Abstract
AIM: To perform a comparative analysis of clinicopathological correlations of cyclooxygenase-2 (COX-2) expression in pancreatic cancer, examined by monoclonal and polyclonal antibodies.
METHODS: The COX-2 expression in 85 resection specimens of pancreatic ductal adenocarcinoma was immunohistochemically examined using both monoclonal and polyclonal antibodies. The final immunoscores were obtained by multiplying the percentage of positive cells with the numeric score reflecting the staining intensity. COX-2 expression levels were classified into three categories (0, 1+, and 2+) and the clinicopathological correlations were statistically evaluated and analyzed.
RESULTS: The positive tumor expression rates of COX-2 were 80.5% using monoclonal antibody and 69.4% using polyclonal antibody. In the Kaplan-Meier analysis, no significant correlations were found between levels of COX-2 expression and overall survival (OS), but trends to longer OS were found in COX-2 negative cases using monoclonal antibody. Significantly longer disease free survival was revealed in COX-2 negative cases using monoclonal antibody (P = 0.019). No correlations between COX-2 expression levels and grade (G), tumor (T) status and nodal (N) status were demonstrated. Low histological grade showed a strong association with a longer OS (P < 0.001). Correlation of survival and T status revealed a shorter OS in T3 tumors, but the results reached only marginal statistical significance (P = 0.070). In the multivariate Cox proportional hazards regression model, histological grade, T and N status remained valuable predictors of a worse survival with borderline significance for T [hazards ratio (HR) = 4.18 for G (if G = 3, P < 0.001); HR = 1.64 for T (if T = 3, P = 0.065); HR = 2.53 for N (if N = 1, P = 0.006)]. Higher grade, T or N status was associated with a worse OS.
CONCLUSION: The immunohistochemically assessed level of COX-2 expression does not seem to represent a valuable independent prognostic factor and is not superior to the conventional prognostic factors.
Keywords: Pancreatic cancer, Cyclooxygenase-2, Immunohistochemistry, Monoclonal antibody, Polyclonal antibody
INTRODUCTION
Invasive ductal adenocarcinoma is the most common neoplasm in the pancreas, constituting about 90% of all pancreatic tumors[1]. Pancreatic cancer represents the fourth leading cause of cancer-related death among men and women in western countries[2,3]. Pancreatic cancer has no early warning signs and symptoms, so most patients present with advanced disease. Despite improved diagnostic and therapeutic modalities, pancreatic cancer still has a very poor prognosis. The incidence of pancreatic cancer almost equals the mortality rate, and it has one of the lowest overall 5-year survival rates (under 5%) among the epithelial cancers[4-6].
The currently accepted model of the development of pancreatic ductal adenocarcinoma (PDAC) understands the oncogenesis of this malignancy as a multistep process, characterized by the progression from the normal ductal epithelium through the spectrum of duct lesions known as pancreatic intraepithelial neoplasia (PanIN) to invasive ductal adenocarcinoma[7,8]. PanINs represent precursor lesions of ductal adenocarcinoma and their classification system distinguishes three grades of PanINs, which harbor a number of well-established molecular events including activation of oncogenes, inactivation of tumor suppressor genes, inactivation of DNA mismatch repair genes, various epigenetic alterations, dysregulation of oncoproteins, and others[9-11]. Mediators of inflammatory pathways [e.g. cyclooxygenase-2 (COX-2), nuclear factor-κB (NF-κB), 5-lipooxygenase (5-LOX), interleukin-8 (IL-8) etc.] are also known to play an important role in carcinogenesis of pancreatic cancer and represent a key link between chronic inflammation and cancer[12-14]. Prostaglandin H2 synthase (COX) represents an enzyme which is involved in the conversion of arachidonic acid to prostaglandins. Two COX isoforms, COX-1 and COX-2, have been identified to date[15]. COX-1 is constitutively expressed in many tissues, and is involved in prostaglandin synthesis under physiological conditions. COX-2 is expressed under certain extracellular or intracellular stimuli, including mitogens, growth factors, proinflammatory cytokines, hormones and infectious agents, and is a component of cellular responses to inflammation. COX-2 is overexpressed in many human solid tumors including pancreatic cancer[16,17]. Several studies have suggested a potential involvement of COX-2 pathways in the regulation of tumor-associated angiogenesis and cell growth in pancreatic cancer. COX-2 has been demonstrated to inhibit apoptosis, promote cell proliferation and to induce the expression of vascular endothelial growth factor[18-21]. COX-2 overexpression has been reported in 56% to 90% of ductal adenocarcinomas[22]. A high level of COX-2 overexpression has also been described in PanIN lesions[23-25], and COX-2 was suggested as a potential therapeutic target for chemoprevention and therapy of pancreatic cancer[22,26].
A significant inverse relationship between COX-2 overexpression and survival rates has been reported in retrospective studies of different types of malignancies[27-32]. Conflicting results have been shown in pancreatic cancer[33-39] and the possible role of primary antibody used for the detection of COX-2 expression has been suggested[33,40]. Comparative analysis has not yet been performed. In our study the expression of COX-2 in resectable pancreatic ductal adenocarcinomas was immunohistochemically examined using both monoclonal and polyclonal antibodies, and analyzed and correlated with clinicopathological parameters.
MATERIALS AND METHODS
Study group and tissue specimens
The study group (summarized in Table 1) consisted of 85 patients [41 males (48.2%) and 44 females (51.8%); median age 61 ± 9.5 years (range 39-85 years)] with resectable pancreatic cancer who had undergone pancreatectomy at the Faculty Hospital Brno and the Masaryk Memorial Cancer Institute between 2000 and 2006. Seventy eight patients underwent hemipancreaticoduodenectomy, 5 patients caudal pancreatectomy and 2 patients total pancreatectomy. No distant metastases were found at initial diagnosis (M0). Selected tumors were histologically confirmed to be invasive ductal adenocarcinomas of the pancreas, and in all patients the formalin fixed paraffin embedded tissues were available for immunohistochemistry. Grading of tumor differentiation was done based on WHO criteria combining the judgment of glandular differentiation including mucin production, mitotic count and nuclear features. Tumor staging was performed according to the International Union Against Cancer TNM System (the 6th edition). The follow-up was available for 75 patients, with five patients alive at the end of the study. Survival data for the patients were obtained from the National Oncological Register of Czech Republic. Resection specimens were fixed in 10% neutral buffered formalin for 24 h and then embedded in paraffin. Hematoxylin-eosin (HE) staining of tissue sections was used to identify representative samples with structures of pancreatic invasive ductal adenocarcinoma (up to three tissue blocks for sectioning were selected for each individual PDAC case). These were selected for immunohistochemical (IH) analysis.
Table 1.
Patient, tumor and treatment characteristics, and levels of COX-2 expression (n = 85)
| n | |
| Gender | |
| Male | 41 |
| Female | 44 |
| Mean age (range) (yr) | 61 ± 9.5 (39-85) |
| Location of tumor | |
| Head, body | 80 |
| Tail | 5 |
| T category | |
| T1 | 6 |
| T2 | 29 |
| T3 | 50 |
| N category | |
| N0 | 23 |
| N1 | 62 |
| M category | |
| M0 | 85 |
| M1 | 0 |
| Surgical procedure | |
| Pancreatoduodenectomy | 78 |
| Caudal pancreatectomy | 5 |
| Total pancreatectomy | 2 |
| Histological differentiation | |
| Well | 8 |
| Moderately | 54 |
| Poorly differentiated | 23 |
| No adjuvant therapy | 30 |
| Adjuvant therapy | 33 |
| Chemotherapy | 23 |
| Chemoradiotherapy | 10 |
| COX-2 expression with monoclonal antibody | |
| COX-2 negative | 16 |
| COX 1+ | 27 |
| COX-2+ | 39 |
| COX-2 expression with polyclonal antibody | |
| COX-2 negative | 26 |
| COX 1+ | 28 |
| COX-2+ | 31 |
COX-2: Cyclooxygenase-2.
Immunohistochemistry
The results of IH analysis of COX-2 expression using polyclonal anti-COX-2 antibody [rabbit polyclonal antibody against COX-2 (H-62), dilution 1:50; Santa Cruz Biotechnology, Inc., Santa Cruz, California, USA] were retrieved from our previous study[38], in which the identical study population was examined.
In the present study, the COX-2 expression was evaluated using mouse monoclonal anti-COX-2 antibody (dilution 1:50, clone CX229; Cayman Chemicals, Ann Arbor, MI, USA). IH detection of COX-2 was performed on 4 μm thick tissue sections applied to positively charged slides. The sections were deparaffinized in xylene and rehydrated through a series of alcohols. Antigen retrieval was performed in the lab microwave (Milestone, Sorisole, Italy) by heating in citrate buffer at pH 6.0 for 20 min at 98°C. The slides were incubated with anti-COX-2 antibodies overnight at 4°C. A streptavidin-biotin peroxidase detection system was used for COX-2 IH using monoclonal antibody (mouse IgG Vectastain Elite Kit, Vector Laboratories, Burlingame, California, USA). The visualization was performed using 3,3’-diaminobenzidine as a substrate (Fluca, Buchs, Swizerland). The slides were counterstained with Gill’s hematoxylin.
Sections from COX-2 strongly positive colon carcinoma (immunoscore 2 - see explanation below) were used as positive controls for COX-2 IH in each run. Additionally, COX-2 immunoreactivity in islets of endocrine pancreas served as an efficient internal positive control[23]. Negative controls of COX-2 IH were performed by incubating samples without the primary antibody.
Evaluation of immunostaining
At least three different representative high-power (× 400) fields of tumor infiltration were examined. Cases with no stated minimal amount of the representative tumor tissue available were excluded. For both monoclonal and polyclonal antibody COX-2 IH, the percentage of positive cells was assessed, and the immunostaining intensity was classified into three categories: numeric score 0, no staining; numeric score 1, weak staining; numeric score 2, moderate and strong staining. The final immunoscore was obtained by multiplying the percentages of positive cells with the numeric score reflecting the staining intensity. Immunoscores were categorized into three levels: 0 (immunoscore < 20); 1 (immunoscore 20-49); 2 (immunoscore 50-200). In cases with heterogeneous expression of COX-2, the average score was counted.
Statistical analysis
Association of categorical parameters was analyzed and presented in contingency tables and tested using Pearson Chi-square test. Kaplan-Meier curves were constructed and median survival times were computed for survival data. Log-rank test was used to test differences between groups. Univariate and multivariate analyses of survival data were performed using Cox regression model to evaluate the predictive value of analyzed parameters (COX-2, histological grade, T status, N status). The level of significance was considered P < 0.05. All analyses were done using Statistica for Windows 8.0.
RESULTS
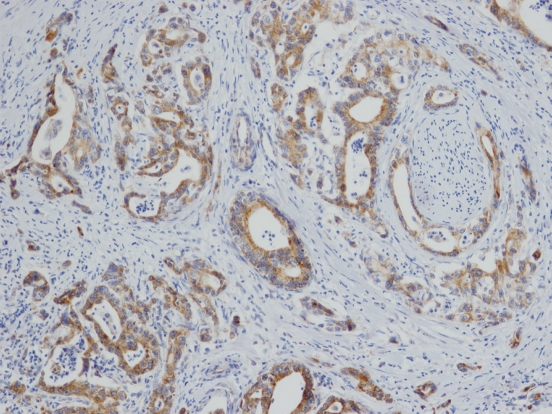
Patient, tumor and treatment characteristics, and levels of COX-2 expression are summarized in Table 1. The immunohistochemical analysis of COX-2 expression displayed considerable heterogeneity in staining intensity and percentage of positive cells between and within individual PDAC cases. In cases with heterogeneous COX-2 expression within a lesion, the average immunoscore for such a case was counted. Using both monoclonal and polyclonal antibodies, the PDAC cells showed diffuse cytoplasmic patterns of expression. Expression of COX-2 (Figure 1), examined using monoclonal anti-COX-2 antibody, was revealed in 66 cases (80.5%); 27 tumors (32.9%) expressed COX-2 at 1+ level, 39 at 2+ level (47.6%), and 16 cases (19.5%) were COX-2 negative (in 3 samples, a sufficient amount of neoplastic tissue was not available for IH analysis, and analysis of COX-2 expression using monoclonal antibody was not performed). Results of immunohistochemical analysis of COX-2 expression by polyclonal antibody were retrieved from our previous study[38] and are included in Table 1 [28 tumors (32.9%) expressed COX-2 at 1+ level, 31 at 2+ level (36.5%), and 26 cases (30.6%) were COX-2 negative].
Figure 1.
Overexpression of cyclooxygenase-2 (COX-2) in pancreatic invasive ductal adenocarcinoma with displayed perineural spreading of the tumor (COX-2 immunohistochemistry, original magnification, × 100).
The median overall survival (OS) in the study population was 1.3 years. There was no significant difference between OS in males and females (median OS 1.1 years in males vs 1.3 years in females; P = 0.143). Regarding nodal (N) status, N1 status was associated with shorter median OS (1.0 year in N1 group vs 1.5 years in N0 group; P = 0.102). Median OS for patients with adjuvant therapy was 1.4 years, and for patients without adjuvant therapy 1.3 years. The median disease free survival (DFS) for patients with adjuvant therapy was 0.8 years, and for patients without adjuvant therapy 0.7 years. Differences did not reach statistical significance.
No correlations between the levels of COX-2 expression (using both monoclonal and polyclonal antibodies) and the histological grade, T or nodal status were revealed.
In the Kaplan-Meier analysis, no significant correlations were found between the levels of COX-2 expression and OS (again using both polyclonal and monoclonal antibodies). However, trends to longer median OS were found in COX-2 negative cases (1.4 years in COX-2 negative vs 1.3 years in low expressors vs 1.0 in high expressors) using monoclonal antibody.
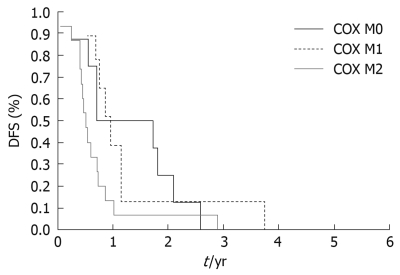
Correlating the levels of COX-2 expression and DFS; using polyclonal antibodies, trends to longer median DFS were found in COX-2 negative cases (1 year in COX-2 negative vs 0.7 years in low expressors vs 0.5 years in high expressors), but these values were not significant (P = 0.211); using monoclonal antibody, a statistically significant correlation was found between COX-2 expression levels and DFS. Significantly longer DFS was revealed in COX-2 negative cases (median DFS 1.2 years in COX-2 negative vs 0.9 year in low expressors vs 0.5 year in high expressors, P = 0.019) (Figure 2).
Figure 2.
Kaplan-Meier disease free survival (DFS) curves for patients with resectable pancreatic cancer stratified by the level of COX-2 expression examined using monoclonal antibody. High expressors of COX-2 (2+, COX M2) had significantly lower survival curve than low expressors of COX-2 (1+, COX M1) or COX-2 negative cases (0, COX M0), P = 0.019.
Low histological grade showed a strong association with a longer OS (P < 0.001). Median OS for patients with grade 3 tumors was 0.7 years vs 1.6 years for grade 2 and 1.8 years for grade 1. Correlation of survival and T status revealed a shorter OS in T3 tumors, but the results reached only marginal statistical significance (P = 0.070). In a multivariate Cox proportional hazards regression model, histological grade (G), T, and nodal status remained valuable predictors of a worse survival with borderline significance for T [hazards ratio (HR) = 4.18 for G (if G = 3, P < 0.001); HR = 1.64 for T (if T = 3, P = 0.065); HR = 2.53 for N (if N = 1, P = 0.006)]. Higher grade, T or N status was associated with a worse OS.
DISCUSSION
Immunohistochemistry has become an integral part of histopathological diagnosis and can also provide important data to predict clinical course of the disease and potential therapeutic responsiveness. Thus, a validation of usefulness of different immunoprofiles and methodological approaches in predictive oncopathology is necessary. Based on a PubMed search, there are 7 papers[33-39] reporting the correlations between COX-2 expression and selected clinicopathological parameters, including survival rates, in pancreatic cancer. The possible role of the COX-2 expression profile used as a prognostic factor for pancreatic ductal adenocarcinomas is largely discussed. Five studies did not show any trends or statistically significant correlations between COX-2 expression and survival rates[35-39]. Our previous study[38] also did not show significant correlations between these parameters, although trends to longer DFS in COX-2 negative cases were demonstrated. Recently, Matsubayashi et al[34] reported that the COX-2 expression in patients with pancreatic ductal adenocarcinoma undergoing a potentially curative pancreaticoduodenectomy is predictive of survival, independent of other known prognostic markers, particularly in cancers ≥ 3 cm. These results were supported by Juuti et al[33] who reported the expression of COX-2 to be associated with poor outcome for pancreatic ductal adenocarcinoma, which was independent of tumor stage, grade, or age in multivariate analysis. Moreover, these results were obtained, even if not only surgically resectable pancreatic cancers were included for analysis. Both Matsubayashi et al[34] and Juuti et al[33], who showed the significant correlation between COX-2 expression and clinical outcome, worked with mouse antihuman monoclonal antibodies. A discussion about the possible role of the selected antibody has been initiated. Antigenic blocking experiments showed higher sensitivity but lower specificity of polyclonal antibodies when compared with monoclonal antibodies[40]. The presented comparative analysis only partially supported the possible prognostic role of COX-2 expression. Results retrieved from our previous study[38], in which rabbit polyclonal antibody was used, were completed with results obtained by examination of COX-2 expression using the same mouse antihuman monoclonal antibody that was used in the studies of Juuti et al[33] and Matsubayashi et al[34]. Using the monoclonal antibody, a significantly shorter DFS was found in patients with COX-2 positive tumors. Trends to shorter DFS in COX-2 expressors were only reported using the polyclonal antibody[38]. No significant results were obtained regarding OS; the only trends to longer OS were demonstrated in COX-2 negative cases using monoclonal antibody. Our results do not sufficiently support the findings of Juuti and Matsubayashi, even when the standardized protocols for immunohistochemical evaluation of COX-2 expression, which should be preventive of known technical and interpretative pitfalls in immunohistochemistry, were used. Except for the mentioned type of antibody, the main factors potentially affecting immunohistochemical staining are the tissue processing, especially the type and length of fixation, and antigen retrieval. All tissues included in the study were routinely fixed in 10% neutral buffered formalin for 24 h, and antigen retrieval was performed using a standardized protocol. To avoid the misinterpretation of results, especially unspecific immunostaining, systems of positive (both external and internal) and negative controls were used in each run. Based on our results, COX-2 expression does not seem to represent a valuable independent prognostic factor, even if using the monoclonal antibody brought statistically significant results when correlating DFS and COX-2 expression levels and when trends to longer median OS were also demonstrated.
Correlating the COX-2 expression with the level of tumor differentiation, no significant relationship was revealed between the level of COX-2 expression and the histological grade of the pancreatic cancer independently of the antibody used. These findings are in agreement with previous studies[23,35,41]. Merati et al[36] described increased expression of COX-2 in well differentiated ductal adenocarcinomas, but this trend was not observed in our study group, and the proposed role of COX-2 in carcinogenesis of pancreatic cancer remains puzzling.
The routinely used grading of the histological differentiation of tumors and evaluation of T and N status were proven to represent efficient prognostic factors. Higher grade of the tumor and N1 status were significantly associated with a shorter survival in patients with resectable pancreatic cancer. T3 status was associated with shorter survival only with marginal significance.
In conclusion, based on these presented results and the previously published data, an immunohistochemical assessment of COX-2 expression is not superior to the conventional prognostic factors such as tumor histological grade, stage, and nodal status. The possible role of COX-2 in potential targeted therapy and chemoprevention of pancreatic cancer using COX-2 inhibitors remain as unanswered questions and need further evaluation.
COMMENTS
Background
Pancreatic cancer is the fourth leading cause of cancer-related deaths. The incidence of pancreatic cancer almost equals the mortality rate and the five-year survival rate does not reach 5%. Mediators of inflammatory pathways are known to play an important role in carcinogenesis and represent a key link between chronic inflammation and cancer.
Research frontiers
Cyclooxygenase-2 (COX-2) represents a key modulatory molecule in inflammation and carcinogenesis. COX-2 is known to have multiple tumorigenic effects. Increased expression of COX-2 has been observed in a variety of tumors including pancreatic cancer. In the literature, the prognostic significance of COX-2 expression including the role of antibody used for an evaluation of COX-2 expression profile have been discussed.
Innovations and breakthroughs
Recent reports have highlighted the role of COX-2 in carcinogenesis of pancreatic cancer and COX-2 has been suggested as a potential therapeutic target. This study confirmed the relationship of COX-2 to biological processes involved in pancreatic cancer, but its potential usefulness as a prognostic marker was not demonstrated.
Applications
The level of COX-2 expression does not seem to be a valuable independent prognostic factor, even if a significantly longer disease free survival and trends to longer overall survival were demonstrated in COX-2 negative cases using monoclonal antibodies. Immunohistochemical assessment of COX-2 expression is not superior to the conventional prognostic factors such as grade, stage and nodal status.
Terminology
COX-1 and COX-2 represent enzymes which are involved in the conversion of arachidonic acid to prostaglandins. COX-1 is expressed in many tissues under physiological conditions, while COX-2 is not constitutively expressed and several studies have suggested its positive role in regulation of growth and angiogenesis as well as inhibition of apoptosis in cancer.
Peer review
In this paper, Hermanova et al analyzed by immunohistochemistry COX-2 in pancreatic adenocarcinoma tissue samples. They concluded that the COX-2 expression does not seem to represent a valuable independent prognostic factor and is not superior to the conventional prognostic factors. Concerning the general view of the manuscript, it is characterized by a well done internal structure and acceptable quality of language. The data are clearly described and the statistical analysis seems to be correct.
Footnotes
Supported by A Grant from the Ministry of Health (IGA), No. NR 9295-3, Czech Republic
Peer reviewer: Evangelos Tsiambas, MD, PhD, Cytopathologist, Lecturer in Molecular Cytopathology, Department of Pathology, Medical School, University of Athens, Ag Paraskevi Attiki, 15341, Greece
S- Editor Wang JL L- Editor Logan S E- Editor Lin YP
References
- 1.Hamilton SR, Aaltonen LA. World Health Organization classification of tumours. Pathology and Genetics of Tumours of Digestive System. Lyon: IARC Press; 2000. pp. 219–252. [Google Scholar]
- 2.Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1997. CA Cancer J Clin. 1997;47:5–27. doi: 10.3322/canjclin.47.1.5. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 4.Bramhall SR, Allum WH, Jones AG, Allwood A, Cummins C, Neoptolemos JP. Treatment and survival in 13,560 patients with pancreatic cancer, and incidence of the disease, in the West Midlands: an epidemiological study. Br J Surg. 1995;82:111–115. doi: 10.1002/bjs.1800820137. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez E, La Vecchia C, Porta M, Negri E, Lucchini F, Levi F. Trends in pancreatic cancer mortality in Europe, 1955-1989. Int J Cancer. 1994;57:786–792. doi: 10.1002/ijc.2910570605. [DOI] [PubMed] [Google Scholar]
- 6.Hruban RH, Yeo CJ, Kern SE. The genetic basis of human cancer. In: Vogelstein B, Kinzler KW, editors. Pancreatic Cancer. New York: McGraw-Hill; 1996. pp. 603–613. [Google Scholar]
- 7.Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Klöppel G, Longnecker DS, et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579–586. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Hruban RH, Wilentz RE, Kern SE. Genetic progression in the pancreatic ducts. Am J Pathol. 2000;156:1821–1825. doi: 10.1016/S0002-9440(10)65054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hruban RH, van Mansfeld AD, Offerhaus GJ, van Weering DH, Allison DC, Goodman SN, Kensler TW, Bose KK, Cameron JL, Bos JL. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol. 1993;143:545–554. [PMC free article] [PubMed] [Google Scholar]
- 10.Rozenblum E, Schutte M, Goggins M, Hahn SA, Panzer S, Zahurak M, Goodman SN, Sohn TA, Hruban RH, Yeo CJ, et al. Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res. 1997;57:1731–1734. [PubMed] [Google Scholar]
- 11.Klöppel G, Lüttges J. The pathology of ductal-type pancreatic carcinomas and pancreatic intraepithelial neoplasia: insights for clinicians. Curr Gastroenterol Rep. 2004;6:111–118. doi: 10.1007/s11894-004-0037-y. [DOI] [PubMed] [Google Scholar]
- 12.Farrow B, Sugiyama Y, Chen A, Uffort E, Nealon W, Mark Evers B. Inflammatory mechanisms contributing to pancreatic cancer development. Ann Surg. 2004;239:763–769; discussion 769-771. doi: 10.1097/01.sla.0000128681.76786.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcea G, Dennison AR, Steward WP, Berry DP. Role of inflammation in pancreatic carcinogenesis and the implications for future therapy. Pancreatology. 2005;5:514–529. doi: 10.1159/000087493. [DOI] [PubMed] [Google Scholar]
- 14.Farrow B, Evers BM. Inflammation and the development of pancreatic cancer. Surg Oncol. 2002;10:153–169. doi: 10.1016/s0960-7404(02)00015-4. [DOI] [PubMed] [Google Scholar]
- 15.Tanabe T, Tohnai N. Cyclooxygenase isozymes and their gene structures and expression. Prostaglandins Other Lipid Mediat. 2002;68-69:95–114. doi: 10.1016/s0090-6980(02)00024-2. [DOI] [PubMed] [Google Scholar]
- 16.Fosslien E. Biochemistry of cyclooxygenase (COX)-2 inhibitors and molecular pathology of COX-2 in neoplasia. Crit Rev Clin Lab Sci. 2000;37:431–502. doi: 10.1080/10408360091174286. [DOI] [PubMed] [Google Scholar]
- 17.Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18:7908–7916. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- 18.Chu J, Lloyd FL, Trifan OC, Knapp B, Rizzo MT. Potential involvement of the cyclooxygenase-2 pathway in the regulation of tumor-associated angiogenesis and growth in pancreatic cancer. Mol Cancer Ther. 2003;2:1–7. [PubMed] [Google Scholar]
- 19.Kong G, Kim EK, Kim WS, Lee KT, Lee YW, Lee JK, Paik SW, Rhee JC. Role of cyclooxygenase-2 and inducible nitric oxide synthase in pancreatic cancer. J Gastroenterol Hepatol. 2002;17:914–921. doi: 10.1046/j.1440-1746.2002.02829.x. [DOI] [PubMed] [Google Scholar]
- 20.Ding XZ, Tong WG, Adrian TE. Blockade of cyclooxygenase-2 inhibits proliferation and induces apoptosis in human pancreatic cancer cells. Anticancer Res. 2000;20:2625–2631. [PubMed] [Google Scholar]
- 21.Eibl G, Bruemmer D, Okada Y, Duffy JP, Law RE, Reber HA, Hines OJ. PGE(2) is generated by specific COX-2 activity and increases VEGF production in COX-2-expressing human pancreatic cancer cells. Biochem Biophys Res Commun. 2003;306:887–897. doi: 10.1016/s0006-291x(03)01079-9. [DOI] [PubMed] [Google Scholar]
- 22.Kokawa A, Kondo H, Gotoda T, Ono H, Saito D, Nakadaira S, Kosuge T, Yoshida S. Increased expression of cyclooxygenase-2 in human pancreatic neoplasms and potential for chemoprevention by cyclooxygenase inhibitors. Cancer. 2001;91:333–338. doi: 10.1002/1097-0142(20010115)91:2<333::aid-cncr1006>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 23.Maitra A, Ashfaq R, Gunn CR, Rahman A, Yeo CJ, Sohn TA, Cameron JL, Hruban RH, Wilentz RE. Cyclooxygenase 2 expression in pancreatic adenocarcinoma and pancreatic intraepithelial neoplasia: an immunohistochemical analysis with automated cellular imaging. Am J Clin Pathol. 2002;118:194–201. doi: 10.1309/TPG4-CK1C-9V8V-8AWC. [DOI] [PubMed] [Google Scholar]
- 24.Albazaz R, Verbeke CS, Rahman SH, McMahon MJ. Cyclooxygenase-2 expression associated with severity of PanIN lesions: a possible link between chronic pancreatitis and pancreatic cancer. Pancreatology. 2005;5:361–369. doi: 10.1159/000086536. [DOI] [PubMed] [Google Scholar]
- 25.Hermanova M, Trna J, Nenutil R, Dite P, Kala Z. Expression of COX-2 is associated with accumulation of p53 in pancreatic cancer: analysis of COX-2 and p53 expression in premalignant and malignant ductal pancreatic lesions. Eur J Gastroenterol Hepatol. 2008;20:732–739. doi: 10.1097/MEG.0b013e3282f945fb. [DOI] [PubMed] [Google Scholar]
- 26.Dannenberg AJ, Altorki NK, Boyle JO, Dang C, Howe LR, Weksler BB, Subbaramaiah K. Cyclo-oxygenase 2: a pharmacological target for the prevention of cancer. Lancet Oncol. 2001;2:544–551. doi: 10.1016/S1470-2045(01)00488-0. [DOI] [PubMed] [Google Scholar]
- 27.Tsubochi H, Sato N, Hiyama M, Kaimori M, Endo S, Sohara Y, Imai T. Combined analysis of cyclooxygenase-2 expression with p53 and Ki-67 in nonsmall cell lung cancer. Ann Thorac Surg. 2006;82:1198–1204. doi: 10.1016/j.athoracsur.2006.04.069. [DOI] [PubMed] [Google Scholar]
- 28.Shi H, Xu JM, Hu NZ, Xie HJ. Prognostic significance of expression of cyclooxygenase-2 and vascular endothelial growth factor in human gastric carcinoma. World J Gastroenterol. 2003;9:1421–1426. doi: 10.3748/wjg.v9.i7.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheehan KM, Sheahan K, O'Donoghue DP, MacSweeney F, Conroy RM, Fitzgerald DJ, Murray FE. The relationship between cyclooxygenase-2 expression and colorectal cancer. JAMA. 1999;282:1254–1257. doi: 10.1001/jama.282.13.1254. [DOI] [PubMed] [Google Scholar]
- 30.Ristimäki A, Sivula A, Lundin J, Lundin M, Salminen T, Haglund C, Joensuu H, Isola J. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62:632–635. [PubMed] [Google Scholar]
- 31.Erkinheimo TL, Lassus H, Finne P, van Rees BP, Leminen A, Ylikorkala O, Haglund C, Butzow R, Ristimäki A. Elevated cyclooxygenase-2 expression is associated with altered expression of p53 and SMAD4, amplification of HER-2/neu, and poor outcome in serous ovarian carcinoma. Clin Cancer Res. 2004;10:538–545. doi: 10.1158/1078-0432.ccr-0132-03. [DOI] [PubMed] [Google Scholar]
- 32.Buskens CJ, Van Rees BP, Sivula A, Reitsma JB, Haglund C, Bosma PJ, Offerhaus GJ, Van Lanschot JJ, Ristimäki A. Prognostic significance of elevated cyclooxygenase 2 expression in patients with adenocarcinoma of the esophagus. Gastroenterology. 2002;122:1800–1807. doi: 10.1053/gast.2002.33580. [DOI] [PubMed] [Google Scholar]
- 33.Juuti A, Louhimo J, Nordling S, Ristimäki A, Haglund C. Cyclooxygenase-2 expression correlates with poor prognosis in pancreatic cancer. J Clin Pathol. 2006;59:382–386. doi: 10.1136/jcp.2005.026831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsubayashi H, Infante JR, Winter J, Klein AP, Schulick R, Hruban R, Visvanathan K, Goggins M. Tumor COX-2 expression and prognosis of patients with resectable pancreatic cancer. Cancer Biol Ther. 2007;6:1569–1575. doi: 10.4161/cbt.6.10.4711. [DOI] [PubMed] [Google Scholar]
- 35.Koshiba T, Hosotani R, Miyamoto Y, Wada M, Lee JU, Fujimoto K, Tsuji S, Nakajima S, Doi R, Imamura M. Immunohistochemical analysis of cyclooxygenase-2 expression in pancreatic tumors. Int J Pancreatol. 1999;26:69–76. doi: 10.1007/BF02781733. [DOI] [PubMed] [Google Scholar]
- 36.Merati K, said Siadaty M, Andea A, Sarkar F, Ben-Josef E, Mohammad R, Philip P, Shields AF, Vaitkevicius V, Grignon DJ, et al. Expression of inflammatory modulator COX-2 in pancreatic ductal adenocarcinoma and its relationship to pathologic and clinical parameters. Am J Clin Oncol. 2001;24:447–452. doi: 10.1097/00000421-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Matsumoto G, Muta M, Tsuruta K, Horiguchi S, Karasawa K, Okamoto A. Tumor size significantly correlates with postoperative liver metastases and COX-2 expression in patients with resectable pancreatic cancer. Pancreatology. 2007;7:167–173. doi: 10.1159/000104241. [DOI] [PubMed] [Google Scholar]
- 38.Hermanova M, Karasek P, Nenutil R, Kyr M, Tomasek J, Baltasova I, Dite P. Clinicopathological correlations of cyclooxygenase-2, MDM2, and p53 expressions in surgically resectable pancreatic invasive ductal adenocarcinoma. Pancreas. 2009;38:565–571. doi: 10.1097/MPA.0b013e31819fef8b. [DOI] [PubMed] [Google Scholar]
- 39.Tonini G, Vincenzi B, Santini D, Scarpa S, Vasaturo T, Malacrino C, Coppola R, Magistrelli P, Borzomati D, Baldi A, et al. Nuclear and cytoplasmic expression of survivin in 67 surgically resected pancreatic cancer patients. Br J Cancer. 2005;92:2225–2232. doi: 10.1038/sj.bjc.6602632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saukkonen K, Nieminen O, van Rees B, Vilkki S, Härkönen M, Juhola M, Mecklin JP, Sipponen P, Ristimäki A. Expression of cyclooxygenase-2 in dysplasia of the stomach and in intestinal-type gastric adenocarcinoma. Clin Cancer Res. 2001;7:1923–1931. [PubMed] [Google Scholar]
- 41.Okami J, Yamamoto H, Fujiwara Y, Tsujie M, Kondo M, Noura S, Oshima S, Nagano H, Dono K, Umeshita K, et al. Overexpression of cyclooxygenase-2 in carcinoma of the pancreas. Clin Cancer Res. 1999;5:2018–2024. [PubMed] [Google Scholar]