Abstract
Objectives
Persons with serious mental illnesses (SMI) have elevated rates of comorbid medical conditions, but may also face challenges in effectively managing those conditions.
Methods
The study team developed and pilot-tested the Health and Recovery Program (HARP), an adaptation of the Chronic Disease Self-Management Program (CDSMP) for mental health consumers. A manualized, six-session intervention, delivered by mental health peer leaders, helps participants become more effective managers of their chronic illnesses. A pilot trial randomized 80 consumers with one or more chronic medical illness to either the HARP program or usual care.
Results
At six month follow-up, participants in the HARP program had a significantly greater improvement in patient activation than those in usual care (7.7% relative improvement vs. 5.7% decline, p=0.03 for group*time interaction), and in rates of having one or more primary care visit (68.4% vs. 51.9% with one or more visit, p=0.046 for group*time interaction). Intervention advantages were observed for physical health related quality of life (HRQOL), physical activity, medication adherence, and, and though not statistically significant, had similar effect sizes as those seen for the CDSMP in general medical populations. Improvements in HRQOL were largest among medically and socially vulnerable subpopulations.
Conclusions
This peer-led, medical self-management program was feasible and showed promise for improving a range of health outcomes among mental health consumers with chronic medical comorbidities. The HARP intervention may provide a vehicle for the mental health peer workforce to actively engage in efforts to reduce morbidity and mortality among mental health consumers.
Keywords: serious mental illness, chronic disease, wellness, recovery, self management
1. Introduction
Persons with serious mental illness (SMI) are at elevated risk for a host of chronic medical conditions, (Jeste DV 1996; Goldman 2000; Dickey et al. 2002; Jones DR 2004; Sokal J 2004; Carney et al. 2006; Carney and Jones 2006; Leucht et al. 2007; Meyer and Nasrallah 2009). At the same time, they also face a series of barriers to effectively managing those illnesses. Physical inactivity (Brown et al. 1999; Daumit et al. 2005), poor diet, (McCreadie R 1998) problems with adherence to somatic medications (Kreyenbuhl et al. 2008), and limited health literacy (Dickerson et al. 2005; Dickerson et al. 2009) may both increase the incidence of illness and raise challenges to managing those conditions after they have developed.
Within the general medical literature, there is a growing recognition of the value of interventions that improve patient self-management of chronic medical conditions (Monninkhof et al. 2003; Chodosh et al. 2005; Effing et al. 2007). These programs work to improve an individual’s ability to manage his or her illness and health behaviors and act as an effective patient. (Hibbard et al. 2004).
Peer specialists make up one of the most rapidly growing segments of the mental health workforce in the US. These peers are trained to work in a variety of different settings to promote mental health recovery and wellbeing (Davidson et al. 1999; Cook 2005; Davidson et al. 2006). Amidst growing concern in the mental health consumer community about elevated morbidity and premature mortality (Parks and Svedsen 2006), mental health consumer leaders are increasingly calling for efforts to incorporate physical health and wellness into existing consumer recovery programs (Fricks 2009). However there are currently no evidence-based interventions available to do so.
This study adapted an established medical disease self-management program to be delivered by, and to, mental health consumers. This manuscript describes the development of the program and results of a pilot study designed to assess its feasibility and potential to improve self-management and health outcomes.
2. Methods
2.1 Overview of the Chronic Disease Self Management Program (CDSMP)
The intervention builds on the Chronic Disease Self Management Program (CDSMP) developed by Lorig et al at the Stanford Patient Education Center (Lorig 1999; Lorig 2006). CDSMP programs are led by two peer educators with chronic medical conditions; any given group typically includes participants with a range of chronic conditions such as diabetes and arthritis. A series of six group sessions addresses self-management tasks that have been found to be common across chronic health conditions (Clark et al. 1991) (Hibbard et al. 2007; Mosen et al. 2007). The elements of the intervention include regular action planning and feedback, modeling of behaviors and problem-solving by participants, reinterpretation of symptoms, and training in specific disease management techniques. In multiple studies, the CDSMP has shown to improve disease self management, health services use, and clinical outcomes (Lorig et al. 1999) (Lorig et al. 2001) (Lorig et al. 2008; Lorig et al. 2009).
2.2 Adapting the Chronic Disease Self Management Program for Mental Health Consumers
The study adapted the CDSMP to mental health consumers using the iterative “ADAPT-ITT” approach for adaptation of evidence-based interventions developed by Wingood and DiClemente (Wingood and DiClemente 2008). An expert panel comprised of mental health consumer leaders, a health educator, and the developer of the CDSMP was convened to consider the specific issues faced by persons with serious mental illness in managing their medical needs, and how these factors should be taken into consideration in modifying the intervention.
Peer leaders led a pre-pilot trial of the unmodified CDSMP with 8 subjects, followed by a series of two focus groups led by the health educator with those participants. The expert panel reviewed the results and then made recommendations about revisions to the manual. The health educator made the appropriate changes, which were reviewed and approved by the expert panel.
While the core structure of the program was retained, several modifications were made to adapt it to the needs and characteristics of mental health consumers. Because of potential gaps in health literacy and cognitive limitations (Dickerson et al. 2005; Dickerson et al. 2009), the manual was simplified to a sixth-grade reading level and a self-management record was added to track disease-specific self-management, medications, upcoming appointments, dietary intake, and physical activity To improve motivation and engagement in care, each participant was paired with a partner from the group, with the two working together toward accomplishing action plans and goals.
Materials were added emphasizing the connection between mind and body, and a section was added about the importance of coordinating information about medications between primary care providers and psychiatrists. The section on medical advanced directives was expanded to also include mental health advanced directives, which specify preferences if a client is unable to make decisions due to psychiatric symptoms.
Finally, the diet and exercise sections were modified to address the high rates of poverty and social disadvantage in this population. The diet section provided strategies for purchasing healthy food on a budget (including using food stamps) and strategies were provided to allow participants to safely exercise in their own homes.
2.3 Randomized Trial
Subsequently, a small randomized trial was conducted at a Community Mental Health Center (CMHC) to establish feasibility, effectiveness, and to inform further studies of the program in this population. All study participants gave written, informed consent, and the study was approved by the University’s Institutional Review Board.
2.3.1 Study Setting
The study was conducted at an urban CMHC. The target population of the facilities is individuals age 18 and older from the area that experience serious and persistent mental illness with or without comorbid addictive disorders.
2.3.2 Sample Recruitment
The sample was recruited through waiting rooms and flyers posted in outpatient clinics at the two facilities. This dual approach has been found to be optimal for recruiting vulnerable populations for health behavior interventions (Harris et al. 2003). To be eligible, subjects had to be on the active patient roster at the CMHC, have a severe mental illness, (National Advisory Mental Health Council 1993) have one or more chronic medical condition, and have the capacity to provide informed consent. Inclusion criteria were kept broad to optimize generalizability.
2.3.3 Measures
Patient activation reflects an individual’s perceived ability to manage his or her illness and health behaviors, and act as an effective patient (Hibbard et al. 2004). This construct was measured using the 13-item Patient Activation Measure (PAM) (Hibbard et al. 2004). Patient activation is calculated on a 0-100 score, with 100 as the highest possible degree of activation.
Disease self-management was assessed using questions about physical activity, health services use, and medication adherence. Questions about physical activity and source of a primary care provider were drawn from the Behavioral Risk Factor Surveillance System (BRFSS) (Stein et al. 1993; Arday et al. 1997; Brownson et al. 1999; Nelson et al. 2001). Medication adherence was assessed using a validated self-report measure of problems in adherence to medication (Morisky et al. 1986).
Health Related Quality of Life (HRQOL) was measured by the SF-36, constructed for use in the Medical Outcomes Study (McHorney et al. 1993; McHorney et al. 1994). A Physical Component Summary (PCS) and Mental Component Summary (MCS) scores were constructed from the survey, scored between 0 (poor health) to 100 (perfect health) (Ware et al. 1995).
In general populations, two groups of factors have been found to place populations at risk of poor self-management and health outcomes: social vulnerability factors including lack of an adequate support network and low SES, and medical vulnerability factors, such as problems in obtaining and maintaining appropriate medical services. (Aday 1994; Gelberg et al. 2000). Thus, exploratory analyses were conducted to see whether the program had differential effects on physical health status among patients who either were medically vulnerable (uninsured or no primary care provider) or socially vulnerable (living alone or unemployed).
2.3.4 Randomization
Using a computerized algorithm, patients were randomized to the intervention or usual care group by the project manager. After randomization, interviews were administered at baseline and again at 6 months post-baseline. Interviewers were blinded to subjects’ randomization status.
2.3.5 Intervention
Two certified mental health peer specialists participated in a community-based, 5-day CDSMP master training course to become master trainers in the CDSMP program. Subsequently, they received 3 additional days of training from the team’s principal investigator and health educator in the Health and Recovery Peer (HARP) program, the adapted version of the CDSMP. The health educator observed the initial sessions and provided detailed feedback to the peer leaders to assess and optimize fidelity to the intervention.
Each subject in the intervention group attended up to 6 group sessions led by mental health peer specialists. Sessions covered following topics related to chronic disease self-management: 1. Overview of self-management 2. Exercise and physical activity 3. Pain and fatigue management 4. Healthy eating on a limited budget 5. Medication management 6. Finding and working with a regular doctor.
During the sessions, peer educators modeled appropriate behaviors and responses, and participation from each group member helped model behavior and improve motivation for other members. Attendees are taught to develop short-term “action plans” for choosing domains of health behavior change. (Lorig et al. 1994) This process involves identifying a problem that is of particular concern, listing ideas for solving the problem, and then developing a plan outlining specific, short-term goals for improvement.
2.3.6 Usual Care
Subjects assigned to usual care continued to receive all medical, mental health, and peer-based services that they were otherwise receiving prior to entry into the study.
2.4. Statistical Analysis
All analyses were conducted as intent-to-treat. Bivariate analyses examined differences between the intervention and usual care groups on demographic and clinical variables at baseline, to assess adequacy of randomization, as well as at the follow-up period. The primary analytic technique for assessing statistically significant changes in outcome variables was random regression. This method makes it possible to compare the difference in change between groups over time, and to conduct intent-to-treat analyses that include subjects with missing data at one or more follow-up periods. Analyses were conducted using the SAS MIXED procedure for continuous variables and PROC GENMOD for binary and ordinal variables. For each outcome measure, the model assessed the outcome as a function of 1) randomization group 2) time since randomization and 3) group*time interaction. The group*time interaction, which reflects the relative difference in change in the parameters over time, was the primary measure of statistical significance.
As a pilot study, the intervention was primarily designed to assess feasibility and effect sizes rather than to assess statistical significance on study outcomes.
3. Results
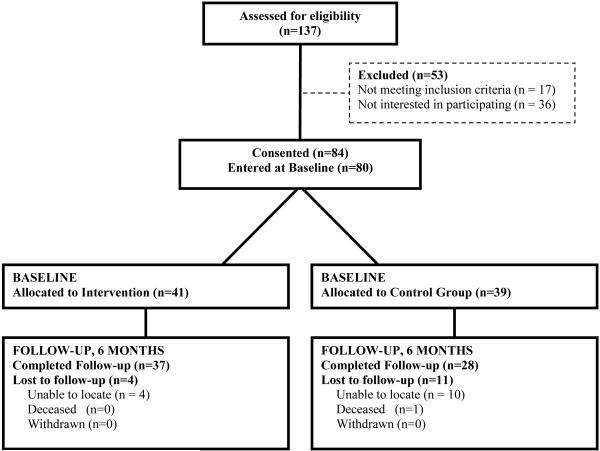
A total of 80 subjects were randomized to either the HARP intervention (n=41) or usual care (n=39). Among those completing baseline assessments, 65 (81.2%) completed the 6-month follow-up. (Figure 1). Participants in the intervention group attended a mean of 4.75 (SD=4.07) and a median of 5 sessions.
Figure 1.
Study Flow Chart
3.1. Baseline Characteristics
The mean age of the population was 48; a majority (82.5%) were African American and most were poor (mean annual income $7,704 ($2,520, $12,306). A total of 20% of participants were uninsured, with the majority having Medicaid and/or Medicare. The most common primary mental diagnoses were bipolar disorder (32.5%), schizophrenia (28.8%), major depression (26.3%) and PTSD (11.3%). The most common medical comorbidities were hypertension (62.5%), arthritis (48.8%), asthma (22.5%), and heart disease (22.5%).
3.2 6- Month Outcomes
At six months, patient activation was clinically and statistically significantly higher in the HARP intervention than the usual care group (52.0 +/−10.1 intervention vs. 44.9 +/−9.6 control, p=0.01). The difference in change over time (7.7% improvement in the intervention vs. 5.7% decline in the usual care) in patient activation was statistically significant in a random effects model (p=0.03).
There was also a significant improvement in the proportion of the sample reporting one or more visit to a primary care provider, with a 8.3% improvement in the HARP group, compared with a 17.1% decline in the usual care group (p=0.04 for the group*time interaction).
At six month follow-up, the intervention group reported an additional 40 minutes per week spent in moderate/vigorous exercise compared to the intervention group (191 vs. 151 minutes/week). While not statistically significant, this 20% relative difference in exercise was nearly identical to the six month effect size for the six month follow-up on the original CDSMP study in a general community sample (111 vs. 91 minutes) (Lorig et al. 1999). This represented a 27% improvement in the intervention group versus a 1.6% decline in the usual care group (group*time interaction not significant).
The scale for medication adherence has a possible range from zero (no problems) to four (more problems). At six month follow-up, the mean scale score for the HARP group was lower (better) than the mean value for the usual care group (1.32 vs. 1.61). This reflected a 14.2% improvement in the intervention group versus a 7.3% decline in the usual care group (group*time interaction not significant).
At six month follow-up the intervention group scored higher on the HRQOL Physical Component Summary than the usual care group (42.9+/−14.2 vs. 40.0+/−13.7). This reflected a twofold larger improvement for the intervention group (16.3% relative increase versus 8.1% improvement for the usual care group, group*time interaction not significant). For the Mental Component Summary, six month scores (36.8+/−10.0 vs. 36.8+/−11.1) and relative improvement (10.5% vs. 8.6%) were smaller (group*time interaction not significant). The HRQOL effect sizes were comparable to those in studies implementing the CDSMP in general medical populations.(Haas et al. 2005)
Exploratory analyses were conducted to see whether the program’s benefits for physical health related quality of life differed across medical and social vulnerability status. Effect sizes for these comparisons indicated substantially greater levels of improvement on the Physical Component Summary in populations with medical vulnerability (23.3% improvement on the PCS for uninsured clients vs. 11.1% improvement for those with insurance; 31% improvement on the PCS for those without a primary care provider vs. 2.9% improvement for those without one). There were also substantially larger effects among the subgroup with social vulnerability (14.3% improvement for those living alone versus 8.9% improvement for those living with a companion; 29.8% improvement for unemployed versus 4.1% for employed). Given the small cell sizes for these moderator analyses, none of these effect sizes were statistically significant.
4. Discussion
The Health and Recovery Peer (HARP) Program, an adapted version of the Chronic Disease Self-Management Program, was feasible to implement and showed promise in improving a range of self-management and health outcome measures, including significant improvements in patient activation and greater likelihood of using primary care medical services. These improvements appeared to be greatest in populations with financial and social disadvantage.
The study was associated with a substantial and statistically significant degree of improvement in patient activation, a measure of an individual’s self-management capacity. (Hibbard et al. 2004; Hibbard et al. 2007; Mosen et al. 2007) There has been increasing attention in the medical literature about the importance of patient activation both in guiding clinical care and predicting outcomes. In longitudinal studies, positive changes in patient activation have been found to be associated with improved self-management behaviors (Hibbard et al. 2007), medication adherence, and outcomes including quality of life (Mosen et al. 2007). Coupled with the other study findings, these results suggest the intervention’s potential to improve other more downstream health outcomes.
Although the focus of this intervention was on improving patient rather than on provider or system-level determinants of health use, there was a significant improvement in the proportion of persons using primary care services. In general populations, the CDSMP has been demonstrated to help direct patients toward appropriate modalities of care and away from more costly services. (Lorig et al. 1999), Future studies should examine how the HARP program affects a broader range of health services such as emergency room visits and inpatient hospitalization as well as its ability to improve quality of medical care.
Across other key study outcomes, the intervention was associated with effect sizes comparable or better to those seen for the CDSMP in general medical populations. The study found larger effect sizes for Health Related Quality of Life than have been reported in similar trials in general clinical populations. (Haas et al. 2005) (Jerant et al. 2009). This suggests considerable potential for these programs to improve care in this population, given their high levels of medical and psychosocial need.
Within the study sample, the intervention appeared to be particularly beneficial among medically and socially disadvantaged subgroups. This finding is consistent with other studies suggesting that mental health quality improvement programs may differentially improve the least well-off individuals, thereby reducing disparities in care. (Wells et al. 2000). Again, this is likely due to the fact that the most disadvantaged groups have the most to gain from efforts to improve their health care and self management skills.
As a pilot study, the study had several limitations, including reliance on self-report outcome measures, a relatively brief follow-up period, and lack of adequate power to assess statistical significance for many of the study outcomes. Further testing using a broader range of outcome measures, longer follow-up periods, and larger sample sizes will be needed to establish the HARP program as an evidence-based practice.
Although medical disease management and the mental health consumer recovery movement grew up independently, there are a number of striking parallels between the two. The core features described for mental health recovery --peer support, holistic orientation, self-direction, and person-centeredness (Substance Abuse and Mental Health Services Administration 2006) – all are also central features of the CDSMP. These similarities suggest the potential for each of these approaches to inform the other. Medical disease self-management programs may increasingly be integrated into peer workforce to improve health and healthcare for mental health consumers. At the same time, the recovery model may provide a useful approach for understanding how all persons with all chronic illnesses – both medical and mental health - can lead fuller and healthier lives.
Table 1. Baseline Characteristics.
| variable | HARP (n=41) | Usual Care (n=39) | p value |
|---|---|---|---|
| Race | 0.07 | ||
| African American | 30 (73.2%) | 36 (92.3%) | |
| White | 10 (24.4%) | 3 (7.7%) | |
| Other | 1 (2.4%) | 0 (0%) | |
| Gender | 0.58 | ||
| Female | 27 (65.9%) | 29 (74.4%) | |
| Male | 14 (34.1%) | 10 (25.6%) | |
| Age | 47.8+/−10.1 | 48.4+/−10.1 | 0.92 |
| Insurance | 0.62 | ||
| No Insurance | 9 (22.0%) | 7 (17.9%) | |
| 30 (73.2%) | 29 (74.4%) | ||
| Medicare/Medicaid | |||
| Private Insurance | 2 (4.9%) | 2 (5.1%) | |
| Annual income | $10,620 (4560- 14,400) |
$7476 (2400-9444) | 0.20 |
| Mental Diagnosis | |||
| Schizophrenia | 11 (26.8%) | 12 (30.8%) | 0.60 |
| Bipolar Disorder | 14 (34.1%) | 12 (30.8%) | 0.70 |
| Major Depression | 9 (22.0%) | 12 (30.8%) | 0.31 |
| PTSD | 7 (17.1%) | 2 (5.1%) | 0.11 |
|
Medical
Comorbidity |
|||
| Hypertension | 25 (60.9%) | 25 (64.1%) | 0.57 |
| Arthritis | 23 (56.1%) | 16 (41.0%) | 0.26 |
| Asthma | 10 (24.4%) | 8 (20.5%) | 0.82 |
| Heart Disease | 10 (24.4%) | 8 (20.5%) | 0.77 |
Table 2. 6-Month Outcomes.
| HARP (n=41) | Usual Care (n=39) |
p value | p value for group*time interaction |
|
|---|---|---|---|---|
| Patient Activation (0-100 ) | 0.03 | |||
| Baseline | 48.3 +/−11.5 | 47.6+/−12.3 | 0.96 | |
| 6-months | 52.0+/−10.1 | 44.9+/−9.6 | 0.01 | |
|
One or More Primary Care
Visit | ||||
| Baseline | 24 (58.5%) | 23 (61.1%) | 0.60 | 0.046 |
| 6-months | 26 (68.4%) | 14 (51.9%) | 0.18 | |
|
Moderate or Vigorous
Physical Activity (minutes/week) | ||||
| Baseline | 150+/−236 | 154+/−194 | 0.88 | 0.40 |
| 6-months | 191+/−278 | 152+/249 | 0.14 | |
|
Medication Adherence
(lower score indicates better adherence) | ||||
| Baseline | 1.5+/−1.2 | 1.5+/−1.4 | 0.78 | 0.22 |
| 6-months | 1.3+/−1.3 | 1.6+/−1.4 | 0.25 | |
|
Physical Health Related
Quality of Life (higher score indicates better health) | ||||
| Baseline | 36.9+/−10.3 | 37.0+/−12.5 | 0.98 | 0.41 |
| 6-months | 42.9+/−14.2 | 40.0+/−13.7 | 0.27 | |
|
Mental Health Related
Quality of Life (higher score indicates better health) | ||||
| baseline | 33.3+/−9.13 | 33.9+/−9.3 | 0.80 | 0.96 |
| 6-months | 36.8+/−10.0 | 37.0+/−11.8 | 0.95 | |
4. Acknowledgements
The study would like to acknowledge the support of the DeKalb Community Service Board, where the trial was conducted.
1. Role of Funding Source.
The study was funded by NIMH R34MH078583. The study sponsor had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
3. Conflict of Interest
Dr. Lorig receives royalties from Bull Publisher for being an author of Living a Healthy Life with Chronic Conditions. This book was written for the self-management course and is used in this study. All other authors declare that they have no conflict of interest.
REFERENCES
- Aday LA. Health status of vulnerable populations. Annu Rev Public Health. 1994;15:487–509. doi: 10.1146/annurev.pu.15.050194.002415. [DOI] [PubMed] [Google Scholar]
- Arday DR, Tomar SL, et al. State smoking prevalence estimates: a comparison of the Behavioral Risk Factor Surveillance System and current population surveys. Am J Public Health. 1997;87(10):1665–9. doi: 10.2105/ajph.87.10.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Birtwistle J, et al. The unhealthy lifestyle of people with schizophrenia. Psychol Med. 1999;29(3):697–701. doi: 10.1017/s0033291798008186. [DOI] [PubMed] [Google Scholar]
- Brownson RC, Eyler AA, et al. Reliability of information on physical activity and other chronic disease risk factors among US women aged 40 years or older. Am J Epidemiol. 1999;149(4):379–91. doi: 10.1093/oxfordjournals.aje.a009824. [DOI] [PubMed] [Google Scholar]
- Carney CP, Jones L, et al. Medical comorbidity in women and men with schizophrenia: a population-based controlled study. J Gen Intern Med. 2006;21(11):1133–7. doi: 10.1111/j.1525-1497.2006.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney CP, Jones LE. Medical comorbidity in women and men with bipolar disorders: a population-based controlled study. Psychosom Med. 2006;68(5):684–91. doi: 10.1097/01.psy.0000237316.09601.88. [DOI] [PubMed] [Google Scholar]
- Chodosh J, Morton SC, et al. Meta-analysis: chronic disease self-management programs for older adults. Ann Intern Med. 2005;143(6):427–38. doi: 10.7326/0003-4819-143-6-200509200-00007. [DOI] [PubMed] [Google Scholar]
- Clark N, Becker M, et al. Self-management of chronic illness. Journal of Aging and Health. 1991;3:3–27. [Google Scholar]
- Cook J. “Patient-Centered” and “Consumer-Directed” Mental Health Services: A Report Prepared for the Institute of Medicine. University of Illinois at Chicago; Chicago, Ill: 2005. [Google Scholar]
- Daumit GL, Goldberg RW, et al. Physical activity patterns in adults with severe mental illness. J Nerv Ment Dis. 2005;193(10):641–6. doi: 10.1097/01.nmd.0000180737.85895.60. [DOI] [PubMed] [Google Scholar]
- Davidson L, Chinman M, et al. Peer support among individuals with severe mental illness: a review of the evidence. Clin Psychol Sci Prac. 1999;6(6):165–187. [Google Scholar]
- Davidson L, Chinman M, et al. Peer support among adults with serious mental illness: a report from the field. Schizophr Bull. 2006;32(3):443–50. doi: 10.1093/schbul/sbj043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson FB, Goldberg RW, et al. Diabetes knowledge among persons with serious mental illness and type 2 diabetes. Psychosomatics. 2005;46(5):418–24. doi: 10.1176/appi.psy.46.5.418. [DOI] [PubMed] [Google Scholar]
- Dickerson FB, Kreyenbuhl J, et al. A 5-year follow-up of diabetes knowledge in persons with serious mental illness and type 2 diabetes. J Clin Psychiatry. 2009;70(7):1057–8. doi: 10.4088/jcp.08l04602. [DOI] [PubMed] [Google Scholar]
- Dickey B, Normand SL, et al. Medical morbidity, mental illness, and substance use disorders. Psychiatr Serv. 2002;53(7):861–7. doi: 10.1176/appi.ps.53.7.861. [DOI] [PubMed] [Google Scholar]
- Effing T, Monninkhof EM, et al. Self-management education for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2007;(4) doi: 10.1002/14651858.CD002990.pub2. CD002990. [DOI] [PubMed] [Google Scholar]
- Fricks L. Consumers take charge of wellness. National Council Magazine. 2009 Winter;:20–22. 2009. [Google Scholar]
- Gelberg L, Andersen RM, et al. The Behavioral Model for Vulnerable Populations: application to medical care use and outcomes for homeless people. Health Serv Res. 2000;34(6):1273–302. [PMC free article] [PubMed] [Google Scholar]
- Goldman L. Comorbid medical illness in psychiatric patients. Curr Psychiatry Rep. 2000;2(3):256–63. doi: 10.1007/s11920-996-0019-x. [DOI] [PubMed] [Google Scholar]
- Haas M, Groupp E, et al. Chronic disease self-management program for low back pain in the elderly. J Manipulative Physiol Ther. 2005;28(4):228–37. doi: 10.1016/j.jmpt.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Harris KJ, Ahluwalia JS, et al. Successful recruitment of minorities into clinical trials: The Kick It at Swope project. Nicotine Tob Res. 2003;5(4):575–84. doi: 10.1080/1462220031000118540. [DOI] [PubMed] [Google Scholar]
- Hibbard JH, Mahoney ER, et al. Do increases in patient activation result in improved self-management behaviors? Health Serv Res. 2007;42(4):1443–63. doi: 10.1111/j.1475-6773.2006.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbard JH, Stockard J, et al. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39(4 Pt 1):1005–26. doi: 10.1111/j.1475-6773.2004.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerant A, Moore-Hill M, et al. Home-based, peer-led chronic illness self-management training: findings from a 1-year randomized controlled trial. Ann Fam Med. 2009;7(4):319–27. doi: 10.1370/afm.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste DV, G J, Lindamer LA, Lacro JP. Medical comorbidity in schizophrenia. Schizophr Bull. 1996;22(3):413–30. doi: 10.1093/schbul/22.3.413. [DOI] [PubMed] [Google Scholar]
- Jones DR, M C, Barreira PJ, Fisher WH, Hargreaves WA, Harding CM. Prevalence, Severity, and Co-occurrence of Chronic Physical Health Problems of Persons With Serious Mental Illness. Psychiatr Serv. 2004;55(11):1250–1257. doi: 10.1176/appi.ps.55.11.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreyenbuhl J, Dixon LB, et al. Does Adherence to Medications for Type 2 Diabetes Differ Between Individuals With Vs Without Schizophrenia? Schizophr Bull. 2008 doi: 10.1093/schbul/sbn106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht S, Burkard T, et al., editors. Physical Illness and Schizophrenia: A Review of the Evidence. Cambridge University Press; New York: 2007. [DOI] [PubMed] [Google Scholar]
- Lorig K. Chronic Disease Self Management Leader’s Manual. Stanford Patient Education Research Center; Palo Alto, CA: 1999. [Google Scholar]
- Lorig K. Chronic Disease Self Management Leader’s Manual. Stanford Patient Education Research Center; Palo Alto, CA: 2006. [Google Scholar]
- Lorig K, Holman H, et al. Living a Healthy Life with Chronic Conditions. Bull Publishing Company; Palo Alto, CA: 1994. [Google Scholar]
- Lorig K, Ritter PL, et al. Community-Based Peer-Led Diabetes Self-management: A Randomized Trial. Diabetes Educ. 2009 doi: 10.1177/0145721709335006. [DOI] [PubMed] [Google Scholar]
- Lorig KR, Ritter P, et al. Chronic disease self-management program: 2-year health status and health care utilization outcomes. Med Care. 2001;39(11):1217–23. doi: 10.1097/00005650-200111000-00008. [DOI] [PubMed] [Google Scholar]
- Lorig KR, Ritter PL, et al. The internet-based arthritis self-management program: a one-year randomized trial for patients with arthritis or fibromyalgia. Arthritis Rheum. 2008;59(7):1009–17. doi: 10.1002/art.23817. [DOI] [PubMed] [Google Scholar]
- Lorig KR, Sobel DS, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: a randomized trial. Med Care. 1999;37(1):5–14. doi: 10.1097/00005650-199901000-00003. [DOI] [PubMed] [Google Scholar]
- McCreadie R, M E, Blacklock C, Tilak-Singh D, Wiles D, Halliday J, Paterson J. Dietary intake of schizophrenic patients in Nithsdale, Scotland: case-control study. BMJ. 1998;317(7161):784–5. doi: 10.1136/bmj.317.7161.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHorney CA, Ware JE, Jr., et al. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32(1):40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- McHorney CA, Ware JE, Jr., et al. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- Meyer J, Nasrallah H, editors. Medical illness and schizophrenia. American Psychiatric Press; Washington, DC: 2009. [Google Scholar]
- Monninkhof EM, van der Valk PD, et al. Self-management education for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2003;(1) doi: 10.1002/14651858.CD002990. CD002990. [DOI] [PubMed] [Google Scholar]
- Morisky DE, Green LW, et al. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- Mosen DM, Schmittdiel J, et al. Is patient activation associated with outcomes of care for adults with chronic conditions? J Ambul Care Manage. 2007;30(1):21–9. doi: 10.1097/00004479-200701000-00005. [DOI] [PubMed] [Google Scholar]
- National Advisory Mental Health Council Health care reform for Americans with severe mental illness: report of the National Advisory Mental Health Council. Am J Psychiatry. 1993;150:1447–1465. doi: 10.1176/ajp.150.10.1447. [DOI] [PubMed] [Google Scholar]
- Nelson DE, Holtzman D, et al. Reliability and validity of measures from the Behavioral Risk Factor Surveillance System (BRFSS) Soz Praventivmed. 2001;46(Suppl 1):S3–42. [PubMed] [Google Scholar]
- Parks J, Svedsen D. Morbidity and Mortality in People with Serious Mental Illness. National Association of State Mental Health Program Directors; Alexandria, VA: 2006. [Google Scholar]
- Sokal J, M E, Dickerson FB, Kreyenbuhl J, Brown CH, Goldberg RW, Dixon LB. Comorbidity of medical illnesses among adults with serious mental illness who are receiving community psychiatric services. J Nerv Ment Dis. 2004;192(6):421–7. doi: 10.1097/01.nmd.0000130135.78017.96. [DOI] [PubMed] [Google Scholar]
- Stein AD, Lederman RI, et al. The Behavioral Risk Factor Surveillance System questionnaire: its reliability in a statewide sample. Am J Public Health. 1993;83(12):1768–72. doi: 10.2105/ajph.83.12.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration National Consensus Statement on Mental Health Recovery. 2009 2006. from http://mentalhealth.samhsa.gov/publications/allpubs/sma05-4129/
- Ware JE, Jr., Kosinski M, et al. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33(4 Suppl):AS264–79. [PubMed] [Google Scholar]
- Wells KB, Sherbourne C, et al. Impact of disseminating quality improvement programs for depression in managed primary care: a randomized controlled trial. JAMA. 2000;283(2):212–20. doi: 10.1001/jama.283.2.212. [DOI] [PubMed] [Google Scholar]
- Wingood GM, DiClemente RJ. The ADAPT-ITT model: a novel method of adapting evidence-based HIV Interventions. J Acquir Immune Defic Syndr. 2008;47(Suppl 1):S40–6. doi: 10.1097/QAI.0b013e3181605df1. [DOI] [PubMed] [Google Scholar]