Abstract
MuRF1 is a member of the RBCC (RING, B-box, coiled-coil) superfamily that has been proposed to act as an atrogin during muscle wasting. Here, we show that MuRF1 is preferentially expressed in type II muscle fibers. Five and 14 days after denervation, MuRF1 protein was further elevated but remained preferentially expressed in type-II muscle fibers. Consistent with a fiber-type dependent function of MuRF1, the tibialis anterior muscle (rich in type-II muscle fibers) was considerably more protected in MuRF1-KO mice from muscle wasting when compared to soleus muscle with mixed fiber-types. We also determined fiber type distributions in MuRF1/MuRF2 double deficient KO (dKO) mice, because MuRF2 is a close homolog of MuRF1. MuRF1/MuRF2 dKO mice showed a profound loss of type-II fibers in soleus muscle. As a potential mechanism we identified the interaction of MuRF1/MuRF2 with myozenin-1, a calcineurin/NFAT regulator and a factor required for maintenance of type-II muscle fibers. MuRF1/MuRF2 dKO mice had lost myozenin-1 expression in tibialis anterior muscle, implicating MuRF1/MuRF2 as regulators of the calcineurin/NFAT pathway. In summary, our data suggest that expression of MuRF1 is required for remodeling of type-II fibers under pathophysiological stress states, whereas MuRF1 and MuRF2 together are required for maintenance of type-II fibers, possibly via the regulation of myozenin-1.
Keywords: Muscle atrophy, MuRF1, Myozenin-1/calsarcin-2, muscle fiber types
INTRODUCTION
Skeletal muscles represent a tissue type that can be functionally remodeled in remarkable ways. For example, upon increasing load, as occurs during exercise, skeletal muscle respond with a large increase in fiber-diameter (hypertrophy); upon unloading, as occurs for example in bedridden patients, muscles rapidly reduce diameter (atrophy). The mechanisms for hypertrophy/atrophy include an up-regulation of the calcineurin-NFAT pathway (stimulated by the calcium influx into the sarcoplasm), activation of protein synthesis via the mTOR/p70S6K pathway (caused by myofibrillar stretch), and activation of intracellular stress/strain-response signaling (including the p38/ERK/MEK kinase pathways), for review see [1]. These adaptations to exercise include not only positive feeback regulation of fiber trophicity but also include metabolic adaptations, such as increase in mitochondrial content [2], and changes in fiber-type [3]. For example, enhanced neuro-muscular activity as induced by chronic low-frequency stimulation, which is experimentally used to mimic exercise, evokes transitions in myofibrillar protein expression, ultimately leading to a conversion of fast into a slower skeletal muscle fiber type [4]. Although the characterization of factors determining fiber type specification are still ongoing, calcineurin, MEF2, CaMK and PGC-1 [5; 6] have been identified as muscle fiber type I promoting factors, whereas thyroid hormone acts as a type II driving factor [7]. One recent factor emerging for the maintenance of fast fiber-types is myozenin-1 (also called calsarcin-2 or FATZ). Functionally, myozenin-1 belongs to the calsarcin protein family whose members inhibit the protein phosphatase calcineurin [8] which in turn activates the transcription factor by dephosphorylating NFAT. Among the calsarcin family, myozenin-1 has a specialized function for muscle fiber-type specification. Recent studies on the myozenin-1 gene deficient mouse model demonstrated that normal expression of myozenin-1 is required for the maintenance of fast fibers [8]. Consistent with having almost pure type I muscle fiber skeletal muscles, myozenin-1 KO mice have a marked capacity for long distance running [8].
The chronic loss of sarcomeres, particularly of type II fibers with increasing age [9] is receiving growing attention because of its clinical importance. During chronic muscle wasting, loss of sarcomeres is accompanied by numerous alterations in the proteome, metabolome and transcriptome, including downregulation of mitochondrial metabolism, and of muscle specific genes. Genome-wide comparisons of healthy and wasting muscle tissues identified a specific set of genes that become up-regulated during diverse states of muscle wasting [10; 11]. These transcriptional changes are not merely a down-regulation of the exercise-stimulated genes and include genes that have been postulated to be causally linked to the development of muscle atrophy, the so-called atrogins [11; 12]. In particular, atrogin-1 (also known as MAFBx) and MuRF1 (muscle ring finger-1) might play key roles in the regulation of muscle remodeling [12]. While gastrocnemius mouse leg muscle loses as much as ~50% mass loss during two weeks after denervation, gastrocnemius muscles in knock-out mice deficient for atrogin-1 or for MuRF1 are significantly spared from mass loss (only 20 % of control) [12]. In conclusion, these knock-out studies suggest that the upregulation of MafBx/atrogin-1 and MuRF1 in wildtype mice after denervation is required for the development of atrophy. MuRF1 has been implicated in amino acids metabolism control [13] and might also be a target of certain amino acids such as leucine, since it has been shown that MuRF1 up regulation during skeletal muscle atrophy is minimized by leucine supplementation [14]. MuRF1 might also be an important player in skeletal muscle longitudinal growth [15].
A direct mechanistic link explaining why up-regulation of atrogin-1 and MuRF1 might trigger the degradation of muscle tissues during atrophy is that both proteins have ubiquitin ligase activities. Atrogin-1 and MuRF1 can potentially catalyze as E3 ubiquitin ligases the specific multi-ubiquitination of target protein, thereby initiating their proteasome-dependent degradation by the ubiquitin protesome system (UPS) [16]. Here, we focus on MuRF1 and MuRF2 (muscle ring finger-2) that share an N-terminal RING finger domain (that contains the ubiquitin E3 ligase activity), followed by a B-box and a central coiled-coil domain. MuRFs are also members of the RBCC superfamily (RING+B-box+coiled-coil) as are TRIM (Tripartite Motif-containing) [17]. With regards to its E3 ligase activity, MuRF1 for example was shown in vitro to multi-ubiquitinate troponin-I [18], myosin [19; 20] and actin [21]. However, in vivo, the mechanisms leading to the targeting of certain muscle proteins are more complex, because atrogin-1 and MuRF1 might have differential accessibility to different proteins within the myocyte. To address this issue, Cohen and colleagues [21] used in a well-designed set of studies an inducible cre-lox mouse model that upon Cre-induction deletes MuRF1’s RING finger domain, thus abolishing its ubiquitination-activity while leaving the remainder of MuRF1 and its potential structural functions for M-line assembly intact [22; 23]. In this model, myosin was not protected from multi-ubiquitination and degradation after denervation, contrasting its previous identification as an in vitro target of MuRF1 [20]. Rather, two other components of the thick filament, myosin light chain-2 (MLC2) and myosin-binding protein-C(MyBp) were protected from degradation, suggesting that their ubiquitination is MuRF1-mediated [21]. Therefore, current research is focused on the identification of the in vivo targets of atrogin-1 and MuRF1 using for example transgenic mouse models and adenoviral gene transfer of selected E3 ubiquitin ligase activities. Accordingly, a recent study used adenoviral based approaches suggested that atrogin-1 and MuRF1 are likely to recognize different targets [24]. Hirner et al. demonstrated by using a transgenic mouse model that the skeletal muscle specific overexpression of MuRF1 was not sufficient to cause muscle wasting and instead resulted in metabolic defects, thereby implicating MuRF1 in metabolism re-routing during muscle atrophy [25].
The above discussed denervation studies were focused on the protection of muscle tissue mass from atrophy following long term denervation (>10 days) and less on other physiological parameters such as force or fiber type composition. Here, we characterized the MuRF1-deficient muscles after denervation with regards to fiber type composition in an attempt to clarify whether MuRF1 deficient muscles are physiologically normal and thus whether removal of MuRF1 activity could indeed present a muscle protective strategy. Surprisingly we found that MuRF1 is not evenly distributed in normal muscle tissue, rather it is specifically expressed in type II fibers. In addition, MuRF1is preferentially induced in type II fibers after denervation. Consistent with a role of MuRF1 in fast fiber type remodeling we found that in MuRF1-KO mice fast fiber-rich muscles are considerably more protected than slow fiber type muscles. The direct physical interaction of MuRF1 with myozenin-1 may provide a mechanistic basis of how MuRF1 is involved in type-II fiber specification, thereby implicating MuRF1 in chronic pathophysiological fiber-type remodeling conditions such as occurs during denervation and ageing.
MATERIALS AND METHODS
This study was conducted with animal care guidelines issued by the German National Research Foundation. All protocols were approved by the Animal Care and Use Committee of Universitätsmedizin Mannheim.
Animals
All mice were bred in-house and genotyped by standard methods. MuRF1 and MuRF2 null mice were on a C57/BL6 background and were made as previously described [26]. MuRF1/2 double null mice (5–6 months) were obtained by breeding double heterozygous mice to avoid any selection of potential modifier genes. The MuRF1-overexpressing transgenic line (MuRF1 TG) was described recently [25]. Animals were housed in standard plastic cages in an animal room with controlled environmental conditions and maintained on standard food and water ad libitum.
Experimental design
MuRF1 mice had their sciatic nerve sectioning as described elsewhere [27]. Briefly, the dorsal skin of the thigh was cut and the posterior muscles divided to show the sciatic nerve. A chronic denervation was obtained by carefully cutting a 10-mm section of the sciatic nerve. After 5 and 14 days tibialis anterior and soleus muscles were excised and the effectiveness of denervation was confirmed by observing the locomotion of the life mouse and also by verifying the discontinuity of the sciatic nerve at the thigh level after the animal was sacrificed.
Immunohistochemistry
The primary antibodies used for immunostaining were: (1) monoclonal mouse anti-myosin heavy chain (MHC) II, clone MY-32 (1:1,000; cat# M4276, Sigma) and (2) monoclonal mouse anti-skeletal myosin MHC I, clone NOQ7.5.4D (1:4,000; cat# M8421, Sigma), (3) monoclonal multi-ubiquitin (1:1,000; cat# ab20239), (4) monoclonal mono-ubiquitin (1:1,000; cat# ab7780) and (5) MuRF1 were detected with specific antibodies recently described [28]; [25]. The secondary antibodies used for immunostaining were: (1) goat anti-mouse IgG-Cyanine Cy2 (1:100; cat# 115–225–174 Jackson Lab), (2) donkey anti-chicken IgY-Cyanine Cy3 (1:1000; cat# 703–165–155 Jackson Lab), (3) donkey anti-mouse Cyanine Cy3 (1:1000; cat# 715–165–150 Jackson Lab) and (4) donkey anti-rabbit Cyanine Cy3 (1:100; cat# 711–165–152 Jackson Lab). Cross-sections of muscles for immunostaining were fixed with 4% paraformaldehyde in 0.2M phosphate buffer (PB) for 10 min at room temperature, washed with PBS 3 times for 3 minutes each and then blocked/permeabilized with 0.1 glycine/0.2% triton X-100 in phosphate-buffered saline (PBS) for 1 hour. Subsequently the slides were incubated with a solution containing the primary antibody, 3% normal goat serum and 0.3% triton X-100/0.1M PB overnight in a moisture chamber (4°C). After washing with 0.1M PBS (3 times for 10 min each), a solution containing the secondary antibody and 0.3% triton X-100/0.1 M PBS were added for 2 h in a dark chamber. The slides were then washed in 0.1M PB (3 times for 10 min each) and mounted with Vectrashield mounting medium for fluorescence with 4′, 6-diamidino-2-phenylindole (DAPI) (cat# H-1200, Vector Labs) and coverslipped.
Quantitative and morphometric analysis
The quantitative and morphometric analysis were evaluated on a microscope (Nikon Eclipse E600©, Fukuoka, Japan) equipped with a digital video camera and image software (Metamorph®, Universal Imaging Corporation©, Downingtown, USA) digitizing unit connected to a computer (Image Pro-plus, Media Cybernetic). For the incidence of muscle fiber type I and II and cross sectional area (CSA) determination, a total of approximately 500 (soleus) and 1,000 fibers (tibialis anterior) per muscle per each group were studies. Three to four cross-sections of the soleus and tibialis anterior muscles from different animals were analyzed in all groups. For classification of type I and type II fibers, positive imunolabeling for MHCI and MHCII antibodies were used respectively. Fibers which were lightly labeled were considered intermediary.
Gel electrophoresis and Western blot
Protein extracts were prepared from frozen samples of tibialis anterior and soleus muscles tissue of wild type and KO mice and for each sample 50 μg of total protein were used. The protein levels of MuRF1 and MuRF2 were determined with specific antibodies recently described [25; 29]. Atrogin/Mafbx (Cat#AP2041, ECM Biosciences) and Myozenin-1 (Cat#ab58704 Abcam) and Myozenin-2 (Cat# 26200 Abcam) were detected with commercially available antibodies. For detection, muscle tissues were homogenized and protein species resolved on 4–10% gradient SDS-acrylamide gels (Invitrogen) followed by transference. After incubation, with primary antibodies, horseradish-peroxidase-conjugated secondary antibody, specific bands were visualized by enzymatic chemiluminescence (Super Signal West Pico; Pierce, Bonn, Germany), and densitometry was quantified using a one-dimensional scan software package (Scanalytics, Rockville, USA). Loading variations were monitored by Coomassie-staining.
Yeast two hybrid protein interaction studies
The human full length MuRF1 and MuRF2 cDNAs were amplified from total human skeletal muscle cDNA and inserted into pGBKT7 (BD Biosciences). For Y2H screens, the recombinant MuRF1,2 baits were transformed into Saccharomyces cerevisiae, strain AH109, that were subsequently co-transformed with cDNA libraries inserted in pGADT7 as described earlier [29]. For mating, myozenin-1 gene fragments were inserted into pGADT7. Myozenin-1 constructs were co-transformed together with pGBKT7-MuRF1 and pGBKT7-MuRF2 clones, respectively, into AH109 cells. The Y2H screens and matings were essentially as described by the manufacturer (Yeastmaker Yeast Transformation System 2, Clontech). Co-transformed cells were incubated for 5 days at 30 °C on SD/Leu-/Trp-/His- plates. Subsequent determination of β-galactosidase activities, further mapping studies by mating of deletion constructs, rescue of plasmid DNA form interacting prey clones and their sequence analysis with AD3 primer was performed as described previously[28].
Statistical analysis
Multiple comparisons of mean values were performed with analysis of variance (ANOVA) and a post-hoc Tukey’s test to compare mean values when appropriate. For comparisons between only two groups, the unpaired t-test was used. For all comparisons, a p<0.05 was considered significant.
RESULTS
Induction of MuRF1 and its associated signaling partners during denervation
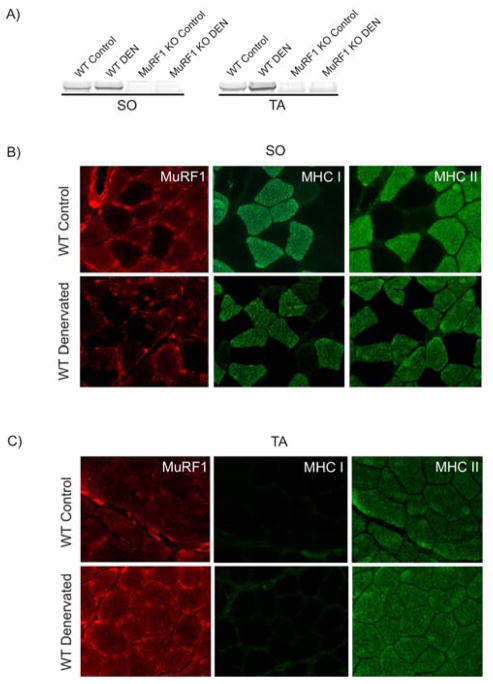
Previous studies on MuRF1 have been limited by the lack of specificity of available MuRF1 antibodies. Here, we tested the various sera raised in our laboratory first by Western blotting of soleus and tibialis anterior extracts, both from WT and MuRF1-KO mice. This confirmed specificity of IgY-type anti-MuRF1 antibodies obtained from chicken (Figure 1A). Next, we examined soleus and tibialis anterior muscles after 14 days after lesion of N. ischiadicus (innervates knee flexors, plantar flexors (i.e. soleus) and plantar extensors (i.e. tibialis anterior)) with these antibodies as well as antibodies specific for type-I (MHC I), and type II (MHC II). In the mixed fiber-type composed soleus, MuRF1 was expressed preferentially in type-II muscle fibers (Figure 1B). In the fast-fiber type tibialis anterior, MuRF1 was expressed in all fibers (wich are type II). After denervation, MuRF1 was still associated with type-II fibers both in soleus and tibialis anterior (Figure 1C), and was expressed at higher levels as indicated by Western blots (Figure 1A).
Figure 1. Fiber-type specific induction of MuRF1 during denervation.
A) Specificity of the used antibody and blotting conditions for MuRF1 as indicated by the absence of MuRF1 signal in MuRF1-KO extracts. In WT, MuRF1 was induced about twofold by denervation (DEN) in both in soleus (SO) and tibialis anterior (TA) muscles.
B) SO and C) TA muscles were examined 14 days after N. ischiadicus lesion with antibodies specific for MuRF1, type-I MHC (MHC I), and type II MHC (MHC II). From left to right, immunohistochemistry with MuRF1, MHC-I, or MHC-II antibdodies. MuRF1 is expressed preferentially in type-II muscle fibers. 400x.
Next, we monitored the induction of MuRF1, MuRF2, and of atrogin-1 (because of their overlapping signaling functions). At 14 days, MuRF1 appears as a doublet, raising the possibility of its post-translational modification or degradation (Figure 2A). The expression level of MuRF2’s 60 kDa isoform is not altered. However, a 50 kDa splice isoform of MuRF2 was induced by denervation (noted by RT-PCR studies and visible on the Western blots as minor bands below the major 60 kDa MuRF2 skeletal isoform, see Figure 2B). Finally, we noted that atrogin-1 was not significantly induced at days 5 and 14 in soleus and tibialis anterior (Figures 2C,D), consistent with a recent report that atrogin is only transiently increased after denervation[30].
Figure 2. Time-course of induction of MuRF1 and MuRF2 and MuRF-associated proteins after denervation as determined by Western blot studies.
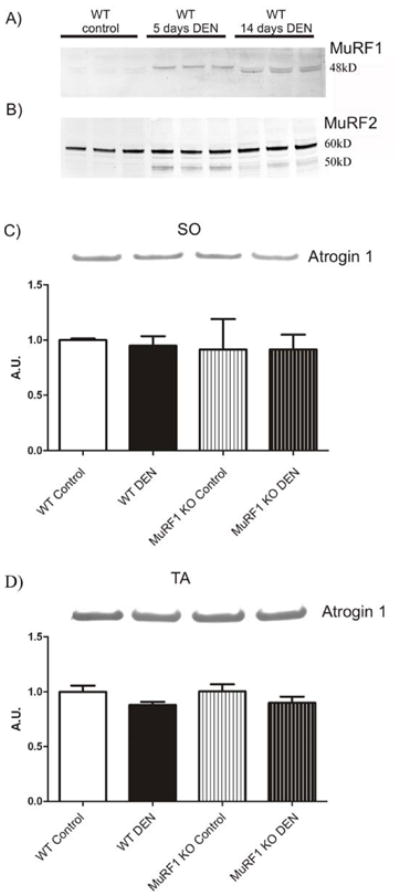
A) Protein expression of MuRF1 and MuRF2 after denervation (DEN) in quadriceps was monitored at 5 and 14 days. MuRF1 is induced 5 days after DEN. At 14 days, MuRF1 is still elevated, but appears as a slightly faster migrating doublet, possibly indicating its degradation.
B) MuRF2’s 60 kDa isoform is not significantly altered in expression before and after DEN. However, a 50 kDa splice isoform is induced by denervation.
C, D) In contrast to MuRF1, atrogin-1 was not significantly altered in expression at day 14 in both in soleus (SO) and tibialis anterior (TA). Histograms show the relative protein expression and bars represent the mean of each group expressed in arbitrary units (A.U.) with indicated the standard deviation.
Differential protection of tibialis anterior and soleus muscle tissues after denervation in MuRF1-KO mice
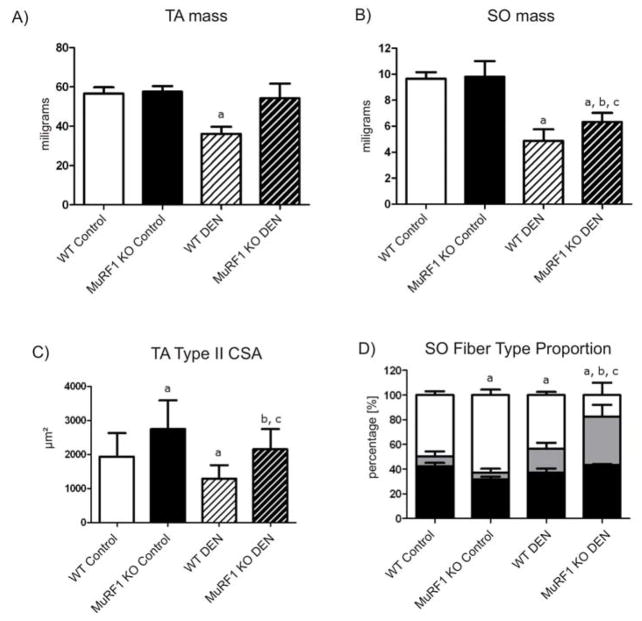
Inactivation of MuRF1 was previously shown to protect muscle from atrophy after denervation [12]. Because of its type-II fiber association we investigated the relative amounts of atrophy protection after denervation in soleus (as a muscle containing both type-I and type-II fibers), and tibialis anterior muscles (a muscle composed of almost exclusively type II fibers). This demonstrated that tibialis anterior from MuRF1-KO mice was essentially protected from atrophy 14 days after denervation, at least with regards to its whole mass: we detected only a trend towards weight loss (the mean weight was reduced by 10% with a p value of 0.062; see Figure 3A). In contrast, soleus from MuRF1-KO mice significantly atrophied with a weight loss of~35% reduction (see Figure 3B). Consistent with a preferential protection of tibialis anterior, CSA of individual type-II fibers was strongly protected after denervation in MuRF1-deficient mice, but we also noted a significant hypertrophy before denervation in MuRF1-KO type-II fibers from tibialis anterior (Figure 3C). Overall, fiber type II CSA in tibialis anterior was reduced 22% after 14 days of denervation. In contrast, muscle fibers in soleus suffered a more pronounced loss (CSA reduction 33% after 14 days of denervation). Somewhat surprisingly, we did not note a difference between type I and type II fibers in soleus (data not shown).
Figure 3. Differential protection of tibialis anterior (TA) and soleus (SO) muscles after denervation in MuRF1-KO mice.
A, B) Comparison of whole muscle mass 14 days after denervation (DEN). TA muscles were strongly protected from atrophy in a MuRF1-KO background and only lost 6% mass during 14 days of denervation. SO atrophied in both WT and MuRF1-KO mice; atrophy protection in the MuRF1-KO mice was 13% when compared to WT. a: p<0.05 vs WT control group; b: p<0.05 vs MuRF1 KO control group and c: p<0.05 vs WT denervated group.
C) Consistent with the total muscle mass protection of TA, single fiber cross sectional areas (CSA) from MuRF1-KO mice remained strongly protected after DEN. Elevated CSAs before DEN contributed to this effect. a: p<0.05 vs WT control group; b: p<0.05 vs MuRF1 KO control group and c: p<0.05 vs WT denervated group.
D) Fiber type composition in SO and its dependence on DEN and MuRF1 expression. Black bars represent type I, white bars type II and grey bars intermediate fiber type occurrence as percentile of total fibers. DEN resulted in the prominent appearance of an intermediate fiber type in the MuRF1-KO background (also detectable in WT after DEN at low frequency). a: p<0.05 vs WT control group; b: p<0.05 vs MuRF1 KO control group and c: p<0.05 vs WT denervated group.
Error bars represent the mean of at least 3 measurements from 3 independent experiments, lines represent standard deviation.
Finally, we noted during our fiber-type analysis that most type-II fibers lost their clear specification after denervation in MuRF1-KO mice: most MHC-II positive fibers co-stained also with MHC-I and were grouped into an intermediate class (Figure 3D). In summary, MURF1 inactivation preferentially protects type-II fibers from atrophy after denervation.
Regulation of myozenin-1/2 expression by MuRF1 and MuRF2
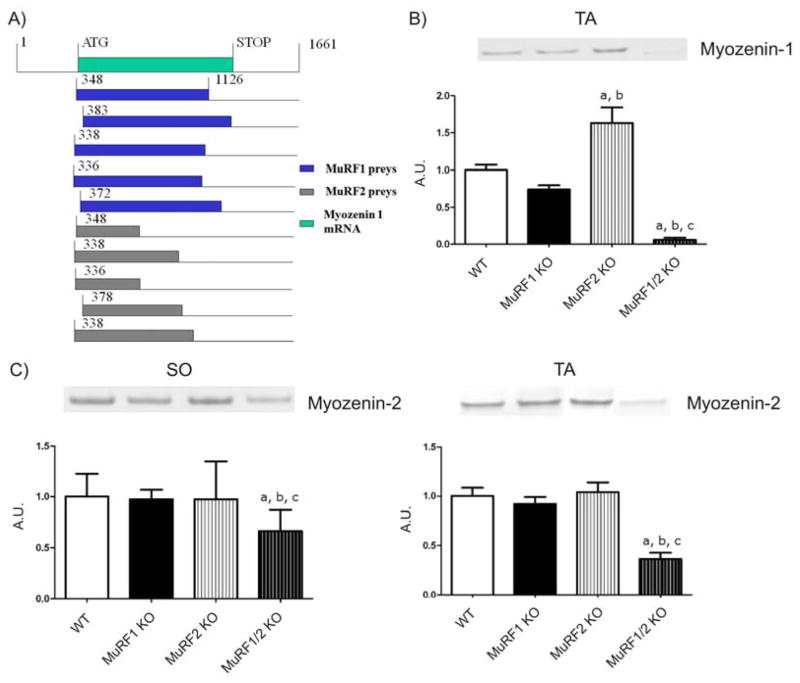
The expression of MuRF1 in type-II fibers and the preferential protection of tibialis anterior muscle suggested a fiber-type dependence of MuRF1 functions. Therefore we surveyed the previously identified 75 MuRF1 interacting prey clones [29] and determined which potential binding partners of MuRF1 might account for fiber-dependent signaling. In this survey we noted that in a set of four independent Y2H screens using MuRF1 and MuRF2 baits, we had fished 10 myozenin-1 gene prey clones from both cardiac and skeletal cDNA libraries (see also [29]). In contrast, none of our Y2H screens identified MYOZ2 prey clones. The sequencing of these ten prey clones assigned the region of interaction to bp 380–1265 in human myozenin-1 gene (Figure 4A). This segment is predicted to interact with both MuRF1 and MuRF2. Next we tested if the deletion of MuRF1 and/or MuRF2 affects the expression of myozenin-1 in vivo in tibialis anterior muscle (in our initial Western blot experiments we could detect myozenin-1 in the fast-twitch tibialis anterior muscle but not in soleus muscle, consistent with Frey 2008). While both single KO models had a moderate effect (less than two-fold change), the combined deletion of MuRF1 and MuRF2 virtually abolished myozenin-1 expression in tibialis anterior (Figure 4B). We noted also a trend of myozenin-2 loss in double-deficient MuRF1/MuRF2 KO mice, both in tibialis anterior and soleus (Figures 4C,D), although this loss was less striking than myozenin-1. In summary, combined deletion of MuRF1 and MuRF2 functionally knocks down both myozenin-1 and myozenin-2, and is therefore predicted to lead to an activation of the calcineurin-NFAT pathway.
Figure 4. Physical and functional interaction of MuRF1 and MuRF2 with the calsarcin members myozenin-1 and 2.
A) Top green bar shows the translated region of the myozenin-1 cDNA (spanning from bp 348 to 1126; accession NM021245). A total of ten myozenin-1 (MYOZ-1) prey clones were identified in the MuRF1 screen (blue), or in the MuRF2 screen (grey). All ten MYOZ1 prey clones represent full-length or close to full-length cDNA clones. This predicts that the region encompassing nucleotides 383 up to the 3′ end is required for the interaction of MYOZ1 with MuRF1 and MuRF2.
B) Western blot analysis of MYOZ-1 expression in MuRF1, MuRF2 and dKO mice. Consistent with Frey et al (2008) we detected MYOZ1 in tibialis anterior (TA) but not soleus (SO) by Western blots. Combined deletion of MuRF1 and MuRF2 results in loss of MYOZ1 expression in TA. a: p<0.05 vs WT group; b: p<0.05 vs MuRF1 KO group and c: p<0.05 vs MuRF2 KO group; A.U. arbitrary units.
C) Myozenin-2 (MYOZ-2) expression was also reduced after MuRF1/MuRF2 deletion, although the effect was less striking (left: SO; right: TA). a: p<0.05 vs WT group; b: p<0.05 vs MuRF1 KO group and c: p<0.05 vs MuRF2 KO group.
Requirement of MuRF1 and MuRF2 for physiological fiber type specification
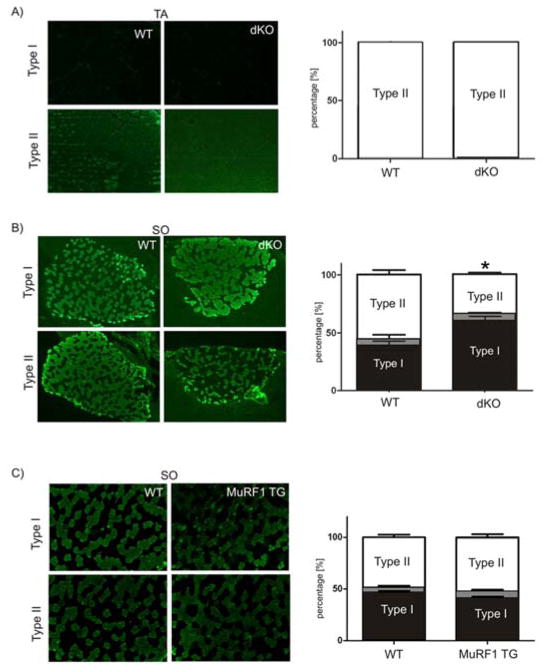
The functional knock-down of myozenin-1 in MuRF1/MuRF2 deficient mice raised the possibility of an impairment of type II fiber specification in dKO mice [8]. Therefore we studied next the distribution of type I and type II fibers in dKO mice. In tibialis anterior muscles, we did not find an effect on fiber-type composition: mouse tibialis anterior is a pure fast-fiber composed muscle [31], and this was also the case in dKO tibialis anterior (Figure 5A). However, in the mixed fiber-type soleus muscle, we noted a marked shift towards type-I fibers (Figure 5B). In contrast, we did not find a significant fiber-type shift in soleus in a MuRF1 overexpressing model (Figure 5C). Similarly, we did not note a significant shift towards type I fibers in the single MuRF2 KO models (data not shown). In summary, we conclude that only the combined inactivation of MuRF1 and MuRF2 caused a significant loss of type-II fibers in soleus.
Figure 5. Effect of combined MuRF1/MuRF2 deletion and MuRF1 overexpression on muscle fiber type distribution.
Type I and type II fibers were detected by immunofluorescence with antibodies specific for MHCI and MHCII, respectively (left), and their relative frequencies were determined in tibialis anterior (TA) and soleus (SO) (right) of WT, dKO, and MuRF1-overexpressing (MuRF1-TG) mice. Black bars represent type I, white bars type II and grey bars intermediate fiber type occurrence as percentile of total fibers.
A) Combined inactivation of MuRF1 and MuRF2 did not affect the pure type-II fiber type found in TA. 100x.
B) SO from dKO mice was markedly enriched in type-I fibers. 100x.
C) Overexpression of MuRF1 did not affect fiber-type distribution (see text for details). 100x.
Data are expressed as mean ± SD; n= 3 whole cross-sections; *p<0.05 vs. WT.
Analysis of the distribution of ubiquitin in skeletal muscle fibers and its dependence on MuRF1/MuRF2 expression
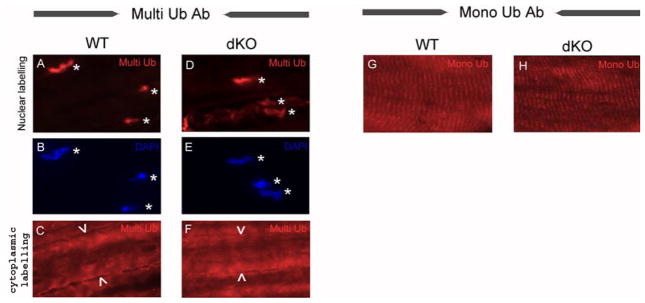
Interaction studies identified numerous interactions of MuRF1 and MuRF2 with sarcomeric proteins (for review, [28]). We therefore tested the ubiquitin distribution in skeletal muscle fibers from tibialis anterior by immunohistochemistry, using antibodies specific for multi-ubiquitin or mono-ubiquitin epitopes. We detected perinuclear and/or nuclear labeling with the multi-ubiquitin specific antibody (it was not possible to accurately distinguish between perinuclear or nuclear labeling by the fluorescence microscopy used here). We detected a cytoplasmic labeling as well (Figure 6A-F). In contrast, when using the mono-ubiquitin specific antibody, we detected a clear striated labeling pattern (Figure 6G). Intriguingly, we did not note any obvious differences between samples prepared from wild type or MuRF1/MuRF2 double knockout mice (both for sections stained with mono- or the multi-ubiquitin specific antibodies; comparisons in Figures 6A and D; 6G and H, respectively). We conclude that the expression of MuRF1/MuRF2 is not required for global ubiquitin distribution within myocytes, at least as detected by the here used methods.
Figure 6. Ubiquitin immunolocalization in skeletal muscle fiber.
Immunofluorescence of longitudinal sections of tibialis anterior muscle using a multi-ubiquitin (A, C, D, F) and a mono-ubiquitin specific antibody (G, H); ubiquitin epitopes were detected in red. Nuclei were labeled with DAPI in blue (see B and E). Multi-ubiquitin and DAPI labelings co-localized both in wild type (WT, A and B) and in MuRF1/MuRF2 double knockout tibialis anterior muscles (dKO, D and E); for co-localization see asterisks in A and D. In addition, the multi-ubiquitin antibody labeled the cytoplasm diffusely, both in WT (C) and dKO (F; arrow heads indicate the limits of one skeletal muscle fiber). The mono-ubiquitin specific antibody detected a striated pattern, both in WT (G) and dKO (H) animals. No differences were noted in ubiquitin distribution between WT and dKO samples (multi-ubiquitin: compare A and D, C and F; mono-ubiquitin: compare G and H).
DISCUSSION
MuRF1 transcription is induced after muscle unloading, for example as induced by denervation, as part of the atrophy-response program. Here we studied MuRF1 and related proteins after 5 and 14 days of denervation. Intriguingly, we found that MuRF1 is expressed in a fiber-type II specific fashion in control muscle and likewise post-induction by denervation. Consistent with a role of MuRF1 for type-II fiber trophicity we detected a differential protection of tibialis anterior muscle (predominantly type-II fibers) as compared to soleus muscle (a mixed muscle with about equal sub-populations of type I and type II fibers): Tibialis anterior muscle was significantly more protected from wasting after unloading. As long as two weeks after denervation, tibialis anterior total mass and CSA remained at about wild type levels. Also, when taking into account that tibialis anterior muscles in MuRF1-KOs were about ~20% hypertrophic before denervation, denervation-induced atrophy was still attenuated (CSA reduction after denervation in tibialis anterior was 22%, see Figure 3C; in soleus 33% (not shown)). Taken together, these results implicate MuRF1 in the physiological regulation of type II fiber trophicity during pathophysiological stress conditions. Finally, we noted in our Western blot studies that MuRF1 protein is expressed at significant levels in normal tibialis anterior muscle (Figure 1), pointing also at physiological roles of MuRF1 in healthy type-II muscle fibers. Possibly, MuRF1 in conjunction with MuRF2 is required for maintaining type-II fiber identity (Figure 3D). Therefore, MuRF1’s functions are likely to extend beyond serving as an atrogene, and this work highlights this E3 ligase as a type II driving factor.
A fiber-type dependence of MuRF1’s function in muscle protection needs to be considered when interpreting atrophy models. For example, fiber-type shifts occurring after denervation will affect distribution of muscle proteins that have a fiber-type dependent expression such as myosin heavy and light chain isoforms and MyBP-C isoforms. Possibly, some previously noted protein shifts after denervation on a MuRF1-KO background might have been partially caused by fiber-type shifts and not by their protection from UPS-degradation. For example, if MuRF1 is unable to target myozenin-1, MHC-I could be protected secondarily as myozenin-1 halts its inhibitory action on the calcineurin pathway, which is involved in MHC-I maintenance. Similarly, the targeting of CARP by MuRF1 (another binding partner of MuRF1/MuRF2, see [29]) might affect MLC-2 transcription as part of the Nkx2.5 pathway (regulated by CARP, see [32]).
Our data also suggest that the in vivo roles of MuRF1 for fiber type-II regulation are complex and depend on its associated signaling partner MuRF2 (Figure 5): MuRF1 and MuRF2 together negatively regulate myozenin-1 and myozenin-2, factors involved in type-II fiber specification. The marked loss of myozenin-1/myozenin-2 in the MuRF1+2- double-deficient background could explain why dKO mice have a pronounced loss of type-II fibers (Figure 4): Recent analysis of a myozenin-1 and myozenin-2-deficient mice model demonstrated a requirement of both proteins for maintenance of type II fibers [8; 33]. Functionally, myozenin-1 and myozenin-2 deficient mice had an upregulation of calcineurin/NFAT activity in their skeletal muscles [8; 33]. In analogy, the removal of MuRF1 and MuRF2 resulting in a functional knock-down of both myozenin-1 and myozenin-2 (Figure 5B, C) is expected to activate the calcineurin-NFAT pathway in tibialis anterior. This in turn can explain the hypertrophy of tibialis anterior muscles before and after denervation (Figures 2A, C).
Future studies will need to clarify why myozenin-1 and myozenin-2 expression is lost in MuRF1/2 double deficient mice. Interestingly, MuRF1/MuRF2 directly interacts with myozenin-1 (Figure 5A). Myozenin-1 and MuRF1/MuRF2 share in common the interactions with Tcap, myotilin, and Z-disc bound filamin (for the interaction of FATZ/myozenin-1, see [34]; for MuRF1/2 interaction with these three proteins, see [28; 29] for the interaction with filamin see [35]). Therefore, it appears likely that myozenin-1 and MuRF1/2 constitute a common protein complex with signalosome functions within the Z-disc region that includes regulation of the calcineurin-NFAT pathway [36]. The fact that we did not detect a direct interaction between MuRFs and myozenin-2 suggests that other players exist, such as intermediate bridging proteins. Within this signaling pathway context, MuRF1 together with MuRF2 could be located upstream of myozenins, and potentially regulate the balance between relatively oxidative versus glycolytic fiber types in fast type II fibers. On the protein level, it remains to be elucidated what the functional consequences of these interactions are, because the dramatic downregulation in the dKO model does not support the idea that MuRF1/MuRF2 downregulates myozenin-1 by multi-ubiquitination as E3 ligases.
Possibly, MuRF1/2 docking to Z-disc bound myozenin-1 [8] mediates the ubiquitination of specific components of this signalosome (for such a possible model, see Figure 7), which could be achieved by specific modifications in the Z-disk lattice. We did find a striated labeling pattern by using a mono-ubiquitin antibody, reinforcing the idea that sarcomeric proteins are targeted by E3 ligases. On the other hand, when we used a multi-ubiquitin antibody, a difuse cytoplasmic labeling was obtained. Combined, our ubiquitin immunofluorescence lozalization data suggest that sarcomeric proteins, (at least in a healthy muscle) are preferentially mono-ubiquitinated, which might be indicate fine tune modifications in sarcomeric protein activity (Figure 6). By using the multi-ubiquitin antibody, we also detected a very strong perinuclear labeling, which has been previously reported (Figure 6). Future studies are required to address if MuRF1/myozenin-1 based modifications of the Z-disc lattice can affect the activity of the myozenin-1/calcineurin complex.
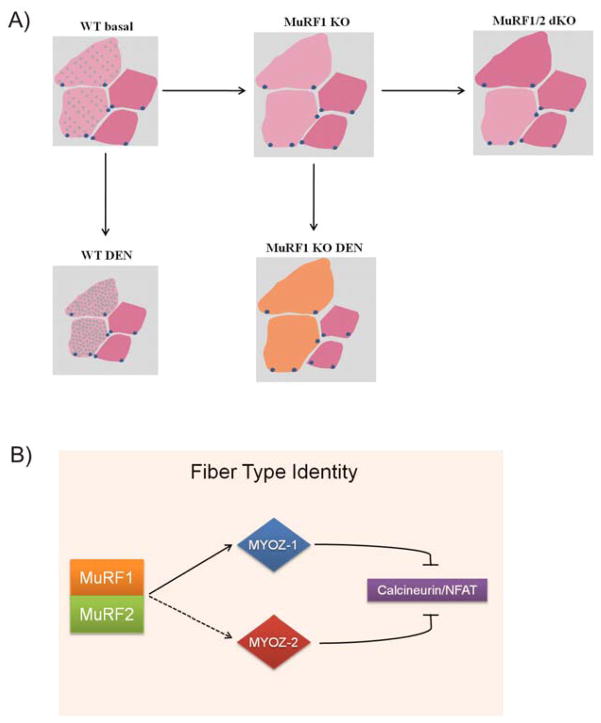
Figure 7. Model for MuRF1/2 dependent type-II fiber trophicity and NFAT signaling control.
A) Drawing summarizing the main findings of the study. In WT mice type II fibers (pink) express MuRF1 (small green dots inside the fiber) in contrast to type I fibers (red) where MuRF1 is absent. After denervation (DEN), MuRF1 expression is further elevated in type II fibers, thereby decreasing their cross sectional area (WT DEN). In myofibrils from MuRF1-KO mice after DEN, type II fibers are preferentially spared from atrophy when compared to type I fiber tissues. In addition, MuRF1-deficient fibers lose their type II fiber identity as unloading evolves, migrating towards an intermediate state (represented by orange color).
B) Proposed model for MuRF1 signaling on type II muscle fibers. Modification of the myozenin-1 (MYOZ-1)/calcineurin and myozenin-2 (MYOZ-2)/calcineurin complex by MuRF1 and MuRF2 results in a downregulation of the calcineurin-NFAT pathway that opposes type-II hypertrophy. Therefore, combined deletion of MuRF1/MuRF2 might result in a diminished calcineurin-NFAT signaling, that interferes with the physiological maintenance of type II fibers.
The two concepts that MuRF1 acts under pathophysiological conditions as a player for causing type II fiber sarcopenia and at the same time is required for physiological maintenance of type II fiber identity are not mutually exclusive. Possibly, in a basal state, MuRF1’s physiological role in type II fibers is to negatively regulate hypertrophy, by down-regulating the NFAT pathway, and protect them during chronic metabolic stress and challenge situations. This model would explain why the pronounced elevation of MuRF1 in the recently described MuRF1-transgenic model does not drive type II fiber sarcopenia under non-challenge conditions. Upon non-physiological and permanent removal of MuRF1 and challenge by disuse, the type II fiber determination function is impaired, and fibers become hypertrophic (Figures 3A,C). As disuse progresses those “protected” fibers lose type II specification and now fall an intermediate classification (Figure 3D). Clearly, future studies involving for example conditional KO models need to address MuRF1’s role in muscle under stress conditions that extent beyond the atrogene concept.
Acknowledgments
We thank Alexander Gasch for support with the protein interaction studies and a critical reading of the manuscript, and Nicole Macha for excellent mouse breeding support. Supported by DFG (La668/13–1 to SL), by NIH HL062881 to H.G and FAPESP (grants# 07/57613–3 and 06/61523–7).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Potthoff MJ, Olson EN, Bassel-Duby R. Skeletal muscle remodeling. Curr Opin Rheumatol. 2007;19:542–9. doi: 10.1097/BOR.0b013e3282efb761. [DOI] [PubMed] [Google Scholar]
- 2.Hood DA. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle. Appl Physiol Nutr Metab. 2009;34:465–72. doi: 10.1139/H09-045. [DOI] [PubMed] [Google Scholar]
- 3.Lee-Young RS, Canny BJ, Myers DE, McConell GK. AMPK activation is fiber type specific in human skeletal muscle: effects of exercise and short-term exercise training. J Appl Physiol. 2009;107:283–9. doi: 10.1152/japplphysiol.91208.2008. [DOI] [PubMed] [Google Scholar]
- 4.Putman CT, Dixon WT, Pearcey JA, Maclean IM, Jendral MJ, Kiricsi M, Murdoch GK, Pette D. Chronic low-frequency stimulation upregulates uncoupling protein-3 in transforming rat fast-twitch skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1419–26. doi: 10.1152/ajpregu.00421.2004. [DOI] [PubMed] [Google Scholar]
- 5.Chin ER, Olson EN, Richardson JA, Yang Q, Humphries C, Shelton JM, Wu H, Zhu W, Bassel-Duby R, Williams RS. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev. 1998;12:2499–509. doi: 10.1101/gad.12.16.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 7.Simonides WS, van Hardeveld C. Thyroid hormone as a determinant of metabolic and contractile phenotype of skeletal muscle. Thyroid. 2008;18:205–16. doi: 10.1089/thy.2007.0256. [DOI] [PubMed] [Google Scholar]
- 8.Frey N, Frank D, Lippl S, Kuhn C, Kogler H, Barrientos T, Rohr C, Will R, Muller OJ, Weiler H, Bassel-Duby R, Katus HA, Olson EN. Calsarcin-2 deficiency increases exercise capacity in mice through calcineurin/NFAT activation. J Clin Invest. 2008;118:3598–608. doi: 10.1172/JCI36277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snijders T, Verdijk LB, van Loon LJ. The impact of sarcopenia and exercise training on skeletal muscle satellite cells. Ageing Res Rev. 2009;8:328–38. doi: 10.1016/j.arr.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. Faseb J. 2004;18:39–51. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]
- 11.Lecker SH, Solomon V, Mitch WE, Goldberg AL. Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J Nutr. 1999;129:227S–237S. doi: 10.1093/jn/129.1.227S. [DOI] [PubMed] [Google Scholar]
- 12.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–8. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 13.Koyama S, Hata S, Witt CC, Ono Y, Lerche S, Ojima K, Chiba T, Doi N, Kitamura F, Tanaka K, Abe K, Witt SH, Rybin V, Gasch A, Franz T, Labeit S, Sorimachi H. Muscle RING-finger protein-1 (MuRF1) as a connector of muscle energy metabolism and protein synthesis. J Mol Biol. 2008;376:1224–36. doi: 10.1016/j.jmb.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 14.Baptista IL, Leal ML, Artioli GG, Aoki MS, Fiamoncini J, Turri AO, Curi R, Miyabara EH, Moriscot AS. Leucine attenuates skeletal muscle wasting via inhibition of ubiquitin ligases. Muscle Nerve. doi: 10.1002/mus.21578. [DOI] [PubMed] [Google Scholar]
- 15.Soares AG, Aoki MS, Miyabara EH, Deluca CV, Ono HY, Gomes MD, Moriscot AS. Ubiquitin-ligase and deubiquitinating gene expression in stretched rat skeletal muscle. Muscle Nerve. 2007;36:685–93. doi: 10.1002/mus.20866. [DOI] [PubMed] [Google Scholar]
- 16.Pickart CM, Eddins MJ. Ubiquitin: structures, functions, mechanisms. Biochim Biophys Acta. 2004;1695:55–72. doi: 10.1016/j.bbamcr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Mrosek M, Meier S, Ucurum-Fotiadis Z, von Castelmur E, Hedbom E, Lustig A, Grzesiek S, Labeit D, Labeit S, Mayans O. Structural analysis of B-Box 2 from MuRF1: identification of a novel self-association pattern in a RING-like fold. Biochemistry. 2008;47:10722–30. doi: 10.1021/bi800733z. [DOI] [PubMed] [Google Scholar]
- 18.Kedar V, McDonough H, Arya R, Li HH, Rockman HA, Patterson C. Muscle-specific RING finger 1 is a bona fide ubiquitin ligase that degrades cardiac troponin I. Proc Natl Acad Sci U S A. 2004;101:18135–40. doi: 10.1073/pnas.0404341102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fielitz J, Kim MS, Shelton JM, Latif S, Spencer JA, Glass DJ, Richardson JA, Bassel-Duby R, Olson EN. Myosin accumulation and striated muscle myopathy result from the loss of muscle RING finger 1 and 3. J Clin Invest. 2007;117:2486–95. doi: 10.1172/JCI32827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clarke BA, Drujan D, Willis MS, Murphy LO, Corpina RA, Burova E, Rakhilin SV, Stitt TN, Patterson C, Latres E, Glass DJ. The E3 Ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab. 2007;6:376–85. doi: 10.1016/j.cmet.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Cohen S, Brault JJ, Gygi SP, Glass DJ, Valenzuela DM, Gartner C, Latres E, Goldberg AL. During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J Cell Biol. 2009;185:1083–95. doi: 10.1083/jcb.200901052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McElhinny AS, Kakinuma K, Sorimachi H, Labeit S, Gregorio CC. Muscle-specific RING finger-1 interacts with titin to regulate sarcomeric M-line and thick filament structure and may have nuclear functions via its interaction with glucocorticoid modulatory element binding protein-1. J Cell Biol. 2002;157:125–36. doi: 10.1083/jcb.200108089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mrosek M, Labeit D, Witt S, Heerklotz H, von Castelmur E, Labeit S, Mayans O. Molecular determinants for the recruitment of the ubiquitin-ligase MuRF-1 onto M-line titin. Faseb J. 2007;21:1383–92. doi: 10.1096/fj.06-7644com. [DOI] [PubMed] [Google Scholar]
- 24.Mearini G, Gedicke C, Schlossarek S, Witt CC, Kramer E, Cao P, Gomes MD, Lecker SH, Labeit S, Willis MS, Eschenhagen T, Carrier L. Atrogin-1 and MuRF1 regulate cardiac MyBP-C levels via different mechanisms. Cardiovasc Res. 2009 doi: 10.1093/cvr/cvp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirner S, Krohne C, Schuster A, Hoffmann S, Witt S, Erber R, Sticht C, Gasch A, Labeit S, Labeit D. MuRF1-dependent regulation of systemic carbohydrate metabolism as revealed from transgenic mouse studies. J Mol Biol. 2008;379:666–77. doi: 10.1016/j.jmb.2008.03.049. [DOI] [PubMed] [Google Scholar]
- 26.Witt CC, Gerull B, Davies MJ, Centner T, Linke WA, Thierfelder L. Hypercontractile properties of cardiac muscle fibers in a knock-in mouse model of cardiac myosin-binding protein-C. J Biol Chem. 2001;276:5353–9. doi: 10.1074/jbc.M008691200. [DOI] [PubMed] [Google Scholar]
- 27.Stockholm D, Herasse M, Marchand S, Praud C, Roudaut C, Richard I, Sebille A, Beckmann JS. Calpain 3 mRNA expression in mice after denervation and during muscle regeneration. Am J Physiol Cell Physiol. 2001;280:C1561–9. doi: 10.1152/ajpcell.2001.280.6.C1561. [DOI] [PubMed] [Google Scholar]
- 28.Witt SH, Granzier H, Witt CC, Labeit S. MURF-1 and MURF-2 target a specific subset of myofibrillar proteins redundantly: towards understanding MURF-dependent muscle ubiquitination. J Mol Biol. 2005;350:713–22. doi: 10.1016/j.jmb.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 29.Witt CC, Witt SH, Lerche S, Labeit D, Back W, Labeit S. Cooperative control of striated muscle mass and metabolism by MuRF1 and MuRF2. Embo J. 2008;27:350–60. doi: 10.1038/sj.emboj.7601952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sacheck JM, Hyatt JP, Raffaello A, Jagoe RT, Roy RR, Edgerton VR, Lecker SH, Goldberg AL. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. Faseb J. 2007;21:140–55. doi: 10.1096/fj.06-6604com. [DOI] [PubMed] [Google Scholar]
- 31.Augusto V, Padovani CR, Campos GER. Skeletal muscle fiber types in C57BL6J mice. Braz J Morphological Sciences. 2004;21(2):89–94. [Google Scholar]
- 32.Zou Y, Evans S, Chen J, Kuo HC, Harvey RP, Chien KR. CARP, a cardiac ankyrin repeat protein, is downstream in the Nkx2–5 homeobox gene pathway. Development. 1997;124:793–804. doi: 10.1242/dev.124.4.793. [DOI] [PubMed] [Google Scholar]
- 33.Frey N, Barrientos T, Shelton JM, Frank D, Rutten H, Gehring D, Kuhn C, Lutz M, Rothermel B, Bassel-Duby R, Richardson JA, Katus HA, Hill JA, Olson EN. Mice lacking calsarcin-1 are sensitized to calcineurin signaling and show accelerated cardiomyopathy in response to pathological biomechanical stress. Nat Med. 2004;10:1336–43. doi: 10.1038/nm1132. [DOI] [PubMed] [Google Scholar]
- 34.Faulkner G, Pallavicini A, Comelli A, Salamon M, Bortoletto G, Ievolella C, Trevisan S, Kojic S, Dalla Vecchia F, Laveder P, Valle G, Lanfranchi G. FATZ, a filamin-, actinin-, and telethonin-binding protein of the Z-disc of skeletal muscle. J Biol Chem. 2000;275:41234–42. doi: 10.1074/jbc.M007493200. [DOI] [PubMed] [Google Scholar]
- 35.Takada F, Vander Woude DL, Tong HQ, Thompson TG, Watkins SC, Kunkel LM, Beggs AH. Myozenin: an alpha-actinin- and gamma-filamin-binding protein of skeletal muscle Z lines. Proc Natl Acad Sci U S A. 2001;98:1595–600. doi: 10.1073/pnas.041609698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frey N, Richardson JA, Olson EN. Calsarcins, a novel family of sarcomeric calcineurin-binding proteins. Proc Natl Acad Sci U S A. 2000;97:14632–7. doi: 10.1073/pnas.260501097. [DOI] [PMC free article] [PubMed] [Google Scholar]