Abstract
During a severe local or systemic inflammatory response, immune mediators target lung tissue. This process may lead to acute lung injury and impaired diffusion of gas molecules. Although several mathematical models of gas exchange have been described, none simulate acute lung injury following inflammatory stress. In view of recent laboratory and clinical progress in the understanding of the pathophysiology of acute lung injury, such a mathematical model would be useful. We first derived a partial differential equations model of gas exchange on a small physiological unit of the lung (325 alveoli), which we refer to as a respiratory unit (RU). We next developed a simple model of the acute inflammatory response and implemented its effects within a RU, creating a single RU model. Linking multiple RUs with various ventilation/perfusion ratios and taking into account pulmonary venous blood remixing yielded our lung-scale model. Using the lung-scale model, we explored the predicted effects of inflammation on ventilation/perfusion distribution and the resulting pulmonary venous partial pressure oxygen level during systemic inflammatory stresses. This model represents a first step towards the development of anatomically faithful models of gas exchange and ventilation under a broad range of local and systemic inflammatory stimuli resulting in acute lung injury, such as infection and mechanical strain of lung tissue.
Keywords: Acute inflammation, Acute Lung Injury, Immunology, Lung Edema
Introduction
There is a substantial literature on mathematical models of gas exchange, that have focused on different aspects of the process of gas transfer and distribution between ambient air and blood, including distribution of ventilation, transport between alveolar air space and pulmonary capillaries, red blood cell rheology, hemoglobin dynamics and acid-base physiology (Ben-Tal, 2006). These models span different anatomical scales and have generally been useful to study airflow distribution, deposition of particulate drugs in airways, lung mechanics and shunt physiology.
New imaging techniques (Moller et al., 2002; Tawhai et al., 2003), cell-type identification tools (Olson et al., 2002) and high-throughput methods are providing a unique opportunity to construct and calibrate increasingly complex and realistic models that integrate the large body of existing knowledge with these newer data and provide modeling tools with significant translation impact into the understanding, and modification of, human disease. In the realm of critical care medicine, acute lung injury (ALI) is an inflammatory process that can be triggered by chemical, infectious or traumatic stimuli. ALI typically requires mechanical ventilatory support, is morbid and the most frequent manifestation of multisystem organ dysfunction, the leading cause of death in the ICU (Rubenfeld et al., 2007). ALI also has long-term consequences in those who survive the acute illness. Substantial public and corporate resources are devoted to prevent and treat ALI. There are also good animal models of ALI (Delclaux et al., 1997) and its pathophysiology is increasingly well understood (Ware, 2006).
To our knowledge, existing modeling efforts have not attempted to describe the progression of ALI following a local or systemic inflammatory stimulus. We therefore describe such an attempt, where the direct effects of inflammatory mediators on lung tissue are modeled, along with their impact on lung volumes, lung water content and gas exchange ability. This is the first step in constructing a truly multiscale representation of gas exchange under inflammatory stress, with an explicit representation of the time evolution of ventilation-perfusion inequalities under such conditions, thus linking cell and tissue level inflammation, physical and physiological alveolar phenonema at the mesoscale, to global gas exchange at the scale of the whole organ. Because this study is the first step in constructing such a framework, the process of model development is described in considerable detail, and the simulations presented do not explore the full flexibility of the model.
Methods
Overview
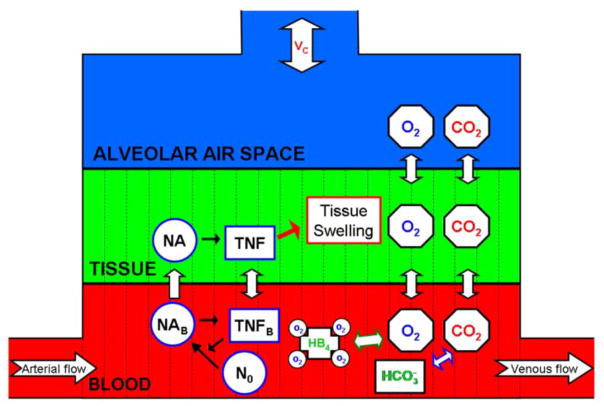
In order to explore the lung during an inflammatory response we modeled gas exchange and the inflammatory response within a small portion of the lung. We consider the local dynamics on a respiratory unit (RU), which consists of 25 alveoli and the capillaries surrounding them (Guyton et al., 2000). We constructed a model of gas exchange within a RU consisting of partial differential equations for oxygen, oxyhemoglobin, carbon dioxide, and bicarbonate. Figure 1 illustrates the interactions included within the single RU model.
Figure 1. Model Schematic for a single RU.
Interactions included within the single RU model. Resting neutrophils, activated neutrophils in the tissue and blood are represented by N0, NA, and NAB, respectively. The subscript B on TNF designates blood TNF. Arrows across the barriers between the compartments represent diffusion. Black arrows represent upregulation. The green arrow between HB4 and O2 represents the binding and unbinding of O2 to HB4. The white/blue arrow between CO2 and represents the enzymatic reactions between CO2 and . The red arrow represents the interactions between the immune and the gas exchange subsystems.
A RU consists of three compartments, the alveolar air space, lung tissue and capillary blood. There are variables expressing the partial pressure of oxygen (PO2) and carbon dioxide (PCO2) in all three compartments, while oxyhemoglobin (HB4) and bicarbonate ( ) are only in the blood. We model the following biological processes, diffusion of oxygen and carbon dioxide, hemoglobin uptake of oxygen, and enzymatic reactions governing carbon dioxide and bicarbonate levels. We assume diffusion occurs across two distinct barriers within the RU, the blood/tissue and tissue/alveolar air space barriers. Tidal breathing regulates alveolar gas pressures of oxygen and carbon dioxide within the alveolar space. Following the development of the gas exchange subsystem of the model, we incorporate a response to an inflammatory stimulus, the inflammation subsystem, leading to the development of ALI. This additional subsystem consists of equations for neutrophils and tumor necrosis factor (TNF), a canonical pro-inflammatory cytokine, in both the blood and tissue compartment. This immune subsystem has fewer details than other inflammation models (Chow et al., 2005; Daun et al., 2008), but has an explicit spatial domain.
The RU is modeled as a one-dimensional spatial domain along the length of the capillary with a uniform grid. Therefore, gas molecules in the blood and tissue and HB4 and in the blood all vary in time and space. We assume alveolar air space to be well mixed. Under this assumption, variables in this compartment are functions of time only. Space along the compartment is modeled as N units of equal length and variables are modeled on each unit. Therefore, the variable names are oxygen/carbon dioxide in blood on unit i, PO2Bi/PCO2Bi, in the tissue on unit i, PO2Ti/PCO2Ti, and in the alveolar air space, PO2A/PCO2A. Oxyhemoglobin and bicarbonate on unit i, are HB4i and , respectively. After developing the gas exchange subsystem, we will derive the immune variables based on their known interactions and effects on gas exchange.
Diffusion
Diffusion, the exchange of molecules and cells across both the alveolar air space/tissue and tissue/blood barriers, is the only form of interaction between the compartments. Previous models for gas exchange depict diffusion as movement from the alveolar space to the blood across a tissue barrier (Ben-Tal, 2006; Hahn et al., 1993; Hill et al., 1973; Keener et al., 1998). However, we consider lung tissue as a compartment, not merely a barrier. We have chosen this approach to better reflect the impact of tissue volume changes caused by inflammation on gas exchange between air and blood. In order to properly model the effects of inflammation, we derive terms for diffusion driven by PO2 and PCO2 gradients across each barrier, tissue/blood and air/tissue.
Gas diffusion across the tissue/blood and tissue/air barriers is described in Equations (1)–(3). Detailed descriptions of the gas exchange parameters are in Table 1. The parameters scale partial pressure to molar concentration, for example, in compartment j. The parameters expressing overall diffusion rates from compartments j to and from k are of the form . The volumes of each compartment blood, tissue, and alveolar air space are represented by VB0, VT0, and VA0, respectively. In order to derive these terms we initially considered the number of oxygen molecules diffusion across the barrier on each unit, which is a dependent on the partial pressure gradient between compartments. For example, expresses the number of oxygen molecules diffusing from tissue to blood per second on the ith unit of space. The diffusion rate is assumed to be the same on each spatial unit. Therefore the rate on a given unit is . To calculate the change in partial pressure due to diffusion across the barrier on each compartment we divided the number of oxygen molecules which are diffusing by volume on the spatial unit (i.e. VB0/N) and σ for their respective compartments. The resulting expression are the first terms of Equations (1) and (2).
Table 1.
Gas Exchange Parameters
| Parameter | Value | Description | Comments | Source | |
|---|---|---|---|---|---|
| L |
|
Length of RU along the capillary | Estimated by assuming that an individual alveolus diameter is 0.02 cm and that as the blood traverses the lung it is in contact with half of the circumference of seven alveoli. | (Guyton et al., 2000) | |
| h |
|
Length of each unit of space along the capillary | We use 20 spatial units in our calculations. | ||
| tin | 1 second | Length of inspiration | Total time for breathing cycle is assumed to be 4 seconds, 15 breaths per minute. The inhalation time is one forth of the total time. | ||
| tout | 3 seconds | Length of expiration | See above comment for tin. | ||
| V0 | 0.29 cm/sec | Speed of blood | We estimated the range for v0 0.01788–0.4398 cm/sec, by assuming transient times for blood between 0.5 to 12.3 seconds with the capillary length calculated above. We took 0.75 seconds to be our transient time under normal conditions. | ||
| PO2B0 | 40 mmHg | Pulmonary arterial PO2 | (Guyton et al., 2000) | ||
| PCO2B0 | 45 mmHg | Pulmonary arterial PCO2 | (Guyton et al., 2000) | ||
| PO2air | 150 mmHg | Air PO2 | (Guyton et al., 2000) | ||
| PCO2air | 0 mmHg | Air PCO2 | (Guyton et al., 2000) | ||
| iHB4 | 1.7×10−3 M | Arterial concentration of saturated hemoglobin | Found by fitting our oxyhemoglobin curve such that hemoglobin is 50% saturated at a PO2 of 27 mmHg and 99% saturated at a PO2 of 100 mmHg and assuming PO2B0 is 40 mmHg | (Keener et al., 1998) | |
|
|
2.4×10−2 M | Arterial concentration of bicarbonate. | (Guyton et al., 2000) | ||
|
|
6.7×10−12 L/s | Rate constant for the diffusion of oxygen between tissue and blood. | |||
|
|
2.4 ×10−12 L/s | Rate constant for the diffusion of oxygen between tissue and air. | |||
|
|
8.5 ×10−11 L/s | Rate constant for the diffusion of carbon dioxide between tissue and blood. | |||
|
|
7.6 ×10−10 L/s | Rate constant for the diffusion of carbon dioxide between tissue and air. | |||
|
|
1.2 ×10−6 M/mmHg | in the blood. | Calculated from the solubility coefficient for oxygen in water. | (Guyton et al., 2000) | |
|
|
1.2 ×10−6 M/mmHg | in the tissue. | Calculated from the solubility coefficient for oxygen in water. | (Guyton et al., 2000) | |
|
|
5.2 ×10−5 M/mmHg | in the air. | Calculated from nRT = PV. | ||
|
|
3.0 ×10−5 M/mmHg | in the blood. | Calculated from the solubility coefficient for carbon dioxide in water. | (Guyton et al., 2000) | |
|
|
3.0 ×10−5 M/mmHg | in the tissue. | Calculated from the solubility coefficient for carbon dioxide in water. | (Guyton et al., 2000) | |
|
|
5.2 ×10−5 M/mmHg | in the air. | Calculated from nRT = PV. | ||
| Nru | 1.2 ×107 | Number of RU’s in the lung. | Calculated assuming that there are 3×108 alveoli in the lung and that each RU consists of 25 alveoli. | (Guyton et al., 2000) | |
| VB0 |
|
Blood volume: blood volume in the capillaries of a RU under normal conditions. | There is between 0.07–0.14L of blood in the lung capillaries. For our simulations, we assume under normal condition that the blood volume is 0.09L. | (Keener et al., 1998) | |
| VT0 |
|
Tissue Volume: tissue volume in a RU under normal conditions. | Calculated assuming that there is 0.5L of tissue in the lung. | ||
| VAmin0 |
|
Minimum of the alveolar air space: volume of the alveolar air space in a RU at the end of expiration under normal conditions. | Calculated assuming that the minimum volume in the lung is 2.3L. | (Guyton et al., 2000) | |
| Vc |
|
Alveolar Ventilation: the volume inhaled into a RU during a breath under normal conditions. | Calculated assuming that alveolar ventilation for the whole lung was 0.35 L. | ||
|
|
1.8×105 | Ratio of the reverse and forward reaction rates for the saturation of hemoglobin. | This ratio was found by fitting the oxyhemoglobin saturation curve as described for iHB4. | ||
| m | 3.6 | Number of oxygen molecules needed to bind to Hbm in order to form Hb4. | This parameter was found by fitting the oxyhemoglobin saturation curve as described for iHB4. | ||
| k+ | 5×10−4/s | Rate at which m oxygen molecules bind to Hbm to form Hb4. | This parameter was chosen to ensure the hemoglobin and oxygen dynamics stabilize in first third of the capillary. | (Guyton et al., 2000) | |
| THB | 2.2×10−3 M | Concentration of total hemoglobin, HB4 + HBm. | (Guyton et al., 2000) | ||
| kcatCB | 8.8×105/s | Maximum rate of the enzymatic reaction with CO2 as the substrate. | Fit such that the carbon dioxide and bicarbonate dynamics stabilize in the first tenth of the capillary. | (Guyton et al., 2000) | |
| kmCB | 0.01 M | Michaelis-Menten Constant, concentration were the reaction occurs at half the maximum rate, for the enzymatic reaction with CO2 as the substrate. | See comment for kcatCB. | ||
| kcatHC | 2.0×105/s | Maximum rate of the enzymatic reaction with as the substrate. | See comment for kcatCB. | ||
| kmHC | 0.02 M | Michaelis-Menten Constant, concentration were reaction occurs at half the maximum rate, for the enzymatic reaction with as the substrate. | See comment for kcatCB. | ||
| DO2T | 3.2×10−5 cm2/s | Oxygen diffusion constant in water. | (MacDougall et al., 1967) | ||
| DCT | 6.5×10−4 cm2/s | The diffusion constant for diffusion of carbon dioxide within the tissue. | Diffusion of carbon dioxide is 20 times faster than that of oxygen. | (Guyton et al., 2000) | |
| σ | 20 | This parameter determines the rise in sh(t) our approximation to the Heaviside function. | Increasing this parameter sharpens the rise in sh(t). | ||
| τ1 | 0.5 s | Time constant for inspiration. | Chosen so that VC0 is inhaled in tin seconds. | ||
| τ2 | 0.7 s | Time constant for expiration. | Chosen so that VC0 is exhaled in tout seconds. |
| (1) |
| (2) |
| (3) |
Diffusion across the tissue/air barrier is more complex because the alveolar air space compartment is assumed to be well mixed while the tissue compartment is not. As in above derivation, is the number of molecules that will diffuse from air to tissue per second on each spatial unit. In the tissue compartment the derviation is the same as above. This term is multiplied by −1, since it appears in the tissue equation and we divided by VT0/N and , in order to calculate the change in PO2Ti. This then simplifies to the second term of Equation (2). For the change in partial pressure for the air compartment one sums over all units to account for exchange with each unit of the tissue and then divides by the volume of alveolar airspace, VA0, and obtaining Equation (3).
Blood/O2
As blood traverses the capillary through a RU, oxygen diffuses into the blood and reacts with hemoglobin. Rather than accounting for the full dynamics of all four oxygen binding sites on hemoglobin tetramers, we consider hemoglobin as being either partially saturated (HBm) or fully saturated (HB4), where m is a Hill factor derived empirically from the oxyhemoglobin dissociation curve (Keener et al., 1998). We refer to HBm as unsaturated hemoglobin. Oxygen binds to HBm to form HB4 at the rate k+ and it detaches at the rate k− from HB4, to return to HBm. The reaction between unsaturated and saturated hemoglobin and oxygen is modeled by Equations (4)–(6).
| (4) |
| (5) |
| (6) |
Total concentration hemoglobin, THB, is conserved. Thus, HBm can be replaced with THB-HB4 and equations (4)–(6) reduce to equations (7) and (8).
| (7) |
| (8) |
The temporal dynamics are much faster than the spatial dynamics. Therefore, we assume quasi steady state for the saturated hemoglobin when determining parameter values. This is necessary to develop an equation for percent saturation of hemoglobin that is dependent on PO2B only. We find that percent saturation of hemoglobin is an increasing sigmoid function of the partial pressure of oxygen, . Using the data for this curve we find that m=3.6 andRH = k−/k+ = 177916 ((Guyton et al., 2000). Incorporating flow of these molecules in the blood and the diffusion terms described earlier yields Equations (9)–(10). The speed of blood (cm/s) is represented by v0. This subsystem now includes both the spatial and temporal dynamics for O2 in the blood. Equations (11) and (12) are the discretized version of (9) and (10), were ∇i is the discrete approximation to the first derivative.
| (9) |
| (10) |
| (11) |
| (12) |
Blood/CO2
As dissolved CO2 and bicarbonate traverses the RU, bicarbonate releases CO2 into the blood stream. This occurs by a catalyzed enzymatic reaction in which bicarbonate is the substrate. The reverse reaction is of the same type, however, with CO2 as the substrate (Bidani et al., 1988). These reactions are modeled by the first two terms of Equations (13)–(14).
These terms are Michaelis-Menten type where the kcat parameters represent the maximum rate of the catalytic reaction and km is the Michaelis constant, the level of the substrate for which the reaction occurs at half kcat. The variable is PCO2, hence the substrate in terms of the equation variables is , the concentration of CO2. Finally, we incorporate blood flow and diffusion between tissue and blood in this subsystem as done previously for O2 in the blood. The CO2/bicarbonate subsystem in the blood including temporal and spatial dynamics is described by Equations (13) and (14). Equations (15) and (16) are the discretized form of this subsystem.
| (13) |
| (14) |
| (15) |
| (16) |
Tissue/O2 & CO2
Within tissue, the only interaction modeled other than the diffusion between compartments is the diffusion of gas molecules along the tissue, expressed by the reaction-diffusion Equations (17)–(18), where is the discrete approximation to the second derivative.
| (17) |
| (18) |
Alveolar O2 and CO2
An average healthy human breathes about 15 times a minute with an inspiration:expiration time ratio of 1:3. We assumed a total tidal breath of 500 ml, with 150 ml of dead space ventilation, yielding an effective alveolar ventilation of 350 ml per breath, with residual total alveolar volume at the end of expiration 2300 ml (Guyton et al., 2000). This corresponds to an influx of 2.92×10−5 ml of new gas per RU per breath, termed VC0 and referred to as alveolar ventilation, and an end expiration (residual) volume of 1.92×10−4 ml per RU (VAmin0). In order to develop equations for the changes in partial pressures of O2 and CO2 in alveolar gas, we first derive equations for the change in alveolar volume on a RU, Equations (19)–(21).
| (19) |
| (20) |
| (21) |
Equation (19) is a smooth heavy side function that is approximately one during inspiration and zero during expiration. The length of inspiration is tin=1 second and length of expiration is tout=3 seconds. The parameter σ controls the abruptness of the function’s switch from one to zero. For our calculations we set σ to 20. The variable y in Equation (20) is a measure of the alveolar volume change. During inspiration there is growth in y and it decays during expiration. The τ’s are chosen such that in tin seconds y reaches VC0 and decays to zero in tout seconds. Therefore, the equation of alveolar air volume, Equation (21), is VAmin0 at end of expiration and VAmin0+VC0 at the end of inspiration.
We are now ready to model the change in alveolar air space partial pressures of O2 and CO2. We will refer to the partial pressure of the O2 and CO2 in the air as PO2air and PCO2air respectively. Under normal conditions, where we are assuming that optimality between the screening effect and alveolar size has been achieved, PO2air is150 mmHg and PCO2air is 0 mmHg (Guyton et al., 2000). In order to properly model the changes in partial pressure, we track the changes in number of molecules and convert to a partial pressure change as we did with diffusion between compartments. The number of oxygen molecules in the alveolar space is the concentration of O2 times the volume in the alveolar space, VA0[O2]. During inspiration the change in number of molecules due to breathing, not diffusion, is Equation (22). This equation simplifies to Equation (23) when we assume that there is no change in outside air concentration. Dividing Equation (23) through by σA we derive the differential equation for the partial pressure for oxygen in the alveolar air space, Equation (24).
| (22) |
| (23) |
with
| (24) |
During expiration we assume that the air within the RU is well mixed; therefore all expired air has the same concentration. With this assumption there is no change in concentration and therefore pressure during expiration. Including diffusion, we model changes in alveolar PO2 during breathing by Equation (25). The equation for CO2 has the same form.
| (25) |
The immune subsystem
We use a simple model of the inflammatory response which includes TNF and neutrophils (Abraham, 2003; Pallister et al., 2002). TNF activates circulating neutrophils in the blood. Once activated, neutrophils can “diffuse” into lung tissue by transendothelial migration, where inflammatory factors cause stickiness of the endothelial surface such that activated neutrophils attach to endothelium and marginate into tissue locally (Petri et al., 2008). Activated neutrophils in both the blood and tissue produce TNF. TNF diffuses between the tissue and blood barrier by passive diffusion (Pappenheimer et al., 1951). Also, since inflammation is function of many factors not included in this model that could sustain inflammatory effects after the levels of TNF and activated neutrophils have dropped, we incorporate into our model a differential equation for inflammation. Inflammation’s growth is dependent on TNF levels. However, it decays at a slower rate than TNF.
We model these biological interactions with Equations (26)–(30). This subsystem consists of equations for TNF in the blood, TNFB, and activated neutrophils on each unit of the blood, NABi, and for both TNF and activated neutrophils on each unit of the tissue compartment, TNFi and NAi. Note that unlike other variables in this subsystem TNFB is not a function of space, since it is well mixed in the blood. The inflammation variable is a function of space and time. It is a measure of the local tissue inflammation and is represented by zi.
| (26) |
| (27) |
| (28) |
| (29) |
| (30) |
The first term of all equations models decay of the variable. The second term of Equation (27) models the activation of resting neutrophils by TNF in the blood. This reaction is modeled by a Hill type equation with an exponent of two. This nonlinearity models the need for multiple TNF molecules to bring about the activation of a neutrophil. A similar term appears as the second term of the TNFB equation, Equation (26), to model the consumption of TNF during this process. The third term in Equations (26)–(27) and the second terms of Equations (28)–(29) model diffusion. These terms do not have the same form because the mechanisms by which neutrophils and TNF diffuse during an inflammatory response are different.
Increasing local inflammation promotes activated neutrophils diffusion, however these effects saturate for higher levels of inflammation, hence there is a maximum rate for the diffusion of neutrophils. Due to these properties of neutrophil diffusion we model this with a hill type function, rv(zi). Note that resting neutrophils are assumed not to diffuse; therefore, there is no activation of neutrophils within the tissue. Once an activated neutrophil diffuses into to the tissue we assume that it does not diffuse back into the blood.
TNF diffusion also increases as inflamed endothelium becomes more porous to all small molecules (Aird, 2005). We assume this dependence to be linear and is model with the function rp(zi). Diffusion for both TNF and activated neutrophils are modeled as with the gas molecules with the rv(zi) or rp(zi) taking the role of the diffusion constant.
The final term in Equation (26) expresses the production of TNF by activated neutrophils. This sum is an numerical estimate for the integral . This integral accounts for TNF produced by the activated neutrophils throughout the entire blood compartment. We denote the length of the capillary with L. The last term of Equation (27) is flow of neutrophils in the blood. The last term of the Equation (28), represents chemotaxis of tissue neutrophils towards TNF. The last final two terms of the tissue TNF equation (29) are production of TNF from neutrophils and diffusion of TNF within the tissue compartment, respectively.
We model local inflammation with Equation (30), which consists of two terms, growth and decay. The growth of inflammation is dependent on the local TNF. We model this dependence using a Hill type function. This nonlinearity is used since there must be substantial levels of TNF in the tissue to trigger inflammatory changes and the growth rate of inflammation saturates. Note that the inflammation variable has a maximum value of one, which corresponds to tissue being severely inflamed.
Single RU model: Effects of inflammation on gas exchange
During an inflammatory response, there is swelling of the tissue layer caused by capillary leak. In terms of the classic Starling description of inter-compartmental fluid shifts (Guyton et al., 2000), the overall permeability of the capillary to water and osmotically active molecules is increased. As a result, the increased tissue volume encroaches on both alveolar and capillary volumes. Our model does not explicitly include Starling mechanisms to drive intercompartmental fluid shifts. Rather, we use a simpler phenomenological description where volumes are directly impacted by the average local intensity of inflammation. Specifically, we replace, VB0, VT0, VAmin0, VC0 and v0 with VB, VT, VAmin, VC and v respectively, where VB, VT, VAmin, and VC are functions of the average of the local inflammation variables, . VB a sigmoidal function that decreases as z̄i increases and has maximum VB0, Equation (31). VAmin is a similar shaped function, but with a maximum of VAmin0, and it a tracks the minimum of the alveolar air space (volume at the end expiration), Equation (33). As z̄i increases the amount volume lost from the blood and alveolar air space is added to the volume of the tissue yielding Equation (32). Note the parameters are set such that mvta>mvtb, since swelling tissue expands more readily into the more compliant alveolar air space than into the blood compartment. In this model as the compartment volumes change we maintain a constant blood flow. Therefore, velocity of blood is a function of the blood volume and it is determined by v = v0VB0/VB.
Inflammation increases lung stiffness reducing the lung’s ability to generate a tidal volume (decrease of VC). We modeled this change in Vc with a sigmoidal function of inflammation, as we did with compartmental volumes, where maximal alveolar ventilation is VC0. The parameter mc determines the effectiveness of inflammation to reduce VC, see Equation (34).
| (31) |
| (32) |
| (33) |
| (34) |
Taking into account these changes due to inflammation, we have the final form of our model, Equations (35)–(48).
BLOOD EQUATIONS
| (35) |
| (36) |
With k− = RHk+
| (37) |
| (38) |
| (39) |
| (40) |
TISSUE EQUATIONS
| (41) |
| (42) |
| (43) |
| (44) |
| (45) |
ALVEOLAR AIR SPACE EQUATIONS
| (46) |
| (47) |
| (48) |
DIFFUSION FUNCTIONS
Explanations of parameter values for the gas exchange and immune subsystems are given in Table 1 and 2, respectively. Most gas exchange parameters are experimentally well characterized. Remaining parameters were chosen to reproduce known biological behaviors of model subsystems. Parameterization of the gas exchange subsystem was such that hemoglobin and oxygen dynamics stabilize in the first third of the capillary while bicarbonate and carbon dioxide reactions stabilize in the first tenth of the capillary (Guyton et al., 2000). For the immune subsystem parameters were estimated, such that there is bistablity between fixed points representing health and non-recovery (Day et al., 2006; Reynolds et al., 2006).
Table 2.
Immune Subsystem Parameters
| Parameter | Value | Description |
|---|---|---|
| μTB | 1.8/s | Decay rate of TNFB (blood). |
| α | 8 | Rate at which TNFB is depleted during the activation of neutrophils. |
| N0 | 1.2 | Amount of resting neutrophils available for activation. |
| ktn | 1 | Hill Constant, the TNFB level at where the activation of neutrophils occurs at half its maximum rate. |
| mp | 1×10−5 | Slope of the linear function of z modeling the change in the diffusion rate of TNF and TNFB during an inflammatory response. |
| b | 1×0−3 | y-intercept of the linear function of z modeling the change in the diffusion rate of TNF and TNFB during an inflammatory response. |
| γB | 3 | Production rate of TNFB from neutrophils in the blood |
| μNB | 0.05/s | Decay rate of activated neutrophils in the blood. |
| β | 12/s | Rate of at which neutrophils are activated by TNF. |
| kv | 1×10−4/s | Maximum diffusion rate for activated neutrophils. |
| k | 0.7 | Hill Constant, the level of inflammation at where the diffusion of neutrophils occurs at half its maximum rate. |
| μT | 1.8/s | Decay rate of TNF. |
| γT | 3 | Production rate of TNF from neutrophils in the tissue. |
| DT | 1 cm2/s | Diffusion constant for diffusion of TNF within the tissue. |
| μN | 0.05/s | Decay rate of neutrophil within the tissue. |
| χ | 1 | Chemotaxis constant for neutrophils. |
| μz | 0.01/s | Decay rate of inflammation. |
| kzg | 0.1/s | Growth rate of inflammation |
| ktz | 2 | Hill Constant, the level of TNF at where growth of inflammation is half its maximum rate. |
| mvtb | 0.4 | Hill Constant, sets the level of inflammation which causes tissue swelling into the blood compartment to reach half its maximum compartment to reach half its maximum. |
| mvta | 1 | Hill Constant, sets the level of inflammation which causes tissue swelling into the tissue compartment to reach half its maximum. mvta>mvtb, since swelling tissue expands more readily into alveolar air space. |
| mc | 10 | Hill Constant, sets the level of inflammation which causes the alveolar ventilation to fall to half its maximum. |
Lung-scale model
We next linked the output of multiple RUs under various physiological conditions by the method depicted in Figure 2. We assumed that initial compartmental volumes were uniform across RUs. Therefore, all blood variable concentrations at the arterial end and initial compartmental volumes, VT0, VB0, and VA0, are the same on each RU. We introduced heterogeneity by ranging ventilation/perfusion ratios from 0.31 to 1.15, following a truncated normal distribution N~(0.73, 0.04) (Neumann et al., 2001). This was achieved by ranging scaled alveolar ventilation from 150 ml and 550 ml using N~(350 ml, 104 ml2), while holding perfusion constant (480 ml/breath cycle). For convenience, we refer to the scaled volume, which are the individual RU volumes scaled by the number of alveoli.
Figure 2. Model schematics for the linking of multiple RUs to create a lung-scale model.

The first column represents concentrations and partial pressures entering the RU. These are the same on all RUs throughout the simulations. All compartmental volumes are uniformly distributed across the RUs. Initial levels used are given in Table 1. The initial alveolar ventilation on the RUs was determined by sampling a normal truncated distribution. Simulations were run for the various RUs and then a weighted average dependent on the distribution was taken of the blood variables values on exiting the RU. This weighted average is used as the initial condition for the ODE system that models venous mixing of the blood exiting the various RUs. The results of this mixing are the output of the full model, which are the variables in the left column.
At the venous end, mixing of the blood exiting the RUs is accounted for by the system of ODEs, Equations (49)–(52). Equations (49) and (50), include the dynamics between hemoglobin and oxygen. Initial conditions for PO2B are calculated by averaging venous blood PO2 level from each RU. Initial HB4 concentration is calculated by determining the percent saturation of pooled blood. Equations (51) and (52) include the dynamics between carbon dioxide and bicarbonate. The initial condition for PCO2B and are calculated, as with PO2B, by averaging over the venous blood PCO2 and level from each RU. The system is simulated to steady state, yielding mixed PO2, HB4, PCO2, and of the multiscale lung-scale model.
| (49) |
| (50) |
| (51) |
| (52) |
Models were developed using XPPAUT (Ermentrout, 2002). Grid implementation of the code was performed using MATLAB® (The MathWorks, Natick, MA) on the computational resources of PittGrid (www.pittgrid.pitt.edu).
Implementing shunting
In severely inflamed alveoli, fluid accumulates in air space and surfactant production is compromised, resulting in increased surface tension in the alveolar wall, leading to alveolar closure. Thus, supply of fresh alveolar air to the tissue is eliminated. In animal models of inflammatory lung injury, shunting has been shown to increase from less than 5% at baseline to more than 50% with injury (Neumann et al., 2001). In order to model this effect, the diffusion rate between the air and tissue compartments is set to zero when scaled alveolar ventilation falls below 40% of VC0 (scaled 140 ml). All other dynamics remain the same and the alveolar air space no longer participates in the RU dynamics. We refer to this critical value as the alveolar closure threshold.
Results
Gas exchange during tidal breathing
Simulations of the model in the absence of inflammation give results similar to those observed experimentally (Guyton et al., 2000). PO2 in the blood increases from 40 mmHg at the pulmonary arterial end (PO2B0) to 101.7 mmHg at the pulmonary venous end (PO2B20) as blood crosses the capillary, which is similar to the levels seen in the tissue compartment (Figure 3A, B). HB4 becomes saturated in the first third of the capillary, Figure 3C. The oscillations arise in Figure 3A, B, C due to varying PO2 in the alveolar air space during breathing. PO2 in alveolar air space oscillates between 96.2 and 101.7 mmHg during the four-second breath cycle, see Figure 3D.
Figure 3. Oxygen and saturated hemoglobin levels under normal conditions.
A. PO2 (mmHg) levels in blood. Zero on the x-axis represents the pulmonary arterial end of the capillary, while, moving right in space, 20 is the pulmonary venous end. The y-axis is time, therefore the columns represent the capillary PO2 levels in a space unit as time progresses. B. PO2 levels in the tissue. C. Saturated hemoglobin in the blood. D. Alveolar PO2 levels with a four second breath cycle, one second inspiration and 3 seconds expiration.
Blood PCO2 drops from 45 to 40.7 mmHg as the blood transverses the capillary, see Figure 4A. Enzymatic reactions between bicarbonate and CO2 stabilize in the first few space units (Figure 4C). PCO2 in the alveolar space oscillates between 40.70 and 43.16 mmHg, similar oscillations occur in the tissue, see Figure 4B, D. The oscillations of PCO2 in the tissue occur over the entire length of the tissue unlike those of PO2 in the tissue. This is due to the higher diffusion coefficient for carbon dioxide in the tissue. Carbon dioxide decreases during inspiration, since inspired air has a PCO2 of zero.
Figure 4. Carbon Dioxide and Bicarbonate levels under normal conditions.
A. PCO2 (mmHg) levels in blood. Zero on the x-axis represents the pulmonary arterial end of the capillary, moving to the right is space along the capillary. The y-axis is time; columns represent PCO2 levels on individual space units as time progresses. B. PCO2 (mmHg) in the tissue. C. Bicarbonate in the blood. D. Alveolus PCO2 levels with a four second breath cycle, one second inspiration and 3 second expiration.
The immune subsystem
The immune subsystem is bistable between health and non-recovery (sustained inflammation). In health, TNFi, TNFB, NAi, NABi and zi return to zero in all compartments. For sustained inflammation, corresponding to an outcome of death, these variables evolve to a persistently elevated value. Given an initial dose of TNFB below 2.23 this subsystem will resolve itself to health and above this level the system will not recover (Eichacker et al., 1991). In our model, inflammation can also be triggered by initializing one of the other immune variables above its threshold for survival (simulations not shown).
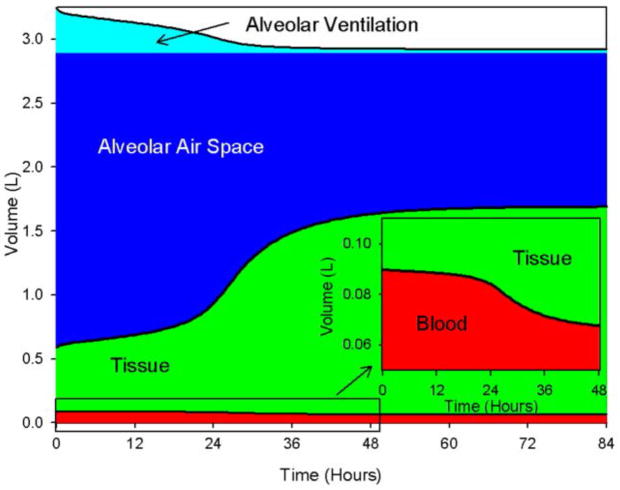
The dynamics of this subsystem determine the volumes of the compartments and the alveolar ventilation in the alveolar space during an inflammatory response. In Figure 5, we display the effects of inflammation on the compartmental volumes and alveolar ventilation: tissue swelling is accompanied by a corresponding reduction in other compartmental volumes. Figure 5 illustrates end-inspiratory volumes during an insult of insult (TNFB(0)=4), the subsystem does not recover. Alveolar ventilation is reduced to 34.5 ml. The inset in Figure 5 is a zoomed view of the box in lower left corner of Figure 5, showing the expansion of the tissue compartment into the blood compartment. Velocity of the blood increases with similar dynamics to those of the tissue volume with a maximum 0.297 cm/sec following the survivable challenge, and 0.400 cm/s following the lethal challenge.
Figure 5. Compartment volumes and alveolar ventilation during inflammation initiated in the blood.
Initial levels of TNF in the blood (TNFB) = 4. The model reaches a steady state where the immune variables remain elevated and lung volumes are impaired. Inset is an enlarged view of the box in the lower left corner of the large plot. This shows in more detail the swelling of the tissue compartment into the blood compartment as inflammation increases.
Simulations of a single RU
Combining both the gas exchange and immune subsystems simulates the effects of inflammation on gas exchange. During a lethal insult (TNFB(0)=4) PO2 drops to 43.3 ml at the venous end, hemoglobin no longer saturates in the capillary, and PCO2 also increases significantly to 41.1 mmHg at the venous end. A non-lethal response (TNFB(0)=2) is accompanied by minimal changes in the gas exchange subsystem.
The model predicts an interesting phenomenon. Following a lethal inflammatory insult, hemoglobin will eventually fail to saturate as it traverses the capillary as PO2 decreases (Figure 6). This is shown in the top curve (red) of Figure 6, which is the percent of total hemoglobin that is fully saturated, 100HB4/THB versus time. More unexpected is the prediction that the percent of the capillary traversed before hemoglobin equilibrates with O2 decreases with time as this equilibrium point moves further away from the full saturation.
Figure 6. The effects of lethal inflammation on hemoglobin saturation.
The bottom curve (blue) is the percent of the capillary traversed before oxygen equilibrates with hemoglobin versus time following the inflammatory insult (see text for details). The top curve (red) is the percent of the total hemoglobin that is fully saturated.
Lung-scale model
The time evolution of the distribution of scaled RU volumes N~(350 ml, 104 ml2) for a lethal insult predicts that a significant proportion of RUs will reach the alveolar closing threshold within 12 hours and all are below closing volume by 24 hours (Figure 7). The overall structure of the distribution remains unchanged as the average decreases, but its variance decreases in time.
Figure 7. Distributions of alveolar ventilations at times 0, 12 and 24 hours following a lethal insult.
Initially, 500 RUs are normally distributed between with scaled alveolar ventilation between 150 and 550 ml (mean = 350 ml) (black bars). After 12 hours the mean scaled volume has dropped to 127 ml (dark gray bars). At 24 hours the mean scaled alveolar ventilation average is 47 ml (pale grey bars). The dotted vertical line at 140 ml represents the scaled alveolar ventilation used as the alveolar closure threshold.
In Figure 8, we display predictions of pulmonary venous PO2 of the lung-scale model, and of transients of single RU with minimum (150 ml), mean (350 ml) and maximum (550 ml) initial scaled alveolar ventilations. The lung-scale model transient is always below the transient with initial alveolar ventilation 350 ml due to mixing of the pulmonary blood in the proximal pulmonary veins, in accordance to experimental observations (Guyton et al., 2000). As we wished to evaluate the relative impact of changes in lung volumes and shunting on gas exchange, we also displayed the prediction of a model where shunting was not implemented, confirming the major role of shunting in the rapid deterioration of gas exchange during a lethal inflammatory stimulus (Neumann et al., 2001).
Figure 8. Dynamics of pulmonary venous O2 following a lethal insult of TNF.
Dashed lines represent dynamics of single RUs with minimum scaled alveolar ventilation, 150 ml (green), mean scaled alveolar ventilation, 350 ml (blue), and maximum scaled alveolar ventilation, 550 ml (red). Following redistribution of blood exiting RUs, the dynamic of pulmonary venous O2 varies whether shunting is present or not, without shunting (black) and with shunting (grey).
In Figure 9 we compare the effect of altering diffusion and shunting during inflammation. We plotted the transients for pulmonary venous PO2 with shunting and diffusion both increased and decreased by fifty percent. For the increased shunting curve (blue) the alveolar closure scaled volume threshold was set to 70 ml and for the decreased shunting curve (red) it was set to 210 ml. As expected the lower closure causes more significant drops in PO2. Initial levels are even reduced, since with the lower threshold even in the absence of inflammation there are RUs in the distribution which have shunted. With the lower threshold the drops in PO2 levels are consistent with the normal parameter set with no shunting until approximately 24 hours. When altering diffusion the across the barrier diffusion rate constants for all the gases and the immune variables were changed simultaneously and the normal closure threshold was used when implementing shunting. For the decreased diffusion (green) curve these rate constants were set to half their normal level and for the increased diffusion (gray) curve they were set to 1.5 their normal level. Decreased diffusion initially results in lower PO2 levels than in the normal case. This is due to less oxygen diffusing into the blood. Overall we see that less diffusion leads to higher PO2 levels, this is because of the decrease in the diffusion of neutrophils. Fewer neutrophils in the tissue causes less tissue swelling. More diffusion has the opposite effect due to the increased diffusion of neutrophils. Therefore, even though the gas molecules diffuse more, the PO2 level is significantly lower due to inflammation.
Figure 9. Varying the diffusion constant and alveolar closure threshold during a lethal insult.

For the diffusion curves the diffusion constants for all variables which diffuse was increased and decreased by fifty percent throughout the simulation. The alveolar closure threshold was increased and decreased by fifty percent for the shunting curves. This threshold was 70 ml for the increased shunting curve (blue) and 210 ml for the decreased shunting curve.
Discussion
Our model for gas exchange contains an immune subsystem and explicit lung tissue layer. This model therefore is used to simulate inflammation within a respiratory unit and determine the effect of inflammation on the ability for gas exchange to occur. A variety of stimuli can trigger inflammation, including infection and activated neutrophils (Delclaux et al., 1997; Domenici-Lombardo et al., 1995; Simons et al., 1987; Song et al., 2001). A mild inflammatory stimulus does not result in a significant change in partial pressure for either oxygen or carbon dioxide. Larger stimuli lead to a significant reduction in partial pressure of oxygen at the pulmonary venous end, and therefore systemic arterial circulation. This effect is driven by (1) impaired gas diffusion caused by tissue swelling, (2) closure of alveoli below a critical respiratory unit volume, and (3) oxygen remixing in the pulmonary veins.
Other mathematical models of the lung have concentrated on lung mechanics, pulmonary circulation, gas exchange with the tissue layer treated as a barrier, airway mechanics, and drug deposition in the tracheo-bronchial tree (Ben-Tal, 2006; Hahn et al., 1993; Hill et al., 1973; Liu et al., 1998; Menges et al., 2001). With the exception of mycobacterial granuloma formation modeling (Marino et al., 2004; Marino et al., 2007), attempts at mathematical simulation of lung injury have been sparse, although pulmonary immunity and injury are of immense clinical interest and relevance. Immune-mediated lung injury has typically only been approached with experimental models. These experiments include both insults from the blood and directly introduced into the lung in the form of lipopolysaccharide instillation, chemical injury, and pathogen deposition (Bergeron et al., 1998; Domenici-Lombardo et al., 1995). The model we present is the first to incorporate a tissue layer allowing the explicit stimulation of immune effector cells or molecular mediators, and their potential modulation for therapeutic purposes. Our formulation is also conducive to the introduction of varying amount of details at different scales of description, depending on the goals of simulations. For example, we included many details regarding molecular mechanisms of gas exchange, and fewer details on the local immune response. The formulation of the RU model is computationally intensive to simulate, owing to the spatial description and the wide range of time-scales involved. The fast time scale of tidal breathing compared to inflammatory dynamics supports temporal averaging of the fast dynamics over the breath cycle to develop a more computationally feasible model. Accordingly, we are developing an approximation to this model without an explicit breathing mechanism that captures the inflammatory effects on gas exchange. Once the numerical accuracy of this approximation is confirmed, we will have derived a computationally efficient model that allows multiple RU’s under heterogeneous conditions seen in the lung to be linked using a physiologically reasonable implementation of diffusion (simulating extension of a process to neighboring RUs) and advection (simulating lymphatic transport of bacteria for example). Though our primary goal was to model pulmonary response to a systemic challenge, our model could capture different modes of local injury, once RUs are explicitly linked by a spatial formulation at that scale, potentially informed by existing structural models of experimental data (Burrowes et al., 2006; Swan et al., 2008). The introduction of more realistic heterogeneity in ventilation and perfusion, in ways that reflect interventions such as positive end-expiratory pressure of positional (prone) therapy in also of high relevance because these intervention specifically target patients that under inflammatory stress and because there is strong evidence that interventions themselves trigger inflammation (Slutsky et al., 2003). Viral infections, chemical pneumonitis and autoimmune lung disease are other ailments where gas exchange is exquisitely linked to pulmonary inflammation, and where a disease-specific, more detailed representation of the pulmonary immune response could be interface with our model. We are particularly interested in the development of influenza-related acute pulmonary failure (Hancioglu, B. et al., 2007; Hancioglu et al., 2007), a hallmark of highly cytopathic strains (Kobasa et al., 2007).
Our model is limited in a number of ways. Although the characterization of physicochemical parameters describing local gas exchange is adequate, quantification of parameters characterizing pulmonary blood flow and alveolar ventilation, and our treatment of ventilation-perfusion heterogeneity, is quite insufficient. We achieved heterogeneity in ventilation-perfusion by allowing ventilation to vary, while keeping perfusion constant on each RU. This resulted in a range of ventilation-perfusion ratio which is typically less than that encountered experimentally (Alfaro et al., 2001; Maurenbrecher et al., 2001; Neumann et al., 2001). The likely impact of a wider range of ventilation-perfusion ratios would be to accelerate the deterioration of gas exchange impairment in mixed pulmonary venous blood. There is a growing body of data that could guide this development (Alfaro et al., 2001; Hoffman et al., 2004; Moller et al., 2002). We made no attempt to link alveolar gas content to inspired gas content. This would be of high relevance if inhalation modes of treatment or disease propagation are contemplated, especially with particulate agents (Sapoval et al., 2002; Weibel et al., 2005). Similarly, our treatment of lung tissue swelling could be greatly improved with a more detail treatment of local hydrostatic and oncotic pressures. This would be of relevance for serious simulations of positional therapy. Yet, there is a dearth of suitable datasets to properly triangulate many of the parameters on which to base such extensions. Such datasets would ideally contain information on local concentrations of relevant immune mediators, counts of activated immune cells and relative volume distributions of alveolar air space, capillary blood and lung tissue. We also assumed fixed boundary conditions for pulmonary arterial gas and acid-base status. Clearly, a closed loop model that included a metabolic component would be preferable, since, for example, decreased oxygen delivery may result in increased extraction and lower input oxygen partial pressures on the pulmonary arterial boundary. Accordingly, we would expect model predictions to be more optimistic than observations for extreme hypoxia. We also assumed an oxyhemoglobin dissociation curve that was independent of blood pH. Both of these simplifications could be addressed in extensions of our models. Interestingly, promising experimental techniques allow less invasive and repetitive measurement of ventilation-perfusion ratios (Rhodes et al., 1989; Roca et al., 1994), offering exciting new possibilities to compare prediction of multiscale organ physiology to experimental paradigms of acute lung injury.
In conclusion, we believe the model presented herein constitutes an important first step in the development of realistic simulations of pulmonary function under inflammatory stress, and of interventions aimed at improving gas exchange in this broadly relevant context. The model is generically multiscale and be improved in its physiologic accuracy and computational load.
Acknowledgments
We thank Dr John Hotchkiss for his insightful comments.
Grant support
This work was supported in part by National Institutes of Health (R01-GM83602) and National Science Foundation (DMS 0817131).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ben-Tal A. Simplified models for gas exchange in the human lungs. J Theor Biol. 2006;238:474–495. doi: 10.1016/j.jtbi.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Moller HE, Chen XJ, Saam B, Hagspiel KD, Johnson GA, Altes TA, de Lange EE, Kauczor HU. MRI of the lungs using hyperpolarized noble gases. Magn Reson Med. 2002;47:1029–1051. doi: 10.1002/mrm.10173. [DOI] [PubMed] [Google Scholar]
- Tawhai MH, Burrowes KS. Developing integrative computational models of pulmonary structure. Anat Rec B New Anat. 2003;275:207–218. doi: 10.1002/ar.b.10034. [DOI] [PubMed] [Google Scholar]
- Olson TS, Singbartl K, Ley K. L-selectin is required for fMLP- but not C5a-induced margination of neutrophils in pulmonary circulation. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1245–R1252. doi: 10.1152/ajpregu.00540.2001. [DOI] [PubMed] [Google Scholar]
- Rubenfeld GD, Herridge MS. Epidemiology and outcomes of acute lung injury. Chest. 2007;131:554–562. doi: 10.1378/chest.06-1976. [DOI] [PubMed] [Google Scholar]
- Delclaux C, Rezaiguia-Delclaux S, Delacourt C, Brun-Buisson C, Lafuma C, Harf A. Alveolar neutrophils in endotoxin-induced and bacteria-induced acute lung injury in rats. Am J Physiol. 1997;273:L104–L112. doi: 10.1152/ajplung.1997.273.1.L104. [DOI] [PubMed] [Google Scholar]
- Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27:337–349. doi: 10.1055/s-2006-948288. [DOI] [PubMed] [Google Scholar]
- Guyton AC, Hall JE. Textbook of Medical Physiology. 10. W.B. Saunders Company; Philadelphia, PA: 2000. [Google Scholar]
- Chow CC, Clermont G, Kumar R, Lagoa C, Tawadrous Z, Gallo D, Betten B, Bartels J, Constantine G, Fink MP, Billiar TR, Vodovotz Y. The acute inflammatory response in diverse shock States. Shock. 2005;24:74–84. doi: 10.1097/01.shk.0000168526.97716.f3. [DOI] [PubMed] [Google Scholar]
- Daun S, Rubin J, Vodovotz Y, Roy A, Parker R, Clermont G. An ensemble of models of the acute inflammatory response to bacterial lipopolysaccharide in rats: results from parameter space reduction. J Theor Biol. 2008;253:843–853. doi: 10.1016/j.jtbi.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Keener JP, Sneyd J. Mathematical Physiology. Springer-Verlag; New York: 1998. [Google Scholar]
- Hill EP, Power GG, Longo LD. Mathematical simulation of pulmonary O2 and CO2 exchange. American Journal of Physiology. 1973;224:904–917. doi: 10.1152/ajplegacy.1973.224.4.904. [DOI] [PubMed] [Google Scholar]
- Hahn CE, Black AM, Barton SA, Scott I. Gas exchange in a three-compartment lung model analyzed by forcing sinusoids of N2O. J. Appl. Physiol. 1993;75:1863–1876. doi: 10.1152/jappl.1993.75.4.1863. [DOI] [PubMed] [Google Scholar]
- Bidani A, Crandall ED. Velocity of CO2 exchanges in the lungs. Annu Rev Physiol. 1988;50:639–652. doi: 10.1146/annurev.ph.50.030188.003231. [DOI] [PubMed] [Google Scholar]
- Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31:S195–S199. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- Pallister I, Dent C, Topley N. Increased neutrophil migratory activity after major trauma: a factor in the etiology of acute respiratory distress syndrome? Crit Care Med. 2002;30:1717–1721. doi: 10.1097/00003246-200208000-00007. [DOI] [PubMed] [Google Scholar]
- Petri B, Phillipson M, Kubes P. The physiology of leukocyte recruitment: an in vivo perspective. J Immunol. 2008;180:6439–6446. doi: 10.4049/jimmunol.180.10.6439. [DOI] [PubMed] [Google Scholar]
- Pappenheimer JR, Renkin EM, Borrero LM. Filtration, Diffusion and Molecular Sieving Through Peripheral Capillary Membranes: A Contribution to the Pore Theory of Capillary Permeability. AJP - Legacy. 1951;167:13–46. doi: 10.1152/ajplegacy.1951.167.1.13. [DOI] [PubMed] [Google Scholar]
- Aird WC. Spatial and temporal dynamics of the endothelium. J Thromb Haemost. 2005;3:1392–1406. doi: 10.1111/j.1538-7836.2005.01328.x. [DOI] [PubMed] [Google Scholar]
- Reynolds A, Rubin J, Clermont G, Day J, Ermentrout B. A reduced mathematical model of the acute inflammatory response: I Derivation of the model and analysis of anti-inflammation. J Theor Biol. 2006;242:220–236. doi: 10.1016/j.jtbi.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Day J, Rubin J, Vodovotz Y, Chow CC, Reynolds A, Clermont G. A reduced mathematical model of the acute inflammatory response II. Capturing scenarios of repeated endotoxin administration J Theor Biol. 2006;242:237–256. doi: 10.1016/j.jtbi.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Neumann P, Hedenstierna G. Ventilation-perfusion distributions in different porcine lung injury models. Acta Anaesthesiol Scand. 2001;45:78–86. doi: 10.1111/j.1399-6576.2001.450113.x. [DOI] [PubMed] [Google Scholar]
- Ermentrout GB. Simulating, analyzing, and animating dynamical systems: A guide to XPPAUT for researchers and students. 1. Soc for Industrial & Applied Math; Philadelphia: 2002. [Google Scholar]
- Eichacker PQ, Hoffman WD, Farese A, Banks SM, Kuo GC, MacVittie TJ, Natanson C. TNF but not IL-1 in dogs causes lethal lung injury and multiple organ dysfunction similar to human sepsis. J Appl Physiol. 1991;71:1979–1989. doi: 10.1152/jappl.1991.71.5.1979. [DOI] [PubMed] [Google Scholar]
- Delclaux C, Rezaiguia-Delclaux S, Delacourt C, Brun-Buisson C, Lafuma C, Harf A. Alveolar neutrophils in endotoxin-induced and bacteria-induced acute lung injury in rats. Am J Physiol. 1997;273:L104–L112. doi: 10.1152/ajplung.1997.273.1.L104. [DOI] [PubMed] [Google Scholar]
- Domenici-Lombardo L, Adembri C, Consalvo M, Forzini R, Meucci M, Romagnoli P, Novelli GP. Evolution of endotoxin induced acute lung injury in the rat. Int J Exp Pathol. 1995;76:381–390. [PMC free article] [PubMed] [Google Scholar]
- Simons RK, Maier RV, Lennard ES. Neutrophil function in a rat model of endotoxin-induced lung injury. Arch Surg. 1987;122:197–203. doi: 10.1001/archsurg.1987.01400140079010. [DOI] [PubMed] [Google Scholar]
- Song Y, Ao L, Raeburn CD, Calkins CM, Abraham E, Harken AH, Meng X. A low level of TNF-alpha mediates hemorrhage-induced acute lung injury via p55 TNF receptor. Am J Physiol Lung Cell Mol Physiol. 2001;281:L677–L684. doi: 10.1152/ajplung.2001.281.3.L677. [DOI] [PubMed] [Google Scholar]
- Liu CH, Niranjan SC, Clark JW, Jr, San KY, Zwischenberger JB, Bidani A. Airway mechanics, gas exchange, and blood flow in a nonlinear model of the normal human lung. J Appl Physiol. 1998;84:1447–1469. doi: 10.1152/jappl.1998.84.4.1447. [DOI] [PubMed] [Google Scholar]
- Menges T, Hermans PW, Little SG, Langefeld T, Boning O, Engel J, Sluijter M, de GR, Hempelmann G. Plasminogen-activator-inhibitor-1 4G/5G promoter polymorphism and prognosis of severely injured patients. Lancet. 2001;357:1096–1097. doi: 10.1016/S0140-6736(00)04311-7. [DOI] [PubMed] [Google Scholar]
- Marino S, Kirschner DE. The human immune response to Mycobacterium tuberculosis in lung and lymph node. J Theor Biol. 2004;227:463–486. doi: 10.1016/j.jtbi.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Marino S, Sud D, Plessner H, Lin PL, Chan J, Flynn JL, Kirschner DE. Differences in reactivation of tuberculosis induced from anti-TNF treatments are based on bioavailability in granulomatous tissue. PLoS Comput Biol. 2007;3:1909–1924. doi: 10.1371/journal.pcbi.0030194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron Y, Ouellet N, Deslauriers AM, Simard M, Olivier M, Bergeron MG. Cytokine kinetics and other host factors in response to pneumococcal pulmonary infection in mice. Infect Immun. 1998;66:912–922. doi: 10.1128/iai.66.3.912-922.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrowes KS, Tawhai MH. Computational predictions of pulmonary blood flow gradients: gravity versus structure. Respir Physiol Neurobiol. 2006;154:515–523. doi: 10.1016/j.resp.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Swan A, Hunter P, Tawhai M. Pulmonary gas exchange in anatomically-based models of the lung. Adv Exp Med Biol. 2008;605:184–189. doi: 10.1007/978-0-387-73693-8_32. [DOI] [PubMed] [Google Scholar]
- Slutsky AS, Imai Y. Ventilator-induced lung injury, cytokines, PEEP, and mortality: implications for practice and for clinical trials. Intensive Care Med. 2003;29:1218–1221. doi: 10.1007/s00134-003-1793-0. [DOI] [PubMed] [Google Scholar]
- Hancioglu B, Swigon D, Clermont G. A dynamical model of human immune response to influenza A virus infection. J Theor Biol. 2007;246:70–86. doi: 10.1016/j.jtbi.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Hancioglu B, Clermont G, Swigon D. Ensemble models for human immune response to influenza A virus infection. Journal of Critical Care. 2007;22:339–340. doi: 10.1016/j.jcrc.2007.10.015. (Abstract) [DOI] [PubMed] [Google Scholar]
- Kobasa D, Jones SM, Shinya K, Kash JC, Copps J, Ebihara H, Hatta Y, Kim JH, Halfmann P, Hatta M, Feldmann F, Alimonti JB, Fernando L, Li Y, Katze MG, Feldmann H, Kawaoka Y. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319–323. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- Alfaro V, Roca-Acin J, Palacios L, Guitart R. Multiple inert gas elimination technique for determining ventilation/perfusion distributions in rat during normoxia, hypoxia and hyperoxia. Clin Exp Pharmacol Physiol. 2001;28:419–424. doi: 10.1046/j.1440-1681.2001.03455.x. [DOI] [PubMed] [Google Scholar]
- Maurenbrecher H, Lamy M, by-Dupont G, Frascarolo P, Hedenstierna G. An animal model of response and nonresponse to inhaled nitric oxide in endotoxin-induced lung injury. Chest. 2001;120:573–581. doi: 10.1378/chest.120.2.573. [DOI] [PubMed] [Google Scholar]
- Hoffman EA, Clough AV, Christensen GE, Lin CL, McLennan G, Reinhardt JM, Simon BA, Sonka M, Tawhai MH, van Beek EJ, Wang G. The comprehensive imaging-based analysis of the lung: a forum for team science. Acad Radiol. 2004;11:1370–1380. doi: 10.1016/j.acra.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Sapoval B, Filoche M, Weibel ER. Smaller is better--but not too small: a physical scale for the design of the mammalian pulmonary acinus. Proc Natl Acad Sci U S A. 2002;99:10411–10416. doi: 10.1073/pnas.122352499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel ER, Sapoval B, Filoche M. Design of peripheral airways for efficient gas exchange. Respir Physiol Neurobiol. 2005;148:3–21. doi: 10.1016/j.resp.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Rhodes CG, Valind SO, Brudin LH, Wollmer PE, Jones T, Hughes JM. Quantification of regional V/Q ratios in humans by use of PET. I Theory. J Appl Physiol. 1989;66:1896–1904. doi: 10.1152/jappl.1989.66.4.1896. [DOI] [PubMed] [Google Scholar]
- Roca J, Wagner PD. Contribution of multiple inert gas elimination technique to pulmonary medicine. 1. Principles and information content of the multiple inert gas elimination technique. Thorax. 1994;49:815–824. doi: 10.1136/thx.49.8.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall JD, McCabe M. Diffusion coefficient of oxygen through tissues. Nature. 1967;215:1173–1174. doi: 10.1038/2151173a0. [DOI] [PubMed] [Google Scholar]