Abstract
The highly conserved DRY motif located at the end of the third transmembrane of G protein-coupled receptors has been described as a key motif for several aspects of GPCR functions. However, in the case of the vertebrate gonadotropin-releasing hormone receptor (GnRHR), the amino acid in the third position in the DRY motif is variable. In the lamprey, a most basal vertebrate, the third amino acid of the “DRY” in GnRHR is His, while it is most often His/Gln in the type II GnRHR. To investigate the functional significance of the substitution of DRY to DRH in the lamprey(l)GnRHR, second messenger signaling, ligand binding and internalization of the wild-type and mutant lGnRH receptors were characterized with site-directed mutagenesis. Treatment of the DRE151 and DRS151 mutant receptors with lamprey GnRH-I significantly reduced inositol phosphate compared to wild-type (DRH151) and DRY151 receptors. The logIC50 of wild-type receptor (−9.554±0.049) was similar to the logIC50 of DRE151, DRS151 and DRX151 mutants, yet these same mutants were shown to significantly reduce cell surface expression. However, the DRY151 mutant compared to the wild-type receptor increased cell surface expression, suggesting that the reduction of IP production was due to the level of the cell surface expression of the mutant receptors. The rate of internalization of DRX151 (35.60%) was reduced compared to wild-type and other mutant receptors. These results suggest that His151 of the lamprey GnRH receptor may play a critical role in the retention of a certain level of cell-surface expression for subsequent cellular second messenger events.
Keywords: GnRH receptor, DRY motif, receptor expression, signaling, site-directed mutagenesis, lamprey
1. Introduction
The hypothalamic-pituitary (HP) system is considered to be a vertebrate innovation and seminal event that emerged prior to or during the differentiation of the ancestral agnathans, reviewed in Sower et al., 2009. In spite of the very diverse patterns of life cycles and reproductive strategies and behaviors, this endocrine system is remarkably conserved throughout the gnathostome line-ages. Lampreys as basal vertebrates are the earliest evolved vertebrates for which there are demonstrated functional roles for two possibly three gonadotropin-releasing hormones (GnRHs) that act via the hypothalamic-pituitary-gonadal axis controlling reproductive processes (Kavanaugh et al., 2008). To date, the biochemical, molecular, immunocytochemical and functional studies on the structure and function of the GnRHs in lamprey have established that similar to all other vertebrates, the lampreys have a hypothalamic-pituitary-gonadal axis and that there is a high conservation of the mechanisms of GnRH action (Sower et al., 2009). From recent data, we propose a modified paradigm in that the neuroendocrine control of reproduction and thyroid functions in an Agnathan, the sea lamprey, exhibits an overlapping, simplified organization represented by one and possibly two glycoprotein hormones putatively interacting with two glycoprotein receptors, a gonadotropin-like receptor and a thyroid stimulating hormone -like receptor (Sower et al., 2009). This modified paradigm now includes the agnathans and can serve as a model for analysis of the evolutionary mechanisms leading to emergence of the highly specialized Gnathostome endocrine axes.
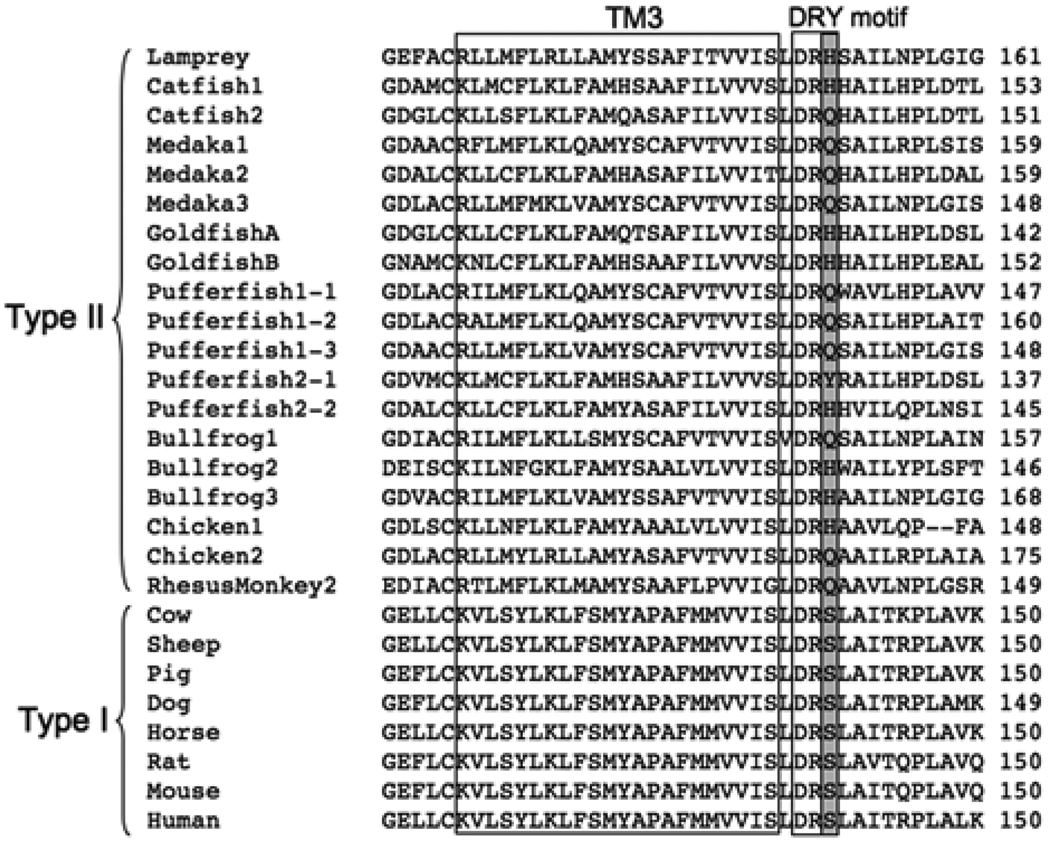
GnRH is a central regulator of reproductive function in vertebrates and acts via the hypothalamic-pituitary-gonadal axis. Its function is mediated through a pituitary GnRH receptor (GnRHR), a class A 7-transmembrane G protein-coupled receptor (GPCR). One of the remarkable characteristics of vertebrate GnRHRs is the absence or presence of the intracellular C-terminal tail. The receptors are generally categorized as two different types: type I receptor, lacking the intracellular C-terminal tail, or type II receptor, retaining the C-terminal tail (Millar et al., 2004). The other notable characteristic of GnRHRs is the variation of a DRY motif of GPCRs that is a highly conserved amino acid triplet at the end or junction of the third transmembrane domain and the second intracellular loop. DRY has been described as a key motif for several aspects of GPCR functions including receptor activation, ligand binding and G-protein coupling (Rovati et al., 2007). There are variable substitutions of the third amino acid in the “DRY” motif of GnRHRs from different classes of vertebrates. This region potentially contributes to GnRHR function. In many cases, type I receptor DRY motif is substituted with DR‘S’, while type II has DR‘H/Q’ (Fig. 1). To date, there are few reports about the functional significance of the Ser in DRS of type I receptors (Arora et al., 1997; Arora et al., 1995; Byrne et al., 1999). Thus, the functional significance of this variation of the DRY motif, particularly the type II GnRHR, is not established.
Figure 1.
Sequence alignment of the proximal region of TM3 and DRY motif in GnRHR from representative vertebrate species. The alignment was arranged with the entire amino acid sequences of the receptors based on Clustal W. The accession numbers for species are followed : Lamprey (AAQ04564), Catfish1 (O42329), Catfish2 (AAM95605), Medaka1 (NP_001098352), Medaka2 (NP_001098392), Medaka3 (NP_001098393), GoldfishA (AAD20001), GoldfishB (AAD20002), Pufferfish1-1 (BAE45695),Pufferfish1-2 (BAE45697), Pufferfish1-3 (BAE45699), Pufferfish2-1 (BAE45701), Pufferfish2-2 (BAE45704), Bullfrog1 (AAG42575), Bullfrog2 (AAG42949), Bullfrog3 (AAG42574), Chicken1(NP_989984), Chicken2 (NP_001012627), RhesusMonkey2 (NP_001028014), Cow (NP_803480), Sheep (NP_001009397), Pig (NP_999438), Dog (NP_001003121), Horse (NP_001075305), Rat (NP_112300), Mouse (NP_034453), Human (NP_000397).
In the sea lamprey, Petromyzon marinus, one of only two extant representatives of the oldest lineage of vertebrates, agnathans, a functional type II GnRH receptor was cloned from the pituitary (Silver et al., 2005). The lamprey GnRH receptor (lGnRHR-1) was shown to activate both the cAMP and IP signaling systems; however, the IP system was activated at an approximately 10 fold lower concentration to both lamprey GnRH-I and lamprey GnRH-III, and was also activated to a greater magnitude of approximately 4.5 fold, compared to ~1.7 fold (lamprey GnRH-I) or ~2.1 fold (lamprey GnRH-III) (Silver and Sower, 2006). These responses of IP3 and cAMP signaling systems are similar to the type I and type II GnRH receptors from different vertebrate species (Arora et al., 1998; Grosse et al., 2000; Liu et al., 2002; Oh et al., 2005; Stanislaus et al., 1998). In addition, the lGnRHR-1 retains conserved structural features and amino acid motifs of other known GnRH receptors. An HFRK motif in the membrane proximal region of the bullfrog type-II GnRH receptor-1 was shown to be required for cAMP signaling, but not for IP signaling (Oh da et al., 2005). Similarly a homologous motif, HVRR, was shown also to be required for cAMP signaling in the lGnRHR-1 (Silver and Sower, 2006). In the lamprey, the third amino acid residue of the DRY motif in GnRH receptor is “His” instead of “Tyr” and in the gnathostome type II GnRH receptors, the third amino acid residue is most often His/Gln instead of Tyr. These identified conserved motifs in the lamprey GnRH receptor are thought to be evolutionarily conserved throughout vertebrates. Thus, the lGnRHR-1, an ancestral form of vertebrate GnRHRs, is a critical model in our understanding of the structural and functional evolution of GnRHR. To investigate the functional significance of the substitution of DRY to DRH in the lGnRHR-1, second messenger signaling, ligand binding and internalization of the wild-type and mutant lGnRHRs were characterized with site-directed mutagenesis. The His151 of DRH was substituted with Tyrosine (DRY151 mutant), Serine (DRS151 mutant ), Glutamate (DRE151 mutant), or point deletion (DRX151 mutant).
2. Materials and methods
2.1. Site-directed mutagenesis
The point mutations were introduced into the pcDNA3.1 vectors (Invitrogen, Carlsbad, CA, USA) including the full coding sequence of lGnRHR-1 by Quikchange site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA) using mutagenic primers as follows: DRY151 mutant was amplified using the sense primer, 5’- GCTTGGACCGCTACTCGGCCATCCTC -3’ and the antisense primer, 5’- GAGGATGGCCGAGTAGCGGTCCAAGC -3’. The sense primer, 5’-GCTTGGACCGCGAGTCGGCCATCCTC -3’ and the antisense primer, 5’-GAGGATGGCCGACTCGCGGTCCAAGC -3’ were used for DRE151 mutant, the sense primer, 5’- GCTTGGACCGCTCATCGGCCATCCTC -3’ and the antisense primer, 5’- GAGGATGGCCGATGAGCGGTCCAAGC -3’ were for DRS151 mutant, and the sense primer, 5’- CAGCTTGGACCGCTCGGCCATCCTCA -3’ and the antisense primer, 5’- TGAGGATGGCCGAGCGGTCCAAGCTG -3’ were for DRX151 mutant. The underlined nucleotides indicate the sequences encoding the substituted amino acids (Fig. 2). PCR was performed with 2 ng of the pcDNA3.1 including the full coding sequence of lGnRHR-1 as the template at 95°C for 30 sec as an initial denaturation followed by 18 cycles of 95°C for 30 sec as denaturation, 55°C for 1min as annealing and 68°C for 6 min as extension. PCR product was subsequently digested by Dpn I restriction enzyme (4 U/PCR mixture) at 37°C for 1h and directly transformed into OneShot Top 10 chemically competent E.coli (Invitrogen, Carlsbad, CA, USA). The desired mutations of the insert sequences were confirmed by DNA sequencing.
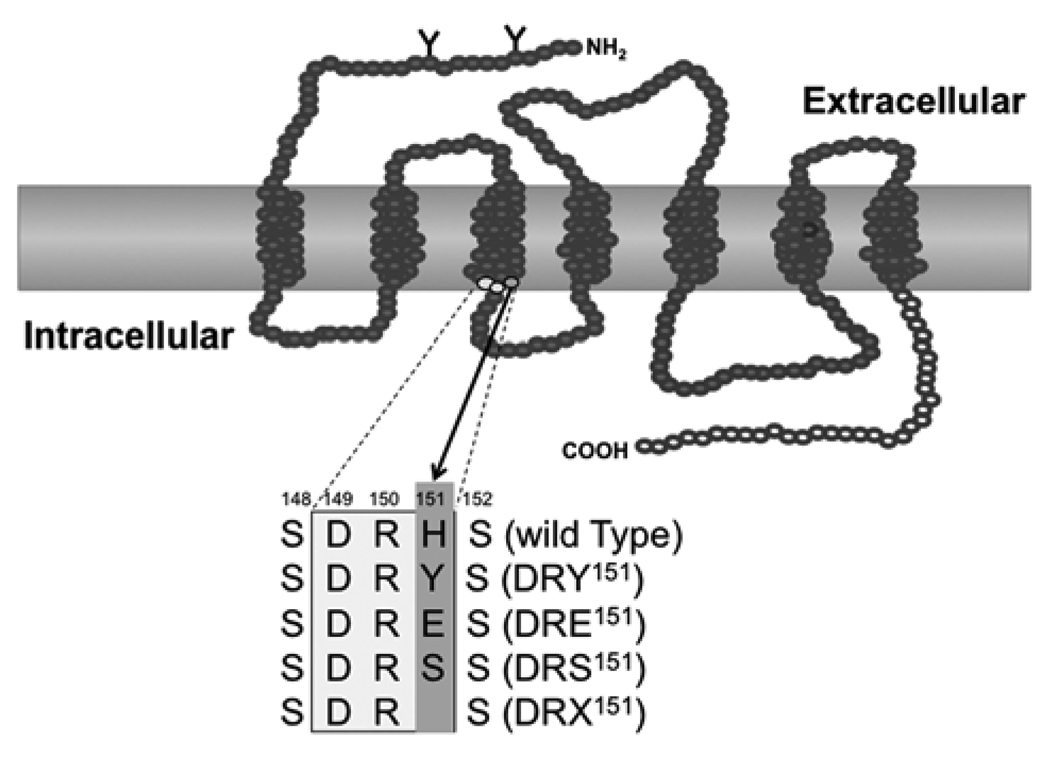
Figure 2.
A schematic diagram of the location of the point mutaions in lGnRHR-1. The rectangle shows the DRH motif and the point substitutions and deletion in lGnRHR-1 corresponding to DRY motif in GPCR. In lGnRHR-1, Tyr of DRY is substituted with His (wild-type), same as most of type II receptors, while Tyr is substituted with Ser in type I receptor. His151 in lGnRHR-1 was substituted with Tyr (DRY151), Glu (DRE151), or Ser (DRS151) or deleted (DRX151).
2.2. Cell culture and transfection
COS7 cells were maintained in 10% fetal bovine serum in DMEM (Invitrogen) at 37°C in 5% CO2 before transfection as previously described (Silver et al., 2005). Each 24 µg of construct DNA: DRH151 (wild-type), DRY151, DRE151, DRS151 and DRX151 mutants, or non-insert vector was diluted in 1.5 ml of Opti-MEM (Invitrogen) and mixed gently, while 60 µl of Lipofectamin 2000 (Invitrogen) was diluted in 1.5 ml of Opti-MEM for each construct DNA and incubated for 5 min at room temperature. After the 5 min incubation, the diluted DNA and the diluted Lipofectamine were combined, mixed gently and incubated for 20 min at room temperature. After the 20 min incubation, each DNA/Lipofectamine/Opti-MEM complex was added into 2.2 × 106 cells cultured with 10% fetal bovine serum in DMEM in 10-cm culture plate and incubated for 24 h at 37°C in 5 % CO2.
2.3. Inositol phosphate assay
The inositol phosphate assay (IP assay) was performed as previously described (Silver et al., 2005). Transfected cells were trypsinized and seeded in 12-well plates (1.0 × 105 cells per well) and cultured in 10% fetal bovine serum in DMEM at 37°C in 5% CO2. Briefly, after the adhesion of the cells (overnight culture), cells were washed with PBS and incubated in 1 ml of Medium 199 (Invitrogen) containing 2% fetal bovine serum and 2µCi Myo [2-3H]inositol (Amersham Pharmacia, Buckinghamshire, UK) per well for 18h. After the 18h incubation, cells were washed and pre-incubated. After pre-incubation, cells were incubated in 1 ml of IP buffer containing concentrations of lGnRH-III (10−6 to 10−14M) per well. The stimulations were stopped and the plates were incubated on ice for 30 min. The extracts from the cells were transferred, neutralized with 5M KOH and 20% perchloric acid and incubated. The supernatant of the extracts were transferred after centrifugation at 5000 rpm at 4°C. Total inositol phosphate was isolated from the extracts by ion exchange chromatography with AG1×8 resin (BioRad, Hercules, CA, USA) and measured. Treatments with each concentration of lGnRH-III were run in duplicate and the data were fitted with sigmoidal dose-response curves by Prism 4 (GraphPad, San Diego, CA, USA).
2.4. Receptor binding assessment
The ligand-binding assay was performed as previously described (Silver and Sower, 2006). Briefly, transfected cells were seeded and after two days, cells were washed with assay buffer (25 mM HEPES-modifed DMEM with 0.1% BSA) and then incubated with assay buffer containing 1 nM 125I-labeled lamprey GnRH-I and decreasing concentrations of unlabeled lGnRH-III (10−6 to 10−12M) for 3.5 h on ice at 4°C. After incubation, cells were washed and solubilized. All total binding and NSB samples were run in triplicate, and each independent experiment was repeated at least three times. The data were fitted with one-site competition binding curves by Prism 4 (GraphPad).
2.5 Receptor-internalization assay
Transfected cells were seeded in 24-well plates at 1.5 × 105 cells per well and maintained for 48 h as previously described (Silver and Sower, 2006). After 48 h, cells were incubated with 1 nM 125I-labeled lamprey GnRH-I at 4°C for 3.5 h and then incubated at 37°C for various periods of time. Subsequently, cells were washed twice for 20 min to remove surface bound 125I-labeled lamprey GnRH-I (Silver and Sower, 2006). Acid resistant (internalized) ligand was solubilized in 0.5 M NaOH. Nonspecific binding was determined by cells transfected with non-insert vector or non-transfected cells at each time point. Surface bound and internalized ligands were counted by γ-counter. The treatments were run in triplicate. The data were fitted with one phase exponential association curves by Prism 4 (GraphPad).
3. Results
3.1. IP response of the DRY151, DRS151, DRE151 mutants, or wild type (DRH151)
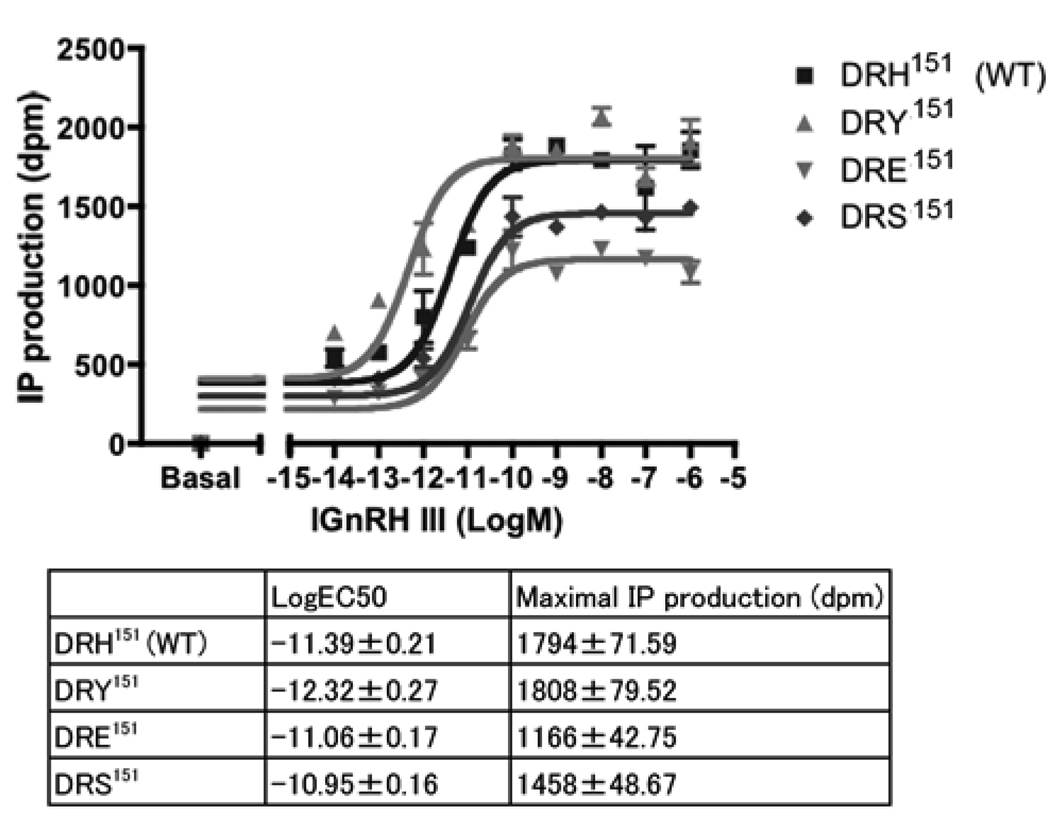
All mutant receptors showed dose-response effects to lGnRH-III from 10−6 to 10−14M and with different Log EC50 values (Fig. 3). Log EC50 (mean ± SEM) of DRE151 mutant (−11.06 ± 0.17) and DRS151 mutant (−10.95 ± 0.16) were higher than that of wild-type (−11.39 ± 0.21), while maximal IP production (mean ± SEM) of DRE151 mutant (1166 ± 42.75) and DRS151 mutant (1458 ± 48.67) was lower than that of wild-type (1794 ± 71.59). On the other hand, log EC50 of DRY151 mutant (−12.32 ± 0.27) was lower than that of wild-type, while the maximal IP production of DRY151 mutant (1808 ± 79.52) was comparable to that of wild-type.
Figure 3.
IP response of wild-type and mutant lGnRHRs. DRH151 (wild-type), DRY151, DRE151 and DRS151 showed different dose-response curves responding to lGnRH-III. The values of IP productions were shown as dpm. The data are mean ± SEM of duplicate in a representative experiment. LogEC50 of DRE151 and DRS151 were higher than DRH151 (wild-type) while the maximal IP productions were lower than DRH151 (wild-type). LogEC50 of DRY151 was lower than DRH151 (wild-type) while the maximal IP production was similar to DRH151 (wild-type).
3.2. Ligand binding of the DRY151, DRS151, DRE151, DRX151 mutants, or wild type
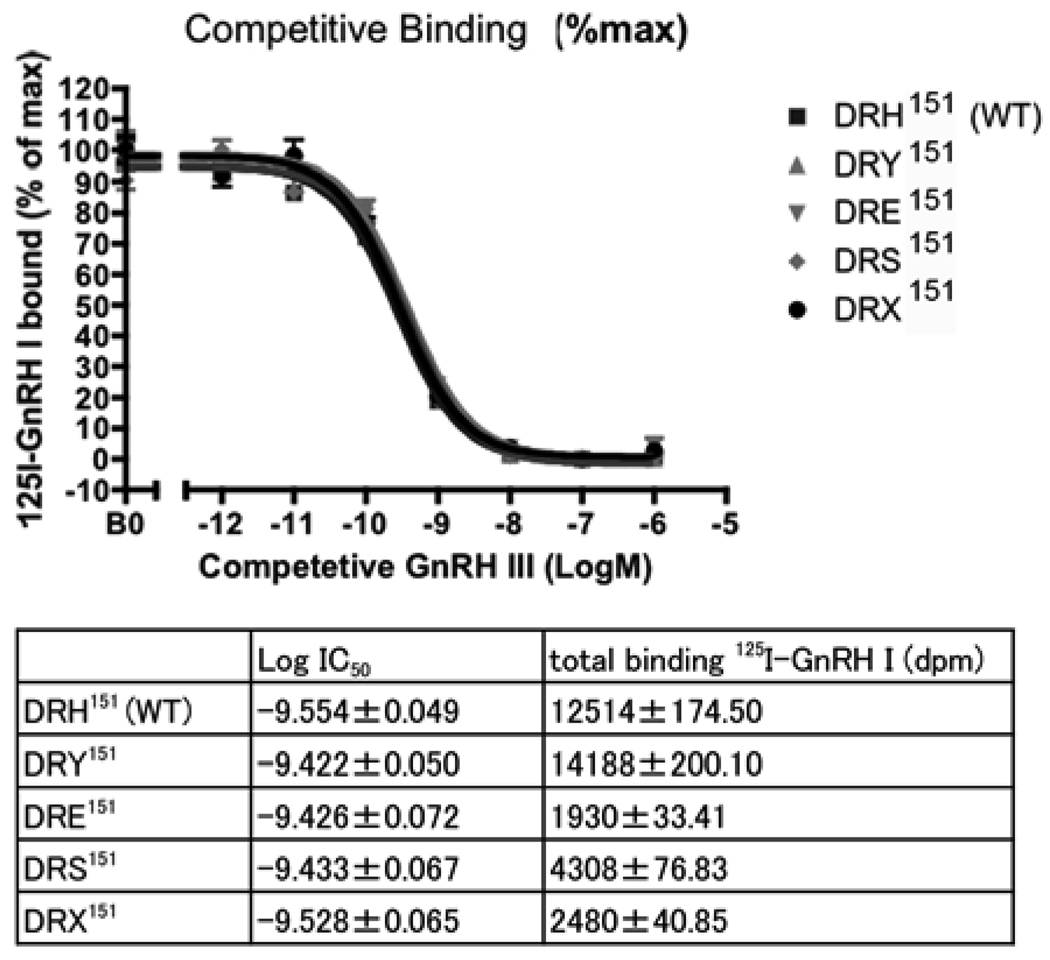
Ligand-binding affinity between all receptors was similar (Fig. 4). The logIC50 of wild-type receptor (−9.554±0.049) was similar to the DRY151 mutant (−9.422±0.050), DRE151 mutant (−9.426±0.072), DRS151 mutant (−9.433±0.067) and DRX151 mutant (−9.528±0.065). However, 125I-lGnRH-I binding varied between wild-type and mutant receptors. The level of the specific binding (%WT) of DRY151 mutant was significantly higher (164%) than WT, and DRE151 (12%), DRS151 (29%) and DRX151 (14%) mutants were significantly lower than WT (Fig 5).
Figure 4.
Ligand binding of wild-type and mutant lGnRHRs. Competitive binding assays of 125I-lGnRHR-1 were performed with decreasing concentrations of lGnRH III. The values of radioligand binding in the graph were shown as the percentages of maximum 125I-lGnRH binding (mean ± SEM in triplicate). The data represents one of three independent experiments with essentially same results. Log IC50 of wild-type and mutant receptors were similar but total bindings of 125I-lGnRH were varied
Figure 5.
Receptor expression of wild-type and mutant lGnRHRs. The data are shown as the percentages of wild-type binding to 125I-lGnRH (1 nM) and each bar represents mean ± SEM of three independent experiments in triplicate. One-way ANOVA was performed with Bonferroni post hoc test (a, P < 0.001 vs WT ; b, P < 0.001 vs DRY )
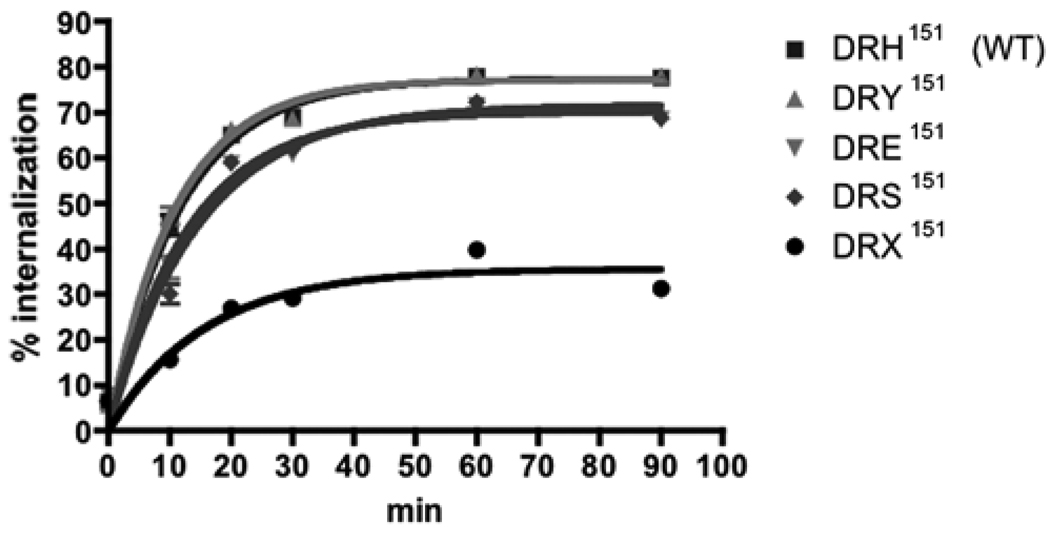
3.3. Wild-type and mutant lGnRHRs internalization
To investigate the effects of His151 point mutations of lGnRHR-1 on endocytotic trafficking in ligand-dependent manner, internalization assays were performed with 125I-lGnRH-I. Wild-type and mutant receptors were rapidly internalized (t1/2 = 7.5–11 min) and each reached a plateau within 60 min. Y max of mutant receptors (DRY151 = 77.19%, DRE151 = 70.13%, DRE151 = 71.65%) except for DRX151 was similar to that of the wild-type receptor (77.36%) (Fig. 6). The rate of internalization of DRX151 (35.60%) was reduced compared with wild-type and the other mutant receptors.
Figure 6.
Internalization of wild-type and mutant lGnRHRs. Percent internalization was expressed as the ratio of internalized radioligand to the total cell-surface radioligand at each time point. Data are shown as mean ± SEM in triplicate, representing at least two independent experiments.
4. Discussion
DRY is a highly conserved amino acid triplet of GPCRs and has been described as a key motif for several functions of GPCR (Rovati et al., 2007). It has been shown that the critical residues for receptor functions of the DRY motif of GPCRs are the first two residues, Asp and Arg, rather than the third residue, Tyr (Flanagan, 2005; Rovati et al., 2007). The GnRH receptor is a class A rhodopsin-like GPCR and one of the receptors which has been characterized physiologically and pharmacologically in mammals and also some nonmammalian vertebrates because of its key role in reproductive function through vertebrates. The third amino acid residue in the DRY motif is variable in the vertebrate gonadotropin-releasing hormone receptor. In the lamprey, a most basal vertebrate, the third amino acid residue of the DRY motif in the GnRH receptor is “His” instead of “Tyr”. In type II GnRH receptors, the third amino acid residue is most often His/Gln instead of Tyr. This is the first study that has examined the third amino acid substitution in the DRY/H/Q of any type II GnRH receptors. Treatment of the DRE151 and DRS151 mutant receptors with lamprey GnRH-I significantly reduced inositol phosphate compared to wild-type (DRH151) and DRY151 receptors. Subsequent studies using ligand binding and receptor expression were analyzed with wild-type and mutant receptors. The binding affinity using log IC50 was similar between wild-type and mutant receptors, which indicates that ligand binding affinity didn’t influence the change of IP response. However, the level of cell-surface expression of the receptors labeled with 125I-GnRH-I was different between wild-type and mutant receptors. Since the competitive binding assays showed that the ligand binding affinity between all mutant and wild-type receptors was not significantly different, the total binding of 125I-GnRH-I to the wild-type and mutant lGnRHRs could be directly compared as the receptor expression level. DRE151, DRS151 and DRX151 mutants significantly reduced cell surface expression while DRY151 mutant increased cell surface expression when compared to the wild-type receptor, suggesting that the reduction of IP production was due to the level of the cell surface expression of the mutant receptors. In addition, the level of internalization was similar between wild-type and mutant receptors except for the DRX151 mutant. The DRX151 mutant induced a decrease in internalization as well as a reduced cell surface expression, suggesting ligand-independent receptor expression, i.e. such as receptor trafficking from the endoplasmic reticulum (ER).
In general, point mutations lead to misfolding that can affect the cell surface expression of proteins involving GPCRs (Duvernay et al., 2005; Garriga et al., 1996; Sitia and Braakman, 2003). It has been described that most of the point mutations in GnRH receptor lead to a decrease in receptor expression including the natural mutations which are linked to human disease, hypogonadotropic hypogonadism (Conn et al., 2007; Millar et al., 2004; Rispoli and Nett, 2005). According to several reports from several types of GPCR, Asp and Arg of DRY are critical for cell surface expression due to the structural instability, impairment of the association with β-arrestin and internalization (Alewijnse et al., 2000; Hawtin, 2005; Wilbanks et al., 2002). Additionally, pharmacoperone, which rescues misfolded mutant proteins to correctly route in the cells, was shown to function in an Arg mutant of the V2 vasopressin receptor suggesting that DRY is important for folding and cell surface trafficking from the ER (Conn et al., 2007). The significance of Tyr residue of DRY was unclear from this vasopression receptor study. In the present study, the lamprey GnRHR and three of the four mutants of lamprey GnRHR all induced a decrease in receptor expression except the mutant DRY151 which induced an increase in receptor expression. Other studies on the DRS motif in mammalian type I GnRHRs revealed that Ser could be involved in ligand binding and receptor internalization events (Arora et al., 1997; Arora et al., 1995; Byrne et al., 1999). Substituting Ser with Tyr increased both the ligand binding affinity and internalization (Arora et al., 1995) but substituting with Ala had no effect (Arora et al., 1997). In our study, substituting His with Tyr increased the cell-surface expression of the receptor but had no effect on ligand binding affinity and did not increase internalization. Thus, mammalian type I and lamprey type II GnRH receptors can both accept the substitution of Tyr and retain the functionality of the original residues which imply Tyr could be primordial through vertebrate GnRHRs, although the specific functions are different between the two types of receptors. These differences between the mammalian and lamprey GnRH receptors may represent a different functionality between type I and type II receptors, which are representative of two evolutionarily distant receptors. For instance, there is not an intracellular c-terminal tail in type I receptor so that the receptor cannot be rapidly internalized. This phenomenon implies that different mechanisms of desensitization in the mammalian type I GnRH receptors compared to the type II GnRH receptors have evolved over the course of the evolution of vertebrates (McArdle et al., 1999; McArdle et al., 2002). The rate of internalization of type II GnRH receptors is much greater compared to type I GnRH receptors suggesting the c-terminal tail is essential for the rapid internalization (Pawson et al., 1998), and seems to be maximized at the biological threshold. This suggests that the type II GnRH receptor mutants may not be capable of augmenting internationalization. In addition, Byrne et al (1999) suggested that the third amino acid position in DRY and the phosphorylation in the site are not essential for internalization. This would support the result of our study that the rate of internalization of the DRE151, DRS151 and DRY151 mutants was similar to that of wild-type because the c-terminal tail was more essential for internalization, although DRX151, point deletion of His151 deletion in DRH, decreased the rate of internalization compared to other mutants and the wild-type receptors. DRX151 mutant possesses Ser152 next to Arg150 (Fig. 2) so that DRX151 could compensate for the lack of His151 with Ser152 and maintain the same amino acid triplet as DRS151. However, the rate of internalization of DRX151 was different from DRS151, suggesting that the conformational change of the receptor caused by His151 might be linked to the internalization event.
The third amino acid of the DRY motif is highly conserved among most of GPCRs but not in the GnRH receptors. Although there are some exceptions in that DRY is found in a few vertebrate and invertebrate GnRH receptors (Hauser et al., 1998; Ikemoto and Park, 2005). Lampreys have two other GnRH receptors that have not yet been published. One of the novel lamprey GnRH receptors (lGnRHR-3, accession number DQ915103) has DRY instead of DRH. The experimental evidence from the current study shows that substitution of DRH with DRY maintains the receptor functions. In examining this motif across invertebrate and vertebrate GnRH receptors, it is suggested that DRY could be an ancestral motif of an ancestral GnRHR since it is also highly conserved in the other types of GPCRs. This motif in GnRHR receptors has perhaps been subjected to selection pressures leading to variable amino acid residues including S, H or Q in the third position of DRY motif during the course of GnRHR molecular evolution.
In summary, the mutation of His151 of the lamprey GnRH receptor altered the cell-surface receptor expression as well as second messenger signaling and internalization. These results suggest that His151 of the lamprey GnRH receptor may play a critical role in the retention of a certain level of cell-surface expression for subsequent cellular second messenger events. Further studies of the lamprey GnRH receptor(s) that have retained key ancestral residues and motifs will be necessary in furthering our understanding of the evolution of the structure and function of the GnRH receptor in vertebrates.
Acknowledgements
This research was supported by a grant of NIH [5R21RR024477-02]. This is Scientific Contribution Number 2386 from the New Hampshire Agricultural Experiment Station. We thank Bernadine Schultz, Mike Wilmot and Allisan Aquilina-Beck for their assistance with iodination. We also thank Dr. Mihael Freamat and Scott I. Kavanaugh for their useful advice and suggestions on the experimental techniques.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alewijnse AE, Timmerman H, Jacobs EH, Smit MJ, Roovers E, Cotecchia S, Leurs R. The effect of mutations in the DRY motif on the constitutive activity and structural instability of the histamine H(2) receptor. Mol Pharmacol. 2000;57:890–898. [PubMed] [Google Scholar]
- Arora KK, Cheng Z, Catt KJ. Mutations of the conserved DRS motif in the second intracellular loop of the gonadotropin-releasing hormone receptor affect expression, activation, and internalization. Mol Endocrinol. 1997;11:1203–1212. doi: 10.1210/mend.11.9.9968. [DOI] [PubMed] [Google Scholar]
- Arora KK, Krsmanovic LZ, Mores N, O'Farrell H, Catt KJ. Mediation of cyclic AMP signaling by the first intracellular loop of the gonadotropin-releasing hormone receptor. J Biol Chem. 1998;273:25581–25586. doi: 10.1074/jbc.273.40.25581. [DOI] [PubMed] [Google Scholar]
- Arora KK, Sakai A, Catt KJ. Effects of second intracellular loop mutations on signal transduction and internalization of the gonadotropin-releasing hormone receptor. J Biol Chem. 1995;270:22820–22826. doi: 10.1074/jbc.270.39.22820. [DOI] [PubMed] [Google Scholar]
- Byrne B, McGregor A, Taylor PL, Sellar R, Rodger FE, Fraser HM, Eidne KA. Isolation and characterisation of the marmoset gonadotrophin releasing hormone receptor: Ser(140) of the DRS motif is substituted by Phe. J Endocrinol. 1999;163:447–456. doi: 10.1677/joe.0.1630447. [DOI] [PubMed] [Google Scholar]
- Conn PM, Ulloa-Aguirre A, Ito J, Janovick JA. G protein-coupled receptor trafficking in health and disease: lessons learned to prepare for therapeutic mutant rescue in vivo. Pharmacol Rev. 2007;59:225–250. doi: 10.1124/pr.59.3.2. [DOI] [PubMed] [Google Scholar]
- Duvernay MT, Filipeanu CM, Wu G. The regulatory mechanisms of export trafficking of G protein-coupled receptors. Cell Signal. 2005;17:1457–1465. doi: 10.1016/j.cellsig.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Flanagan CA. A GPCR that is not DRY. Mol Pharacol. 2005;68:1–3. doi: 10.1124/mol.105.014183. [DOI] [PubMed] [Google Scholar]
- Garriga P, Liu X, Khorana H. Structure and function in rhodopsin: Correct folding and misfolding in point mutants at and in proximity to the site of the retinitis pigmentosa mutation Leu-125 Arg in the transmembrane helix C. Proc. Natl. Acad. Sci. U S A. 1996:4560–4564. doi: 10.1073/pnas.93.10.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse R, Schmid A, Schoneberg T, Herrlich A, Muhn P, Schultz G, Gudermann T. Gonadotropin-releasing hormone receptor initiates multiple signaling pathways by exclusively coupling to G(q/11) proteins. J Biol Chem. 2000;275:9193–9200. doi: 10.1074/jbc.275.13.9193. [DOI] [PubMed] [Google Scholar]
- Hauser F, Sondergaard L, Grimmelikhuijzen CJ. Molecular cloning, genomic organization and developmental regulation of a novel receptor from Drosophila melanogaster structurally related to gonadotropin-releasing hormone receptors for vertebrates. Biochem Biophys Res Commun. 1998;249:822–828. doi: 10.1006/bbrc.1998.9230. [DOI] [PubMed] [Google Scholar]
- Hawtin SR. Charged Residues of the Conserved DRY Triplet of the Vasopressin V1a Receptor Provide Molecular Determinants for Cell Surface Delivery and Internalization. Mol Pharmacol. 2005;68:1172–1182. doi: 10.1124/mol.105.013359. [DOI] [PubMed] [Google Scholar]
- Ikemoto T, Park MK. Identification and molecular characterization of three GnRH ligands and five GnRH receptors in the spotted green pufferfish. Mol Cell Endocrinol. 2005;242:67–79. doi: 10.1016/j.mce.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Kavanaugh SI, Nozaki M, Sower SA. Origins of gonadotropin-releasing hormone (GnRH) in vertebrates: identification of a novel GnRH in a basal vertebrate, the sea lamprey. Endocrinology. 2008;149:3860–3869. doi: 10.1210/en.2008-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Usui I, Evans LG, Austin DA, Mellon PL, Olefsky JM, Webster NJ. Involvement of both G(q/11) and G(s) proteins in gonadotropin-releasing hormone receptor-mediated signaling in L beta T2 cells. J Biol Chem. 2002;277:32099–32108. doi: 10.1074/jbc.M203639200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle CA, Davidson JS, Willars GB. The tail of the gonadotrophin-releasing hormone receptor: desensitization at, and distal to, G protein-coupled receptors. Mol Cell Endocrinol. 1999;151:129–136. doi: 10.1016/s0303-7207(99)00024-6. [DOI] [PubMed] [Google Scholar]
- McArdle CA, Franklin J, Green L, Hislop JN. Signalling, cycling and desensitisation of gonadotrophin-releasing hormone receptors. J Endocrinol. 2002;173:1–11. doi: 10.1677/joe.0.1730001. [DOI] [PubMed] [Google Scholar]
- Millar RP, Lu ZL, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR. Gonadotropin-releasing hormone receptors. Endocr Rev. 2004;25:235–275. doi: 10.1210/er.2003-0002. [DOI] [PubMed] [Google Scholar]
- Oh DY, Song JA, Moon JS, Moon MJ, Kim JI, Kim K, Kwon HB, Seong JY. Membrane-proximal region of the carboxyl terminus of the gonadotropin-releasing hormone receptor (GnRHR) confers differential signal transduction between mammalian and nonmammalian GnRHRs. Mol Endocrinol. 2005;19:722–731. doi: 10.1210/me.2004-0220. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Katz A, Sun YM, Lopes J, Illing N, Millar RP, Davidson JS. Contrasting internalization kinetics of human and chicken gonadotropin-releasing hormone receptors mediated by c-ternminal tail. J. Endocrinol. 1998;156:R9–R12. doi: 10.1677/joe.0.156r009. [DOI] [PubMed] [Google Scholar]
- Rispoli LA, Nett TM. Pituitary gonadotropin-releasing hormone (GnRH) receptor: structure, distribution and regulation of expression. Anim Reprod Sci. 2005;88:57–74. doi: 10.1016/j.anireprosci.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Rovati GE, Capra V, Neubig RR. The highly conserved DRY motif of class A G protein-coupled receptors: beyond the ground state. Mol Pharmacol. 2007;71:959–964. doi: 10.1124/mol.106.029470. [DOI] [PubMed] [Google Scholar]
- Silver MR, Nucci NV, Root AR, Reed KL, Sower SA. Cloning and characterization of a functional type II gonadotropin-releasing hormone receptor with a lengthy carboxy-terminal tail from an ancestral vertebrate, the sea lamprey. Endocrinology. 2005;146:3351–3361. doi: 10.1210/en.2005-0305. [DOI] [PubMed] [Google Scholar]
- Silver MR, Sower SA. Functional characterization and kinetic studies of an ancestral lamprey GnRH-III selective type II GnRH receptor from the sea lamprey, Petromyzon marinus. J Mol Endocrinol. 2006;36:601–610. doi: 10.1677/jme.1.02005. [DOI] [PubMed] [Google Scholar]
- Sitia R, Braakman I. Quality control in the endoplasmic reticulum protein factory. Nature. 2003;426:891–894. doi: 10.1038/nature02262. [DOI] [PubMed] [Google Scholar]
- Sower SA, Freamat M, Kavanaugh SI. The Origins of the Vertebrate Hypothalamic-Pituitary-Gonadal (HPG) & Hypothalamic-Pituitary Thyroid (HPT) Endocrine Systems: New Insights From Lampreys. General Comparative Endocrinology. 2009;161:16120–16129. doi: 10.1016/j.ygcen.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Stanislaus D, Ponder S, Ji TH, Conn PM. Gonadotropin-releasing hormone receptor couples to multiple G proteins in rat gonadotrophs and in GGH3 cells: evidence from palmitoylation and overexpression of G proteins. Biol Reprod. 1998;59:579–586. doi: 10.1095/biolreprod59.3.579. [DOI] [PubMed] [Google Scholar]
- Wilbanks AM, Laporte LM, Bohn LM, Barak LS, Caron MG. Apparent Loss-of-Function Mutant GPCRs Revealed as Constitutively Desensitized Receptors. Biochemistry. 2002;41:11981–11989. doi: 10.1021/bi020275m. [DOI] [PubMed] [Google Scholar]