Abstract
Background. Only a few studies have reported on the effect of high-dose insulin (HDI) infusion on cardiac function in healthy volunteers.
Methods. We studied ten healthy volunteers with low-dose dobutamine (LDD, 10 μg/kg/min) echocardiography and HDI echocardiography (insulin administration for one hour) by volume and Doppler analysis.
Results. During LDD, cardiac output increased from 5.7±1.3 l/min to 9.0±2.1 l/min (p<0.001) and during HDI from 5.5±1.2 l/min to 6.2±1.1 l/min (p=0.048). Increase was not only due to increase in frequency, which was only present in the LDD study, but also due to increase in stroke volume (from 82±15 ml to 110±23 ml, p<0.001 during LDD and from 82±16 ml to 93±24 ml, p=0.014 during HDI). The increase in stroke volume was the result of a decrease in end-systolic volume with an unchanged end-diastolic volume.
Conclusion. High-dose insulin infusion results in increased cardiac output by improving systolic myocardial function. (Neth Heart J 2010;18:183–9.)
Keywords: Insulin Infusion Systems; Ventricular Function, Left; Echocardiography; Dobutamine
Several studies observed improvement of LV function during glucose-insulin-potassium (GIK) administration in (post)ischaemic myocardium. Most of these studies were performed in animal models1-5 but also in patients undergoing cardiac surgery.6-10
Dobutamine enhances overall myocardial performance and contraction, and high-dose insulin (HDI) infusion improves left ventricular systolic function in patients with left ventricular impairment due to ischaemic coronary disease.11-15 However, the effect of high-dose insulin infusion is less clear in normal subjects; one study with no effects on myocardial function in normal, healthy subjects has been published.16
We compared wall motion improvement during low-dose dobutamine infusion with high-dose insulin infusion in ten normal subjects, and observed an increase in stroke volume during high-dose insulin infusion which was not significantly different from the increase in stroke volume during low-dose dobutamine stimulation. The increase in stroke volume was due to a reduced end-systolic volume with an unchanged end-diastolic volume.
Subjects and methods
Ten healthy male volunteers were studied. These subjects were healthy with regard to the absence of cardiac complaints, normal physical examination and unremarkable ECG and baseline echocardiogram.
Echocardiography
On two separate occasions, the volunteers underwent echocardiography using Sonos 5500 equipment (Philips, Best, the Netherlands). After baseline echocardiography, two types of infusions were applied: on the first occasion, low-dose dobutamine infusion and on the second occasion, high-dose insulin infusion (see below). The echocardiographic examination consisted of a standard parasternal and apical examination with M-mode measurements and Doppler measurements of flow over the aortic valve, the left ventricular outflow tract, mitral valve, and pulmonary veins. This examination was repeated during pharmacological intervention, and examinations were stored for off-line analysis.
Low-dose dobutamine infusion
Low-dose dobutamine infusion was started at a rate of 5 μg/kg/min. After five minutes in this stage, echocardiography was repeated and after completion of echocardiography, the infusion was increased to 10 μg/kg/min. Again after five minutes, the final echocardiographic examination was recorded.
High-dose insulin infusion
To obtain supraphysiological insulin levels, an euglycaemic hyperinsulinaemic clamp (EHC) procedure was used as described previously.17 Cannulas were introduced into large veins in both arms. One cannula was used for blood sampling, the other cannula was used for infusion of an insulin solution (15 U insulin per m2 body surface area added to 50 ml NaCl 0.65%) and a glucose-potassium solution (500 ml glucose 20% with 20 ml potassium 10% added). After determination of baseline blood glucose level, insulin infusion was started at a rate of 60 ml/h for four minutes, then decreased to 30 ml/h for four minutes and then continued at 13 ml/h for 60 minutes. Every ten minutes, blood samples were taken for determination of blood glucose levels (GlucoTouch, Beerse, Belgium) and the glucose infusion was adjusted to maintain blood glucose levels between 4.5 and 6.0 mmol/l. Echocardiography was performed before start and after sixty minutes of insulin infusion.
Physical response to infusions
Blood pressure and heart rate were recorded at baseline and during low-dose dobutamine infusion at each stage and every ten minutes during the clamp procedure.
Blood sampling
At baseline and at the end of low-dose dobutamine infusion, as well as at baseline and at the end of the clamp procedure, blood samples were taken and levels of glucose, insulin, free fatty acids, sodium, potassium, phosphate, calcium, albumin, epinephrine, and norepinephrine were determined.
Analysis of echocardiography
Echocardiographic images were reviewed by two experienced observers, side-by-side, without knowledge of the type of pharmacological intervention. Wall motion was scored according to a 13-segment model with a five-point scale (-1=hyperkinesia, 0=normokinesia, 1=hypokinesia, 2=akinesia, 3=dyskinesia).18 Left ventricular volumes in end-diastole and end-systole were determined, using a system for off-line analysis (ImageVue, Nova Microsonics Inc., NJ, USA) using Simpson’s biplane method.19 Doppler measurements were also analysed with this system.
Calculations
Stroke volume was calculated as the difference between end-diastolic and end-systolic volume (SV=EDV-ESV). Ejection fraction was calculated as the percentage reduction of the end-diastolic volume (EF=SV/EDV*100%). Cardiac output was calculated as stroke volume multiplied by heart rate (CO=SV*HR). Mean arterial pressure was calculated from (systolic blood pressure + twice diastolic blood pressure) divided by 3 (MAP=(SBP+2*DBP)/3). Peripheral vascular resistance (PVR) was calculated (with the assumption that central venous pressure was zero) by dividing mean arterial pressure by the cardiac output (PVR=MAP/CO).
Statistical analysis
Data are presented as mean ± SD. Continuous data were compared with Student’s t-test, using paired t-tests where appropriate.
Results
The ten volunteers were 36.9±7.4 years old. Mean height was 185±5.5 cm, mean weight 81.4±12.1 kg, body surface area 2.07±0.2 m2.
Haemodynamic response
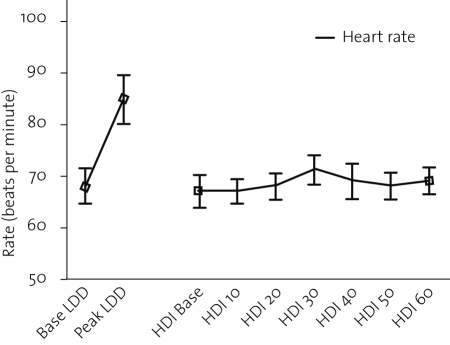
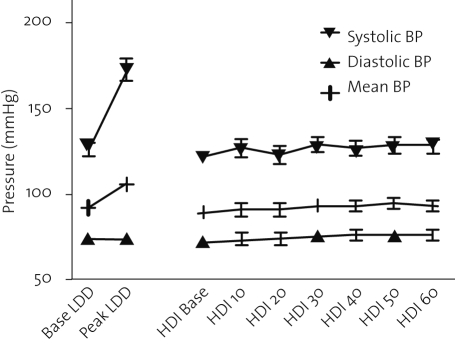
The haemodynamic response is shown in table 1 and depicted in figures 1 and 2. The increase in heart rate and blood pressure during low-dose dobutamine was significant. In contrast, heart rate and blood pressure remained unchanged during high-dose insulin infusion.
Table 1.
Haemodynamic response to low-dose dobutamine and high-dose insulin infusion.
| LDD baseline | LDD peak | Difference | P | HDI baseline | HDI 60 min | Difference | P | |
|---|---|---|---|---|---|---|---|---|
| Heart rate (BPM) | 68±11 | 85±15† | 17±18 | 0.013 | 67±10 | 69±8 | 2±4 | 0.24 |
| Systolic BP (mmHg) | 126±12 | 173±19† | 47±17 | <0.001 | 122±9 | 128±13 | 6±9 | 0.08 |
| Diastolic BP (mmHg) | 74±8 | 73±7 | -1±9 | 0.73 | 72±7 | 76±10 | 4±7 | 0.13 |
| MAP (mmHg) | 92±7 | 106±7* | 15±10 | 0.002 | 89±6 | 93±10 | 4±7 | 0.08 |
| RPP (*103 mmHg/min) | 8.6±1.6 | 14.6±2.5† | 6.1±2.1 | <0.001 | 8.2±1.1 | 8.7±0.9 | 0.6±1.0 | 0.09 |
BPM=beats per minute, BP=blood pressure, MAP=mean arterial pressure, RPP=rate-pressure product, LDD=low-dose dobutamine, HDI=high-dose insulin. * p<0.05 compared with HDI 60 min, † p<0.01 compared with HDI 60 min.
Figure 1.
Response of heart rate to low-dose dobutamine (LDD) and high-dose insulin (HDI) administration.
Figure 2.
Response of blood pressure to low-dose dobutamine (LDD) and high-dose insulin (HDI) administration.
Biochemical response
Biochemical response is displayed in table 2. Levels of glucose, sodium, potassium, magnesium and calcium did not change significantly from baseline. Levels of phosphate decreased significantly during high-dose insulin infusion and levels of albumin decreased during both low-dose dobutamine and high-dose insulin infusion. Insulin levels were unchanged during low-dose dobutamine but raised significantly to supraphysiological levels during high-dose insulin infusion. FFA levels increased significantly during low-dose dobutamine but were not significantly changed by high-dose insulin. Finally, neither epinephrine nor norepinephrine levels were changed by either intervention.
Table 2.
Biochemical response to low-dose dobutamine and high-dose insulin infusion.
| LDD baseline | LDD peak | P | HDI baseline | HDI 60 min | P | |
|---|---|---|---|---|---|---|
| Sodium (mmol/l) | 142±1 | 142±2 | 1.00 | 142±1 | 141±2 | 0.46 |
| Potassium (mmol/l) | 3.8±0.2 | 3.9±0.3 | 0.32 | 3.8±0.2 | 3.8±0.2 | 0.60 |
| Chloride (mmol/l) | 105±1 | 105±1 | 0.48 | 105±2 | 106±2 | 0.14 |
| Calcium (mmol/l) | 2.30±0.04 | 2.33±0.05† | 0.19 | 2.28±0.06 | 2.24±0.05 | 0.08 |
| Magnesium (mmol/l) | 0.85±0.08 | 0.87±0.08 | 0.48 | 0.84±0.05 | 0.86±0.07 | 0.57 |
| Phosphate (mmol/l) | 0.99±0.08 | 0.92±0.10† | 0.09 | 1.05±0.07 | 0.80±0.06 | <0.001 |
| Glucose (mmol/l) | 5.3±0.8 | 5.0±0.6 | 0.34 | 5.0±1.1 | 4.8±1.1 | 0.68 |
| Albumin (g/l) | 37±2 | 40±2† | 0.007 | 37±3 | 35±2 | 0.034 |
| FFA (mmol/l) | 0.16±0.12 | 0.73±0.35† | <0.001 | 0.23±0.25 | 0.10±0.06 | 0.13 |
| Insulin (pmol/l) | 104±76 | 93±67† | 0.74 | 109±90 | 783±164 | <0.001 |
| Norepineprine (nmol/l) | 3.0±0.9 | 3.0±1.3 | 0.95 | 2.9±0.9 | 3.5±1.4 | 0.34 |
| Epinephrine (nmol/l) | 0.3±0.1 | 0.3±0.1 | 0.08 | 0.2±0.1 | 0.3±0.1 | 0.55 |
FFA=free fatty acids, LDD=low-dose dobutamine, HDI=high-dose insulin. †p<0.01 compared with HDI 60 min.
Echocardiographic segmental response
In all volunteers, all segments showed normal wall motion at baseline and became hyperkinetic during both dobutamine stimulation and insulin administration.
Changes in echocardiographic parameters
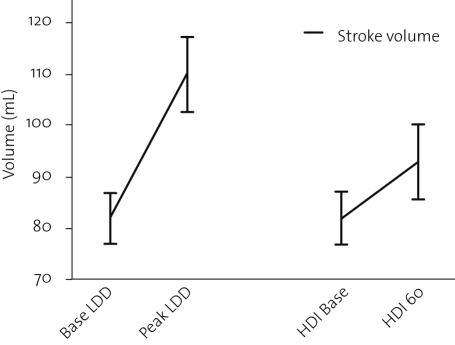
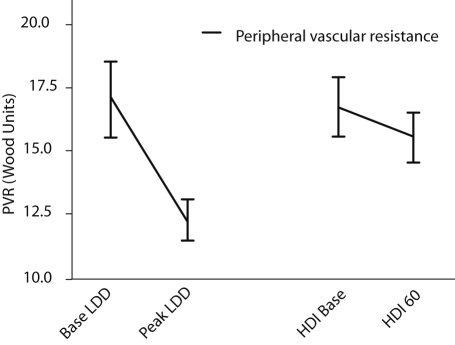
In table 3, changes in echocardiographic parameters are shown. End-diastolic volumes remained unchanged, and end-systolic volumes were significantly reduced, both during low-dose dobutamine and high-dose insulin infusions. Thus stroke volume and ejection fraction increased during both low-dose dobutamine infusion and high-dose insulin infusion. This was confirmed by an increase of the volume-time integral over the aortic valve. The increased stroke volume was reflected in an increased cardiac output during both infusions. The change in stroke volume is graphically depicted in figure 3. Remarkably, peripheral vascular resistance was reduced during dobutamine but unchanged during high-dose insulin infusion, as shown in figure 4.
Table 3.
Changes in left ventricular volumes and Doppler measurements.
| Baseline LDD | Peak LDD | Difference | P | HDI baseline | HDI 60 min | Difference | P | |
|---|---|---|---|---|---|---|---|---|
| Heart rate (BPM) | 69±8 | 83±16† | 14±18 | 0.038 | 68±9 | 68±8 | 0±6 | 1.00 |
| EDV (ml) | 133±23 | 136±31 | 3±10 | 0.36 | 133±25 | 129±32 | -4±16 | 0.46 |
| ESV (ml) | 51±11 | 26±10† | -25±5 | <0.001 | 52±12 | 36±11 | -16±7 | <0.001 |
| Stroke volume (ml) | 82±15 | 110±23† | 28±11 | <0.001 | 82±16 | 93±24 | 12±12 | 0.014 |
| Ejection fraction (%) | 62±4 | 81±4† | 19±3 | <0.001 | 61±4 | 72±5 | 11±4 | <0.001 |
| Cardiac output (l/min) | 5.7±1.3 | 9.0±2.1† | 3.3±1.9 | <0.001 | 5.5±1.2 | 6.2±1.1 | 0.7±1.0 | 0.048 |
| VTI Aorta (cm) | 25.6±4.5 * | 32.3±5.9 | 6.7±8.0 | 0.026 | 23.0±3.6 | 28.3±4.1 | 5.4±2.0 | <0.001 |
| PVR (Woods units) | 17.0±4.7 | 12.3±2.7† | -4.6±5.1 | 0.018 | 16.7±3.4 | 15.5±3.2 | -1.2±3.3 | 0.27 |
| Peak E (cm/s) | 63±7 | 82±14† | 19±10 | <0.001 | 56±6 | 55±7 | -1±9 | 0.72 |
| E/A ratio | 1.3±0.2 | 1.4±0.4 | 0.1±0.4 | 0.40 | 1.3±0.2 | 1.2±0.2 | -0.1±0.2 | 0.28 |
| E deceleration time (ms) | 139±13 | 139±34 | -1±36 | 0.97 | 146±22 | 160±23 | 14±29 | 0.16 |
EDV=end-diastolic volume, ESV=end-systolic volume, VTI=volume-time integral, PVR=peripheral vascular resistance, E=mitral valve inflow E wave velocity, A=mitral valve inflow A wave velocity, LDD=low-dose dobutamine, HDI=high-dose insulin, *p<0.05 compared with HDI baseline, †p<0.001 compared with HDI 60 min.
Figure 3.
Change in stroke volume in response to low-dose dobutamine (LDD) and high-dose insulin (HDI) administration.
Figure 4.
Change in calculated peripheral vascular resistance (PVR) in response to low-dose dobutamine (LDD) and high-dose insulin (HDI) administration.
Discussion
This study showed that high-dose insulin infusion in normal, healthy volunteers resulted in an increase in stroke volume and ejection fraction, comparable with the effect of low-dose dobutamine infusion in same subjects. The increase in stroke volume was the result of a decrease in left ventricular end-systolic volume, while end-diastolic volume remained unchanged. In contrast to low-dose dobutamine infusion, the increase in LV stroke volume during high-dose insulin infusion was not accompanied by changes in blood pressure, heart rate or peripheral resistance. Because heart rate did not increase during high-dose insulin infusion, the increase in cardiac output was lower than during low-dose dobutamine infusion, despite a similar improvement in stroke volume, but cardiac output was still significantly higher than at baseline.
To the best of our knowledge, this is the first study performed using high-dose insulin infusion and Doppler echocardiography to assess left ventricular systolic and diastolic function in normal subjects. Because the volunteers had a normal baseline LV function, increase in stroke volume and ejection fraction could not be related to the number of segments that showed improvement of function.
Biochemical response to low-dose dobutamine and high-dose insulin showed an expected increase in insulin levels during high-dose insulin infusion. Unexpectedly, the FFA level was not reduced to nearly zero after one hour of high-dose insulin, and was raised significantly by low-dose dobutamine. Epinephrine and norepinephrine levels did not change, a finding similar to that of Airakasinen,16 excluding a stress effect of lowering glucose levels by high-dose insulin administration and secondary improvement of left ventricular function.
High-dose insulin infusion with tailored glucose administration was chosen to study the effect of insulin instead of a fixed combination of glucose-insulin-potassium (as in many of the myocardial infarction studies) or a glucose infusion with tailored insulin administration. This clamp technique, although often mistaken for GIK, is often used to improve glucose uptake in the myocardium before 18F-fluorodeoxyglucose (18F-FDG) in nuclear viability studies administration. The advantage of the approach is that a target administration of insulin is obtained of around 100 mU/kg/h, and that volume administration is limited to the need of glucose administration. Using this protocol, it is improbable that increase in serum osmolality improves left ventricular function, as has been suggested in the past in dogs.20 Also, it was improbable that volume load was the cause of left ventricular improvement. In a study in post myocardial infarction patients by our group, administration of similar amounts of NaCl as usually administered in glucose administration had no effect on myocardial function.14
High-dose insulin and enhancement of LV function
In perfused animal hearts with induced ischaemia, insulin administration during ischaemia resulted in better global LV function after reperfusion than hearts that did not receive insulin.1,21 In intact animals, high-dose insulin administration preserved regional LV function during3 and post ischaemia.3-5 One study attributed the effect solely to insulin.1
In patients undergoing cardiac surgery, GIK infusion resulted in higher postoperative cardiac indices compared with patients receiving standard treatment.6,8-10 Cardiac index and LV stroke work index improved both during dobutamine and GIK, but the rate-pressure product and the tension-time index only increased in the dobutamine group.9 Whole body oxygen consumption increased with dobutamine while it remained unchanged in GIK infusion.9 GIK infusion resulted in a lower systemic vascular resistance resulting in a decreased afterload.8,10
Investigations in normal volunteers are sparse. Ferrannini et al. observed in a study with healthy volunteers that blood pressure, heart rate and LV end-diastolic pressure and myocardial oxygen consumption remained unchanged during GIK infusion.22 A transition was observed in myocardial substrate usage from FFA towards glucose, lactate and pyruvate, with a rise in net myocardial CO2 production, thus indicating a change towards carbohydrate utilisation. Because data on cardiac output were not reported, a conclusion about a net left ventricular work can not be drawn. Airaksinen did not observe any effect of moderate hyperinsulinaemia (540±130 pmol/l vs. 783±164 in this study) on myocardial function or contractility in healthy volunteers.16
The vasodilator effect of insulin described previously could explain the increase in ejection fraction while blood pressure and heart rate remain unchanged.8,10,25 However, we did not observe a vasodilator effect using the calculations that are classically used to determine peripheral vascular resistance. If the effect were only due to decreased afterload, we would expect a decrease in blood pressure and an increase in heart rate, as a decrease in resistance has to be compensated by an increase in flow to keep the blood pressure constant. To increase blood flow an increase in contractility is required. In this study, contractility is not enhanced by increased stretch of myocardial muscle fibres with constant end-diastolic volumes, but by other factors. Possibly, the volume load of high-dose insulin echocardiography contributed to improved LV function and increased ejection fraction via the Frank-Starling mechanism. However, end-diastolic volume did not change significantly and infusion of similar amounts of saline did not result in functional improvement in a previous study from our institution.14 Therefore we do not consider this to be the mechanism responsible for increased performance.
Mechanism of action: insulin and increased contractility
The increase in contractility during high-dose insulin fusion has been subscribed to several mechanisms. These include the enhanced availability of glucose to the cell and/or reduction of plasma FFA levels (resulting in a decrease in myocardial oxygen consumption), effects on Na+/K+ ATP-ase, improved Ca2+ handling,24 as well as a direct effect on contractility via phosphatidylinositol-3-kinase (PI3K).25,26 During high-dose insulin infusion, the availability of substrate for glycolysis to cells is improved, thus providing a possibility to increase myocardial efficiency (more adenosine triphosphatase (ATP) production per molecule oxygen used). In isolated perfused rat hearts, lactate production was higher during low-flow ischaemia in hearts perfused with glucose-insulin solution than in control hearts, indicating increased glycolysis.27 Cytosolic ATP content was higher during late ischaemia and anorganic phosphate (Pi), the degradation product of ATP, was unchanged in the glucose-insulin treated hearts. In addition, a lower end-diastolic pressure during the ischaemic period and a substantially increased systolic function at reperfusion was observed with glucose insulin.27 ATP obtained from glycolysis, taking place in the cytosol and not in the mitochondria, is preferentially used to maintain membrane functions, such as ATP-sensitive K+ channels28 and the sarcolemmal Ca2+ pump29,30 used to maintain Ca2+ homeostasis, important to both contraction and relaxation of the myofilaments in the cardiomyocyte. The functional impairment (both systolic and diastolic) observed in post-ischaemic myocardium is related to cellular Ca2+ overload.30 In this view, GIK infusion is able to reduce the Ca2+ overload present in post-hypoxic myocardial cells, resulting in increased contractility and relaxation. However, these mechanisms may apply to ischaemic cells but not to assumed normally perfused myocardium from volunteers. Therefore another explanation for the inotropic effect of insulin must be present, which may be found in the direct effect on sarcolemmal calcium pumps and the effect via PI3K.
Limitations of the study
Echocardiography was used to measure the effects of the two types of infusion, to keep the comparison restricted to the types of intervention rather than combining two types of intervention with two types of measurements, with their respective limitations.
The assessment of LV function by volume measurements using biplane echocardiography is based on assumptions regarding the geometry of the left ventricle. These assumptions are valid in the investigated group of volunteers and therefore less prone to errors than when used in patients with sustained myocardial infarction. Furthermore, not only stroke volume as determined by volume measurements was increased during both interventions, but also VTI over the aortic valve, a volume-independent Doppler measurement.
Conclusion and clinical implications
The results of the present study indicate that high-dose insulin infusion has positive inotropic effects in healthy volunteers. It confirms previous results from studies applying high-dose insulin infusion in heart failure, showing improved wall motion.
References
- 1.Doenst T, Richwine RT, Bray MS, Goodwin GW, Frazier OH, Taegtmeyer H. Insulin improves functional and metabolic recovery of reperfused working rat heart. Ann Thorac Surg. 1999;67:1682-8. [DOI] [PubMed] [Google Scholar]
- 2.Apstein CS, Gravino FN, Haudenschild CC. Determinants of a protective effect of glucose and insulin on the ischemic myocardium. Effects on contractile function, diastolic compliance, metabolism, and ultrastructure during ischemia and reperfusion. Circ Res. 1983;52:515-26. [DOI] [PubMed] [Google Scholar]
- 3.Lazar HL, Zhang X, Rivers S, Bernard S, Shemin RJ. Limiting ischemic myocardial damage using glucose-insulin-potassium solutions. Ann Thorac Surg. 1995;60:41-6. [DOI] [PubMed] [Google Scholar]
- 4.Zhu P, Lu L, Xu Y, Greyson C, Schwartz GG. Glucose-insulin-potassium preserves systolic and diastolic function in ischemia and reperfusion in pigs. Am J Physiol. 2000;278:H595-H603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed SS, Lee CH, Oldewurtel HA, Regan TJ. Sustained effect of glucose-insulin-potassium on myocardial performance during regional ischemia. Role of free fatty acid and osmolality. J Clin Invest.1978;61:1123-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazar HL, Philippides G, Fitzgerald C, Lancaster D, Shemin RJ, Apstein C. Glucose-insulin-potassium solutions enhance recovery after urgent coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1997;113:354-60. [DOI] [PubMed] [Google Scholar]
- 7.Bellows SD, Kloner RA. Glucose-Insulin-Potassium does not reduce myocardial infarct size in an ischemic/reperfusion rabbit model. J Thromb Thrombolysis. 1998;5:25-7. [DOI] [PubMed] [Google Scholar]
- 8.Coleman GM, Gradinac S, Taegtmeyer H, Sweeney M, Frazier OH. Efficacy of metabolic support with glucose-insulin-potassium for left ventricular pump failure after aortocoronary bypass surgery. Circulation. 1989;80:I9-6. [PubMed] [Google Scholar]
- 9.Hiesmayr M, Haider WJ, Grubhofer G, Heilinger D, Keznickl FP, Mares P, et al. Effects of dobutamine versus insulin on cardiac performance, myocardial oxygen demand, and total body metabolism after coronary artery bypass grafting. J Cardiothorac Vasc Anesth. 1995;9:653-8. [DOI] [PubMed] [Google Scholar]
- 10.Jeppsson A, Ekroth R, Kirno K, Milocco I, Nilsson B, Nilsson F, et al. Insulin and amino acid infusion after cardiac operations: effects on systemic and renal perfusion. J Thorac Cardiovasc Surg. 1997;113:594-602. [DOI] [PubMed] [Google Scholar]
- 11.Alan S, Ulgen M, Dedeoglu I, Kaya H, Toprak N. Long-term glucose insulin potassium infusion improves systolic and diastolic function in patients with chronic ischemic cardiomyopathy. Swiss Med Wkly. 2003;26;133:419-22. [DOI] [PubMed] [Google Scholar]
- 12.Cottin Y, Lhuillier I, Gilson L, Zeller M, Bonnet C, Toulouse C et al. Glucose insulin potassium infusion improves systolic function in patients with chronic ischemic cardiomyopathy. Eur J Heart Fail. 2002;4:18-4. [DOI] [PubMed] [Google Scholar]
- 13.Khoury VK, Haluska B, Prins J, Marwick TH. Effects of glucose-insulin-potassium infusion on chronic ischaemic left ventricular dysfunction. Heart. 2003;89:6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein LJ, van Campen LC, Sieswerda GT, Kamp O, Visser CA, Visser FC. Glucose-insulin-potassium echocardiography detects improved segmental myocardial function and viable tissue shortly after acute myocardial infarction. J Am Soc Echocardiogr. 2006;19:763-71. [DOI] [PubMed] [Google Scholar]
- 15.Yetkin E, Senen K, Ileri M, Atak R, Battaoglu B, Yetkin O, et al. Identification of viable myocardium in patients with chronic coronary artery disease and myocardial dysfunction: comparison of low-dose dobutamine stress echocardiography and echocardiography during glucose-insulin-potassium infusion. Angiology. 2002;53:67-6. [DOI] [PubMed] [Google Scholar]
- 16.Airaksinen J, Lahtela JT, Ikaheimo MJ, Sotaniemi EA, Takkunen JT. Intravenous insulin has no effect on myocardial contractility or heart rate in healthy subjects. Diabetologia. 1985;28:649-52. [DOI] [PubMed] [Google Scholar]
- 17.van der Weerdt AP, Klein LJ, Visser CA, Visser FC, Lammertsma AA. Use of arterialised venous instead of arterial blood for measurement of myocardial glucose metabolism during euglycaemic-hyperinsulinaemic clamping. Eur J Nucl Med Mol Imaging. 2002;29:663-9. [DOI] [PubMed] [Google Scholar]
- 18.Kan G, Visser CA, Koolen JJ, Dunning AJ. Short and long term predictive value of admission wall motion score in acute myocardial infarction. A cross sectional echocardiographic study of 345 patients. Br Heart J. 1986;56:422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nijland F, Kamp O, Verheugt FW, Veen G, Visser CA. Long-term implications of reocclusion on left ventricular size and function after successful thrombolysis for first anterior myocardial infarction. Circulation. 1997;95:11-7. [DOI] [PubMed] [Google Scholar]
- 20.Bronsveld W, Van Lambalgen AA, Van den Bos GC, Teule GJ, Thijs LG. Ventricular function, hemodynamics, and oxygen consumption during infusions of blood and glucose-insulin-potassium (GIK) in canine endotoxin shock. Circ Shock. 1982;9:145-56. [PubMed] [Google Scholar]
- 21.Eberli FR, Weinberg EO, Grice WN, Horowitz GL, Apstein CS. Protective effect of increased glycolytic substrate against systolic and diastolic dysfunction and increased coronary resistance from prolonged global underperfusion and reperfusion in isolated rabbit hearts perfused with erythrocyte suspensions. Circ Res. 1991;68:466-81. [DOI] [PubMed] [Google Scholar]
- 22.Ferrannini E, Santoro D, Bonadonna R, Natali A, Parodi O, Camici PG. Metabolic and hemodynamic effects of insulin on human hearts. Am J Physiol. 1993;264:E308-15. [DOI] [PubMed] [Google Scholar]
- 23.Cardillo C, Kilcoyne CM, Nambi SS, Cannon RO, III, Quon MJ, Panza JA. Vasodilator response to systemic but not to local hyperinsulinemia in the human forearm. Hypertension. 1998;32:740-5. [DOI] [PubMed] [Google Scholar]
- 24.Apstein CS. Glucose-insulin-potassium for acute myocardial infarction: remarkable results from a new prospective, randomized trial. Circulation. 1998;98:2223-6. [DOI] [PubMed] [Google Scholar]
- 25.Zaha V, Francischetti I, Doenst T. Insulin improves postischemic recovery of function through PI3K in isolated working rat heart. Mol Cell Biochem. 2003;247:229-32. [DOI] [PubMed] [Google Scholar]
- 26.von Lewinski D, Bruns S, Walther S, Kogler H, Pieske B. Insulin causes [Ca2+]i-dependent and [Ca2+]i-independent positive inotropic effects in failing human myocardium. Circulation. 2005;111:2588-95. [DOI] [PubMed] [Google Scholar]
- 27.Cave AC, Ingwall JS, Friedrich J, Liao R, Saupe KW, Apstein CS, et al. ATP synthesis during low-flow ischemia: influence of increased glycolytic substrate. Circulation. 2000;101:2090-6. [DOI] [PubMed] [Google Scholar]
- 28.Weiss J, Hiltbrand B. Functional compartmentation of glycolytic versus oxidative metabolism in isolated rabbit Heart. J Clin Invest. 1985;75:436-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu KY, Zweier JL, Becker LC. Functional coupling between glycolysis and sarcoplasmic reticulum Ca2+ transport. Circ Res. 1995;77:88-97. [DOI] [PubMed] [Google Scholar]
- 30.Jeremy RW, Koretsune Y, Marban E, Becker LC. Relation between glycolysis and calcium homeostasis in postischemic myocardium. Circ Res. 1992;70:1180-90. [DOI] [PubMed] [Google Scholar]