Abstract
Background
Sudden cardiac death (SCD) can be the first manifestation of cardiovascular disease. Development of screening methods for higher / lower risk is critical.
Methods
The Cardiovascular Healthy Study (CHS) is a population-based study of risk factors for coronary heart disease and stroke those ≥65 years. N=49 (of 1649) with usable Holters and in normal sinus rhythm, suffered SCD during follow up and were matched with 2 controls, alive at the time of death of the case and not suffering SCD on follow up. Univariate and multivariate conditional logistic regression determined the association of Holter-based information and SCD.
Results
In univariate models, the upper half of VPC counts, abnormal heart rate turbulence, decreased normalized low frequency power, increased T-wave alternans (TWA) and decreased DFA1 (short-term fractal scaling exponent) were associated with SCD, but time domain HRV was not. In multivariate models, the upper half of VPC counts (OR=6.6) and having TWA ≥37µV on Ch2 (OR=4.8) were independently associated with SCD. Also, the upper half of VPC counts (OR=6.9) and having DFA1 <1.05 (OR=5.0) were independently associated with SCD. When additive effects were explored: having both higher VPCs and higher TWA was associated with an OR of 8.2 for SCD compared to 2.6 for having either. Also, having both higher VPCs and lower DFA1 was associated with an OR of 9.6 for SCD compared to 3.1 for having either.
Conclusions
Results support a potential role for 24-hour Holter recordings to identify older adults at increased or lower risk of SCD.
Keywords: Sudden death, risk stratification, ambulatory ECG monitoring, heart rate variability, heart rate turbulence, T-wave alternans, arrhythmias, population-dwelling elderly
Introduction
Sudden cardiac death (SCD) is a common first manifestation of significant cardiovascular disease and has been reported to be responsible for 50% of cardiovascular mortality in the US.1 Therefore, identification of those in the population at highest and lowest risk for SCD is of critical importance. Twenty-four hour ambulatory ECG (i.e., Holter) monitoring, which is noninvasive and easily performed in a naturalistic outpatient setting that more accurately reflects daily life, is an attractive source of information that might help identify higher and lower risk people. Among data potentially available for risk stratification and obtainable from Holter recordings are: counts of ventricular premature beats, standard and novel heart rate variability measures, heart rate turbulence and measurement of T-wave alternans.
An increased frequency of ventricular premature beats has been associated with risk of SCD among the general population2 and in patients with known cardiovascular disease.3 Decreased HRV, reflecting abnormalities in cardiac autonomic modulation, is clearly associated with both all-cause and cardiac mortality in post-infarction patients, but the case for HRV as a predictor of SCD is far less clear.4 Decreased values for the short-term fractal scaling exponent, a “non-linear” HRV measure that captures the underlying pattern of the heart rate time series, have been linked to mortality post-MI5 and also in the Cardiovascular Health Study,6 but not explicitly linked to risk of SCD. Abnormal heart rate turbulence, which quantifies the heart rate responses to ventricular premature contractions, appears to reflect baroreflex function, predicts total and cardiovascular mortality in cardiac patients,7 and we have shown that it independently predicts cardiovascular mortality in the Cardiovascular Health Study.6 Abnormal heart rate turbulence has also been linked to risk of SCD post-MI and in patients with heart failure.8,9
“Electrical alternans,” the phenomenon of changing amplitude of the repolarization portion of the ECG that repeats on every other heart beat, has been shown to be a substrate for lethal ventricular arrhythmias. Microvolt T-wave alternans (TWA) seen during treadmill or bicycle stress testing has identified patients at increased risk of SCD.10,11,12,13 The modified moving average (MMA) method has been shown to be suitable for use during ambulatory ECG monitoring.14,15,16 We have recently shown that TWA from Holter recordings was associated with risk of SCD in patients with a recent MI and severe left ventricular dysfunction. 17
We therefore examined these potential Holter-based risk markers: ventricular premature beat counts, time domain, frequency domain and non-linear HRV, heart rate turbulence and TWA in a nested case-control study of well-characterized population-dwelling older adults who died suddenly compared to 2:1 matched controls who were alive at the time of the death of the case who did not subsequently suffer SCD. Participants were from the Cardiovascular Health Study (CHS), an ongoing epidemiological study that seeks to identify risk factors for cardiovascular disease in older adults.
Methods
Participants
The Cardiovascular Health Study (CHS) is an NIH-sponsored population-based longitudinal study to identify risk factors that relate to the onset and course of coronary heart disease and stroke in 5,201 men and women aged 65 years and older.18 Participants underwent extensive testing, at baseline (starting in 1989) and during a second examination cycle (starting in 1996), to identify the presence and severity of cardiovascular risk factors and the presence of overt disease. A less extensive follow up examination was performed annually, and telephone data were collected semi-annually. Follow up was up to 13 years. Ambulatory ECG (Holter) monitoring was performed on a subset of 1429 volunteers at baseline (yr2 cohort) and repeated in 864 during the second Holter examination (yr7 cohort). Recordings were also made during the second Holter examination in an additional 385 participants who entered the study during exam year 5 (minority cohort).
Of the 1814 individuals who had at least one Holter recording in the CHS, 1649 were in normal sinus rhythm had at least 18 hours of analyzable Holter data. N=49 (3%) of them suffered SCD during up to 14 years of follow up. SCD was defined as a sudden pulseless condition (presumably, but not definitely, ventricular fibrillation) due to a cardiac etiology in an otherwise stable patient. All individuals who had their event either out of the hospital or in the emergency room were included, but patients who were already hospitalized and nursing home patients who often have multiple comorbidities were excluded. SCD was carefully ascertained by a cardiologist’s record review of all cardiac deaths.
Each case was matched on age within 5 years, gender, beta-blocker use, history of MI and use of hypoglycemic medications (oral or insulin) with 2 control participants alive at the time of death of the case and not suffering sudden death on follow up. This nested case: control approach was chosen because measurement of T-wave alternans required reloading and completely reanalyzing the Holter recordings, and it was not feasible to do this for all of the recordings in the cohort. The primary matching variables were chosen as ones that would strongly impact HRV and would also be associated with risk of sudden death. There were 49 cases and 97 matching controls with usable Holter recordings. Where two recordings were available for the same case, the second recording was analyzed and the same Holter recording examination cycle recording was used for their controls. Matches were based on clinical and demographic factors at the time of the Holter recording.
Data Analysis
Tapes were recorded on Del Mar Avionics recorders with a calibrated timing signal. Scanning was performed at the Washington University School of Medicine Heart Rate Variability Laboratory using a MARS 8000 Holter scanner (GE Medical Systems, Milwaukee, WI). After the scanner automatically detected and labeled all QRS complexes, data were reviewed and edited by the technicians. Results were reviewed in detail by one of us (PKS) with attention paid to ensuring that only normal-to-normal (N-N) beats with uniformly detected onsets, within each recording, were included in the HRV analysis. The longest and shortest true N-N intervals were identified for each recording. Intervals outside of these limits and ectopic beats were excluded from HRV calculations. Twenty-four-hour time domain, frequency domain and non-linear HRV analysis and calculation of heart rate turbulence were performed on a Sun Enterprise 450 server (Sun Microsystems, Palo Alto, CA) using validated research software.
Time Domain HRV
Time domain indices of HRV are derived from statistical calculations performed on the set of normal-to-normal (N-N) intervals. Average heart rate was determined. Time domain parameters measured in this study included: SDNN (standard deviation of all N-Ns in ms) and SDANN (standard deviation of 5-min averages of N-N intervals in ms) which are primarily influenced by circadian rhythms.19 The HRV measures SDNNIDX (average standard deviation of 5-min N-Ns) and the coefficient of variance (standard deviation of 5-min N-Ns/average of 5-min N-Ns) reflect changes over periods <5 minutes and are believed to reflect a combination of sympathetic and parasympathetic influences on HR.19 Short-term HRV indices, rMSSD (the root mean square of successive differences between N-Ns) and pNN50 (the percent of N-Ns >50 ms different from the prior one) reflect beat-to-beat changes in HR, mediated primarily by changes in parasympathetic modulation.19
Frequency Domain HRV
Traditional frequency domain HRV is based on power spectral analysis,20 and quantifies the amount of variance in N-N intervals at different underlying frequencies. Power spectral analysis was performed using standard methods.20 Ultra low frequency power (ULF) reflects variance in heart rate with a period of between 5 min and 24 hours and primarily reflects circadian HR patterns and long-term activities.21 Very low frequency power (VLF, variations from 20 s to 5 min cycles) is believed to reflect the activity of the renin-angiotensin and parasympathetic systems, although it is exaggerated by periodic breathing patterns.22 Low frequency power (LF, variations from 3–9 cycles/min) reflects the combined activity of the sympathetic and parasympathetic nervous systems..21 Beat-to-beat HR changes are quantified by high frequency power (HF, variations at respiratory frequencies) and primarily reflect parasympathetic modulation of HR.21 In addition, there are various ratio-based HRV measures that are proposed as measures of relative autonomic balance.21 These include normalized low and high frequency power and the low-to-high frequency power ratio.
Non-Linear HRV
Non-linear HRV quantifies the structure of the HR time series. Power law slope characterizes the fractal (i.e., self-similar) qualities of HRV occurring on time scales ranging from about a minute to several hours. More negative values are associated with worse outcomes among cardiac patients.23 Detrended fluctuation analysis (DFA) quantifies the fractal scaling properties of the short-term R-R interval time series.24,25 DFA1 (the short-term fractal scaling exponent) quantifies these properties on a scale of 4–11 beats. Higher values indicate less complexity and more periodicity in the HR time series, lower values indicate more random fluctuations.
Heart Rate Turbulence
Heart rate turbulence quantifies the response of the sinus node to VPCs.26 Two indices are calculated, turbulence onset (TO) and turbulence slope (TS). In healthy hearts, there is generally a brief and subtle sinus tachycardia after a ventricular premature contraction (VPC). TO measures the magnitude of this tachycardia (if any) as the percent change in N-N interval of the sinus two beats after the VPC compared to the two beats before. In healthy hearts this index is negative or zero. Thus, a TO>0 is abnormal (no tachycardia or bradycardia). TS quantifies the oscillation in heart rate (tachycardia, bradycardia then return to baseline) that follows a VPC as the largest fitted slope of the N-N intervals between any 5 beats within 15 beats of the VPC. These indices require 5 VPCs for calculation and are determined from a signal average of the N-N beats before and after the VPCs on the recording. Heart rate turbulence indices are usually analyzed as categorical variables. The optimal cutpoint for abnormal TS was <3 ms/beat and that for TO >0 based on prior results from the CHS.6
T-wave Alternans
TWA analysis software was not yet available at the time of the original scanning of the CHS recordings. Therefore, for measurement of TWA, a subset of Holter tapes were reloaded and rescanned on a MARSPC Holter Analyzer (GE Medical Systems, Milwaukee, WI), and TWA was measured using software version 7.1.1. The software identified periods of possible TWA using the modified moving average (MMA) algorithm.14 The details for the MMA algorithm have been previously published.14 Briefly, the stream of heart beats is divided into two bins such that alternating beats are in different bins (ABABAB). The morphology of the beats is averaged and continuously updated for each bin, in this case for groups of 8. The magnitude of TWA is the maximum difference in amplitude between the average of the A beats and the average of the B beats.
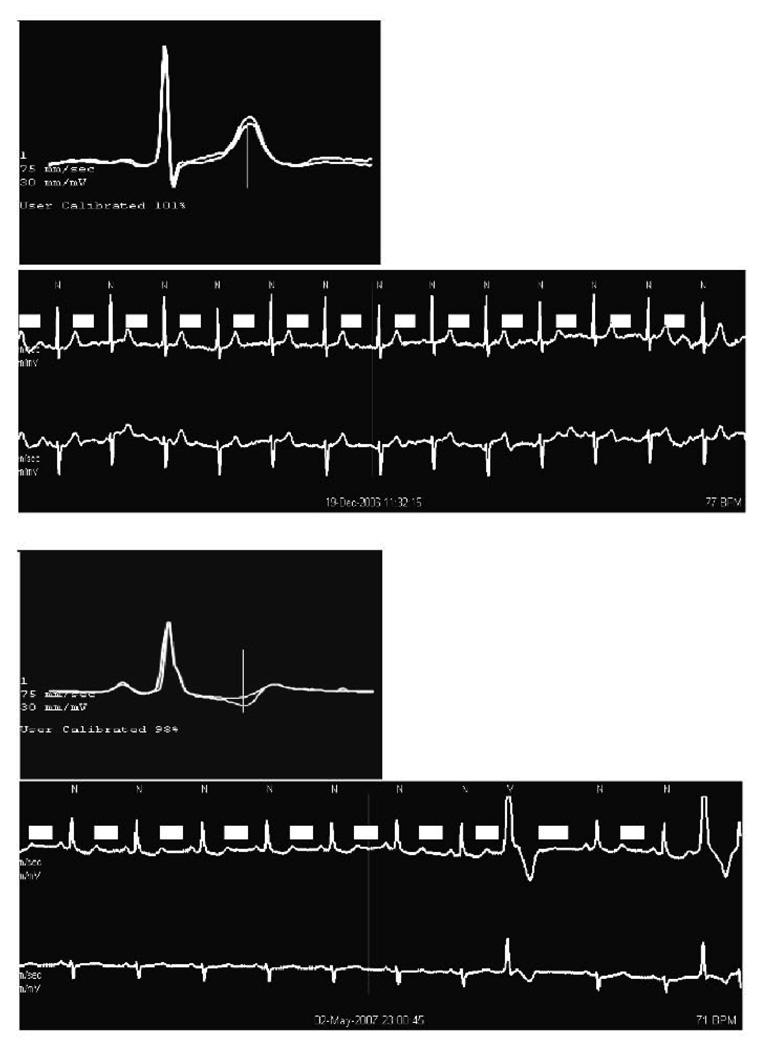
TWA measurements were made from normal beats with accurately detected beat onsets only. In a manner similar to that used for routine Holter-based ST segment analysis, the greatest TWA magnitudes were separately examined for each of the two leads, starting at the maximum value detected. For each value of TWA, the associated ECG strip and superimposed A and B waveforms from the MMA algorithm, were displayed. TWA measurements were rejected if the ECG was noisy or if the T waves could not be clearly seen during the measurement period. TWA measurements were also rejected and the next highest value taken if the QRS complexes of the superimposed TWA measurements (see the small window of Figure 1) did not line up due to uneven detection of the fiducial points of different beats by the scanner software. This was rarely a problem however, because of careful attention to this issue during the scanning process. This process was repeated until the 3 periods with the highest accepted TWA measurements were identified. Magnitude of TWA, heart rate, and time of the episode were recorded. The average of the 3 highest TWA values was determined and used as the “peak TWA” for that lead. For verification, the screenshot for each TWA determination and the screenshot of the period immediately prior were printed out and overread by one of us (PKS). Figures 1 show the superimposed A and B waveforms at maximum TWA and the associated ECG strip for channels 1 and 2, in two CHS participants. Although we were unable to find anyone who could tell us the specifics of the CHS Holter hookup, we believe that channel 1 corresponds to V5 lead and that channel 2 corresponds to aVR.
Figure 1.
Holter-based T-wave alternans in two CHS participants. Small boxes are the QRS-aligned templates generated by modified moving average analysis, which show the measurement of the maximum difference between successive sinus beats. The associated ECG strip is shown below it. TWA is 58 µV in the top figure and 61 µV in the bottom one.
Statistics
Heart rate, heart rate variability and the averages of the highest three TWA magnitude in each lead, heart rates at maximum TWA and times of each TWA event for each lead were first compared between those who suffered SCD and controls using t-tests. Heart rate turbulence categorized as TS and TO normal vs. TS and/or TO abnormal was compared for cases and controls using Chi-square analysis. Cut points for significant TWA on either channel and for DFA1 were explored using conditional logistic regression and the cut point that best separated cases and controls determined.
The unadjusted relationship of Holter-based factors different between cases and controls and sudden death was tested via conditional logistic regression. All participants had usable values for the number of VPCs, all but 2 (one case, one control) for HRT, and all but one (control) had usable TWA on at least one channel. Fewer had usable TWA on channel 2 (46 cases, 87 controls), and still fewer had usable frequency domain and non-linear HRV (38 cases, 90 controls) due to noisy Holter recordings. We therefore reported three different multivariate conditional logistic regression models for SCD: the first using the entire cohort, the second using those with TWA values on channel 2 and the third involving those with usable frequency domain and non-linear HRV data. Finally, we developed multivariate models using combined risk markers, i.e., increased VPCs combined with TWA above the cutpoint and increased VPCs combined with DFA1 below the cutpoint, comparing SCD between those with no abnormalities, one factor abnormal and both abnormal. Software was SPSS 15.0 (SPSS, Chicago, IL). Significance was set at p=0.05.
The authors had full access to the data and take responsibility for its integrity.
Results
Subjects were 73±5 yrs, 99 males, 47 females and were followed for up to 13 years. Time from Holter to SCD was 4.7±3.0 (range 0.2–10.4) yrs. Time from Holter to death for controls that died was 7.5 ± 2.6 yrs, (range 2.3 –11.6 yrs, 33% mortality). N=107 were white and N=39 were non-white. There was no ethnic difference in the prevalence of SCD. Based on qualitative echocardiography, 108 participants had normal left ventricular (LV) function and 28 had borderline or abnormal LV function.27 There was a trend (p=0.061) for those with SCD to be categorized as having borderline or abnormal LV function (54% SCD vs. 46% no SCD), but LV function did not enter any of the multivariate models for prediction of SCD described below.
Ventricular Premature Contraction Counts (Table 1 and Table 2)
Table 1.
Holter-Based Parameters Significantly Different Between SCD and Controls.
| SCD | Controls | p-value | |
|---|---|---|---|
| VPCs in Quartiles | N=49 | N=97 | <0.001 |
| 1st quartile (≤3) | 1 (4%) | 23 (96%) | |
| 2nd quartile (4–26) | 8(23.5%) | 26 (76.5%) | |
| 3rd quartile (31–232) | 17 (40.5%) | 23(59.5%) | |
| 4th quartile (>285) | 23 (50%) | 23(50%) | |
| HRT By Category | (N=48) | (N=96) | |
| TS Abnormal | 21 (46%) | 25 (54%) | 0.026 |
| Either TS or TO Abnormal | 27 (44%) | 35 (56%) | 0.019 |
| TWA |
(N=48 Ch 1)
(N=46 Ch 2) |
(N=96 Ch 1)
(N=87 Ch2) |
|
| TWA >46 µV in Ch1 | 17 (46%) | 20(54%) | 0.047 |
| TWA>37 µV in Ch2 | 18 (51%) | 17 (49%) | 0.026 |
| Max TWA Ch 1 | 44.2±20.5 | 38.0±13.3 | 0.059 |
| Max TWA Ch 2 | 39.1±28.1 | 31.5±12.8 | 0.087 |
| Frequency Domain HRV | (N=38) | (N=90) | |
| Normalized LF power | 55.4±2.8 | 62.6±1.4 | 0.002 |
| Normalized HF power | 29.1±11.1 | 25.0±0.6 | 0.038 |
| DFA1 | 0.97±0.21 | 1.11±0.20 | 0.001 |
| (N=38) | (N=90) | ||
| DFA1 ≥1.015 | 23 (51%) | 22 (49%) | <0.001 |
| DFA1 <1.015 | 67 (82%) | 15 (18%) |
Table Legend: VPCs=ventricular premature contractions, HRT=Heart Rate Turbulence, TS=Turbulence Slope, TO=Turbulence Onset, TWA=T-Wave Alternans, Normalized LF Power=Normalized low frequency power (% of averaged 5-min spectral power at frequencies of 0.04–0.15 Hz), Normalized HF power=Normalized high frequency power (% of averaged 5-min spectral power at frequencies of 0.15–0.4 Hz), DFA1=detrended fractal scaling exponent.
Table 2.
Unadjusted Conditional Logistic Regression for SCD.
| OR (95% CI) |
p-value | |
|---|---|---|
| Full Cohort (N=146, 49 SCD) | ||
| Lower half of VPCs (≤26, N=58, 9 SCD) |
1.0 | |
| Upper half of VPCs (>30, N=88, 40 SCD) |
4.61 (1.96–10.84) |
<0.001 |
| Cohort with HRT (N=144, 48 SCD) | ||
| Normal HRT | 1.0 | |
| Abnormal HRT | 2.60 (1.16–5.60) |
0.019 |
| Cohort with Usable TWA in Ch 2 (N=133, 46 SCD) | ||
| TWA>37 µV in Ch2 (N=35, 14 SCD) |
2.78 (1.08–7.14) |
0.034 |
| Cohort with Usable Frequency Domain HRV (N=127, 37 SCD) | ||
| Normalized LF power | 0.96 (0.92–0.99) |
0.015 |
| DFA1 | 0.08 (0.010–0.6) |
0.012 |
| DFA1 ≥1.015 (N=82) | 1.0 | |
| DFA1 <1.015 (N=45) | 5.61 (2.05–15.3) |
0.001 |
|
Cohort with Usable TWA in Ch 2 and Usable Frequency Domain
HRV (N=127, 33 SCD) | ||
| TWA>37 µV in Ch2 | 3.47 (0.95–12.6) |
0.053 |
Table Legend: See Legend Table 1.
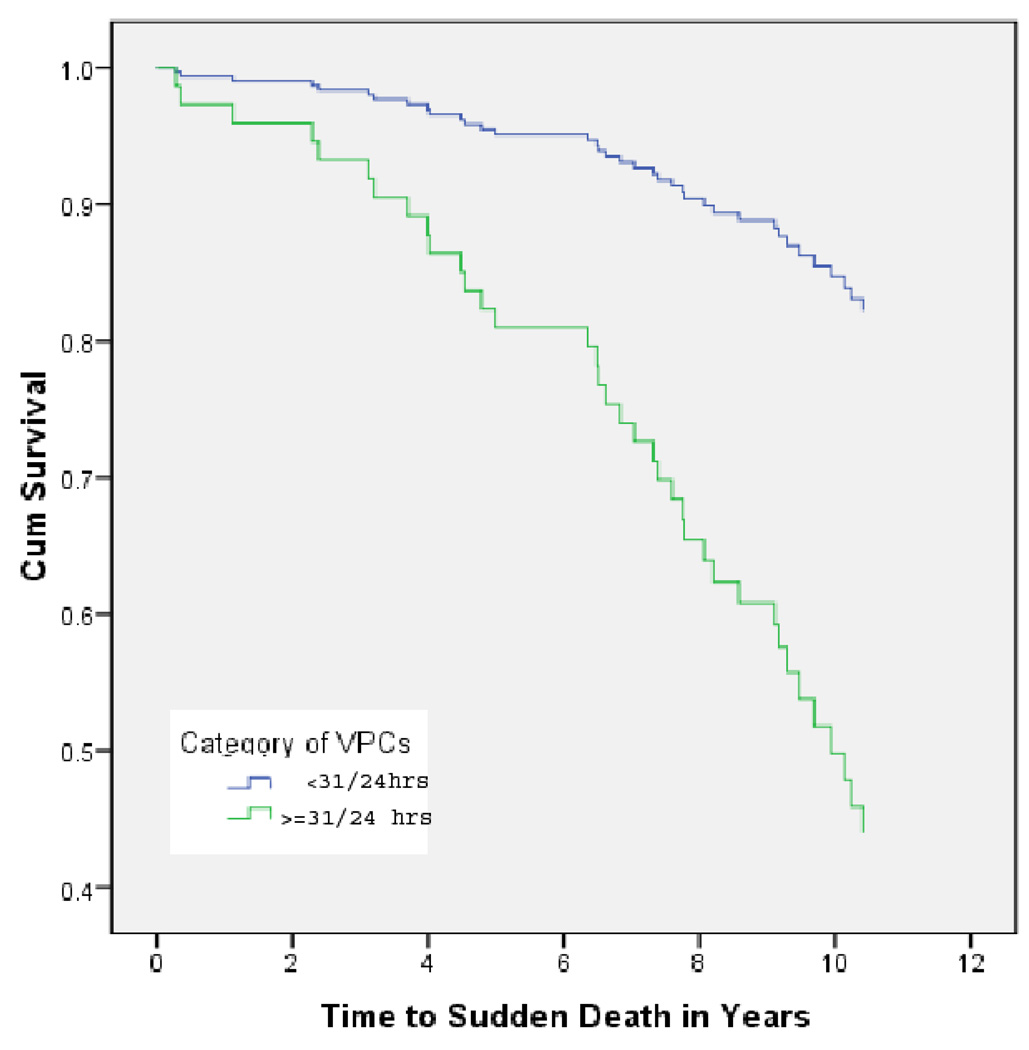
When the number of ventricular premature contractions (VPCs) was compared by quartiles, only one participant with and 23 participants without SCD were in the lowest quartile (≤3 VPCs in 24 hours). Quartile cutpoints were based on VPC counts for the entire CHS baseline Holter cohort. The number of participants with SCD was so small in the first quartile that to permit statistical analysis, the risk of SCD was analyzed using the upper and lower halves of VPC counts. Those with SCD were much less likely to be in the lower half of 24-hour VPC counts. Table 2 shows that risk of SCD was 4.6 times higher among those in the upper compared to the lower half of 24-hour VPC counts. Figure 2 shows age-adjusted survival curves for SCD in the higher and lower halves of 24-hour VPC counts.
Figure 2.
Age-adjusted survival curves for SCD for participants with VPCs in the lower two vs. the upper two quartiles of the CHS cohort.
Heart Rate Variability (Table 1 and Table 2)
When heart rate and time domain HRV were compared between cases and controls, there were no significant differences for any measure, e.g., SDNN=119 ± 36 for controls and 117 ± 34 ms for cases, p=0.78). When frequency domain HRV was compared between cases and controls, normalized low frequency power was significantly lower (55.4±12.8% for SCD, 62.6±11.4% controls, p=0.002) and normalized high frequency power significantly higher (29.1% ± 11.1 for SCD vs. 25.0 ± 10.6% for controls, p=0.038), while none of the other the frequency domain comparisons were significant. For economy of space, non-significant time and frequency domain results are not described.
When non-linear HRV measures were compared between cases and controls, DFA1 was significantly lower (0.97 ± 0.21 for cases and 1.11 ± 0.20 for controls, p=0.001). Upon univariate conditional logistic regression, decreased DFA1 and normalized LF power were each associated with SCD (Table 2), but increased normalized HF power (not shown) was no longer significant. Because the relationship of DFA1 to SCD was nominally stronger than that for normalized LF power, it was chosen for further analysis.
To clarify the relationship of lower DFA1 with SCD, the cutpoint that maximally separated those who died of SCD and those who did not was determined. Maximal separation occurred at DFA1=1.015. Those with DFA1 below this cut point were 5.6 times more likely to suffer SCD.
Heart Rate Turbulence (Table 1 and Table 2)
On Chi-square analysis, having abnormal heart rate turbulence slope alone was associated with SCD (p=0.026). The combination of both abnormal HR turbulence slope and onset added slightly to the prediction of SCD (p=0.019). As shown in Table 2, the relationship of having abnormal HRT and SCD remained significant upon unadjusted conditional logistic regression analysis (p=0.019).
T-Wave Alternans (Table 1 and Table 2)
Although maximum TWA on each channel tended to be higher for cases than for controls, e.g., 44 ± 21 vs. 38± 13 µV for ch1 (V5) and 39 ±28 vs. 32 ± 13 µV on channel 2 (aVR), this difference was not significant (p>0.087). Time of maximal TWA and heart rate at maximal TWA were not different between groups.
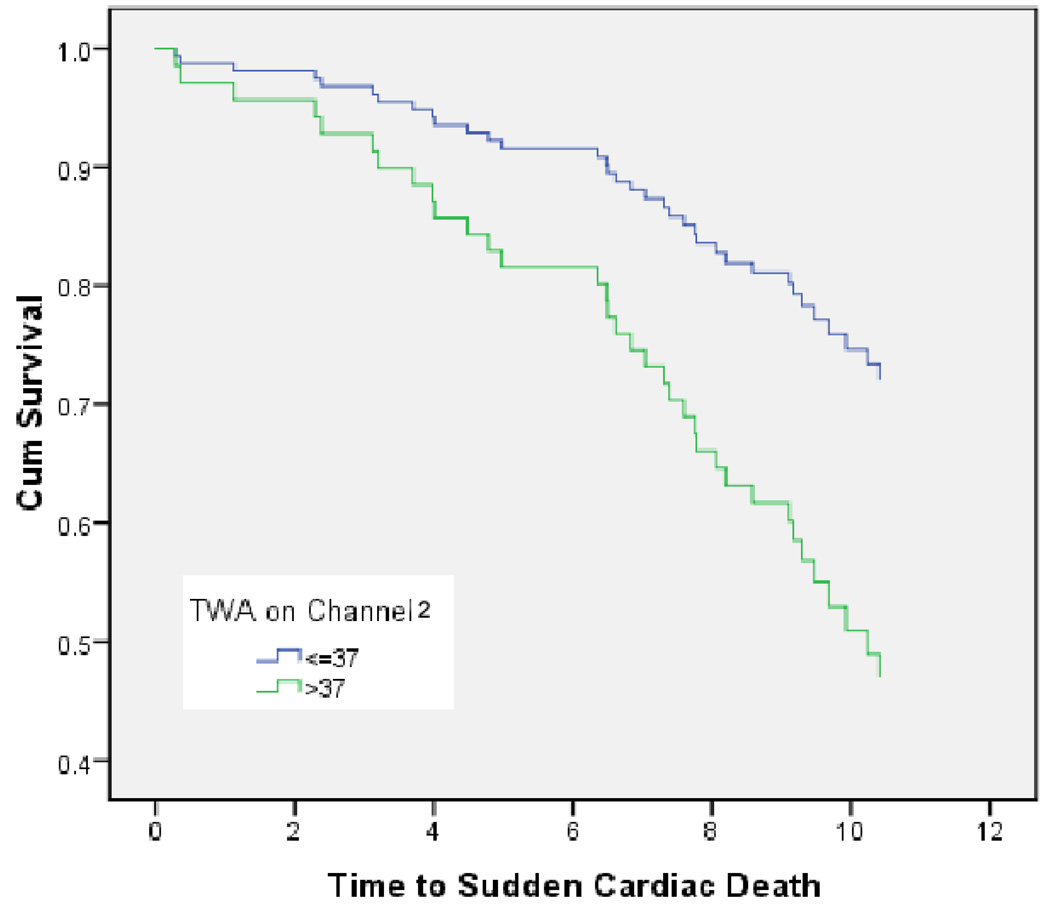
While having TWA >46 µV on ch1 or having TWA above 37 µV on ch2 were each significantly associated with a higher prevalence of SCD by chi-square analysis, only having TWA above 36–38 µV on channel 2 was significantly associated with SCD using conditional logistic regression analysis (Table 2). Having TWA above this ch2 cutpoint was associated with a nearly 3-fold increase in the unadjusted risk of SCD. Figure 3 shows age-adjusted survival curves for SCD in participants with TWA above and below 37 µV in channel 2. As can be seen from the figure, survival curves continue to diverge on follow up.
Figure 3.
Age-adjusted survival curves for SCD for participants with Holter TWA above and below the cutpoint of 37 µV on channel 2.
Combined Risk Models for SCD (Table 3)
Table 3.
Multivariate Conditional Logistic Regression Models for SCD.
| OR (95% CI) |
p-value | |
|---|---|---|
| Model 1. Usable TWA in Ch 2 and VPCs (N=133, 46 SCD) | ||
| Lower half of VPCs (≤30, N=58, 9 SCD) |
1.0 | NA |
| Upper half of VPCs (>30, N=88, 40 SCD) |
6.62 (2.30–19.06) |
<0.001 |
| TWA>37 µV in Ch2 (N=35, 14 SCD) |
4.84 (1.48–15.81) |
0.009 |
| Model 2. Usable Frequency Domain HRV with VPCs (N=128, 38 SCD) | ||
| Lower half of VPCs (≤30, N=58, 9 SCD) |
1 | |
| Upper half of VPCs (>30, N=86, 40 SCD) |
6.91 (2.02–26.7) |
0.002 |
| DFA1 <1.015 (N=45, 22 SCD) | 5.00 (1.8–16.6) |
0.003 |
| Model 3. Combined VPCs and TWA (N=133, 46 SCD) | ||
| Lower half of VPCS or TWA TWA ≤37 µV in Ch2 (N=51, 9 SCD) |
1 | NA |
| Either upper half of VPCs or TWA>37 µV in Ch2 (N=62, 21 SCD) |
2.59 (1.17–6.00) |
0.027 |
| Both upper half of VPCs and TWA>37 µV in Ch2 (N=20, 14 SCD) |
8.23 (3.25–20.84) |
<0.001 |
| Model 4. Combined VPCs and DFA1 (N=128, 38 SCD) | ||
| Lower half of VPCs and DFA1≥1.015 (N=51,6 SCD) |
1 | NA |
| Upper half of VPCs or DFA1
<1.015 (N=50, 14 SCD) |
3.10 (1.19–8.09) |
0.021 |
| Both upper half of VPCs and DFA1 <1.015 (N=26, 17 SCD) |
9.59 (3.74–24.56) |
<0.001 |
Table Legend: See Legend Table 1.
When HRT was combined with VPCs in the multivariate model, HRT was not independently associated with SCD (data not shown). However, increased TWA remained significantly associated with SCD in model 1. Among participants with Holter recordings adequate for the calculation of 24-hour frequency domain and non-linear HRV (model 2) decreased values DFA1 were also independently associated with SCD when combined with VPC categories. DFA1 was chosen for the model because it was nominally stronger in distinguishing between SCD and controls the t-tests (Table 1) and with conditional logistic regression (Table 2). Normalized low frequency power was highly correlated with DFA1 (r=0.90, p<0.001) and therefore entered model 3 in a similar fashion. Normalized high frequency power was not significant (p=0.295). TWA did not add to risk of SCD in the model with VPCs and DFA1.
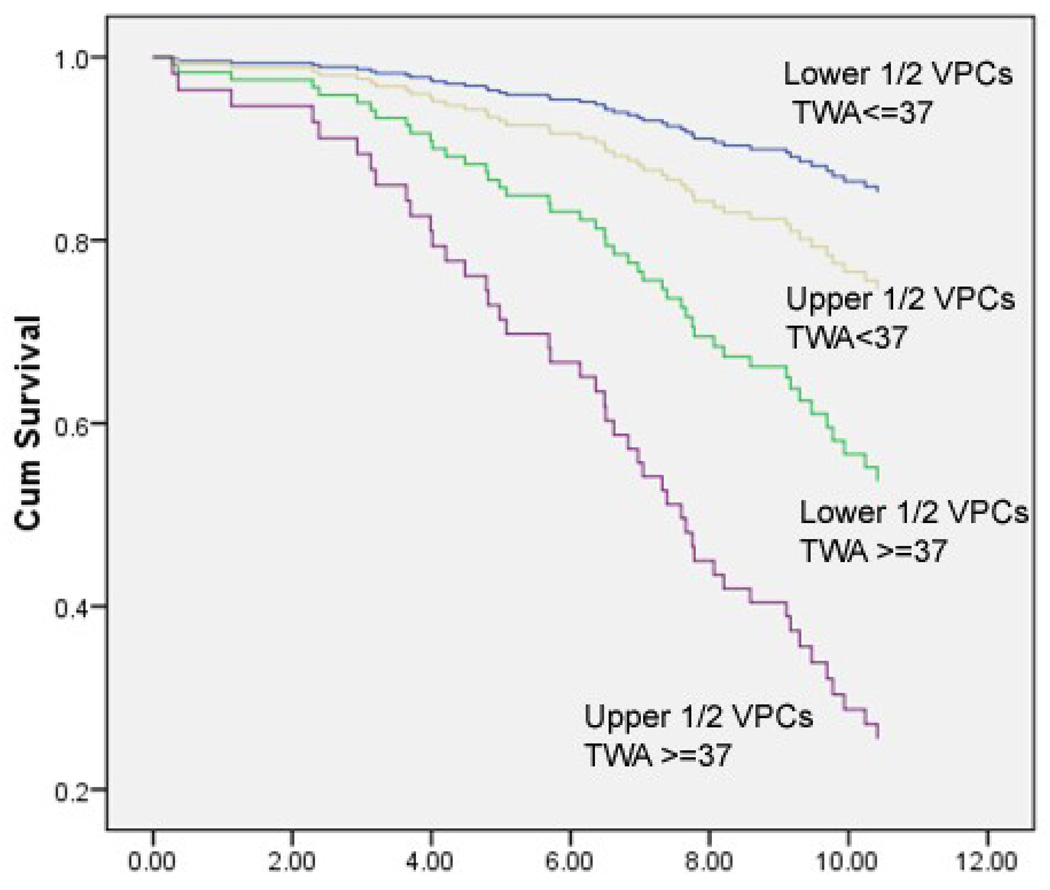
The additive effect of these risk factors was explored in Models 3 and 4 in Table 3. Compared to being in the lower half of VPCs and below the cut point in TWA, having either alone was associated with a 2.6 odds ratio for SCD compared to having neither, but having both risk factors was associated with an odds ratio of SCD of 8.2 compared to having neither. This result is shown in Figure 4. It is of interest that the one participant in the first quartile of VPC counts who died of SCD had abnormal TWA.
Figure 4.
Age-adjusted survival curves for the combined risk for SCD of having VPCs in the upper half and/or with Holter TWA above the cutpoint of 37 µV on channel 2.
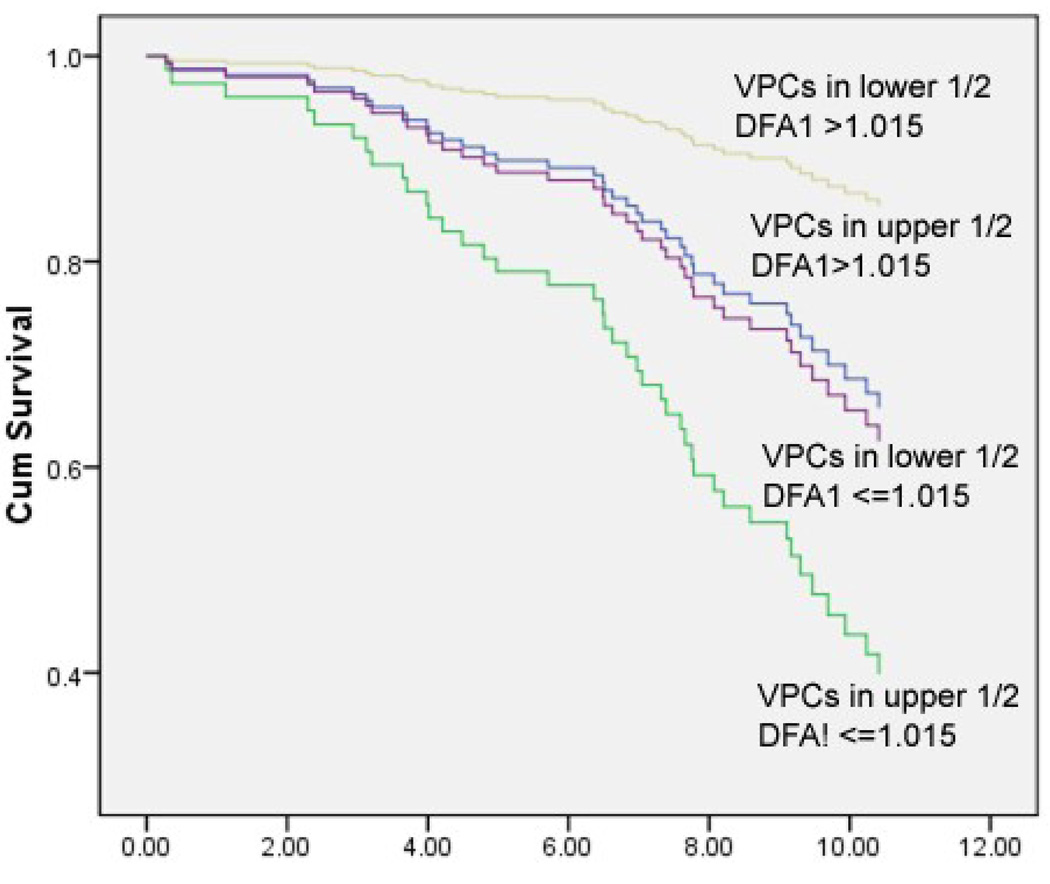
Using VPCs and DFA1 together also identified groups at higher risk of SCD (Figure 5). Begin categorized as abnormal on only one of these risk factors was associated with a 3.1 odds ratio of SCD compared to having both normal, but having both abnormal increased the odds ratio to 9.6.
Figure 5.
Age-adjusted survival curves for the combined risk of SCD for participants with VPCs in the upper half and/or DFA1 <1.015.
When DFA1 and TWA were combined, the risk of SCD for having either one or both abnormal was similar (OR=3.89, p=0.004 for having either, OR=4.7, p=0.026 for having both, model not shown).
Discussion
Our results suggest that information 24-hour Holter monitoring could identify people at increased and markedly decreased risk of SCD. Although heart rate and time domain HRV did not distinguish population-dwelling older adults at increased or decreased risk of SCD, Holter-based measures including: increased frequency of ventricular premature beats, more abnormal heart rate turbulence slope, higher TWA and lower values for the short-term fractal scaling exponent (DFA1) were seen among those who later died suddenly. Moreover, those with both abnormal DFA1 and more frequent VPCs were at an almost 10-fold risk of SCD compared to those with neither.
Our finding that an increased number of ventricular beats on 24-hour Holter monitoring was associated with SCD has not previously been reported in a population study. Furthermore, those with virtually no VPCs on 24-Holter monitoring were at especially low risk of SCD. However, Abdalla et al reported that the presence of any VPC on a 2-min rhythm strip among 15,637 healthy white men being screened for the Multiple Risk Factor Intervention Trial (MRFIT) was associated with a 3-fold relative risk of SCD but no increased risk of nonsudden cardiovascular death over 7.5 years of follow up.2 Although the MRFIT population was younger (35–57 years) than the CHS (≥ 65 years), both results support the potential of this simple measure from 24-hour Holter monitoring to identify adults at higher and lower risk of SCD.
We have previously reported that abnormal heart rate turbulence, which reflects a loss of the adaptive capacity in the cardiovascular system, was associated with increased risk of cardiovascular mortality in the Cardiovascular Health Study; however, the current study did not have the power to detect a specific risk, if any, of abnormal HRT for SCD.
T-wave alternans (TWA) reflects uneven repolarization of the cells of the ventricles of the heart that is believed to create conditions favorable for the development of lethal arrhythmias.28 We had previously reported that Holter-based TWA is a significant risk for sudden cardiac death in high risk patients with a recent MI.17 Results of the current study are promising in as much as MMA-based TWA was significantly associated with SCD even after adjustment for VPC counts. Tapes were in good condition and despite their age (up to 20 yrs), although the original choice of lead placement made channel 2 TWA less generally usable. Importantly, survival curves continued to diverge on follow up suggesting that the predictive value of TWA on Holter may not be limited to events in the short term. The specific cutpoint of 37 µV that maximized separation between cases and controls is lower than that reported in other studies of cardiac patients in whom the follow up period was much shorter than 13 years. 16,17 Further validation in a larger study is needed to determine if the cutpoint for TWA associated with excess risk of SCD in any specific Holter lead would be different among community-dwelling older adults.
Neither heart rate nor any time domain HRV measure was associated with SCD. This has generally also been the case among post-MI patients, where time domain HRV has predicted overall mortality but not SCD. However, careful adjudication of cause of death has been problematic in these studies.29 In the Autonomic Tone and Reflexes After Myocardial Infarction (ATRAMI) study, SDNN <70 ms, reflecting reduced overall HRV, was part of a composite index for predicting a combined endpoint of fatal and non-fatal cardiac arrest.8
An important finding of the current study is that decreased values of the frequency domain ratio measure normalized low frequency power were independently associated with SCD. Our interpretation of this finding is discussed below. The relationship of decreased normalized low frequency power and SCD has not previously been reported, although decreased values for low frequency power by itself (not different between cases and controls in the current study) were associated with SCD in some heart failure trials.29 Furthermore, decreased values for normalized LF power have also predicted mortality in heart failure patients.30
Notably, the strongest relationship of HRV to SCD was found in decreased values for the short-term fractal scaling exponent (DFA1). Decreased DFA1 reflects a higher degree of randomness (i.e., increased sinus arrhythmia of non-respiratory origin) in heart rate patterns. This randomness, which we have called “erratic rhythm,” may reflect sino-atrial node dysfunction, abnormal autonomic balance or even subtle atrial arrhythmias that cannot be identified from a two-channel Holter recording.31 However, it must be noted that we have not found any relationship between detected atrial arrhythmias and cardiovascular mortality in the CHS. Decreased DFA1 has strongly predicted mortality in post-MI patients5,32 and in patients with heart failure.33
We believe that the relationship of decreased normalized low frequency power with SCD is related to its strong correlation with DFA1 in this population. The meaning of normalized low frequency power depends on its context. Decreased normalized low frequency power in the presence of decreased normalized high frequency power, as found for example in patients with type I diabetes34 is a clear marker for autonomic dysfunction. However, decreased normalized low frequency power in the presence of increased normalized high frequency power, was found in the current study. This increase in normalized high frequency power correlates the presence of erratic rhythm and confounds HRV measures by making them appear to be higher (i.e., more normal) than they are.31 Therefore, the decrease in normalized (i.e., relative) low frequency power appears to be a direct consequence of the contribution of abnormal beat-to-beat variability to total HRV (the denominator of normalized low frequency power) and this abnormal beat-to-beat variability appears to associated with risk of SCD.
Strengths of the current study include: carefully adjudicated determination of SCD and 100% follow up among the well-characterized participants in the Cardiovascular Health Study. Recordings were analyzed to research standards. VPC counts, the major predictor of SCD, are available on any commercial Holter recording system.
Limitations include: the fact that Holter recordings were performed in a subset of the Cardiovascular Health Study, although clinical and demographic characteristics of those who did and did not have Holter recordings were similar. The nested case:control design was necessitated by the limited number of SCD cases and by the requirement to completely rescan the recordings in order to measure T-wave alternans. T-wave alternans could be measured in channel 1 (V5) on all but one recording, but the quality of the signals in the aVR lead was not adequate for this measurement on channel 2 (aVR) of all recordings. Although sudden deaths were carefully adjudicated and were determined to be cardiovascular, it was not possible to determine whether the cause of SCD was arrhythmic. Also, the use of the optimal cut point to estimate risk in the same cohort increases the chance that this risk will be overestimated so results need to be tested in a different group. Finally, measurement of DFA1 requires careful editing of Holter recordings, and DFA1 is not current available on any commercial Holter system, although the algorithm is free and downloadable from PhysioNet.35 However, normalized low frequency power, which is often available, provided similar risk stratification at least in the presence of careful Holter scanning.
In conclusion, results support a potential relationship of measures of abnormalities in cardiac functioning detected on 24-hour Holter recordings with higher and lower risk of sudden cardiac death in community-dwelling older adults.
Acknowledgments
The research reported in this article was supported by contract numbers N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, grant number U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm. In addition this research was supported by by R0-1 HL62181 from the National Heart, Lung, and Blood Institute. The T-wave alternans analysis was supported by a grant from GE Medical Systems.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: All authors declare no conflicts of interest and have nothing to declare financially.
References
- 1.Myerberg R. Sudden Cardiac Death: Exploring the limits or our knowledge. Journal of Cardiovascular Electrophysiology. 2001;12:369–381. doi: 10.1046/j.1540-8167.2001.00369.x. [DOI] [PubMed] [Google Scholar]
- 2.Abdalla IS, Prineas RJ, Neaton JD, Jacobs DR, Jr, Crow RS. Relation between ventricular premature complexes and sudden cardiac death in apparently healthy men. Am J Cardiol. 1987;60:1036–1042. doi: 10.1016/0002-9149(87)90348-1. [DOI] [PubMed] [Google Scholar]
- 3.Moss AJ, Davis HT, DeCamilla J, Bayer LW. Ventricular ectopic beats and their relation to sudden and nonsudden cardiac death after myocardial infarction. Circulation. 1979;60:998. doi: 10.1161/01.cir.60.5.998. [DOI] [PubMed] [Google Scholar]
- 4.Zareba W, Moss AJ. Noninvasive risk stratification in postinfarction patients with severe left ventricular dysfunction and methodology of the MADIT II noninvasive electrocardiology substudy. J Electrocardiol. 2003;36 Suppl:101–108. doi: 10.1016/j.jelectrocard.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 5.Mäkikallio TH, Høiber S, Køber L, Torp-Pedersen C, Peng CK, Goldberger AL, Huikuri HV. Fractal analysis of heart rate dynamics as a predictor of mortality in patients with depressed left ventricular function after acute myocardial infarction. TRACE Investigators. TRAndolapril Cardiac Evaluation. Am J Cardiol. 1999;83:836–839. doi: 10.1016/s0002-9149(98)01076-5. [DOI] [PubMed] [Google Scholar]
- 6.Stein PK, Barzilay JI, Chaves PHM, Mistretta SQ, Domitrovich PP, Gottdiener JS, Rich MW, Kleiger RE. Novel Measures of Heart Rate Variability Predict Cardiovascular Mortality in Older Adults Independent of Traditional Cardiovascular Risk Factors: The Cardiovascular Health Study. J Cardiovascular Electrophysiology. doi: 10.1111/j.1540-8167.2008.01232.x. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Francis J, Watanabe MA, Schmidt G. Heart Rate Turbulence: A New Predictor for Risk of Sudden Cardiac Death. Annals of Noninvasive Electrocardiology. 2005;10:102–109. doi: 10.1111/j.1542-474X.2005.10102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.La Rovere MT, Pinna GD, Hohnloser SH, Marcus FI, Mortara A, Nohara R, Bigger JT, Jr, Camm AJ, Schwartz PJ for the ATRAMI Investigators. Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias. Implications for clinical trials. Circulation. 2001;103:2072–2077. doi: 10.1161/01.cir.103.16.2072. [DOI] [PubMed] [Google Scholar]
- 9.Cygankiewicz I, Zareba W, Vazquez R, Vallverdu M, Gonzalez-Juanatey JR, Valdes M, Almendral J, Cinca J, Caminal P, de Luna AB Muerte Subita en Insuficiencia Cardiaca Investigators. Heart rate turbulence predicts all-cause mortality and sudden death in congestive heart failure patients. Heart Rhythm. 2008;5:1095–1102. doi: 10.1016/j.hrthm.2008.04.017. Epub 2008 May 2. [DOI] [PubMed] [Google Scholar]
- 10.Weber S, Tillmanns H, Waldecker B. Prevalence of t wave alternans in healthy subjects. PACE. 2003;26(Pt. I):49–52. doi: 10.1046/j.1460-9592.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- 11.Nieminen T, Lehtimäki T, Viik J, Lehtinen R, Nikus K, Kööbi T, Niemelä K, Turjanmaa V, Kaiser W, Huhtala H, Verrier RL, Huikuri H, Kähönen M. T-wave alternans predicts mortality in a population undergoing a clinically indicated exercise test. Eur Heart J. 2007;28:2332–2337. doi: 10.1093/eurheartj/ehm271. [DOI] [PubMed] [Google Scholar]
- 12.Minkkinen M, Kähönen M, Viik J, Nikus K, Lehtimäki T, Lehtinen R, Kööbi T, Turjanmaa V, Kaiser W, Verrier RL, Nieminen T. Enhanced predictive power of quantitative TWA during routine exercise testing in the Finnish Cardiovascular Study. J Cardiovasc Electrophysiol. 2009;20:408–415. doi: 10.1111/j.1540-8167.2008.01325.x. [DOI] [PubMed] [Google Scholar]
- 13.Osman AF, Gold MR. T wave alternans for ventricular arrhythmia risk stratification. Curr Opin Cardiol. 2002;17:1–5. doi: 10.1097/00001573-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Nearing BD, Verrier RL. Modified moving average analysis of t-wave alternans to predict ventricular fibrillation with high accuracy. J Appl Physiol. 2002;92:541–549. doi: 10.1152/japplphysiol.00592.2001. [DOI] [PubMed] [Google Scholar]
- 15.Verrier RL, Kumar K, Nearing BD. Basis for sudden cardiac death prediction by T-wave alternans from an integrative physiology perspective. Heart Rhythm. 2009;6:416–422. doi: 10.1016/j.hrthm.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakaki K, Ikeda T, Miwa Y, Miyakoshi M, Abe A, Tsukada T, Ishiguro H, Mera H, Yusu S, Yoshino H. Time-domain T-wave alternans measured from Holter electrocardiograms predicts cardiac mortality in patients with left ventricular dysfunction: A prospective study. Heart Rhythm. 2009;6:332–337. doi: 10.1016/j.hrthm.2008.12.011. 2009. [DOI] [PubMed] [Google Scholar]
- 17.Stein PK, Sanghavi D, Domitrovich PP, Mackey RA, Deedwania P, et al. Ambulatory ECG-Based T-Wave Alternans Predicts Sudden Cardiac Death in High-Risk Post-MI Patients with Left Ventricular Dysfunction In the EPHESUS Study. J Cardiovascular Electrophysiol. 2008;19:1037–1042. doi: 10.1111/j.1540-8167.2008.01225.x. Epub 2008 Jun 28. [DOI] [PubMed] [Google Scholar]
- 19.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 18.Kleiger RE, Stein PK, Bosner MS. Time domain measurements of heart rate variability. Cardiology Clinics of North America. 1992;10:478–487. [PubMed] [Google Scholar]
- 20.Rottman JN, Steinman RC, Albrecht P, Bigger JT, Jr, Rolnitzky LM, Fleiss JL. Efficient estimation of the heart period power spectrum suitable for physiologic or pharmacologic studies. Am J Cardiol. 1990;66:1522–1524. doi: 10.1016/0002-9149(90)90551-b. [DOI] [PubMed] [Google Scholar]
- 21.Kleiger RE, Stein PK, Bigger JT., Jr Heart Rate Variability: Measurement and Clinical Utility. A.N.E. 2005;10(1):1–14. doi: 10.1111/j.1542-474X.2005.10101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor JA, Carr DL, Myers CW, Eckberg DL. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation. 1998;98:547–555. doi: 10.1161/01.cir.98.6.547. [DOI] [PubMed] [Google Scholar]
- 23.Bigger JT, Jr, Steinman RC, Rolnitzky LM, Fleiss JL, Albrecht P, Cohen RJ. Power law behavior of RR-interval variability in healthy middle-aged persons, patients with recent acute myocardial infarction, and patients with heart transplants. Circulation. 1996;93:2142–2151. doi: 10.1161/01.cir.93.12.2142. [DOI] [PubMed] [Google Scholar]
- 24.Peng CK, Havlin S, Stanley HE, Goldberger AL. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos. 1995;5:82–87. doi: 10.1063/1.166141. [DOI] [PubMed] [Google Scholar]
- 25.Iyengar N, Peng CK, Morin R, Goldberger AL, Lipsitz LA. Age-related alterations in the fractal scaling of cardiac interbeat interval dynamics. Am J Physiol. 1996;271:R1078–R1084. doi: 10.1152/ajpregu.1996.271.4.R1078. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt G, Malik M, Barthel P, Schneider R, Ulm K, Rolnitzky L, Camm AJ, Bigger JT, Jr, Schömig A. Heart-rate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction. Lancet. 1999;353:1390–1396. doi: 10.1016/S0140-6736(98)08428-1. [DOI] [PubMed] [Google Scholar]
- 27.Gardin JM, Siscovick D, Anton-Culver H, Lynch JC, Smith VE, Klopfenstein HS, Bommer WJ, Fried L, O'Leary D, Manolio TA The Cardiovascular Health Study. Sex, age, and disease affect echocardiographic left ventricular mass and systolic function in the free-living elderly. Circulation. 1995;15:1739–1748. doi: 10.1161/01.cir.91.6.1739. [DOI] [PubMed] [Google Scholar]
- 28.Gold MR, Spencer W. T wave alternans for ventricular arrhythmia risk stratification. Curr Opin Cardiol. 2003;18:1–5. doi: 10.1097/00001573-200301000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Sandercock GRH, Brodie DA. The Role of Heart Rate Variability in Prognosis for Different Modes of Death in Chronic Heart Failure. Pacing and Clinical Electrophysiology. 2006;29:892–904. doi: 10.1111/j.1540-8159.2006.00457.x. [DOI] [PubMed] [Google Scholar]
- 30.Guzzetti S, Mezzetti S, Magatelli R, et al. Linear and non-linear 24 h heart rate variability in chronic heart failure. Auton Neurosci. 2000;(86):14–19. doi: 10.1016/S1566-0702(00)00239-3. [DOI] [PubMed] [Google Scholar]
- 31.Stein PK, Domitrovich PP, Hui N, et al. Sometimes Higher Heart Rate Variability is Not Better Heart Rate Variability: Results of Graphical and Non-Linear Analyses. Journal of Cardiovascular Electrophysiology. 2005;16:1–6. doi: 10.1111/j.1540-8167.2005.40788.x. [DOI] [PubMed] [Google Scholar]
- 32.Huikuri HV, Mäkikallio TH, Peng CK, et al. Fractal correlation properties of R-R interval dynamics and mortality in patients with depressed left ventricular function after an acute myocardial infarction. Circulation. 2000;101:47–53. doi: 10.1161/01.cir.101.1.47. [DOI] [PubMed] [Google Scholar]
- 33.Makikallio TH, Huikuri H, Hintze U, et al. Fractal analysis and time- and frequency-domain measures of heart rate variability as predictors of mortality in patients with heart failure. Am J Cardiol. 2001;87:178–182. doi: 10.1016/s0002-9149(00)01312-6. [DOI] [PubMed] [Google Scholar]
- 34.Mäkimattila S, Schlenzka A, Mäntysaari M, et al. Predictors of abnormal cardiovascular autonomic function measured by frequency domain analysis of heart rate variability and conventional tests in patients with type 1 diabetes. Diabetes Care. 2000;23:1686–1693. doi: 10.2337/diacare.23.11.1686. [DOI] [PubMed] [Google Scholar]
- 35.Goldberger AL, Amaral LAN, Glass L, et al. PhysioBank, PhysioToolkit, and PhysioNet: Components of a New Research Resource for Complex Physiologic Signals. Circulation. 2000;101:e215–e220. doi: 10.1161/01.cir.101.23.e215. [Circulation Electronic Pages; http://circ.ahajournals.org/cgi/content/full/101/23/e215] [DOI] [PubMed] [Google Scholar]