Abstract
Dry eye (DE) is a multifactorial condition that affects the surface of the eye and induces an inflammatory response. Corneal nerves play an important role in the maintenance of a healthy ocular surface. Here we review corneal structure, nerve architecture, DE conditions, and nerve regeneration following corneal surgery and discuss how n-3 fatty acids affect the health of the cornea. Animal studies show that resolvins, compounds derived from eicosapentaenoic acid (EPA), increase tear volume and decrease inflammation induced by DE. After corneal surgery in rabbits, treatment with nerve growth factor (NGF) or pigment epithelial derived factor (PEDF) in conjunction with docosahexaenoic acid (DHA) increase nerve density and corneal epithelial cell proliferation. Increased synthesis of the novel docosanoid, neuroprotectin D1 (NPD1), was found in corneas after the animals were treated with PEDF and DHA. Topical application of these lipids derived from n-3 fatty acids could be useful in treating DE and prevent clinical complications such as cornea erosion and ulcerations.
1. Structure of the cornea
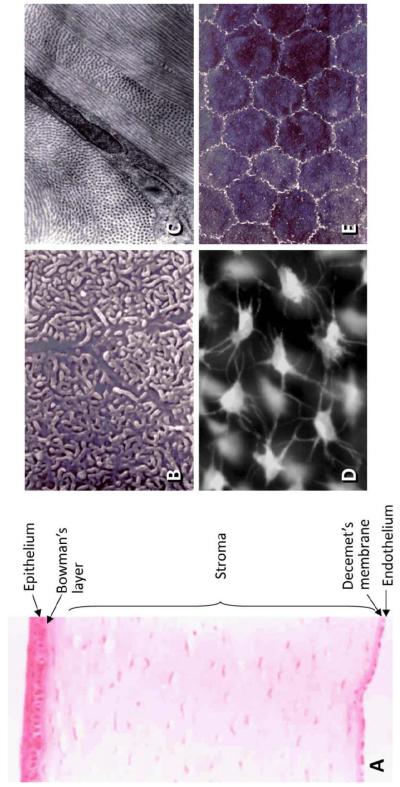
The cornea, an avascular and transparent tissue localized on the surface of the eye, serves as a barrier to protect the eye’s inner contents and as a lens to transmit and refract light. Histologically, the cornea can be divided into 5 layers: the epithelium, Bowman’s layer, the stroma, Descemet’s layer, and the endothelium (Fig.1A).
Fig. 1.
A) Cornea structure. B) Projections of the superficial flattened epithelial cells taken with scanning electron microscope. C) Transmission electron micrograph showing a flat keratocyte embedded in the collagen lamellae of the stroma. D) Immunofluorescence image of keratocytes stained with a live fluorescent dye (calcein acetoxymethyl) shows the dendritic processes that touche the neighboring cells, forming a mesh between the lamellae. E) Scanning electron microscope image of the endothelium shows that the cells have a hexagonal, regular shape and that they make contact through tight junctions.
The epithelium consists of three to four layers of flattened superficial cells (Fig.1B), one to three layers of wing cells, and a single layer of columnar basal cells. Among the epithelial cells, only the basal cells have mitotic activity, and therefore they also serve as a source for both wing and superficial cells.
The Bowman’s layer is an acellular membrane-like zone present at the interface between the epithelium and the stroma. A postulated function of this layer is to prevent close contact between epithelial and stromal cells.
The corneal stroma takes up 90% of the cornea’s volume. It is composed of a highly organized and uniquely transparent extracellular matrix (ECM) of collagen fibrils and proteoglycans that provide both the refractive shape and the tensile strength of the corneal tissue. Keratocytes are the principal cells of the stroma, and are responsible for the synthesis and maintenance of the ECM components. In normal adult corneas, keratocytes appear as a population of flat and dendritic cells, which reside between the collagen lamellae and connect to each other through a network of extensive processes (Fig. 1C, D). These are mitotically quiescent cells that have a very low metabolic and turnover rate. The homeostatic characteristics of stroma contribute to corneal transparency. After injury, cells at the wound periphery become metabolically activated, migrate into the damaged area, and transform into active fibroblasts and myofibroblasts. These cells proliferate and produce a disorganized and fibrotic ECM to repair the damaged area [1-5]. Myofibroblasts are distinct due to their expression of α-smooth muscle actin (α-SMA).
The Decemet’s membrane is the basement membrane of the endothelium and consists of a number of proteins, including laminin, fibronectin, collagen type IV and VIII, and proteoglycans that contain heparan sulfate, dermatan sulfate and keratan sulfate.
The corneal endothelium is a single layer of cells covering the posterior of Decemet’s membrane in a well-organized mosaic pattern (Fig.1E). This cell layer contains ion transport pumps and plays an essential role in maintaining corneal transparency. In the normal human cornea, the endothelial cells have a uniform hexagonal shape. Human corneal endothelial cells do not proliferate in vivo but do proliferate in cell culture systems, suggesting that the cells themselves have a mitotic capability [6]. Studies reveal that the corneal endothelium in vivo is arrested in the G1 phase of the cell cycle [7]. In mild cases of endothelial damage, the cells lose their shape and increase their area to maintain contact and cover the entire posterior portion of the cornea. In severe cases of endothelial damage (e.g. corneal alkali burn), the endothelium is replaced by the multilayered-retrocorneal membrane made up of α-SMA-positive cells [5].
2. Dry eye and n-3 fatty acids
The ocular surface is covered by a very thin layer of tear fluid, called tear film, that is comprised of three layers: lipids, water, and mucous. Lipid analysis of the human tear film has been difficult and still is not precise. This is due to several factors, such as the complex lipid composition of this film, the small amount of available material, and the limitations of the analytical procedures [8]. The upper lipid layer (0.11 μm) consists of a non-polar phase containing lipids, such as cholesteryl esters, triglycerides, hydrocarbons, and wax esters that represent about 60% of the total lipids. The polar phase contains small amounts of phosphatidylcholine and phosphatidylethanolamine with unsaturated fatty acids that give fluidity to the lipid layer. The main unsaturated fatty acids are oleic, linoleic and α-linolenic acids [8]. This lipid layer reduces the rate of tear evaporation and assists in uniform tear spreading. The middle aqueous layer (7 μm), secreted by the lacrimal gland, is comprised of water and takes up more than 98% of total tear volume. The lower mucous layer (0.02-0.05 μm), which is derived from the conjunctiva goblet cells and the ocular surface epithelium, is formed mainly by mucins and serves as an anchor for the tear film, helping it adhere to the superficial epithelium [9].
In addition, the tear film contains many biologically important factors, including electrolytes, glucose, immunoglobulins, lactoferrin, lysozyme and albumin, and many other bioactive substances, such as histamine, prostaglandins, growth factors, and interleukins [10-12]. Hence, the tear film acts not only as a lubricant and nutritional source, but also as the source of the regulatory factors for the maintenance and repair of the corneal epithelium.
Dry eye (DE) is a multifactorial condition that results in symptoms of discomfort, visual disturbance, and instability of the tear film that induce inflammation and damage of the ocular surface [13]. Epidemiological studies have revealed the prevalence of DE ranging from 5% to 34% for different ages [14], with a higher incidence occurring in older patients. In the United States, an estimated 3.23 million women and 1.68 millionmen, a total of 4.91 million people, aged 50 years and older are affected [14].
DE, which usually affects both eyes, may be characterized by several signs and symptoms: a stinging, burning or scratchy sensation; stringy mucus in or around the eyes; increased eye irritation from smoke or wind; eye fatigue after short periods of reading; sensitivity to light; difficulty wearing contact lenses; tearing; blurred vision, often worsening at the end of the day or after visually focusing for a prolonged period on a nearby task.
Some clinical studies in women found that the amount, type, and ratio of essential fatty acids in the diet may play a key role in the prevention of DE [15, 16]. Among other findings, it has been shown that women with the highest levels of n-3 fatty acids in their diets reduced their risk of DE by 20%, compared with women with the lowest levels of n-3 fatty acids in their diet. A dietary ratio of n-6 to n-3 fatty acids greater than 15:1 was associated with a 2.5-fold increased risk of DE [15]. Recent studies in animal models of DE have shown beneficial effects of supplementation with a mix of eicosapentaenoic acid (20:5n-3; EPA), docosahexaenoic acid (22:6n-3; DHA) and γ-linolenic acid (18:3n-6; GLA) for 2 months [17]. In a mouse model, topical application of the n-3 fatty acid α-linoleic acid (18:3n-3; ALA) produces a significant decrease in epithelial damage, expression of inflammatory cytokines and macrophage infiltration [16].The beneficial effects could be due to the action of ALA, to the enlongation and desaturation products, EPA and DHA, or to new derivatives of these fatty acids, such as resolvins and neuroprotectins [18-21].
Resolvins were discovered in inflammatory exudates during the resolution phase of inflammation in animals treated with EPA and aspirin [18]. The main metabolite is resolvin E1 (RvE1; 5(S), 12(R), 18(R)-trihydroxyeicosapentaenoic acid), which inhibits PMN infiltration and expression of cytokines [19].
Since the pathology of DE has an inflammatory component [22, 23], we tested the action of two derivatives of RvE1 administrated topically as methyl esters in a mouse model. Both compounds increased tear flow, promoted a healthy epithelium, and decreased cyclooxygenase-2 (COX-2) and α-smooth muscle actin (α-SMA) expression as well as macrophages infiltration, demonstrating that these derivatives of the n-3 fatty acid EPA could have a therapeutic role in DE treatment [24].
3. Regulation of tear fluid by corneal nerves
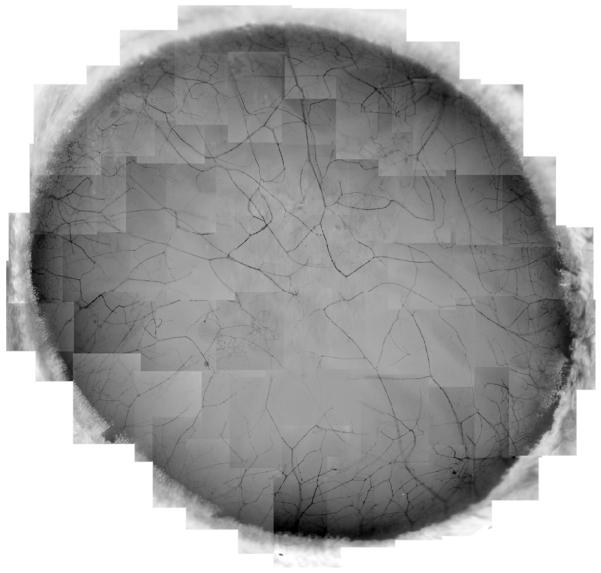
One important characteristic of the cornea is that it is densely packed with sensory nerves derived from the ophthalmic division of the trigeminal ganglion, autonomic parasympathetic fibers from the ciliary ganglion, and a small number of sympathetic nerves from the superior cervical ganglion [25, 26]. Recently, we have developed methods of immunofluorescence staining and imaging, which, for the first time, allowed for the construction of the whole corneal nerve architecture of both the epithelium and stroma of the human cornea [27]. We found that stromal nerves enter the tissue in a radiating pattern and run towards the anterior stroma, subsequently dividing into smaller branches. The tips of the stromal nerve branches, which are predominantly present within the peripheral area, penetrate the basement membrane into the epithelium and give place to long bundles. In addition, some branches link with each other in the center (Fig.2).
Fig. 2.
Wholemount view of human corneal stromal nerve distribution. The images, taken from the right eye of a 45-year-old male donor, show that the nerves are equally distributed around the limbus. These nerves divide into several branches at the peripheral cornea. Some of the branches connect with each other to form the stromal nerve network, but most of them penetrate upwards into the epithelia to give rise to the long bundles to innervate the central epithelium.
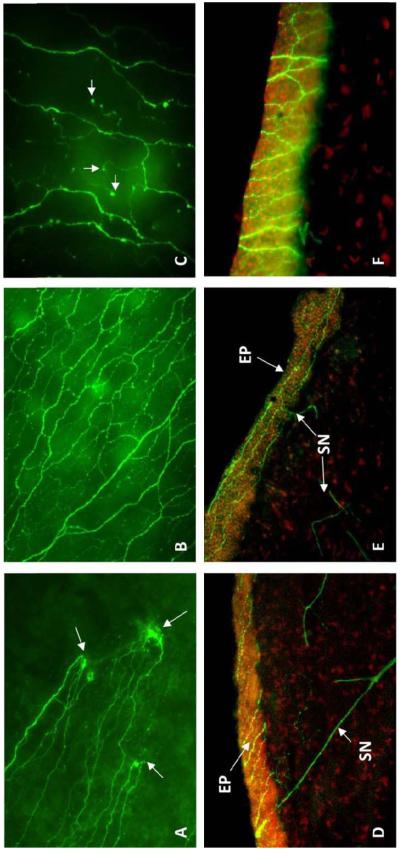
The long bundles in the intra- or sub-epithelium run like waving lines from the periphery to the center (Fig.3A, D, E) and merge into an area within the central cornea. Along its course, the long bundles divide into numerous smaller branches, which connect to each other and constitute a delicate nerve network within the epithelium (Fig.3B, F). Fine terminals or free endings budding from the network innervate the epithelial cells (Fig.3C).
Fig. 3.
Immunofluorescence images show the architecture of epithelial nerves in human cornea. A) Wholemount image shows the penetrating sites (arrows) of stromal nerve branches at the peripheral cornea where several epithelial nerve bundles give rise. B) Wholemount image shows the epithelial nerve network in the central cornea. C) Wholemount image at high magnification shows the terminals (arrows). D) Transected image shows the stromal nerve (SN) branches penetrate into epithelium (EP). E) Transected image shows the long bundles running throughout the epithelium. F) Transected image at high magnification shows the nerve network located intraepithelially.
In our study, we found that the density of epithelial nerves together with the number of terminals is significantly higher in the center than in the periphery. There is no difference in the epithelial nerve density between genders, but there is a progressive reduction in epithelial nerve densities with increased aging; a significant decline was found in humans 70 years of age and older.
All of these nerves play an important role in maintaining a healthy corneal surface, and interruption of corneal innervation may cause altered epithelial morphology and function, poor tear film production, and delayed wound healing [26]. Damage to the epithelium could produce epithelial defects, such as reduced transparency, or even more serious consequences, including corneal melting and complete opacity.
Secretion of the lacrimal glands is controlled by a neural reflex arc of the sensory afferent nerves (trigeminal sensory fibers) of the cornea and conjunctiva. Under normal conditions, stimulation of the nerve-free endings in the corneal epithelium generates afferent nerve impulses that activate the efferent nerves in the lacrimal gland and stimulate tear secretion to the surface of the eye. As such, changes in the ocular surface are detected by the nerve endings, which thus initiate a rapid response from the lacrimal gland.
4. Dry eye after refractive surgery
As mentioned above, damage to the corneal sensory nerves impair lacrimal gland function and can cause DE. Corneal refractive surgeries such as photorefractive keratectomy (PRK) and laser assisted in situ keratomileusis (LASIK), two of the most common refractive surgeries currently performed to correct vision, result in damage to the corneal nerves. In PRK, the removal of the epithelium injures the epithelial nerves, and the application of the laser damages the anterior stromal nerves in the ablation zone. During LASIK procedures, epithelial nerve bundles and superficial stromal nerve branches in the flap interface are cut by a microkeratome. Additional damage to the stromal nerves is caused by excimer laser photo ablation. Therefore, one of the most common complications after refractive surgery is DE [28, 29].
It had been reported that 60% of LASIK patients experience DE 1 month after the surgery [30], and 50% of these patients still have symptoms of ocular dryness and irritation after 6 months [31]. Although there are a number of possible causes for LASIK-associated DE, the damage to corneal nerves during the creation of the flap is thought to be the main cause. The impairment of corneal nerves interrupts the cornea reflex arc, affecting tear production [32].
5. DHA potentiates the effect of NGF in corneal nerve regeneration after PRK
In vivo confocal microscopy studies allow for observation of nerve regeneration after PRK in the living cornea. Regenerating subbasal nerves have been detected one week after PRK [33]. Partial, and in some cases, total recovery of subbasal nerves has been reported to occur between 8-12 months after PRK [34, 35]. However, even 5 years after PRK is performed, some corneas do not achieve a normal pattern of subbasal nerve morphology [36]. A prospective longitudinal study showed an 80% recovery of nerve density 2 years after myopic PRK, and these levels remained stable during the 5-year follow-up [37]. Therefore, a proper regrowth of corneal nerves into the ablated area after PRK is essential for the recovery of a normal corneal surface.
Several growth factors have been shown to promote neurite extension and survival [38-40], among which nerve growth factor (NGF), a neurotrophic and immunomodulatory mediator, is responsible for the growth, differentiation, and survival of sensory neurons and acceleration of wound healing [41-44]. Corneal keratocytes, as well as epithelial and endothelial cells, synthesize NGF, and epithelial cells also express NGF receptors. Injury to the cornea induces an upregulation of NGF [45], and topical NGF promotes healing of corneal neurotrophic ulcers. Thus, it has been postulated that NGF plays a role in the modulation of epithelial–stromal communication, which is essential in the induction of stromal healing [46, 47]. In addition, corneal sensitivity after LASIK has been enhanced by the administration of topical NGF [48].
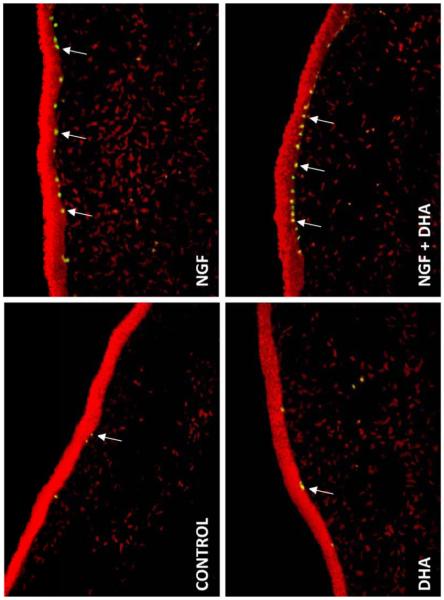
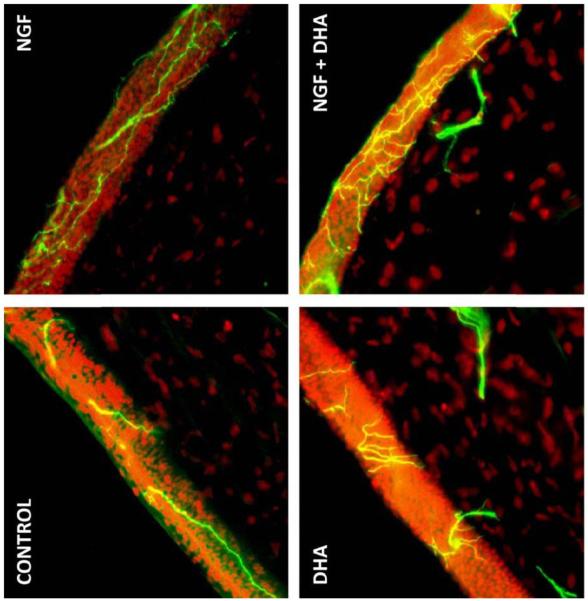
We investigated the effect of NGF in conjunction with the n-3 fatty acid DHA on corneal nerve regeneration after PRK [49] in a rabbit model. βIII tubulin was used to stain the nerves. Eight weeks after the surgery, βIII tubulin-positive epithelial nerve area was significantly higher in the DHA plus NGF group than in animals untreated or treated with NGF or DHA alone (Fig.4). More importantly, there was a higher percentage of epithelial cell proliferation as identified by Ki-67 immunodetection in the basal epithelium of corneas treated with the combination of DHA and NGF compared to NGF-treated group; DHA did not have an effect on cell proliferation (Fig.5). This demonstrates that treatment with NGF and DHA promotes a healthy epithelium after PRK. We proposed that the mechanisms could be through the action of the DHA-derived lipid mediator, neuroprotectin D1 (NPD1). This docosanoid has potent anti-inflammatory and neuroprotective actions [21, 50-53]. The enhancement of corneal nerve regeneration may yield a faster anatomic and functional recovery after PRK or LASIK, thus preventing DE and neurotrophic keratopathies.
Fig. 4.
Images of rabbit corneal sections taken 8 weeks after PRK surgery and treated with NGF, DHA or NGF plus DHA. Controls were treated with PBS. The sections were immunostained with a monoclonal antibody for βIII-tubilin. DAPI (4′-6′-diamino-2phenylindole) was used to stain the cell nuclei. Photographs were taken with a fluorescent microscope
Fig 5.
Ki-67 staining of the basal corneal epithelial cells (arrows) of rabbits 8 weeks after surgery and treated with NGF, DHA or NGF plus DHA. Controls were treated with PBS. DAPI was used to stain the nuclei.
6. PEDF plus DHA induces corneal nerve regeneration and NPD1 synthesis
NPD1 synthesis requires the activation of 15-lipoxygenase-1, which converts DHA to NPD1 and is stimulated by several growth factors. It has been found that pigment epithelial derived factor (PEDF) is ten times more potent than NGF in stimulating NPD1 synthesis in retinal pigment epithelial cells [52]. PEDF is a potent and broad-acting neurotrophic and neuroprotective factor that regulates processes associated with angiogenesis, neuronal cell survival, and cell differentiation [54, 55]. In chick spinal cord cultures, PEDF exerts a strong neurotrophic effect, increasing cell survival and length of neuritis [56]. In the cornea it is expressed most strongly by epithelial cells [55].
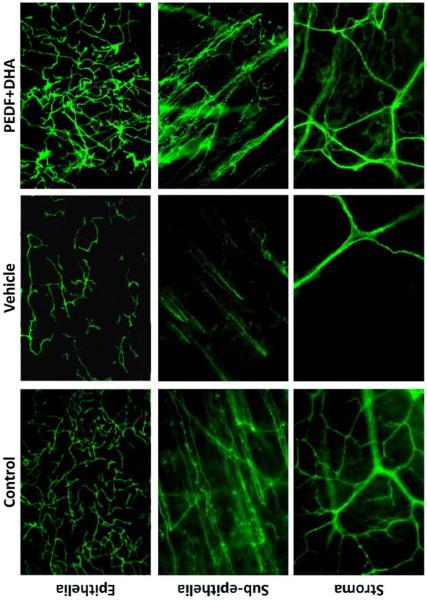
To determine if NPD1 is involved in corneal nerve regeneration, we used a rabbit model of lamellar keratectomy. In this model, we severed the stromal nerves to investigate the effect of DHA plus PEDF on the regeneration of the nerves, and then assessed the synthesis of NPD1. There was a 2.5-fold increase in corneal nerve area in PEDF plus DHA-treated animals, compared with vehicle-treated animals at 2 and 4 weeks post-surgery [57]. Eight weeks after surgery, fewer nerves were found in the epithelial, subepithelial and stromal areas compared to normal corneas without surgery. The nerve density in the treated animals was similar to corneas without surgery (Fig. 6). When rabbits were treated with PEDF or DHA alone, there was no significant nerve regeneration compared to vehicle-treated animals.
Fig 6.
βIII-tubulin immunoestainig of rabbit nerves in corneal wholemounts. The images of epithelial, subpithelial and stromal nerves were taken at 8 weeks after corneal lamellar keratectomy that damaged the stromal nerves. The animals were treated for 6 weeks with PEDF plus DHA or vehicle. Controls were normal corneas.
To determine if the treatment increased NPD1 production, corneal tissue of animals treated with PEDF plus DHA were analyzed by mass spectrometry [57]. No NPD1 synthesis occurred in non-treated corneas. One week after treatment, there was a peak corresponding to NPD1 in the treated corneas. Corneas contain very small amounts of DHA in their phospholipids [58]. One of the interesting findings of this study was that supplementation with DHA may be necessary to allow PEDF to stimulate the synthesis of NPD1. Therefore, this signaling mechanism up-regulates corneal nerve regeneration and may be targeted in neurotrophic keratitis, DE after refractive surgery, and other corneal diseases.
7. Summary and Conclusion
DE is one of the most common pathologies that affects the ocular surface. This condition worsens with age and after refractive surgery and has both inflammatory and neuromodulatory components. In this review, we discuss some of the most recent research from our laboratory concerning the action of n-3 fatty acids and their derivatives, namely resolvins and neuroprotectins. Resolvins decrease inflammation and increase tear production, and the combination of NGF and PEDF with DHA improves nerve regeneration after injury. Although DHA is a minute component of lipids in the cornea [58], this tissue can synthesize NPD1 when treated with a combination of DHA and PEDF. Therefore, this docosanoid could have therapeutic value useful for preventing serious consequences of nerve damage, such as DE, epithelial erosions, and corneal ulcerations.
Acknowledgments
The authors’ work described here was supported by National Institutes of Health, National Eye Institute grant R01 EY19465, by a Translational Research Initiative grant from the Lousiana State University Health Sciences Center, New Orleans, and by a grant from Resolvyx Pharmaceuticals, Bedford, Massachusetts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ottino P, He J, Axelrad TW, Bazan HE. PAF-induced furin and MT1-MMP expression is independent of MMP-2 activation in corneal myofibroblasts. Invest Ophthalmol Vis Sci. 2005;46:487–496. doi: 10.1167/iovs.04-0852. [DOI] [PubMed] [Google Scholar]
- 2.He J, Bazan HE. Synergistic Effect of Platelet-activating Factor and Tumor Necrosis Factor-α on Corneal Myofibroblast Apoptosis. Invest Ophthalmol Vis Sci. 2006;47:883–891. doi: 10.1167/iovs.05-0581. [DOI] [PubMed] [Google Scholar]
- 3.He J, Bazan HE. Epidermal growth factor (EGF) Synergism with TGF-β1 via PI-3 kinase activity in corneal keratocyte differentiation. Invest Ophthalmol Vis Sci. 2008;49:2936–2945. doi: 10.1167/iovs.07-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esquenazi S, He J, Bazan NG, Bazan HE. Comparison of corneal wound healing response in PRK and LASEK. J Cataract Refract Surg. 2005;31:1632–1639. doi: 10.1016/j.jcrs.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 5.He J, Bazan NG, Bazan HE. Alkali-induced corneal stromal melting prevention by a novel platalet activating factor (PAF) receptor antagonist. Arch Ophthalmol. 2006;124:70–78. doi: 10.1001/archopht.124.1.70. [DOI] [PubMed] [Google Scholar]
- 6.Kakazu AH, He J, Bazan NG, Bazan HE. Aspirin-triggered lipoxin-A4 (epi-LxA4) is an important mediator for maintaining the integrity of human Corneal Endothelial cells. ARVO, E-abstract. 2009:1818–A486. [Google Scholar]
- 7.Joyce NC, Harris DL, Zieske JD. Mitotic inhibition of corneal endothelium in neonatal rats. Invest Ophthalmol Vis Sci. 1998;39:2572–2583. [PubMed] [Google Scholar]
- 8.Butovich IA. Cholesteryl esters as a depot for very long chain fatty acids in human meibum. J Lipid Res. 2009;50:501–513. doi: 10.1194/jlr.M800426-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holly FJ, Lemp MA. Tear physiology and dry eye. Surv Ophthalmol. 1977;22:69–87. doi: 10.1016/0039-6257(77)90087-x. [DOI] [PubMed] [Google Scholar]
- 10.van Setten G, Schultz G. Transforming growth factor-alpha is a constant component of human tear fluid. Graefes Arch Clin Exp Ophthalmol. 1994;232:523–526. doi: 10.1007/BF00181994. [DOI] [PubMed] [Google Scholar]
- 11.Afonso A, Sobrin L, Monroy DC, Selzer M, Lokeshwar B, Pflugfelder SC. Tear fluid gelatinase B activity correlates with IL-1alpha concentration and fluorescein clearance in ocular rosacea. Invest Ophthalmol Vis Sci. 1999;40:2506–2512. [PubMed] [Google Scholar]
- 12.Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42:2283–2292. [PubMed] [Google Scholar]
- 13.Lemp AM. The definition and classification of dry eye disease: report of the Definition and Classification Subcommitte of the International Dry Eye Workshop. Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. and the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007) 2007. [DOI] [PubMed] [Google Scholar]
- 14.Smith JA. The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007;5:93–107. doi: 10.1016/s1542-0124(12)70082-4. and the Epidemiology Subcommittee of the International Dry Eye Workshop (2007) 2007. [DOI] [PubMed] [Google Scholar]
- 15.Miljanovic B, Trivedi KA, Dana MR, Gilbard JP, Buring JE, Schaumberg DA. Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. Am J Clin Nutr. 2005;82:887–893. doi: 10.1093/ajcn/82.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rashid S, Jin Y, Ecoiffier T, Barabino S, Schaumberg DA, Dana MR. Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Arch Ophthalmol. 2008;126:219–225. doi: 10.1001/archophthalmol.2007.61. [DOI] [PubMed] [Google Scholar]
- 17.Viau S, Maire MA, Pasquis B, et al. Efficacy of a 2-month dietary supplementation with polyunsaturated fatty acids in dry eye induced by scopolamine in a rat model. Graefes Arch Clin Exp Ophthalmol. 2009;247:1039–1050. doi: 10.1007/s00417-009-1080-z. [DOI] [PubMed] [Google Scholar]
- 18.Serhan CN, Hong S, Gronert K, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arita M, Yoshida M, Hong S, et al. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci U S A. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butovich IA. On the structure and synthesis of neuroprotectin D1, a novel anti-inflammatory compound of the docosahexaenoic acid family. J Lipid Res. 2005;46:2311–2314. doi: 10.1194/jlr.C500015-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci USA. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pflugfelder SC, Jones D, Ji Z, Afonso A, Monroy D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjögren’s syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999;19:201–211. doi: 10.1076/ceyr.19.3.201.5309. [DOI] [PubMed] [Google Scholar]
- 23.Lobefalo L, D’Antonio E, Colangelo L, et al. Dry eye in allergic conjunctivitis: role of inflammatory infiltrate. Int J Immunopathol Pharmacol. 1999;12:133–137. [PubMed] [Google Scholar]
- 24.Li N, He J, Bazan HEP. The resolvin E1 analogs, RX-10065 and RX-10005, improve tear production and decrease inflammation in a mouse dry eye model. ARVO, E- abstract. 2008 [Google Scholar]
- 25.Zander E, Weddell G. Observations on the innervation of the cornea. J Anat. 1951;85:68–99. [PMC free article] [PubMed] [Google Scholar]
- 26.Müller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, content and function. Exp Eye Res. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 27.He J, Bazan NG, Bazan HEP. Mapping the Whole Human Corneal Nerve Architecture. ARVO, E-abstract. 2009:2603–D1023. [Google Scholar]
- 28.Linna TU, Vesaluoma MH, Perez-Santonja JJ, et al. Effect of myopic LASIK on corneal sensitivity and morphology of subbasal nerves. Invest Ophthalmol Vis Sci. 2000;41:393–397. [PubMed] [Google Scholar]
- 29.Albietz JM, Lenton LM, McLennan SG. Effect of Laser in situ keratomileusis for hypopia on tear film and ocular surface. J Cataract Refract Surg. 2002;18:113–123. doi: 10.3928/1081-597X-20020301-02. [DOI] [PubMed] [Google Scholar]
- 30.Yu EY, Leung A, Rao S, Lam DS. Effect of laser in situ keratomileusis on tear stability. Ophthalmology. 2000;107:2131–2135. doi: 10.1016/s0161-6420(00)00388-2. [DOI] [PubMed] [Google Scholar]
- 31.Hovanesian JA, Shah SS, Maloney RK. Symptoms of dry eye and recurrent erosion syndrome after refractive surgery. J Cataract Refract Surg. 2001;27:577–584. doi: 10.1016/s0886-3350(00)00835-x. [DOI] [PubMed] [Google Scholar]
- 32.Stern ME, Gao J, Siemasko KF, Beuerman RW, Pflugfelder SC. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004;78:409–416. doi: 10.1016/j.exer.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Linna T, Tervo T. Realtime confocal microscopic observations on human corneal nerves and wound healing after excimer laser photorefractive keratectomy. Curr Eye Res. 1997;16:640–649. doi: 10.1076/ceyr.16.7.640.5058. [DOI] [PubMed] [Google Scholar]
- 34.Corbett MC, Prydal JI, Verma S, Oliver KM, Pande M, Marshall J. An in vivo investigation of the structures responsible for corneal haze after photorefractive keratectomy and their effect on visual function. Ophthalmology. 1996;103:1366–1380. doi: 10.1016/s0161-6420(96)30495-8. [DOI] [PubMed] [Google Scholar]
- 35.Kauffmann T, Bodanowitz S, Hesse L, Kroll P. Corneal reinnervation after photorefractive keratectomy and laser in situ keratomileusis: an in vivo study with a confocal videomicroscope. Ger J Ophthalmol. 1996;5:508–512. [PubMed] [Google Scholar]
- 36.Moilanen JA, Vesaluoma MH, Muller LJ, Tervo TM. Long term corneal morphology after PRK by in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 2003;44:1064–1069. doi: 10.1167/iovs.02-0247. [DOI] [PubMed] [Google Scholar]
- 37.Erie JC, McLaren JW, Hodge DO, Bourne WM. Recovery of corneal subbasal nerve density after PRK and LASIK. Am J Ophthalmol. 2005;140:1059–1064. doi: 10.1016/j.ajo.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 38.Chan KY, Haschke RH. Action of a trophic factor(s) from rabbit corneal epithelial culture on dissociated trigeminal neurons. J Neurosci. 1981;1:1155–1162. doi: 10.1523/JNEUROSCI.01-10-01155.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan KY, Haschke RH. Isolation and culture of corneal cells and their interactions with dissociated trigeminal neurons. Exp Eye Res. 1982;35:137–156. doi: 10.1016/s0014-4835(82)80062-6. [DOI] [PubMed] [Google Scholar]
- 40.Emoto I, Beuerman RW. Stimulation of neurite growth by epithelial implants into corneal stroma. Neurosci Lett. 1987;23:140–144. doi: 10.1016/0304-3940(87)90118-2. [DOI] [PubMed] [Google Scholar]
- 41.Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1997;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 42.Riaz SS, Tomlinson DR. Neurotrophic factors in peripheral neuropathies: pharmacological strategies. Prog Neurobiol. 1996;49:125–143. doi: 10.1016/0301-0082(96)00010-x. [DOI] [PubMed] [Google Scholar]
- 43.Mearow KM, Dril Y, Diamond J. Increased NGF mRNA expression in denervated rat skin. Neuroreport. 1993;4:351–354. doi: 10.1097/00001756-199304000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Verge VM, Merlio JP, Grondin J, et al. Colocalization of NGF binding sites, trk mRNA, and low affinity NGF receptor mRNA in primary sensory neurons: responses to injury and infusion of NGF. J Neurosci. 1992;12:4011–4022. doi: 10.1523/JNEUROSCI.12-10-04011.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lambiase A, Manni L, Bonini S, Rama P, Micera A, Aloe L. Nerve growth factor promotes corneal healing: structural, biochemical, and molecular analyses of rat and human corneas. Invest Ophthalmol Vis Sci. 2000;41:1063–1069. [PubMed] [Google Scholar]
- 46.Lambiase A, Rama P, Bonini S, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. N Engl J Med. 1998;338:1174–1180. doi: 10.1056/NEJM199804233381702. [DOI] [PubMed] [Google Scholar]
- 47.Bonini S, Lambiase A, Rama P, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology. 2000;107:1347–1351. doi: 10.1016/s0161-6420(00)00163-9. [DOI] [PubMed] [Google Scholar]
- 48.Joo MJ, Yuhan KR, Hyon JY, et al. The effect of nerve growth factor on corneal sensitivity after laser in situ keratomileusis. Arch Ophthalmol. 2004;122:1338–1341. doi: 10.1001/archopht.122.9.1338. [DOI] [PubMed] [Google Scholar]
- 49.Esquenazi S, Bazan HE, Bui V, He J, Kim DB, Bazan NG. Topical combination of NGF and DHA increases rabbit corneal nerve regeneration after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2005;46:3121–3127. doi: 10.1167/iovs.05-0241. [DOI] [PubMed] [Google Scholar]
- 50.Bazan NG, Rodriguez de Turco EB. Review: pharmacological manipulation of docosahexaenoic-phospholipid biosynthesis in photoreceptor cells: implications in retinal degeneration. J Ocul Pharmacol. 1994;10:591–604. doi: 10.1089/jop.1994.10.591. [DOI] [PubMed] [Google Scholar]
- 51.Bazan NG. Homeostatic regulation of photoreceptor cell integrity: significance of the potent mediator neuroprotectin D1 buisynthesized from docosahexaenoic acid: the Proctor Lecture. Invest Ophthalmol Vis Sci. 2007;48:4866–4881. doi: 10.1167/iovs.07-0918. [DOI] [PubMed] [Google Scholar]
- 52.Mukherjee PK, Marcheselli VL, Barreiro S, Hu J, Bok D, Bazan NG. Neurotrophins enhance retinal pigment epithelial cell survival through neuroprotectin D1 signaling. Proc Natl Acad Sci USA. 2007;104:13152–13157. doi: 10.1073/pnas.0705949104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marcheselli VL, Hong S, Lukiw WJ, et al. Novel docosanoids inhibit brain ischemia reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 54.Tombran-Tink J, Barnstable CJ. PEDF: A multifaceted neurotrophic factor. Nat Rev Neurosci. 2003;4:628–636. doi: 10.1038/nrn1176. [DOI] [PubMed] [Google Scholar]
- 55.Becerra SP. Focus on molecules: pigment epithelium-derived factor (PEDF) Exp Eye Res. 2006;82:739–749. doi: 10.1016/j.exer.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 56.Houenou LJ, D’Costa AP, Li L, et al. Pigment epithelium-derived factor promotes the survival and differentiation of developing spinal motor neurons. J Comp Neurol. 1999;412:506–514. [PubMed] [Google Scholar]
- 57.Cortina MS, He J, Li N, Bazan NG, Bazan HE. PEDF plus DHA Induces Neuroprotectin D1 Synthesis and Corneal Nerve Regeneration after Experimental Surgery. Invest Ophthalmol Vis Sci. 2009 doi: 10.1167/iovs.09-3641. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bazan HE, Bazan NG. Composition of phospholipids and free fatty acids and incorporation of labeled arachidonic acid in rabbit cornea. Comparison of epithelium, stroma and endothelium. Curr Eye Res. 1984;3:1313–1319. doi: 10.3109/02713688409007418. [DOI] [PubMed] [Google Scholar]