The Notch intracellular domain and β-catenin team up with RBP-J to regulate gene transcription and promote the development of arterial endothelial cells.
Abstract
Molecular mechanisms controlling arterial–venous specification have not been fully elucidated. Previously, we established an embryonic stem cell differentiation system and demonstrated that activation of cAMP signaling together with VEGF induces arterial endothelial cells (ECs) from Flk1+ vascular progenitor cells. Here, we show novel arterial specification machinery regulated by Notch and β-catenin signaling. Notch and GSK3β-mediated β-catenin signaling were activated downstream of cAMP through phosphatidylinositol-3 kinase. Forced activation of Notch and β-catenin with VEGF completely reconstituted cAMP-elicited arterial EC induction, and synergistically enhanced target gene promoter activity in vitro and arterial gene expression during in vivo angiogenesis. A protein complex with RBP-J, the intracellular domain of Notch, and β-catenin was formed on RBP-J binding sites of arterial genes in arterial, but not venous ECs. This molecular machinery for arterial specification leads to an integrated and more comprehensive understanding of vascular signaling.
Introduction
One of the earliest occurrences in organogenesis is the development of the vascular system. The vascular system is first formed as a primitive vascular network by differentiation and assembly of vascular progenitor cells. Molecular differences between arterial and venous endothelial cells (ECs) become apparent at this stage before circulation begins (Wang et al., 1998; Adams et al., 1999; Zhong et al., 2001). Although acquisition of arterial and venous EC identities from progenitors is a crucial step for establishing the complete circulation system, cellular and molecular processes that regulate arterial–venous specification are not fully elucidated.
Notch is a single-pass transmembrane receptor known for its function in controlling cell fate decisions and creating boundaries through cell–cell communication (Lai, 2004). Ligand binding to Notch leads to cleavage and release of the Notch intracellular domain (NICD), and NICD translocates to the nucleus and associates with the transcription factor RBP-J (also called CSL, CBF-1 in mammals, Suppressor of Hairless [Su(H)] in Drosophila, and LAG-1 in Caenorhabditis elegans; Christensen et al., 1996; Kidd et al., 1998; Artavanis-Tsakonas et al., 1999). Notch (Notch 1, 4) and its cell surface ligands (Delta like-1 [Dll1], Dll4, and Jagged1 and 2) are expressed in arteries but not in veins (Villa et al., 2001; Sörensen et al., 2009). Genetic studies of Notch signaling components have shown that these arterial EC markers are essential for proper formation of the developing vasculature (Xue et al., 1999; Lawson et al., 2001; Duarte et al., 2004; Krebs et al., 2004). Thus, Notch signaling plays an important role in arterial specification.
Wnt/β-catenin signaling regulates embryogenesis and is involved in the pathogenesis of a variety of diseases (Nusse, 2005; Clevers, 2006; Grigoryan et al., 2008). Recent studies suggested that Wnt/β-catenin signaling also plays a key role in vascular biology (Goodwin and D’Amore, 2002). Mice deficient for Wnt2 displayed vascular abnormalities including defective placental vasculature (Monkley et al., 1996). Knockout mice for the Wnt receptor gene, Frizzled-5, died in utero due to defects in yolk sac angiogenesis (Ishikawa et al., 2001). Defects of the β-catenin gene in ECs caused aberrant vascular patterning and increased vascular fragility (Cattelino et al., 2003). Nevertheless, the role of Wnt/β-catenin signaling in arterial–venous development is unknown.
We previously demonstrated that Flk1+ (also designated as VEGF receptor 2) cells derived from embryonic stem (ES) cells serve as vascular progenitors and can constructively reproduce the early vascular organization processes including differentiation of both endothelial and mural cells (MCs; vascular smooth muscle cells and pericytes) and vascular structure formation (Yamashita et al., 2000; Yamamizu et al., 2009). We also reported that activation of the adrenomedullin/cAMP pathway induced differentiation of arterial ECs from Flk1+ cells. Activation of the cAMP pathway induced Notch activation in differentiating ECs. Although inhibition of Notch signaling abolished arterial EC induction, activation of Notch using a NICD–estrogen receptor fusion protein together with VEGF treatment did not induce arterial ECs (Yurugi-Kobayashi et al., 2006). These results indicated that Notch signaling is essential but not sufficient for arterial EC induction, suggesting that other factors are involved in this process.
In this study, we investigated signal transduction events downstream of the cAMP pathway with the use of our ES cell differentiation system and found novel molecular machinery for arterial EC induction. That is, not single, but dual activation of Notch and β-catenin signaling together with VEGF successfully reconstituted arterial EC induction from vascular progenitors. RBP-J, NICD, and β-catenin formed a protein complex specifically in arterial but not venous ECs both from ES cells and in vivo. Moreover, dual induction of NICD and β-catenin enhanced promoter activity of target genes in vitro and arterial gene expression during in vivo angiogenesis in adults. Thus, Notch and β-catenin signaling converge via formation of a single protein complex which should form a core molecular machinery that induces arterial fate in ECs.
Results
PI3K is involved in arterial EC induction downstream of cAMP
Previously, we reported that two vascular cell types, ECs (positive for CD31/vascular endothelial [VE]–cadherin/endothelial nitric oxide synthase [eNOS]/Claudin5) and MCs (positive for smooth muscle actin [SMA]/Calponin/SM22α), were selectively induced (Yamashita et al., 2000; Yamamizu et al., 2009) when purified ES cell–derived Flk1+ cells were cultured with VEGF and serum. Under these culture conditions, only these two cell types (i.e., ECs and MCs), but not blood cells such as CD45+ cells, were specifically induced (Yamamizu et al., 2009). Most of the CD31+ ECs induced with VEGF and serum had a venous phenotype, which did not express ephrin B2, an arterial EC marker. Simultaneous stimulation of VEGF and cAMP signaling by addition of a cAMP analogue, 8bromo-cAMP, successfully induced ephrinB2+ arterial ECs, indicating that the cAMP pathway regulates arterial EC induction (Fig. 1 A; Yurugi-Kobayashi et al., 2006).
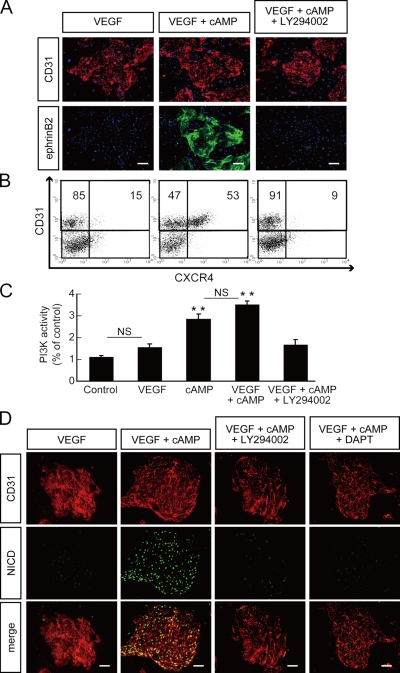
Figure 1.
Inhibitory effect of PI3K inhibitor LY294002 on arterial EC induction from Flk1+ cells. (A) Double-fluorescent staining for CD31 and ephrinB2 after a 3-d culture of Flk1+ cells (Flk-d3). Top panels: CD31 (pan-ECs, red) and DAPI (blue). Bottom panels: EphB4-Fc (ephrinB2+ arterial ECs, green) and DAPI (blue). Flk1+ cells stimulated with VEGF alone (50 ng/ml; left panels), VEGF and 8bromo-cAMP (0.5 mM; middle panels), or VEGF, 8bromo-cAMP, and a PI3K inhibitor, LY294002 (7.5 µM; right panels). Bars: 100 µm. (B) Flow cytometry for CD31 and CXCR4 expression at Flk-d3. Percentages of CXCR4+/CD31+ arterial ECs and CXCR4−/CD31+ venous ECs in total ECs (CD31+ cells) are indicated. (C) PI3K activity at Flk-d3. Flk1+ cells stimulated with vehicle, VEGF, 8bromo-cAMP, VEGF and 8bromo-cAMP, or VEGF, 8bromo-cAMP, and LY294002 (7.5 µM; n = 3; **, P < 0.01 vs. vehicle; NS: not significant). (D) Double-fluorescent immunostaining for cleaved Notch intracellular domain (NICD) and CD31 at Flk-d3. Left panels, CD31 (pan-ECs, red). Middle panels, cleaved NICD (green). Right panels, merged image. Flk1+ cells stimulated with VEGF alone, VEGF and 8bromo-cAMP, VEGF, 8bromo-cAMP and LY294002, or VEGF, 8bromo-cAMP, and a γ-secretase inhibitor, DAPT (2.5 µM). Bars: 100 µm.
We then investigated the downstream targets of cAMP. First we examined various kinase inhibitors (Fig. S1). Among them, LY294002, a phosphatidylinositol-3 kinase (PI3K) inhibitor, potently and specifically inhibited the cAMP-elicited induction of ephrinB2+ arterial ECs (ephrinB2+/CD31+), but not total EC (CD31+) appearance from Flk1+ progenitor cells (Fig. 1 A and Fig. S1). We further quantitatively evaluated arterial EC induction at the cellular level with flow cytometry. We used a chemokine receptor, CXCR4, as an arterial EC marker (Tachibana et al., 1998; Ara et al., 2005; Yurugi-Kobayashi et al., 2006). CXCR4−/CD31+ venous ECs were mainly induced by VEGF treatment alone. CXCR4+/CD31+ arterial ECs were induced in the presence of 8bromo-cAMP together with VEGF. Addition of LY294002 almost completely inhibited CXCR4+ arterial EC induction, but not total CD31+ cell appearance (Fig. 1 B).
PI3K is known as one of the downstream molecules of VEGF signaling in adult ECs (Dayanir et al., 2001; Shiojima and Walsh, 2002). Although VEGF treatment alone induced no significant activation of PI3K in Flk1+ cells, treatments with 8bromo-cAMP significantly activated PI3K (Fig. 1 C). These results indicated that cAMP signaling, but not VEGF, can activate PI3K in vascular progenitors or differentiating ECs and contributes to arterial EC induction.
Notch signaling is known to have important functions during arterial–venous specification (Xue et al., 1999; Lawson et al., 2001; Villa et al., 2001; Duarte et al., 2004; Krebs et al., 2004; Sörensen et al., 2009). Previously we demonstrated that addition of 8bromo-cAMP together with VEGF induced Notch activation in differentiating ECs (Fig. 1 D and Fig. S2; Yurugi-Kobayashi et al., 2006). Addition of LY294002 virtually abolished cAMP-induced Notch activation (Fig. 1 D), indicating that PI3K acts downstream of cAMP to activate Notch signaling in differentiating ECs.
GSK3β is negatively involved in arterial EC induction
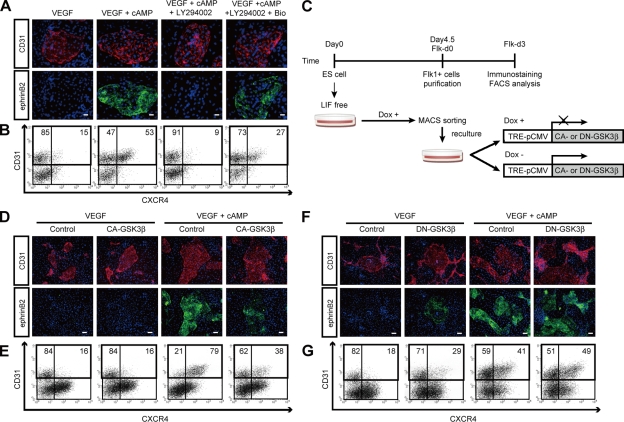
We previously reported in our ES cell system that Notch signaling is essential but not sufficient for arterial EC induction (Yurugi-Kobayashi et al., 2006). We next investigated other downstream targets of the cAMP and PI3K pathways involved in arterial EC induction. When activation of glycogen synthase kinase (GSK) 3β, one of the downstream targets of PI3K (Cross et al., 1995), was blocked by addition of a GSK3β inhibitor, Bio, the inhibitory effects of LY294002 on ephrinB2+ arterial EC induction was partially restored (Fig. 2 A). The inhibitory effect of LY294002 on CXCR4+/CD31+ arterial EC appearance was also partially reversed by the addition of Bio (Fig. 2 B). We generated ES cell lines expressing constitutively active (CA) or dominant-negative (DN) mutants of GSK3β using a tetracycline-regulatable system (Tet-Off; Fig. S3; Summers et al., 1999; Rommel et al., 2001; Yamamizu et al., 2009). We then induced expression of the CA- or DN-GSK3β in Flk1+ cell cultures by depleting doxycycline (Dox), a tetracycline analogue (Fig. 2 C). CA-GSK3β expression in Flk1+ progenitor cells inhibited arterial EC induction by VEGF and cAMP treatment (Fig. 2, D and E). On the other hand, DN-GSK3β expression in Flk1+ progenitor cells weakly induced arterial ECs with VEGF treatment alone (Fig. 2 F and G), indicating that GSK3β negatively regulates arterial EC induction downstream of PI3K.
Figure 2.
Inhibitory effect of GSK3β on arterial EC differentiation. (A) Double-fluorescent staining for CD31 and ephrinB2 at Flk-d3. Top panels, CD31 (pan-ECs, red) and DAPI (blue). Bottom panels, EphB4-Fc (ephrinB2+ arterial ECs, green) and DAPI (blue). Flk1+ cells stimulated with VEGF alone (50 ng/ml), VEGF and 8bromo-cAMP (0.5 mM), VEGF, 8bromo-cAMP, and LY294002 (7.5 µM), or VEGF, 8bromo-cAMP, LY294002, and a GSK3β inhibitor, Bio (100 nM). Bars: 100 µm. (B) Flow cytometry for CD31 and CXCR4 expression at Flk-d3. Percentages of CXCR4+/CD31+ arterial ECs and CXCR4−/CD31+ venous ECs in total ECs (CD31+ cells) are indicated. (C) Experimental system for GSK3β expression. ES cell line expressing constitutive active (CA) form or dominant-negative (DN) form of GSK3β by tetracycline-inducible expression system (Tet-Off) were established. Doxycycline (Dox) was added during the first 4.5 d of culture of ES cell differentiation to Flk1+ cells. Subsequently, Flk1+ cells were sorted by MACS and plated on type IV collagen-coated dishes, and cells were cultured in the presence or absence of 1 µg/ml Dox. (D and E) Induction of CA-GSK3β. (F and G) Induction of DN-GSK3β. (D and F) Double-fluorescent staining for CD31 and ephrinB2 at Flk-d3. Top panels, CD31 (pan-ECs, red) and DAPI (blue). Bottom panels, EphB4-Fc (ephrinB2+ arterial ECs, green) and DAPI (blue). Flk1+ cells were cultured with VEGF alone, or VEGF and 8bromo-cAMP, in the presence or absence of Dox. Bars: 200 µm. (E and G) Flow cytometry for CD31 and CXCR4 expression at Flk-d3. Percentages of CXCR4+/CD31+ arterial ECs and CXCR4−/CD31+ venous ECs in total ECs (CD31+ cells) are indicated.
Activation of β-catenin and Notch signaling induces arterial ECs
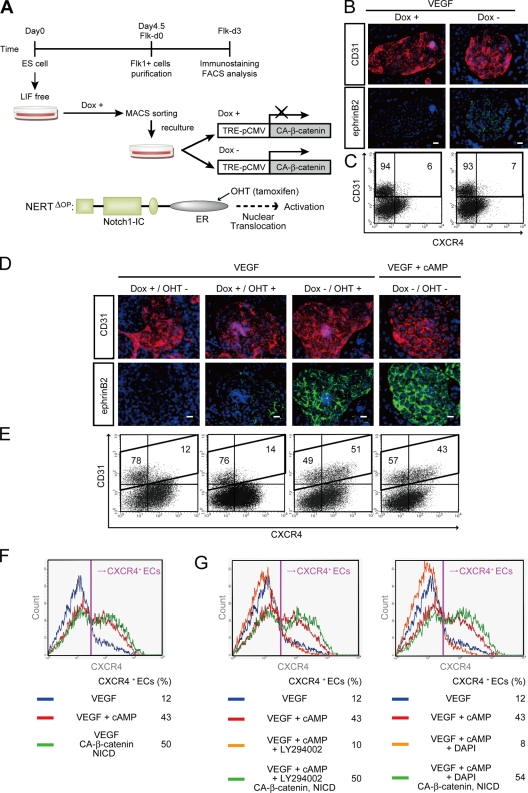
Next, we investigated whether β-catenin, a negatively regulated downstream target of GSK3β (Nusse, 2005), is involved in arterial EC induction. We generated an ES cell line expressing CA-β-catenin regulated by the Tet-Off system (Fig. S3; Hirabayashi et al., 2004; Yamamizu et al., 2009). Flk1+ cells were sorted and recultured with VEGF in the presence or absence of 1 µg/ml Dox (Dox+ or Dox−; Fig. 3 A). CA-β-catenin expression (Dox−) together with VEGF stimulation showed almost no induction of ephrinB2+ arterial ECs (Fig. 3 B) as well as CXCR4+/CD31+ arterial ECs (Fig. 3 C), indicating that β-catenin signaling alone is not sufficient for arterial EC induction.
Figure 3.
Arterial EC induction by dual activation of β-catenin and Notch signaling. (A) Experimental system for dual activation of Notch and β-catenin signaling. ES cell lines carrying CA-β-catenin regulated by Tet-Off system, and a fusion protein of N1ICD and estrogen receptor (ER), NERTΔOP, were established. CA-β-catenin was induced by depletion of Dox, and Notch activation was induced by nuclear translocation of NERTΔOP with addition of 4-hydrotamoxifen (OHT). 1 µg/ml Dox was added during the first 4.5 d of culture of ES cell differentiation to Flk1+ cells. After Flk1+ cells were sorted by MACS and plated on type IV collagen-coated dishes, cells were treated with or without Dox and/or OHT (150 ng/ml). (B and C) Activation of β-catenin together with VEGF. (B) Double-fluorescent staining for CD31 and ephrinB2 at Flk-d3. Left panels, Dox treatment. Right panels, Dox free (expression of CA-β-catenin). (C) Flow cytometry for CD31 and CXCR4 expression at Flk-d3. Percentages of CXCR4+/CD31+ arterial ECs and CXCR4−/CD31+ venous ECs in total ECs (CD31+ cells) are indicated. (D–F) Dual activation of Notch and β-catenin signaling. (D) Double-fluorescent staining for CD31 and ephrinB2 at Flk-d3. Top panels, CD31 (pan-ECs, red) and DAPI (blue). Bottom panels, EphB4-Fc (ephrinB2+ arterial ECs, green) and DAPI (blue). Flk1+ cells were treated with VEGF alone (50 ng/ml), together with Dox+ (control), Dox+/OHT+ (Notch activated), or Dox−/OHT+ (dual activated) condition. VEGF and 8bromo-cAMP (0.5 mM) treatment in Dox+ condition is shown as positive control. Bars: 100 µm. (E) Flow cytometry for CD31 and CXCR4 expression. Percentages of CXCR4+/CD31+ arterial ECs and CXCR4−/CD31+ venous ECs in total ECs (CD31+ cells) are indicated. (F and G) Expression profile of CXCR4 in CD31+ ECs by flow cytometry. Percentages of CXCR4+ arterial ECs in total ECs are indicated. (F) VEGF treatment alone (blue line), VEGF and 8bromo-cAMP (red line), and VEGF together with dual activation of β-catenin and Notch activation (Dox−, OHT+; green line) are shown. (G) VEGF treatment alone (blue line), VEGF and 8bromo-cAMP (red line), VEGF, 8bromo-cAMP, and LY294002 (7.5 µM; left panel), DAPT (2.5 µM; right panel, orange line), VEGF, 8bromo-cAMP, and LY294002 (left panel), or DAPT (right panel) together with dual activation of β-catenin and Notch activation (Dox−, OHT+; green line) are shown.
Previously we established an inducible Notch activation system in ES cells using a fusion protein (NERTΔOP) of the intracellular domain of murine Notch1 (N1ICD) and the estrogen receptor (ER), which allows regulated nuclear translocation of N1ICD with an ER ligand, 4-hydroxytamoxifen (OHT; Fig. 3 A; Schroeder et al., 2006; Yurugi-Kobayashi et al., 2006). We subsequently generated an ES cell line expressing both the NERTΔOP fusion protein and tetracycline-regulated CA-β-catenin. Activation of Notch by addition of 150 ng/ml OHT together with VEGF induced only a faint arterial EC induction, compatible with our previous results (Yurugi-Kobayashi et al., 2006). However, remarkable appearance of ephrinB2+ ECs was clearly observed after dual activation of Notch and β-catenin signaling by the addition of OHT and depletion of Dox, respectively, even in the absence of cAMP (Fig. 3 D). FACS analysis further demonstrated that the dual activation of Notch and β-catenin signaling completely reproduced the effects of cAMP on arterial EC induction (Fig. 3, E and F). Moreover, though LY294002 or a γ-secretase inhibitor, DAPT, almost completely abolished arterial EC induction by cAMP, the dual activation of Notch and β-catenin signaling completely reversed their inhibitory effects (Fig. 3 G). These results indicate that the dual activation of Notch and β-catenin is sufficient to reconstitute the roles of cAMP in arterial EC induction.
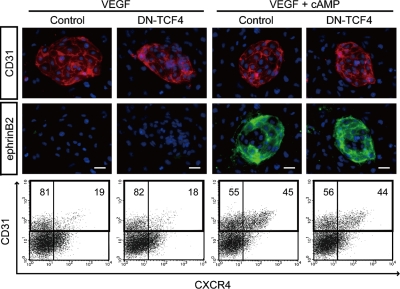
Cytoplasmic β-catenin translocates into the nucleus where it forms a complex with transcription factors of the TCF/LEF family and activates target molecules (Orsulic and Peifer, 1996; Nusse, 2005). To investigate whether the TCF transcription factor is involved in arterial EC induction, we generated an ES cell line expressing DN-TCF4 regulated by the Tet-Off system (Fig. S3; van de Wetering et al., 2002; Yamamizu et al., 2009). Even when we expressed DN-TCF4 in Flk1+ vascular progenitors, cAMP-elicited arterial EC induction was not affected (Fig. 4, A and B). Thus, TCF did not appear to be involved in the arterial specification process.
Figure 4.
Effects of DN-TCF4 on arterial EC induction from Flk1+ cells. (Top) Double-fluorescent staining for CD31 and ephrinB2 at Flk-d3. CD31 panels, CD31 (pan-ECs, red) and DAPI (blue). ephrinB2 panels, EphB4-Fc (ephrinB2+ arterial ECs, green) and DAPI (blue). Flk1+ cells induced from DN-TCF4 ES cell line were cultured with VEGF alone (50 ng/ml) or VEGF and 8bromo-cAMP (0.5 mM), in the presence or absence of 1 µg/ml Dox. Bars: 100 µm. (Bottom) Flow cytometry for CD31 and CXCR4 expression at Flk-d3. Percentages of CXCR4+/CD31+ arterial ECs and CXCR4−/CD31+ venous ECs in total ECs (CD31+ cells) are indicated.
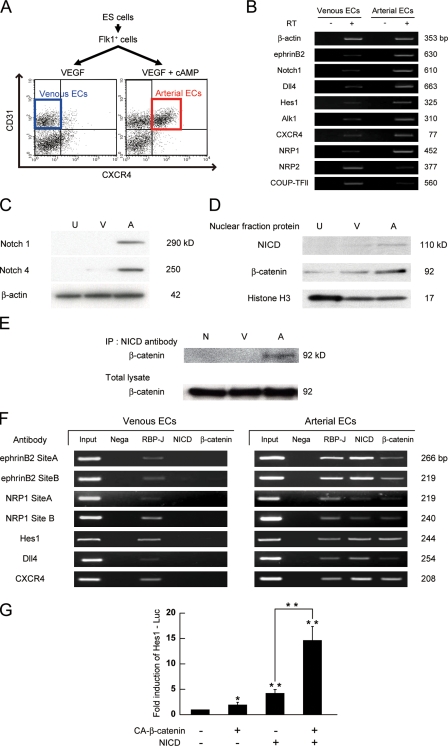
A protein complex of RBP-J, NICD, and β-catenin is formed specifically in arterial ECs
We further investigated how Notch and β-catenin signaling pathways interact to induce arterial ECs. We first attempted to examine the expression and molecular interaction of these two molecules in purified arterial and venous ECs induced from ES cells (Fig. 5 A; Yurugi-Kobayashi et al., 2006). Purified arterial and venous ECs showed a distinct mRNA expression pattern of arterial EC markers (ephrinB2, Notch1, Dll4, Hes1, Alk1, CXCR4, and NRP1) and venous EC markers (NRP2 and COUP-TFII), respectively (Fig. 5 B). Western blot analysis revealed that Notch1 and 4 protein expression was specifically detected in arterial ECs but not in venous ECs (Fig. 5 C), suggesting that cAMP regulates both Notch induction and activation. Furthermore, the NICD was present almost specifically in the nuclear fraction of arterial ECs but not in that of venous ECs. Although β-catenin was observed in the nuclear fraction of both arterial and venous ECs, arterial ECs showed a higher nuclear β-catenin expression than venous ECs (Fig. 5 D). Immunoprecipitation (IP) assays revealed that the arterial-expressed NICD formed a protein complex with β-catenin (Fig. 5 E). We further confirmed the formation of a NICD/β-catenin protein complex on several arterial genes by chromatin-IP (ChIP) assays. Recently, RBP-J binding sites in mouse ephrinb2 or hes1 genes were reported to regulate their expression in response to Notch activation (Grego-Bessa et al., 2007; Shimizu et al., 2008). We further performed in silico investigations of RBP-J binding sites within other arterial-specific genes, and found conserved RBP-J binding sites in both the mouse and human genome in neuropilin1, dll4, and cxcr4 genes (Fig. S4). ChIP assays demonstrated that although the RBP-J protein was present on these RBP-J binding sites in both arterial and venous ECs, NICD and β-catenin formed a protein complex with RBP-J only in arterial ECs (Fig. 5 F). We further confirmed the functional relevance of the dual activation of NICD and β-catenin on target gene expression in Flk1+ cells. Compared with NICD alone, dual expression of NICD and β-catenin synergistically increased Hes1 gene promoter activity by approximately threefold (n = 3; P < 0.002; Fig. 5 G). Taken together, these results suggest that Notch and β-catenin signaling converge into a single protein complex with RBP-J, NICD, and β-catenin (arterial protein complex) on RBP-J binding sites specifically in arterial ECs, and that this heterotrimeric arterial protein complex synergistically activates target gene expressions in Flk1+ vascular progenitors and induces arterial EC specification.
Figure 5.
Arterial-specific formation of protein complex with RBP-J, NICD, and β-catenin. (A) Purification of arterial and venous ECs from ES cells. CXCR4+/CD31+ cells at Flk-d3 induced by VEGF (50 ng/ml) with 8bromo-cAMP (0.5 mM) and CXCR4−/CD31+ cells induced by VEGF alone were purified as arterial and venous ECs, respectively. (B) RT-PCR for mRNA expression of arterial and venous EC markers in purified arterial and venous ECs induced from ES cells as indicated in panel A. (C) Western blot for protein expression of Notch1 and Notch4 in purified arterial and venous ECs. U, undifferentiated ES cells; V, venous ECs; A, arterial ECs. (D) Nuclear localization of NICD and β-catenin. A representative result of Western blot analysis for NICD and β-catenin using nuclear fraction of purified arterial and venous ECs. Anti-histone H3: nuclear fraction control. (E) Immunoprecipitation assay. Immunoblot with anti–β-catenin antibody for total cell lysates or cell lysates immunoprecipitated with anti-NICD antibody. N: negative control, immunoprecipitated with normal rabbit-IgG antibody. (F) ChIP assays for RBP-J, NICD, and β-catenin on RBP-J binding sites of arterial markers in ECs from ES cells. Input: PCR products generated using DNA from nonimmunoprecipitated chromatin as a template. Negative control: immunoprecipitated with normal rabbit-IgG antibody. RBP-J, NICD, β-catenin: immunoprecipitated chromatin with antibodies for corresponding proteins. (G) Hes1 Luciferase reporter assay. A Notch signaling reporter, Hes1-Lucifearse plasmid was transiently transfected to MACS-purified Flk1+ cells together with CA-β-catenin and/or NERTΔOP activation. After 24 h, the luciferase activities were measured (n = 3; *, P < 0.05; **, P < 0.01 vs. control or between corresponding values).
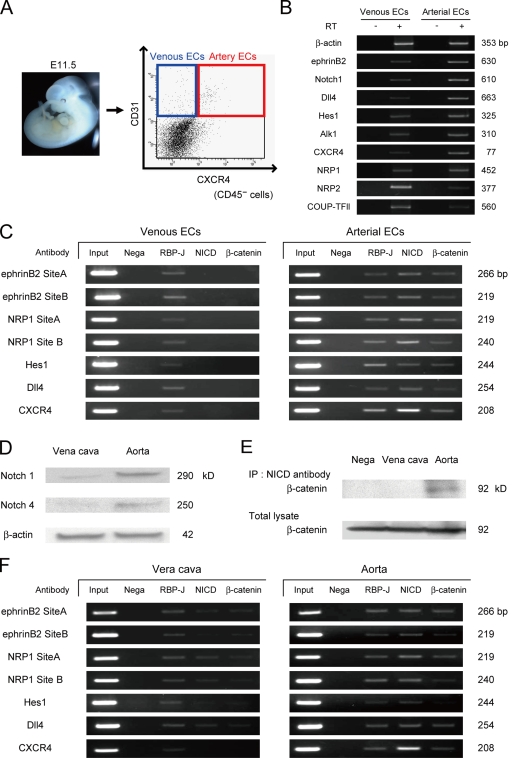
The arterial protein complex is also formed in embryonic and adult vessels
We purified arterial (CXCR4+/CD31+/CD45−) and venous (CXCR4−/CD31+/CD45−) ECs from embryonic day (E) 11.5 mouse embryos (Fig. 6 A) and performed ChIP assays for the RBP-J binding sites in ephrinb2, neuropilin1, dll4, hes1, and cxcr4 genes. Purified arterial and venous ECs showed distinct mRNA expression patterns of arterial and venous EC markers, respectively (Fig. 6 B). Similar to ECs derived from ES cells, the arterial protein complex (NICD/β-catenin/RBP-J protein complex) was formed on RBP-J binding sites of arterial marker genes specifically in arterial ECs, but not in venous ECs in the embryo (Fig. 6 C). Moreover, we investigated whether the arterial protein complex is also formed in adult mice using isolated aorta and vena cava. Notch1 and 4 proteins were specifically detected in the aorta (Fig. 6 D). IP assays revealed that NICD and β-catenin formed a protein complex only in the aorta (Fig. 6 E). ChIP assays further demonstrated that the arterial protein complex was evidently identified in the aorta rather than the vena cava (Fig. 6 F). Together, these results indicate the existence of the same molecular interaction in both embryos and adults.
Figure 6.
Formation of the arterial protein complex in the embryo and adult vessels. (A) Purification of arterial and venous ECs from the mouse embryo. Arterial ECs (CXCR4+/CD31+/CD45−) and venous ECs (CXCR4−/CD31+/CD45−) were isolated from E11.5 embryos. (B) RT-PCR for mRNA expression of arterial and venous EC markers in purified arterial and venous ECs from E11.5 mouse embryo. (C) ChIP assays for RBP-J, NICD, and β-catenin on RBP-J binding sites of arterial markers in ECs from embryos. (D) Western blot for Notch1 and Notch4 in isolated aorta and vena cava. (E) Immunoprecipitation assay for isolated aorta and vena cava. Immunoblot with anti–β-catenin antibody for total cell lysates or cell lysates immunoprecipitated with anti-NICD antibody. (F) ChIP assays for RBP-J, NICD, and β-catenin on RBP-J binding sites of arterial markers in the aorta and vena cava.
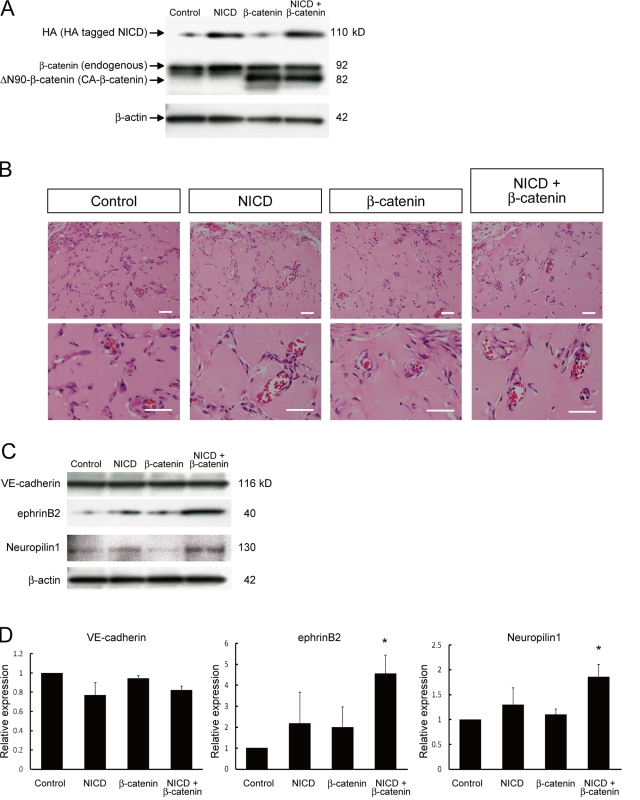
Dual activation of Notch and β-catenin signaling enhances arterial gene expression during in vivo angiogenesis
Finally, to investigate whether Notch and β-catenin signaling is involved in arterial specification during angiogenesis in adults, we examined in vivo angiogenesis using a gel plug assay (Kim et al., 2002). Adenoviral vectors for NICD and/or CA-β-catenin were mixed into Matrigel plugs with VEGF (100 ng/ml) and heparin (10 units/ml). Transgene products were successfully induced in gel plugs at 1 wk after transplantation (Fig. 7 A). Formation of vascular structures and the presence of blood cells within the vascular lumen occurred in gel plugs to a similar extent under all experimental conditions tested (Fig. 7 B). Although expression of a pan-EC marker, VE-cadherin, was not altered, expression of the arterial markers ephrinB2 and Neuropilin1 was significantly increased in gel plugs only with the dual induction of NICD and CA-β-catenin (Fig. 7, C and D). These results indicate that dual activation of Notch and β-catenin signaling also functions in arterial EC induction during in vivo angiogenesis in adults.
Figure 7.
Enhancement of arterial gene expression through dual activation of Notch and β-catenin during in vivo angiogenesis. Matrigel containing VEGF (100 ng/ml), heparin (10 units/ml), and adenoviral vectors (vehicle [control], HA-tagged N1ICD [NICD], and/or CA-β-catenin) were injected subcutaneously in mice. After 7 d, the mice were sacrificed and plugs were excised. (A) Western blot for HA-tagged N1ICD and CA-β-catenin in recovered cells from Matrigel plugs. (B) Hematoxylin and eosin staining of Matrigel sections. Overall appearances were not different. Invasion of blood vessels with vascular lumen and blood cells were observed. Bars: 200 µm. (C) Representative result of Western blot for VE-cadherin, ephrinB2, and Neuropilin1 in recovered cells from Matrigel plugs. (D) Quantitative evaluation of VE-cadherin (left graph), ephrinB2 (middle graph), and Neuropilin1 (right graph) protein expression in Matrigels. Relative expression normalized with β-actin expression is shown. (n = 3; *, P < 0.05 vs. control).
Discussion
Here, we demonstrated that simultaneous activation of Notch and β-catenin signaling can constructively reproduce the induction processes of arterial ECs from Flk1+ vascular progenitors through the formation of an arterial-specific protein complex. cAMP, PI3K, Notch, and β-catenin signaling interact and converge during EC differentiation to specify arterial cell fate. These findings provide novel insights into vascular signaling necessary for cell differentiation and diversification as well as into molecular mechanisms of cell fate determination.
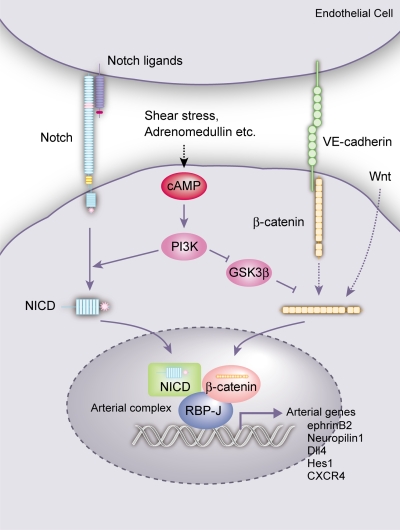
In ECs, Notch (Notch1, 4) activation can be induced by various Notch ligands, including Dll1, Dll4, and Jagged2, which are expressed by arterial ECs, and Jagged1, which is expressed in ECs and mural cells (Villa et al., 2001; Sörensen et al., 2009). All of this Notch signaling is considered to be mediated by the NICD and RBP-J transcription factor. On the other hand, β-catenin signaling in ECs can be activated through Wnt ligands as well as VE-cadherin. Thus, Wnt ligands such as Wnt2, 5a, and 10b, expressed in fetal blood vessels, are involved in EC differentiation (Monkley et al., 1996; Goodwin and D’Amore, 2002). VE-cadherin is heavily tyrosine phosphorylated and is linked to β-catenin. When adherens junctions mature, tyrosine residues in VE-cadherin tend to be dephosphorylated and β-catenin is partially released from the complex (Dejana et al., 1999), allowing nuclear translocation of β-catenin and activation of downstream signaling cascades. As VE-cadherin and β-catenin are broadly expressed in ECs and mice with EC-specific disruption of β-catenin show broad vascular phenotypes (Cattelino et al., 2003), β-catenin should possess both common roles in ECs and specific roles in arterial ECs. Adrenomedullin, which is mainly secreted from vascular smooth muscle cells, activates the cAMP pathway and induces arterial ECs in the ES cell system (Yurugi-Kobayashi et al., 2006). Many other factors, such as fluid shear stress, should be involved in cAMP activation in ECs. All of this signaling in blood vessels should finally converge into the single arterial protein complex (NICD/β-catenin/RBP-J protein complex) and contribute to induce arterial ECs (Fig. 8).
Figure 8.
Molecular mechanisms of arterial EC specification. cAMP signaling, which could be induced by adrenomedullin, shear stress, and so on, activates Notch and β-catenin signaling through PI3K (and GSK3β) in vascular progenitors (as well as differentiating ECs). Notch and β-catenin signaling subsequently converges into a single protein complex with RBP-J, NICD, and β-catenin (arterial complex) on arterial genes. Notch signaling from Notch ligand binding and β-catenin signaling from Wnt and VE-cadherin should also participate in forming the complex. The arterial complex should play a central role in the specification of arterial cell fate in ECs.
In addition to induction of arterial ECs, Notch and β-catenin could also be potentially involved in maintenance of the arterial EC phenotype. When activation of Notch and β-catenin signaling was ceased in arterial ECs from Flk-d3, ephrinB2 expression was attenuated and disappeared until Flk-d12 (Fig. S5). On the other hand, When Notch and β-catenin signaling was activated at Flk-d3 in venous ECs induced by VEGF alone, ephrinB2 expression could still be induced (Fig. S5), suggesting that ECs at the early differentiation stage still possess a plasticity for arterial–venous specification. The existence of the arterial protein complex in the adult vessels (Fig. 6) and synergistic effect of dual activation of Notch and β-catenin signaling on arterial gene expression during in vivo angiogenesis (Fig. 7) provide further supporting evidence that the complex should be involved in the induction as well as the maintenance of arterial phenotypes. Recently, Shin et al. (2009) reported that activation of Notch or β-catenin signaling during early mesoderm differentiation in the chick embryo induces separation of smooth muscle and blood/endothelial progenitor lineages. Notch and β-catenin signaling should play distinct roles in cell fate determination depending on each differentiation stage.
Interaction of Notch and β-catenin signaling has been reported in various contexts. Though Notch and β-catenin signaling pathways were reported to produce synergistic effects on maintenance of hematopoietic stem cells (Duncan et al., 2005), hair follicles (Estrach et al., 2006), and intestinal stem cells (Radtke and Clevers, 2005; van Es et al., 2005), direct molecular interaction of Notch and β-catenin was not clear from these reports. Direct interaction of Notch and β-catenin has been reported in Drosophila, in which the Notch C-terminal region to the cdc10/ankyrin repeats is associated with Armadillo/β-catenin and negatively regulates β-catenin/TCF transcription (Hayward et al., 2005). In our study, TCF4 was not involved in arterial specification, and β-catenin exerted its arterializing effect through a TCF-independent manner. Recently, Shimizu et al. (2008) showed that the Notch/β-catenin/RBP-J complex suppresses differentiation of neural precursor cells independent of TCF. The protein complex that directly converges Notch and β-catenin signaling may play a critical role in cell fate determination in various organs.
Until now, individual roles of VEGF, Flk1, PI3K, Notch, and β-catenin have been suggested in various studies, namely, VEGF and Flk1 in EC differentiation (Sakurai et al., 2005), PI3K and β-catenin in vascular formation (Monkley et al., 1996; Dayanir et al., 2001; Ishikawa et al., 2001; Goodwin and D’Amore, 2002; Shiojima and Walsh, 2002; Cattelino et al., 2003), and Notch in arterial specification (Xue et al., 1999; Lawson et al., 2001; Villa et al., 2001; Zhong et al., 2001; Duarte et al., 2004; Krebs et al., 2004; Sörensen et al., 2009). We constructively reproduced the cellular processes of arterial EC induction in vitro, which can lead to integrate these signaling pathways and offer comprehensive understanding of how these molecules interact during EC differentiation and arterial specification. The novel insights into the molecular machinery of cell differentiation and diversification would provide clues for clinical strategies with vascular-specific manipulation and drug discovery targeting ischemic disease and cancer.
Materials and methods
Generation of stable cell lines with inducible cDNAs
cDNA for CA-β-catenin (ΔN90-β-catenin; a gift from Dr. Y. Gotoh, University of Tokyo, Tokyo, Japan; Hirabayashi et al., 2004), CA-GSK3β (S9A-GSK; a gift from Dr. M.J. Birnbaum, University of Pennsylvania School of Medicine, Philadelphia, PA; Summers et al., 1999), DN-GSK3β (a gift from Dr. J.R. Woodgett, Samuel Lunenfeld Research Institute, Toronto, Canada; Rommel et al., 2001), and DN-TCF4 (a gift from Dr. H. Clevers, University Medical Center, Utrecht, Netherlands; van de Wetering et al., 2002) were introduced into the downstream region of tetracycline responsive element (TRE)–regulatable CMV promoter of Exchange vector (Yamamizu et al., 2009). We previously generated ES cells carrying recombination sites of loxP and a mutant loxP, loxP511, in ROSA locus and tetracycline-transactivator gene (EStTA-ROSA; Yamamizu et al., 2009). Stable ES cell lines that express the desired cDNA under the control of the TRE-regulatable CMV promoter were generated by introducing Exchange vectors and pBS185 (Cre expression vector) to EStTA-ROSA cells using mouse ES cell Nucleofector kit (Lonza). Hygromycin- and ganciclovir-resistant colonies were selected and subjected to further studies (cDNA EStTA cells; Yamamizu et al., 2009). For DN-TCF4–expressing ES cells, total hygromycin- and ganciclovir-resistant colonies were collected and used. Tet-inducible expression and/or function of these cDNAs were confirmed by Western blotting and Wnt/β-catenin signaling reporter assays using TOP-FLASH TCF reporter plasmid (Millipore; Fig. S3, A–D).
An ES cell line carrying both tet-regulatable CA-β-catenin gene and OHT-regulated Notch activation system with NERTΔOP, a fusion protein of N1ICD and ER (Yurugi-Kobayashi et al., 2006), was generated by introducing NERTΔOP plasmid carrying puromycin-resistant gene to CA-β-catenin EStTA cells using mouse ES cell Nucleofector kit (Lonza). Cells were then plated on 10-cm dishes containing 1 µg/ml doxycycline (Dox+). After 1 d, the medium was changed to Dox+ medium containing 200 µg/ml hygromycin and 1 µg/ml puromycin. Hygromycin- and puromycin-resistant colonies were selected and subjected to further studies (CA-β-catenin/NERTΔOP EStTA cells).
Cell culture
ES cell lines, D3, EStTA-ROSA, and various EStTA derivatives were maintained as described previously (Yurugi-Kobayashi et al., 2006; Yamamizu et al., 2009). Differentiation was induced in these ES cell lines using differentiation medium (DM) (α-minimal essential medium, MEM; Invitrogen) supplemented with 10% fetal calf serum (FCS; Japan Bioserum) and 5 × 10−5 M 2-mercaptoethanol (2-ME; Invitrogen) as described previously (Yamashita et al., 2000; Yurugi-Kobayashi et al., 2006; Yamamizu et al., 2009). In brief, undifferentiated ES cells were cultured on type IV collagen-coated dishes without leukemia inhibitory factor at a cell density of 0.75–103 cells/cm2 for 96–108 h. Cultured cells were harvested and subjected to magnetic cell sorting (MACS) purification. Purified Flk1+ cells were then plated onto type IV collagen-coated dishes (BD) at a density of 0.75–104 cells/cm2 in DM. After 3 d of Flk1+ cell differentiation (Flk-d3), induced ECs were examined by immunohistochemistry and flow cytometric analyses. Various reagents were occasionally added to the Flk1+ cell culture, including human VEGF165 (R&D Systems), 8-bromoadenosine-3′, 5′-cyclic monophosphate sodium salt (8-bromo-cAMP’ Nacalai Tesque), γ-secretase inhibitor, DAPT, PI3K inhibitor, LY294002, GSK3β inhibitor, Bio, Akt inhibitor, TAT-Akt-inh, PKA inhibitor, PKI or H89, p38 inhibitor, SB202190, MEK inhibitor, PD98059, PKCαβ inhibitor, PKCη inhibitor, and PKCζ inhibitor (EMD). Dox was added to ES cells during the first 4.5 d of ES cell differentiation culture for the tetracycline-regulated cDNA expression experiments. Subsequently, purified Flk1+ cells were plated on type IV collagen-coated dishes and cultured in the presence or absence of 1 µg/ml Dox. 1 µg/ml Dox did not affect EC differentiation, proliferation, or arterial–venous specification in control ES cells (Yamamizu et al., 2009). For OHT-induced Notch activation, 150 ng/ml of OHT was added 24 h after Flk1+ cell plating. OHT did not affect arterial–venous specification in control ES cells.
Immunocytochemistry
Immunostaining of cultured cells was conducted as described previously (Yurugi-Kobayashi et al., 2006, Yamamizu et al., 2009). ECs were fixed with 5% dimethyl sulfoxide/methanol. For double staining of ephrinB2 and CD31, fixed culture slides were incubated with EphB4-human immunoglobulin (Ig) Fc portion chimeric protein (EphB4-Fc; 1:50; R&D Systems) followed by human IgG Fc peroxidase-conjugated goat IgG fraction (1:500; ICN Biomedicals, Inc.). TSA biotin system (PerkinElmer) was used to amplify the signal for EphB4-Fc staining. EphrinB2P+P cells were visualized with streptavidin/Alexa Fluor 488 conjugate (Invitrogen; Yurugi-Kobayashi et al., 2006). CD31+ cells were stained with phycoerythrin (PE)-conjugated monoclonal antibody (mAb) for CD31 (Mec13.3; 1:300; BD). Cleaved Notch intracellular domain (NICD) staining was performed with TSA Biotin System using anti-cleaved Notch1 antibody (1:300; Cell Signaling Technology), followed by peroxidase-labeled anti–rabbit IgH antibody (1:500; Vector Laboratories; Yurugi-Kobayashi et al., 2006). Stained cells were photographed with an inverted fluorescent microscope (Eclipse TE2000-U; Nikon) with 10× and 20× objectives, Plan Fluor (Nikon), a digital camera system, AxioCam HRc (Carl Zeiss, Inc.), and AxioVision software (Carl Zeiss, Inc.). All images were taken at room temperature.
PI3K activity assay
Flk1+ cells (104 cells/ml) were incubated on 96-well plates and stimulated with vehicle, VEGF (50 ng/ml), 8-bromo-cAMP (0.5 mM), VEGF with 8-bromo-cAMP, or VEGF with 8-bromo-cAMP and LY294002 (7.5 µM). After a 3-d culture of Flk1+ cells, PI3K activity was evaluated using FACE PI3 Kinase p85 ELISA kit (Active Motif) according to the manufacturer’s instructions. The concentration was normalized by cell numbers stained with crystal violet. PI3K activity was measured with a microplate reader (ARVO MX; PerkinElmer).
Cell purification and FACS analysis
After the induction of Flk1+ cells, they were harvested and stained with allophycocyanin (APC)-conjugated AVAS12 antibody (Yamashita et al., 2000). Flk1+ cells were sorted with auto MACS (Miltenyi Biotec) using anti-APC MicroBeads (Miltenyi Biotec) and recultured for EC differentiation. For the arterial and venous EC FACS analysis, cultured cells were harvested at Flk-d3 and stained with a combination of PE-conjugated anti-CD31 mAb (Mec13.3; BD) and biotin-conjugated anti-CXCR4 mAb (BD) followed by streptavidin-conjugated APC (BD), and then subjected to analysis using FACSVantage or FACSAria (BD). Arterial and venous ECs in vivo were isolated from embryos at E11.5. Embryos were diced, digested with dispase II (Roche), and hemolyzed. Arterial and venous ECs negative for CD45 were obtained using FACSVantage or FACSAria.
RT-PCR
Total RNA was isolated from arterial and venous ECs with RNeasy (QIAGEN) according to the manufacturer’s instructions and reverse transcribed with the SuperScript III First-Strand Synthesis System (Invitrogen). RT-PCR was performed as described previously (Yamashita et al., 2005) using the indicated primers (Table S1).
Subcellular proteome extraction and Western blotting
Sorted arterial and venous ECs were subjected to protein extraction according to their subcelluar localization using the ProteoExtract Subcelluar Proteome Extraction kit (EMD). Nuclear extraction or total lysates were subjected to SDS–PAGE using gradient gels (Atto Co.) followed by electrophoretic transfer onto nitrocellulose membranes. The blots were incubated for 1 h in Blocking One blocking agents (Nacalai Tesque). Then, the membranes were incubated overnight with the respective first antibodies (1:1,000) for β-catenin (BD), NICD (Cell Signaling Technology), histone H3 (Abcam), Notch1 (Abcam), Notch4 (Santa Cruz Biotechnology, Inc.), ephrinB2 (R&D Systems), neuropilin1 (R&D Systems), and β-actin (Sigma-Aldrich). Horseradish peroxidase (HRP)–conjugated anti–mouse Ig antibody (Invitrogen), HRP-conjugated anti–goat Ig antibody (Invitrogen), or HRP-conjugated anti–rabbit Ig antibody (Vector Laboratories) were used as secondary antibodies (1:10,000). The Can Get Signal Immunoreaction Enhancer Solution kit (Toyobo) was used to enhance the signal. Immunoreactivity was detected with the Chemi-Lumi One Enhanced Chemiluminescence kit (Nacalai Tesque). Signal intensity was calculated with Scion Image software (Scion Corp.).
Immunoprecipitation and chromatin immunoprecipitation assays
Arterial and venous EC cell lysates were subjected to immunoprecipitation using Protein G HP SpinTrap (GE Healthcare) and anti-NICD antibody, and then immunoblotted with antibody specific to β-catenin (see previous section).
Arterial and venous ECs were subjected to cross-linking with 3.7% formaldehyde. The aorta and vena cava were isolated from adult mice (8–10 wk old) and were also subjected to cross-linking with 3.7% formaldehyde, followed by the ChIP assay using a ChIP assay kit (Millipore). Chromatin was sheared to an average length of 0.4–1.0 kb. Antibodies to RBP-J (K0043; Tokusyu-meneki Laboratory, Tokyo, Japan), NICD (Cell Signaling Technology), and β-catenin (BD) were used for immunoprecipitation. PCR amplifications were performed in 20 µl with primers specific for the promoter analysis, as shown below. The sensitivity of the PCR amplifications was evaluated with serial dilutions of total DNA collected after sonication (input fraction). Amplified DNA was separated on 2% agarose gels and visualized with ethidium bromide. Sets of primers were used to amplify DNA sequences containing the conserved RBP-J binding sites (GTGGGAA) of the mouse ephrinB2 and Hes1 gene according to Grego-Bessa et al. (2007) and Shimizu et al. (2008), respectively (Table S2). Moreover, we performed in silico investigations of the cis-acting elements within various reported arterial-specific genes using VISTA browser (Mayor et al., 2000) and ClustalW (Thompson et al., 1994). We analyzed the RBP-J binding sites conserved between the mouse and human genomes in ±10 kb of each gene. We found conserved RBP-J binding sites in the neuropilin1, dll4, and cxcr4 genes (Fig. S4 and Table S2). PCR amplification was conducted with a variable number of cycles (94°C for 30 s, 60°C for 30 s, and 72°C for 30 s).
Luciferase reporter assay
FACS-purified Flk1+ cells were transfected with a Notch signaling reporter, Hes1-Luciferase plasmid carrying the firefly luciferase gene under the control of the Hes1 promoter region (−467 to +46; a gift from Drs. R. Kageyama and T. Ohtsuka, Kyoto University, Kyoto, Japan; Ohtsuka et al., 1999) using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instruction. After 24 h, luciferase activity was assayed using a luminometer (Lumat LB 9507; Berthold Technologies). The luciferase activity was normalized with that of control Renilla luciferase.
Construction and preparation of adenovirus vectors
HA-tagged N1ICD (generated by deleting the ER sequence from the NERTΔOP plasmid; Yurugi-Kobayashi et al., 2006) or CA-β-catenin was introduced into pENTR (Invitrogen), and LR recombination reactions were performed between the pAd/CMV/V5-DEST (Invitrogen) and pENTR with HA-tagged NICD or CA-β-catenin to generate full-length adenoviral vectors.
Plasmid DNAs encoding the full-length adenoviral vectors were linearized and transfected into 293 cells using Lipofectamine 2000 (Invitrogen). The resultant adenovirus vectors were propagated by serial passages on 293 cells and purified by CsCl2 density gradient ultracentrifugation, as described previously (Ng and Graham, 2002).
In vivo Matrigel plug assay and cell recovery
In vivo induction of arterial ECs was evaluated with the Matrigel plug assay (Kim et al., 2002). VEGF (final concentration, 100 ng/ml; R&D Systems) and heparin (final concentration, 10 units/ml; Nacalai Tesque) were dissolved in BD Matrigels Matrix High Concentration Solution. Adenoviral vector solutions for vehicle, NICD, and/or CA-β-catenin were mixed with the Matrigel solution on ice (total 8 × 108 plaque-forming units [p.f.u.] of virus [20 µl]/500 µl Matrigel; control: 8 × 108 p.f.u. of vehicle; NICD: 4 × 108 p.f.u. of NICD and 4 × 108 p.f.u. of vehicle; β-catenin: 4 × 108 p.f.u. of CA-β-catenin and 4 × 108 p.f.u. of vehicle; NICD + β-catenin: 4 × 108 p.f.u. of NICD and 4 × 108 p.f.u. of CA-β-catenin). Matrigel solution (500 µl) containing growth factors and adenovirus was injected subcutaneously near the mid-abdomen of anesthetized nude mice, and the mice were sacrificed 7 d after injection. All animal experiments were performed in accordance with the guidelines for Animal Experiments of Kyoto University, which conforms to the Guide for the Care and Use of Laboratory Animals in Japan. Gel plugs were excised and fixed in 4% paraformaldehyde, subjected to an ethanol dehydration series, and embedded in paraffin. Serial sections were deparaffinized and stained with hematoxylin and eosin. Stained sections were photographed using a microscope (model BX51; Olympus) with 4× objectives, UPlanFLN (Olympus), and DP2-BSW software (Olympus). All images were taken at room temperature. For cell recovery, the excised Matrigel plugs were minced with a sterile scalpel, passed 10 times through an 18-gauge needle, treated with BD Cell Recovery Solution for 1 h at 4°C, centrifuged, and subjected to Western blotting.
Statistical analysis
Comparisons among values for all groups were performed with an analysis of variance (ANOVA). At least three independent experiments were performed. A p-value less than 0.05 was considered significant. Values are reported as means ± SDs.
Online supplemental material
Fig. S1 shows pharmacological studies for cAMP downstream targets on arterial EC induction. Fig. S2 shows activation Notch signaling by cAMP together with VEGF. Fig. S3 shows generation of stable ES cell lines with inducible CA-β-catenin, CA-GSK3β, or DN-TCF4. Fig. S4 shows in silico investigations of the cis-acting elements within arterial-specific genes. Fig. S5 shows plasticity for arterial–venous phenotypes in early ECs. Table S1 shows the primer list for RT-PCR. Table S2 shows the primer list for ChIP. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200904114/DC1.
Acknowledgments
We are grateful to Y. Gotoh for the CA-β-catenin plasmid, M.J. Birnbaum for the CA-GSK3β plasmid, J.R. Woodgett for the DN-GSK3β plasmid, H. Clevers for the DN-TCF4 plasmid, R. Kageyama and T. Ohtsuka for the Hes1-luciferase plasmid, Y. Toda for histological analyses, and Y. Hirabayashi for preparation of adenovirus. We thank M. Takahashi for critical reading of the manuscript. K. Yamamizu performed all experiments and wrote the manuscript; T. Matsunaga helped with ES cell differentiation, cell sorting, and studies for DN-TCF4; T. Matsunaga, H. Uosaki, H. Fukushima and M. Hiraoka-Kanie helped experiments mainly with plasmid construction; K. Mitani helped to purify adenovirus; S. Katayama helped with immunostaining experiments; and J.K. Yamashita supervised all experiments and wrote the paper.
This study was supported by grants from the Ministry of Education, Science, Sports and Culture of Japan; the Ministry of Health, Labor and Welfare; the New Energy Industrial Development Organization (NEDO) of Japan; the Project for Realization of Regenerative Medicine; and Japan Society for the Promotion of Science.
The authors declare no conflict of interest.
Footnotes
Abbreviations used in this paper:
- CA
- constitutive active
- ChIP
- chromatin immunoprecipitation
- CXCR4
- CXC chemokine receptor 4
- Dll
- Delta like
- DN
- dominant negative
- Dox
- doxycycline
- EC
- endothelial cell
- ER
- estrogen receptor
- ES
- embryonic stem
- Flk1
- fetal liver kinase 1
- GSK3
- glycogen synthase kinase 3
- NICD
- Notch intracellular domain
- NRP
- neuropilin
- OHT
- 4-hydroxytamoxifen
- PI3K
- phosphatidylinositol-3-kinase
- RBP-J
- recombination signal sequence binding protein J
- TCF4
- transcription factor 4
- Tet-Off
- tetracycline-regulatable system
References
- Adams R.H., Wilkinson G.A., Weiss C., Diella F., Gale N.W., Deutsch U., Risau W., Klein R. 1999. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 13:295–306 10.1101/gad.13.3.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ara T., Tokoyoda K., Okamoto R., Koni P.A., Nagasawa T. 2005. The role of CXCL12 in the organ-specific process of artery formation. Blood. 105:3155–3161 10.1182/blood-2004-07-2563 [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S., Rand M.D., Lake R.J. 1999. Notch signaling: cell fate control and signal integration in development. Science. 284:770–776 10.1126/science.284.5415.770 [DOI] [PubMed] [Google Scholar]
- Cattelino A., Liebner S., Gallini R., Zanetti A., Balconi G., Corsi A., Bianco P., Wolburg H., Moore R., Oreda B., et al. 2003. The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J. Cell Biol. 162:1111–1122 10.1083/jcb.200212157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen S., Kodoyianni V., Bosenberg M., Friedman L., Kimble J. 1996. lag-1, a gene required for lin-12 and glp-1 signaling in Caenorhabditis elegans, is homologous to human CBF1 and Drosophila Su(H). Development. 122:1373–1383 [DOI] [PubMed] [Google Scholar]
- Clevers H. 2006. Wnt/beta-catenin signaling in development and disease. Cell. 127:469–480 10.1016/j.cell.2006.10.018 [DOI] [PubMed] [Google Scholar]
- Cross D.A., Alessi D.R., Cohen P., Andjelkovich M., Hemmings B.A. 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 378:785–789 10.1038/378785a0 [DOI] [PubMed] [Google Scholar]
- Dayanir V., Meyer R.D., Lashkari K., Rahimi N. 2001. Identification of tyrosine residues in vascular endothelial growth factor receptor-2/FLK-1 involved in activation of phosphatidylinositol 3-kinase and cell proliferation. J. Biol. Chem. 276:17686–17692 10.1074/jbc.M009128200 [DOI] [PubMed] [Google Scholar]
- Dejana E., Bazzoni G., Lampugnani M.G. 1999. Vascular endothelial (VE)-cadherin: only an intercellular glue? Exp. Cell Res. 252:13–19 10.1006/excr.1999.4601 [DOI] [PubMed] [Google Scholar]
- Duarte A., Hirashima M., Benedito R., Trindade A., Diniz P., Bekman E., Costa L., Henrique D., Rossant J. 2004. Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev. 18:2474–2478 10.1101/gad.1239004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan A.W., Rattis F.M., DiMascio L.N., Congdon K.L., Pazianos G., Zhao C., Yoon K., Cook J.M., Willert K., Gaiano N., Reya T. 2005. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat. Immunol. 6:314–322 10.1038/ni1164 [DOI] [PubMed] [Google Scholar]
- Estrach S., Ambler C.A., Lo Celso C., Hozumi K., Watt F.M. 2006. Jagged 1 is a beta-catenin target gene required for ectopic hair follicle formation in adult epidermis. Development. 133:4427–4438 10.1242/dev.02644 [DOI] [PubMed] [Google Scholar]
- Goodwin A.M., D’Amore P.A. 2002. Wnt signaling in the vasculature. Angiogenesis. 5:1–9 10.1023/A:1021563510866 [DOI] [PubMed] [Google Scholar]
- Grego-Bessa J., Luna-Zurita L., del Monte G., Bolós V., Melgar P., Arandilla A., Garratt A.N., Zang H., Mukouyama Y.S., Chen H., et al. 2007. Notch signaling is essential for ventricular chamber development. Dev. Cell. 12:415–429 10.1016/j.devcel.2006.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan T., Wend P., Klaus A., Birchmeier W. 2008. Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev. 22:2308–2341 10.1101/gad.1686208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward P., Brennan K., Sanders P., Balayo T., DasGupta R., Perrimon N., Martinez Arias A. 2005. Notch modulates Wnt signalling by associating with Armadillo/beta-catenin and regulating its transcriptional activity. Development. 132:1819–1830 10.1242/dev.01724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi Y., Itoh Y., Tabata H., Nakajima K., Akiyama T., Masuyama N., Gotoh Y. 2004. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 131:2791–2801 10.1242/dev.01165 [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Tamai Y., Zorn A.M., Yoshida H., Seldin M.F., Nishikawa S., Taketo M.M. 2001. Mouse Wnt receptor gene Fzd5 is essential for yolk sac and placental angiogenesis. Development. 128:25–33 [DOI] [PubMed] [Google Scholar]
- Kidd S., Lieber T., Young M.W. 1998. Ligand-induced cleavage and regulation of nuclear entry of Notch in Drosophila melanogaster embryos. Genes Dev. 12:3728–3740 10.1101/gad.12.23.3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.S., Skurk C., Thomas S.R., Bialik A., Suhara T., Kureishi Y., Birnbaum M., Keaney J.F., Jr., Walsh K. 2002. Regulation of angiogenesis by glycogen synthase kinase-3beta. J. Biol. Chem. 277:41888–41896 10.1074/jbc.M206657200 [DOI] [PubMed] [Google Scholar]
- Krebs L.T., Shutter J.R., Tanigaki K., Honjo T., Stark K.L., Gridley T. 2004. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev. 18:2469–2473 10.1101/gad.1239204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai E.C. 2004. Notch signaling: control of cell communication and cell fate. Development. 131:965–973 10.1242/dev.01074 [DOI] [PubMed] [Google Scholar]
- Lawson N.D., Scheer N., Pham V.N., Kim C.H., Chitnis A.B., Campos-Ortega J.A., Weinstein B.M. 2001. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 128:3675–3683 [DOI] [PubMed] [Google Scholar]
- Mayor C., Brudno M., Schwartz J.R., Poliakov A., Rubin E.M., Frazer K.A., Pachter L.S., Dubchak I. 2000. VISTA : visualizing global DNA sequence alignments of arbitrary length. Bioinformatics. 16:1046–1047 10.1093/bioinformatics/16.11.1046 [DOI] [PubMed] [Google Scholar]
- Monkley S.J., Delaney S.J., Pennisi D.J., Christiansen J.H., Wainwright B.J. 1996. Targeted disruption of the Wnt2 gene results in placentation defects. Development. 122:3343–3353 [DOI] [PubMed] [Google Scholar]
- Ng P., Graham F.L. 2002. Construction of first-generation adenoviral vectors. Methods Mol. Med. 69:389–414 [DOI] [PubMed] [Google Scholar]
- Nusse R. 2005. Wnt signaling in disease and in development. Cell Res. 15:28–32 10.1038/sj.cr.7290260 [DOI] [PubMed] [Google Scholar]
- Ohtsuka T., Ishibashi M., Gradwohl G., Nakanishi S., Guillemot F., Kageyama R. 1999. Hes1 and Hes5 as notch effectors in mammalian neuronal differentiation. EMBO J. 18:2196–2207 10.1093/emboj/18.8.2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsulic S., Peifer M. 1996. Cell-cell signalling: Wingless lands at last. Curr. Biol. 6:1363–1367 10.1016/S0960-9822(96)00731-2 [DOI] [PubMed] [Google Scholar]
- Radtke F., Clevers H. 2005. Self-renewal and cancer of the gut: two sides of a coin. Science. 307:1904–1909 10.1126/science.1104815 [DOI] [PubMed] [Google Scholar]
- Rommel C., Bodine S.C., Clarke B.A., Rossman R., Nunez L., Stitt T.N., Yancopoulos G.D., Glass D.J. 2001. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat. Cell Biol. 3:1009–1013 10.1038/ncb1101-1009 [DOI] [PubMed] [Google Scholar]
- Sakurai Y., Ohgimoto K., Kataoka Y., Yoshida N., Shibuya M. 2005. Essential role of Flk-1 (VEGF receptor 2) tyrosine residue 1173 in vasculogenesis in mice. Proc. Natl. Acad. Sci. USA. 102:1076–1081 10.1073/pnas.0404984102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder T., Meier-Stiegen F., Schwanbeck R., Eilken H., Nishikawa S., Häsler R., Schreiber S., Bornkamm G.W., Nishikawa S., Just U. 2006. Activated Notch1 alters differentiation of embryonic stem cells into mesodermal cell lineages at multiple stages of development. Mech. Dev. 123:570–579 10.1016/j.mod.2006.05.002 [DOI] [PubMed] [Google Scholar]
- Shimizu T., Kagawa T., Inoue T., Nonaka A., Takada S., Aburatani H., Taga T. 2008. Stabilized beta-catenin functions through TCF/LEF proteins and the Notch/RBP-Jkappa complex to promote proliferation and suppress differentiation of neural precursor cells. Mol. Cell. Biol. 28:7427–7441 10.1128/MCB.01962-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin M., Nagai H., Sheng G. 2009. Notch mediates Wnt and BMP signals in the early separation of smooth muscle progenitors and blood/endothelial common progenitors. Development. 136:595–603 10.1242/dev.026906 [DOI] [PubMed] [Google Scholar]
- Shiojima I., Walsh K. 2002. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ. Res. 90:1243–1250 10.1161/01.RES.0000022200.71892.9F [DOI] [PubMed] [Google Scholar]
- Sörensen I., Adams R.H., Gossler A. 2009. DLL1-mediated Notch activation regulates endothelial identity in mouse fetal arteries. Blood. 113:5680–5688 10.1182/blood-2008-08-174508 [DOI] [PubMed] [Google Scholar]
- Summers S.A., Kao A.W., Kohn A.D., Backus G.S., Roth R.A., Pessin J.E., Birnbaum M.J. 1999. The role of glycogen synthase kinase 3beta in insulin-stimulated glucose metabolism. J. Biol. Chem. 274:17934–17940 10.1074/jbc.274.25.17934 [DOI] [PubMed] [Google Scholar]
- Tachibana K., Hirota S., Iizasa H., Yoshida H., Kawabata K., Kataoka Y., Kitamura Y., Matsushima K., Yoshida N., Nishikawa S., et al. 1998. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 393:591–594 10.1038/31261 [DOI] [PubMed] [Google Scholar]
- Thompson J.D., Higgins D.G., Gibson T.J. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680 10.1093/nar/22.22.4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering M., Sancho E., Verweij C., de Lau W., Oving I., Hurlstone A., van der Horn K., Batlle E., Coudreuse D., Haramis A.P., et al. 2002. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 111:241–250 10.1016/S0092-8674(02)01014-0 [DOI] [PubMed] [Google Scholar]
- van Es J.H., van Gijn M.E., Riccio O., van den Born M., Vooijs M., Begthel H., Cozijnsen M., Robine S., Winton D.J., Radtke F., Clevers H. 2005. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 435:959–963 10.1038/nature03659 [DOI] [PubMed] [Google Scholar]
- Villa N., Walker L., Lindsell C.E., Gasson J., Iruela-Arispe M.L., Weinmaster G. 2001. Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mech. Dev. 108:161–164 10.1016/S0925-4773(01)00469-5 [DOI] [PubMed] [Google Scholar]
- Wang H.U., Chen Z.F., Anderson D.J. 1998. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 93:741–753 10.1016/S0092-8674(00)81436-1 [DOI] [PubMed] [Google Scholar]
- Xue Y., Gao X., Lindsell C.E., Norton C.R., Chang B., Hicks C., Gendron-Maguire M., Rand E.B., Weinmaster G., Gridley T. 1999. Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum. Mol. Genet. 8:723–730 10.1093/hmg/8.5.723 [DOI] [PubMed] [Google Scholar]
- Yamamizu K., Kawasaki K., Katayama S., Watabe T., Yamashita J.K. 2009. Enhancement of vascular progenitor potential by protein kinase A through dual induction of Flk-1 and Neuropilin-1. Blood. 114:3707–3716 10.1182/blood-2008-12-195750 [DOI] [PubMed] [Google Scholar]
- Yamashita J., Itoh H., Hirashima M., Ogawa M., Nishikawa S., Yurugi T., Naito M., Nakao K., Nishikawa S. 2000. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 408:92–96 10.1038/35040568 [DOI] [PubMed] [Google Scholar]
- Yamashita J.K., Takano M., Hiraoka-Kanie M., Shimazu C., Peishi Y., Yanagi K., Nakano A., Inoue E., Kita F., Nishikawa S.I. 2005. Prospective identification of cardiac progenitors by a novel single cell-based cardiomyocyte induction. FASEB J. 19:1534–1536 [DOI] [PubMed] [Google Scholar]
- Yurugi-Kobayashi T., Itoh H., Schroeder T., Nakano A., Narazaki G., Kita F., Yanagi K., Hiraoka-Kanie M., Inoue E., Ara T., et al. 2006. Adrenomedullin/cyclic AMP pathway induces Notch activation and differentiation of arterial endothelial cells from vascular progenitors. Arterioscler. Thromb. Vasc. Biol. 26:1977–1984 10.1161/01.ATV.0000234978.10658.41 [DOI] [PubMed] [Google Scholar]
- Zhong T.P., Childs S., Leu J.P., Fishman M.C. 2001. Gridlock signalling pathway fashions the first embryonic artery. Nature. 414:216–220 10.1038/35102599 [DOI] [PubMed] [Google Scholar]