Abstract
The monoclonal antibody trastuzumab and the EGFR/HER2 tyrosine kinase inhibitor lapatinib improve the clinical outcome of patients with HER2-overexpressing breast cancer. However, the majority of metastatic cancers will eventually progress suggesting the need for other therapies. Because HER2 overexpression persists, we hypothesized that the anti-HER2 immune response induced by cancer vaccines would be an effective strategy for treating trastuzumab and lapatinib-refractory tumors. Furthermore, we hypothesized that the antibody response could synergize with lapatinib to enhance tumor inhibition. We developed a recombinant adenoviral vector expressing a kinase-inactive HER2 (Ad-HER2-ki) to use as a cancer vaccine. Vaccine-induced polyclonal HER2-specific anti-serum was analyzed for receptor internalization and signaling effects alone and in combination with lapatinib. Ad-HER2-ki vaccine induced potent T cell and antibody responses in mice and the vaccine-induced polyclonal HER2-specific anti-serum mediated receptor internalization and degradation much more effectively than trastuzumab. Our in vitro studies demonstrated that HER2-vaccine induced antibodies effectively caused a decrease in HER2 expression, but when combined with lapatinib caused significant inhibition of HER2 signaling, decreased pERK and pAKT levels, and reduced breast tumor cell proliferation. In addition, a known mechanism of resistance to lapatinib, induction of survivin, was inhibited. The combination of Ad-HER2-ki plus lapatinib also showed superior anti-tumor efficacy in vivo. Based on these results, we feel clinical studies using this approach to target HER2-overexpressing breast cancer, including trastuzumab- and lapatinib-resistant tumors is warranted.
Keywords: HER2, antitumor immunity, immunization, breast cancer
INTRODUCTION
The human epidermal growth factor receptor 2 (HER2), overexpressed in 20–30% of breast cancers, is associated with more aggressive tumors and inferior overall survival (1). Combinations of the anti-HER2 antibody trastuzumab and chemotherapy lengthen survival in metastatic HER2-overexpressing breast cancer (2). However, progressive disease typically occurs within one year. Lapatinib, a potent reversible inhibitor of HER2 and EGFR tyrosine kinases (3), in conjunction with chemotherapy, enhances time to progression in these patients (4). Unfortunately, responses to lapatinib are generally short-lived, and progression remains a significant clinical problem.
Intriguingly, the overexpression of HER2 persists in trastuzumab and lapatinib-refractory tumors (5, 6), and thus, targeting HER2 with T cells and antibodies induced by cancer vaccines is a potentially effective strategy. More than a dozen phase I and II studies of cancer vaccines have been conducted in breast cancer patients (7). These vaccines have included proteins, peptides, modified tumor cells, and dendritic cells loaded with breast tumor antigens. In these studies, HER2 has been demonstrated to be immunogenic, with a suggestion that immunized patients had an improved clinical outcome (8–12). While we saw promising results following immunization with HER2-protein loaded dendritic cells (13), such approaches are limited to specialized centers with cell processing expertise, and a more feasible approach to immunize patients would be the use of recombinant viral vectors encoding HER2.
Among the many vectors being studied, recombinant adenovirus (Ad) is the most widely used in clinical gene therapy applications including vaccines, having demonstrated the ability to induce immune responses in many clinical studies (14–17). The commonly used Ad5 serotype has an extensive safety profile in the vaccine setting (18–20). Although we wished to incorporate the HER2 gene into Ad vectors for therapeutic vaccination against HER2, there is potential concern that the full length molecule may be oncogenic (21, 22). Therefore, we generated a recombinant adenovirus encoding full length human HER2 with a kinase-inactivating mutation (Ad-HER2-ki) and demonstrated that it was non-oncogenic and activated HER2-specific T cells and polyclonal antisera (called HER2-vaccine induced antibodies) with potent anti-tumor activity in murine models (unpublished data). Interestingly, HER2 specific polyclonal antisera mediate receptor internalization and degradation much more effectively than trastuzumab, the monoclonal antibody targeting HER2 (unpublished data).
Since lapatinib is now commonly used to treat patients with advanced HER2 overexpressing breast cancer that has become refractory to trastuzumab and because of our promising preclinical data suggesting that HER2-specific antibodies were synergistic when combined with lapatinib (23), it was our intention to develop a vaccine strategy that could be used synergistically with lapatinib in humans. Therefore, we studied the effect of lapatinib combined with HER2-vaccine induced antibodies on HER2-expressing cell lines. We then evaluated whether lapatinib would affect the induction of an immune response in vivo. Next, we assessed the antitumor activity of the Ad-HER2-ki vaccine in conjunction with lapatinib. We observed that the HER2-vaccine induced antibodies had enhanced anti-signaling and anti-proliferative activity in the presence of lapatinib, that Ad-HER2-ki could induce potent HER2-specific immune responses when administered with lapatinib, and the combination resulted in greater antitumor activity in vivo than either agent alone.
METHODS
Reagents
Lapatinib was obtained from the Duke University Medical Center Pharmacy. The tablets were pulverized and then mixed with water at a concentration of 5mg/ml. Similarly, trastuzumab and rituximab (as an antibody control) were obtained from the pharmacy and used as reconstituted. HER2 peptide mixes were synthesized by Jerini Peptide Technologies (Berlin, Germany) as 15-mers overlapping by 11 amino acids.
Cell lines
The human breast cancer cell line Au565 (Her2+) was obtained from American Type Culture Collection (ATCC, Manassas, VA) and cultured in RPMI medium 1640 with 10% heat-inactivated FBS. Human breast cancer cell lines BT474 (Her2+) and SK-BR-3 (Her2+) were obtained from the Duke University Comprehensive Cancer Center Cell Culture Facility and were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS. The mouse breast cancer cell line 4T1 was purchased from ATCC. 4T1-HER2 was kindly provided by Dr. Michael Kershaw (Cancer Immunology Program, Peter MacCallum Cancer Centre, Victoria, Australia)(24) and maintained in DMEM with penicillin/streptomycin, and 10% FBS.
Adenovirus Vector Preparation
Construction of the E1-, E3- Ad vector containing the human full length HER2 with an inactivating mutation in the kinase domain (Ad-HER2-Ki-) or beta-gal Lac-Z antigen under the control of human CMV promoter/enhancer elements was performed as previously described (25). The LTR-2/erbB2 plasmid was provided by Dr. L. E. Samelson, (NCI, Bethesda, MD, USA) and the HER2-ki sequence with a K753A mutation to a key residue in the ATP binding region to render the tyrosine kinase inactive (26) was created using Quik-Change mutagenesis (Stratagene, La Jolla, CA).
Mice
C57BL/6 and BALB/c mice were purchased from Jackson Labs (Bar Harbor, ME. All work was conducted in accordance with Duke IACUC-approved protocols.
Induction of HER2-vaccine induced antibodies
C57BL/6 mice were vaccinated via footpad injection with Ad-Lac-Z or Ad-HER2-ki vectors (2.6×1010 particles/mouse). Fourteen days later, mice were euthanized and sera were collected and stored at −80°C.
MTT assay to detect cell proliferation
To assess VIA effects on proliferation, HER2+ cells (Au565 at 5,000 cells per well in a 96-well plate) were cultured with purified HER2-ki-VIA or control serum (1:50 dilution) for 3 days and assessed by 3- (4, 5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazoliumbromide (MTT) assay. Trastuzumab (Herceptin) (10 μg/ml) was used as a positive control and sera from mice receiving Ad-LacZ vaccine or saline were used as negative controls.
Western Blotting to analyze pathway inhibition
AU565 cell extracts were prepared by scraping cells off petri dishes, washing cell pellets 2x in phosphate buffered saline (PBS), and then re-suspending pellets in two-packed-cell volumes of RIPA buffer (150 mM NaCl, 50 mM Tris-HCl, pH 7.5, 0.25% (w/v) deoxycholate, 1% NP-40, 5 mM sodium orthovanadate, 2 mM sodium fluoride, and a protease inhibitor cocktail). Protein concentrations were determined using a modification of the Bradford method (Bio-Rad Labs, Hercules, CA). Equal amounts of proteins (50 ug) were resolved by 4–15% gradient SDS polyacrylamide gel electrophoresis. After transfer, the membranes were then probed with specific antibodies recognizing target proteins (pTyr (Sigma), HER2, Akt, pAkt, Erk 1/2, pErk1/2, (Cell Signaling, Beverly, MA) survivin, and actin (Sigma, St. Louis, MO)) and IRDye 800 conjugated anti- rabbit or mouse IgG or Alexa Fluor 680 anti-rabbit IgG and were visualized using the Odyssey Infrared Imaging System (LI-COR, Lincoln, NE).
Immunogenicity of lapatinib plus Ad-HER2-ki
Eight (8) wk old female C57BL/6 mice received lapatinib (75mg/kg/d) by oral gavage or vehicle (saline) daily beginning on d 0. Beginning on day 7, mice were vaccinated with 2.6×1010 particles of Ad-HER2-ki or Ad-LacZ. Fourteen days post-injection, mice were euthanized and splenocytes and sera were collected for analysis by ELISPOT and flow cytometry.
ELISPOT analysis
IFN-γ ELISPOT assays (Mabtech Inc., Cincinnati, OH) performed according to the manufacturer’s instructions. Splenocytes (500,000 cells/well) were added to the well, and HER2 peptide mix (2.6 μg/ml: BD Bioscience, San Jose, CA) was used as a stimulating antigen. HIV peptide mix (BD Bioscience) was used as a negative control, and a mixture of PMA (50 ng/ml) and Ionomycin (1 μg/ml) was a positive control of the assay.
Analysis of anti-HER2 antibody binding by ELISA
Human breast tumor cell lines (BT474, SKBR3, MCF-7) were harvested, washed and 3× 105 cells were suspended in 100 μl 1% BSA-PBS and incubated with HER2-vaccine induced antibodies or LacZ-vaccine induced antibodies (1:100, 1:100, 1:5000) for 30 min at 4°C. Cells were washed twice with 2 ml of 1% PBS-BAS and stained with HRP-conjugated anti-human IgG (#109-035-088; Jackson ImmunoResearch Labs, Inc., West Grove, PA) (1:5000) in 100 μl 1% BSA-PBS for 30 min at 4°C. After two times washing with 1% BSA-PBS, 150 μl TMB substrate was added and cells were incubated for 15 min at room temperature. 100 μl of supernatant was transferred in to a 96 well-plate. The absorbance was measured on plate reader at 660 nm.
Analysis of anti-HER2 antibody binding by Flow cytometry
We have adapted a methodology reported by Wei et al. to measure anti-HER2 vaccine induced antibodies in vaccinated mouse serum by flow cytometry (27). Briefly, 3 × 105 cells (mouse 4T1-HER2, HER2+; mouse 4T1, HER2−) were incubated with diluted (1:100, 1:1000, 1:10,000) mouse serum (HER2-vaccine induced antibodies or LacZ-vaccine induced antibodies) for 1h at 4°C, washed with 1% BSA-PBS, stained with PE-conjugated anti-mouse IgG (Dako, Cat # R0480) for 30 minutes at 4°C, and washed again. Samples were analyzed on a BD LSRII flow cytometer with results represented as histograms.
Complement dependent cytotoxicity assay
The HER2-vaccine induced antibodies or LacZ-vaccine induced antibodies in sera from mice immunized as above was diluted (1:100) and co-incubated with target cells (4T1 and 4T1-HER2) at 37°C for 1h and 1:100 diluted rabbit serum as the source of complement. After 2.5 h incubation, cytotoxicity was measured using the CytoTox 96 Non Radioactive Cytotoxicity Assay (Promega; per manufacturer’s instructions) to measure LDH release in the culture media as evidence of cytotoxicity. Percent cell lysis is denoted with error bars representing Standard Deviation.
Measuring antibody dependent cellular cytotoxicity (ADCC)
Effector cells for the ADCC assays were obtained by mincing murine spleens, passing the cells through a nylon sieve, lysing the red blood cells, and culturing the remaining cells in RPMI 1640 containing mouse IL-2 (1000 U/ml) for 3 days. Non-adherent cells were removed by washing the flask gently with PBS twice. The adherent cells were supplemented with fresh RPMI 1640 medium containing IL-2 and cultured for 3 additional days. The adherent cells were then harvested and used as effector cells for ADCC assay. 1 million target (4T1-HER2) cells were labeled with 100 μCi of 51Chromium at 37°C for 1h. The labeled target cells were washed three times with culture medium, counted and plated (104/well in 100ul medium) into V-bottomed 96-well microtiter plates, then incubated with either HER2-vaccine induced antibodies (1:100), control LacZ-vaccine induced antibodies (1:100), or trastuzumab (20mg/ml) at 4°C for 20 minutes. Effector cells were add to the plates containing target cells and incubated for another 4 hr. The Effector : target (E:T) ratio was 20:1. After incubation the plates were centrifuged for 5 minutes at 500g and 100 μl supernatant was removed from each well for counting of radioactivity in a spectrometer (Auto-gamma; Packard, Meriden, CT). The cytotoxicity of each sample was determined as follows: Lysis (%) = (experimental − target spontaneous)/(target maximum−target spontaneous)*100%.
Assessment of HER2 localization and internalization
Construction of fluorescent HER2 construct: The HER2-YFP was constructed by using a LTR-2/erbB-2(HER2) construct as PCR template(21) and pcDNA3.1-mYFP construct as vector (gift from Roger Y. Tsien, University of California at San Diego). HER2 was PCR amplified by using the primers 5′-CCCAAGCTTAGCACCATGGAGCTGGCGGCC-3′ and 5-CCGCTCGAGCACTGGCACGTCCAGACCCAG-3′, and inserted into the vector by Hind III and XhoI restriction sites. The authentication of HER2 cDNA was verified by sequencing. HEK293 cells were maintained in MEM medium supplemented with 10% fetal bovine serum and 100 units of penicillin and streptomycin. The day before transfection, 0.3 million HEK293 cells were seeded into Fibronectin-coated 35mm Glass bottom dishes (MatTek). HER2YFP DNA was transfected into cells using FuGENE 6 (Roche). Twenty-four hours after transfection, cells were treated with 100 μg/ml of HER2-vaccine induced antibodies, LacZ-vaccine induced antibodies, or trastuzumab in culture medium for live cell imaging using Zeiss laser scanning microscopy (LSM-510).
Murine model of antitumor activity of lapatinib plus Ad-HER2-ki
Eight (8) wk old female BALB/c mice were implanted with 30,000 4T1-HER2 mouse mammary tumor cells expressing human HER2 on d 0. Mice received lapatinib (75mg/kg/d) by oral gavage daily beginning on d 0 and they were randomized (n=8 or 9 mice per group) to be vaccinated weekly with 2.6×1010 particles of Ad-HER2-ki or Ad-LacZ on d 4, 11, and 18. Tumor volume was measured, once it became palpable, every 2 days using calipers and is reported for day 29 when mice were euthanized in accordance with humane endpoints for tumor size as stated in the Duke IACUC policy.
Statistical analyses
To analyze tumor volume measurements, a cubic root transformation was applied to stabilize the variance such that residuals are normally distributed (data not shown)(28). An ANOVA test was used to assess statistical differences in Day 29 volume measurements, and step-down Student t-tests were applied to 5 pair-wise treatment comparisons of interest, using Bonferroni corrected p-values. Longitudinal growth models were estimated for changes in tumor volume across time, using mixed effects models. The covariance structure was estimated with a time-continuous autoregressive model, that was determined to be optimal by the Bayesian Information Criteria, BIC. Fixed effects were considered for the interaction of Treatment with a quadratic trend across Day, and the likelihood ratio test was highly significant (χ2 = 51.5 with 8 d.f. ; p < 0.0001), such that one concludes there is a distinct treatment difference in tumor growth over time. Wald-type tests are reported for the linear and quadratic trends within treatment. Analyses were performed using R version 2.8.1. For all tests, statistical significance was set at p < 0.05.
RESULTS
Ad vector encoding kinase-inactivated HER2 induces potent T cell and antibody responses
We have developed a recombinant adenoviral vector expressing full length human HER2 with a single amino acid mutation that eliminates kinase activity (Ad-HER2-ki) but retains the kinase domain to enhance T cell immunogenicity conferred by the intracellular domain. When wild type C57BL6 mice were vaccinated with Ad-HER2-ki, splenocytes from vaccinated mice were demonstrated by ELISpot to recognize an overlapping human HER2 peptide mix, while splenocytes from mice receiving control Ad-LacZ vaccine or saline showed no reactivity to the HER2 peptide mix (Fig. 1a). To measure HER2-specific antibody responses, binding of vaccine induced antibodies in mouse serum was tested against HER2 strongly expressing (BT474, SKBR3) and weakly expressing (MCF-7) cell lines (Fig. 1b). The serum of mice vaccinated with the Ad-HER2-ki had binding titers of 1:5000, while the serum of mice receiving the control Ad-LacZ vaccine showed only background levels of binding. The HER2-vaccine induced antibodies recognized greater than 14 epitopes in the intracellular and extracellular domain (data not shown; unpublished data) demonstrating that the antibody responses are polyclonal.
Figure 1.

(a) HER2-specific T cell responses in vaccinated mice. Pooled splenocytes obtained from C57BL/6 mice (n=8 per treatment group) 14 days after injection of Ad-HER2-ki, Ad-LacZ, or saline, were tested in an interferon-gamma ELISPOT and the number of responding T cells (+/− std deviation) was determined. The number of responding cells per 500,000 splenocytes in response to the antigen stimulus (x-axis) is shown. (b) HER2-specific T cell responses in vaccinated mice. Serum obtained from the same mice as in A at 14 days post injection of Ad-HER2-ki or Ad-LacZ was mixed with the listed cell lines shown on the x-axis. Binding of anti-HER2 antibodies was detected by ELISA. Absorbance was measured at 660 nm. (c) Polyclonal anti-HER2 Vaccine Induced Antibodies (Ad-HER2-VIA) mediates CDC in vitro. Pooled serum (8 mice per group) from C57BL/6 mice immunized with Ad-HER2-ki or Ad-LacZ, or, trastuzumab (10 μg/ml), was mixed with HER2 overexpressing cell lines (BT474 and SKBR3) or a HER2 negative cell line (MDA-231) and then rabbit complement was added. The percentage lysis of the cells (+/− std dev) was determined by Chromium release assay. (d) Polyclonal anti-HER2 Vaccine Induced Antibodies (Ad-HER2-VIA) mediates ADCC in vitro. NK cells derived from C57BL/6 mice were cultured with the anti-HER2 vaccine induced antibodies, anti-LacZ vaccine induced antibodies, or trastuzumab, and the cell line 4TI-HER2. The percentage lysis of the cells (+/− std dev) was determined by Chromium release assay. (e) Anti-proliferative effect of HER2-vaccine induced antibodies on HER2+ SKBR3 proliferation. HER2+ SKBR3 cells were cultured with HER2-vaccine induced antibodies, LacZ-vaccine induced antibodies, Trastuzumab (10 μg/ml), or saline and an MTT assay was used to determine proliferation. Statistical analysis comparing samples to the vehicle alone treatment: * trastuzumab p= 0.010; ** HER2-VIA p> 0.0001.
Vaccine induced antibodies against HER2 (HER2-VIA) lyse HER2+ breast tumor cells
Direct antibody-mediated tumor cell killing is a powerful potential mechanism of action of vaccine induced antibodies. We evaluated the capacity of vaccine induced antibodies against HER2 to mediate complement dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (ADCC). Trastuzumab did not mediate CDC but the HER2-vaccine induced antibodies exhibited strong CDC against SKBR3 and BT474 human breast tumor cells, while control LacZ-vaccine induced antibodies showed no effect (Fig. 1c). The effect was HER2-specific because there was no CDC against the HER2 negative cell line MDA-231. In order to evaluate ADCC, we cultured mouse NK cells with HER2-VIA or LacZ-VIA and the human HER2-expressing 4T1 mammary tumor line (4T1-HER2) as a target. HER2-vaccine induced antibodies and trastuzumab mediated significant and equivalent levels of ADCC (Fig. 1d). These data demonstrate that the Ad-HER2-ki induced polyclonal sera contain polyclonal antibodies with an extended spectrum of activity compared with trastuzumab.
Vaccine induced antibodies against HER2 inhibit proliferation of HER2+ cell lines
Although immunization with Ad-HER2-ki was able to efficiently induce humoral immunity in vivo, we also wished to determine whether the antibodies could inhibit HER2+ tumor cell proliferation as has been ascribed to trastuzumab. We found that when highly HER2+ human breast cancer cells (SKBR3) were cultured with HER2-vaccine induced antibodies from the sera of Ad-HER2-ki vaccinated mice, their proliferation was significantly inhibited compared to cells cultured with control LacZ-vaccine induced antibodies (Fig. 1e). Indeed, the inhibition of proliferation was greater than with trastuzumab. Similar results were obtained with other HER2+ human breast cancer cell lines, BT474 and AU565 (data not shown), thus demonstrating the anti-proliferative effect of the vaccine induced antibodies against HER2 in vitro.
Vaccine induced antibodies against HER2 mediate HER2 receptor internalization
Growth factor receptor downregulation has been proposed as a mechanism for the inhibition of tumor growth mediated by monoclonal antibodies. To ascertain whether receptor downregulation was caused by HER2-vaccine induced antibodies, we next investigated HER2 expression levels in highly HER2+ SKBR3 cells after exposure to serum vaccine induced antibodies against HER2. Analysis by Western blotting revealed a decrease in HER2 protein levels in cells exposed to HER2-vaccine induced antibodies relative to untreated cells or cells exposed to LacZ-vaccine induced antibodies (Fig. 2a). This loss of HER2 expression suggested that HER2 was being internalized and degraded after exposure to HER2-vaccine induced antibodies. To confirm this, we sought to visualize HER2 receptor internalization. Using fluorescently labeled endogenous HER2 in SKBR3 cells, we observed dramatic internalization and aggregation of the receptor within 1 hour after exposure to HER2-vaccine induced antibodies, but not with exposure to trastuzumab or control LacZ-vaccine induced antibodies (Fig. 2b).
Figure 2. Polyclonal anti-HER2 Vaccine Induced Antibodies (Ad-HER2-VIA) lead to HER2 receptor internalization and degradation.

(a) Western blots of ErbB2, in SKBR3 cells at 72 hours after designated treatments. (1 = no treatment, 2 = lapatinib 0.2 micro-Molar, 3 = HER2-VIA (1:50), 4 = LacZ-VIA (1:50). (b) HER2-GFP transfected HEK293 cells were plated onto collagen-coated plastic dishes with glass bottoms and cultured overnight. Confocal images of cells expressing HER 2-GFP were acquired using Zeiss laser scanning microscopy (LSM-510). Cells were left untreated (top row) or treated for 1h with HER2-vaccine induced antibodies (HER2-VIA, bottom left), LacZ-vaccine induced antibodies (LacZ-VIA, bottom middle), or trastuzumab (bottom right). Representative images of three independent experiments are shown.
HER2-Vaccine induced antibodies enhance the anti-signaling effect of lapatinib
Because of our published evidence of synergy between vaccine induced antibodies and small molecule inhibition of HER2, we performed Western blot analysis on the human HER2+ breast tumor cell line Au565 treated with lapatinib and serum from HER2 immunized mice with the Ad-HER2-ki vaccine to evaluate the downstream effects of this combination (Fig. 3a). As expected, lapatinib reduced pTyr (as an indicator of pHER2), pErk, and pAKT levels, but did not alter HER2 expression. Trastuzumab had a minimal effect on HER2 expression, even in the presence of lapatinib. In contrast, serum HER2-vaccine induced antibodies reduced the level of HER2 protein and the combination of lapatinib and serum vaccine induced antibodies against HER2 reduced HER2 protein and pTyr, pErk, and pAKT expression. In addition, the combination of lapatinib plus the HER2-vaccine induced antibodies resulted in loss of survivin expression. Similar effects were observed in experiments with the cell lines SKBR3 and BT474 (data not shown). These data demonstrate that lapatinib and the polyclonal HER2-vaccine induced antibodies induced by immunization against HER2 have different effects on HER2+ breast cancer cell lines, and that combining the two agents results in additional perturbation of tumor cell signaling.
Figure 3. HER2-VIA enhances anti-signaling and anti-proliferative effects of lapatinib.


(a) AU565 breast cancer cells were incubated with lapatinib, HER2-vaccine induced antibodies, LacZ-vaccine induced antibodies, and trastuzumab with or without lapatinib. Lysates were analyzed by Western blot for HER2, pztyr (all phosphorylated tyrosines), ERK1/2, pERK, pAKT, AKT1/2, and survivin. Vaccine induced antibodies were used at a dilution of 1:50; lapatinib was at 0.1 μM; and trastuzumab was at 10 μg/ml. (b) Au565 cells (1 × 104) cells were treated with vaccine induced antibodies (1:50 dilution) with or without lapatinib (0.1 μM) for 3 days. Live cells were measured using a thiazolyl blue (MTT) cell proliferation assay by reading A485 and the mean percent inhibition of proliferation +/− standard deviation (SD) is represented. All treatments were performed in triplicate.
HER2-Vaccine induced antibodies enhance the anti-proliferative effect of lapatinib
Having demonstrated that the HER2-vaccine induced antibodies inhibited proliferation of HER2-expressing cell lines (Fig. 1e), we wanted to determine whether there would be additional benefit for the addition of lapatinib to the HER2-vaccine induced antibodies, we cultured the AU565 and BT474 cells with HER2-vaccine induced antibodies and lapatinib. As demonstrated in figure 3b, lapatinib plus the HER2-vaccine induced antibodies resulted in greater inhibition of proliferation of AU565 than lapatinib alone at this sub-maximal dose. Similar results were obtained for the BT474 (data not shown). These data demonstrate that the HER2-vaccine induced antibodies contain antibodies that synergize with lapatinib to reduce the proliferation of HER2-expressing cell lines.
Immune responses to the Ad-HER2-ki vaccine are not impaired by lapatinib
Little is known about the effect of lapatinib on the immune response to cancer vaccines in vivo. BALB/c mice were treated with lapatinib or vehicle for 21 days by oral gavage daily beginning on day 0 and were vaccinated at day 7 with Ad-HER2-ki, Ad-LacZ, or vehicle. The magnitude of the day 21 HER2-specific T cell response to Ad-HER2-ki, measured by an interferon gamma ELISPOT using mouse splenocytes incubated with a HER2 polypeptide mix, was identical, irrespective of whether mice were receiving lapatinib or vehicle (Fig. 4a). These data demonstrate that concurrent lapatinib does not diminish T cell responses to the Ad-HER2-ki vaccine. Similarly, we studied the induction of anti-HER2 antibody responses in the setting of lapatinib. The HER2-vaccine induced antibodies bound to HER2 expressing tumor cells to the same extent, regardless of whether activated in the presence, or absence, of lapatinib (Fig. 4b). Furthermore, HER2-vaccine induced antibodies from mice treated with lapatinib or vehicle and vaccinated with the Ad-HER2-ki were tested for complement-dependent cytotoxicity (CDC) and antibody dependant cellular cytotoxicity (ADCC) in vitro. Lapatinib administration had no effect on the ability of the Ad-HER2-ki to induce VIA capable of lysing HER2+ 4T1 tumor cells by CDC (Fig. 4c) or ADCC (Fig. 4d). These data indicate no negative effect of lapatinib on induction of antibody responses to Ad-HER2-ki.
Figure 4. T cell, antibody and cytolytic responses to immunization with Ad-HER2-ki vaccine are not impaired by lapatinib treatment.



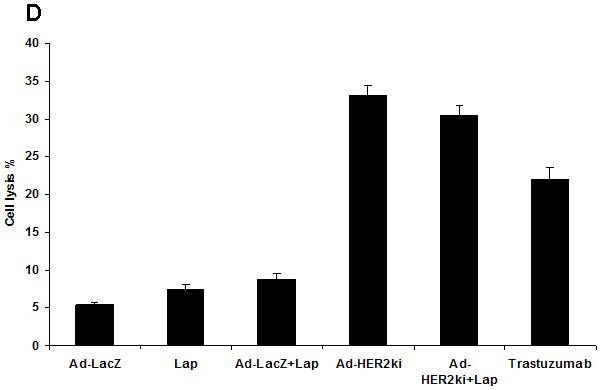
(a) Wild type C57BL/6 mice, treated with lapatinib (75mg/kg/d) or vehicle (PBS), were vaccinated with Ad-HER2-ki, Ad-LacZ, or vehicle (PBS). The magnitude of the immune response to HER2 was measured by IFNγ ELISPOT. The mean number of IFNγ producing cells per 500,000 splenocytes is represented +/− SD. (b) Mouse 4T1-HER2 (HER2+) and 4T1 (HER2−) were incubated with diluted HER2-vaccine induced antibodies or LacZ-vaccine induced antibodies, stained with PE-conjugated anti-mouse IgG and samples analyzed by flow cytometer. The histogram representing the mean fluorescent intensity (MFI) for each treatment is shown. (c) 4T1 or 4T1-HER2 cells were incubated mouse HER2-vaccine induced antibodies (from mice treated with or without lapatinib as in figure 1A) or LacZ-vaccine induced antibodies (1:50) along with rabbit serum as a source of complement. Supernatant from the cultures were taken for the CDC assay. The percentage of lysis was calculated by the following formula: lysis(%)=(experimental−target spontaneous)/(target maximum−target spontaneous)*100%. (d) NK cells derived from C57BL6 mice were cultured with the cell line SKBR3 and serum from mice treated with Lapatinib, Ad-LacZ, Ad-LacZ plus lapatinib, Ad-HER2-ki, Ad-HER2-ki plus lapatinib. Trastuzumab was used as a control for ADCC activity. The percentage lysis of the tumor cells (+/− std dev) was determined by Chromium release assay.
Ad-HER2-ki vaccine plus lapatinib leads to greater tumor regression than either therapy alone in a treatment model
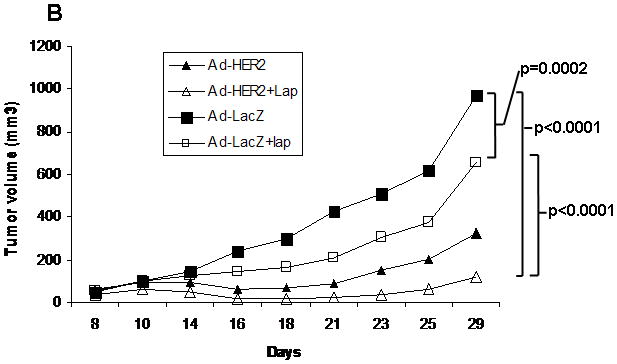
In order to demonstrate efficacy of a combination of lapatinib and vaccination, we administered lapatinib simultaneously with Ad-HER2-ki immunizations to mice bearing HER2 expressing breast tumor cells and evaluated tumor growth over time (Fig. 5a–c). In vehicle-treated animals, tumor growth reached an average volume of 968 mm3 at Day 29, and treatment arms with Ad-HER2-ki vaccine and lapatinib showed general tumor control (ANOVA F4,37 = 4.84, p = 0.003). While the step-down tests of single agent anti-tumor activity were not statistically significant at the α = 0.05 level after adjusting for multiple comparisons (t = 0.94 and 2.68, for lapatinib and Ad-HER2-ki respectively), the combination of both resulted in the greatest tumor control (t = 4.42, adjusted p = 0.003). Distinct differences are observed in the average tumor growth profile of each treatment over time (Fig. 5b), as indicated by the highly significant interaction between treatment and a quadratic trend across Day (P < 0.0001). Among mice receiving vehicle-control, tumor growth increased linearly on the cube-root scale (slope = 0.3, P = 0.019), while mice receiving Ad-HER2-ki vaccine alone or in combination with lapatinib showed a significant attenuation in volume from Day 10 to Day 20 before similar growth patterns returned (P = 0.015 and 0.02, respectively). These data demonstrate synergistic anti-tumor activity for the combination of Ad-HER2-ki and lapatinib on tumor growth in treating established tumors. Comparing final tumor volume at day 29 (Fig. 5c), when the study reached humane endpoints, there was a highly significant decrease in tumor volume comparing the lapatinib plus Ad-LacZ control with the lapatinib plus Ad-HER2-ki vaccine (P=0.0009).
Figure 5. Synergistic anti-tumor effect of the administration of Ad-HER2-ki and lapatinib treatment on the growth of established 4T1-huHER2 tumors in BALB/c mice.


(a) Schema for murine tumor therapy experiment. (b) BALB/c mice implanted with 30,000 4T1 mouse mammary tumor cells expressing human HER2 received lapatinib (100mg/kg/d). Mice were vaccinated weekly for 3 weeks with 2.6×1010 particles of Ad-HER2-ki or Ad-LacZ. Tumor volume was measured, once tumors became palpable, every 2 days using calipers. Mean tumor volume is represented for each treatment group. (c) Data is shown for the various treatment groups at day 29 when mice were euthanized in accordance with humane endpoints for tumor size, per Duke IACUC policy. Bars denote mean tumor size for each group. Circles denote individual mice. Lap = lapatinib.
DISCUSSION
Tumors that progress on trastuzumab and lapatinib continue to express high levels of HER2, leading us to propose targeting HER2+ tumors using a cancer vaccine strategy. We have developed an adenoviral vector vaccine expressing a kinase-inactive, full length human HER2 gene (Ad-HER2-ki), which we have demonstrated is non-oncogenic (unpublished data). We now establish that this vector induces HER2-specific T cell responses and polyclonal antibody responses capable of mediating ADCC and CDC. In addition to these classical immune functions, the antibodies induced by Ad-HER2-ki had potent anti-proliferative effects on HER2-expressing tumor cells. We hypothesized that this might be due to receptor downregulation and subsequently demonstrated that the serum HER2-vaccine induced antibodies produced significant receptor internalization that did not occur when tumor cells were treated with trastuzumab, distinguishing the polyclonal serum antibodies from conventional monoclonal antibody approaches. Finally, because the monoclonal HER2 targeting antibody trastuzumab synergizes with lapatinib, we tested whether vaccine induced antibodies induced by vaccinations against HER2 would synergize with lapatinib in vitro and whether combining lapatinib and Ad-HER2-ki immunization would lead to enhanced control of breast tumors in vivo. Our results establish that the combination was superior to either agent alone in vitro and in vivo.
Several observations in this study require additional commentary. First, much of the activity observed for the Ad-HER2-ki is likely related to the induction of a polyclonal immune response. For example, the HER2-vaccine induced antibodies stimulated by the Ad-HER2-ki mediate both CDC and ADCC. It is widely reported that trastuzumab mediates ADCC but not CDC. Whether these multiple activities of the serum vaccine induced antibodies against HER2 are due to different antibodies or are functions of one antibody will be evaluated in future studies aiming to identify the different components of the polyclonal sera. Another activity likely related to the polyclonal characteristics of the sera is the internalization of HER2 induced by the HER2-vaccine induced antibodies, a function neither we, nor others (29–31), have observed for trastuzumab. Combining two monoclonal antibodies targeting different epitopes on HER2 has been observed to cause HER2 internalization (30, 32) and there is other evidence that supports the ability of multiple antibodies to different epitopes being more efficient at internalizing receptors (32–34). We have identified 14 epitopes recognized by the HER2-vaccine induced antibodies (unpublished data). The importance of the internalization lies in the possibility that internalized receptors may meet one of two fates, either being recycled to the cell surface or degraded. Receptors recycled to the cell surface may continue to stimulate tumor growth, while receptor degradation would block growth factor signaling and clearly be the more desirable outcome for an anti-tumor strategy. Our preliminary evidence supports the latter for HER2 receptor internalized by HER2-vaccine induced antibody treatment (unpublished data).
The second major observation is that the lapatinib could be administered along with the Ad-HER2-ki and the lapatinib did not affect the immune response to immunization. We are not aware of any other data regarding the effect of lapatinib on the immune response. Some other tyrosine kinase inhibitors have demonstrated negative effects on the immune response, such as sorafenib (which targets raf in the EGFR pathway as well as other targets) while others, such as sunitinib, have had no detrimental effects(35, 36).
The third major observation is that there was synergy between the lapatinib and the HER2-VIA activated by the Ad-HER2-ki. Although lapatinib and HER2-VIA target the same molecule, their effects on signaling are different. Alone, the HER2-vaccine induced antibodies had their greatest effect on HER2 protein levels. As expected, lapatinib interrupted signaling through HER2 and thus the phosphorylation of downstream molecules. The combination of the two reagents resulted additionally in a reduction in levels of the anti-apoptotic protein survivin, which would result in enhanced tumor cell apoptosis. We previously reported in vitro results combining polyclonal antisera from rabbits immunized with a HER2 fusion protein with lapatinib (23). Thus, although combining lapatinib and trastuzumab has shown favorable clinical results (37), it is possible that the combination of lapatinib and a polyclonal anti-HER2 antibody response will be superior because of the additional effects provided by polyclonal antibodies over a monoclonal antibody targeting a single epitope. It is also intriguing that lapatinib treatment can lead to stabilization and accumulation of HER2, enhancing trastuzumab-mediated cytotoxicity (38). We expect similarly that it will potentiate the activity of vaccines targeting HER2.
Collectively, our results strongly support the assessment of Ad-HER2-ki in human clinical trials. The potential benefits of a vaccine strategy over a MAb approach, with the induction of both T cell and polyclonal antibody responses, and multiple mechanisms of action resulting from polyclonal antibody induction, encourage the use of vaccine strategies. And there is increasing evidence that cancer vaccines can improve patient survival, renewing enthusiasm for cancer vaccine approaches. (13, 39–41). The synergy seen with the vaccine plus lapatinib suggests that there use in combination should also be evaluated clinically. Clinical trials to evaluate the combination are scheduled to open in 2010. These clinical studies will determine if similar levels of cellular and humoral immune response can be induced in breast cancer patients to those seen in our animal model, and whether the vaccine results in clinical efficacy. More broadly, we believe our results suggest that targeting receptor molecules using vaccines as a means to perturb signaling offers new opportunities to target cancer beyond the conventional lytic killing of tumors by the immune system.
Acknowledgments
FUNDING
This work was supported by grants from the National Cancer Institute [NCI P50 CA89496-01 and 5P50CA068438 to HKL, NCI R01 CA95447 to TMC]; Department of Defense Breast Cancer Research Program Clinical Translational Research Award [BC050221 to TMC]; Department of Defense [W81XWH-07-1-0392 GRD]; and Susan G. Komen Foundation Postdoctoral Fellowship Award [KG080627 to ZH].
References
- 1.Paik S, Hazan R, Fisher ER, Sass RE, Fisher B, Redmond C, Schlessinger J, Lippman ME, King CR. Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: prognostic significance of erbB-2 protein overexpression in primary breast cancer. J Clin Oncol. 1990;8(1):103–112. doi: 10.1200/JCO.1990.8.1.103. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 3.Xia W, Mullin RJ, Keith BR, Liu LH, Ma H, Rusnak DW, Owens G, Alligood KJ, Spector NL. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002;21(41):6255–6263. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]
- 4.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, Skarlos D, Campone M, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 5.Ritter CA, Perez-Torres M, Rinehart C, Guix M, Dugger T, Engelman JA, Arteaga CL. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res. 2007;13(16):4909–4919. doi: 10.1158/1078-0432.CCR-07-0701. [DOI] [PubMed] [Google Scholar]
- 6.Martin AP, Miller A, Emad L, Rahmani M, Walker T, Mitchell C, Hagan MP, Park MA, Yacoub A, Fisher PB, Grant S, Dent P. Lapatinib resistance in HCT116 cells is mediated by elevated MCL-1 expression and decreased BAK activation and not by ERBB receptor kinase mutation. Mol Pharmacol. 2008;74(3):807–822. doi: 10.1124/mol.108.047365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emens LA, Reilly RT, Jaffee EM. Breast cancer vaccines: maximizing cancer treatment by tapping into host immunity. Endocr Relat Cancer. 2005;12(1):1–17. doi: 10.1677/erc.1.00671. [DOI] [PubMed] [Google Scholar]
- 8.Peoples GE, Gurney JM, Hueman MT, Woll MM, Ryan GB, Storrer CE, Fisher C, Shriver CD, Ioannides CG, Ponniah S. Clinical trial results of a HER2/neu (E75) vaccine to prevent recurrence in high-risk breast cancer patients. J Clin Oncol. 2005;23(30):7536–7545. doi: 10.1200/JCO.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 9.Disis ML, Schiffman K, Guthrie K, Salazar LG, Knutson KL, Goodell V, dela Rosa C, Cheever MA. Effect of dose on immune response in patients vaccinated with an her-2/neu intracellular domain protein--based vaccine. J Clin Oncol. 2004;22(10):1916–1925. doi: 10.1200/JCO.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Murray JL, Gillogly ME, Przepiorka D, Brewer H, Ibrahim NK, Booser DJ, Hortobagyi GN, Kudelka AP, Grabstein KH, Cheever MA, Ioannides CG. Toxicity, immunogenicity, and induction of E75-specific tumor-lytic CTLs by HER-2 peptide E75 (369–377) combined with granulocyte macrophage colony-stimulating factor in HLA-A2+ patients with metastatic breast and ovarian cancer. Clin Cancer Res. 2002;8(11):3407–3418. [PubMed] [Google Scholar]
- 11.Salazar LG, Fikes J, Southwood S, Ishioka G, Knutson KL, Gooley TA, Schiffman K, Disis ML. Immunization of cancer patients with HER-2/neu-derived peptides demonstrating high-affinity binding to multiple class II alleles. Clin Cancer Res. 2003;9(15):5559–5565. [PubMed] [Google Scholar]
- 12.Disis ML, Gooley TA, Rinn K, Davis D, Piepkorn M, Cheever MA, Knutson KL, Schiffman K. Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J Clin Oncol. 2002;20(11):2624–2632. doi: 10.1200/JCO.2002.06.171. [DOI] [PubMed] [Google Scholar]
- 13.Morse MA, Hobeika A, Osada T, Niedzwiecki D, Marcom PK, Blackwell KL, Anders C, Devi GR, Lyerly HK, Clay TM. Long Term Disease-Free Survival and T Cell and Antibody Responses in Women with High-Risk HER2+ Breast Cancer Following Vaccination Against HER2+ J Transl Med. 2007;5(1):42–51. doi: 10.1186/1479-5876-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson JM. Adenoviruses as gene-delivery vehicles. N Engl J Med. 1996;334(18):1185–1187. doi: 10.1056/NEJM199605023341809. [DOI] [PubMed] [Google Scholar]
- 15.Barouch DH, Nabel GJ. Adenovirus vector-based vaccines for human immunodeficiency virus type 1. Hum Gene Ther. 2005;16(2):149–156. doi: 10.1089/hum.2005.16.149. [DOI] [PubMed] [Google Scholar]
- 16.Tatsis N, Ertl HC. Adenoviruses as vaccine vectors. Mol Ther. 2004;10(4):616–629. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kozarsky KF, Wilson JM. Gene therapy: adenovirus vectors. Curr Opin Genet Dev. 1993;3(3):499–503. doi: 10.1016/0959-437x(93)90126-a. [DOI] [PubMed] [Google Scholar]
- 18.Reyes-Sandoval A, Fitzgerald JC, Grant R, Roy S, Xiang ZQ, Li Y, Gao GP, Wilson JM, Ertl HC. Human immunodeficiency virus type 1-specific immune responses in primates upon sequential immunization with adenoviral vaccine carriers of human and simian serotypes. J Virol. 2004;78(14):7392–7399. doi: 10.1128/JVI.78.14.7392-7399.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hensley SE, Cun AS, Giles-Davis W, Li Y, Xiang Z, Lasaro MO, Williams BR, Silverman RH, Ertl HC. Type I interferon inhibits antibody responses induced by a chimpanzee adenovirus vector. Mol Ther. 2007;15(2):393–403. doi: 10.1038/sj.mt.6300024. [DOI] [PubMed] [Google Scholar]
- 20.Barouch DH, Pau MG, Custers JH, Koudstaal W, Kostense S, Havenga MJ, Truitt DM, Sumida SM, Kishko MG, Arthur JC, Korioth-Schmitz B, Newberg MH, et al. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J Immunol. 2004;172(10):6290–6297. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- 21.Di Fiore PP, Pierce JH, Kraus MH, Segatto O, King CR, Aaronson SA. erbB-2 is a potent oncogene when overexpressed in NIH/3T3 cells. Science. 1987;237(4811):178–182. doi: 10.1126/science.2885917. [DOI] [PubMed] [Google Scholar]
- 22.Hudziak RM, Schlessinger J, Ullrich A. Increased expression of the putative growth factor receptor p185HER2 causes transformation and tumorigenesis of NIH 3T3 cells. Proc Natl Acad Sci U S A. 1987;84(20):7159–7163. doi: 10.1073/pnas.84.20.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia W, Gerard CM, Liu L, Baudson NM, Ory TL, Spector NL. Combining lapatinib ( GW572016), a small molecule inhibitor of ErbB1 and ErbB2 tyrosine kinases, with therapeutic anti-ErbB2 antibodies enhances apoptosis of ErbB2-overexpressing breast cancer cells. Oncogene. 2005;24(41):6213–6221. doi: 10.1038/sj.onc.1208774. [DOI] [PubMed] [Google Scholar]
- 24.Kershaw MH, Jackson JT, Haynes NM, Teng MW, Moeller M, Hayakawa Y, Street SE, Cameron R, Tanner JE, Trapani JA, Smyth MJ, Darcy PK. Gene-engineered T cells as a superior adjuvant therapy for metastatic cancer. J Immunol. 2004;173(3):2143–2150. doi: 10.4049/jimmunol.173.3.2143. [DOI] [PubMed] [Google Scholar]
- 25.Hodges BLEH, Everett RS, Ding EY, Serra D, Amalfitano A. Adenovirus vectors with the 100K gene deleted and their potential for multiple gene therapy applications. J Virol. 2001;75:5913–5920. doi: 10.1128/JVI.75.13.5913-5920.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akiyama T, Matsuda S, Namba Y, Saito T, Toyoshima K, Yamamoto T. The transforming potential of the c-erbB-2 protein is regulated by its autophosphorylation at the carboxyl-terminal domain. Mol Cell Biol. 1991;11(2):833–842. doi: 10.1128/mcb.11.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piechocki MP, Pilon SA, Wei WZ. Quantitative measurement of anti-ErbB-2 antibody by flow cytometry and ELISA. J Immunol Methods. 2002;259(1–2):33–42. doi: 10.1016/s0022-1759(01)00487-2. [DOI] [PubMed] [Google Scholar]
- 28.Vlotides G, Siegel E, Donangelo I, Gutman S, Ren SG, Melmed S. Rat prolactinoma cell growth regulation by epidermal growth factor receptor ligands. Cancer Res. 2008;68(15):6377–6386. doi: 10.1158/0008-5472.CAN-08-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Longva KE, Pedersen NM, Haslekas C, Stang E, Madshus IH. Herceptin-induced inhibition of ErbB2 signaling involves reduced phosphorylation of Akt but not endocytic down-regulation of ErbB2. Int J Cancer. 2005;116(3):359–367. doi: 10.1002/ijc.21015. [DOI] [PubMed] [Google Scholar]
- 30.Hommelgaard AM, Lerdrup M, van Deurs B. Association with membrane protrusions makes ErbB2 an internalization-resistant receptor. Mol Biol Cell. 2004;15(4):1557–1567. doi: 10.1091/mbc.E03-08-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Austin CD, De Maziere AM, Pisacane PI, van Dijk SM, Eigenbrot C, Sliwkowski MX, Klumperman J, Scheller RH. Endocytosis and sorting of ErbB2 and the site of action of cancer therapeutics trastuzumab and geldanamycin. Mol Biol Cell. 2004;15(12):5268–5282. doi: 10.1091/mbc.E04-07-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedman LM, Rinon A, Schechter B, Lyass L, Lavi S, Bacus SS, Sela M, Yarden Y. Synergistic down-regulation of receptor tyrosine kinases by combinations of mAbs: implications for cancer immunotherapy. Proc Natl Acad Sci U S A. 2005;102(6):1915–1920. doi: 10.1073/pnas.0409610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roepstorff K, Grovdal L, Grandal M, Lerdrup M, van Deurs B. Endocytic downregulation of ErbB receptors: mechanisms and relevance in cancer. Histochem Cell Biol. 2008;129(5):563–578. doi: 10.1007/s00418-008-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirisits A, Pils D, Krainer M. Epidermal growth factor receptor degradation: an alternative view of oncogenic pathways. Int J Biochem Cell Biol. 2007;39(12):2173–2182. doi: 10.1016/j.biocel.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Zhao W, Gu YH, Song R, Qu BQ, Xu Q. Sorafenib inhibits activation of human peripheral blood T cells by targeting LCK phosphorylation. Leukemia. 2008;22(6):1226–1233. doi: 10.1038/leu.2008.58. [DOI] [PubMed] [Google Scholar]
- 36.Hipp MM, Hilf N, Walter S, Werth D, Brauer KM, Radsak MP, Weinschenk T, Singh-Jasuja H, Brossart P. Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood. 2008;111(12):5610–5620. doi: 10.1182/blood-2007-02-075945. [DOI] [PubMed] [Google Scholar]
- 37.O’Shaughnessy KLBJ, Burstein H, Storniolo AM, Sledge G, Baselga J, Koehler M, Laabs S, Florance A, Roychowdhury D. A randomized study of lapatinib alone or in combination with trastuzumab in heavily pretreated HER2+ metastatic breast cancer progressing on trastuzumab therapy. Journal of Clincal Oncology. 2008;26(May 20 suppl) abstr 1015. [Google Scholar]
- 38.Scaltriti M, Verma C, Guzman M, Jimenez J, Parra JL, Pedersen K, Smith DJ, Landolfi S, Ramon y Cajal S, Arribas J, Baselga J. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene. 2009;28(6):803–814. doi: 10.1038/onc.2008.432. [DOI] [PubMed] [Google Scholar]
- 39.Schlom J, Gulley JL, Arlen PM. Paradigm Shifts in Cancer Vaccine Therapy. Exp Biol Med (Maywood) 2008;233(5):522–534. doi: 10.3181/0708-MR-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.News in brief. Nat Rev Drug Discov. 2009;8(5):346–347. [Google Scholar]
- 41.Disis ML, Strickler JH, Wallace D, Goodell V, Salazar LG, Higgins D, Childs J, Tietje K, Dang Y, Slota M. Cellular immune parameters associated with improved long-term survival in advanced stage breast cancer patients after active immunization with a HER2-specific vaccine. J Clin Oncol. 2008;26(15S) abstr. 3015. [Google Scholar]



