Abstract
Purpose
To compare corneal high-order aberrations and visual acuity after LASIK with the flap created by a femtosecond laser (bladeless) to LASIK with the flap created by a mechanical microkeratome.
Design
Prospective, randomized, paired-eye study.
Methods
Fellow eyes of 21 patients with myopia or myopic astigmatism were randomized by ocular dominance. Corneal topography and visual acuity were measured before and at 1, 3, 6, 12 and 36 months after LASIK. Wavefront errors from the anterior corneal surface were calculated from the topography data over 4- and 6-mm-dimater pupils and decomposed into Zernike polynomials to the 6th order.
Results
There were no differences in corneal total high-order aberrations, spherical aberration, coma or trefoil between methods of flap creation at any examination over 4-and 6-mm-diameter pupils. Over a 6 mm pupil, total high-order aberrations increased by 1 month after LASIK with both treatments (p≤0.001) and remained increased through 36 months (p≤0.001). Uncorrected and best-corrected visual acuity did not differ between methods at any examination and remained stable postoperatively through 3 years; the minimum detectable difference in visual acuity between treatments was ≤0.1 logMAR (≤1 line of vision, α=0.05/6, β=0.20, n=21).
Conclusions
The planar configuration of the femtosecond laser flap did not offer any advantage in corneal high-order aberrations or visual acuity through 3 years after LASIK. Corneal high-order aberrations remain stable through 3 ears after LASIK.
INTRODUCTION
Laser in situ keratomileusis (LASIK) is the most common corneal refractive surgery for the correction of myopia,1 and involves photoablation of the corneal stroma deep to an anterior corneal flap. Flap creation is the main surgical step of this procedure and can result in complications.2 Flaps have traditionally been created with mechanical microkeratomes, but femtosecond laser technology has emerged as an alternative for flap creation.3 Femtosecond lasers use ultrafast pulses to induce photodisruption of tissue with minimal surrounding tissue damage.4, 5 LASIK flaps created with the femtosecond laser have a planar configuration; flap thickness is uniform in contrast to flaps created by a microkeratome, in which the center of the flap is thinner than the periphery.6–8 The planar flap configuration has been suggested to confer an optical advantage, possibly reducing high-order aberrations.8 Several studies have described the short-term clinical outcomes of patients who underwent LASIK with the flap created by a femtosecond laser versus a mechanical microkeratome; some have found little difference in outcome between the techniques,9, 10 whereas others have suggested more favorable outcomes with the femtosecond laser.7, 8, 11–14
LASIK has also been associated with long-term deficits of keratocytes in the flap stroma.15 The clinical effects of anterior keratocyte deficits have not been studied, but one consequence might be instability of postoperative corneal wavefront errors resulting from changes in the anterior corneal surface. In this randomized, contralateral eye study, we investigated differences between, and the stability of, corneal high-order aberrations and visual acuity through 3 years after LASIK with the flap created by either a femtosecond laser (bladeless) or a mechanical microkeratome. We have previously reported short-term visual outcomes for this trial.9
MATERIALS AND METHODS
Subjects
Twenty-one subjects were recruited from patients attending the refractive surgery service at Mayo Clinic. All patients had myopia or myopic astigmatism, were > 21 years old, and were determined to be suitable candidates for LASIK after a rigorous screening examination. Subjects were excluded if they had any corneal abnormalities, a history of ocular disease, trauma, or surgery, diabetes mellitus or other systemic disease known to affect the eye, or if they used ocular medications. Systemic medications were permitted unless they were known to affect the cornea or anterior segment. Subject age at surgery was 38 ± 10 years (mean ± standard deviation; range, 22–54 years). Patients who developed new ocular conditions during the follow-up period were excluded from subsequent analysis if it was determined that the new condition would interfere with the outcomes. Fellow eyes of unoperated normal myopic controls were examined concurrently; age of controls was 43 ± 7 years (range, 29–55 years). This study complied with the Health Insurance Portability and Accountability Act and was approved by the Mayo Clinic Institutional Review Board. Informed consent was obtained from all subjects after explanation of the nature and possible consequences of the study.
Randomization
Patients were stratified by ocular dominance and then one eye of each patient was randomized to LASIK with the flap created by a femtosecond laser, and the other eye to LASIK with the flap created by a mechanical microkeratome. Ocular dominance was tested by asking patients to use both hands to frame a distant target while an observer determined with which eye the target was aligned.
LASIK Procedure
Bladeless flaps were created with a 15-kHz femtosecond laser (IntraLase FS, IntraLase Corp., Irvine, CA). All flaps had a superior hinge and intended thickness of 120 µm. Raster line and spot separation were 9 and 11 µm, respectively; raster energy was 2.3 µJ, and side-cut energy was 2.5 µJ. Flaps created by the mechanical microkeratome (Hansatome, Bausch & Lomb, Rochester, NY) had a superior hinge with intended thickness of 180 µm. Non-wavefront-guided ablation of the stromal bed was performed with a VISX Star S4 excimer laser (VISX, Santa Ana, CA) with radiant exposure of 160 mJ/cm2. Emmetropia was attempted in all cases by using an ablation zone that ranged from 6.5 × 6.5 mm for spherical corrections to 6.5 × 5.0 mm for astigmatic corrections. Both eyes of each patient were treated on the same day. All procedures followed a standard protocol: the bladeless flap was created first on the eye randomized to the femtosecond laser; the fellow eye then received a full LASIK procedure with the flap created by the mechanical microkeratome; finally, LASIK was completed on the first eye by separating and lifting the flap created by the femtosecond laser and ablating the stroma. It was not possible to mask patients as to which treatment was received in each eye. Postoperative topical medication regimens were identical for each eye and consisted of ciprofloxacin ophthalmic solution 4 times per day for 5 days, and fluorometholone 0.1% 4 to 8 times daily with a taper over 3 weeks.
Outcome Measures
Patients were examined before LASIK and at 1, 3, 6, 12 and 36 months after LASIK. At each examination, corneal topography, high-contrast visual acuity, low-contrast visual acuity, and manifest refraction were recorded. Whole eye aberrometry was introduced to the study protocol during the enrollment phase and thus data were not available for all eyes at every examination; low-contrast visual acuity data were only available at 12 and 36 months after LASIK. Measurements were made by observers masked as to which treatment was received in each eye.
Corneal topography was recorded by using a Humphrey Atlas Corneal Topography System (Humphrey Systems, Pleasanton, CA). Two to 4 topographic maps were recorded for each eye, centered over the line of sight. The videokeratography maps of each cornea were examined by one masked observer, and the map with the most complete image and the smallest non-digitized areas was selected for assessment of wavefront errors from the anterior corneal surface. Aberrations for the whole eye were measured with a Hartman-Shack aberrometer (VISX Wavescan, Santa Ana, CA). Two to 4 whole eye wavefront analyses were acquired for each eye, and the exam with the highest quality and largest pupil diameter, as indicated by the aberrometry software, was selected by a masked observer.
High-contrast visual acuity was measured by using the electronic Early Treatment of Diabetic Retinopathy Study testing protocol.16 Uncorrected visual acuity (UCVA) and best-spectacle corrected visual acuity (BCVA) were recorded as letter scores, which were converted to logarithm of the minimum angle of resolution (logMAR).
Best spectacle-corrected low-contrast visual acuity (LCVA) was measured by using a backlit 10% Sloan Translucent Low Contrast Chart (Precision Vision, La Salle, IL) with a testing distance of 4 meters. LCVA was measured in a darkened room and recorded as letter scores under photopic (screen brightness, 139 cd/m2) and mesopic (screen brightness, 1.1 cd/m2) conditions; mesopic conditions were achieved by placing a neutral density (2 ND) filter in front of the low-contrast chart. ETDRS letter scores were converted to logMAR and Snellen equivalent.
Wavefront errors
From the corneal topography (anterior corneal surface) and whole eye aberrometry data, the wavefront errors over 4-mm and 6-mm pupils were calculated by using VOLCT (Sarver and Associates, Inc., Carbondale, IL) and decomposed into Zernike polynomials to the 6th order. All high-order aberrations were summarized for Zernike orders 3 – 6 as:
where is the Zernike coefficient of radial order n and angular frequency m. Spherical aberration was expressed as , coma as , and trefoil as . Anterior corneal surface wavefront errors were calculated by assuming that the average refractive index of the eye was 1.3375 at a wavelength of 555 nm. For whole eye aberrometry wavefront errors were calculated over a 6 mm pupil and data were excluded if the measured pupil diameter was less than 6 mm.
Statistical Analysis
The study was powered a priori to detect a difference of 0.15 logMAR in UCVA or BCVA at 3 years after LASIK by assuming that the standard deviation of the difference in visual acuity would be 0.15 logMAR. This required a minimum sample size of 16 subjects (α=0.05/6, β=0.20, paired test). Differences between treatments at each examination, and differences between preoperative and postoperative examinations for each treatment, were assessed by using 2-tailed paired t tests if the data were distributed normally and Wilcoxon signed-rank tests if they were not. P-values were adjusted for multiple comparisons by using the Bonferroni method, and p<0.05 was considered statistically significant. All statistical analyses were performed with Statistical Analysis System Version 9.1.3 (SAS Institute Inc., Cary, NC). Minimum detectable differences were calculated post-hoc for non-significant differences assuming α=0.05/5 or 0.05/6 (depending on the comparison) and β=0.20.
Incomplete whole eye wavefront error data and no LCVA data were available in patients before LASIK. To determine if differences in whole eye wavefront errors and LCVA exist between fellow unoperated eyes, we compared these between fellow eyes of the normal myopic controls.
RESULTS
All subjects were included for analysis through 12 months of follow-up after LASIK. After 1 year, 4 eyes of 2 patients required enhancement procedures for mild under-corrections, which were similar in the fellow eyes; data for these eyes were retained in the analysis at 36 months. Visual acuity and whole eye aberrometry data were excluded in both eyes of 1 patient at 36 months because of the presence of visually-significant nuclear sclerotic cataracts; corneal topography data for this patient were included. One eye of one patient experienced trauma-induced recurrent erosions between 13 and 22 months after surgery; no erosions occurred after that time and data for this eye were included at 36 months.
Topography-derived wavefront error
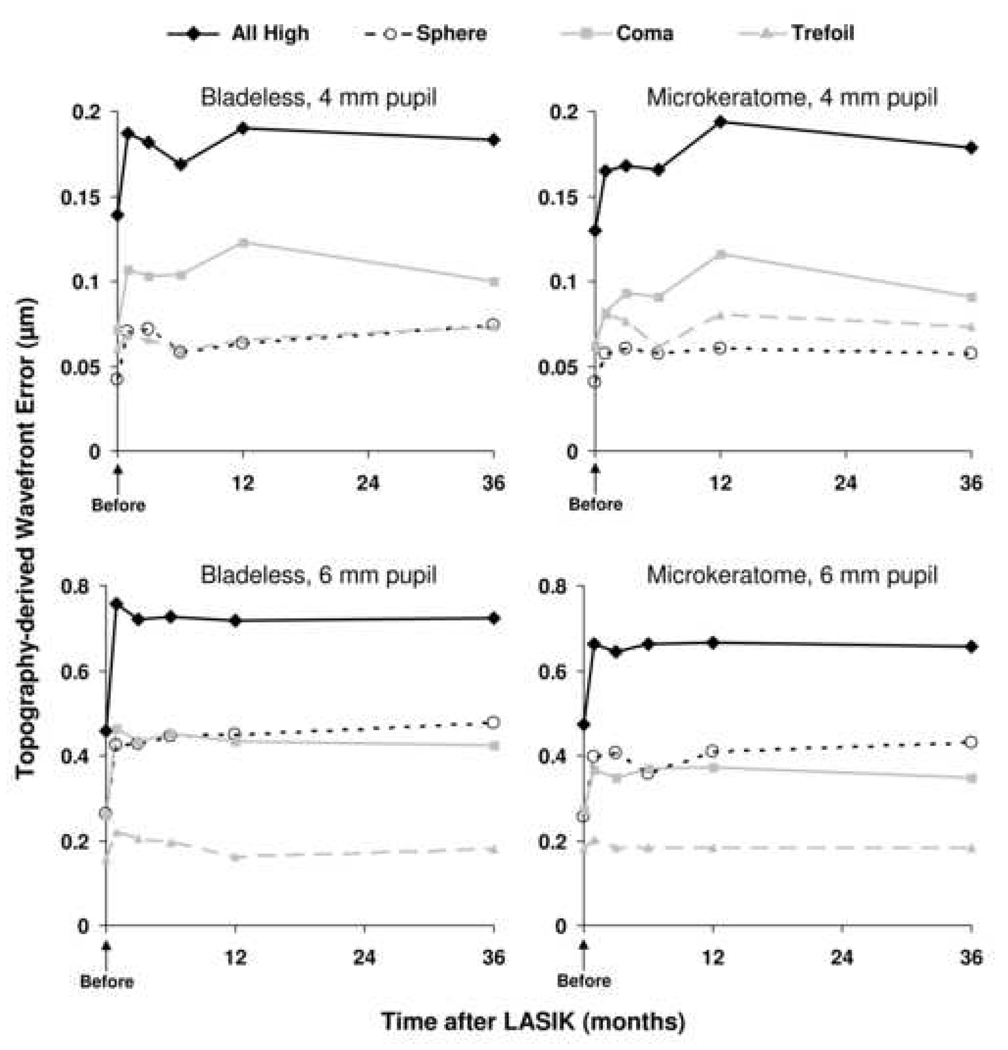
There were no differences in high-order aberrations derived from the anterior corneal surface between bladeless and microkeratome treatments at any examination over 4-mm and 6-mm pupil diameters (Table 1 and Table 2). Over a 4 mm pupil, total high-order aberrations increased by 1 month after LASIK in both treatment groups compared to preoperative (bladeless, p=0.03; microkeratome, p=0.007) and remained elevated through 36 months in the microkeratome group (p≤0.01) but not in the bladeless group (minimum detectable difference between 36 months and preoperative was 0.06 µm (α=0.05/5, β=0.20, n=21, paired analysis) (Table 1). Over a 6 mm pupil, total high-order aberrations increased by 1 month after LASIK in both treatment groups compared to preoperative (p<0.002) and remained elevated through 36 months (p≤0.001) (Table 2).
Table 1.
Topography-derived corneal wavefront errors after LASIK (4 mm diameter pupil).
| Wavefront Error (Root Mean Square, µm) | Mean MDD between treatments (µm) |
||||||
|---|---|---|---|---|---|---|---|
| Before LASIK |
1 month | 3 months | 6 months | 12 months | 36 months | ||
| All High Order Aberrations | |||||||
| Bladeless | 0.14 ± 0.07 | 0.19 ± 0.05a | 0.18 ± 0.05 | 0.17 ± 0.04 | 0.19 ± 0.07 | 0.18 ± 0.04 | 0.05 |
| Microkeratome | 0.14 ± 0.04 | 0.16 ± 0.04b | 0.17 ± 0.05c | 0.16 ± 0.05d | 0.19 ± 0.08b | 0.18 ± 0.05b | |
| Spherical | |||||||
| Bladeless | 0.04 ± 0.02 | 0.07 ± 0.04 | 0.07 ± 0.03c | 0.06 ± 0.03 | 0.06 ± 0.03 | 0.07 ± 0.03b | 0.03 |
| Microkeratome | 0.04 ± 0.02 | 0.06 ± 0.03 | 0.06 ± 0.03 | 0.06 ± 0.03 | 0.06 ± 0.04 | 0.06 ± 0.03 | |
| Coma | |||||||
| Bladeless | 0.07 ± 0.06 | 0.11 ± 0.06 | 0.10 ± 0.06 | 0.10 ± 0.06 | 0.12 ± 0.06 | 0.10 ± 0.06 | 0.05 |
| Microkeratome | 0.07 ± 0.03 | 0.08 ± 0.04 | 0.09 ± 0.05d | 0.09 ± 0.04c | 0.11 ± 0.07b | 0.09 ± 0.04d | |
| Trefoil | |||||||
| Bladeless | 0.06 ± 0.05 | 0.07 ± 0.04 | 0.06 ± 0.03 | 0.06 ± 0.04 | 0.06 ± 0.05 | 0.07 ± 0.04 | 0.04 |
| Microkeratome | 0.06 ± 0.03 | 0.08 ± 0.04 | 0.07 ± 0.04 | 0.06 ± 0.03 | 0.08 ± 0.04 | 0.07 ± 0.04 | |
Data are presented as mean ± standard deviation; n=21.
There were no differences between femtosecond laser (bladeless) and microkeratome treatments at any time; the mean minimum detectable difference (mean MDD) between treatments is indicated (α=0.05/6, β=0.20, n=21, paired analysis).
p=0.04,
p<0.01,
p=0.01,
p=0.02,
p=0.03,
p=0.05, versus preoperative; P-values were adjusted for 5 comparisons by using the Bonferroni technique.
Table 2.
Topography-derived corneal wavefront errors after LASIK (6 mm diameter pupil).
| Wavefront Error (Root Mean Square, µm) | Mean MDD between treatments (µm) |
||||||
|---|---|---|---|---|---|---|---|
| Before LASIK |
1 month | 3 months | 6 months | 12 months | 36 months | ||
| All High Order Aberrations | |||||||
| Bladeless | 0.46 ± 0.18 | 0.76 ± 0.25 a | 0.72 ± 0.21 a | 0.73 ± 0.20 a | 0.72 ± 0.20 a | 0.72 ± 0.21 b | 0.13 |
| Microkeratome | 0.47 ± 0.22 | 0.66 ± 0.21 b | 0.64 ± 0.20 b | 0.66 ± 0.23 b | 0.66 ± 0.18 a | 0.65 ± 0.18 b | |
| Spherical | |||||||
| Bladeless | 0.26 ± 0.09 | 0.42 ± 0.16 a | 0.43 ± 0.15 a | 0.43 ± 0.12 a | 0.45 ± 0.13 a | 0.47 ± 0.14 a | 0.08 |
| Microkeratome | 0.26 ± 0.08 | 0.40 ± 0.15 a | 0.40 ± 0.13 a | 0.40 ± 0.12 a | 0.41 ± 0.13 a | 0.43 ± 0.12 a | |
| Coma | |||||||
| Bladeless | 0.26 ± 0.19 | 0.46 ± 0.26 c | 0.43 ± 0.24c | 0.45 ± 0.25d | 0.43 ± 0.26e | 0.42 ± 0.24 | 0.14 |
| Microkeratome | 0.27 ± 0.23 | 0.36 ± 0.25 | 0.35 ± 0.25 | 0.35 ± 0.27 | 0.38 ± 0.23 | 0.35 ± 0.22 | |
| Trefoil | |||||||
| Bladeless | 0.16 ± 0.08 | 0.22 ± 0.2 | 0.20 ± 0.14 | 0.19 ± 0.11 | 0.16 ± 0.12 | 0.18 ± 0.09 | 0.11 |
| Microkeratome | 0.18 ± 0.10 | 0.20 ± 0.10 | 0.18 ± 0.10 | 0.18 ± 0.15 | 0.18 ± 0.10 | 0.18 ± 0.11 | |
Data are presented as mean ± standard deviation; n=21.
There were no differences between femtosecond laser (bladeless) and microkeratome treatments at any time; the mean minimum detectable difference (mean MDD) between treatments is indicated (α=0.05/6, β=0.20, n=21, paired analysis).
p<0.001,
p<0.01,
p=0.02,
p=0.03,
p=0.04, versus preoperative; P-values were adjusted for 5 comparisons by using the Bonferroni technique.
Whole eye aberrations
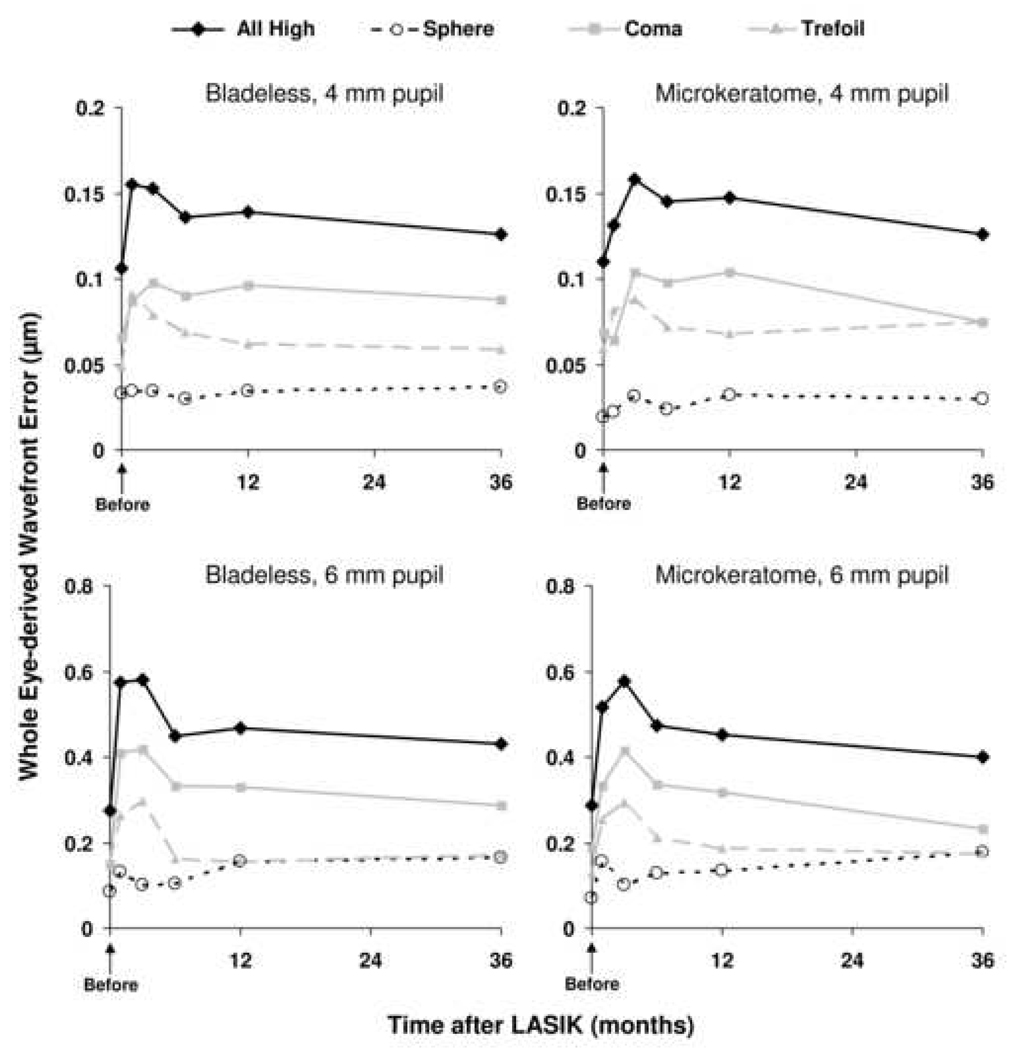
There were no differences in high-order aberrations derived from the whole eye between bladeless and microkeratome treatments at any examination over 4-mm and 6-mm pupil diameters (Table 3 and Table 4). The difference in total high-order aberrations after LASIK compared to preoperative were not statistically significant in either group over 4-mm and 6-mm pupil diameters; the mean minimum detectable differences were approximately 0.1 µm and 0.4 µm for 4-mm and 6-mm pupils respectively (α=0.05/5, β=0.20, paired analyses). Wavefront errors did not differ between fellow eyes of normal unoperated myopic controls (Table 5).
Table 3.
Whole eye wavefront errors after LASIK (4 mm diameter pupil).
| Wavefront Error (Root Mean Square, µm) | Mean MDD between treatments (µm) |
||||||
|---|---|---|---|---|---|---|---|
| Before LASIK n=10 |
1 month n=8 |
3 months n=12 |
6 months n=17 |
12 months n=21 |
36 months n=20 |
||
| All High Order Aberrations | |||||||
| Bladeless | 0.10 ± 0.05 | 0.15 ± 0.06 | 0.15 ± 0.05 | 0.13 ± 0.05 | 0.14 ± 0.04 | 0.12 ± 0.04 | 0.07 |
| Microkeratome | 0.11 ± 0.03 | 0.13 ± 0.06 | 0.16 ± 0.06 | 0.14 ± 0.05 | 0.15 ± 0.04 | 0.12 ± 0.05 | |
| Spherical | |||||||
| Bladeless | 0.03 ± 0.03 | 0.03 ± 0.01 | 0.03 ± 0.03 | 0.03 ± 0.03 | 0.03 ± 0.02 | 0.04 ± 0.02 | 0.03 |
| Microkeratome | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.02 | 0.02 ± 0.02 | 0.03 ± 0.02 | 0.03 ± 0.02 | |
| Coma | |||||||
| Bladeless | 0.06 ± 0.05 | 0.09 ± 0.07 | 0.10 ± 0.06 | 0.09 ± 0.05 | 0.09 ± 0.05 | 0.09 ± 0.04 | 0.07 |
| Microkeratome | 0.07 ± 0.03 | 0.06 ± 0.03 | 0.10 ± 0.04 | 0.10 ± 0.04 | 0.10 ± 0.03 | 0.07 ± 0.04 | |
| Trefoil | |||||||
| Bladeless | 0.05 ± 0.01 | 0.09 ± 0.05 | 0.08 ± 0.03 | 0.07 ± 0.03 | 0.06 ± 0.03 | 0.06 ± 0.03 | 0.05 |
| Microkeratome | 0.06 ± 0.02 | 0.08 ± 0.07 | 0.08 ± 0.05 | 0.07 ± 0.04 | 0.06 ± 0.04 | 0.07 ± 0.04 | |
Data are presented as mean ± standard deviation.
There were no differences between femtosecond laser (bladeless) and microkeratome treatments at any time; the mean minimum detectable difference (mean MDD) between treatments is indicated (α=0.05/6, β=0.20, paired analysis).
p=0.05 versus preoperative; P-values were adjusted for 5 comparisons by using the Bonferroni technique.
Table 4.
Whole eye wavefront errors after LASIK (6 mm diameter pupil).
| Wavefront Error (Root Mean Square, µm) | Mean MDD between treatments (µm) |
||||||
|---|---|---|---|---|---|---|---|
| Before LASIK n=7 |
1 month n=5 |
3 months n=5 |
6 months n=10 |
12 months n=12 |
36 months n=16 |
||
| All High Order Aberrations | |||||||
| Bladeless | 0.28 ± 0.06 | 0.57 ± 0.17 | 0.58 ± 0.10 | 0.45 ± 0.14 | 0.47 ± 0.12 | 0.43 ± 0.11 | 0.21 |
| Microkeratome | 0.29 ± 0.08 | 0.51 ± 0.11 | 0.58 ± 0.13 | 0.47 ± 0.16 | 0.45 ± 0.12 | 0.40 ± 0.12 | |
| Spherical | |||||||
| Bladeless | 0.08 ± 0.06 | 0.11 ± 0.10 | 0.10 ± 0.07 | 0.10 ± 0.08 | 0.15 ± 0.08 | 0.16 ± 0.09 | 0.11 |
| Microkeratome | 0.07 ± 0.06 | 0.15 ± 0.07a | 0.10 ± 0.07b | 0.12 ± 0.08 | 0.13 ± 0.10 | 0.18 ± 0.10c | |
| Coma | |||||||
| Bladeless | 0.15 ± 0.08 | 0.40 ± 0.18 | 0.42 ± 0.16 | 0.33 ± 0.16 | 0.33 ± 0.14 | 0.29 ± 0.14 | 0.27 |
| Microkeratome | 0.18 ± 0.08 | 0.33 ± 0.10c | 0.41 ± 0.14 | 0.33 ± 0.15 | 0.31 ± 0.13 | 0.23 ± 0.12 | |
| Trefoil | |||||||
| Bladeless | 0.15 ± 0.07 | 0.26 ± 0.19 | 0.29 ± 0.07 | 0.16 ± 0.13 | 0.15 ± 0.11 | 0.17 ± 0.10 | 0.19 |
| Microkeratome | 0.13 ± 0.05 | 0.25 ± 0..22 | 0.29 ± 0.14 | 0.21 ± 0.13 | 0.18 ± 0.10 | 0.17 ± 0.08 | |
Data are presented as mean ± standard deviation.
There were no differences between femtosecond laser (bladeless) and microkeratome treatments at any time; the mean minimum detectable difference (mean MDD) between treatments is indicated (α=0.05/6, β=0.20, paired analysis).
p=0.03,
p=0.01,
p=0.02,
p<0.01, versus preoperative; P-values were adjusted for 5 comparisons by using the Bonferroni technique.
Table 5.
Whole eye wavefront errors (4 mm diameter pupil) and low-contrast visual acuity in fellow eyes of unoperated normal myopic controls.
| Right Eye | Left Eye | P | MDD | |
|---|---|---|---|---|
| Whole Eye Wavefront Error (Root Mean Square, µm) | ||||
| All high-order | 0.11 ± 0.05 | 0.11 ± 0.04 | 0.86 | 0.04 |
| Spherical | 0.03 ± 0.02 | 0.03 ± 0.03 | 0.36 | 0.01 |
| Coma | 0.07 ± 0.05 | 0.06 ± 0.04 | 0.86 | 0.04 |
| Trefoil | 0.07 ± 0.03 | 0.06 ± 0.03 | 0.55 | 0.02 |
| Low Contrast Visual Acuity (logMAR) | ||||
| Photopic | 0.25 ± 0.13 | 0.23 ± 0.15 | 0.54 | 0.08 |
| Mesopic | 0.60 ± 0.10 | 0.60 ± 0.18 | 0.91 | 0.09 |
Data are presented as mean ± standard deviation; n=18 for all comparisons.
MDD, minimum detectable difference between eyes (α=0.05, β=0.20, n=18, paired analysis).
Visual Acuity and Refractive Error
No differences in high-contrast UCVA or BCVA were found between eyes that received a bladeless flap or eyes that received a microkeratome flap at any examination through 6 months, as previously reported,9 or at 12 or 36 months (Table 6). At 36 months, both eyes of 1 patient were excluded from analysis because of nuclear sclerotic cataract formation. Post-hoc analysis showed the minimum detectable differences for high-contrast visual acuity at 12 and 36 months were ≤0.1 log MAR (≤1 line of vision, α=0.05/6, β=0.20, n=21).
Table 6.
High-contrast visual acuity before and after LASIK
| Visual Acuity (LogMar) (Snellen Equivalent) |
|||
|---|---|---|---|
| Before | 1 year | 3 years* | |
| Uncorrected | |||
| 0.93 ± 0.33 | 0.02 ± 0.11 | −0.01 ± 0.14 | |
| Bladeless | (20/170) | (20/21) | (20/20) |
| 0.98 ± 0.35 | −0.01 ± 0.10 | 0.004 ± 0.15 | |
| Microkeratome | (20/191) | (20/20) | (20/20) |
| P | 0.67 | >0.99 | >0.99 |
| MDD (logMAR) | 0.10 | 0.07 | 0.10 |
| Best spectacle- corrected |
|||
| −0.07 ± 0.07 | −0.04 ± 0.08 | −0.08 ± 0.09 | |
| Bladeless | (20/17) | (20/18) | (20/17) |
| −0.08 ± 0.05 | −0.05 ± 0.07 | −0.06 ± 0.08 | |
| Microkeratome | (20/17) | (20/18) | (20/17) |
| P | >0.99 | >0.99 | >0.99 |
| MDD (logMAR) | 0.05 | 0.06 | 0.06 |
Data are presented as mean ± standard deviation; n=21 unless stated. P-values (paired t-tests) compare femtosecond laser (bladeless) and microkeratome treatments and were adjusted for 6 comparisons by the Bonferroni technique (comparisons at 1, 3 and 6 months are not shown but were reported previously9).
MDD= minimum detectable difference (paired data, α=0.05/6, β=0.20).
n=20, both eyes of 1 patient excluded because of the presence of cataracts.
For both methods of flap creation, there were no differences in best spectacle-corrected visual acuity before and after LASIK, and no differences in uncorrected visual acuity after LASIK.
Photopic LCVA was 0.17 ± 0.09 logMAR (Snellen equivalent, 20/30) in the bladeless eyes and 0.17 ± 0.10 logMAR (Snellen equivalent, 20/30) in the microkeratome eyes at 12 months (p>0.99), and 0.18 ± 0.10 logMAR (Snellen equivalent, 20/30) in the bladeless eyes and 0.18 ± 0.11 logMAR (Snellen equivalent, 20/30) in the microkeratome eyes at 36 months (p>0.99). Mesopic LCVA was 0.55 ± 0.13 logMAR (Snellen equivalent, 20/71) in the bladeless eyes and 0.53 ± 0.13 logMAR (Snellen equivalent, 20/68) in the microkeratome eyes at 12 months (p>0.99), and 0.55 ± 0.10 logMAR (Snellen equivalent, 20/71) in the bladeless eyes and 0.50 ± 0.10 logMAR (Snellen equivalent, 20/63) in the microkeratome eyes at 36 months (p=0.11). Photopic and mesopic LCVA did not differ between fellow eyes of normal unoperated myopic controls (Table 5).
Manifest refractive error did not differ between treatments before or after LASIK (Table 7).
Table 7.
Manifest refractive error before and after LASIK
| Refractive Error (D) | |||
|---|---|---|---|
| Before | 1 year | 3 yearsa | |
| Manifest Sphere | |||
| Bladeless | −4.02 ± 1.61 | −0.31± 0.32 | −0.35 ± 0.37 |
| Microkeratome | −4.15 ± 1.62 | −0.25 ± 0.33 | −0.29 ± 0.35 |
| P | >0.99 | >0.99 | >0.99 |
| MDD (D) | 0.54 | 0.25 | 0.24 |
| Manifest Cylinder | |||
| Bladeless | 0.70 ± 0.82 | 0.19 ± 0.26 | 0.10 ± 0.25 |
| Microkeratome | 0.77 ± 0.88 | 0.10 ± 0.20 | 0.14 ± 0.25 |
| P | >0.99 | >0.99b | >0.99 |
| (MDD) | 0.41 | 0.18 | 0.23 |
Data are presented as mean ± standard deviation; n=21 unless stated. P-values (paired t-tests unless stated) compare femtosecond laser (bladeless) and microkeratome treatments and were adjusted for 6 comparisons by the Bonferroni technique (comparisons at 1, 3 and 6 months are not shown but were reported previously9).
MDD= minimum detectable difference (paired data, α=0.05/6, β=0.20).
n=20, both eyes of 1 patient excluded because of the presence of cataracts.
Wilcoxon signed-rank test.
DISCUSSION
Femtosecond laser technology enables the creation of geometrically precise LASIK flaps with uniform thickness across the flap, predictable hinge lengths with lamellar dissection under the hinge, and steep side-cuts for improved flap realignment.6–8, 17 In contrast to LASIK flaps created with mechanical microkeratomes, the geometrically planar configuration of bladeless flaps has been suggested to confer advantages over microkeratome flaps, including the induction of fewer high-order aberrations,7, 8 and lesser astigmatism.13, 14 In this randomized, paired-eye study, we did not find any differences in outcomes between eyes that received a bladeless flap compared to eyes that received a flap created by a mechanical microkeratome through three years after LASIK.
The main goals of this study were to compare corneal high-order aberrations and visual acuity between bladeless and microkeratome treatments. High-order aberrations from the anterior corneal surface did not differ between the two methods of flap creation at any examination, indicating no optical advantage of the planar flap in our study. Total high-order aberrations increased by 1 month after LASIK, and although they remained statistically elevated through 3 years in the microkeratome group only, the small sample size resulted in insufficient statistical power to detect small changes within their bladeless group. Montés-Micó et al. compared corneal high-order aberrations in consecutive series of LASIK with flaps created with a femtosecond laser or with a microkeratome and found that aberrations increased after both treatments11; however, the postoperative high-order aberrations were not statistically compared between treatments, but appeared similar. In a non-randomized study, Buzzonetti et al. found that corneal high-order aberrations were higher after LASIK with the flap created by a microkeratome (Hansatome) than after LASIK with a femtosecond laser (IntraLase),18 which is in contrast to our results. The eyes in our study had less myopia than those treated by Buzzonetti et al., and this might account for the discrepancy between the studies. Preoperative corneal high-order aberrations in our study were similar to those reported by other investigators using different Placido-based and Scheimpflug topography instruments11, 18, 19; nevertheless, the data must be compared with caution because differences might be the result of using different instruments and converting data to conform to a specific optical zone diameter.
Aberrations from the anterior corneal surface did not change between 1 month and 3 years after LASIK in either treatment group, which might be expected given the long-term stability in vision after this procedure.20 Nevertheless, the cornea does undergo many pathophysiologic changes after LASIK, including epithelial thickening,21, 22 loss of anterior keratocytes,15 and delayed reinnervation,23 that could result in subtle changes in corneal properties, including topography. The epithelium thickens within a month of LASIK21, 22 and thus topographic changes related to this early remodeling of the epithelium would not have been detected in the present study. In a previous study, we found that stromal keratocyte density decreased in the flap during the early years after LASIK,15 and we have replicated these results in the present study cohort (Patel SV et al., Keratocyte density three years after LASIK: bladeless versus microkeratome; IOVS 2009, ARVO E-Abstract #4524). Keratocytes are the cells that maintain the corneal stroma, and remodel the wounded cornea by producing new collagen,24–26 and their loss might affect the ultrastructural properties of the cornea. The architecture of the anterior cornea, which is critical for maintaining the anterior corneal curvature,27 is disrupted after LASIK, and possibly altered by chronic keratocyte deficits; nevertheless, these slow changes do not appear to affect corneal topography during the first 3 years after LASIK. Extended follow-up of this cohort is planned to determine any longer-term effects.
Similar to the topography-derived aberrations, we did not find any differences in aberrations for the whole eye between treatments at any examination. Our results through 6 months were limited by the small sample sizes because collection of these data was implemented after study enrollment commenced, but our data at 1 and 3 years after LASIK were complete. With our limited preoperative sample size, comparisons of postoperative to preoperative had low statistical power, but postoperative increases in high-order aberrations after LASIK are well known28, 29 with some increase in aberrations being attributed to flap creation alone.30, 31 Several studies have suggested that microkeratome flaps induce more whole-eye aberrations than bladeless flaps but the differences have frequently not been clinically or statistically significant. In a randomized, paired-eye study, Chan et al. suggested less spherical, coma and trefoil aberrations with bladeless flaps than with microkeratome flaps after wavefront-guided LASIK, but the differences were not statistically significant at 6 and 12 months.10 Lim et al. compared a series of eyes receiving either bladeless or microkeratome flaps and found that spherical aberration was higher in microkeratome flaps at 3 months, but total high-order aberrations did not differ.12 Similarly, Medeiros et al. found that the increases in total high-order and spherical aberrations were lower after wavefront-guided LASIK with bladeless flaps that with microkeratome flaps.7 Tran et al. compared high-order aberrations before and after flap creation and found that they increased with mechanical microkeratome flaps; their small sample might have resulted in insufficient statistical power to show a difference with bladeless flaps, and post-flap high-order aberrations were not compared between treatments.8 Our data after non-wavefront-guided LASIK suggest that even if differences in high-order aberrations do exist between methods of flap creation, the differences are small and likely to be clinically insignificant. Despite our limited preoperative data, the absence of differences in whole eye wavefront errors between fellow eyes of controls indicated that differences between fellow eyes of the LASIK patients before surgery were also unlikely (Table 5).
In this study, both the anterior corneal aberrations and the whole eye aberrations were reported over 4 mm and 6 mm-diameter optical zones. Anterior corneal aberrations calculated from topography were determined from estimates of elevation at several thousand points across the cornea; in contrast, whole eye aberrations were derived from a few hundred data because of the limited number of lenslets in our aberrometer. As a result, our estimates of corneal aberrations were more robust than our estimates of whole eye aberrations. Nevertheless, not all aberrations after LASIK arise from the anterior corneal surface, and thus, whole eye aberrations do provide additional data. Although we excluded whole eye aberrations from both eyes of one subject at 36 months because of cataract formation, the whole eye data from the other subjects might include subtle changes in wavefront error from subclinical changes in the crystalline lens.
High-contrast visual acuity remained stable postoperatively with both treatments through 3 years, with no change in manifest refractive error. Our results are similar to other comparative studies that also found no difference in visual acuity between the treatments as long as 1 year after surgery.10–12 Although our study was powered to detect a difference in BCVA or UCVA of 0.15 logMAR between treatment, a post-hoc analysis indicated that the smallest difference we could have detected was in fact 0.07 logMAR at 12 months and 0.10 logMAR at 36 months. LCVA also did not differ between treatments, and although we did not have preoperative LCVA data in our study, we showed that LCVA does not differ between fellow eyes of controls, indicating that LCVA before was unlikely to be different in the eyes before LASIK..Similarly, Chan et al. found no difference in LCVA between treatments at 12 months.10
Although the intended flap thicknesses for femtosecond and microkeratome treatments were different, the achieved flap thicknesses were similar (femtosecond laser, 143 ± 16 µm; microkeratome, 138 ± 22 µm),9 eliminating any possible confounding effect. All femtosecond laser treatments in this study were performed with the 15 kHz system because our laser had not been upgraded to 30 kHz or 60 kHz systems when the procedures were performed in 2004 and 2005. The upgrades enable less energy to be delivered to the cornea and do not change the geometric configuration of the flap, and thus we would not expect the anterior corneal aberrations data in this study to be different from those obtained with the upgraded systems.
In summary, in this randomized paired-eye trial, we found no difference in high-order aberrations or visual acuity after LASIK in eyes with the flap created by a femtosecond laser compared to eyes with the flap created by a mechanical microkeratome. The planar configuration of the bladeless flap did not offer any advantage in visual outcome in our study. Corneal high-order aberrations and visual acuity remained stable through 3 years after LASIK.
Figure 1. Topography-derived corneal wavefront errors after LASIK.
Corneal high-order aberrations did not differ between bladeless or microkeratome flap creation at any examination before or after LASIK (n=21 at every examination, paired analysis). Total high-order aberrations increased immediately after LASIK and remained elevated through 36 months in both groups, except for the 4 mm pupil in the bladeless group (minimum detectable difference between 36 months and preoperative in the bladeless group was 0.06 µm [α=0.05/5, β=0.20, n=21, paired analysis]).
Figure 2. Whole eye wavefront errors after LASIK.
There were no differences in high-order aberrations derived from the whole eye between bladeless and microkeratome treatments at any examination over 4-mm and 6-mm pupil diameters. The number of data (paired eyes) varied for each examination: 4-mm pupil, n=10 before LASIK, n=8 at 1 month, n=12 at 3 months, n=17 at 6 months, n=21 at 12 months, and n=20 at 36 months; 6-mm pupil, n=7 before LASIK, n=5 at 1 month and 3 months, n=10 at 6 months, n=12 at 12 months, and n=16 at 36 months.
Acknowledgments
-
a.
Funding/Support: National Institutes of Health EY 02037 (WMB), Bethesda, MD; Research to Prevent Blindness, Inc., New York, NY (SVP as Olga Keith Wiess Special Scholar, and an unrestricted departmental grant), and Mayo Foundation, Rochester, MN.
-
c.
Contributions of authors in each of these areas: design of the study (JWM, WMB, SVP); conduct of the study (JWM, WMB, SVP); Collection (RC, JWM, SVP), management (RC, JWM, SVP), analysis (RC, JWM, DOH, SVP), and interpretation (RC, JWM, DOH, WMB, SVP) of the data; and preparation (RC, SVP), review (RC, JWM, DOH, WMB, SVP), and approval (RC, JWM, DOH, WMB, SVP) of the manuscript.
-
d.
Conformity with author information: This study was approved by the Mayo Clinic Institutional Review Board and complied with HIPAA regulations; informed consent was obtained from all subjects after discussion of the risks and consequences of the study. The trial was registered at www.clinicaltrials.gov with identifier #NCT00350246.
-
e.
Other acknowledgments: Dr. Ramon Calvo is currently affiliated with the University General Hospital, Valencia, Spain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
b. Financial disclosures: None (all authors).
References
- 1.Sugar A, Rapuano CJ, Culbertson WW, et al. Laser in situ keratomileusis for myopia and astigmatism: safety and efficacy: A report by the American Academy of Ophthalmology. Ophthalmology. 2002;109:175–187. doi: 10.1016/s0161-6420(01)00966-6. [DOI] [PubMed] [Google Scholar]
- 2.Stulting RD, Carr JD, Thompson KP, Waring GO, Wiley WM, Walker JG. Complications of laser in situ keratomileusis for the correction of myopia. Ophthalmology. 1999;106:13–20. doi: 10.1016/S0161-6420(99)90000-3. [DOI] [PubMed] [Google Scholar]
- 3.Sugar A. Ultrafast (femtosecond) laser refractive surgery. Curr Opin Ophthalmol. 2002;13:246–249. doi: 10.1097/00055735-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Juhasz T, Kastis GA, Suarez C, Bor Z, Bron WE. Time-resolved observations of shock waves and cavitation bubbles generated by femtosecond laser pulses in corneal tissue and water. Lasers Surg Med. 1996;19:23–31. doi: 10.1002/(SICI)1096-9101(1996)19:1<23::AID-LSM4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 5.Lubatschowski H, Maatz G, Heisterkamp A, et al. Application of ultrashort laser pulses for intrastromal refractive surgery. Graefes Arch Clin Exp Ophthalmol. 2000;238:33–39. doi: 10.1007/s004170050006. [DOI] [PubMed] [Google Scholar]
- 6.von Jagow B, Kohnen T. Corneal architecture of femtosecond laser and microkeratome flaps imaged by anterior segment optical coherence tomography. J Cataract Refract Surg. 2009;35:35–41. doi: 10.1016/j.jcrs.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Medeiros FW, Stapleton WM, Hammel J, Krueger RR, Netto MV, Wilson SE. Wavefront analysis comparison of LASIK outcomes with the femtosecond laser and mechanical microkeratomes. J Refract Surg. 2007;23:880–887. doi: 10.3928/1081-597X-20071101-03. [DOI] [PubMed] [Google Scholar]
- 8.Tran DB, Sarayba MA, Bor Z, et al. Randomized prospective clinical study comparing induced aberrations with IntraLase and Hansatome flap creation in fellow eyes: Potential impact on wavefront-guided laser in situ keratomileusis. J Cataract Refr Surg. 2005;31:97–105. doi: 10.1016/j.jcrs.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 9.Patel SV, Maguire LJ, McLaren JW, Hodge DO, Bourne WM. Femtosecond laser versus mechanical microkeratome: A randomized controlled study. Ophthalmology. 2007;114:1482–1490. doi: 10.1016/j.ophtha.2006.10.057. [DOI] [PubMed] [Google Scholar]
- 10.Chan A, Ou J, Manche EE. Comparison of the Femtosecond Laser and Mechanical Keratome for Laser In Situ Keratomileusis. Arch Ophthalmol. 2008;126:1484–1490. doi: 10.1001/archopht.126.11.1484. [DOI] [PubMed] [Google Scholar]
- 11.Montés-Micó R, Rodríguez-Galietero A, Alió JL. Femtosecond Laser versus Mechanical Keratome LASIK for Myopia. Ophthalmology. 2007;114:62–68. doi: 10.1016/j.ophtha.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 12.Lim T, Yang S, Kim M, Tchah H. Comparison of the IntraLase femtosecond laser and mechanical microkeratome for laser in situ keratomileusis. Am J Ophthalmol. 2006;141:833–839. doi: 10.1016/j.ajo.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 13.Kezirian GM, Stonecipher KG. Comparison of the IntraLase femtosecond laser and mechanical keratomes for laser in situ keratomileusis. J Cataract Refr Surg. 2004;30:804–811. doi: 10.1016/j.jcrs.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Durrie DS, Kezirian GM. Femtosecond laser versus mechanical keratome flaps in wavefront-guided laser in situ keratomileusis: prospective contralateral eye study. J Cataract Refr Surg. 2005;31:120–126. doi: 10.1016/j.jcrs.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 15.Erie JC, Patel SV, McLaren JW, Hodge DO, Bourne WM. Corneal keratocyte deficits after photorefractive keratectomy and laser in situ keratomileusis. Am J Ophthalmol. 2006;141:799–809. doi: 10.1016/j.ajo.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135:194–205. doi: 10.1016/s0002-9394(02)01825-1. [DOI] [PubMed] [Google Scholar]
- 17.Binder PS. Flap dimensions created with the IntraLase FS laser. J Cataract Refr Surg. 2004;30:26–32. doi: 10.1016/S0886-3350(03)00578-9. [DOI] [PubMed] [Google Scholar]
- 18.Buzzonetti L, Petrocelli G, Valente P, et al. Comparison of corneal aberration changes after laser in situ keratomileusis performed with mechanical microkeratome and IntraLase femtosecond laser: 1-year follow-up. Cornea. 2008;27:174–179. doi: 10.1097/ICO.0b013e31815a50bf. [DOI] [PubMed] [Google Scholar]
- 19.Read SA, Collins MJ, Iskander DR, Davis BA. Corneal topography with Scheimpflug imaging and videokeratography: Comparative study of normal eyes. J Cataract Refr Surg. 2009;35:1072–1081. doi: 10.1016/j.jcrs.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 20.Alió JL, Muftuoglu O, Ortiz D, et al. Ten-Year Follow-up of Laser In Situ Keratomileusis for Myopia of up to −10 Diopters. Am J Ophthalmol. 2008;145:46–54. doi: 10.1016/j.ajo.2007.09.010. e1. [DOI] [PubMed] [Google Scholar]
- 21.Patel SV, Erie JC, McLaren JW, Bourne WM. Confocal microscopy changes in epithelial and stromal thickness up to 7 years after LASIK and photorefractive keratectomy for myopia. J Refract Surg. 2007;23:385–392. doi: 10.3928/1081-597X-20070401-11. [DOI] [PubMed] [Google Scholar]
- 22.Ivarsen A, Fledelius W, Hjortdal JO. Three-Year Changes in Epithelial and Stromal Thickness after PRK or LASIK for High Myopia. Invest Ophthalmol Vis Sci. 2009;50:2061–2066. doi: 10.1167/iovs.08-2853. [DOI] [PubMed] [Google Scholar]
- 23.Erie JC, McLaren JW, Hodge DO, Bourne WM. Recovery of corneal subbasal nerve density after PRK and LASIK. Am J Ophthalmol. 2005;140:1059–1064. doi: 10.1016/j.ajo.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 24.Birk DE, Lande MA, Fernandez-Madrid FR. Collagen and glycosaminoglycan synthesis in aging human keratocyte cultures. Exp Eye Res. 1981;32:331–339. doi: 10.1016/0014-4835(81)90038-5. [DOI] [PubMed] [Google Scholar]
- 25.Lande MA, Birk DE, Nagpal ML, Rader RL. Phagocytic properties of human keratocyte cultures. Invest Ophthalmol Vis Sci. 1981;20:481–489. [PubMed] [Google Scholar]
- 26.Netto MV, Mohan RR, Ambrosio R, Jr, Hutcheon AE, Zieske JD, Wilson SE. Wound healing in the cornea: a review of refractive surgery complications and new prospects for therapy. Cornea. 2005;24:509–522. doi: 10.1097/01.ico.0000151544.23360.17. [DOI] [PubMed] [Google Scholar]
- 27.Muller LJ, Pels E, Vrensen GF. The specific architecture of the anterior stroma accounts for maintenance of corneal curvature. Br J Ophthalmol. 2001;85:437–443. doi: 10.1136/bjo.85.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moreno-Barriuso E, Lloves JM, Marcos S, Navarro R, Llorente L, Barbero S. Ocular Aberrations before and after Myopic Corneal Refractive Surgery: LASIK-Induced Changes Measured with Laser Ray Tracing. Invest Ophthalmol Vis Sci. 2001;42:1396–1403. [PubMed] [Google Scholar]
- 29.Oshika T, Klyce SD, Applegate RA, Howland HC, El Danasoury MA. Comparison of corneal wavefront aberrations after photorefractive keratectomy and laser in situ keratomileusis. Am J Ophthalmol. 1999;127:1–7. doi: 10.1016/s0002-9394(98)00288-8. [DOI] [PubMed] [Google Scholar]
- 30.Porter J, MacRae S, Yoon G, Roberts C, Cox IG, Williams DR. Separate effects of the microkeratome incision and laser ablation on the eye's wave aberration. Am J Ophthalmol. 2003;136:327–337. doi: 10.1016/s0002-9394(03)00222-8. [DOI] [PubMed] [Google Scholar]
- 31.Pallikaris IG, Kymionis GD, Panagopoulou SI, Siganos CS, Theodorakis MA, Pallikaris AI. Induced optical aberrations following formation of a laser in situ keratomileusis flap. J Cataract Refr Surg. 2002;28:1737–1741. doi: 10.1016/s0886-3350(02)01507-9. [DOI] [PubMed] [Google Scholar]