Abstract
Objective
To test the safety, tolerability, and pharmacokinetics of the anti- TNF-α monoclonal antibody, infliximab, in subjects with intravenous immunoglobulin (IVIG)-resistant Kawasaki disease (KD).
Study design
We conducted a multicenter, randomized, prospective trial of second IVIG infusion (2 g/kg) versus infliximab (5 mg/kg) in 24 children with acute KD and fever following initial treatment with IVIG. Primary outcome measures were infliximab safety, tolerability, and pharmacokinetics. Secondary outcome measures were duration of fever and changes in markers of inflammation.
Results
Study drug infusions were associated with cessation of fever within 24 hours in 11 of 12 subjects treated with infliximab and 8 of 12 subjects retreated with IVIG. No infusion reactions or serious adverse events were attributed to either study drug. No significant differences were observed between treatment groups in the change from baseline for laboratory variables, fever, or echocardiographic assessment of coronary arteries.
Conclusion
Both infliximab and a second IVIG infusion were safe and well-tolerated in subjects with KD who were resistant to standard IVIG treatment. The optimal management of patients resistant to IVIG remains to be determined.
Keywords: TNF-α, cytokine, monoclonal antibody, coronary artery aneurysm, infliximab, Kawasaki disease
Kawasaki disease (KD) is a self-limited illness that results in coronary artery aneurysms in up to 25% of untreated children. Administration of high-dose intravenous immunoglobulin (IVIG) in combination with aspirin (ASA) results in dramatic clinical improvement and reduced incidence of coronary artery aneurysms in the majority of patients with KD (1). However, a subset of patients has persistence or recrudescence of fever following IVIG treatment (2). Common practice is to administer a second dose of IVIG (2 g/kg) to patients who fail to become afebrile within 24 to 48 hours after completion of the first infusion, even though the benefits of this approach have never been formally evaluated (3). Fever persists in approximately half of the patients treated with a second dose of IVIG (2 g/kg) (2). Because KD is a self-limited illness from which most children eventually recover with no specific therapy, it is unclear whether retreated patients become afebrile as a result of the additional therapy or simply as a function of time and the natural history of their disease process. Patients resistant to IVIG patients are at increased risk of coronary artery damage as a result of the acute vasculitis; therefore, some form of additional therapy is warranted (2).
Serum levels of the pro-inflammatory cytokine tumor necrosis factor-alpha (TNF-α) are elevated in patients with acute KD, with the highest levels observed in patients who develop coronary artery abnormalities (4). We postulated that TNF-α blockade may be effective in the control of inflammation in patients with KD who fail to respond to IVIG. Infliximab (Remicade®, Centocor, Inc., Malvern, PA) is a chimeric murine/human immunoglobulin G (IgG)1 monoclonal antibody that binds specifically to human TNF-α-1(5). Infliximab is indicated for the treatment of immune-modulated inflammatory disorders, including pediatric Crohn's disease (6). In a recent report of infliximab treatment in patients with KD who failed to respond to repeated IVIG infusions, patients demonstrated dramatic improvement and no infliximab-related adverse events (7). Other case reports support this finding (8-11). The objective of this pilot, multicenter, randomized, prospective trial was to assess the safety, tolerability, and pharmacokinetics of infliximab versus a second dose of IVIG in pediatric patients with IVIG-resistant KD.
Methods
Pediatric subjects diagnosed with acute KD and treated with IVIG on or before the 14th day of fever were eligible if the fever was persistent or recrudescent (oral or rectal temperature ≥ 38.0°C) 48 hours to 7 days after the completion of the initial IVIG infusion. At the time of KD diagnosis, subjects must have been febrile for at least three days but not more than 14 days and must have met four of the five standard clinical criteria for KD or met three of five criteria with at least one dilated coronary artery (internal diameter ≥2.5 standard deviations from the mean normalized for body surface area (Z score)) as determined by echocardiography (12). Exclusion criteria included a history of tuberculosis (TB), histoplasmosis, or coccidiomycosis, recent TB exposure, a chest radiograph suggestive of pulmonary infection obtained within one week of study enrollment, immunization with Bacille Camille Guerin in the previous 6 months, and steroid or other immunomodulatory treatment within the week prior to study enrollment. Informed parental consent and subject assent were obtained as appropriate, and all local Institutional Review Boards approved the study protocol.
All subjects received an initial IVIG infusion (2g/kg) prior to study enrollment. At study entry, all subjects had a chest radiograph to screen for latent mycobacterial or fungal infection. A Mantoux skin test for tuberculosis was placed on all subjects and the results recorded at 48 h. However, randomization was not delayed waiting for the results of the skin test. Subjects were assigned 1:1 to receive intravenous (IV) infliximab 5 mg/kg or IVIG 2 g/kg using randomly permuted blocks stratified by age (<1 yr. old or ≥1 yr. old). Family members and investigators were aware of the treatment assignment. Acetaminophen (15 mg/kg orally) and diphenhydramine (1 mg/kg orally or IV) were administered before study-drug infusion. ASA was administered orally at 80-100 mg/kg/day in 4 doses until the first clinical evaluation at week 1, when the ASA dose was reduced to 3-5 mg/kg/day. All other antipyretic or anti-inflammatory therapies were prohibited. AEs were classified according to severity and possible association to KD or study treatment. Recrudescent or persistent fever was classified separately and was not considered an SAE. An independent Safety Monitoring Board reviewed clinical data after 6, 12, 18, and 24 subjects completed the study protocol.
Pharmacokinetic analyses
A sample size of 24 subjects (12 subjects randomized to infliximab with at least 6 subjects less than 1 yr. old) was planned to allow assessment of infliximab pharmacokinetics by standard non-compartmental methods (WinNonlin Professional, version 5.0.1 [Pharsight, Inc. Mountain View, CA]). Samples were collected before and at 2 and 24 hours, 1 week (5-9 days), 2 weeks (12-16 days), and 4 weeks (26-30 days) after infliximab infusion. Subjects with detectable infliximab concentrations at week 4 had another sample drawn at week 10 (68-72 days). For concentrations that were below the level of detection, the value was set to 0.1 mcg/mL (the detection limit of the assay) for analysis. Concentration data were analyzed by direct inspection to determine maximum plasma concentration (Cmax) and the corresponding time (Tmax). The area under the curve from time 0 to the last measurable concentration (AUC0-last) was estimated using the trapezoidal rule up to the last measurable concentration. The extrapolated area under the curve after the last measurable concentration was estimated as Clast/λz, where λz was the terminal slope of the curve. AUC from time 0 to infinity (AUC0-∞) was calculated as AUC0-last+Clast/λz. Clearance (CL) from plasma was calculated as dose/AUC. Half-life (T½) was calculated as 0.693/ λz, and the estimated volume of distribution (V) was determined by CL/λz.
Serum antibodies to infliximab were measured using a bridging enzyme-linked immunosorbent assay as previously described (13, 14). The assay detection limit for purified positive control antibody was 630 ng/mL in blood. Subject samples diluted 1:10 were tested for antibodies to infliximab at baseline and either 4 weeks or 10 weeks post infusion. Samples with assay results above 0.25 optical density units with at least a 2-fold increase from baseline were considered positive, confirmed in a separate assay, and the titer was measured.
Laboratory assessments were performed at baseline (pre-randomization) and sequentially thereafter at 2h, 24h, 1 week, 2 weeks, and 4 weeks in all study subjects. Laboratory analyses included a complete blood count and concentrations of C-reactive protein (CRP), IgG, cytokines, and infliximab (limit of detection 0.1mcg/mL). For CRP, a value of 0.07 mg/dL was assigned for values below the limit of detection. Alanine aminotransferase (ALT) was only measured at baseline and again at 4 weeks post-randomization. Serum levels of interleukins (IL)-1β, IL-8, soluble TNF-α receptor-I (sTNFRI) and -II, and IL-6 were measured at all five study timepoints using the Beadlyte Human Multi-Cytokine Detection System (Upstate Biotechnology, Lake Placid NY) and the Luminex100 plate reader (Luminex Corporation, Austin, TX) according to manufacturer's instructions. Because direct measurement of TNF-α in plasma is unreliable, we measured soluble TNF-α receptor (sTNFR1) as a proxy (15). Quantification of cytokines was performed by regression analysis from a standard curve generated from cytokine standards included in the kit with a lower limit of detection of 10 pg/mL for all cytokines evaluated. For statistical analysis, samples with optical density readings below the limit of the standard regression curve were assigned a value half that of the limit of detection level.
Temperature was measured either orally or rectally on all subjects at least every 6 hours. For analysis of days of fever following study treatment, a day was defined as each 24 hour-period following the end of the first study drug infusion.
An echocardiogram was performed at baseline (within 48 h of study entry) and again between 2 and 4 weeks after study-drug administration. Each center interpreted its own echocardiograms. The internal lumen diameters of the left anterior descending and right coronary arteries adjusted for body surface area and expressed as standard deviation units (Z scores) were recorded (16). Arteries were classified as normal (Z < 2.5), dilated (Z ≥ 2.5), or aneurysmal (Z ≥ 4.0 with focal dilatation of the vessel ≥ 1.5 times the adjacent segment). Analyses included comparison between groups for Z Max defined as the maximal Z score of any vessel (right or left anterior descending coronary arteries) measured on any echocardiogram at any time point and the change in the absolute measurement of the internal diameter between baseline (pre-study treatment) and 2-4 weeks post-study entry.
Statistical Analyses
Subjects who crossed over to a second study drug were analyzed separately for the 1-4 week timepoints. Primary endpoints were pharmacokinetics and safety/tolerability of a single infliximab infusion (5 mg/kg). Secondary endpoints were cessation of fever within 24 hours after the first study-drug infusion, change in markers of inflammation including cytokine and CRP levels, white blood cell (WBC) count, percent neutrophils, and percent bands between baseline and all subsequent timepoints. Pharmacokinetic variables of infliximab summarized by median, interquartile range, minimum and maximum were compared between subjects < or ≥1 yr old with Wilcoxon Rank-Sum tests for differences in medians. AEs and SAEs were compared between groups using Fisher's exact tests. Descriptive analyses were performed using median and interquartile range. Temperature was analyzed by computing the area under the temperature curve (AUCtemp) from study entry until 36 hours after study-drug administration and by comparing these values between groups with the Wilcoxon Rank-Sum test. The chi-square and Fisher exact tests were used to compare dichotomous variables. The Wilcoxon Rank-Sum test was used to compare the absolute laboratory values, the coronary artery Z scores, and the changes from baseline in these values between groups. A repeated-measures ANOVA was used to assess differences in coronary artery Z scores over time between groups. This trial was not powered to detect differences in coronary artery outcome. For the cytokine panel, the Friedman test for multiple data points was used to test for significant changes. The Kruskal-Wallis or Mann-Whitney tests were used for comparisons at each timepoint. All statistical tests were two-tailed. A p-value of <0.05 was considered significant. The corporate study sponsor (Centocor, Inc[h2].) performed the determinations of infliximab levels and the assays for anti-infliximab antibodies.
Results
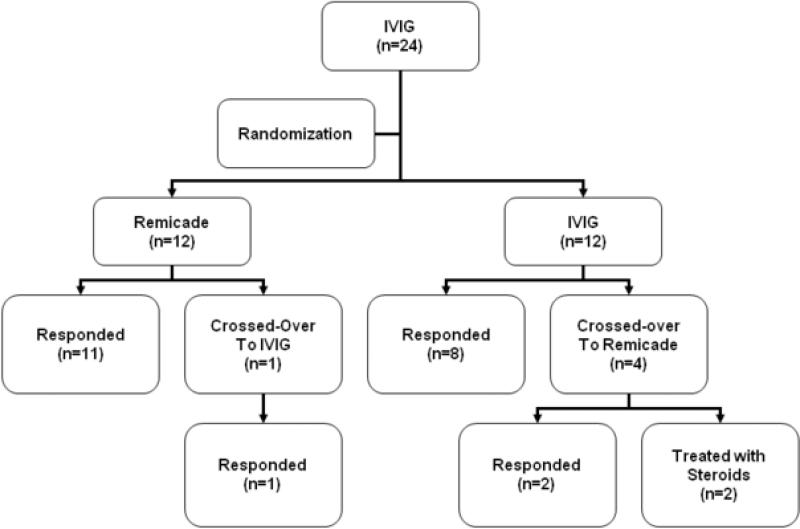
Twenty-four subjects were enrolled between September 2004 and September 2006 at 6 study sites in the United States. Four sites enrolled one subject each, one site enrolled 5 subjects (4 treated with infliximab), and the remaining site enrolled 15 subjects (11 treated with infliximab). At baseline, demographics and disease characteristics, laboratory data, coronary artery dimensions, and incidence of coronary artery abnormalities were similar between groups (Table I; available at www.jpeds.com), with the exception of higher sTNFRI concentrations in the infliximab-treated group (Table II; available at www.jpeds.com). In the 12 subjects randomized to a second IVIG infusion, fever resolved in 8 subjects and persisted or recrudesced in 4 subjects. Fever resolved in 2 of these 4 subjects who crossed over to infliximab (Figure 1; available at www.jpeds.com). The 2 remaining subjects required additional therapy with steroids (methylprednisolone followed by oral prednisone) or steroids plus methotrexate. In the 12 subjects randomized to infliximab, fever resolved in all but 1 subject whose temperature normalized after crossing over to receive a second IVIG infusion. Thus, of the 16 subjects who received infliximab as either their first or second study medication, 3 of 16 (18.7%) required additional therapy versus 4 of 13 (30.7%) subjects who received a second IVIG infusion. No subject experienced an infusion reaction to either study medication. No subject had a positive skin test for tuberculosis.
Table 1.
Demographic, laboratory, and echocardiographic parameters at study entry*.
| IVIG | Infliximab | |
|---|---|---|
| n=12 | n=12 | |
| Age (months) | 22** | 20 |
| 10 - 38 | 8 - 37 | |
| 3 - 85 | 2.4 - 75 | |
| Female sex - no. of subjects (%) | 3 (25) | 4 (33) |
| Race - no. of subjects (%) | ||
| Asian | 3 (25) | 0 (0) |
| Black | 1 (8) | 2 (17) |
| Indian | 1 (8) | 0 (0) |
| White | 5 (42) | 9 (75) |
| Mixed | 2 (17) | 1 (8) |
| Ethnicity - no. of subjects (%) | ||
| Hispanic/Latino | 4 (33) | 4 (33) |
| Non-Hispanic/Non-Latino | 8 (67) | 8 (67) |
| Illness Day at Initial IVIG | 6# | 5.5 |
| 5 - 7.8 | 4 – 7.3 | |
| 3 - 14 | 3 - 9 | |
| Illness Day at Study Entry | 9.5 | 7.5 |
| 7.8 - 10.8 | 6 - 12 | |
| 5 - 17 | 6 - 15 | |
| CRP (mg/dL) | 8.2 | 7.6 |
| 4.3 - 13 | 5.7 - 12.9 | |
| <0.3 - 19.4 | 4.7 - 30.7 | |
| ALT (IU/L) | 48 | 40.5 |
| 34 - 87 | 36 - 89 | |
| 23 - 146 | 17 - 390 | |
| ZIgG | 7.1 | 8.1 |
| 5.9 - 9.3 | 5.7 - 9.7 | |
| 4.1 - 16.6 | 3.5 - 19.5 | |
| IgG (mg/dL) | 1910 | 1855 |
| 1775 - 1993 | 1733 - 2183 | |
| 1320 - 2810 | 1209 - 2374 | |
| WBC (× 103/mm3) | 13.5 | 16.1 |
| 10.6 - 20.5 | 14.5 - 19.4 | |
| 6.7 - 21.8 | 6.8 - 36.5 | |
| Neutrophils (%) | 54 | 58 |
| 42 - 60 | 45 - 62 | |
| 21 - 68 | 32 - 84 | |
| Calculated ANC (× 103/mm3) | 8.3 | 10.9 |
| 6.5 - 10.7 | 8.5 - 14 | |
| 3.9 - 17.1 | 5.4 - 27.4 | |
| Bands (%) | 7 | 10 |
| 2.5 - 19 | 5.5 - 22.5 | |
| 0 - 40 | 1 - 31 | |
| ZHgb | −2.7 | −2.6 |
| −3.6 – −1.5 | −3.5 – −2 | |
| −4.8 - 0 | −3.9 - 0.2 | |
| Platelets (× 103/mm3) | 505 | 471 |
| 387 - 583 | 388 - 615 | |
| 352 - 718 | 70 - 1183 | |
| LAD Z score | 1.8 | 1.5 |
| 1.3 – 2.4 | 0.7 – 2.7 | |
| −0.6 – 5.3 | −0.7 –4.1 | |
| RCA Z score | 1.3 | 1.1 |
| 0.7 – 1.8 | 1.1 – 1.4 | |
| −0.8 – 4.3 | 0.1 – 2.4 |
P > 0.1 for comparison of all variables between groups
Median, interquartile range, range
Illness Day 1= first day of fever
Abbr. IVIG, intravenous gamma globulin; no., number; CRP, C-reactive protein; ALT, alanine amino transferase; ZIgG, standard deviation units from the mean for age-specific serum immunoglobulin G values; ZHgb, standard deviation units from the mean for age-specific hemoglobin values; Z score, internal diameter of the left anterior descending (LAD) and right coronary artery (RCA) normalized for body surface area and expressed in standard deviation units from the mean
Table 2.
Serum cytokine and cytokine receptor concentrations at study entry
|
IVIG (n=11) |
Infliximab (n=12) |
P value* | |
|---|---|---|---|
| IL-1b | 182.5** (85.4-531.7) |
434.8 (192.7-3,812.3) |
0.11 |
| IL-6 | 346.5 (118.4-581.8) |
649.03 (315.5-1,437.2) |
0.09 |
| IL-8 | 175.4 (79.8-238.0) |
376.5 (115.0-872.0) |
0.19 |
| sTNF-RI | 8,146.0 (6,819.3-10,070.2) |
12,428.0 (10,929.7-20,589.6) |
0.009 |
| sTNF-RII | 4,405.5 (3,955.8-7,297.8) |
5,784.0 (4,236.8-7,110.0) |
0.6 |
Mann-Whitney U test.
Median pg/ml, (interquartile range)
Abbr.: IVIG, intravenous gamma globulin; IL, interleukin; sTNF-R, soluble tumor necrosis factor receptor
Figure 1.
Flow chart of subjects after randomization. Numbers in parentheses indicate number of subjects. The two subjects who failed to respond to the second study treatment were treated off-protocol with steroids.
In this pilot trial, 5 subjects (3 infliximab, 2 IVIG) experienced one or more SAEs. All SAEs were possibly related to KD or to antibiotics administered before disease diagnosis, and none were directly attributable to either study medication (Table III; available at www.jpeds.com). Both of the subjects treated with IVIG crossed over to infliximab because of persistent fever that continued, requiring additional (off study) anti-inflammatory therapy; both patients developed coronary artery abnormalities (dilatation or aneurysms).
Table 3.
Serious adverse events.
| Patient age (mos.) | Study treatment(s) (additional treatment off study) | Comments |
|---|---|---|
| 9 | IVIG, infliximab (steroids, methotrexate) | Persistent fever treated with IV methylprednisilone, oral steroids, and methotrexate; dilated coronary arteries (Z max RCA:4.6;Z max LAD: 3.3), dilatation resolved |
| 4 | infliximab (infliximab, IVIG) | Enlarging aneurysms (Z max LAD: 4.9; Z max RCA: 7.5); re-admitted for repeat infliximab and IVIG infusion; aneurysms resolved |
| 48 | Infliximab | Drug-related rash with eosinophilia and systemic symptoms (DRESS) attributed to azithromycin administered prior to study entry (reported to FDA), required hospitalization for supportive care; normal coronary arteries |
| 10 | IVIG, infliximab (steroids) | Persistent fever plus rising CRP treated with IV methylprednisilone; aneurysms of RCA and LAD (Z max RCA: 7.2; Zmax LAD: 8.1) |
| 74 | Infliximab | Acute cerebellar ataxia attributed to KD(resolved); giant aneurysms (> 8mm) treated with warfarin, (Z max RCA: 15.8; Z max LAD: 7.44) |
IVIG: intravenous immunoglobulin; RCA: right coronary artery; LAD: left anterior descending coronary artery; Z max: maximal internal lumen diameter expressed in standard deviation units from the mean (Z score) normalized for body surface area; FDA: Food and Drug Administration
Eighteen subjects (9 infliximab, 4 IVIG, 1 infliximab crossover to IVIG, 4 IVIG crossovers to infliximab) experienced one or more adverse events. In these subjects, 30 of 48 AEs including eczema, coronary artery aneurysms, diastolic dysfunction, and pericardial effusion were attributable to KD. Of the 10 subjects with 18 adverse events not attributable to KD, 5 were treated with infliximab, 1 with IVIG, 3 with IVIG followed by infliximab, and 1 with infliximab followed by IVIG. Of these AEs, only transient hepatomegaly was deemed possibly related to study drug infusion in all 6 subjects.
Transient hepatomegaly was reported as an AE at week 2 in 5 subjects who received infliximab (as first or second study medication) and at week 1 in 1 subject who received IVIG. Hepatomegaly had resolved by the subsequent study visit in 4 of the infliximab subjects, and by the second subsequent study visit in the remaining 2 subjects. The presence of hepatomegaly before study drug administration could not be determined because of right upper quadrant guarding possibly due to gallbladder hydrops on the initial examination. ALT levels were measured as part of routine care (not required by the study protocol) in all 6 subjects prior to ASA administration and the first (pre-study) IVIG infusion and were elevated (range 55-217 IU/L) in 5 of these 6 subjects. ALT levels were elevated (146 and 390 IU/L) in 2 of 4 subjects after first (pre-study) IVIG infusion and immediately before infliximab infusion. All study subjects had normal ALT levels (< 45 IU/L) at week 4, including the 6 with transient hepatomegaly. Analysis of both median ALT at baseline and week 4 and the change from baseline in the 16 subjects who received infliximab ever versus the 8 subjects who received only second IVIG showed no difference between the groups (data not shown).
Pharmacokinetics of infliximab and IVIG
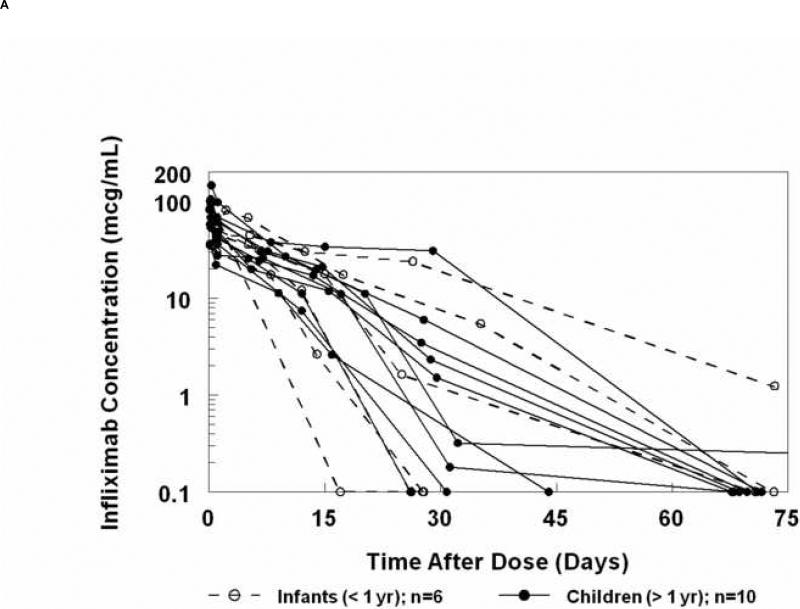
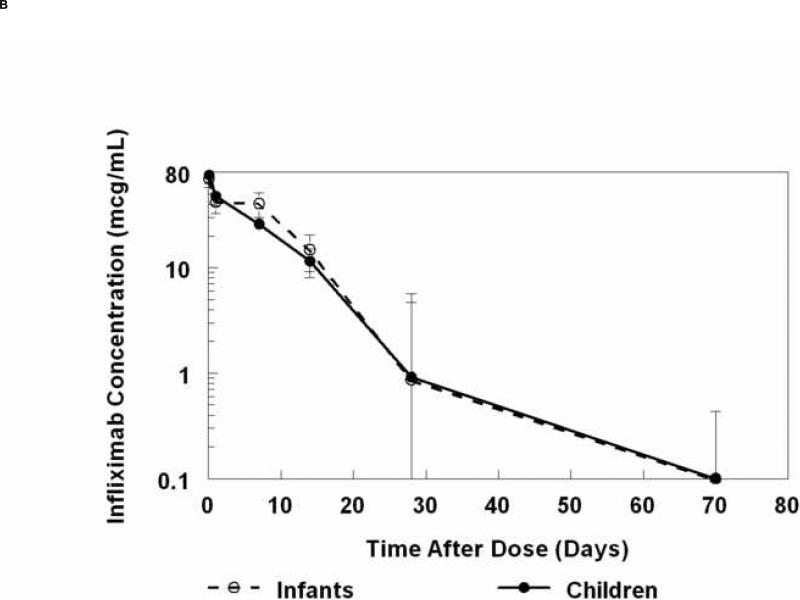
The mean peak serum concentration measured 2 hours after the end of infliximab infusion (5mg/kg) was 78 ± 28 mcg/mL (Figure 2A). No significant age-dependent differences were noted in the decreasing serum concentrations over time (Figure 2B, Table IV; available at www.jpeds.com). Concentrations at 4 weeks were highly variable but were similar among subjects of all ages. The subject with the most rapid elimination was an infant who received a second IVIG dose prior to infliximab (crossover subject). However, no similar trend was observed for the other 3 crossover subjects who received infliximab as the second study medication. Infliximab concentrations decreased to below the level of detection in 6 of 16 subjects by week 4, and 9 additional subjects had undetectable concentrations by week 10. A single subject had detectable infliximab at week 10, with a concentration of 1.24 mcg/mL.
Figure 2.
A, Concentration of infliximab versus time curves for each individual subject. B, Median (± standard error of the median) infliximab concentrations versus time after dose in infants and children.
Table 4.
Infliximab pharmacokinetics in 16 IVIG-resistant Kawasaki disease subjects.
| Parameter |
Infants (n=6) |
Children (n=10) |
Overall (n=16) |
|---|---|---|---|
| AUC (mcg•day/mL) | |||
| Median | 746* | 547 | 619 |
| IQR | 537 – 1093 | 429 – 834 | 458 – 891 |
| Range |
276 – 1517 |
251 – 1812 |
251 – 1812 |
| CL (mL/hr/kg) | |||
| Median | 0.11 | 0.15 | 0.14 |
| IQR | 0.08 – 0.16 | 0.1 – 0.19 | 0.09 – 0.18 |
| Range |
0.05 – 0.3 |
0.05 – 0.33 |
0.05 – 0.33 |
| ke (days−1) | |||
| Median | 0.175 | 0.095 | 0.095 |
| IQR | 0.091 – 0.263 | 0.092 – 0.123 | 0.092 – 0.215 |
| Range |
0.053 – 0.287 |
0.025 – 0.246 |
0.025 – 0.287 |
| V (mL/kg) | |||
| Median | 53 | 81 | 67 |
| IQR | 37 – 68 | 65 – 109 | 48 – 97 |
| Range |
22 – 88 |
30 – 202 |
22 – 202 |
| Cmax (mcg/mL) | |||
| Median | 81 | 75 | 81 |
| IQR | 64 – 82 | 60 – 99 | 58 – 97 |
| Range |
35 – 100 |
36 – 147 |
35 – 147 |
| T ½ (days) | |||
| Median | 5.1 | 7.3 | 7.3 |
| IQR | 2.6 – 7.6 | 5.7 – 7.5 | 3.2 – 7.6 |
| Range | 2.4 – 13 | 2.8 – 27.9 | 2.4 – 27.9 |
Abbr.: IVIG, intravenous gamma globulin; AUC, area under the concentration-time curve; CL, clearance; ke, elimination rate constant; V, volume of distribution; Cmax, maximum concentration; T ½, half-life; IQR, interquartile range
p > 0.05 for comparison of all variables between groups
Antibodies to infliximab were measured at 4 or 10 weeks post-infusion. Three male subjects aged 3, 34, and 74 months developed low titer (1:10-20) antibodies to infliximab. All three subjects received infliximab as the only study medication. All became afebrile following their infusion and had infliximab AUCs at or below the median for this trial population. Two of the three subjects had abnormal echocardiograms at study entry and developed coronary artery aneurysms. The remaining subject developed transient dilatation of the left anterior descending (LAD) coronary artery.
Clinical response to treatment
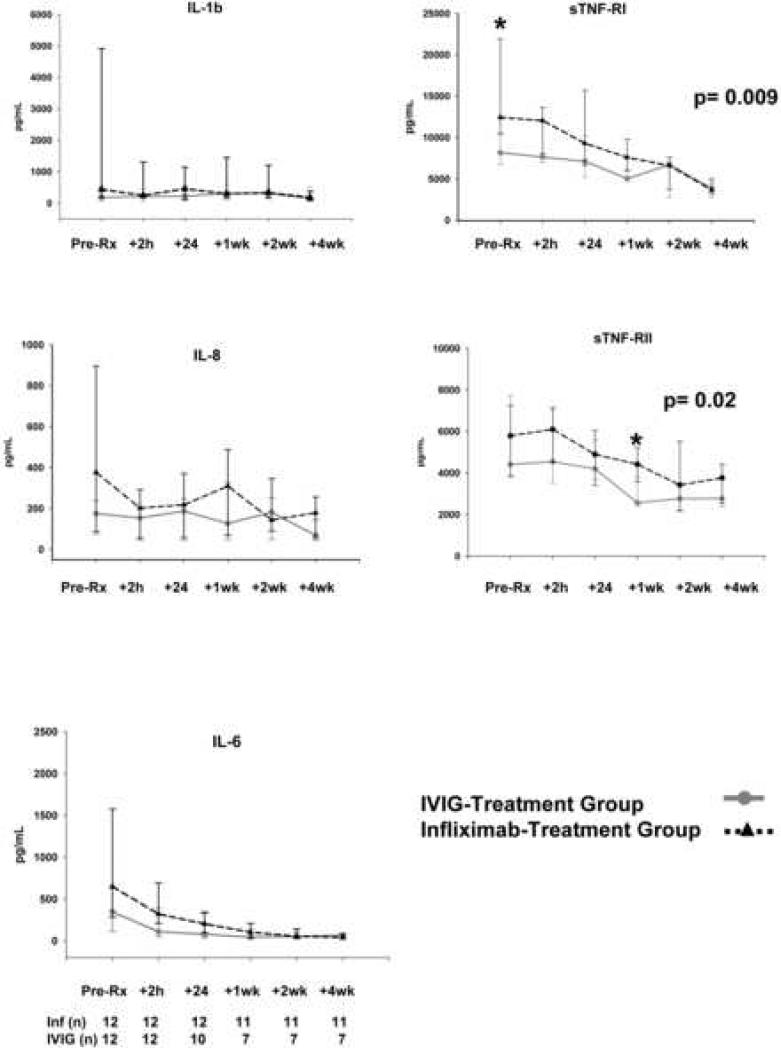
The AUC for temperature was similar between the two treatment groups (Table V; available at www.jpeds.com). However, the AUCtemp at 36 hours after study drug infusion was higher (p=0.003) in the five subjects who crossed over (mean temperature = 37.6°C) as compared with subjects who did not crossover (mean temperature = 36.9°C). In terms of days of fever post-study drug administration, 1 subject in the infliximab-treated group had 2 days of fever, and in the second IVIG-treated group, 2 subjects had 2 days of fever and 2 subjects had >2 days for fever and were treated off- protocol with methylprednisolone. Absolute values for laboratory tests and changes from baseline were similar between the two groups at 24 hours after the end of study drug infusion with the exception of IgG levels, which were higher in the group receiving a second infusion of IVIG (Table VI; available at www.jpeds.com). From weeks 1 to 4, the white blood cell and platelet counts were higher in the infliximab-treated group (p=0.02 and p=0.03, respectively). The percent neutrophils and absolute neutrophil count remained elevated in the infliximab-treated group at week 4. However, the difference between baseline and week 4 values for the two groups was different only for platelet count (p=0.04, data not shown). No differences were observed between groups for CRP at any time point or for ALT (measured only at week 4). Serum concentrations of IL1-β, IL-8, and IL-6 were similar between groups at all timepoints evaluated (Figure 3; available at www.jpeds.com). Concentrations of sTNFRI and sTNFRII were higher in the infliximab group at all timepoints, but only reached statistical significance at study entry for sTNFRI and at week 1 for sTNFRII (Figure 3 and Table II). Because of the skewed enrollment of subjects by site, a post-hoc analysis of baseline inflammatory markers for the 15 subjects enrolled at a single site was performed. These subjects had higher levels of inflammatory markers compared with subjects at other sites (data not shown).
Table 5.
Comparison of treatment groups for duration of fever. Subjects were randomized to receive IVIG or infliximab. Responsive subjects were those who remained afebrile following infusion of their first study drug. Subjects who failed to become afebrile crossed over to receive the second study treatment (cross-over subjects).
| |
Randomized to 2nd IVIG (n=12) |
Randomized to infliximab (n=12) |
Cross-over (n=5) |
Non-cross-over (n=19) |
|---|---|---|---|---|
| AUC0-36 (°C*hr) | ||||
| Median | 1331 | 1333 | 1352* | 1327 |
| IQR | 1322 - 1349 | 1323 - 1341 | 1349 - 1354 | 1319 - 1338 |
| Range | 1286 – 1367 | 1313 – 1354 | 1341 – 1367 | 1286 – 1356 |
p=0.003 for cross-over vs. non-cross-over subjects
Abbr.: IVIG: intravenous immunoglobulin; AUC0-36: area under temperature curve from start of first study drug infusion (time 0) to 36 hour later; IQR: interquartile range, 25th – 75th percentile
Table 6.
Laboratory values 24-hours after completion of study drug infusion.
| Characteristic | IVIG (n=11) | Infliximab (n=12) | P-value |
|---|---|---|---|
| CRP (mg/dL) | 4.8* | 6.2 | 0.34 |
| 2.7 - 10.1 | 4.5 - 13.2 | ||
| <0.3 - 23.9 | 2.7 - 35.8 | ||
| ZIgG | 16.1 | 7.4 | 0.001 |
| 14.5 - 19 | 5.3 - 9.6 | ||
| 8.5 - 27.7 | 4.4 - 17 | ||
| IgG (mg/dL) | 3467 | 1809 | 0.0001 |
| 3210 - 3730 | 1645 - 1945 | ||
| 1930 - 4330 | 1110 - 2710 | ||
| WBC (× 103/mm3) | 12.8 | 14.9 | 0.44 |
| 7.7 - 17.2 | 12.2 - 18.7 | ||
| 4.7 - 27.6 | 7.7 - 30.9 | ||
| Neutrophils (%) | 37 | 45 | 0.21 |
| 25 - 53 | 41 - 55 | ||
| 16 - 75 | 30 - 66 | ||
| Calculated ANC (× 103/mm3) | 5.1 | 7.2 | 0.37 |
| 2.8 - 10.9 | 5.5 - 10.8 | ||
| 2.1 - 21.8 | 4.5 - 20 | ||
| Bands (%) | 8 | 4.5 | 0.25 |
| 4.3 - 9.8 | 2 - 10 | ||
| 3 - 63 | 1 - 14 | ||
| Lymphocytes (%) | 35 | 37 | 1 |
| 22 - 43 | 30 - 40 | ||
| 5 - 62 | 21 - 43 | ||
| ZHgb | −3 | −3.2 | 0.8 |
| −4.6 – −2 | −3.6 – −2.8 | ||
| −5.2 – 0.2 | −4.3 – −1.5 | ||
| Hemoglobin (g/dL) | 9.8 | 9.6 | 0.5 |
| 8.8 – 10.7 | 8.9 - 10 | ||
| 8 - 12 | 7.8 - 11 | ||
| Platelet count (× 103/mm3) | 626 | 574 | 0.8 |
| 507 - 735 | 505 - 727 | ||
| 338 - 998 | 149 - 1173 |
Median; interquartile range; range
Abbr. IVIG, intravenous gamma globulin; no., number; CRP, C-reactive protein; ALT, alanine amino transferase; ZIgG, standard deviation units from the mean for age-specific serum immunoglobulin G values; WBC, white blood cell; ANC, absolute neutrophil count; ZHgb, standard deviation units from the mean for age-specific hemoglobin values
Figure 3.
Time course of serum cytokine and soluble TNF receptors in subjects treated with IVIG (solid lines) or infliximab (dashed lines). The analysis after the 24h time point excludes those patients who crossed over to receive other study drug (n=5). The asterisk (*) denotes timepoints for which the difference between median values for the two treatment groups was significant. Abbr. IL, interleukin; sTNFR, soluble tumor necrosis factor-α receptor; Pre-Rx, before study drug treatment.
Coronary artery outcome
Overall, 11 subjects (46%) had normal echocardiograms at all timepoints, eight subjects (33%) had at least one dilated coronary artery segment (right coronary artery (RCA) or LAD Z score ≥2.5), and five subjects (21%) developed coronary artery aneurysms: four randomized to infliximab (one with giant aneurysms) and one randomized to the IVIG who crossed over to infliximab. Of these five subjects with aneurysms, four had coronary artery abnormalities (either aneurysm or dilatation) documented by echocardiogram at the time of study enrollment. The remaining subject had a normal echocardiogram at study entry and subsequently developed a giant aneurysm of the proximal right coronary artery (8.2 mm in largest dimension). A composite variable of the maximal Z scores for each coronary artery segment at any timepoint (Z Max) and the difference in Z scores from baseline (Δ Z) were similar between groups (Table VI; available at www.jpeds.com). Because IVIG has only been shown to prevent aneurysms when used on or before Illness Day 10, we analyzed the results both including and excluding the 4 subjects (2 each randomized to infliximab or second IVIG infusion) who received their initial IVIG infusion after Illness Day 10. Analyses of within-subject changes over time in coronary artery z scores or absolute internal dimensions showed no differences between groups.
Discussion
In this study, the pharmacokinetics of infliximab did not differ according to age. This is the first time that infliximab has been used in a clinical trial for patients < 12 months old. Standard dosing based on weight demonstrated peak concentrations similar to previous reports, regardless of the age. Peak and trough serum concentrations of infliximab following a 6mg/kg dose were reported in 62 children age 4-17 years with pauciarticular juvenile rheumatoid arthritis (17). The peak concentrations were similar to those observed in this study. The single dose of 5 mg/kg used in this study demonstrated comparable systemic infliximab exposure to that previously reported in adolescents and adults (18, 19).
Low-titer antibodies to infliximab were detected in three of sixteen subjects (18.7%), which is similar to the published experience in pediatric patients with Crohn's disease (12.5%) and polyarticular juvenile rheumatoid arthritis (12.2%) (17, 20) . Although antibodies have been associated with an increased incidence of infusion reactions in patients receiving multiple doses of infliximab, this was not an issue for our subjects who only received a single dose.
One limitation of this study is the small sample size, which was based solely on power calculations for the primary objective of describing the pharmacokinetics in this study population. Five subjects who received infliximab and one who received IVIG developed transient hepatomegaly, but the available data were insufficient to explore the extent to which this finding might be related to infliximab infusion. In addition, although this was a multicenter study, 15 of the 24 subjects were enrolled at a single site and these included 11 of the 16 subjects who received infliximab as either their first or second study drug. The fact that one-third of the subjects randomized to second IVIG infusion went on to receive infliximab may have obscured differences between the groups.
Infliximab infusion was safe and well-tolerated in this small population of pediatric subjects with IVIG-resistant KD. The pharmacokinetics of infliximab was similar in subjects regardless of age. Until future clinical trials establish best clinical practice, infliximab can be considered as a potential alternative to additional IVIG infusions or IV pulse methylprednisolone for patients with IVIG-resistant KD.
Table 7.
Comparison of coronary artery internal dimensions measured by echocardiography and normalized for body surface area (Z score).
| |
IVIG (n=12) |
Infliximab (n=12) |
|---|---|---|
| Z Max | ||
| Median | 2.2 | 3.5 |
| IQR | 1.7 – 3.6 | 1.1 – 4.9 |
| Range |
0.9– 7.7 |
0.3 – 14.3 |
| Δ Z – RCA | ||
| Median | −0.2 | −0.5 |
| IQR | −0.9 – 0.6 | −1.3 – 0.3 |
| Range |
−1.7 – 3.2 |
−2.4 – 6.2 |
| ΔZ – LAD | ||
| Median | −0.3 | −0.05 |
| IQR | −1.2 – 1 | −0.9 – 1.2 |
| Range | −3.2 – 2.5 | −3.3 – 11.5 |
p>0.1 for all comparisons
Abbr.: IVIG, intravenous gamma globulin; Z Max: Maximal Z score of any vessel (right or left anterior descending coronary arteries) measured on any echocardiogram at any time point; Δ Z: change in Z score between baseline (pre-study treatment) and 2-4 weeks post-study entry; the Z score for the 2-4 week measurement was subtracted from the baseline Z score; RCA: right coronary artery; LAD: left anterior descending; IQR: interquartile range
Acknowledgments
The authors thank Joan M. Pancheri R.N.BSN, CCRC, Michael Farrell R.N., Christina D. Rains, RN, BSN, Deborah A. Town, RN, BS, CCRC, Christy L. Wilkerson, RN, BSN, Noreen A. Montemayor, RN, BSN for their contributions to this study. We also thank the members of the Data Safety Monitoring Board: Hal M. Hoffman, M.D., Paul Grossfeld, M.D., and James A. Gonzalez, Pharm.D.
This investigator-initiated clinical trial was supported by a grant from Centocor, Inc.and by the National Institute for Child Health and Human Development, Pediatric Pharmacology Research Unit (PPRU) Network (5U10 HD031318). Centocor Inc. performed the determinations of infliximab levels and the assays for anti-infliximab antibodies. Dr. Gregory F. Keenan and Khalid Mamun participated in editing the manuscript. The authors declare[h1] no potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Newburger JW, Takahashi M, Beiser AS, Burns JC, Bastian J, Chung KJ, et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. 1991;324(23):1633–9. doi: 10.1056/NEJM199106063242305. [DOI] [PubMed] [Google Scholar]
- 2.Burns JC, Capparelli EV, Brown JA, Newburger JW, Glode MP. Intravenous gamma-globulin treatment and retreatment in Kawasaki disease. US/Canadian Kawasaki Syndrome Study Group. Pediatr Infect Dis J. 1998;17(12):1144–8. doi: 10.1097/00006454-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Sundel RP, Burns JC, Baker A, Beiser AS, Newburger JW. Gamma globulin re-treatment in Kawasaki disease. J Pediatr. 1993;123(4):657–9. doi: 10.1016/s0022-3476(05)80972-2. [DOI] [PubMed] [Google Scholar]
- 4.Matsubara T, Furukawa S, Yabuta K. Serum levels of tumor necrosis factor, interleukin 2 receptor, and interferon-gamma in Kawasaki disease involved coronary-artery lesions. Clin Immunol Immunopathol. 1990;56(1):29–36. doi: 10.1016/0090-1229(90)90166-n. [DOI] [PubMed] [Google Scholar]
- 5.Knight DM, Trinh H, Le J, et al. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Molecular Immunology. 1993;30:1443–53. doi: 10.1016/0161-5890(93)90106-l. [DOI] [PubMed] [Google Scholar]
- 6.Baldassano R, Braegger CP, Escher JC, DeWoody K, Hendricks DF, Keenan GF, et al. Infliximab (REMICADE) therapy in the treatment of pediatric Crohn's disease. Am J Gastroenterol. 2003;98(4):833–8. doi: 10.1111/j.1572-0241.2003.07343.x. [DOI] [PubMed] [Google Scholar]
- 7.Burns JC, Mason WH, Hauger SB, Janai H, Bastian JF, Wohrley JD, et al. Infliximab treatment for refractory Kawasaki syndrome. J Pediatr. 2005;146(5):662–7. doi: 10.1016/j.jpeds.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 8.Zulian F, Zanon G, Martini G, Mescoli G, Milanesi O. Efficacy of infliximab in long-lasting refractory Kawasaki disease. Clin Exp Rheumatol. 2006;24(4):453. [PubMed] [Google Scholar]
- 9.Saji T, Kemmotsu Y. Infliximab for Kawasaki syndrome. J Pediatr. 2006;149(3):426. doi: 10.1016/j.jpeds.2005.07.039. author reply. [DOI] [PubMed] [Google Scholar]
- 10.Stenbog EV, Windelborg B, Horlyck A, Herlin T. The effect of TNFalpha blockade in complicated, refractory Kawasaki disease. Scand J Rheumatol. 2006;35(4):318–21. doi: 10.1080/03009740600588228. [DOI] [PubMed] [Google Scholar]
- 11.Weiss JE, Eberhard A, Chowdhury D, Gottlieb BS. Infliximab as a novel therapy for refractory Kawasaki Disease. Journal of Rheumatology. 2004;31:808–10. [PubMed] [Google Scholar]
- 12.Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110(17):2747–71. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]
- 13.Hanauer SB, Wagner CL, Bala M, Mayer L, Travers S, Diamond RH, et al. Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn's disease. Clin Gastroenterol Hepatol. 2004;2(7):542–53. doi: 10.1016/s1542-3565(04)00238-1. [DOI] [PubMed] [Google Scholar]
- 14.Maini RN, Breedveld FC, Kalden JR, Smolen JS, Davis D, Macfarlane JD, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998;41(9):1552–63. doi: 10.1002/1529-0131(199809)41:9<1552::AID-ART5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 15.Higuchi M, Aggarwal BB. TNF induces internalization of the p60 receptor and shedding of the p80 receptor. J Immunol. 1994;152(7):3550–8. [PubMed] [Google Scholar]
- 16.McCrindle BW, Li JS, Minich LL, Colan SD, Atz AM, Takahashi M, et al. Coronary artery involvement in children with Kawasaki disease: risk factors from analysis of serial normalized measurements. Circulation. 2007;116(2):174–9. doi: 10.1161/CIRCULATIONAHA.107.690875. [DOI] [PubMed] [Google Scholar]
- 17.Ruperto N, Lovell DJ, Cuttica R, Wilkinson N, Woo P, Espada G, et al. A randomized, placebo-controlled trial of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum. 2007;56(9):3096–106. doi: 10.1002/art.22838. [DOI] [PubMed] [Google Scholar]
- 18.Ternant D, Mulleman D, Degenne D, Willot S, Guillaumin JM, Watier H, et al. An enzyme-linked immunosorbent assay for therapeutic drug monitoring of infliximab. Ther Drug Monit. 2006;28(2):169–74. doi: 10.1097/01.ftd.0000189901.08684.4b. [DOI] [PubMed] [Google Scholar]
- 19.St Clair EW, Wagner CL, Fasanmade AA, Wang B, Schaible T, Kavanaugh A, et al. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46(6):1451–9. doi: 10.1002/art.10302. [DOI] [PubMed] [Google Scholar]
- 20.Hyams J, Crandall W, Kugathasan S, Griffiths A, Olson A, Johanns J, et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn's disease in children. Gastroenterology. 2007;132(3):863–73. doi: 10.1053/j.gastro.2006.12.003. quiz 1165-6. [DOI] [PubMed] [Google Scholar]
- 21.Tobon GJ, Canas C, Jaller JJ, Restrepo JC, Anaya JM. Serious liver disease induced by infliximab. Clin Rheumatol. 2007;26(4):578–81. doi: 10.1007/s10067-005-0169-y. [DOI] [PubMed] [Google Scholar]