Abstract
The plasma membrane is a major resource for production of bioactive lipids and contains a large proportion of the cellular sphingomyelin (SM) content. Consequently, the regulation of SM levels at the plasma membrane by enzymes such as sphingomyelinase (SMase) and SM synthase 2 (SMS2) can have profound effects – both on biophysical properties of the membrane, but also on cellular signaling. Over the past twenty years, there has been considerable research into the physiological and cellular functions associated with regulation of SM levels, notably with regards to the production of ceramide. In this review, we will summarize this research with particular focus on the SMases and SMS2. We will outline what biological functions are associated with SM metabolism/production at the PM, and discuss what we believe are major challenges that need to be addressed in future studies.
Keywords: sphingolipids, plasma membrane, sphingomyelinase, sphingomyelin synthase
Introduction
The plasma membrane (PM) of the cell has the important function of separating the inner cellular environment from the extracellular space. However, it also has a crucial role in communication between cells and their environments, being involved in processes such as subcellular trafficking, signal transduction, and metabolite exchange. Like all membranes, the PM is comprised of a lipid bilayer with a hydrophobic core and hydrophilic surfaces – in this case, an extracellular surface and a cytosolic surface. Although conceived primarily (but not exclusively) as a single homogeneous domain in the classic fluid mosaic model [1], the PM is now thought to contain membrane microdomains termed ‘lipid rafts’. Comprised of laterally segregated sphingomyelin (SM) and cholesterol tightly packed into liquid-ordered domains that exist in the surrounding ‘liquid disordered’ phase, it has been suggested that rafts have an important role in signal transduction and other cellular processes. This is discussed in more depth in the following reviews [2,3]. However, despite the abundance of literature investigating rafts, their existence and functional roles remain an issue of heated discussion.
As in most organelles, there is also an asymmetry within the PM lipid bilayer such that the compositions of the extracellular and cytosolic leaflets are significantly different. The outer leaflet is reported to be enriched in phosphatidylcholine (PC), SM and glycosphingolipids whereas the inner leaflet is abundant in phosphoinositides, phosphatidylserine (PS), phosphatidylethanolamine and phosphatidic acid [4,5]. Classically, the PM cholesterol is thought to be portioned relatively evenly between the two layers [5]; however, a recent study suggests the preponderance of cholesterol localizes to the inner leaflet [6]. Thus, with such distinct lipid environments, it is not difficult to imagine that metabolism of lipids within each leaflet may produce distinct physiological outcomes.
The PM contains a large portion of the cellular SM [7]. Not only is it a component of lipid rafts, but SM can be metabolized to ceramide, a bioactive lipid in its own right, but also a precursor molecule to other signaling lipids and a central hub of the sphingolipid network (reviewed in [8]). Conversely, the production of SM from ceramide and PC by the enzyme SM synthase can serve to regulate signal transduction through reducing ceramide levels, but also increasing levels of DAG and SM. Thus, both sphingomyelinase (SMases) and SM synthases can act as signaling switches at the PM. Moreover, as SM and ceramide have differing properties, the metabolism of SM to ceramide or production of SM from ceramide can consequently also have important biophysical effects on the membrane itself. Here, we will outline the current literature related to SM metabolism at the PM with particular focus on the sphingomyelinases and SM synthase 2. In addition, we will review the biological functions associated with SM metabolism/production at the PM, and will briefly discuss the major challenges to be addressed in the future.
Bacterial Sphingomyelinase
Many pathogenic bacteria produce proteins with neutral SMase activity despite the fact that they are unable to synthesize sphingolipids themselves. The bacterial SMases (bSMases) are secretory proteins and are part of a larger family of phospholipases released into the environment where they are able to utililize host cell lipids to exert cytotoxic effects [9]. Importantly, the identification and purification of bSMases provided the sphingolipid field with useful tools with which to study the effects of endogenous ceramide generation through exogenous addition of bSMases to cell culture, a practice still in use today. However, it should be cautioned that the effects of exogenous bSMases on PM lipids can extend beyond SM, causing hydrolysis of other membrane lipids such as PC. Thus, these effects should be taken into account when interpreting results.
Properties
Currently, bSMase genes have been identified from a number of bacteria such as B. cereus, S. aureus, L. ivanovii, Le. interrogans and the Pseudomonas sp. Strain TK4. In addition, bSMase activities have been characterized from H. pylori and M. tuberculosis (see [10] and references therein). It should also be noted that a number of other members of the bacterial phospholipase family often possess both phospholipase C (PLC) and SMase activities such as PlcHR from P. aerugonosa [11], α-toxin from C. perfringens [12], and PlcB from L. monocytogenes [13]. Many of the identified bacterial SMases share common properties with molecular masses of between 33-38kD although there are exceptions such as the bSMases from TK4 (58kD) and Le. interrogans (63kD) (see [10]). The SMase activity of all these proteins has neutral pH optima and is dependent on divalent cations such as Mg2+ and Mn2+, whereas Ca2+ and Sr2+ are both ineffective for hydrolysis [14]. Based on the homology of the active site residues, the bSMases were classified as members of the DNAse 1 superfamily and this was subsequently confirmed with the recent solving of the crystal structures of bSMases from B. cereus, L. ivanovii and beta-toxin from S. aureus [14-16]. These structures also shed light on the catalytic mechanisms, and it was proposed that SM hydrolysis proceeds by acid base catalysis through a pentavalent phosphorus transition state. For a more detailed description of the proposed mechanism, the reader is referred to [14]. Notably, the conserved catalytic site residues throughout many of the N-SMases in higher organisms suggest a common catalytic mechanism. Finally, bSMases from B. cereus, S. aureus and L. ivanovii all contain a hydrophobic β hairpin structure that is necessary for interaction of the bSMases with cell membranes and liposomes and, presumably, functions to bring the SMase into close proximity to its substrate on the outer membrane [14]. However, it should also be noted that this region is not wholly conserved in the bSMases from Le. Interrogans, the Pseudomonas sp. strain TK4 or S. epidermis which suggests that there are additional mechanisms for bSMase interaction with the membrane [15,16].
Bacterial SMase and Hemolysis
The best characterized action of bSMases is their ability to induce hemolysis – the breaking open of red blood cells with subsequent release of hemoglobin into the surrounding fluid. Thus far, hemolytic activity has been reported for all known bSMases [9,10] and many studies have observed that the susceptibility of erythrocytes to undergo hemolysis correlates with their cellular SM content i.e. sheep erythrocytes (∼50% SM) readily undergo bSMase-induced hemolysis whereas, for example, horse erythrocytes (∼10% SM) are much more resistant [9]. In addition, kinetic analysis of the bSMase from S. schleiferi found that the SMase activities and hemolytic activities were closely related and showed identical Michaelis-Mentin kinetics [17]. Thus, it was inferred that SMase activity plays a crucial role in hemolysis. Subsequent confirmation of this came from genetic studies of the beta toxin from S. aureus where it was found that mutation of key catalytic residues abolished SMase activity, and prevented all hemolytic activity [16]. Moreover, similar studies in α-toxin from C. perfringens and PlcHR2 from P. aeruginosa, proteins that possesses both PLC and SMase activities, have found that the SMase activities of these enzymes are more crucial for hemolysis than the PLC activities [12,18]. Taken together, these results suggest that bSMase-mediated hydrolysis of SM at the plasma membrane is crucial for their hemolytic activity.
It is well established that the sensitivity of erythrocytes to hemolysis to certain agents increases when cells are cooled to 4°C following incubation with the hemolytic agents at 37°C [18] although the mechanism underlying this phenomenon termed ‘hot-cold hemolysis’ has remained unclear. Given the crucial role of SMase activity in hemolysis as described above, it was suggested that SM metabolism at the membrane is important for hot-cold hemolysis. Indeed, an early study postulated that such SM hydrolysis generated ‘fragile’ erythrocytes so that, on cooling, the altered membrane properties caused sufficient stress to result in the lysis [19]. More recent studies have begun to probe this question further. A recent study that analyzed erythrocyte ghost membranes following hemolysis induced by PlcHR2 from P. aeruginosa and found the presence of large ceramide-rich domains; importantly, these domains were fluid at 37°C but rigid at 4°C [18]. Moreover, ceramidase treatment reduced the presence of these domains and, consequently, reduced hot-cold hemolysis induced by PlcHR2 [18]. Taken together, these results indicate that the formation of ceramide-rich domains by a bacterial SMase activity plays an important role in the phenomenon of hot-cold hemolysis.
Bacterial SMase and other mammalian cell types
In addition to their deleterious effects on erythrocytes, studies have reported that bSMases are also cytotoxic to other mammalian cell types albeit to varying degrees. An early study found that bSMases from S. aureus, B. cereus, and Streptomyces Sp. were selectively cytotoxic to human monocytes yet had little effects on viability of human granulocytes, fibroblasts, or lymphocytes despite being able to degrade SM efficiently in these cells [20]. In contrast, studies with Sph2 from L. interrogans reported cytotoxic effects on mouse lymphocytes, mouse macrophages, and human liver cells in addition to its hemolytic properties [21] while a more recent study with SMase (β-toxin) from S. aureus reported cytoxicity against proliferating T-cells; importantly, both hemolysis and leukotoxicity were dependent on enzymatic activity [16]. Moreover, PlcHr2 from P. aeruginosa, which is both a SMase and PC-PLC, was reported to be selectively cytotoxic to endothelial cells yet was weakly cytotoxic to HeLa and A549 cells [22]. Notably, the cytotoxic effect of PlcHr2 on endothelial cells resulted in suppression of angiogenesis; however, it was not clear which enzymatic activities were important for this effect [11]. In contrast to this, beta-hemoylsin from S. aureus was found to suppress production of interleukin-8 from endothelial cells, but did not have effects on cell viability; with regards to the former effect, this was postulated to be a result of SM hydrolysis as exogenous ceramide addition produced comparable effects [23]. Taken together, these data suggest that bSMase-mediated hydrolysis of SM at the PM can have adverse effects on a number of mammalian cell types. However, it should be emphasized that this is not limited to cytotoxic effects.
Genetic deletion of bSMase
In addition to the studies outlined above, further evidence for the important role of SMases in bacterial virulence has come from studies utilizing knockout strains. For L. ivanovii, genetic knockout of its SMase, SmcL resulted in a notably weaker hemolysis on blood agar. More importantly, the knockout strains displayed a marked decrease in virulence compared to wild-type strains when injected into mice [13]. Moreover, intracellular proliferation of the knockout strain in MDCK cells was markedly reduced, and it was found that SmcL played an important role in allowing the bacteria to enter the cytoplasm though disruption of the phagosomal compartment [13]. This important role of SmcL in L. ivanovii was further underscored when it was reported to be part of a larger pathogenicity locus, LIPI-2; interestingly, LIPI-2 appeared to be species specific to L. ivanovii [24]. Similar knockout studies with S. aureus have reported that the SMase functions as a virulence factor in infection models of the mouse mammary gland [25] and the rabbit cornea [26] with knockout out strains showing reduced pathogenicity in both cases. More recent research using intranasally infected mice found that SMase plays a crucial role in lung injury induced by S. aureus by enhancing neutrophil influx into the lung and alveolar space, and vascular leakage of serum proteins into the extravascular spaces [27].
Secretory Sphingomyelinase
The existence of a secreted SMase in mammalian systems was first postulated following observations of SMase activity in serum plasma [28]. However, unlike the many bSMases that display homology to the mammalian neutral SMases, the mammalian secreted SMase (S-SMase) was found to be an alternatively trafficked form of the lysosomal acid SMase (L-SMase), which is responsible for the hereditary storage disorder, Niemann-Pick disease (the reader is referred to other reviews on this enzyme). Whereas L-SMase is targeted to the lysosomes through mannose-phosphorylation, S-SMase is not mannose-phosphorylated and is instead trafficked out of the cell by means of the default Golgi secretory pathway [29]. Although there is a paucity of studies on S-SMase, there has been suggestion of regulated S-SMase secretion but the manner in which this occurs remains poorly understood [30].
Properties
As might be expected, S-SMase has some distinct characteristics compared to its lysosomal counterpart. Although both enzymes are zinc hydrolases, L-SMase becomes tightly co-ordinated to Zn2+ ion en route to the lysosome whereas S-SMase requires an external source of Zn2+ for full activity [29]. In addition, although both proteins are N-glycosylated, S-SMase exhibits a complex type pattern in comparison with the high mannose N-glycan composition of L-SMase [29]. Unlike its lysosomal relative, S-SMase has also been demonstrated to hydrolyze lipoprotein-bound SM at neutral pH; while this is perhaps surprising, given the neutral pH of the extracellular milieu, the molecular basis for this in S-SMase remains poorly understood, particularly given the reported narrow pH sensitivity of L-SMase [31]. Thus, mammalian S-SMase seems to function in an extracellular environment and, consequently, could function to act in both an autocrine or paracrine fashion to metabolize SM at the outer leaflet of the PM. As of yet, however, there have been very few studies directed at understanding the specific functional roles of S-SMase.
Elevation of Serum S-SMase activity
Despite the paucity of information on the functional roles of S-SMase, a number of studies have reported elevations of serum S-SMase activity in pathological states including type II diabetes [32], sepsis [33], chronic heart failure [34], and hypercytokinemia [35]. This has also been observed in response to ionizing radiation therapy in cancer patients [36]. However, in all cases, the mechanisms that underlie secretion of the S-SMase protein are poorly understood. Moreover, research thus far has not addressed either the cellular consequences or the subsequent biological implications associated with enhanced S-SMase secretion.
S-SMase and LDL sphingomyelin
Although beyond the scope of this review, it should also be mentioned that there is considerable evidence implicating S-SMase in the aggregation of low density lipoproteins (LDL), an early event in atherosclerosis, through mediating hydrolysis of LDL-bound SM. This is covered in more detail in [37].
Translocation of L-SMase
In addition to the S-SMase discussed above, it is also worth noting that there are reports of metabolism of SM to ceramide at the PM resulting from an acute translocation of L-SMase, presumably from the lysosomes to the outer leaflet of the PM. This has been reported in response to a number of stimuli such as UV radiation, Fas ligand/CD95, cisplatin, and phorbol ester [38-41]. Mechanistically, this was reported to require phosphorylation of L-SMase on serine 508 by protein kinase C-δ in response to UV and cisplatin [38,39].
Ceramide generated by acute translocation of L-SMase has been implicated in cytoskeletal reorganization [38], activation of pro-apoptotic proteins such as JNK [40], ‘capping’ of Fas receptors in T cells [42] and, consequently, apoptosis induced by both UV and Fas ligand [41,42] Despite this, there is still limited information as to how ceramide generated in the PM exerts its effects. Studies have suggested this is through direct downstream signaling effectors such as protein phosphatases [38]. In contrast, other studies have centered around the effects of ceramide generation on the organization of the PM – specifically through modulation of the detergent-insoluble membrane microdomains termed ‘lipid rafts’ [43,44]. Notably, a number of studies have indicated that hydrolysis of SM in rafts leads to the formation of more stable, ceramide-enriched domains and this has been reported to occur in response to a variety of stimuli including UV, CD40, Fas ligand/CD95, viral and bacterial infection amongst others. Moreover, many of these studies have demonstrated a necessity for L-SMase in this process (see [44] and references therein). Thus, in addition to reported roles for L-SMase in modulating lysosomal enzymes such as cathepsin D [45], there is growing evidence indicating functional roles for L-SMase through modulating SM levels at the PM. However, it should also be noted that it is unclear if translocation of L-SMase to the PM is part of a broader relocalization of lysosomal enzymes, or a consequence of specific regulation of the L-SMase enzyme itself. Additionally, the acute translocation of L-SMase to the PM could account for a Zn2+-independent acid SMase activity that has been reported in some studies [32]. However, as the molecular identity of protein responsible for this activity is unknown, strong conclusions cannot be made.
Neutral sphingomyelinase-2 (nSMase2)
Neutral sphingomyelinases (N-SMases) are a family of Mg2+-dependent enzymes that catalyze the hydrolysis of SM into ceramide at pH 7.4. Of the three recently cloned mammalian N-SMases (we use here N-SMase as generic indication of neutral SMases), only nSMase2 and nSMase3 display in vivo activity. However, nSMase3 is reported to localize to the endoplasmic reticulum and the Golgi compartment [46,47]. Thus, in this review, we will focus on nSMase2.
Properties
First purified from mammalian brain, nSMase2 is a membrane-bound protein of 71 kDa with a C-terminal catalytic domain and two predicted hydrophobic segments near the N-terminus [48]. In addition, nSMase2 possesses two palmitoylation sites, one located between the putative hydrophobic regions and the other found within the catalytic region, respectively [49]. Although these palmitoylation sites were reported to be important for PM localization, the subcellular localization of nSMase2 remains not fully determined. It was first described as a Golgi-associated protein potentially involved in the Golgi secretory vesicle trafficking [50] and, indeed, the endogenous nSMase2 was reported to colocalize with Golgi markers in both SH-SY5Y neuroblastoma cells and rat PC-12 cells [48]. However, other studies have indicated a PM localization of overexpressed GFP-, FLAG- or V5-tagged-nSMase2 in various cancer cell lines [51-53] [54]. Furthermore, stimuli such as TNFα, H202 and cell confluence were reported to induce nSMase2 translocation from the Golgi to the PM [55,56]. Despite these reported differences, the SM-rich PM most likely represents the major site of action of nSMase2. Moreover, nSMase2 is activated by phosphatidylserine which is enriched in the inner leaflet of the plasma membrane [57]. Interestingly, the catalytic domain of nSMase2 is predicted to be located in the cytosolic leaflet of the PM suggesting the presence of SM in the intracellular side of the membrane [58]. Although this is contrary to reported lipid asymmetry (see above), this is very consistent with previous studies reporting a pool of signaling SM located at the inner PM leaflet [59,60]. Within the PM, N-SMase activity has been detected in detergent-insoluble fractions of the plasma membrane, ie, rafts/caveolae which are enriched in cholesterol and SM. A possible direct molecular interaction between nSMase and caveolin-1 has also been suggested as the caveolin-scaffolding domain of caveolin-1 was reported to inhibit N-SMase activity [61]. Consistent with this, caveolin-1 overexpression inhibited N-SMase activity and decreased apoptosis in response to staurosporine [62]. Furthermore, this and other chemotherapeutic agents such as daunorubicin and cytosine arabinoside augmented N-SMase activity in raft fractions in human myeloblastic and neuronal cells lines [63-65]. Taken together, this suggests that caveolae/rafts are important for regulation of N-SMase activity, although the identity of the specific N-SMase in these studies is unclear. However, as Goswami et al. recently reported an increase in the activity of overexpressed nSMase2 in the raft fraction in staurosporine-stimulated oligodendroglioma cells (HOG cells), nSMase2 is a good candidate for the caveolae/raft N-SMase enzyme [66].
nSMase2 in cell death
Given the reported role of ceramide in programmed cell death, it is perhaps unsurprising that a number of studies have focused on the role of N-SMase in this process. Previously, N-SMase activation has been reported in apoptosis induced by the death receptor Fas (CD95) and tumor necrosis factor receptor I (TNF-RI). Notably, both receptors are redistributed to the caveolae/raft fraction of the PM upon stimulation by their respective ligands. However, although N-SMase activation seems to participate in the execution phase of apoptosis in response to CD95 engagement [67], it appears to act upstream of mitochondrial events in the TNFR-I signaling pathway [68]. Although the identity of the N-SMase involved is unclear, more recent studies have indicated that nSMase2 may participate in the early events of TNFα-induced apoptosis. By utilizing a mutant TNF-R1, Neumeyer et al, reported a role for nSMase-2 in caspase-3 activation and the subsequent induction of apoptosis by TNFα [69]. Additionally, the reconstitution of nSMase2 in a mouse osteosarcoma cell line lacking the endogenous enzyme restored sensitivity to TNFα [51]. Also, Goswami et al. reported that overexpression of nSMase2 in staurosporine-stimulated oligodendroglioma cells (HOG cells) led to enhanced cell death [66]. Finally, Tani & Hannun found that nSMase2 overexpression, through a tetracycline-inducible expression system, potentiated TNF-α-induced cell death of MCF-7 cells (unpublished observations).
Despite these data, the exact mechanism by which ceramide generation by nSMase2 leads to the activation of caspases and cell death remains to be elucidated. The increase of ceramide in lipid rafts may enhance the recruitment of TNF-RI to the rafts and potentiate the activation of the receptor. However, N-SMase activation can also serve as a source for other bioactive sphingolipids. In particular, a study in nSMase2-deficient human osteosarcoma cells found that both lactosylceramide production and apoptosis were reduced in response to TNF-α [70]. This suggests that nSMase2 is upstream of LacCer in the TNFR signaling pathway.
nSMase2 in trafficking
In addition to the evidence implicating nSMase2 in TNFα-induced cell death, recent studies have also supported a role for nSMase2 in membrane trafficking. Using both the pharmacological inhibitor of nSMase2, GW4869, and neuronal cells from the fro/fro mice (possessing an inactive nSMase2), nSMase2 was implicated in the TNF-α-induced clustering of NMDA receptors in lipid rafts of hippocampal neuronal cells. In this study, it was speculated that rapid ceramide generation at the PM is important for the fusion of vesicles and insertion of the NMDA receptor into the lipid rafts [71]. Notably, this is consistent with studies of the PLC/SMase PlcHR2 (see above) where its hydrolytic activity caused fusion of vesicles in vitro [22]. In addition, ceramide was found to be enriched in exosomes purified from cell culture medium of mouse oligodendroglial cells. Notably, inhibition of nSMase2 with GW4869 or by siRNA reduced exosome release. Although this suggests a role for nSMase2 in the secretory pathway, it was shown that addition of bSMase to giant unilamellar vesicles (GUVs) resulted in formation of intraluminal vesicles [72]. Thus, it was suggested that nSMase2 may act in the endosomal compartments giving rise to multivesicular endosomes through formation of intravesicular membranes. Given the reported localization of nSMase2, this implies intracellular trafficking of nSMase2 from the PM or Golgi to the recycling endosomes. Although we have recently found evidence that nSMase2 may traffic from the PM to the recycling endosomes (D. Milhas and Y.A. Hannun, unpublished observations), in some cell types the budding of exosome vesicles occurs in an endosome-like domain of the PM [73]. Thus, nSMase2 could also be involved in exosome biogenesis from the plasma membrane.
nSMase2 in inflammation
As reviewed recently, nSMase2 plays a role in the inflammation process during aging and in response to IL-1β. Specifically, nSMase2 activation enhances IL-1β signaling by preventing the phosphorylation and ubiquitination of IRAK-1 in a manner dependent on the ceramide-activated protein phosphatase 2 (PP2A) while a decline in hepatic GSH content associated with oxidative stress increases nSMase2 activity during aging [74]. Also, TNF-α-induced nSMase2 activation is involved in vascular inflammatory responses such as the induction of vascular cell adhesion molecule (VCAM) and intracellular adhesion molecule (ICAM) in lung epithelial cells [55]. In both cases, the ceramide generated at the plasma membrane is suggested to be a 2nd messenger in the inflammatory signaling pathway.
In contrast, TNF-α-induced endothelial nitric oxide synthase (eNOS) in human endothelial cells involves the sequential activation of nSMase2 and sphingosine kinase-1 [75]. Thus, it is implied that the nSMase2-generated ceramide is converted to sphingosine-1-phosphate through the actions of a ceramidase and sphingosine kinase 1 (SK1) although the nature of the ceramidase involved remains to be defined. Consistent with this, nSMase2 was also described upstream of SK1 in TNFα-induced proliferation of smooth muscle cells and fibroblasts [76]. Interestingly, in this model matrix metalloproteinases (MT1-MMP1 and MMP-2) are involved in the activation of nSMase2 which is consistent with the action of N-SMase at the PM. Finally, nSMase2 was suggested to be involved in acute hypoxic pulmonary vasoconstriction whereby the N-SMase-derived ceramide could act on the K+ or Ca2+ channels to regulate cell membrane polarization [77]. Taken together, these studies indicate that the production of ceramide by nSMase2 at the PM can have dual effects – both as a source of other bioactive sphingolipids, but also as a signal in its own right.
Sphingomyelin synthase 2 (SMS2)
The sphingomyelin synthases are expressed ubiquitously and catalyze the bidirectional reaction of choline phosphotransferase converting ceramide and PC into SM and diacylglycerol (DAG). Two mammalian isoforms have been cloned, SMS1 and SMS2 [78]. However, as SMS1 localizes solely in the Golgi apparatus whereas SMS2 is found both at the PM and in the Golgi, the focus of this part of the review will be on SMS2.
Properties
SMS2 is an integral membrane protein with six predicted transmembrane domains and a C-terminal catalytic site located on the exoplasmic leaflet of the PM [78]. This implies the production of SM extracellularly from ceramide generated there and, thus, SMS2 could participate in the SM cycle potentially terminating ceramide signals produced from SMase-mediated hydrolysis of SM. Consistent with this, SMS2 was found responsible for the hydrolysis of cell surface-associated SM in HeLa cells [79]. Moreover, siRNA downregulation of SMS2 decreased the level of SM in raft-like domains of the PM [80] while its overexpression induces the opposite effect [81]. However, the PM is not the sole site of SMS2 action as studies have reported the presence of active SMS2 in the Golgi [82] and implicated SMS2 in de novo biosynthesis of SM [79,80]. Notably, these results can also explain the importance of the S-palmitoylation of SMS2 with regards to the PM localization of the enzyme but not for its activity [83].
SMS2 in cell death
Based on its participation in the regulation of lipid-raft structures SMS2 at the PM has been suggested to be involved in signal transduction. More specifically, roles for SMS2 in cell death have been proposed, although this remains controversial. The generation of SM at the PM by the overexpression of SMS2 in CHO cells was reported to enhance TNF-α-induced apoptosis; it was suggested that SM generation in lipid rafts may increase the exposure of the TNF-R at the surface [81]. Additionally, siRNA downregulation of SMS2 protected THP-1 derived macrophages from LPS-induced apoptosis and this was attributed to a decrease of TLR-4 expression at the cell surface [81]. Thus, the level of SM at the PM and specifically in raft microdomains seems to regulate these events by modulating the cell surface expression of receptors. In contrast to these data, siRNA against SMS2 potentiated caspases activation and cell death upon photodynamic therapy in Jurkat cells [84], potentially through increasing ceramide levels. Thus, it seems that SMS2 activity could be pro- or anti-apoptotic depending on the cell type and the stimulus. However, it should be noted that SMS activity regulates both the level of the pro-apoptotic ceramide and the pro-survival DAG and in opposite directions. Consequently, siRNA knockdown of SMS2 reduced growth of HeLa cells, with a slight reduction in cell viability, but this was not restored by the addition of external SM [79]. Thus, decreased SM may not be the only cause of a growth defect; in particular, a decrease in DAG may participate to this effect.
SMS2 in atherosclerosis
More recent data have suggested a promoting role for SMS2 in atherosclerosis in mice. In this study, SMS2 overexpression in a transgenic mouse model induced a pro-atherogenic phenotype characterised by increased plasma non-HDL-SM which induced an aggregation of non-HDL particles after SMase treatment, an upregulation of the HDL receptor SR-BI, a decrease of cholesterol efflux and a reduction of plasma apoE level. Reciprocally, SMS2 knockout mice displayed the opposite effects [85,86]. Mechanistically, SM generation and decreased ceramide in PM rafts consequent to SMS2 activity could be responsible for modulating the expression of the receptors SR-BI and Apo-E at the cell surface. However, the effects of SMS2 activity on the level of other sphingolipids such as sphingosine and sphingosine-1-phosphate may also be involved in the development of atherosclerosis [86]. Finally, the reported role of SMS2 in NFκB activation could also contribute to its pro-atherogenic potential. Notably, the downregulation of SMS2 significantly prevented NF-κB activation in response to LPS in macrophages and upon TNFα stimulation of HEK 293 cells [87]. As suggested for the inhibitory effect of SMS2 on cell death induced by these agonists [81], defects in the recruitment of the TLR-4-MD2 complex and of TNF-RI to lipid rafts was suggested to be the underlying mechanism responsible for decreased NF-κB activation.
Conclusions
The PM represents the primary source of SM which is first biosynthesized in the Golgi compartment by the action of the two isoforms of SMS (SMS1 and SMS2) and then delivered to the PM by vesicular transport. At the PM, the levels of SM can be regulated by multiple enzymes including S-SMase and SMS2 acting in the outer leaflet and nSMase2 located in the inner leaflet. Specifically, these enzymes regulate the levels of both SM and ceramide which can also be metabolized to other bioactive sphingolipids such as sphingosine and S1P as shown schematically in Figure 1. Moreover, SMS2 also modulates the levels of PC and DAG. Thus, the effects of these enzymes may extend far beyond their immediate effects on SM and ceramide alone. Consistent with the proposed role of lipid rafts in signal transduction at the PM, both SMS2 and nSMase2 activities in these microdomains appear to be involved in the modulation of receptor-induced cell death and inflammatory signaling pathways. Thus, the levels of both SM and ceramide at the PM seem to be important for cellular signaling.
Figure 1. Schematic of SM metabolism and production at the plasma membrane.
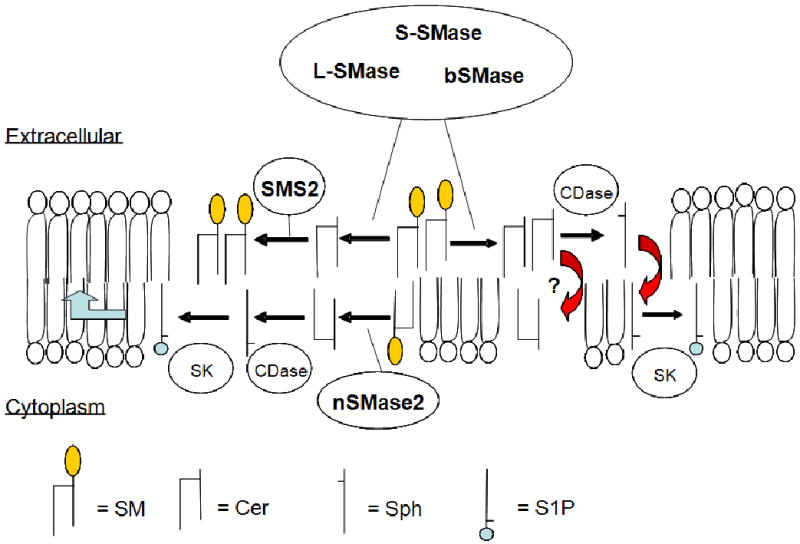
S-SMase, acid SMase (L-SMase) and bSMase can act upon extracellular SM to produce ceramide (Cer) which, in turn can be metabolized back to SM by SMS2. Alternatively, ceramide may potentially flip to the inner leaflet (and vice versa) or, by the actions of ceramidase (CDase), can be converted to sphingosine which can also flip (and vice versa can ‘flop’). In contrast, nSMase2 on the inner leaflet can hydrolyze SM to ceramide. This also can be converted to sphingosine (Sph) by CDase and, potentially, on to sphingosine-1-phosphate (S1P) by the actions of sphingosine kinase (SK). Additionally, sphingosine that has flipped from the outer leaflet may also be converted to sphingosine-1-phosphate by SK. Moreover, sphingosine-1-phosphate on the inner leaflet may be pumped out of the cell to act extracellularly. Thus, small effects on the four major SM-regulating enzymes can have broader implications on the levels of many bioactive lipids.
The manipulation of SM and ceramide level at PM by the overexpression or downregulation of the enzymes involved in their synthesis and/or hydrolysis, ie SMase and SMS has allowed the identification of roles of SMS2 and nSMase2 in specific biological functions. However, some contradictory effects have been reported as, for example, with the role of SMS2 in apoptosis. Although cell type and stimuli dependence can explain these discrepancies, we also think that it is important to consider how the extent of changes in SM and ceramide levels as well as further metabolism may exert different outcome on the cellular responses. For instance, a complete depletion of ceramide or SM may profoundly disturb the structure of the PM and could be deleterious for the cell as reported by the cytotoxic effect of bSMases. In contrast, a moderate decrease of lipid content may modulate the signaling pathways initiated at the PM. We strongly feel that this should be taken into account when interpreting results.
Finally, a major challenge for the future is to gain a full understanding of the specific SM/Cer species present within the outer and inner leaflets of the membrane, and, more importantly, if the production of distinct species, or lipids within specific leaflets has distinct metabolic and physiological consequences for the cell. This will be imperative if we are to obtain an understanding of the distinct roles of S-SMase, and SMS2 acting on the extracellular side of the PM and nSMase2 on the inner leaflet. Moreover, as modulation of PM lipids is an early event in the response to many stimuli, a greater understanding of the lipids at the PM, and the enzymes that regulate them, is essential for us to fully appreciate the intricacies of signal transduction.
List of Abbreviations
- bSMase
Bacterial sphingomyelinase
- LDL
Low density lipoprotein
- L-SMase
Lysosomal acid sphingomyelinase
- N-SMase
Neutral sphingomyelinase
- PC
Phosphatidylcholine
- PLC
Phospholipase C
- PM
Plasma membrane
- SK
Sphingosine kinase
- SM
Sphingomyelin
- SMase
Sphingomyelinase
- SMS1
Sphingomyelinase synthase 1
- SMS2
Sphingomyelin synthase 2
- S-SMase
Secretory sphingomyelinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–31. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 2.Lajoie P, Goetz JG, Dennis JW, Nabi IR. Lattices, rafts, and scaffolds: domain regulation of receptor signaling at the plasma membrane. J Cell Biol. 2009;185:381–5. doi: 10.1083/jcb.200811059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pike LJ. The challenge of lipid rafts. J Lipid Res. 2009;50(Suppl):S323–8. doi: 10.1194/jlr.R800040-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikeda M, Kihara A, Igarashi Y. Lipid asymmetry of the eukaryotic plasma membrane: functions and related enzymes. Biol Pharm Bull. 2006;29:1542–6. doi: 10.1248/bpb.29.1542. [DOI] [PubMed] [Google Scholar]
- 5.Daleke DL. Regulation of phospholipid asymmetry in the erythrocyte membrane. Curr Opin Hematol. 2008;15:191–5. doi: 10.1097/MOH.0b013e3282f97af7. [DOI] [PubMed] [Google Scholar]
- 6.Mondal M, Mesmin B, Mukherjee S, Maxfield FR. Sterols are mainly in the cytoplasmic leaflet of the plasma membrane and the endocytic recycling compartment in CHO cells. Mol Biol Cell. 2009;20:581–8. doi: 10.1091/mbc.E08-07-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–24. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartke N, Hannun YA. Bioactive sphingolipids: metabolism and function. J Lipid Res. 2009;50(Suppl):S91–6. doi: 10.1194/jlr.R800080-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Titball RW. Bacterial phospholipases C. Microbiol Rev. 1993;57:347–66. doi: 10.1128/mr.57.2.347-366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke CJ, Snook CF, Tani M, Matmati N, Marchesini N, Hannun YA. The extended family of neutral sphingomyelinases. Biochemistry. 2006;45:11247–56. doi: 10.1021/bi061307z. [DOI] [PubMed] [Google Scholar]
- 11.Vasil ML, Stonehouse MJ, Vasil AI, Wadsworth SJ, Goldfine H, Bolcome RE, 3rd, Chan J. A complex extracellular sphingomyelinase of Pseudomonas aeruginosa inhibits angiogenesis by selective cytotoxicity to endothelial cells. PLoS Pathog. 2009;5:e1000420. doi: 10.1371/journal.ppat.1000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urbina P, Flores-Diaz M, Alape-Giron A, Alonso A, Goni FM. Phospholipase C and sphingomyelinase activities of the Clostridium perfringens alpha-toxin. Chem Phys Lipids. 2009;159:51–7. doi: 10.1016/j.chemphyslip.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Zorn B, Dominguez-Bernal G, Suarez M, Ripio MT, Vega Y, Novella S, Vazquez-Boland JA. The smcL gene of Listeria ivanovii encodes a sphingomyelinase C that mediates bacterial escape from the phagocytic vacuole. Mol Microbiol. 1999;33:510–23. doi: 10.1046/j.1365-2958.1999.01486.x. [DOI] [PubMed] [Google Scholar]
- 14.Ago H, Oda M, Takahashi M, Tsuge H, Ochi S, Katunuma N, Miyano M, Sakurai J. Structural basis of the sphingomyelin phosphodiesterase activity in neutral sphingomyelinase from Bacillus cereus. J Biol Chem. 2006;281:16157–67. doi: 10.1074/jbc.M601089200. [DOI] [PubMed] [Google Scholar]
- 15.Openshaw AE, Race PR, Monzo HJ, Vazquez-Boland JA, Banfield MJ. Crystal structure of SmcL, a bacterial neutral sphingomyelinase C from Listeria. J Biol Chem. 2005;280:35011–7. doi: 10.1074/jbc.M506800200. [DOI] [PubMed] [Google Scholar]
- 16.Huseby M, Shi K, Brown CK, Digre J, Mengistu F, Seo KS, Bohach GA, Schlievert PM, Ohlendorf DH, Earhart CA. Structure and biological activities of beta toxin from Staphylococcus aureus. J Bacteriol. 2007;189:8719–26. doi: 10.1128/JB.00741-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linehan D, Etienne J, Sheehan D. Relationship between haemolytic and sphingomyelinase activities in a partially purified beta-like toxin from Staphylococcus schleiferi. FEMS Immunol Med Microbiol. 2003;36:95–102. doi: 10.1016/S0928-8244(03)00089-0. [DOI] [PubMed] [Google Scholar]
- 18.Montes LR, Lopez DJ, Sot J, Bagatolli LA, Stonehouse MJ, Vasil ML, Wu BX, Hannun YA, Goni FM, Alonso A. Ceramide-enriched membrane domains in red blood cells and the mechanism of sphingomyelinase-induced hot-cold hemolysis. Biochemistry. 2008;47:11222–30. doi: 10.1021/bi801139z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomita M, Taguchi R, Ikezawa H. Adsorption of sphingomyelinase of Bacillus cereus onto erythrocyte membranes. Arch Biochem Biophys. 1983;223:202–12. doi: 10.1016/0003-9861(83)90586-6. [DOI] [PubMed] [Google Scholar]
- 20.Walev I, Weller U, Strauch S, Foster T, Bhakdi S. Selective killing of human monocytes and cytokine release provoked by sphingomyelinase (beta-toxin) of Staphylococcus aureus. Infect Immun. 1996;64:2974–9. doi: 10.1128/iai.64.8.2974-2979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang YX, Geng Y, Yang JW, Guo XK, Zhao GP. Cytotoxic activity and probable apoptotic effect of Sph2, a sphigomyelinase hemolysin from Leptospira interrogans strain Lai. BMB Rep. 2008;41:119–25. doi: 10.5483/bmbrep.2008.41.2.119. [DOI] [PubMed] [Google Scholar]
- 22.Montes LR, Ibarguren M, Goni FM, Stonehouse M, Vasil ML, Alonso A. Leakage-free membrane fusion induced by the hydrolytic activity of PlcHR(2), a novel phospholipase C/sphingomyelinase from Pseudomonas aeruginosa. Biochim Biophys Acta. 2007;1768:2365–72. doi: 10.1016/j.bbamem.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 23.Tajima A, Iwase T, Shinji H, Seki K, Mizunoe Y. Inhibition of endothelial interleukin-8 production and neutrophil transmigration by Staphylococcus aureus beta-hemolysin. Infect Immun. 2009;77:327–34. doi: 10.1128/IAI.00748-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dominguez-Bernal G, Muller-Altrock S, Gonzalez-Zorn B, Scortti M, Herrmann P, Monzo HJ, Lacharme L, Kreft J, Vazquez-Boland JA. A spontaneous genomic deletion in Listeria ivanovii identifies LIPI-2, a species-specific pathogenicity island encoding sphingomyelinase and numerous internalins. Mol Microbiol. 2006;59:415–32. doi: 10.1111/j.1365-2958.2005.04955.x. [DOI] [PubMed] [Google Scholar]
- 25.Bramley AJ, Patel AH, O'Reilly M, Foster R, Foster TJ. Roles of alpha-toxin and beta-toxin in virulence of Staphylococcus aureus for the mouse mammary gland. Infect Immun. 1989;57:2489–94. doi: 10.1128/iai.57.8.2489-2494.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Callaghan RJ, Callegan MC, Moreau JM, Green LC, Foster TJ, Hartford OM, Engel LS, Hill JM. Specific roles of alpha-toxin and beta-toxin during Staphylococcus aureus corneal infection. Infect Immun. 1997;65:1571–8. doi: 10.1128/iai.65.5.1571-1578.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashida A, Bartlett AH, Foster TJ, Park PW. Staphylococcus aureus beta-toxin induces lung injury through syndecan-1. Am J Pathol. 2009;174:509–18. doi: 10.2353/ajpath.2009.080394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spence MW, Byers DM, Palmer FB, Cook HW. A new Zn2+-stimulated sphingomyelinase in fetal bovine serum. J Biol Chem. 1989;264:5358–63. [PubMed] [Google Scholar]
- 29.Schissel SL, Keesler GA, Schuchman EH, Williams KJ, Tabas I. The cellular trafficking and zinc dependence of secretory and lysosomal sphingomyelinase, two products of the acid sphingomyelinase gene. J Biol Chem. 1998;273:18250–9. doi: 10.1074/jbc.273.29.18250. [DOI] [PubMed] [Google Scholar]
- 30.Marathe S, Schissel SL, Yellin MJ, Beatini N, Mintzer R, Williams KJ, Tabas I. Human vascular endothelial cells are a rich and regulatable source of secretory sphingomyelinase. Implications for early atherogenesis and ceramide-mediated cell signaling. J Biol Chem. 1998;273:4081–8. doi: 10.1074/jbc.273.7.4081. [DOI] [PubMed] [Google Scholar]
- 31.Callahan JW, Jones CS, Davidson DJ, Shankaran P. The active site of lysosomal sphingomyelinase: evidence for the involvement of hydrophobic and ionic groups. J Neurosci Res. 1983;10:151–63. doi: 10.1002/jnr.490100205. [DOI] [PubMed] [Google Scholar]
- 32.Gorska M, Baranczuk E, Dobrzyn A. Secretory Zn2+-dependent sphingomyelinase activity in the serum of patients with type 2 diabetes is elevated. Horm Metab Res. 2003;35:506–7. doi: 10.1055/s-2003-41810. [DOI] [PubMed] [Google Scholar]
- 33.Claus RA, Bunck AC, Bockmeyer CL, Brunkhorst FM, Losche W, Kinscherf R, Deigner HP. Role of increased sphingomyelinase activity in apoptosis and organ failure of patients with severe sepsis. FASEB J. 2005;19:1719–21. doi: 10.1096/fj.04-2842fje. [DOI] [PubMed] [Google Scholar]
- 34.Doehner W, Bunck AC, Rauchhaus M, von Haehling S, Brunkhorst FM, Cicoira M, Tschope C, Ponikowski P, Claus RA, Anker SD. Secretory sphingomyelinase is upregulated in chronic heart failure: a second messenger system of immune activation relates to body composition, muscular functional capacity, and peripheral blood flow. Eur Heart J. 2007;28:821–8. doi: 10.1093/eurheartj/ehl541. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi T, Abe T, Sato T, Miura K, Takahashi I, Yano M, Watanabe A, Imashuku S, Takada G. Elevated sphingomyelinase and hypercytokinemia in hemophagocytic lymphohistiocytosis. J Pediatr Hematol Oncol. 2002;24:401–4. doi: 10.1097/00043426-200206000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Sathishkumar S, Boyanovsky B, Karakashian AA, Rozenova K, Giltiay NV, Kudrimoti M, Mohiuddin M, Ahmed MM, Nikolova-Karakashian M. Elevated sphingomyelinase activity and ceramide concentration in serum of patients undergoing high dose spatially fractionated radiation treatment: implications for endothelial apoptosis. Cancer Biol Ther. 2005;4:979–86. doi: 10.4161/cbt.4.9.1915. [DOI] [PubMed] [Google Scholar]
- 37.Jenkins RW, Canals D, Hannun YA. Roles and regulation of secretory and lysosomal acid sphingomyelinase. Cell Signal. 2009;21:836–46. doi: 10.1016/j.cellsig.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeidan YH, Jenkins RW, Hannun YA. Remodeling of cellular cytoskeleton by the acid sphingomyelinase/ceramide pathway. J Cell Biol. 2008;181:335–350. doi: 10.1083/jcb.200705060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeidan YH, Hannun YA. Activation of Acid Sphingomyelinase by Protein Kinase Cδ-mediated. Phosphorylation J Biol Chem. 2007;282:11549–11561. doi: 10.1074/jbc.M609424200. [DOI] [PubMed] [Google Scholar]
- 40.Charruyer A, Grazide S, Bezombes C, Muller S, Laurent G, Jaffrezou JP. UV-C light induces raft-associated acid sphingomyelinase and JNK activation and translocation independently of a nuclear signal. J Biol Chem. 2005;280 doi: 10.1074/jbc.M412867200. [DOI] [PubMed] [Google Scholar]
- 41.Rotolo JA, Zhang J, Donepudi M, Lee H, Fuks Z, Kolesnick R. Caspase-dependent and -independent activation of acid sphingomyelinase signaling. J Biol Chem. 2005;280:26425–26434. doi: 10.1074/jbc.M414569200. [DOI] [PubMed] [Google Scholar]
- 42.Cremesti A, Paris F, Grassme H, Holler N, Tschopp J, Fuks Z, Gulbins E, Kolesnick R. Ceramide Enables Fas to Cap and Kill. J Biol Chem. 2001;276 doi: 10.1074/jbc.M101866200. [DOI] [PubMed] [Google Scholar]
- 43.Smith EL, Schuchman EH. The unexpected role of acid sphingomyelinase in cell death and the pathophysiology of common diseases. FASEB J. 2008;22:3419–31. doi: 10.1096/fj.08-108043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grassme H, Riethmuller J, Gulbins E. Biological aspects of ceramide-enriched membrane domains. Prog Lipid Res. 2007;46:161–70. doi: 10.1016/j.plipres.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 45.Heinrich M, Neumeyer J, Jakob M, Hallas C, Tchikov V, Winoto-Morbach S, Schneider-Brachert W, Trauzoid A, Hethke A, Schutze S. Cathepsin D links TNF-induced acid sphingomyelinase to Bid-mediated caspase-9 and -3 activation. Cell Death Differ. 2004;11:550–563. doi: 10.1038/sj.cdd.4401382. [DOI] [PubMed] [Google Scholar]
- 46.Corcoran CA, He Q, Ponnusamy S, Ogretmen B, Huang Y, Sheikh MS. Neutral sphingomyelinase-3 is a DNA damage and nongenotoxic stress-regulated gene that is deregulated in human malignancies. Mol Cancer Res. 2008;6:795–807. doi: 10.1158/1541-7786.MCR-07-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krut O, Wiegmann K, Kashkar H, Yazdanpanah B, Kronke M. Novel tumor necrosis factor-responsive mammalian neutral sphingomyelinase-3 is a C-tail-anchored protein. J Biol Chem. 2006;281:13784–93. doi: 10.1074/jbc.M511306200. [DOI] [PubMed] [Google Scholar]
- 48.Hofmann K, Tomiuk S, Wolff G, Stoffel W. Cloning and characterization of the mammalian brain-specific, Mg2+-dependent neutral sphingomyelinase. Proc Natl Acad Sci U S A. 2000;97:5895–900. doi: 10.1073/pnas.97.11.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tani M, Hannun YA. Neutral sphingomyelinase 2 is palmitoylated on multiple cysteine residues: Role of palmitoylation in subcellular localization. J Biol Chem. 2007;282:10047–56. doi: 10.1074/jbc.M611249200. [DOI] [PubMed] [Google Scholar]
- 50.Stoffel W, Jenke B, Block B, Zumbansen M, Koebke J. Neutral sphingomyelinase 2 (smpd3) in the control of postnatal growth and development. Proc Natl Acad Sci U S A. 2005;102:4554–9. doi: 10.1073/pnas.0406380102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim WJ, Okimoto RA, Purton LE, Goodwin M, Haserlat SM, Dayyani F, Sweetser DA, McClatchey AI, Bernard OA, Look AT, Bell DW, Scadden DT, Haber DA. Mutations in the neutral sphingomyelinase gene SMPD3 implicate the ceramide pathway in human leukemias. Blood. 2008;111:4716–22. doi: 10.1182/blood-2007-10-113068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karakashian AA, Giltiay NV, Smith GM, Nikolova-Karakashian MN. Expression of neutral sphingomyelinase-2 (NSMase-2) in primary rat hepatocytes modulates IL-beta-induced JNK activation. Faseb J. 2004;18:968–70. doi: 10.1096/fj.03-0875fje. [DOI] [PubMed] [Google Scholar]
- 53.Rutkute K, Asmis RH, Nikolova-Karakashian MN. Regulation of neutral sphingomyelinase-2 by GSH: a new insight to the role of oxidative stress in aging-associated inflammation. J Lipid Res. 2007;48:2443–52. doi: 10.1194/jlr.M700227-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marchesini N, Hannun YA. Acid and neutral sphingomyelinases: roles and mechanisms of regulation. Biochem Cell Biol. 2004;82:27–44. doi: 10.1139/o03-091. [DOI] [PubMed] [Google Scholar]
- 55.Clarke CJ, Truong TG, Hannun YA. Role for neutral sphingomyelinase-2 in tumor necrosis factor alpha-stimulated expression of vascular cell adhesion molecule-1 (VCAM) and intercellular adhesion molecule-1 (ICAM) in lung epithelial cells: p38 MAPK is an upstream regulator of nSMase2. J Biol Chem. 2007;282:1384–96. doi: 10.1074/jbc.M609216200. [DOI] [PubMed] [Google Scholar]
- 56.Levy M, Castillo SS, Goldkorn T. nSMase2 activation and trafficking are modulated by oxidative stress to induce apoptosis. Biochem Biophys Res Commun. 2006;344:900–5. doi: 10.1016/j.bbrc.2006.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marchesini N, Luberto C, Hannun YA. Biochemical properties of mammalian neutral sphingomyelinase 2 and its role in sphingolipid metabolism. J Biol Chem. 2003;278:13775–83. doi: 10.1074/jbc.M212262200. [DOI] [PubMed] [Google Scholar]
- 58.Tani M, Hannun YA. Analysis of membrane topology of neutral sphingomyelinase 2. FEBS Lett. 2007;581:1323–8. doi: 10.1016/j.febslet.2007.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andrieu N, Salvayre R, Levade T. Comparative study of the metabolic pools of sphingomyelin and phosphatidylcholine sensitive to tumor necrosis factor. Eur J Biochem. 1996;236:738–45. doi: 10.1111/j.1432-1033.1996.00738.x. [DOI] [PubMed] [Google Scholar]
- 60.Linardic CM, Hannun YA. Identification of a distinct pool of sphingomyelin involved in the sphingomyelin cycle. J Biol Chem. 1994;269:23530–7. [PubMed] [Google Scholar]
- 61.Veldman RJ, Maestre N, Aduib OM, Medin JA, Salvayre R, Levade T. A neutral sphingomyelinase resides in sphingolipid-enriched microdomains and is inhibited by the caveolin-scaffolding domain: potential implications in tumour necrosis factor signalling. Biochem J. 2001;355:859–68. doi: 10.1042/bj3550859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu P, Qi B, Zhu H, Zheng Y, Li F, Chen J. Suppression of staurosporine-mediated apoptosis in Hs578T breast cells through inhibition of neutral-sphingomyelinase by caveolin-1. Cancer Lett. 2007;256:64–72. doi: 10.1016/j.canlet.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 63.Grazide S, Maestre N, Veldman RJ, Bezombes C, Maddens S, Levade T, Laurent G, Jaffrezou JP. Ara-C- and daunorubicin-induced recruitment of Lyn in sphingomyelinase-enriched membrane rafts. FASEB J. 2002;16:1685–7. doi: 10.1096/fj.01-0794fje. [DOI] [PubMed] [Google Scholar]
- 64.Kilkus J, Goswami R, Testai FD, Dawson G. Ceramide in rafts (detergent-insoluble fraction) mediates cell death in neurotumor cell lines. J Neurosci Res. 2003;72:65–75. doi: 10.1002/jnr.10549. [DOI] [PubMed] [Google Scholar]
- 65.Testai FD, Landek MA, Dawson G. Regulation of sphingomyelinases in cells of the oligodendrocyte lineage. J Neurosci Res. 2004;75:66–74. doi: 10.1002/jnr.10816. [DOI] [PubMed] [Google Scholar]
- 66.Goswami R, Ahmed M, Kilkus J, Han T, Dawson SA, Dawson G. Differential regulation of ceramide in lipid-rich microdomains (rafts): antagonistic role of palmitoyl:protein thioesterase and neutral sphingomyelinase 2. J Neurosci Res. 2005;81:208–17. doi: 10.1002/jnr.20549. [DOI] [PubMed] [Google Scholar]
- 67.Tepper AD, Ruurs P, Wiedmer T, Sims PJ, Borst J, van Blitterswijk WJ. Sphingomyelin hydrolysis to ceramide during the execution phase of apoptosis results from phospholipid scrambling and alters cell-surface morphology. J Cell Biol. 2000;150:155–64. doi: 10.1083/jcb.150.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luberto C, Hassler DF, Signorelli P, Okamoto Y, Sawai H, Boros E, Hazen-Martin DJ, Obeid LM, Hannun YA, Smith GK. Inhibition of tumor necrosis factor-induced cell death in MCF7 by a novel inhibitor of neutral sphingomyelinase. J Biol Chem. 2002;277:41128–39. doi: 10.1074/jbc.M206747200. [DOI] [PubMed] [Google Scholar]
- 69.Neumeyer J, Hallas C, Merkel O, Winoto-Morbach S, Jakob M, Thon L, Adam D, Schneider-Brachert W, Schutze S. TNF-receptor I defective in internalization allows for cell death through activation of neutral sphingomyelinase. Exp Cell Res. 2006;312:2142–53. doi: 10.1016/j.yexcr.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 70.Martin SF, Williams N, Chatterjee S. Lactosylceramide is required in apoptosis induced by N-Smase. Glycoconj J. 2006;23:147–57. doi: 10.1007/s10719-006-7920-8. [DOI] [PubMed] [Google Scholar]
- 71.Wheeler D, Knapp E, Bandaru VV, Wang Y, Knorr D, Poirier C, Mattson MP, Geiger JD, Haughey NJ. Tumor necrosis factor-alpha-induced neutral sphingomyelinase-2 modulates synaptic plasticity by controlling the membrane insertion of NMDA receptors. J Neurochem. 2009;109:1237–49. doi: 10.1111/j.1471-4159.2009.06038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–7. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 73.Booth AM, Fang Y, Fallon JK, Yang JM, Hildreth JE, Gould SJ. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol. 2006;172:923–35. doi: 10.1083/jcb.200508014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nikolova-Karakashian M, Karakashian A, Rutkute K. Role of neutral sphingomyelinases in aging and inflammation. Subcell Biochem. 2008;49:469–86. doi: 10.1007/978-1-4020-8831-5_18. [DOI] [PubMed] [Google Scholar]
- 75.De Palma C, Meacci E, Perrotta C, Bruni P, Clementi E. Endothelial nitric oxide synthase activation by tumor necrosis factor alpha through neutral sphingomyelinase 2, sphingosine kinase 1, and sphingosine 1 phosphate receptors: a novel pathway relevant to the pathophysiology of endothelium. Arterioscler Thromb Vasc Biol. 2006;26:99–105. doi: 10.1161/01.ATV.0000194074.59584.42. [DOI] [PubMed] [Google Scholar]
- 76.Tellier E, Negre-Salvayre A, Bocquet B, Itohara S, Hannun YA, Salvayre R, Auge N. Role for furin in tumor necrosis factor alpha-induced activation of the matrix metalloproteinase/sphingolipid mitogenic pathway. Mol Cell Biol. 2007;27:2997–3007. doi: 10.1128/MCB.01485-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cogolludo A, Moreno L, Frazziano G, Moral-Sanz J, Menendez C, Castaneda J, Gonzalez C, Villamor E, Perez-Vizcaino F. Activation of neutral sphingomyelinase is involved in acute hypoxic pulmonary vasoconstriction. Cardiovasc Res. 2009;82:296–302. doi: 10.1093/cvr/cvn349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huitema K, van den Dikkenberg J, Brouwers JF, Holthuis JC. Identification of a family of animal sphingomyelin synthases. Embo J. 2004;23:33–44. doi: 10.1038/sj.emboj.7600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tafesse FG, Huitema K, Hermansson M, van der Poel S, van den Dikkenberg J, Uphoff A, Somerharju P, Holthuis JC. Both sphingomyelin synthases SMS1 and SMS2 are required for sphingomyelin homeostasis and growth in human HeLa cells. J Biol Chem. 2007;282:17537–47. doi: 10.1074/jbc.M702423200. [DOI] [PubMed] [Google Scholar]
- 80.Li Z, Hailemariam TK, Zhou H, Li Y, Duckworth DC, Peake DA, Zhang Y, Kuo MS, Cao G, Jiang XC. Inhibition of sphingomyelin synthase (SMS) affects intracellular sphingomyelin accumulation and plasma membrane lipid organization. Biochim Biophys Acta. 2007;1771:1186–94. doi: 10.1016/j.bbalip.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ding T, Li Z, Hailemariam T, Mukherjee S, Maxfield FR, Wu MP, Jiang XC. SMS overexpression and knockdown: impact on cellular sphingomyelin and diacylglycerol metabolism, and cell apoptosis. J Lipid Res. 2008;49:376–85. doi: 10.1194/jlr.M700401-JLR200. [DOI] [PubMed] [Google Scholar]
- 82.Villani M, Subathra M, Im YB, Choi Y, Signorelli P, Del Poeta M, Luberto C. Sphingomyelin synthases regulate production of diacylglycerol at the Golgi. Biochem J. 2008;414:31–41. doi: 10.1042/BJ20071240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tani M, Kuge O. Sphingomyelin synthase 2 is palmitoylated at the COOH-terminal tail, which is involved in its localization in plasma membranes. Biochem Biophys Res Commun. 2009;381:328–32. doi: 10.1016/j.bbrc.2009.02.063. [DOI] [PubMed] [Google Scholar]
- 84.Separovic D, Semaan L, Tarca AL, Awad Maitah MY, Hanada K, Bielawski J, Villani M, Luberto C. Suppression of sphingomyelin synthase 1 by small interference RNA is associated with enhanced ceramide production and apoptosis after photodamage. Exp Cell Res. 2008;314:1860–8. doi: 10.1016/j.yexcr.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dong J, Liu J, Lou B, Li Z, Ye X, Wu M, Jiang XC. Adenovirus-mediated overexpression of sphingomyelin synthases 1 and 2 increases the atherogenic potential in mice. J Lipid Res. 2006;47:1307–14. doi: 10.1194/jlr.M600040-JLR200. [DOI] [PubMed] [Google Scholar]
- 86.Liu J, Zhang H, Li Z, Hailemariam TK, Chakraborty M, Jiang K, Qiu D, Bui HH, Peake DA, Kuo MS, Wadgaonkar R, Cao G, Jiang XC. Sphingomyelin synthase 2 is one of the determinants for plasma and liver sphingomyelin levels in mice. Arterioscler Thromb Vasc Biol. 2009;29:850–6. doi: 10.1161/ATVBAHA.109.185223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hailemariam TK, Huan C, Liu J, Li Z, Roman C, Kalbfeisch M, Bui HH, Peake DA, Kuo MS, Cao G, Wadgaonkar R, Jiang XC. Sphingomyelin synthase 2 deficiency attenuates NFkappaB activation. Arterioscler Thromb Vasc Biol. 2008;28:1519–26. doi: 10.1161/ATVBAHA.108.168682. [DOI] [PubMed] [Google Scholar]


