) |
2.21 (1.90 – 2.57) |
<0.0001 | N/A | N/A | N/A | 3.86 (3.20 – 4.65) |
2.27 (1.94 – 2.65) |
<0.0001 |
| Body mass index (BMI) Kg/m2 |
N/A | N/A | N/A | 1.007 (0.995 – 1.019) |
1.001 (0.991 – 1.010) |
0.35 | 0.993 (0.980 – 1.006) |
0.986 (0.976 – 0.996) |
0.37 |
| Hypertension | N/A | N/A | N/A | 2.47 (1.98 – 3.07) |
2.57 (2.19 – 3.04) |
0.75 | 2.51 (1.99 – 3.15) |
2.63 (2.21 – 3.12) |
0.74 |
| Diabetes | N/A | N/A | N/A | 1.28 (1.06 – 1.54) |
1.44 (1.24 – 1.66) |
0.33 | 1.13 (0.93 – 1.38) |
1.32 (1.14 – 1.53) |
0.23 |
P value for comparison of the odds ratios in the 7-Lead group vs. the 12-lead group
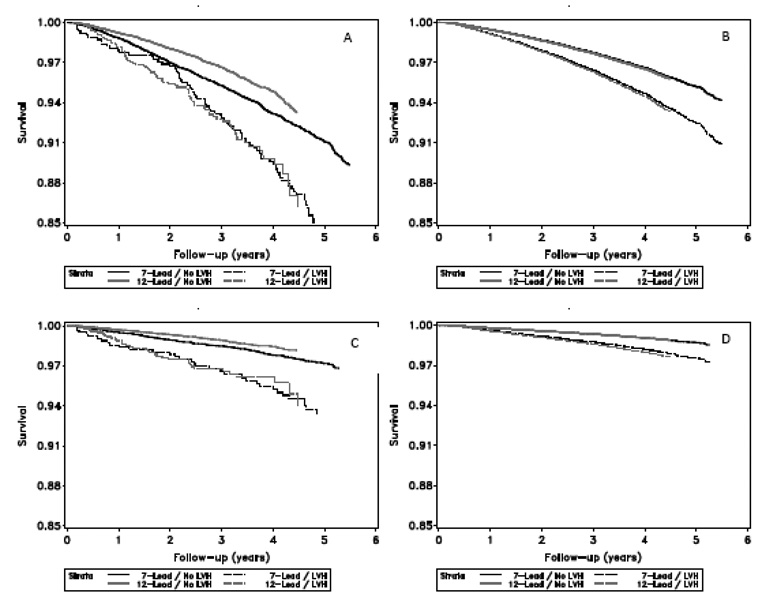
Table 4 shows the association between ECG-LVH (calculated from each of the 7-lead and 12-lead ECGs) and all cause mortality and CVD mortality, separately, in Cox proportional hazards analysis. In all models, regardless of the ECG recording method, ECG-LVH was significantly associated with all cause mortality and CVD mortality. Notably, there were no statistically significant differences in the HRs associated with ECG-LVH detected between the two ECG recording methods. As shown in Figure 1, the multivariable adjusted event free survival curves of ECG-LVH by the modified Cornell (7-lead group) are overlaying those curves of the LVH by the conventional Cornell voltage method for both all cause mortality and for CVD mortality.
Table 4.
Hazards ratios and 95% Confidence interval (HR: 95%) of all cause mortality and cardiovascular mortality stratified by ECG recording method in a multivariable Cox Proportional Hazards Analysis
| All Cause Mortality | Cardiovascular Mortality | |||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| Age + Race + Sex | Model 1+ hypertension + diabetes + history of heart disease + history of stroke/ TIA |
Model 2+ Socioeconomic status |
Age + Race + Sex |
Model 1+ hypertension + diabetes + history of heart disease + history of stroke/ TIA |
Model 2+ Socioeconomic status |
|
| 7-Lead ECG | 1.65 (1.31 – 2.06) |
1.54 (1.22 – 1.95) |
1.50 (1.19 – 1.90) |
2.27 (1.59 – 3.23) |
1.92 (1.32 – 2.85) |
1.87 (1.28 – 2.73) |
| 12-Lead ECG | 1.82 (1.31 – 2.05) |
1.59 (1.25 – 2.02) |
1.60 (1.26 – 2.04) |
2.57 (1.80 – 3.68) |
2.16 (1.49 – 3.11) |
2.21 (1.53 – 3.19) |
| P-value* | 0.54 | 0.85 | 0.70 | 0.60 | 0.65 | 0.51 |
P value for comparison of the Hazard ratios in the 7-Lead ECG group vs. the 12-Lead ECG group
Figure 1.
All-cause mortality and cardiovascular (CVD) mortality shown by ECG recording method and presence/absence of ECG-LVH. Survival estimates are shown “crude” or unadjusted, and after adjustment for age, race, sex, hypertension, diabetes, history of heart disease, and history of previous stroke or TIA. A: Unadjusted all-cause mortality, B: adjusted all-cause mortality, C: unadjusted CVD mortality, D: adjusted CVD mortality.
DISCUSSION
The ECG is the oldest method for detection of LVH (9). Although newer imaging techniques provide a more accurate assessment of ventricular myocardial mass than does the ECG, they do not obviate the clinical use of the ECG. A number of ECG abnormalities have been shown to have independent clinical prognostic value (10) including ECG-detected LVH. The current epidemiological significance of ECG-LVH is primarily in the realm of risk evaluation and prediction (11) rather than providing crude estimates of prevalence/incidence. Therefore, in this analysis we validated the proposed modified Cornell voltage ECG-LVH (in which SV from a mid-sternal lead was used) by comparing it to the ECG-LVH calculated by the conventional Cornell voltage (in which the standard SV3 was used) in terms of associations rather than in terms of crude prevalence estimates. This included associations with demographic characteristics and LVH risk factors as well as associations with overall mortality and CVD mortality. As shown in the results, there were no statistically significant differences between the associations of ECG-LVH and other factors, when LVH was determined by either method. The higher odds ratio of ECG-LVH associated with male sex in the 7-lead group compared to the 12-lead group is possibly due to the effect of female breast that might have attenuated SV3 in the standard 12-lead. Nevertheless, ECG-LVH by either method was associated with male sex as expected.
Based on the results of this analysis, S wave in the mid-sternal lead (SV) could be used as an alternative to S wave in V3 (SV3) to calculate the Cornell voltage formula. This finding would enable those studies that are using single mid-sternal lead instead of the standard set of chest leads to detect ECG-LVH by Cornell voltage. Noteworthy, we are not here advocating ECG recording using a single mid-sternal lead. Recording standard 12-lead ECG should be always sought in epidemiologic studies. This analysis should be read in the context of providing a solution to the inability to calculate Cornell voltage from non-standard ECGs that lack SV3. Without this solution, it would not be possible to measure ECG-LVH (and subsequently Framingham risk score) in a study like REGARDS in which 8,330 participants (29% of the study population) have only mid-sternal chest lead. Although there are other methods for calculating LVH from limb leads only (12–17), all these methods are not superior to Cornell voltage (18) in terms of association with cardiovascular events.
Using SV instead of SV3 without any significant impact on the ECG-LVH by Cornell voltage, as shown in the results, confirms what has been reported as redundancy in the 12-lead ECG, especially the 6 chest leads (8, 19). Actually, missing chest leads could be reconstructed from only 2 chest leads if data from limb leads is included. (4–7). However, including more chest leads would provide more accuracy in reconstructing the missing chest leads. Given the accumulating evidence of redundancy of chest leads, it may be the time to rethink the number/site of chest electrodes. Any modifications in the number/sites of chest electrodes, however, would be challenged by the inability to compare ECGs recorded using modified chest leads and those using the current standard.
The assessment of the performance of the 7-lead versus 12-lead ECG to establish LVH would be strengthened by an experiment where ECG results were contrasted against a “gold standard” such as LVH assessment by echocardiography or magnetic Resonance Imaging (MRI). While this approach seems reasonable on the surface, the cost of performing echocardiograms or MRI in such a large cohort makes the approach infeasible. Given this constraint, we have taken the alternative approach of assessing whether presence/absence of LVH defined by the two approaches has either different associations with risk factors where associations are strongly anticipated, or with outcomes that we anticipate are associated with the presence of LVH. Our finding that the performance of these two outcomes are so similar in such a large study suggests that either approach is reasonable and that it is acceptable to define LVH under either approach (i.e., if LVH defined by a 7-lead protocol “walks like a duck, and quacks like a duck, we may be safe in thinking that it is a duck”!). A cohort with both sets of leads (12 and 7 leads) that are simultaneously recorded would be ideal to further confirm our findings.
Noteworthy, we are not advocating the use of the non-standard ECG recording in epidemiologic studies. We believe that non-standard ECG recording such as using mid-sternal electrode should be used only if it is not feasible to record standard 12-lead ECG. In such exceptional circumstances, using S wave in mid-sternal lead instead of the standard SV3 in the Cornell voltage formula would be appropriate.
ACKNOWLEDGEMENTS
This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. The authors acknowledge the participating investigators and institutions for their valuable contributions: The University of Alabama at Birmingham, Birmingham, Alabama (Study PI, Statistical and Data Coordinating Center, Survey Research Unit): George Howard DrPH, Leslie McClure PhD, Virginia Howard PhD, Libby Wagner MA, Virginia Wadley PhD, Rodney Go PhD, Monika Safford MD, Ella Temple PhD, Margaret Stewart MSPH, David Rhodes RN; University of Vermont (Central Laboratory): Mary Cushman MD; Wake Forest University (ECG Reading Center): Ron Prineas MD, PhD; Alabama Neurological Institute (Stroke Validation Center, Medical Monitoring): Camilo Gomez MD, Susana Bowling MD; University of Arkansas for Medical Sciences (Survey Methodology): LeaVonne Pulley PhD; University of Cincinnati (Clinical Neuroepidemiology): Brett Kissela MD, Dawn Kleindorfer MD; Examination Management Services, Incorporated (In-Person Visits): Andra Graham; Medical University of South Carolina (Migration Analysis Center): Daniel Lackland, DrPH; Indiana University School of Medicine (Neuropsychology Center): Frederick Unverzagt, PhD; National Institute of Neurological Disorders and Stroke, National Institutes of Health (funding agency): Claudia Moy, Ph.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
None
References
- 1.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The REasons for Geographic and Racial Differences in Stroke Study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 2.Casale PN, Devereux RB, Kligfield P, Eisenberg RR, Miller DH, Chaudhary BS, Phillips MC. Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol. 1985;6(3):572–580. doi: 10.1016/s0735-1097(85)80115-7. [DOI] [PubMed] [Google Scholar]
- 3.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 4.Nelwan SP, Crater SW, Meij SH, van Dam TB, Kors JA, Simoons ML, Krucoff MW. Simultaneous comparison of three derived 12-lead ECGs with standard ECG at rest and during percutaneous coronary occlusion. J Electrocardiol. 2004;37:171. doi: 10.1016/j.jelectrocard.2004.08.051. [DOI] [PubMed] [Google Scholar]
- 5.Drew BJ, Pelter MM, Brodnick DE, Yadav AV, Dempel D, Adams MG. Comparison of a new reduced lead set ECG with the standard ECG for diagnosing cardiac arrhythmias and myocardial ischemia. J Electrocardiol. 2002;35:13. doi: 10.1054/jelc.2002.37150. [DOI] [PubMed] [Google Scholar]
- 6.Wei D. Deriving the 12-lead electrocardiogram from four standard leads using information redundancy in the 12-lead system. Int J Bioelectromagn. 2002;4:127. [Google Scholar]
- 7.Nelwan SP, Kors JA, Meij SH. Minimal lead sets for reconstruction of the 12-lead electrocardiogram. J Electrocardiol. 2000;33:163. doi: 10.1054/jelc.2000.20296. [DOI] [PubMed] [Google Scholar]
- 8.Finlay DD, Nugent CD, Kors JA, van Herpen G, Donnelly MP, McCullagh PJ, Black ND. Optimizing the 12-lead electrocardiogram: a data driven approach to locating alternative recording sites. J Electrocardiol. 2007;40:292–299. doi: 10.1016/j.jelectrocard.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Frohlich ED, Tarazi RC, Dustan HP. Clinical physiological correlation in the development of hypertensive heart disease. Circulation. 1971 doi: 10.1161/01.cir.44.3.446. 44z44~55. [DOI] [PubMed] [Google Scholar]
- 10.Hancock EW, Deal BJ, Mirvis DM, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119:e251–e261. doi: 10.1161/CIRCULATIONAHA.108.191097. [DOI] [PubMed] [Google Scholar]
- 11.Rautaharjue P, Rautaharjue F. Investigative electrocardiography in epidemiological studies and clinical trials. 1st Ed. Springer-Verlag London Limited; 2007. [Google Scholar]
- 12.Lewis T. Observations upon ventricular hypertrophy with special reference to preponderance of one or the other chamber. Heart. 1914;5:367–402. [Google Scholar]
- 13.Gubner RS, Ungerlieder HE. Electrocardiographic criteria of left ventricular hypertrophy: factors determining the evolution of the electrocardiographic patterns in hypertrophy and bundle branch block. Arch Intern Med. 1943;72:196–209. [Google Scholar]
- 14.Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar and limb leads. Am Heart J. 1949;37:161–186. doi: 10.1016/0002-8703(49)90562-1. [DOI] [PubMed] [Google Scholar]
- 15.Goldberger E. Unipolar Lead Electrocardiography and Vectorcardiography, Including Standard Leads, Augmented Unipolar Extremity Leads and Multiple Unipolar Precordial Leads, and a Section on Cardiac Arrhythmias. 2nd ed. Philadelphia, Pa: Lea & Febiger; 1949. [Google Scholar]
- 16.Schack JA, Rosenman RH, Katz LN. The aV limb leads in the diagnosis of ventricular strain. Am Heart J. 1950;40:696–705. doi: 10.1016/0002-8703(50)90200-6. [DOI] [PubMed] [Google Scholar]
- 17.Romhilt DW, Estes EH. A point score system for the ECG diagnosis of left ventricular hypertrophy. Am Heart J. 1968;75:752–758. doi: 10.1016/0002-8703(68)90035-5. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh BP, Pham MX, Froelicher VF. Prognostic value of electrocardiographic criteria for left ventricular hypertrophy. Am Heart J. 2005;150(1):161–167. doi: 10.1016/j.ahj.2004.08.041. [DOI] [PubMed] [Google Scholar]
- 19.Kors JA, van Herpen G. How many electrodes and where: a Poldermodel for electrocardiography. J Electrocardiol. 2002;35:7. doi: 10.1054/jelc.2002.37149. [DOI] [PubMed] [Google Scholar]