Abstract
OBJECTIVE
The purpose of this study was to investigate the feasibility of diagnostic breast imaging using a flat-panel detector-based cone-beam CT system.
CONCLUSION
Imaging of 12 mastectomy specimens was performed at 50–80 kVp with a voxel size of 145 or 290 μm. Our study shows that cone-beam breast CT images have exceptional tissue contrast and can potentially reduce examination time with comparable radiation dose.
Keywords: breast neoplasm, CT radiography, mastectomy, radiation dose, specimen radiography
Mammography is an important tool for the screening, diagnosis, and management of breast cancers. The effectiveness of mammography is compromised by fundamental problems including radiation scatter, noise, and the over-lapping of cancers with breast anatomy. Tomosynthesis may partially overcome this limitation, but its image quality suffers from crude depth resolution and associated artifacts [1]. Cone-beam CT, on the other hand, can provide true 3D breast images with isotropic resolution (145 μm or smaller) and radiation dose comparable to two-view mammography [2]. Conventional fan-beam CT applied to breast cancer imaging in the 1970s suffered from limitations including high patient dose, low spatial resolution, long scanning time, large slice thickness, and cardiac and respiratory motion [3, 4]. The advantages of cone-beam CT include a flatpanel digital detector, true 3D images with isotropic resolution, reduced motion artifacts, breast-only exposure to radiation, greater efficiency in use of the X-ray beam, no overlapping structures in the breast, and high contrast resolution. We constructed a flat-panel detector-based cone-beam CT system to investigate the feasibility for dedicated breast imaging [5].
Materials and Methods
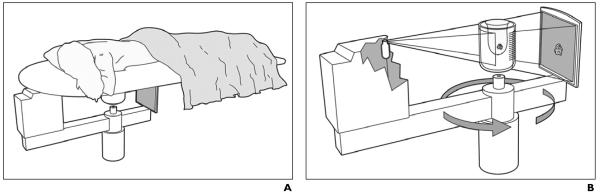
The experimental system consists of a general radiography tube pointing at a 30 × 40 cm amorphous silicon (a-Si/CsI) flat-panel digital detector (Paxscan 4030CB, Varian Medical Systems). A motor-driven rotation stage is used to position and rotate the specimen to simulate dedicated breast CT in which the patient would lie on a table in the prone position with one breast drawn downward through an opening to allow the X-ray tube and detector to rotate around and scan the breast beneath the table (Fig. 1).
Fig. 1.
Drawings show flat-panel detector-based cone-beam CT system.
A, Patient lies prone with one breast drawn downward through opening in scanning device.
B, X-ray tube and detector are mounted on rotating gantry and rotated around breast during acquisition.
A total of 12 mastectomy specimens were acquired fresh from the pathology laboratory in our institution with institutional review board approval. Each specimen was placed in a receptacle formulated from an inverted soda bottle, and the holder was placed on the rotation stage. Scanning was performed at 50–80 kVp with reconstructed voxel size of 145 or 290 μm. The exposures in air at the isocenter of the cone-beam CT system were measured with a pencil-probe ion chamber, resulting in calculated dose levels equivalent to 6–24 mGy for the breast, which correspond to 1–4 times the mean glandular dose limits for two-view mammograms of a 5-cm-thick compressed breast.
Results
The mean scanning time was 12 seconds for low-resolution (binning) mode, which was adequate for visualizing tissue structures, and 48 seconds for high-resolution (nonbinning) mode, necessary for visualizing small calcifications. Artifacts encountered from high-density metallic tumor marker coils and surgical clips were removed or reduced by postprocessing techniques without compromising tissue detail (Fig. 2). Structured noise was minimal because of the absence of overlapping tissue. Breast anatomy was well resolved on all images as skin, adipose, and glandular regions. Image noise was visible but low compared with the tissue contrast. Microcalcifications within cancers were clearly shown. The detection of cancers based on morphologic assessment of tissue structures may be improved compared with mammography because of the lack of overlapping glandular tissue. Cancer was visualized as an irregular spiculated mass with associated microcalcifications or overlying skin thickening (Figs. 3 and 4) or an area of focal asymmetry. Multifocal breast cancer was shown on coronal slices of one of the mastectomy specimens as small irregular masses. Benign lesions including simple cysts were identified on CT as circumscribed oval isodense masses.
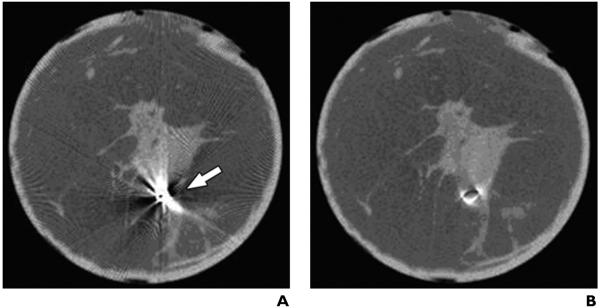
Fig. 2.
51-year-old woman with invasive ductal cancer.
A, Cone-beam breast CT image shows metallic artifacts caused by stereotactic clip within breast (arrow).
B, No metallic artifacts after modified algorithm to correct beam hardening.
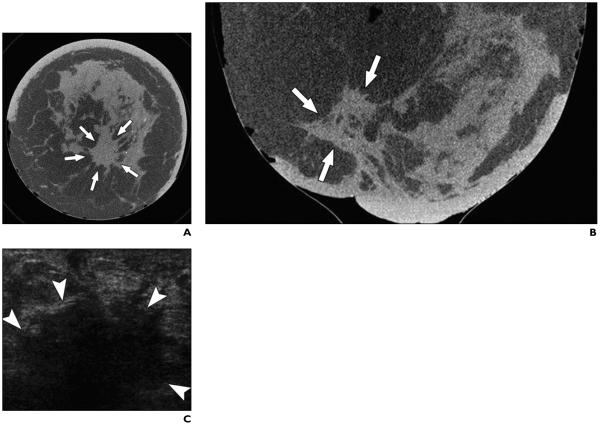
Fig. 3.
51-year-old woman with invasive ductal carcinoma of left breast occupying area of 6 × 5 cm.
A and B, Coronal (A) and axial (B) CT scans of left breast show irregular mass with spiculated margins (arrows). C, Transverse sonogram shows irregular solid hypoechoic mass in left retroareolar position with angular margins and dense posterior acoustic shadowing (arrowheads).
Fig. 4.
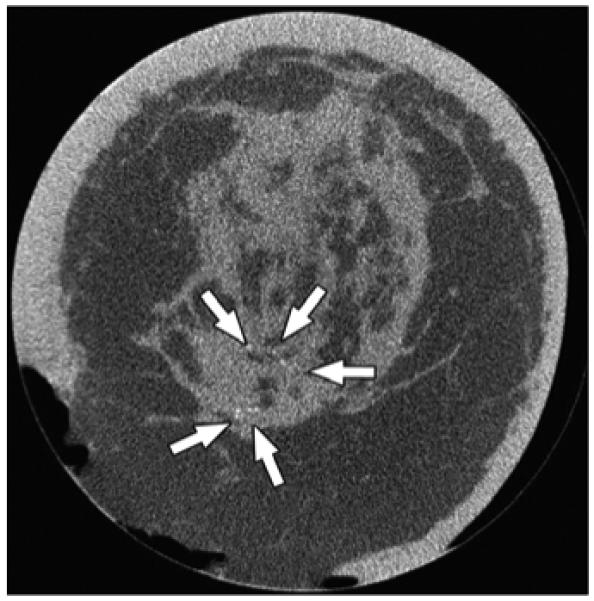
63-year-old woman with invasive ductal carcinoma of left breast. Coronal CT image shows microcalcifications within area of architectural distortion representing known cancer (arrows). Pathology showed ductal carcinoma in situ associated with microcalcifications.
Discussion
Conventional screening mammography produces 2D images with limited contrast and spatial resolution. Therefore, superimposed parenchymal structures can obscure and prevent the detection of small cancers. Conebeam CT overcomes the problem of overlapping tissue by generating 3D images and is a promising technique in diagnosing tumors at an early stage [6]. Studies are ongoing that focus on improving this new technology in breast imaging and investigating its potential role in screening women with a family history of breast cancer or a genetic predisposition, most of whom have dense breasts [7].
The rationale in choosing a specific scanning technique is governed by general radiologic imaging principles. Higher resolution and greater tissue detail are achieved at higher patient dose, whereas lower radiation dose leads to lower resolution images. The selection of exposure technique is a compromise between patient dose and resolution. Boone et al. [7] showed that high tissue contrast can be achieved with an isocenter dose of 6 mGy for a 10-cm-diameter breast. Lai et al. [8] reported that the visibility of microcalcifications with a size of 348, 288, and 257 μm can be achieved with a radiation dose of 3, 6, and 12 mGy, respectively, for a 10-cm-diameter breast. The spatial resolution of the images obtained with our cone-beam CT system is 140 μm, providing potentially greater detail and higher resolution when compared with tomosynthesis. This reflects inherent differences between both imaging techniques. Cone-beam CT renders a true 3D acquisition, whereas tomosynthesis provides a projection image. A direct comparison between cone-beam CT and tomosynthesis would be useful in evaluating the relative merits of each method.
Imaging a single breast with each acquisition may not be efficient use of patient and scanning time. However, dedicated breast CT includes more tissue and better optimizes the resulting image quality when imaging one breast. Imaging both breasts simultaneously may compromise the image quality and ability of the system to accommodate more tissue.
There is no theoretic limit in imaging large breasts. However, larger breasts would require higher radiation dose (mAs and kVp) to penetrate thicker tissue. The ability of the current design of dedicated conebeam CT to visualize and evaluate deeper portions of the breast including the axilla may be limited because of the prone nature of the study and patient positioning. Various different designs and positioning techniques are being investigated to resolve this issue. A possibility is to use the “arm through hole” technique, which is widely practiced when performing stereotactic biopsies of lesions that are close to the chest wall.
An expected advantage of cone-beam breast CT is in imaging the postoperative breast with deformity, in which it is expected that a true 3D acquisition will overcome problems of overlapping structures, overlying skin thickening, scarring with resultant obscuration of masses, or the simulation of pseudomasses.
Loss of data with beam hardening from clips in patients with prior surgery, after biopsy, and with tumor markers is a problem with the current technique and may require modification with a different algorithm to correct beam hardening artifacts from clips. Such algorithms have been developed by our institution [9] and other groups to minimize the artifacts and obtain slice images of reasonable quality.
The expected radiation dose to structures within the chest as a result of scatter when using cone-beam breast CT has not been studied but is expected to be significant near the breast, with a sharp decreasing gradient within the first 2 cm from the breast because of attenuation by tissues in the chest immediately outside the field of the X-ray beam. We expect that the potential value of MDCT in breast imaging is limited because the entire chest is included in the field of view when using this technique, resulting in image quality degradation from higher scatter and lower resolution. Furthermore, structures within the chest will be subjected to unnecessary exposure and increased dose from direct exposure and scattered radiation.
The anticipated number of images that the cone-beam CT mammogram will contain is dependent on breast size. There may be between 700 and 1,200 slice images available for review of a breast ranging in size from 10 to 17 cm thick (nipple to chest wall). Although this number may seem daunting, it is expected that current monitor viewing facilities and the opportunity to use multiplanar reconstructions and displays will overcome the potential negative impact on radiologist productivity.
Our preliminary study, concordant with the preliminary results in the literature, shows that cone-beam CT of the breast can potentially reduce examination time with comparable radiation dose, eliminates the need for compression and additional workup views that are routine with mammography, and shows exceptional tissue contrast. The potential uses of cone-beam CT can be extended to aid imaging-guided surgery and interventional procedures including biopsy or needle localization [7]. Further study of cone-beam breast CT with larger series is needed to better understand its potential role in screening, diagnosis, and management of breast cancers.
Acknowledgments
Supported by research grants EB-00117 from the National Institute of Biomedical Imaging and Bioengineering and CA104759 from the National Cancer Institute.
Footnotes
Presented in part at the 2005 San Antonio Breast Cancer Symposium and the 2007 annual meeting of the American Roentgen Ray Society, Orlando, FL.
References
- 1.Nikalson LT, Christian BT, Nikalson LE, et al. Digital tomosynthesis in breast imaging. Radiology. 1997;205:399–406. doi: 10.1148/radiology.205.2.9356620. [DOI] [PubMed] [Google Scholar]
- 2.Boone JM, Nelson TR, Lindfors KK, Seibert JA. Dedicated breast CT: radiation dose and image quality evaluation. Radiology. 2001;221:657–667. doi: 10.1148/radiol.2213010334. [DOI] [PubMed] [Google Scholar]
- 3.Chang CH, Sibala JL, Lin F, Jewell WR, Templeton AW. Preoperative diagnosis of potentially precancerous breast lesions by computed tomography breast scanner: preliminary study. Radiology. 1978;129:209–210. doi: 10.1148/129.1.209. [DOI] [PubMed] [Google Scholar]
- 4.Gisvold JJ, Reese DF, Karsell PR. Computed tomographic mammography (CTM) AJR. 1979;133:1143–1149. doi: 10.2214/ajr.133.6.1143. [DOI] [PubMed] [Google Scholar]
- 5.Shaw CC, Chen L, Altuntas M, et al. Cone beam breast CT with a flat panel detector: simulation, implementation and demonstration. Conf Proc IEEE Eng Med Biol Soc. 2005;4:4461–4464. doi: 10.1109/IEMBS.2005.1615457. [DOI] [PubMed] [Google Scholar]
- 6.Gong XJ, Glick S, Liu B, Vedula AA, Thacker S. A computer simulation study comparing lesion detection accuracy with digital mammography, breast tomosynthesis, and cone beam CT breast imaging. Med Phys. 2006;33:1041–1052. doi: 10.1118/1.2174127. [DOI] [PubMed] [Google Scholar]
- 7.Boone JM, Kwan ALC, Yang K, Burkett GW, Lindfors KK, Nelson TR. Computed tomography for imaging the breast. J Mammary Gland Biol Neoplasia. 2006;11:103–111. doi: 10.1007/s10911-006-9017-1. [DOI] [PubMed] [Google Scholar]
- 8.Lai CJ, Shaw CC, Chen L, et al. Visibility of microcalcification in cone beam breast CT: effects of X-ray tube voltage and radiation dose. Med Phys. 2007;34:2995–3004. doi: 10.1118/1.2745921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen LY, Shaw CC, Altunbas MC, et al. In: Kalender W, Hahn EG, Schulte AM, editors. Removal of metal object artifacts in cone beam breast CT; Proceedings of the 14th International Conference of Medical Physics; Nuremberg, Germany: International Organisation of Medical Physics. 2005.pp. 352–353. [Google Scholar]