Abstract
AIM: To evaluate the induction of remission and maintenance effects of probiotics for ulcerative colitis.
METHODS: Information was retrieved from MEDLINE, EMBASE, and the Cochrane Controlled Trials Register. The induction of remission and promotion of maintenance were compared between probiotics treatment and non-probiotics treatment in ulcerative colitis.
RESULTS: Thirteen randomized controlled studies met the selection criteria. Seven studies evaluated the remission rate, and eight studies estimated the recurrence rate; two studies evaluated both remission and recurrence rates. Compared with the non-probiotics group, the remission rate for ulcerative colitis patients who received probiotics was 1.35 (95% CI: 0.98-1.85). Compared with the placebo group, the remission rate of ulcerative colitis who received probiotics was 2.00 (95% CI: 1.35-2.96). During the course of treatment, in patients who received probiotics for less than 12 mo compared with the group treated by non-probiotics, the remission rate of ulcerative colitis was 1.36 (95% CI: 1.07-1.73). Compared with the non-probiotics group, the recurrence rate of ulcerative colitis patients who received probiotics was 0.69 (95% CI: 2.47-1.01). In the mild to moderate group who received probiotics, compared to the group who did not receive probiotics, the recurrence rate was 0.25 (95% CI: 0.12-0.51). The group who received Bifidobacterium bifidum treatment had a recurrence rate of 0.25 (95% CI: 0.12-0.50) compared with the non-probiotics group.
CONCLUSION: Probiotic treatment was more effective than placebo in maintaining remission in ulcerative colitis.
Keywords: Inflammatory bowel disease, Ulcerative colitis, Probiotics, Meta-analysis
INTRODUCTION
Inflammatory bowel disease is a chronic recurrent disease, which mainly consists of ulcerative colitis and Crohn’s disease, and whose causes are as yet unclear. It is still assumed that inflammatory bowel disease is caused by integrative factors, such as genetic susceptibility, an imbalance between gastrointestinal probiotics and pathogens, an impairment of intestinal epithelial cells, and host immune dysfunctions. Many clinical and research studies have indicated that intestinal flora dysbacteriosis contributes to the pathogenesis of ulcerative colitis. Probiotics are non-pathogenic beneficial flora, which have important effects on maintaining the balance of intestinal flora[1]. Probiotics can adjust the metabolic activity of the intestinal flora and their components by preventing bacterial overgrowth and by maintaining the integrity of the intestinal mucosal barrier, thereby adjusting and stabilizing the intestinal environment[2,3].
Inducing remission and preventing recurrence and complications are the primary goals of inflammatory bowel disease treatment[4]. Many ulcerative colitis patients experience a short period of remission after induction but then must be treated by surgery after recurrent attacks. Postoperative pouchitis is always caused by the reduction of Lactobacillus lactis and Bifidobacterium. Many studies have discussed the positive effects of probiotics for treating stomach and intestine diseases, including ulcerative colitis[5-7]. However, the sample size has been relatively small, such that there is no definitive evidence as to whether probiotics are helpful. Thus, this paper systematically evaluates probiotics’ curative effects for treating ulcerative colitis based on existing random control trials.
MATERIALS AND METHODS
Retrieval strategy
Data was retrieved from the databases: We searched the data from MEDLINE (1966 to August 2009), EMBASE (1980 to August 2009), and the Cochrane Controlled Trials Register (1995 to August 2009). The keywords were used below: probiotics, inflammatory bowel disease, ulcerative colitis, Escherichia coli, Lactobacillus, Bifidobacterium, and yeasts. Language was limited to studies published in English. Moreover, abstracts presented at main international gastrointestinal disease meetings (including Digestive Disease Week of the American Gastroenterological Association, the World Congress of Gastroenterology) for the past five years were analyzed by joint manual retrieval. The retrieval results were reviewed by two evaluators, and a third person was consulted if the two evaluators’ opinions were different.
Selection criteria: All the random control experiments that compared probiotics with ulcerative colitis treatment medicine or placebo were collected, including abstracts and full texts. The selection criteria were as follows: (1) adult and pediatric studies were included; (2) the experiments compared the curative effect of probiotics with standard therapy for ulcerative colitis or placebo; (3) all were random control tests; (4) abstracts and meeting presentations were selected; and (5) patients had a definite diagnosis of ulcerative colitis using definite diagnosis standards. Reviews and case reports were excluded.
Data retrieval and quality assessment
Two researchers selected the papers after reading the titles, abstracts, and full texts. They evaluated the quality of each selected paper and retrieved the data. If they had different opinions, a third researcher helped them to determine the applicability of the paper in question. The quality of the methods used by the selected random control experiments was assessed by the Cochrane Reviewer Handbook 5.0 random control experiment quality assessment standard. The following methods were evaluated: (1) Random method: random method right, random method not described, random method error; (2) Allocation concealment: concealment method right, concealment method not described, concealment method error, concealment method not used; (3) Blind method: whether the evaluator was blinded to the conditions of the experiment; and (4) Whether lost or exit: if there are lost or exit conditions, the reasons are indicated clearly or not, and whether intention-to-treat analysis was used. According to these four quality criteria, all answers that were “right or enough”, where the possibilities for bias are the least; the assessment was level “A”. If one or more criteria were not described, it means that the assessment was partially satisfied. In this condition, there is a possibility of relative bias, and the assessment was designated as level “B”. If one or more criteria were erroneous or not used, there was a high possibility of relative bias, and the assessment was designated as level “C”.
Statistical analysis
The relative risk and 95% confidence interval (95% CI) was calculated based on the data. Statistical analysis was performed with Cochrane Collaboration’s Revman 5.0. Heterogeneity analyses were performed on the experimental results. If there was obvious heterogeneity, the random effects model was chosen, if not, the fixed effects model was chosen.
Whether there was publication bias could be observed by an inverted funnel plot, and the bias level was assessed by the formula:
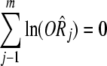 |
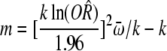 |
Where k is the number of selected papers; m is the least unpublished number of reports that yield a combined effect size with no statistical significance;
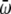 |
is the average weight of k published reports (reciprocal of variance); and
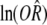 |
is the estimate value logarithm of the combined effect size.
RESULTS
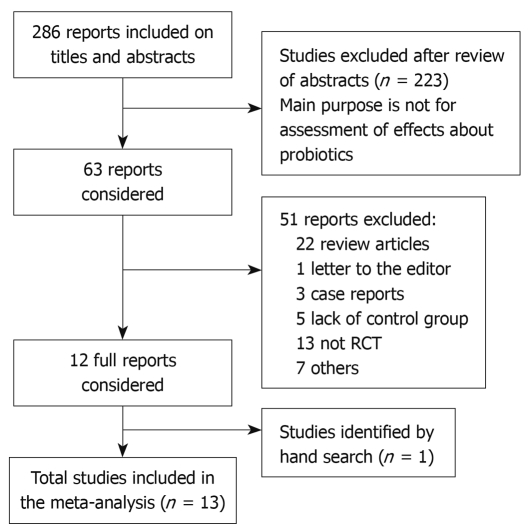
A total of 286 papers were retrieved. After a thorough screening of the titles, abstracts, or full texts and excluding reviews, uncontrolled tests, and basic research, 13 papers were ultimately selected[5-11] (Figure 1). One of the 13 papers was published as an abstract, 12 of were published as full text; seven of them evaluated the remission rate, eight papers assessed the recurrence rate, and two papers evaluated both the remission rate and recurrence rate. The span of the research or the duration of the follow-up visits was 1 to 12 mo. All the papers were published in English. The general conditions of the selected research are shown in Table 1[8-20].
Figure 1.
Study selection process. A flowchart was present in this figure and the flowchart summarizes the selection of studies including numbers and reasons of studies excluded.
Table 1.
Characteristics of included studies
| Study | Disease severity | Probiotic | Control group | Dose of pro-biotic/day) | Treatment duration | N (probiotic/control group) | induction or maintenance of remission N (probiotic/control group) |
| Miele et al[13], 2009 | Mild-to-moderate | VSL#3 | Placebo | 450-1800 × 109 | 12 mo | 14/15 | Induction of remission 13/4; Maintenance of remission 11/4 |
| Sood et al[12], 2009 | Mild-to-moderate | VSL#3 | Placebo | 3.6 × 1012 | 12 wk | 77/70 | Induction of remission 33/11 |
| Henker et al[15], 2008 | Inactive UC | E. coli Nissle 1917 | 5-ASA | 5 × 1010 | 12 mo | 24/10 | Maintenance of remission 18/7 |
| Zocco et al[20], 2006 | Inactive UC | Lactobacillus GG | Mesalazine | 18 × 109 | 12 mo | 65/60/62 | Maintenance of remission 55/48/52 |
| Matthes et al[11], 2006 | Mild-to-moderate | E. coli Nissle 1917 | Placebo | 10-40 × 108 | 4 wk | 46/11 | Induction of remission 20/2 |
| Furrie et al[9], 2005 | Active | Synbiotic (Bifidobacterium longum + inulin-oligofructose) | Potato starch and sachet of 6 g powdered maltodextrose | 4 × 1011 | 4 wk | 9/9 | Induction of remission 5/3 |
| Tursi et al[8], 2004 | Mild-to-moderate | Balsalazide/VSL#3 | Mesalazine/balsalazide | 900 × 108 | 8 wk | 30/30/30 | Induction of remission 24/21/16 |
| Kruis et al[18], 2004 | Inactive | E. coli Nissle 1917 | Mesalazine | 2.5-25 × 109 | 12 mo | 162/165 | Maintenance of remission 89/104 |
| Kato et al[10], 2004 | Mild-to-moderate | Bifidobacterium breve strain Yakult Bifidobacterium bifidum strain Yakult Lactobacillus acidophilus | BFM without B. bifidum and L. acidophilus | 109 | 12 wk | 10/10 | Induction of remission 4/3 |
| Cui et al[16], 2004 | Active | Bifidobacteria | Starch | 1.26 g/d | 8 wk | 15/15 | Maintenance of remission 12/1 |
| Ishikawa et al[17], 2003 | Mild-to-moderate | Bifidobacterium Breve Bifidobacterium Bifidum Lactobacillus acidophilus YIT 0168 | BFM without these Bifidobacteria | 10 × 108 | 12 mo | 11/10 | Maintenance of remission 8/1 |
| Rembacken et al[14], 1999 | Active | E. coli Nissle 1917 serotype O6: K5: H1 | Mesalazine | 5 × 1010 | 12 mo | 57/59 | Induction of remission 39/44; Maintenance of remission 31/27 |
| Kruis et al[19], 1997 | Inactive UC | E. coli Nissle 1917 serotype O6: K5: H1 | Mesalazine | 50 × 109 | 12 wk | 50/53 | Maintenance of remission 42/51 |
E. coli: Escherichia coli.
Quality assessment of selected papers
The quality assessment of the 13 selected papers is shown in Table 2. Three reports were level A, eight were level B, and two papers were level C.
Table 2.
Methodological quality of the 13 RCTs
| Study | Random method | Allocation concealment | Blind method | Lost or exit | Quality assessment | Inclusion/exclusion criteria | Outcome measurement |
| Miele et al[13], 2009 | Right | Right | Double blind | Yes, use ITT analysis | A | Both | CAI by Lichtiger; EI score; HI score |
| Sood et al[12], 2009 | Right | Right | Double blind | Yes, use ITT analysis | A | Both | DAI by Sutherland |
| Henker et al[15], 2008 | Right | Non-described | Non-described | No | B | Inclusion criteria | CAI by Rachmilewitz |
| Zocco et al[20], 2006 | Right | Non-described | Non-described | No | B | Both | CAI by Rachmilewitz; EI by Baron; HI by Truelove-Richard |
| Matthes et al[11], 2006 | Right | Not used | Double blind | Yes, use ITT analysis | C | Not mentioned | DAI by Sutherland |
| Furrie et al[9], 2005 | Right | Non-described | Double blind | No | B | Inclusion criteria | CAI by Walmsley; SI by Baron |
| Tursi et al[8], 2004 | Right | Non-described | Non-described | Yes, use ITT analysis | B | Both | CAI by Lennard; EI score; HI score |
| Kruis et al[18], 2004 | Right | Right | Double blind | Yes, use ITT analysis | A | Both | Scores according to Rachmilewitz |
| Kato et al[10], 2004 | Right | Non-described | Non-described | No | B | Both | CAI by Lichtiger; EI by Harig, Scheppach; HI by Matts |
| Cui et al[16], 2004 | Right | Not used | Non-described | No | C | Not mentioned | Not mentioned |
| Ishikawa et al[17], 2003 | Right | Non-described | Non-described | No | B | Inclusion criteria | Exacerbation of clinical symptoms |
| Rembacken et al[14], 1999 | Right | Non-described | Double blind | Yes, use ITT analysis | B | Inclusion criteria | Scores according to Rachmilewitz |
| Kruis et al[19], 1997 | Right | Non-described | Double blind | Yes, use ITT analysis | B | Both | The same of the CAI score under the E. coli and mesalazine; Scores according to Rachmilewitz |
CAI: Clinical activity index; SI: Sigmoidoscopy index; EI: Endoscopy; HI: Histology index.
Induction of remission of ulcerative colitis by probiotics
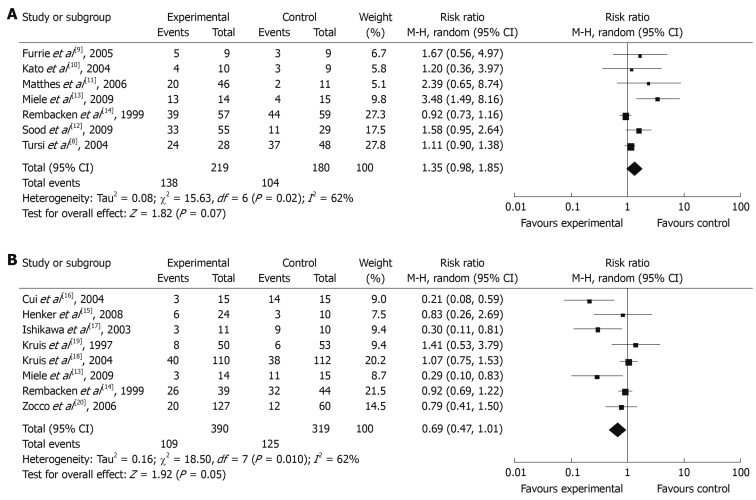
Seven reports evaluated the remission rate, which involved a total of 399 patients. Among the 399 patients, probiotics were used as an auxiliary therapy in 219 patients, and 180 patients were treated by standard therapy or placebo. Comparing the probiotics auxiliary therapy group with non-probiotics auxiliary therapy group, the remission rates were not significantly different (remission rate: 1.35, 95% CI: 0.98-1.85, P = 0.07), but there was an obvious heterogeneity in the results (P = 0.02, I2 = 62%). The total remission rate of the probiotics auxiliary therapy group was 68.2%, and for the non-probiotics auxiliary therapy group it was 60.4% (Figure 2A).
Figure 2.
Remission rate (A) and relapse rate (B) of probiotic group vs control group.
Sub-group analysis
Due to the heterogeneity of the total remission rate, subgroups were analyzed based on the severity of disease, placebo or not, different kinds of probiotics, and the span of probiotics therapy. The selected reports were first separated into a mild to middle subgroup and an active phase subgroup. The remission rate of the mild to middle sub-group was 1.64 (95% CI: 0.98-2.72) and heterogeneity was obvious (P = 0.02, I2 = 66%). The remission rate for the active phase subgroup was 0.97 (95% CI: 0.77-1.22), and heterogeneity existed (P = 0.28, I2 = 13%). Secondly, the selected reports were divided into a placebo controlled sub-group and a non-placebo controlled sub-group. The remission rates of these two sub-groups were 2.00 (95% CI: 1.35-2.96) and 1.00 (95% CI: 0.85-1.18), respectively. The remission rate of the probiotics auxiliary therapy sub-group was significantly better than the placebo subgroup, and there were obvious heterogeneities in these two sub-groups (P = 0.46, I2 = 0%). The selected reports were then separated into a VSL#3 subgroup, an E. coli subgroup, and a Bifidobacterium longum subgroup. The remission rate of the VSL#3 subgroup was 1.66 (95% CI: 0.87-3.15), and heterogeneity was obvious (P = 0.006, I2 = 80%). The remission rate of the E. coli subgroup was 1.02 (95% CI: 0.80-1.30), and heterogeneity was obvious (P = 0.12, I2 = 59%). The remission rate of the Bifidobacterium longum subgroup was 1.43 (95% CI: 0.64-3.19), and heterogeneity was not apparent (P = 0.69, I2 = 0%). Finally, the selected research was divided into groups that received treatment for at least 12 mo or less than 12 mo, based on the course of treatment. The remission rate for the 12 mo sub-group was 1.69 (95% CI: 0.43-6.67), and heterogeneity was not apparent (P = 0.002, I2 = 90%). The remission rate of the sub-group that received treatment for less than 12 mo was 1.36 (95% CI: 1.07-1.73). In the subgroup that received treatment for less than 12 mo and was treated by probiotics auxiliary therapy, the remission rate was obviously better than the control group, and heterogeneity existed (P = 0.33, I2 = 14%).
Sensitivity analysis
Seven reports evaluated the remission rate. In one of the studies, the patients were treated by probiotic enema formulations but not by humatin, so this research was excluded. Sensitivity analysis indicated that the total remission rate was 1.30 (95% CI: 0.94-1.78), and that the statistical heterogeneity was significant (P = 0.02, I2 = 63%). There was no significant differences in the remission rates between the groups that received probiotics or non-probiotics.
Effect of probiotics on the recurrence rate in maintenance treatment of ulcerative colitis
Eight reports assessed the recurrence rate, which involved a total of 709 patients, 390 of them used probiotics for maintenance treatment, and 319 patients used control medicine or a placebo. There was a significant difference between the total recurrence rates between the probiotics maintenance therapy group and placebo maintenance therapy group (recurrence rate: 0.69, 95% CI: 0.47-1.01, P = 0.05). The total recurrence rate for the probiotics maintenance therapy group was 27.9%, while the recurrence of the control group was 39.2%. The heterogeneity of the total recurrence rate was found to be significant by meta-analysis (P = 0.01, I2 = 62%).
Subgroup analysis
Due to the significant heterogeneity of the total recurrence rate, it was necessary to analyze the subgroups to explain and/or reduce the heterogeneity. The selected studies were first divided into an active phase subgroup, a remission stage subgroup, and a mild to middle subgroup. The remission rate of the active phase subgroup was 0.48 (95% CI: 0.10-2.26) with obvious heterogeneity (P = 0.003, I2 = 89%). The remission rate of the remission stage subgroup was 1.01 (95% CI: 0.14-0.61) without heterogeneity (P = 0.96, I2 = 0%). The recurrence rate of the probiotics therapy group was much lower than the non-probiotics therapy group. The selected research was then separated into a placebo control subgroup and a non-placebo control subgroup. The remission rate of the placebo control sub-group was 0.25 (95% CI: 0.12-0.51) without obvious heterogeneity (P = 0.68, I2 = 0%). The non-placebo control subgroup’s remission rate was 0.92 (95% CI: 0.75-1.14) with visible heterogeneity (P = 0.26, I2 = 24%), which was obviously higher than the placebo control sub-group. The remission rate of the probiotics therapy group was not significantly different from the non-placebo control sub-group.
The selected research was then grouped into an E. coli subgroup, a Bifidobacterium subgroup, and a VSL#3 Lactobacillus subgroup. The remission rate of the E. coli subgroup was 1.02 (95% CI: 0.81-1.29), and there was no obvious heterogeneity (P = 0.76, I2 = 0%). The remission rate of the Bifidobacterium subgroup was 0.25 (95% CI: 0.12-0.51) without visible heterogeneity (P = 0.63, I2 = 0%). The VSL#3 lactobacillus subgroup remission rate was 0.59 (95% CI: 0.35-1.01) with significant heterogeneity (P = 0.11, I2 = 60%). Finally, the selected studies were divided into patients that had received treatment for 12 mo and those that had received treatment for less than 12 mo. The remission rate of the 12 mo subgroup was 0.83 (95% CI: 0.68-1.03) with significant heterogeneity (P = 0.07, I2 = 51%), while the remission rate of the group that had received treatment for less than 12 mo was 0.55 (95% CI: 0.09-3.51) with significant heterogeneity (P = 0.009, I2 = 85%) (Figure 2B).
Publication bias assessment
The publication bias could not only be qualitatively analyzed using a funnel plot to observe whether a bias existed, but we could also quantitatively count the bias level using a formula to determine the extent of the publication bias.
Quantification of publication bias: In the above analysis concerning probiotic treatment and its effect on the remission rate and recurrence rate of ulcerative colitis, the publication bias was analyzed quantitatively; however, quantification of the publication bias requires positive results, thus the total recurrence rate of ulcerative colitis affected by probiotics only was assessed.
To compare the recurrence rate between the probiotics group and non- probiotics group, the values used were as follows: ln (R) = -0.3711, W = 101.59, m = 8 × (0.3711/1.96)2 × (101.59/8) - 8 = 21. The result showed the analysis results could not be reversed unless 21 non-statistically significant papers were added. This indicated that publication bias had little effect on the results in the analysis of recurrence rate of probiotics group and non-probiotics group. Therefore, the results were reliable.
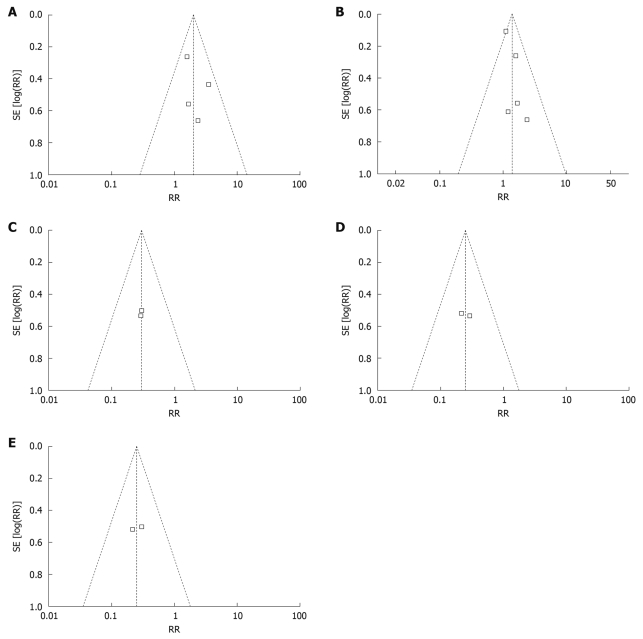
Qualitative evaluation of publication bias: Inverted funnel plot analysis can analyze the results from a fixed effect model. Figure 3 shows that the inverted funnel plot had an uneven distribution and apparent asymmetry. This meant that in the patients who were treated for less than 12 mo, there was a publication bias in the relative of remission rate between the probiotics group and the non-probiotics group. The inverted funnel plots that were symmetrical indicated that there was no visible publication bias.
Figure 3.
Inverted funnel plot analysis of the relative of remission rate. A: Inverted funnel plot analysis of the relative of remission rate between probiotics group and placebo group; B: Less than 12 mo group: Inverted funnel plot analysis of the relative of remission rate between probiotics group and non-probiotics group; C: Mild and middle UC group: Inverted funnel plot analysis of the relative of remission rate between probiotics group and non-probiotics group; D: Inverted funnel plot analysis of the relative of remission rate between probiotics group and placebo group; E: Inverted funnel plot analysis of the relative of remission rate between Bifidobacterium longum therapy group and non-probiotics therapy group.
DISCUSSION
This study shows that using probiotics provides no additional benefit in inducing remission of ulcerative colitis, but probiotics auxiliary therapy is much better than non-probiotics therapy for maintenance therapy. Research has discussed the various effects of probiotics as an ulcerative colitis therapy. The mechanism of probiotics in ulcerative colitis therapy mainly involves the following: (1) to prevent pathogen infiltration by restraining bacterial adherence and bacteria translocation, or to produce anti-bacterial substances that inhibit pathogenic bacteria; (2) to improve the intestinal mucosal barrier function, and the permeability and stability of the mucosal barrier; and (3) to regulate the mucosal immune response. Probiotics cause modifications of the mucosal immune response, improve activities of macrophages and NK cells, stimulate the production of antibodies, regulate the nuclear factor-κB (NF-κB) pathway, induce the apoptosis of T cells, and reduce the secretion of proinflammatory factors[21]. However, most of the studies on the effects of probiotics in ulcerative colitis therapy were performed in vitro or in animal models. Although probiotic effects are closely related to applied clinical conditions, there are some disputes between the results from basic research and clinical research. In addition, the inconsistency of research baselines has also caused disagreement among clinical researchers.
Although this study shows that using probiotics provides no additional benefit in the induction of remission for ulcerative colitis, there was a visible heterogeneity in the total remission rate. This effect might be related to the different methods used by the selected papers, such as the kinds of probiotics used, the treatment time, the types of ulcerative colitis, medication compliance of patients, whether patients were treated by antibiotics simultaneously, difference of the control groups, the analysis of the results, and the sample size. Some research shows that there are significantly different effects yielded by different kinds of bacteria and yeasts used as therapies[22,23]. Subgroup analysis demonstrated that the probiotics group was more likely to be in remission than the placebo group of patients with ulcerative colitis. In the subgroup that received treatment for less than 12 mo, the remission rate of the probiotics group was obviously higher than the non-probiotics group. The results suggested that probiotics auxiliary therapy could be used as another choice to induce the remission of ulcerative colitis, which has a better remission effect than the non-probiotics therapy group after 1 year. Some research has shown that the protective effects of probiotics act during the initial stage of ulcerative colitis impairment, but not during the refractory period. This result still needs to be confirmed using additional random control tests.
In the maintenance therapy stage, the probiotics auxiliary therapy was obviously better than non-probiotic therapy; that is, the recurrence rate of probiotics auxiliary therapy group was significantly lower than non-probiotics therapy group during the maintenance therapy stage, and this difference was heterogeneous. Subgroup analysis showed that the effect of probiotic maintenance therapy was obviously better than non-probiotic therapy in the mild to middle subgroup, placebo subgroup, and Bifidobacterium subgroup, and this difference was statistically significant. The results demonstrated that probiotics auxiliary therapy was more suitable in ulcerative colitis patients. Compared with standard treatments, such as 5-ASA or mesalazine, the effect of probiotics auxiliary therapy was not significantly different, but was obviously better than placebo therapy. The recurrence rate of the Bifidobacterium group was much lower than the non-probiotics therapy group, and the difference was statistically significant.
The main advantage of probiotics therapy is that it does not affect the regeneration of normal mucosa during active therapy[23]. To overcome gastrointestinal tract infections, probiotics must be non-pathogenic and resistant to antibiotics. In addition, probiotics can resist stomach acid, bile, and antibiotics and can modify immune processes to destroy invading microorganisms[24].
In this meta-analysis, the evaluation criteria of the results were not totally consistent. To a certain extent, this condition added to the heterogeneity of the systemic review.
In conclusion, whether the use of probiotics is better than non-probiotics therapy during the remission induction stage and maintenance stage of ulcerative colitis still needs further clinical trials, including variations of bacterial species, applied dosage, treatment timing, and the course of treatment.
COMMENTS
Background
Ulcerative colitis is a chronic recurrent colon disease whose pathogenesis is not clear. Whether probiotics should be used in treatment remains in dispute. This paper compares the effects of probiotics and drug treatment of ulcerative colitis or placebo in remission induction and maintenance of ulcerative colitis.
Research frontiers
Many studies indicated probiotics have positive effects in treating gastrointestinal diseases, including ulcerative colitis. However, the data comes from small sample studies and does not provide enough evidence of probiotics’ advantage.
Innovations and breakthroughs
Thirteen randomized controlled experiments with relatively larger sample sizes, systematically evaluated the probiotics’ remission induction and maintenance effects in treating ulcerative colitis.
Applications
Through meta-analysis, probiotics should be considered as an auxiliary medicine in the remission induction stage and maintenance stage of ulcerative colitis.
Peer review
This is a meta-analysis of the effect of probiotics on ulcerative colitis, which might provide some useful information. It is a nice, well-conducted study.
Footnotes
Peer reviewers: Tauseef Ali, MD, Assistant Professor, Section of Digestive Diseases and Nutrition, University of Oklahoma Health Sciences Center, 920 SL Young Blvd, Oklahoma, OK 73104, United States; Xiaofa Qin, MD, PhD, Department of Surgery, UMDNJ-New Jersey Medical School, 185 South Orange Avenue, Newark, NJ 07103, United States
S- Editor Wang JL L- Editor Stewart GJ E- Editor Lin YP
References
- 1.Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989;66:365–378. [PubMed] [Google Scholar]
- 2.Shanahan F. Probiotics in inflammatory bowel disease--therapeutic rationale and role. Adv Drug Deliv Rev. 2004;56:809–818. doi: 10.1016/j.addr.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Bengmark S. Ecological control of the gastrointestinal tract. The role of probiotic flora. Gut. 1998;42:2–7. doi: 10.1136/gut.42.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rutgeerts P, Vermeire S, Van Assche G. Mucosal healing in inflammatory bowel disease: impossible ideal or therapeutic target? Gut. 2007;56:453–455. doi: 10.1136/gut.2005.088732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saggioro A. Probiotics in the treatment of irritable bowel syndrome. J Clin Gastroenterol. 2004;38:S104–S106. doi: 10.1097/01.mcg.0000129271.98814.e2. [DOI] [PubMed] [Google Scholar]
- 6.Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–1633. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Madsen KL. The use of probiotics in gastrointestinal disease. Can J Gastroenterol. 2001;15:817–822. doi: 10.1155/2001/690741. [DOI] [PubMed] [Google Scholar]
- 8.Tursi A, Brandimarte G, Giorgetti GM, Forti G, Modeo ME, Gigliobianco A. Low-dose balsalazide plus a high-potency probiotic preparation is more effective than balsalazide alone or mesalazine in the treatment of acute mild-to-moderate ulcerative colitis. Med Sci Monit. 2004;10:PI126–PI131. [PubMed] [Google Scholar]
- 9.Furrie E, Macfarlane S, Kennedy A, Cummings JH, Walsh SV, O'neil DA, Macfarlane GT. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trial. Gut. 2005;54:242–249. doi: 10.1136/gut.2004.044834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kato K, Mizuno S, Umesaki Y, Ishii Y, Sugitani M, Imaoka A, Otsuka M, Hasunuma O, Kurihara R, Iwasaki A, et al. Randomized placebo-controlled trial assessing the effect of bifidobacteria-fermented milk on active ulcerative colitis. Aliment Pharmacol Ther. 2004;20:1133–1141. doi: 10.1111/j.1365-2036.2004.02268.x. [DOI] [PubMed] [Google Scholar]
- 11.Matthes H, Krummenerl T, Giensch M, Wollf C, Schulze J. Treatment of mild to moderate acute attacks of distal ulcer- ative colitis with rectally-administered E. coli Nissle 1917: dose-dependent efficacy. Gastroenterology. 2006;130(Suppl 2):A119. [Google Scholar]
- 12.Sood A, Midha V, Makharia GK, Ahuja V, Singal D, Goswami P, Tandon RK. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2009;7:1202–1209, 1209.e1. doi: 10.1016/j.cgh.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Miele E, Pascarella F, Giannetti E, Quaglietta L, Baldassano RN, Staiano A. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am J Gastroenterol. 2009;104:437–443. doi: 10.1038/ajg.2008.118. [DOI] [PubMed] [Google Scholar]
- 14.Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon AT. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet. 1999;354:635–639. doi: 10.1016/s0140-6736(98)06343-0. [DOI] [PubMed] [Google Scholar]
- 15.Henker J, Müller S, Laass MW, Schreiner A, Schulze J. Probiotic Escherichia coli Nissle 1917 (EcN) for successful remission maintenance of ulcerative colitis in children and adolescents: an open-label pilot study. Z Gastroenterol. 2008;46:874–875. doi: 10.1055/s-2008-1027463. [DOI] [PubMed] [Google Scholar]
- 16.Cui HH, Chen CL, Wang JD, Yang YJ, Cun Y, Wu JB, Liu YH, Dan HL, Jian YT, Chen XQ. Effects of probiotic on intestinal mucosa of patients with ulcerative colitis. World J Gastroenterol. 2004;10:1521–1525. doi: 10.3748/wjg.v10.i10.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishikawa H, Akedo I, Umesaki Y, Tanaka R, Imaoka A, Otani T. Randomized controlled trial of the effect of bifidobacteria-fermented milk on ulcerative colitis. J Am Coll Nutr. 2003;22:56–63. doi: 10.1080/07315724.2003.10719276. [DOI] [PubMed] [Google Scholar]
- 18.Kruis W, Fric P, Pokrotnieks J, Lukás M, Fixa B, Kascák M, Kamm MA, Weismueller J, Beglinger C, Stolte M, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617–1623. doi: 10.1136/gut.2003.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kruis W, Schütz E, Fric P, Fixa B, Judmaier G, Stolte M. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 1997;11:853–858. doi: 10.1046/j.1365-2036.1997.00225.x. [DOI] [PubMed] [Google Scholar]
- 20.Zocco MA, dal Verme LZ, Cremonini F, Piscaglia AC, Nista EC, Candelli M, Novi M, Rigante D, Cazzato IA, Ojetti V, et al. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 2006;23:1567–1574. doi: 10.1111/j.1365-2036.2006.02927.x. [DOI] [PubMed] [Google Scholar]
- 21.Fedorak RN, Madsen KL. Probiotics and the management of inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:286–299. doi: 10.1097/00054725-200405000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Rioux KP, Fedorak RN. Probiotics in the treatment of inflammatory bowel disease. J Clin Gastroenterol. 2006;40:260–263. doi: 10.1097/00004836-200603000-00019. [DOI] [PubMed] [Google Scholar]
- 23.McFarland LV. Meta-analysis of probiotics for the prevention of traveler's diarrhea. Travel Med Infect Dis. 2007;5:97–105. doi: 10.1016/j.tmaid.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Van Niel CW, Feudtner C, Garrison MM, Christakis DA. Lactobacillus therapy for acute infectious diarrhea in children: a meta-analysis. Pediatrics. 2002;109:678–684. doi: 10.1542/peds.109.4.678. [DOI] [PubMed] [Google Scholar]