Abstract
Since embryonic stem cell-derived neural progenitors (NPs) have the potential to be used in cell replacement therapy, an understanding of the signaling mechanisms that regulate their terminal differentiation is imperative. In previous studies, we discovered the presence of functional μ opioid receptors (MOR) and κ opioid receptors (KOR) in mouse embryonic stem cells and NPs. Here, MOR and KOR immunoreactivity was detected in NP-derived oligodendrocytes during three stages of their maturation in vitro. Moreover, we examined the modulation of retinoic acid-induced NP differentiation to astrocytes and neurons by μ, [D-ala2, mephe4, gly-ol5] enkephalin, or κ, U69, 593, opioids. Both opioid agonists inhibited NP-derived neurogenesis and astrogenesis via their corresponding receptors as determined by immunocytochemistry. By administering selective inhibitors, we found that opioid inhibition of NP-derived astrogenesis was driven via extracellular-signal regulated kinase (ERK), while the p38 MAP kinase pathway was implicated in opioid attenuation of neurogenesis. In addition, μ and κ opioids stimulated oligodendrogenesis from NP-derived NG2+ oligodendrocyte progenitors via both ERK and p38 signaling pathways. Accordingly, both opioids induced ERK phosphorylation in NG2+ cells. These results indicate that small molecules, such as MOR and KOR agonists may play a modulatory role in NP terminal differentiation.
Keywords: mouse embryonic stem cells, differentiation, neural progenitors, oligodendrocyte progenitors, opioid receptors, MAP kinases, neurogenesis, astrogenesis, oligodendrogenesis
A major challenge that remains to be resolved in stem cell biology is how to direct and control neural differentiation of embryonic stem cells (ESCs)†. A potential mechanism that is poorly understood entails the role of endogenous hormones, growth factors and neurotransmitters in influencing stem cell fate decisions. Therefore, it is important to identify the intrinsic and/or extrinsic factors that regulate the underlying molecular mechanisms involved in ESC self-renewal, proliferation and neural differentiation. Since various NPs express functional neurotransmitter receptors, including G protein coupled receptors (GPCRs), they may regulate NP’s fate.
Our earlier findings provided evidence for the occurrence of MOR-1 and KOR-1 mRNA and protein in blastocyst-derived ESCs (Kim et al., 2006). MOR gene targeting frequencies were studied in 129/Sv and c57BL/6 ESCs (Zhou et al., 2001). MOR, KOR and δ OR were found in P19 embryonic carcinoma cells and RA modulated the expression of these genes (Chen at al., 1999; Park et al., 2005). KOR expression was also found in subcellular fractions of GTR1 ESCs (Ventura et al., 2000).
MAPK signaling plays a major role in the differentiation of stem cells. Some studies suggest a specific requirement for the ERK pathway in the neural lineage commitment of ESCs (Stavridis et al., 2007). ERK2-null ESCs failed to undergo neural and mesodermal differentiation (Kunath et al., 2007). Nevertheless, it was found that RA mediated inhibition of leukemia inhibitory factor (LIF) signaling in ESCs is ERK independent (Tighe and Gudas, 2004). RA-induced ESC differentiation may be achieved by restricting nuclear entry of phospho ERK (Smith et al., 2004). In addition to ERK, p38 is also activated during early ESC differentiation (Lee et al., 2006). Thus, it is likely that distinct ESC lineage commitment programs may be regulated by the integrated action of multiple signaling mechanisms (Rajan and McKay, 1998; Binetruy et al., 2007). We discovered that opioids induced an ERK dependent mouse ESC division leading to the appearance of NPs (Kim et al., 2006).
It has been known for some time that opioids modulate neural stem cell proliferation and neural differentiation. In early studies, acute morphine activated cell growth in the subventricular zone of adult rat brains (Miller et al., 1982). In contrast, chronic morphine suppressed neurogenesis in the adult hippocampal and subgranular zone in vivo (Eisch et al., 2000). Chronic morphine acting via MOR also exerted antiproliferative actions on cerebellar external granular layer neuroblasts or enhanced HIV-1 Tat protein mediated cytotoxicity of glial precursors (Hauser, 1992; Hauser et al., 2000; Hauser et al., 2007; Buch et al., 2007). Recently, MOR was shown to be expressed on radial glia and morphine signaling was found to play a role in cell cycle progression of progenitors in the developing brain (Sargeant et al., 2007). In adult hippocampal progenitors, the endogenous opioid peptide, β-endorphin stimulated cell proliferation and induced oligodendrogenesis via ERK in vitro (Persson et al., 2003, 2006). Activation of δ OR plays a crucial role in neurogenesis and neuroprotection of neural stem cells via PI3K/ERK signaling (Narita et al., 2006). Human fetal neural precursor cells express functional KOR that stimulates proliferation and migration of these cells (Sheng et al., 2007). Most of these studies are performed with brain-derived stem cells or NPs, but little if anything is known about opioid regulation of ESC neural differentiation.
Here, we studied the role of μ and κ agonists and their signaling to ERK and/or p38 MAPK on the regulation of RA induced NP terminal differentiation to neurons, astrocytes and oligodendrocytes. We found that DAMGO, a MOR specific agonist and U69,593, a KOR specific agonist inhibited neurogenesis and astrogenesis but stimulated oligodendrogenesis in ESC-derived NPs. These opioid effects were regulated by ERK and/or p38 signaling.
EXPERIMENTAL PROCEDURES
Reagents
Chemicals were purchased from Sigma Chemical Co. (St. Louis, MO) with exceptions: CTAP, DAMGO and norbinaltorphimine (NorBNI) were from NIDA Drug Supply (Research Triangle, NC); Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were from ATCC (Manassas, VA); rabbit polyclonal MOR-1 (H-80, N-terminus), goat polyclonal KOR (C-20, C-terminus) and rabbit polyclonal ERK Abs were from Santa Cruz (Santa Cruz, CA); rabbit polyclonal glial fibrillary acidic protein (GFAP) Ab was from ImmunoStar, Inc. (Hudson, WI); mouse monoclonal TuJ-1 (IgG2a) and O4 (IgM) Abs were from Neuromics (Edina, MN); mouse monoclonal NG2 (clone 132.38) Ab was from Upstate (Charlottesville, VA); mouse monoclonal phospho-ERK1/2 (against phospho Thr202/Tyr204, clone E10) Ab was from Cell Signaling Technology (Beverly, MA); goat anti-mouse IgG Alexa Fluor 594, goat anti-rabbit IgG Alexa Fluor 488 and goat anti-mouse IgM Alexa Fluor 594 were from Invitrogen/Molecular Probes (Carlsbad, CA); HRP-conjugated goat anti-mouse-IgG and HRP-conjugated goat anti-rabbit-IgG were from Sigma (St. Louis, MO) and VECTASHIELD Mounting Medium was from Vector Laboratories, Inc. (Burlingame, CA).
Opioid treatments
MOR selective agonist DAMGO or KOR selective agonist U69,593 were added to cells for various times. In some experiments, cells were pre-incubated with opioid antagonists, MOR selective CTAP or KOR selective nor-BNI, (1 μM, 60 min) and then treated with corresponding opioid agonist (1 μM).
Mouse ESCs and their neural differentiation
D3 ESCs (ATCC) were maintained in DMEM containing 10% FBS, LIF (100 ng/ml) and 0.1 mM 2-mercaptoethanol (ME) in gelatin-coated flasks as described (Kim et al., 2006). These growth conditions are known to prevent ESCs from differentiating (Bain et al., 1995). Here, cells grown under these conditions will be described as “undifferentiated, self-renewing ESCs”.
The neural induction of ESCs entails the “4−/4+ protocol” which has two phases (Bain et al., 1995; Kim et al., 2006). In the first phase, cells are cultured in non-adhesive Petri dishes in 10% FBS containing DMEM (without LIF and ME) in the absence of RA for 4 days. During this phase, embryoid bodies (EBs) appear. In the second phase, cells are grown in RA (1 μM) containing DMEM + 10% FBS for an additional 4 days. Differentiation of these cells is also a two-phase process: early differentiation, during which Sox-1/nestin-positive cells develop and their terminal differentiation. For this purpose, EBs are dissociated and cultured in eight well chambers in 10% FBS containing DMEM. Cells grown for an additional 2–3 days are referred to here as NPs.
Terminal differentiation of progenitors to neurons, astrocytes and oligodendrocytes
NPs differentiate into neurons and astrocytes upon growth of the cells in 10% FBS containing media for at least 5 days. ESC-derived oligodendrogenesis is a three stage process in vitro as described (Billon et al., 2002; Glaser et al., 2005) with some modifications. Briefly, EBs grown for 2 days in RA and 10% FBS containing media are dissociated and plated on poly-L-lysine coated chambers or six-well plates. Glial progenitors form from these NPs upon growth in DMEM supplemented with N2 (25 μg/ml insulin, 100 μg/ml transferrin, 20 nM progesterone, 100 nM putrescine and 30 nM selenium dioxide, all from Sigma, St. Louis, MO) + 20 ng/ml epidermal growth factor (EGF) + 10 ng/ml basic fibroblast growth factor (bFGF) for 3–5 days (stage 1). Glial progenitors proliferate for 2 days in media + 10 ng/ml bFGF + 10 ng/ml platelet-derived growth factor (PDGF). NG2+ oligodendrocyte progenitors (OPs) appear upon growth in DMEM/N2 containing 3,3,5-tri-iodothyronine hormone (T3, 30 nM) for 2–3 days (stage 2). Finally, OPs are cultured in DMEM supplemented with 0.5 % FBS + 30 nM T3 + 10 ng/ml bFGF + 10 ng/ml PDGF for an additional 2–4 days to engender O4+ immature oligodendrocytes (stage 3).
Immunofluorescence analysis of terminally differentiated cells in the presence of opioids
RA induced NPs or glial progenitors were treated with DAMGO or U69,593 +/− MEK or p38 inhibitors. Fresh agonists +/− inhibitors were added daily in their corresponding growth media for 5 days (NPs and glial progenitors) or 9 days (glial progenitors). Both MAPK inhibitors were dissolved in DMSO and the same amount of solvent was added to control cells. Cells were washed with PBS, fixed in 4% paraformaldehyde for 20 min at 24°C and permeabilized with 0.1% Triton-X/PBS for 5 min at 24°C. Subsequently, cells were blocked with PBS containing 0.5% BSA and 0.1% Tween 20 for 30 min at 24°C. Then cells were incubated in the same blocking solution for 2–3 h at 24°C or overnight at 4 C with one or two of the following primary Abs: a rabbit polyclonal anti-GFAP Ab (1:1, specific marker for astrocytes), a mouse monoclonal anti-TuJ-1 Ab (1:100, neuron-specific class III β-tubulin) or a mouse monoclonal anti-O4 (IgM) Ab (5 μg/mL, an antigen found in immature oligodendrocytes). The following conjugated secondary Abs were added to cells for 1–2 h at 24°C: goat anti-rabbit IgG Alexa Fluor 594, goat anti-mouse IgG Alexa Fluor 488 or goat anti-mouse IgM Alexa Fluor 594. All secondary Abs were used at 1:1000 concentrations. DAPI (1:250) was added together with secondary Abs. After 3 washes with PBS, the slides were treated with anti-fade reagent and examined for immunofluorescence with an OLYMPUS AH3 microscope attached to a CCD Soft Imaging Systems F-view II digital camera that has simultaneous recording capability of dual/triple fluorescence label images. Stained cells were counted.
Detection of MOR and KOR immunoreactivity by fluorescence microscopy
Glial progenitors, OPs and immature oligodendrocytes were washed, fixed in 4% paraformaldehyde for 20 min at 24°C and permeabilized with 0.1% Triton-X/PBS for 5 min at 24°C. Subsequently, cells were blocked with PBS containing 0.5% BSA and 0.1% Tween 20 for 30 min at 24°C. Then, cells were double stained with polyclonal anti-rabbit Abs: MOR (1:2500) or KOR (1:50) and one of the two monoclonal Abs: NG2 (an antigen found on OPs, 1 μg/mL) or O4 (2 μg/mL) overnight at 4°C. Goat anti-mouse IgG/IgM Alexa Fluor 594 and goat anti-rabbit IgG Alexa Fluor 488 secondary Abs (1:700) were applied + DAPI (1:250) for 1 h at 24°C. Cells were examined for immunofluorescence as described above.
ERK1/2 MAPK assays
ERK1/2 phosphorylation was measured by immunoblotting as described (Kim et al., 2006). Cells were lysed in 20 mM HEPES buffer + 10 mM EGTA/40 mM β-glycerophosphate/2.5 mM MgCl2/2 mM sodium vanadate/1% Nonidet-40/1 mM PMSF/20 μg/ml aprotinin/20 μg/ml leupeptin. Cell lysates were centrifuged and protein concentration of the supernatants was determined. Samples (10 μg protein/lane) were separated by 10% SDS-PAGE. Proteins were blotted on Immobilon P™ PVDF membranes. Nonspecific sites were blocked with 5% milk in Tris-buffered saline + 0.2% Tween 20 (TBST). Blots were washed with TBST and incubated with anti-phospho-ERK1/2 Ab (1:2000 dilution) for at least 15 h at 4°C. After washes, blots were incubated with 1:2000 diluted HRP-conjugated goat anti-mouse-IgG for 1 h at 24°C. For assurance of equivalent total ERK1/2 protein per lane, blots were stripped and exposed to anti-ERK1/2 Ab (1:1000), followed by 1:20000 diluted HRP-conjugated goat anti-rabbit-IgG. Bands were visualized using an ECL chemiluminescence detection system. Band intensities were determined by densitometry using a Kodak DC120 digital camera (Scientific Imaging Systems) and analyzed with NIH ImageJ version 1.41m software.
Quantification and statistical analyses of the data
Images of photographed cells were counted using NIH ImageJ software. We counted 700–3000 cells/treatment group, 10–20 fields/well, 30–60 fields/slide and 3–8 slides/treatment group. The number of positively stained cells with stage specific markers were determined as % of the total number of cells (DAPI stained cells) or as % control (untreated cells). Control values were calculated as follows: a mean value was determined and was made equal to 100% and all the other values were calculated as % of the mean value. Data from ERK assays were calculated as phospho ERK/ERK ratios. ERK stimulation in opioid-treated cells was expressed as fold change over basal levels in untreated cells. Statistical determinations were made by one-way ANOVA analysis and Bonferroni’s multiple comparison test or Student’s t test using GraphPad Prism software. Data shown are the mean +/− SEM of at least three independent experiments.
RESULTS
MOR and KOR specific agonists inhibit ESC-derived neurogenesis and astrogenesis via their receptors
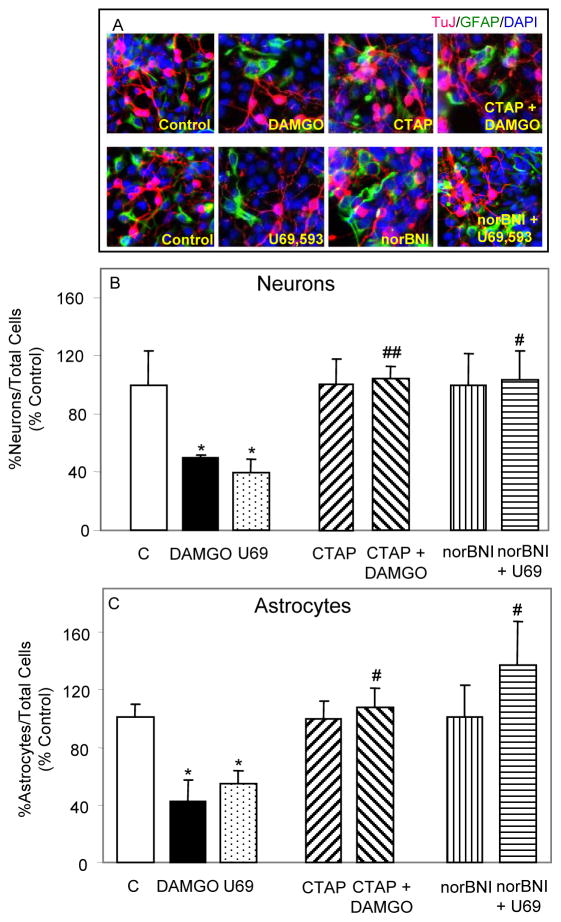
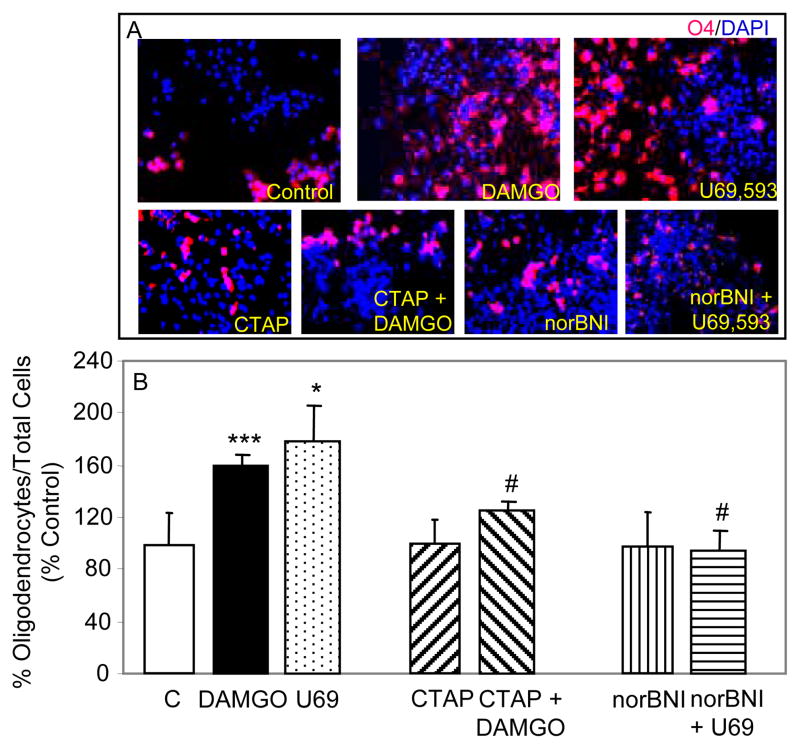
Once NPs are committed to neural differentiation by RA, they normally develop into three types of cells in vitro: neurons, astrocytes and oligodendrocytes (Bain et al., 1995). Since MOR and KOR opioids inhibited the proliferation of RA-induced NPs in our previous studies (Kim et al., 2006), we determined their effect on terminal differentiation of NPs into astrocytes and neurons (Fig. 1). Addition of DAMGO to growth media of RA-generated NPs for 5 days induced a 50% reduction in formation of neurons compared to controls and reduced astrogenesis by 58%. A similar 5 day treatment with U69,593 caused a 60% reduction in neurogenesis and a 46% reduction in astrogenesis from RA-induced NPs.
Fig. 1. μ and κ agonists inhibit ESC-derived neurogenesis and astrogenesis via their receptors.
RA generated NPs, grown for 1–2 days in glass chambers were treated for 5 days with either 1 μM DAMGO or 1 μM U69,593. In some experiments, 1 μM CTAP or 1 μM nor-BNI were added to cells 1 h prior and during opioid agonist treatment. Fresh agonists and antagonists were added on a daily basis. Cells were fixed, permeabilized and then incubated overnight in blocking solution with primary and secondary Abs as described in Experimental Procedures for this and the following two figures. DAPI (1:250) was added together with secondary Abs. (A) Representative images of DAPI+ (blue), TuJ+ (red) and GFAP+ (green) cells are shown. (B&C) Counting data are expressed as % controls (%TuJ+ or %GFAP+ cells vs. total number of DAPI+ cells). N = 3–7. *P<0.05 vs. corresponding controls; #P<0.05, ##P<0.01 vs. agonist alone.
To determine whether μ and κ opioids inhibit neurogenesis and astrogenesis via their own receptors, RA-induced NPs were pretreated with either the MOR specific antagonist CTAP or the KOR specific antagonist norBNI and then treated with corresponding agonists DAMGO or U69,593 for 5 days. The results suggest that antagonist treatments alone did not significantly affect basal levels of neurogenesis and astrogenesis (Fig. 1). Moreover, CTAP and norBNI reversed the actions of DAMGO and U69,593 suggesting that the agonists are acting via their corresponding receptors to inhibit neurogenesis and astrogenesis from RA induced NPs.
MOR and KOR opioids inhibit ESC-derived neurogenesis and astrogenesis via different signaling mechanisms
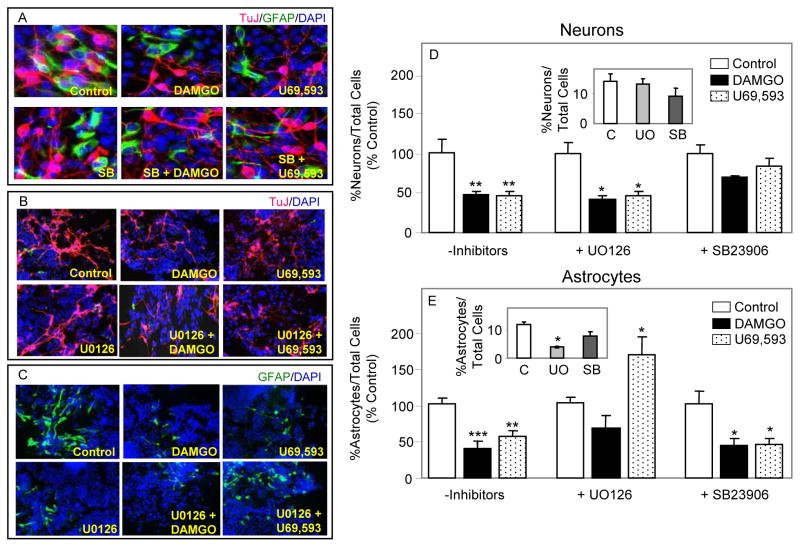
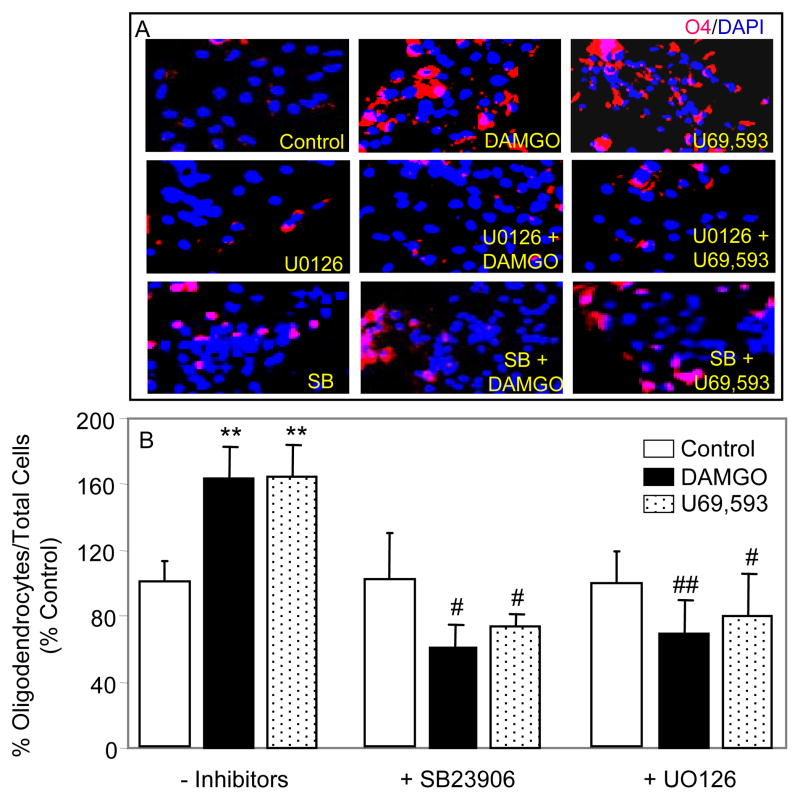
To determine the MAPK signaling pathway(s) involved in opioid inhibition of neurogenesis and astrogenesis from RA induced NPs, U0126 (which blocks MEK activation of ERK) and SB23906 (p38 inhibitor) were used (Fig. 2). These pathways have been found to regulate neurogenesis and/or astrogenesis in other studies (see Introduction). Treating RA/serum-generated NPs with U0126 alone for 5 days had no effect on differentiation into neurons, suggesting that ERK signaling is not required for this process (Fig. 2D inset). Administration of opioids + U0126 did not reverse the inhibition of neurogenesis, implying that DAMGO or U69,593 inhibit terminal differentiation of RA-generated NPs to neurons by an ERK independent mechanism (Fig. 2D). A MAPK signaling pathway other than ERK could elicit opioid inhibitory effects and p38 MAPK was such a candidate. Therefore, RA-induced NPs were treated with SB23906 +/− DAMGO or U69,593 for 5 days. SB23906 alone did not alter the neuronal differentiation of NPs (Fig. 2D inset) suggesting that p38 signaling is not mediating RA/serum-induced neurogenesis. Nevertheless, co-administration of SB23906 with DAMGO or U69,593 reversed opioid effects indicating that p38 is the major signaling pathway involved in opioid inhibition of NP-derived neurogenesis.
Fig. 2. MOR and KOR opioids inhibit the terminal differentiation of RA-induced NPs into astrocytes and neurons via different signaling mechanisms.
NPs were treated for 5 days with 1 μM DAMGO or 1 μM U69,593. U0126 (1 μM) or SB23906 (500 nM) were added to cells 1 h prior and during opioid treatment. (A, B & C) Shown are representative images of DAPI+ (blue), TuJ+ (red) and GFAP+ (green) cells. (D&E) Counting data are expressed as in figure 1. The data in insets show inhibitor effects and are expressed as % neurons or % astrocytes vs. total number of cells. N = 3–8. *P<0.05, **P<0.01, ***P<0.001 vs. corresponding controls.
Addition of U0126 for 5 days to growth media of RA/serum-generated NPs significantly inhibited the formation of astrocytes by 67% (Fig. 2E inset). The data indicate that astrogenesis may be mediated by an ERK pathway. These low levels of astrogenesis were not further reduced upon DAMGO or U69,593 treatments of cells in the presence of UO126. Interestingly, under these conditions U69,593 restored astrocyte numbers to their basal levels, seen in the absence UO126 (Fig. 2E). Astrogenesis in the presence or absence of opioids was unchanged by SB23906, suggesting that p38 signaling may not be involved. In summary, our findings support the idea that μ and κ opioids inhibit terminal differentiation of RA-induced NPs into astrocytes and neurons via different MAPK signaling pathways.
MOR and KOR are detected at all stages of ESC-derived oligodendrogenesis
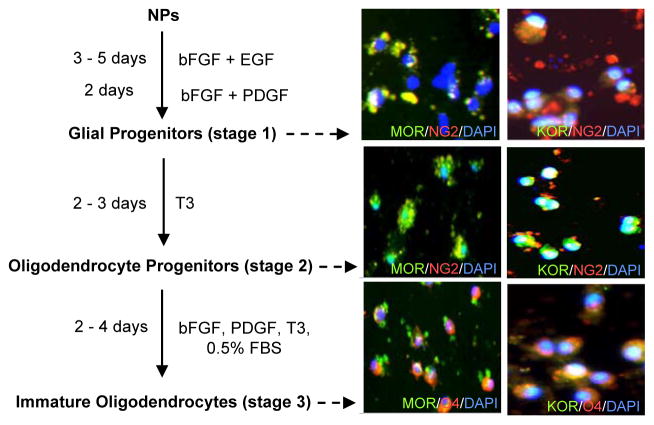
Opioid inhibition of neurogenesis and astrogenesis (Figs. 1& 2) may occur by diverting NPs to oligodendrogenesis. Therefore, we examined the actions of opioids on differentiation of NPs into oligodendrocytes. For this purpose, RA-generated NPs were induced to differentiate to a glial/oligodendrocyte lineage following previously described methods with some modifications (Billon et al., 2002; Glaser et al., 2005). As seen in figure 3, NPs can be converted by treatment with bFGF, EGF and/or PDGF to glial progenitors which in turn differentiate into NG2+ OPs by maintaining them in the presence of T3. Finally, stage 3 entails NG2+ OPs differentiation to O4+ immature oligodendrocytes. NG2 is a chondroitin sulphate proteoglycan that identifies OPs and O4 is a marker for late stage precursors and immature oligodendrocytes (Dawson et al., 2000).
Fig. 3. Detection of MOR and KOR immunoreactivity during NP-derived oligodendrogenesis.
Oligodendrogenesis is a three step process (left). MOR and KOR immunoreactivity is detected at all stages (right). Representative images show MOR+/KOR+ (green), NG2+/O4+ (red) and DAPI+ (blue) staining. Some yellow/orange staining appears when the markers overlap. N=4.
Upon dual staining with OR Abs and oligodendrocyte stage specific markers, we detected MOR and KOR immunoreactivity at all three stages of ESC-derived oligodendrogenesis. Since glial precursors isolated from embryonic spinal cord neural cells expressed MOR and KOR not earlier than 5 DIV (Buch et al., 2007), our glial progenitors (stage 1) were tested for OR presence on day 5 in vitro. At this early stage of oligodendrogenesis, about 40–50% of the cells are NG2+ progenitors and only some of these cells expressed MOR and KOR. At the second stage of ESC-derived oligodendrogenesis, OR immunoreactivity was detected in most of the NG2+ progenitors. The O4+ immature oligodendrocytes expressed high levels of MOR and KOR (Fig. 3).
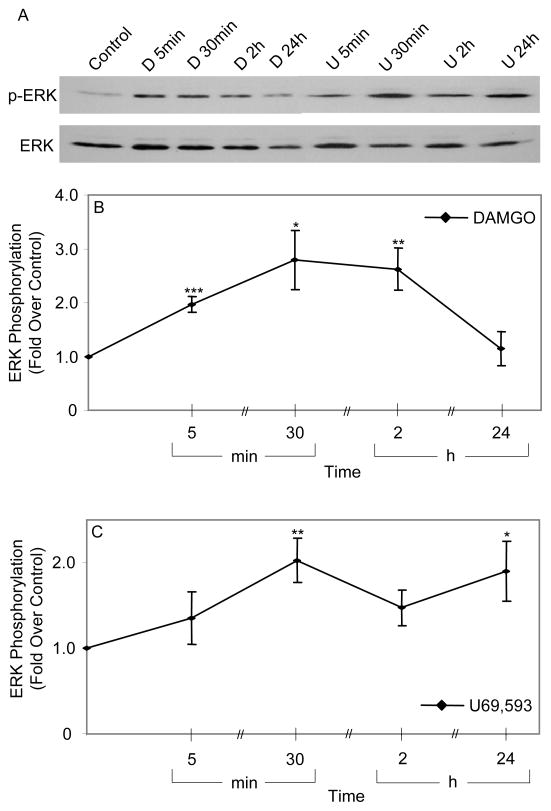
μ and κ opioids stimulate ERK MAPK activity in NG2+ OPs
To investigate opioid modulation of ERK activity, NG2+ OPs were exposed to opioids in N2/growth factor deprived media and ERK phosphorylation was measured by immunoblotting after different intervals. Under these conditions, both DAMGO and U69,593 (both at 1 μM) induced a sustained activation of ERK signaling up to 2 h or 24 h, respectively (Fig. 4). For both ligands, there was at least a 2-fold activation of ERK at all time points, except for DAMGO at 24 h. (Fig. 4). Previously performed time dependent ERK phosphorylation experiments revealed that the same opioid agonists induced a biphasic ERK activation profile in NPs, but maintained a sustained ERK activation in undifferentiated ESCs (Kim et al., 2006).
Fig. 4. Opioid stimulation of ERK phosphorylation in NG2+OPs.
After growth in DMEM/N2 + EGF/bFGF/PDGF for 6–7 days, OPs were treated in media deprived of growth factors with DAMGO (D, 1 μM) or U69,593 (U, 1 μM) for up to 24 h. (A) Representative gels of phospho-ERK and ERK (B&C) Curves of quantified ERK phosphorylation (phospho-ERK/ERK ratios). N = 4. *P<0.05, **P<0.01, ***P<0.001 vs. controls.
Opioids promote oligodendrogenesis from ESC-derived NPs via ERK and p38 MAPK signaling
To study μ and κ opioid modulation of NP differentiation to oligodendrocytes, glial progenitors that were grown for 4 days in N2/EGF/bFGF containing media were treated with opioids for 5 or 9 days. During the 5 day opioid treatment regimen, cells were grown in the presence of DAMGO or U69,593 for 1 day in DMEM/N2 + EGF/bFGF (growth in bFGF/PDGF media was omitted), 2 days in T3 containing media and for the final 2 days in bFGF/PDGF/T3/FBS supplemented media (Fig. 3 left panel). Immunofluorescence cytochemical analysis with O4 Ab revealed that, a 5 day exposure to μ or κ opioids did not induce significant changes in the number of O4+ oligodendrocytes (data not shown). As discussed earlier, the same MOR and KOR agonists were able to inhibit neurogenesis and astrogenesis from NPs after 5 days of opioid treatment (Figs. 1 & 2). Nevertheless, β-endorphin acting via MOR, promoted oligodendrogenesis from adult NPs after a 10 day treatment regimen (Persson et al., 2006).
For experiments involving 9 days of opioid treatment, glial progenitors were grown in the presence of DAMGO or U69,593 for 2 days in DMEM/N2 + EGF/bFGF, 2 days in bFGF/PDGF media, 2 days in T3 containing media and for the final 3 days in bFGF/PDGF/T3/FBS supplemented media. Under these conditions, DAMGO induced a 59% increase in the number of O4+ oligodendrocytes over controls, while U69,593 elicited a 78% increase over basal levels (untreated cells, Fig. 5). The rate of oligodendrogenesis was not altered upon CTAP or nor-BNI treatment of cells for 9 days, suggesting that the antagonists did not significantly affect basal levels of oligodendrogenesis (Fig. 5). Upon preincubation of cells with the corresponding antagonists and then treatment with DAMGO or U69,593 for 9 days, agonist stimulation of oligodendrogenesis was significantly reduced, thereby implicating MOR and KOR.
Fig. 5. μ and κ opioid agonists stmulate ESC-derived oligodendrogenesis via their receptors.
Glial progenitors were treated with DAMGO (1 μM) or U69,593 (1 μM) for 9 days. In some experiments, 1 μM CTAP or 1 μM nor-BNI were added to cells 1 h prior and during opioid agonist treatment. Fresh agonists and antagonists were added on a daily basis. Then cells were fixed, permeabilized and labeled with a mouse monoclonal anti-O4 Ab (5 μg/mL), followed by labeling with a mouse IgM Alexa Fluor 594 (1: 1000) + DAPI (1:250). (A) Representative images of O4+ (red) and DAPI+ (blue) cells. (B) O4+ cell counting data are expressed as % controls (%oligodendrocytes vs. total number of DAPI+ cells). N = 3. *P<0.05, ***p< 0.001 vs. controls; #P<0.05 vs. corresponding agonist alone.
To determine MAPK involvement in opioid stimulation of oligodendrogenesis from RA generated NPs, inhibitors of ERK and p38 signaling were used as described above for opioid modulation of neurogenesis and astrogenesis (Fig. 2). Addition of UO126 or SB23906 alone did not significantly change the number of O4+ oligodendrocytes compared to controls, implying that growth factor/T3 induced differentiation of glial/oligodendrocyte progenitors to O4+ oligodendrocytes is not mediated by MAPK signaling (Fig. 6). In contrast, co-administration of UO126 or SB23906 with either DAMGO or U69,593 significantly reduced the elevation in the number of O4+ oligodendrocytes seen with opioids alone, suggesting the involvement of both, ERK and p38 pathways in opioid stimulatory effects (Fig. 6).
Fig. 6. Opioids stimulate oligodendrogenesis from ESC-derived glial progenitors via ERK and p38 pathways.
Glial progenitors were exposed to DAMGO (1 μM) or U69,593 (1 μM) +/− UO126 (1 μM) or SB23906 (500 nM) for 9 days. The inhibitors were added to cells 1 h prior and during opioid treatment. Fresh agonists and inhibitors were added on a daily basis. Then cells were fixed, permeabilized and labeled with a mouse monoclonal anti-O4 Ab, secondary Ab and DAPI as described in figure 5. (A) Representative images of O4+ (red) and DAPI+ (blue) cells. (B) O4+ cell counting data are expressed as %oligodendrocytes vs. total number of DAPI+ cells. N = 3–6. **P<0.01 vs. controls; #P<0.05, ##P<0.01 vs. agonist alone.
DISCUSSION
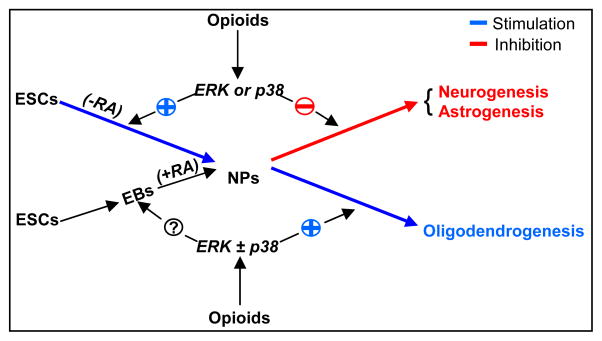
A major finding of this study is that DAMGO and U69,593 stimulate NPs to undergo oligodendrogenesis by a mechanism that entails both ERK and p38 signaling pathways. Accordingly, MOR and KOR immunoreactivity was detected in NG2+ OPs and O4+ oligodendrocytes and these receptors are functional as treatment with both μ and κ opioids induced ERK phosphorylation in NG2+ OPs. In potentiating the oligodendrocytic lineage differentiation, opioids appear to divert ESC-derived NPs from neurogenesis and astrogenesis (Fig. 7). Moreover, DAMGO and U69,593 inhibited astrogenesis and neurogenesis from RA induced NPs via ERK or p38 signaling, respectively.
Fig. 7. The signaling pathways involved in opioid regulation of ESC neural differentiation.
In the absence of an inducing agent, such as RA, μ and κ agonists support neural differentiation of ESCs to NPs by promoting an ERK dependent ESC division. Opioids inhibit the conversion of RA-induced NPs to neurons via p38, they inhibit astrogenesis involving ERK signaling and stimulate oligodendrogenesis by both MAPK signaling pathways. Opioid effects on RA induced neural commitment of ESCs remain to be determined.
Our finding that MOR and KOR are present in NG2+ and O4+ cells represents an addition to the list of neurotransmitter receptor systems identified at different stages of oligodendrocyte development. Glutamate, AMPA, GABAA and dopamine D3 receptors are all expressed in oligodendrocytes and/or their precursors (Von Blankenfeld et al., 1991; Gallo et al., 1996; Bergles et al., 2000). Glial progenitors and O4+ oligodendrocytes were found to express MOR and KOR in vitro and while MOR enhanced DNA synthesis, KOR regulated differentiation of these cells (Knapp et al., 1998; 2001).
Recent electrophysiological and morphological evidence revealed that some adult NG2+ cells are capable of forming synaptic-like excitatory and inhibitory connections with axons, challenging the dogma that neurons are the sole excitable cells in CNS (Lin and Bergles, 2004; Karadottir et al., 2008; Gallo et al., 2008). It was also found that NG2+ progenitors form synapses while they are actively dividing and transfer them to their progeny (Kerkley et al., 2007). As NG2+ progenitors are the most proliferative cells in the postnatal and adult brain, it is possible that the synaptic contacts that they receive may regulate their differentiation potential in vivo (Gallo et al., 2008).
In addition to their neurotransmission role, the GPCRs expressed in NG2+ OPs may play a stimulatory and/or inhibitory role in OPs differentiation to mature oligodendrocytes suggesting that the fine balance between the actions of these receptors may regulate the oligodendrocyte progenitors’ fate. In support of this idea, spontaneous oligodendrogenesis from endogenous precursors has been observed in the adult CNS (McTigue and Tripathi, 2008). Since we found that μ and κ opioids induced a sustained activation of ERK in OPs (Fig. 4), it was not surprising to establish that ERK and p38 MAPK signaling pathways were both involved in opioid stimulation of OP-derived oligodendrogenesis (Fig. 6). Several groups have also found that ERK signaling plays an essential role in the regulation of oligodendrogenesis (Bhat and Zhang, 1996; Hu et al., 2004). Interestingly, opioids also stimulated cell proliferation of NG2+ OPs partially via an ERK dependent mechanism (data not shown). There is a good correlation between these results and trends seen in our previous studies, wherein opioids stimulated proliferation of self-renewing ESCs via ERK signaling and induced the differentiation of these cells to NPs, while opioids inhibited proliferation of more mature NPs and their differentiation to neurons and astrocytes (Kim et al., 2006).
We also addressed the question of opioid signaling pathway(s) involved in neuronal and astrocytic lineage selection and found that μ and κ agonists inhibited the terminal differentiation of NPs to neurons and astrocytes via different MAPKs (Fig. 2). The data imply that opioids may sustain the inhibitory action on astrogenesis by avoiding high levels of ERK activity. In support of this hypothesis, our previous studies established that μ and κ opioids stimulated an oscillating ERK activity in RA generated NPs in contrast to the opioid induced sustained activation of ERK seen in undifferentiated, self-renewing ESCs (Kim et al., 2006) and in OPs (Fig. 4). The unprecedented finding that upon blockade of ERK signaling, U69,593 stimulates astrogenesis (Fig. 2) suggests that κ opioids may have dual effects on this process depending on ERK duration/activation. The signaling pathway involved in KOR stimulatory effects remains to be determined but KOR stimulates proliferation of mature type 1 and type 2 astrocytes via ERK signaling in vitro and in vivo (Xu et al., 2007; McLennan et al., 2008). In contrast, acute morphine can protract the length of GM2/M phase transition of radial glia and basal progenitor cells in vivo (Sargeant et al., 2008) and chronic morphine can inhibit EGF-stimulated type 1 and type 2 astrocytes in vitro (Miyatake et al., 2009). From these results, it is clear that opioids play diverse regulatory roles depending upon the astroglial lineage. This is also consistent with data indicating that GPCR signaling is cell-type specific.
Since our results suggest that ERK/MAPK signaling does not regulate neurogenesis from RA induced NPs, we searched for a role of p38 signaling pathway in opioid inhibition of neurogenesis. p38 signaling has been involved in neuronal differentiation of ESCs and hippocampal NPs (Faigle et al., 2004; Lee et al., 2006). Genetic and biochemical approaches demonstrated that when p38 signaling is off, ESCs are committed to neurogenesis (Aouadi et al., 2006). We proposed that by maintaining p38 activated, MOR/KOR agonists may inhibit neuron formation from RA induced NPs. In fact, our data suggest that spontaneous formation of neurons from RA generated NPs is not dependent on p38 signaling but μ and κ opioid inhibition of this developmental process is regulated predominantly via p38 pathway (Fig. 2).
Our results on opioid modulation of NPs terminal differentiation to astrocytes and neurons (Figs. 1 & 2) are based on studies in which NP treatment with opioids occurred when cells are already committed to neural fate by RA. As discussed, RA induced ESC neural differentiation in vitro is a two step process, one for commitment including the formation of EBs and the other for terminal differentiation. Opioid action on RA-induced ESC neural fate commitment has yet to be investigated (Fig. 7). Some in vitro studies suggest that ERK signaling plays an essential role at the early steps of stem cell commitment to neurogenesis (Rajan and McKay, 1998; Menard et al., 2002; Samuels et al., 2008). In contrast, other studies indicate that ERK is disengaged from the initial neuronal differentiation (Enarsson et al., 2002). Our data suggest that ERK signaling has no role in ESC-derived neurogenesis at a step when NPs are already committed to neural lineage by RA (Fig. 2). Nevertheless, ESC differentiation to neurons is a multi step process and different signaling pathways may regulate these steps. Our previous data indicated that opioids promoted ESC neural commitment in the absence of an inducing agent, such as RA (Kim et al., 2006). Therefore, we hypothesize that opioids may support the induced ESC commitment to neural fate and opioid modulated ERK signaling may turn on neurogenesis (Fig. 7). This remains to be proven. In addition, this hypothesis does not contradict our data on opioid inhibition of neurogenesis (Figs. 1 & 2). The inhibitory actions of opioids appear at a later time during ESC development. This observation underscores the complexity of the multi-step process of terminal differentiation in ESCs.
Here and in our previous study (Kim et al., 2006), we have applied pharmacological doses of opioid agonists (0.1–1 μM) for maximal results. Agonist concentration dependence experiments will be necessary to determine the optimal opioid dose for the observed effects. We cannot rule out the possibility that endogenous opioid peptides (≤ nM concentrations) normally do not play a role in ESC and/or NP fate decision during development. Consistent with this notion is the failure to observe developmental defects in OR, dynorphin and enkephalin knockout mice. Nevertheless, there is evidence for redundancy of opioid peptides and receptors that may mask deficiencies in such knockouts. Accordingly, physiological roles of opioids and their receptors may exist during development but are not detectable except under conditions of stress in mutant mice. Future studies can now be pursued on developmental roles of opioids.
Collectively, our signaling results suggest that the ERK pathway is a major mediator in opioid inhibition of astrogenesis, p38 is the pathway involved in opioid inhibition of neurogenesis and both MAPK pathways may share an equal role in opioid stimulation of oligodendrogenesis (Fig. 7). Moreover, opioids appear to be novel small molecule candidates that could impact neural fate of progenitors. The two opioid agonists studied here may facilitate practical applications of stem cells/progenitors not only in signaling research but also provide support for their use in therapy as found for other small molecules that modulate stem cell fate (Ding et al., 2003; Chen et al., 2006).
Acknowledgments
Supported in part by the following grants: grant from the National Institutesof Health DA-05412 (CJC), seed grant from Pediatric Research Institute, St. Louis University (MMB) and research grant from American Paraplegia Society (MMB).
Footnotes
The abbreviations used are: Ab, antibody; DAMGO, [D-ala2, mephe4, gly-ol5] enkephalin; DMEM, Dulbeco’s Modified Eagles media; EB, embryoid body; EGF, epidermal growth factor; ESCs, embryonic stem cells; ERK, extracellular signal regulated protein kinase; bFGF, basic fibroblast growth factor; GFAP, glial fibrillary acidic protein; GPCR, G protein coupled receptor; KOR, κ opioid receptor; LIF, leukemia inhibitory factor; MAPK, mitogen-activated protein kinase; ME, 2-mercaptoethanol; MOR, μ opioid receptor; nor-BNI, nor-binaltorphimine; NP, neural progenitor; OP, oligodendrocyte progenitor; OR, opioid receptor; PDGF, platelet-derived growth factor; PI3K, phosphoinositide-3 kinase; RA, retinoic acid.
References
- Aouadi M, Bost F, Caron L, Laurent K, Le Marchand-Brustel Y, Binetruy B. p38 mitogen-activated protein kinase activity commits embryonic stem cells to either neurogenesis or cardiomyogenesis. Stem Cells. 2006;24:1399–406. doi: 10.1634/stemcells.2005-0398. [DOI] [PubMed] [Google Scholar]
- Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. Embryonic stem cells express neuronal properties in vitro. Dev Biol. 1995;168:342–57. doi: 10.1006/dbio.1995.1085. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–91. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- Bhat NR, Zhang P. Activation of mitogen-activated protein kinases in oligodendrocytes. J Neurochem. 1996;66:1986–94. doi: 10.1046/j.1471-4159.1996.66051986.x. [DOI] [PubMed] [Google Scholar]
- Billon N, Jolicoeur C, Ying QL, Smith A, Raff M. Normal timing of oligodendrocyte development from genetically engineered, lineage-selectable mouse ESCs. J Cell Sci. 2002;115:3657–65. doi: 10.1242/jcs.00049. [DOI] [PubMed] [Google Scholar]
- Binetruy B, Heasley L, Bost F, Caron L, Aouadi M. Concise review: Regulation of embryonic stem cell lineage commitment by mitogen-activated protein kinases. Stem Cells. 2007;25:1090–5. doi: 10.1634/stemcells.2006-0612. [DOI] [PubMed] [Google Scholar]
- Bongarzone ER, Howard SG, Schonmann V, Campagnoni AT. Identification of the dopamine D3 receptor in oligodendrocyte precursors: potential role in regulating differentiation and myelin formation. J Neurosci. 1998;18:5344–53. doi: 10.1523/JNEUROSCI.18-14-05344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch SK, Khurdayan VK, Lutz SE, Knapp PE, El-Hage N, Hauser KF. Glial-restricted precursors: Patterns of expression of opioid receptors and relationship to human immunodeficiency virus-1 Tat and morphine susceptibility in vitro. Neuroscience. 2007;146:1546. doi: 10.1016/j.neuroscience.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Wei LN, Loh HH. Expression of mu-, kappa- and delta-opioid receptors in P19 mouse embryonal carcinoma cells. Neuroscience. 1999;92:1143–55. doi: 10.1016/s0306-4522(99)00030-5. [DOI] [PubMed] [Google Scholar]
- Chen S, Do JT, Zhang Q, Yao S, Yan F, Peters EC, Scholer HR, Schultz PG, Ding S. Self-renewal of embryonic stem cells by a small molecule. Proc Natl Acad Sci USA. 2006;103:17266–71. doi: 10.1073/pnas.0608156103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MR, Levine JL, Reynolds R. NG2-expressing cells in the central nervous system: Are they oligodendroglial progenitors? J Neurosci Res. 2000;67:471–479. doi: 10.1002/1097-4547(20000901)61:5<471::AID-JNR1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Ding S, Wu TYH, Brinker A, Peters EC, Hur W, Gray NS, Schultz PG. Synthetic small molecules that control stem cell fate. Proc Natl Acad Sci USA. 2003;100:7632–7637. doi: 10.1073/pnas.0732087100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Nat Acad Sci USA. 2000;97:7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enarsson M, Erlandsson A, Larsson H, Forsberg-Nilsson K. Extracellular signal-regulated protein kinase signaling is uncoupled from initial differentiation of central nervous system stem cells to neurons. Mol Canc Res. 2002;1:147–154. [PubMed] [Google Scholar]
- Faigle R, Brederlau A, Elmi M, Arvidsson Y, Hamazaki TS, Uramoto H, Funa K. ASK1 inhibits astroglial development via p38 mitogen-activated protein kinase and promotes neuronal differentiation in adult hippocampus-derived progenitor cells. Mol Cell Biol. 2004;24:280–93. doi: 10.1128/MCB.24.1.280-293.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Mangin JM, Kukley M, Dietrich D. Synapses on NG2-expressing progenitors in the brain: multiple functions? J Physiol. 2008;586:3767–81. doi: 10.1113/jphysiol.2008.158436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Zhou JM, McBain CJ, Wright P, Knutson PL, Armstrong RC. Oligodendrocyte progenitor cell proliferation and lineage progression are regulated by glutamate receptor-mediated K+ channel block. J Neurosci. 1996;16:2659–70. doi: 10.1523/JNEUROSCI.16-08-02659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser T, Perez-Bouza A, Klein K, Brϋstle O. Generation of purified oligodendrocyte progenitors from embryonic stem cells. FASEB J. 2005;19:112–4. doi: 10.1096/fj.04-1931fje. [DOI] [PubMed] [Google Scholar]
- Hauser KF. Morphine regulates DNA synthesis in rat cerebellar neuroblasts in vitro. Brain Res Dev Brain Res. 1992;70:291–7. doi: 10.1016/0165-3806(92)90210-n. [DOI] [PubMed] [Google Scholar]
- Hauser KF, Houdi AA, Turbek CS, Elde RP, Maxson W. Opioids intrinsically inhibit the genesis of mouse cerebellar granule neuron precursors in vitro: Differential impact of mu and delta receptor activation on proliferation and neurite elongation. Eur J Neurosci. 2000;12:1281–1293. doi: 10.1046/j.1460-9568.2000.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, El-Hage N, Stiene-Martin A, Maragos WF, Nath A, Persidsky Y, Volsky DJ, Knapp PE. HIV-1 neuropathogenesis: Glial mechanisms revealed through substance abuse. J Neurochem. 2007;100:567–86. doi: 10.1111/j.1471-4159.2006.04227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Jin L, Feng L. ERK1/2 but not PI3K pathway is required for neurotrophin 3-induced oligodendrocyte differentiation of post-natal neural stem cells. J Neurochem. 2004;90:1339–47. doi: 10.1111/j.1471-4159.2004.02594.x. [DOI] [PubMed] [Google Scholar]
- Karadottir R, Hamilton NB, Bakiri Y, Attwell D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci. 2008;11:450–6. doi: 10.1038/nn2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Clark AL, Kiss A, Hahn JW, Wesselschmidt R, Coscia CJ, Belcheva MM. Mu and kappa opioids induce the differentiation of embryonic stem cells to neural progenitors. J Biol Chem. 2006;281:33749–60. doi: 10.1074/jbc.M603862200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp PE, Maderspach K, Hauser KF. Endogenous opioid system in developing normal and jimpy oligodendrocytes: Mu and kappa opioid receptors mediate differential mitogenic and growth responses. Glia. 1998;22:189–201. doi: 10.1002/(sici)1098-1136(199802)22:2<189::aid-glia10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Knapp PE, Itkis OS, Zhang L, Spruce BA, Bakalkin G, Hauser KF. Endogenous opioids and oligodendroglial function: Possible autocrine/paracrine effects on cell survival and development. Glia. 2001;35:156–65. doi: 10.1002/glia.1080. [DOI] [PubMed] [Google Scholar]
- Kukley M, Kiladze M, Tognatta R, Hans M, Swandulla D, Schramm J, Dietrich D. Glial cells are born with synapses. FASEB J. 2007;22:2957–2969. doi: 10.1096/fj.07-090985. [DOI] [PubMed] [Google Scholar]
- Kunath T, Saba-El-Leil MK, Almousailleakh M, Wray J, Meloche S, Smith A. FGF stimulation of the ERK1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development. 2007;134:2895–902. doi: 10.1242/dev.02880. [DOI] [PubMed] [Google Scholar]
- Lee ER, McCool KW, Murdoch FE, Fritsch MK. Dynamic changes in histone H3 phosphoacetylation during early embryonic stem cell differentiation are directly mediated by mitogen- and stress-activated protein kinase 1 via activation of MAPK pathways. J Biol Chem. 2006;281:21162–21172. doi: 10.1074/jbc.M602734200. [DOI] [PubMed] [Google Scholar]
- Lin SC, Bergles DE. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat Neurosci. 2004;7:24–32. doi: 10.1038/nn1162. [DOI] [PubMed] [Google Scholar]
- McLennan GP, Kiss A, Miyatake M, Belcheva MM, Chambers KT, Pozek JJ, Mohabbat Y, Moyer RA, Bohn LM, Coscia CJ. Kappa opioids promote the proliferation of astrocytes via Gβγ and β-arrestin2-dependent MAPK-mediated pathways. J Neurochem. 2008;107:1753–1765. doi: 10.1111/j.1471-4159.2008.05745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. J Neurochem. 2008;107:1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- Menard C, Hein P, Paquin A, Savelson A, Yang XM, Lederfein D, Barnabe-Heider F, Mir AA, Sterneck E, Peterson AC, et al. An essential role for a MEK-C/EBP pathway during growth factor-regulated cortical neurogenesis. Neuron. 2002;36:597–610. doi: 10.1016/s0896-6273(02)01026-7. [DOI] [PubMed] [Google Scholar]
- Miller CR, O’Steen WK, Deadwyler SA. Effect of morphine on 3H-thymidine incorporation in the subependyma of the rat: An autoradiographic study. J Comp Neurol. 1982;208:209–14. doi: 10.1002/cne.902080209. [DOI] [PubMed] [Google Scholar]
- Miyatake M, Rubinstein TJ, McLennan GP, Belcheva MM, Coscia CC. Inhibition of EGF-induced ERK/MAP kinase mediated astrocyte proliferation by μ opioids: Integration of G protein and β-Arrestin 2-dependent pathways. J Neurochem. 2009;110:662–674. doi: 10.1111/j.1471-4159.2009.06156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Kuzumaki N, Miyatake M, Sato F, Wachi H, Seyama Y, Suzuki T. Role of delta-opioid receptor function in neurogenesis and neuroprotection. J Neurochem. 2006;97:1494–505. doi: 10.1111/j.1471-4159.2006.03849.x. [DOI] [PubMed] [Google Scholar]
- Park SW, Huq MD, Loh HH, Wei LN. Retinoic acid-induced chromatin remodeling of mouse kappa opioid receptor gene. J Neurosci. 2005;25:3350–7. doi: 10.1523/JNEUROSCI.0186-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson AI, Thorlin T, Bull C, Eriksson PS. Opioid-induced proliferation through the MAPK pathway in cultures of adult hippocampal progenitors. Mol Cell Neurosci. 2003;23:360–372. doi: 10.1016/s1044-7431(03)00061-7. [DOI] [PubMed] [Google Scholar]
- Persson AI, Bull C, Eriksson PS. Requirement for Id1 in opioid-induced oligodendrogenesis in cultured adult rat hippocampal progenitors. Eur J Neurosci. 2006;23:2277–88. doi: 10.1111/j.1460-9568.2006.04764.x. [DOI] [PubMed] [Google Scholar]
- Rajan P, McKay RD. Multiple routes to astrocytic differentiation in the CNS. J Neurosci. 1998;18:3620–9. doi: 10.1523/JNEUROSCI.18-10-03620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels IS, Karlo JC, Faruzzi AN, Pickering K, Herrup K, Sweatt JD, Saitta SC, Landreth GE. Deletion of ERK2 mitogen-activated protein kinase identifies its key roles in cortical neurogenesis and cognitive function. J Neurosci. 2008;28:6983–6995. doi: 10.1523/JNEUROSCI.0679-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargeant T, Day DJ, Mrkusich EM, Foo DF, Miller JH. Mu opioid receptors are expressed on radial glia but not migrating neuroblasts in the late embryonic mouse brain. Brain Res. 2007;1175:28–38. doi: 10.1016/j.brainres.2007.07.091. [DOI] [PubMed] [Google Scholar]
- Sheng WS, Hu S, Herr G, Ni HT, Rock RB, Gekker G, Lokensgard JR, Peterson PK. Human neural precursor cells express functional kappa opioid receptors. J Pharmacol Exp Ther. 2007;322:957–963. doi: 10.1124/jpet.107.121988. [DOI] [PubMed] [Google Scholar]
- Smith ER, Smedberg JL, Rula ME, Xu XX. Regulation of Ras-MAPK pathway mitogenic activity by restricting nuclear entry of activated MAPK in endoderm differentiation of embryonic carcinoma and stem cells. J Cell Biol. 2004;164:689–699. doi: 10.1083/jcb.200312028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavridis MP, Lunn JS, Collins BJ, Storey KG. A discrete period of FGF-induced ERK1/2 signalling is required for vertebrate neural specification. Development. 2007;134:2889–94. doi: 10.1242/dev.02858. [DOI] [PubMed] [Google Scholar]
- Tighe AP, Gudas LJ. Retinoic acid inhibits leukemia inhibitory factor signaling pathways in mouse embryonic stem cells. J Cell Phys. 2004;198:223–229. doi: 10.1002/jcp.10424. [DOI] [PubMed] [Google Scholar]
- Xu M, Bruchas MR, Ippolito DL, Gendron L, Chavkin C. Sciatic nerve ligation-induced proliferation of spinal cord astrocytes is mediated by kappa opioid activation of p38 mitogen-activated protein kinase. J Neurosci. 200;27:2570–2581. doi: 10.1523/JNEUROSCI.3728-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura C, Zinellu E, Maninchedda E, Maioli M. Dynorphin B is an agonist of nuclear opioid receptors coupling nuclear protein kinase C activation to the transcription of cardiogenic genes in GTR1 embryonic stem cells. Circ Res. 2003;92:623–9. doi: 10.1161/01.RES.0000065169.23780.0E. [DOI] [PubMed] [Google Scholar]
- Von Blankenfeld G, Trotter J, Kettenmann H. Expression and developmental regulation of a GABAA receptor in cultured murine cells of the oligodendrocyte lineage. Eur J Neurosci. 1991;3:310–316. doi: 10.1111/j.1460-9568.1991.tb00817.x. [DOI] [PubMed] [Google Scholar]
- Zhou L, Rowley DL, Mi QS, Sefcovic N, Matthes HW, Kieffer BL, Donovan DM. Murine inter-strain polymorphisms alter gene targeting frequencies at the mu opioid receptor locus in embryonic stem cells. Mamm Genome. 2001;12:772–8. doi: 10.1007/s00335-001-1003-8. [DOI] [PubMed] [Google Scholar]