A biosensor for cyclin B–Cdk1 activity shows that it uses an unconventional yet simple mechanism for nuclear accumulation.
Abstract
The cyclin B–Cdk1 kinase triggers mitosis in most eukaryotes. In animal cells, cyclin B shuttles between the nucleus and cytoplasm in interphase before rapidly accumulating in the nucleus at prophase, which promotes disassembly of the nuclear lamina and nuclear envelope breakdown (NEBD). What triggers the nuclear accumulation of cyclin B1 is presently unclear, although the prevailing view is that the Plk1 kinase inhibits its nuclear export. In this study, we use a biosensor specific for cyclin B1–Cdk1 activity to show that activating cyclin B1–Cdk1 immediately triggers its rapid accumulation in the nucleus through a 40-fold increase in nuclear import that remains dependent on Cdk1 activity until NEBD. Nevertheless, a substantial proportion of cyclin B1–Cdk1 remains in the cytoplasm. The increase in nuclear import is driven by changes in the nuclear import machinery that require neither Plk1 nor inhibition of nuclear export. Thus, the intrinsic link between cyclin B1–Cdk1 activation and its rapid nuclear import inherently coordinates the reorganization of the nucleus and the cytoplasm at mitotic entry.
Introduction
Mitosis in most animal cells is thought to be triggered by the activation of the cyclin B1–associated kinase, Cdk1 (for review see Lindqvist et al., 2009). Once activated, cyclin B1–Cdk1 can phosphorylate a plethora of substrates, thereby promoting the substantial reorganization of the cell architecture upon which mitosis and cytokinesis depend. These substrates are both nuclear and cytoplasmic, including components of the cytoskeleton microtubule (Andersen et al., 1997; Vasquez et al., 1999; Liakopoulos et al., 2003; Moore and Miller, 2007), actin (Yamashiro et al., 1991; Yamashiro et al., 2001) and intermediate filament networks (Chou et al., 1990; Yamaguchi et al., 2005), caspases (Allan and Clarke, 2007), the Golgi apparatus (Lowe et al., 1998; Draviam et al., 2001; Wang et al., 2003; Preisinger et al., 2005), the nucleolus (Peter et al., 1990a; Klein and Grummt, 1999; Sirri et al., 2002), nuclear lamins (Peter et al., 1990b; Lüscher et al., 1991), nuclear pore complexes (Onischenko et al., 2005; Lusk et al., 2007; for review see Kutay and Hetzer, 2008), and the anaphase-promoting complex/cyclosome (the E3 ligase that eventually degrades cyclin B1 in mitosis; Rudner and Murray, 2000; Kraft et al., 2003). Thus, Cdk1 has been described as the workhorse of the mitotic cell (Nigg, 1991). These substrates are crucial to the generation of the mitotic spindle, chromosome condensation, and nuclear envelope breakdown (NEBD) that characterize mitosis in most animal cells. Yet, despite its importance, the means by which cyclin B1–Cdk1 coordinates the dramatic changes in the nucleus and the cytoplasm remains unknown.
One mechanism that is likely to be important to coordinate entry to mitosis is that cyclin B1–Cdk1 is rapidly imported into the nucleus just before NEBD (Pines and Hunter, 1991; Ookata et al., 1992), but how this is triggered and how it relates to the activation of cyclin B1–Cdk1 is unknown. In interphase, cyclin B1 shuttles between the nucleus and the cytoplasm but is mostly cytoplasmic because its rate of nuclear export exceeds its rate of import (Hagting et al., 1998; Toyoshima et al., 1998; Yang et al., 1998). The nuclear export sequence (NES) binds to the exportin Crm1 (Yang et al., 1998) and is located in the N terminus of cyclin B1, where it is surrounded by phosphorylation sites, including sites for the Plk1 kinase, either in the NES on Ser147 (Toyoshima-Morimoto et al., 2001) or nearby on Ser133 (Hagting et al., 1999; Yuan et al., 2002; Jackman et al., 2003). Substituting the Ser in the NES with glutamic acid reduces cyclin B1 nuclear export in Xenopus laevis oocytes and impairs its ability to bind to Crm1 in vitro (Yang et al., 2001). Thus, the prevailing view is that the sudden nuclear accumulation of cyclin B1 is caused by Plk1 phosphorylating cyclin B1 at Ser147 in the NES and inhibiting its export (Toyoshima-Morimoto et al., 2001). This implicates Plk1 as the key regulator to coordinate the reorganization of the nucleus and the cytoplasm at mitosis. However, in conflict with this, we and others have shown that Plk1 phosphorylates cyclin B1 on Ser133 not Ser147 (Yuan et al., 2002; Jackman et al., 2003) and that overexpressing Plk1 does not cause cyclin B1 to move into the nucleus in interphase (Jackman et al., 2003). Similarly, Lénárt et al. (2007) found that a Plk1 inhibitor did not block the nuclear import of cyclin B1 at mitosis.
We previously showed that cyclin B1–Cdk1 autophosphorylates two sites in its own N terminus (Ser126 and 128) that could be detected first at centrosomes, indicating that cyclin B1–Cdk1 is activated in the cytoplasm (Jackman et al., 2003). Mutating all of these phosphorylation sites and both putative Plk1 phosphorylation sites in the N terminus to glutamic acid enhanced the nuclear import of cyclin B1–GFP in vitro and in interphase cells (Hagting et al., 1999; Yang et al., 2001), whereas mutating them to Ala appeared to delay the timing of cyclin B1 import in prophase. Thus, we suggested that the rapid accumulation of cyclin B1 into the nucleus at prophase was caused by a phosphorylation-dependent nuclear import signal in its N terminus triggered by a combination of cyclin B1–Cdk1 and Plk1 kinase activities (Hagting et al., 1999), but the temporal relationship between the activation of cyclin B1–Cdk1 and its nuclear import, and the role of Plk1 and the inhibition of nuclear export remained unresolved.
We have recently developed a biosensor that can be used to measure specifically cyclin B1–Cdk1 activity in living cells. In this study, we use this probe to show that as soon as cyclin B1–Cdk1 is activated, it rapidly accumulates in the nucleus, and this import depends on continual cyclin B1–Cdk1 activity but is independent of the Plk1 kinase. Furthermore, we show that the change in cyclin B1 localization is generated almost exclusively by a strong increase in import rate through a general change in nuclear import machinery and not through generating a nuclear import signal on cyclin B1. Furthermore, we show that a substantial amount of cyclin B1–Cdk1 remains in the cytoplasm even at the peak of its nuclear accumulation just before NEBD. Thus, through the coupling of cyclin B1–Cdk1 activity to its nuclear import, we strengthen the evidence that cyclin B1–Cdk1 must be activated in the cytoplasm and provide an elegantly simple molecular mechanism for how nuclear and cytoplasmic events are coordinated at the entry to mitosis.
Results
Cyclin B1–Cdk1 activity drives its nuclear import
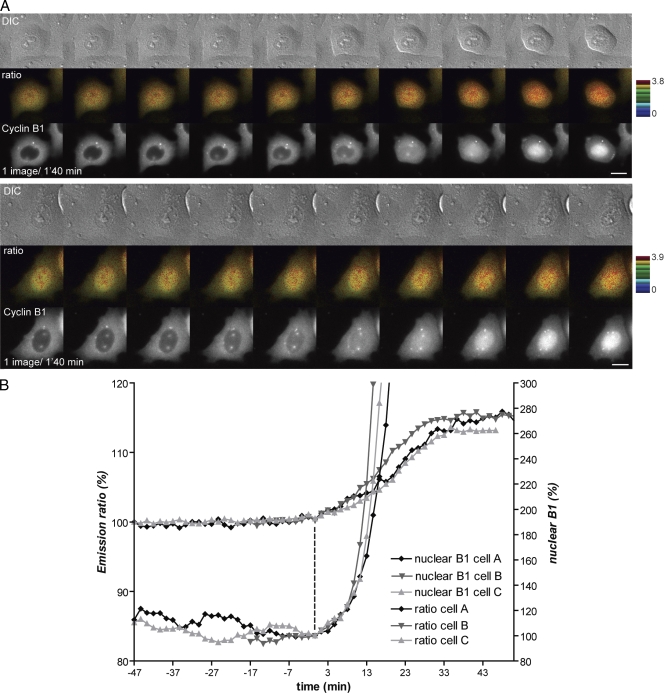
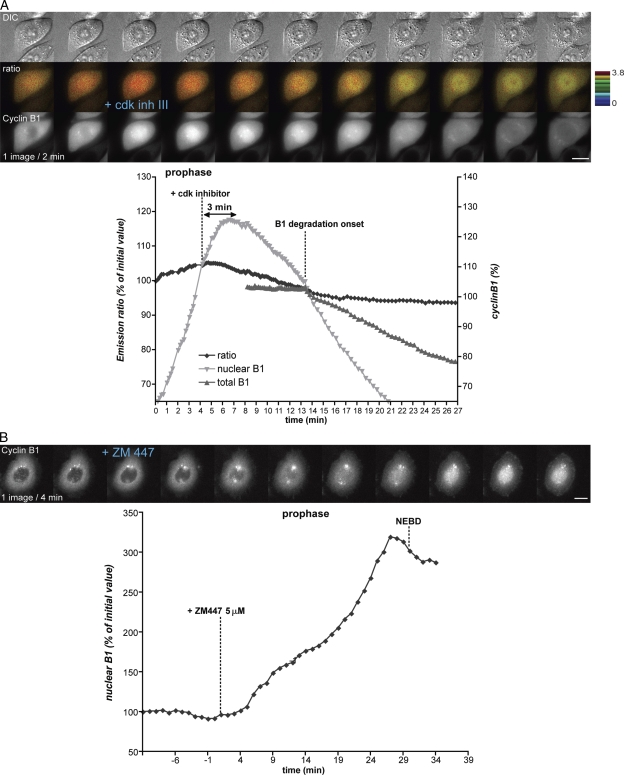
We recently developed a biosensor to detect cyclin B1–Cdk1 activity in living cells (Gavet and Pines, 2010). This biosensor is specifically phosphorylated by cyclin B1–Cdk1 and not by cyclin A– or cyclin E–Cdk complexes, and upon phosphorylation, exhibits an increase in Förster resonance energy transfer (FRET) between its constituent cyan and yellow fluorescent proteins. With this biosensor, we found that cyclin B1–Cdk1 was activated ∼27 min before NEBD in HeLa and ∼18 min before NEBD in retinal pigment epithelial cells. In both cell types, activation was immediately followed by centrosome separation and cell rounding (Gavet and Pines, 2010). Because cyclin B1 accumulated in the nucleus before NEBD, we investigated the relation between cyclin B1–Cdk1 activation and its nuclear transport in prophase. Using HeLa cells expressing the cyclin B1–Cdk1 biosensor and a fluorescently tagged cyclin B1, cyclin B1–mCherry, we found an excellent correlation between the initial activation of cyclin B1–Cdk1 and its rapid nuclear import (Fig. 1 and Video 1). Cyclin B1 began to accumulate in the nucleus an average of 30 s after the FRET signal began to increase (27 ± 7 min before NEBD; n = 18). This result indicated that cyclin B1–Cdk1 activation might trigger its nuclear accumulation; therefore, to test this hypothesis, we simultaneously recorded the FRET ratio and the nuclear accumulation of cyclin B1 in early prophase and added a potent Cdk1 inhibitor (Cdk1/2 inhibitor III) a few minutes after cyclin B1 began to move into the nucleus. We observed an immediate reduction in cyclin B1 import rate followed by its export from the nucleus (Fig. 2 A and Video 2). Correlating this with cyclin B1–Cdk1 activity showed that the FRET signal started to decrease 0.5–1 min after adding the drug and was closely followed by the nuclear export of cyclin B1 ∼1–2 min later (n = 6). Adding the inhibitor even in late prophase triggered cyclin B1 export and completely inhibited NEBD and entry to mitosis. We also observed that the total level of cyclin B1–mCherry fluorescence started to decrease ∼9–10 min after adding the Cdk inhibitor, most likely as a consequence of activating the anaphase-promoting complex/cyclosome (Di Fiore and Pines, 2008). Identical results were obtained using another Cdk inhibitor (RO3306; unpublished data). As a control, we tested the effect of adding an aurora B inhibitor (ZM447439), and this had no effect on the nuclear accumulation of cyclin B1 (Fig. 2 B). Thus, we conclude that cyclin B1–Cdk1 activation triggers its own nuclear import in prophase.
Figure 1.
Activation and nuclear accumulation of cyclin B1–Cdk1 are coincident. (A) HeLa cells coexpressing the cyclin B1–Cdk1 activity FRET sensor and cyclin B1–mCherry were recorded at one image every 1 min 40 s by time-lapse fluorescence and differential interference contrast (DIC) microscopy as cells entered mitosis. Two cells entering mitosis are displayed for DIC (top), FRET efficiency determined by emission ratio (middle), and cyclin B1–mCherry (bottom). Bars, 10 µm. (B) Quantification of the nuclear accumulation of cyclin B1 and emission ratio in three different cells. The vertical dashed line indicates when signals started to increase.
Figure 2.
Nuclear accumulation of cyclin B1–Cdk1 depends on Cdk activity. (A) HeLa cells coexpressing the cyclin B1–Cdk1 activity FRET sensor and cyclin B1–mCherry were recorded at one image every 2 min as cells entered mitosis. After cyclin B1 began to move into the nucleus, 300 nM Cdk1/2 inhibitor III was added. The quantification curves correspond to the cell displayed. Note the immediate decrease in the nuclear accumulation rate of cyclin B1 just after addition of the Cdk inhibitor before its reexport ∼3 min later. (B) Cells analyzed as in A were treated with 5 µM aurora B inhibitor ZM447439 in late G2 phase (n = 6). Bars, 10 µm.
Cyclin B1–Cdk1 is active in both the nucleus and the cytoplasm in prophase
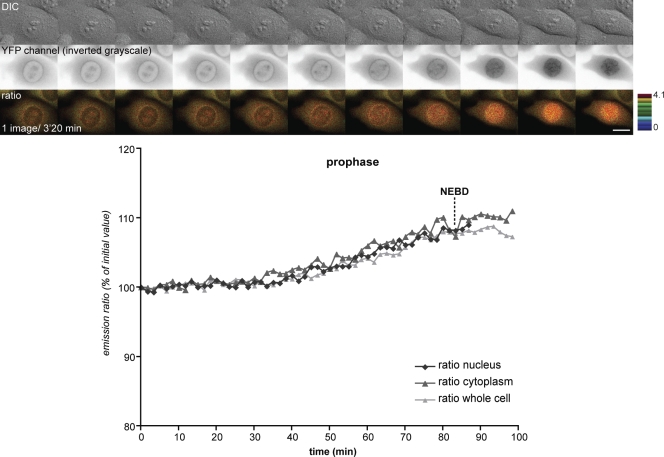
Because activating cyclin B1–Cdk1 immediately triggered its import into the nucleus, it was possible that this would deplete active cyclin B1–Cdk1 from the cytoplasm until NEBD. To test this, we compared the activation of cyclin B1–Cdk1 in the cytoplasm and the nucleus using modified versions of the cyclin B1–Cdk1 biosensor. We targeted the biosensor to the cytoplasm by tagging it with an NES and targeted it to chromosomes by fusing it with histone H2B (Fig. S1 A). Fluorescence loss in photobleaching and FRAP assays confirmed that the H2B fusion protein was stably attached to chromosomes and did not diffuse into the cytoplasm (Fig. S1 B and not depicted). Monitoring dividing cells by time-lapse microscopy showed that the change in FRET efficiency of the biosensor was very similar between the cytoplasm and the nucleus in all cells analyzed, indicating that cyclin B1–Cdk1 became progressively more active in both the cytoplasm and the nucleus as cells entered mitosis (n = 16 for three independent experiments; Fig. 3 and Video 3). These results agree with the conclusions of Lindqvist et al. (2007), who found by immunofluorescence analysis that some active cyclin B1–Cdk1 remains in the cytoplasm in prophase, whereas the rest moves into the nucleus. Thus, activation in the cytoplasm allied with the immediate and rapid nuclear import of a proportion of cyclin B1–Cdk1 inherently synchronizes mitotic events in the cytoplasm and the nucleus.
Figure 3.
Active cyclin B1–Cdk1 kinase is located both in the cytoplasm and nucleus during prophase. HeLa cells coexpressing cytoplasmic and nuclear targeted cyclin B1–Cdk1 biosensors (fused to an NES and histone H2B, respectively) were recorded as cells entered mitosis (one image every 1 min 40 s). DIC (top), YFP emission (inverted grayscale; middle), and emission ratio (bottom) images are shown. Quantifications of the emission ratio in the whole cell, the nucleus, and the cytoplasm correspond to the cell displayed. Bar, 10 µm.
Cyclin B1–Cdk1 accumulation in the nucleus does not require Plk1 activity or phosphorylation on cyclin B1
The coupling between cyclin B1–Cdk1 activation and its rapid import into the nucleus appeared to be the key to synchronizing nuclear and cytoplasmic changes in cell architecture as cells entered mitosis, but the mechanism behind this was unclear. Our previous results indicated that it was caused by an increase in nuclear import generated by phosphorylation of the N terminus of cyclin B1 (Hagting et al., 1999), but other studies had concluded that phosphorylation, particularly phosphorylation by Plk1, inhibited the export of cyclin B1 (Yang et al., 1998; Toyoshima-Morimoto et al., 2001).
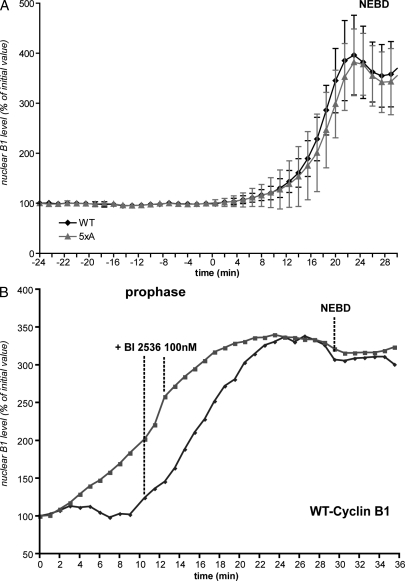
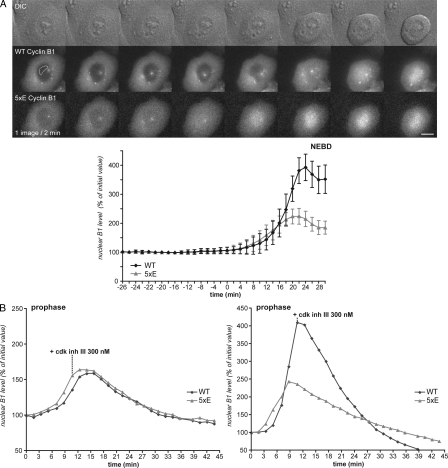
To establish the mechanism, we first addressed the importance of phosphorylation in the N terminus of cyclin B1. We compared the nuclear import in prophase of wild-type cyclin B1 with mutants carrying mutations to Ala in the autophosphorylation sites (Ser126 and 128), the consensus Plk1 site (Ser133), the NES (Ser147), or in all sites together (Ser116, 126, 128, 133, and 147). We first determined that the rate of cyclin B1–GFP import was not affected by the level of its expression (Fig. S2). When coexpressed in HeLa cells, the nuclear import in prophase of all the cyclin B1 mutants linked to EGFP was indistinguishable in timing or rate from wild-type cyclin B1–mCherry (Fig. 4 A, Fig. S3, and Video 4). Thus, neither autophosphorylation nor phosphorylation by Plk1 was required to trigger the nuclear accumulation of cyclin B1 in prophase. However, it was possible that Plk1 could trigger the nuclear accumulation of cyclin B1 by a mechanism separate from phosphorylating cyclin B1. Therefore, we tested the consequence of adding a potent Plk1 inhibitor (BI 2536) to cells in prophase. As shown in Fig. 4 B, inhibiting Plk1 had no effect on the nuclear accumulation of cyclin B1 (Fig. 4 B and Video 5), which is in agreement with a previous study (Lénárt et al., 2007). The potency of the inhibitor was confirmed because all cells were subsequently blocked in prometaphase after NEBD (Lénárt et al., 2007), and those cells in anaphase immediately failed cytokinesis when the inhibitor was added (Video 5; Burkard et al., 2007; Petronczki et al., 2007). Thus, we can conclude that Plk1 is not required to activate cyclin B1–Cdk1, for the nuclear import of cyclin B1, nor for the synchrony of nuclear and cytoplasmic changes in prophase.
Figure 4.
Nuclear accumulation of cyclin B1 in prophase is independent of its phosphorylation and of Plk1 activity. (A) Cells coexpressing wild-type (WT) cyclin B1–mCherry and 5xA (Ser116, 126,128,133, and 147A)–cyclin B1–GFP were assayed, and the nuclear accumulation of the proteins was quantified. Mean curves of quantifications in different cells are displayed (n = 5). (B) Cells expressing wild-type cyclin B1–mCherry were assayed, and100 nM Plk1 inhibitor (BI 2536) was added during the nuclear import of cyclin B1 in prophase. Two examples are displayed (one image/minute). Error bars show SEM.
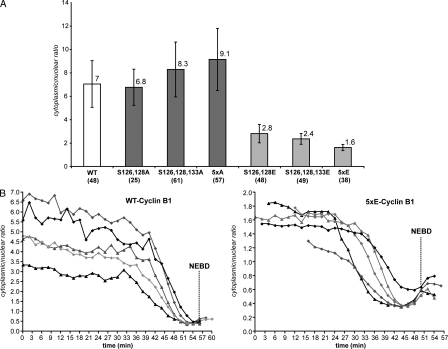
Previously, we and others have shown that substituting glutamic acids at the phosphorylation sites in the N terminus of cyclin B1 created a nuclear import signal and reduced the binding between cyclin B1 and the exportin Crm1 in vitro (Hagting et al., 1999; Yang et al., 2001). Therefore, we compared the behavior of these mutants with wild-type cyclin B1 through the cell cycle. First, we quantified optical sections obtained with a spinning-disk confocal microscope to measure their distribution between the cytoplasm and the nucleus in interphase. This showed that substitution with glutamic acids progressively displaced the equilibrium toward the nucleus, whereas replacing the Ser with Ala shifted the equilibrium toward the cytoplasm (Fig. 5 A).
Figure 5.
The nuclear/cytoplasmic distribution of cyclin B1 is only affected by phosphorylation in its N terminus domain in interphase. (A) The nuclear/cytoplasmic ratio of wild-type (WT) cyclin B1–GFP, Ser126– and 128A–cyclin B1–GFP, Ser126–,128A–, and 133A–cyclin B1–GFP, 5xA (Ser116,126,128,133, and 147A)–cyclin B1–GFP, Ser126– and 128E–cyclin B1–GFP, Ser126–,128A–, and 133E–cyclin B1–GFP, and 5xE (Ser116,126,128,133, and 147E) cyclin B1–GFP was quantified on optical sections of asynchronous interphase HeLa cells. Mean values ± SEM and numbers of cells assayed are displayed. (B) Cells expressing either wild-type or 5xE cyclin B1–GFP were recorded. Real time changes in the nuclear/cytoplasmic ratio were quantified on optical sections as cells entered mitosis. Five examples are displayed for each experiment.
Next, we measured the change in distribution between the cytoplasm and nucleus of 5xE and wild-type cyclin B1 during prophase (Fig. 6 A). We observed that the timing and rate of nuclear accumulation were the same, although the degree of nuclear accumulation of the 5xE mutant was only ∼60% of wild-type cyclin B1 (n = 11; Fig. 6 A). This might be a direct consequence of the difference in their equilibrium distribution between the cytoplasm and the nucleus in interphase (Fig. 5 A). Therefore, we measured the changes in the distribution between the cytoplasm and nucleus of wild-type and 5xE cyclin B1 on optical sections of cells progressing from G2 phase to mitosis (Fig. 5 B). Wild-type cyclin B1 changed from approximately five times more concentrated in the cytoplasm in late G2 phase to about two times more concentrated in the nucleus just before NEBD (cytoplasm/nucleus, ∼0.4 ± 0.06; n = 6; Fig. 5 B, left). Thus, the cytoplasm still contained substantial levels of cyclin B1 at the end of prophase. Notably, the 5xE mutant cyclin B1 reached the same distribution just before NEBD (cytoplasm/nucleus, ∼0.45 ± 0.07; n = 5; Fig. 5 B, right). Thus, we conclude that phosphorylation of the N-terminal region of cyclin B1 modulates its interaction with the import machinery, but this effect is only significant in interphase.
Figure 6.
The nuclear accumulation of wild-type and 5xE mutant of cyclin B1 in prophase is concurrent. (A) The nuclear accumulation of cyclin B1 in prophase was assayed in cells coexpressing wild-type (WT) cyclin B1–mCherry and 5xE cyclin B1–GFP. DIC (top), mCherry (middle), and GFP (bottom) images are shown. The region of interest used for quantification of the nuclear signal is displayed. Mean curves of quantifications in different cells are displayed (n = 6). Bar, 10 µm. (B) Cells were assayed as in A, and 300 nM Cdk inhibitor (Cdk1/2 inhibitor III) was added during prophase. Two examples are displayed. Error bars show SEM.
Cyclin B1–Cdk1 activation stimulates its nuclear import rate
A previous study had reported that the nuclear accumulation of cyclin B1 was driven by phosphorylation in the NES at Ser147, inhibiting its nuclear export (Toyoshima-Morimoto et al., 2001). Therefore, we tested whether substituting Ser147 with glutamic acid inhibited cyclin B1 export at prophase. We added a Cdk inhibitor, as wild-type or the 5xE mutant cyclin B1 began to accumulate in the nucleus in prophase and observed that both forms of cyclin B1 were rapidly reexported and returned to the original equilibrium distribution with the same timing, provided that at the beginning of the experiment they had accumulated in the nucleus to a similar extent (Fig. 6 B, left). Wild-type cyclin B1 was exported more rapidly if its relative accumulation in the nucleus was higher than the 5xE mutant at the end of prophase (Fig. 6 B, right; and Video 6). Thus, the introduction of glutamic acids into the N terminus of cyclin B1 did not inhibit its nuclear export.
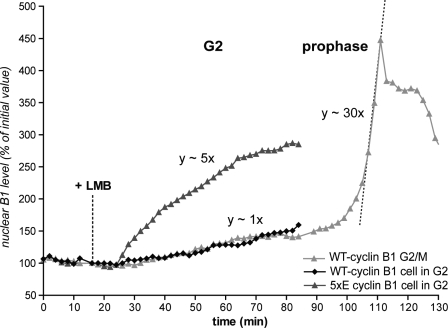
To determine whether cyclin B1–Cdk1 activation in prophase stimulated its nuclear import, inhibited its export, or both, we inhibited the Crm1 exportin with leptomycin B (LMB). In agreement with our previous results (Hagting et al., 1999), LMB induced a slow and progressive nuclear accumulation of wild-type cyclin B1 in G2 phase cells (Fig. 7 and Fig. S4), whereas under the same conditions, the 5xE mutant was imported about five times faster than wild type. This explained the difference observed in their relative nuclear/cytoplasmic ratios in interphase (Fig. 5 A). However, when cells treated with LMB reached prophase, the nuclear import rate of both wild-type and 5xE cyclin B1 suddenly increased by up to 40-fold (Fig. 7 and Fig. S4). The timing, rate, and extent of nuclear accumulation of cyclin B1 during prophase were very similar in the presence or absence of LMB (control cells, n = 26: increase in rate, 20–54-fold; mean, 38-fold; LMB-treated cells, n = 9: increase in rate, 24–55-fold; mean, 39-fold; Fig. S4). Thus, the nuclear accumulation of cyclin B1 in prophase is almost exclusively caused by a strong stimulation of its import rate.
Figure 7.
Cyclin B1 nuclear import rate increases significantly at mitotic entry. Cells expressing either wild-type (WT) or 5xE cyclin B1–GFP were recorded, and the nuclear import of wild-type and 5xE cyclin B1 were quantified after treating G2 phase cells with 20 nM LMB to inhibit nuclear export. Note the sudden and strong increase of the wild-type cyclin B1 nuclear import rate when the cell enters mitosis.
Cyclin B1–Cdk1 activation regulates the nuclear transport machinery
Because the rapid rise in nuclear import of cyclin B1 in prophase was not caused by Plk1 or by phosphorylation of cyclin B1, we first investigated whether active cyclin B1–Cdk1 altered soluble import factors in prophase. Cyclin B1 binds directly to the N terminus of importin β1 (residues 1–462; Moore et al., 1999), and this domain contains a unique S/T-P sequence (Ser12) that is phosphorylated in mitotic cell extracts (Dephoure et al., 2008). Therefore, we asked whether this phosphorylation might induce the nuclear import of cyclin B1–Cdk1, but overexpressing wild-type importin β1 or mutants with this site changed to Ala or Glu did not alter the nuclear/cytoplasmic distribution of cyclin B1 in interphase (Fig. S5).
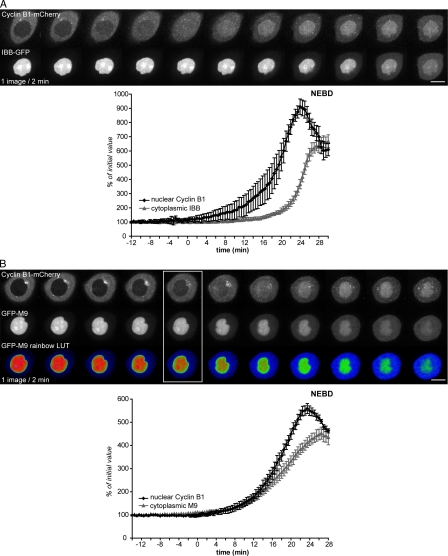
Several proteins of the nuclear pore complex are phosphorylated by cyclin B1–Cdk1 (Macaulay et al., 1995; Blethrow et al., 2008; for review see Kutay and Hetzer, 2008), making us consider the possibility that active cyclin B1–Cdk1 might modify the permeability (i.e., size exclusion limit) and/or functional properties of the nuclear pores. To assay this, we used two different markers: an importin β1–binding domain of importin α (IBB) and a Gly-rich sequence recognized by importin β2 (M9), both fused to GFP (Lénárt et al., 2003). We coexpressed IBB-GFP or GFP-M9 with wild-type cyclin B1–mCherry, assayed their localization by spinning-disk confocal microscopy, and quantified optical sections of cells as they entered mitosis. As shown in Fig. 8 A, IBB-GFP began to move into the cytoplasm in late prophase ∼10 min before NEBD, most likely reflecting the increase in the size exclusion limit of nuclear pores (Lénárt et al., 2003), whereas cyclin B1 started to accumulate in the nucleus several minutes before this (cyclin B1, mean of 21 ± 5 min before NEBD; IBB, mean of 10 ± 1.5 min; n = 7; Video 7). Surprisingly, we observed a very similar timing between cyclin B1 accumulation in the nucleus and GFP-M9 export to the cytoplasm, although on average, cyclin B1 began to accumulate in the nucleus ∼3 min before M9 began to move out (cyclin B1, mean of 26.5 ± 2 min before NEBD; M9, 24 ± 3 min before NEBD; n = 6; Fig. 8 B and Video 8).
Figure 8.
The nuclear accumulation of cyclin B1–Cdk1 in prophase correlates with a change in nuclear transport machinery. (A and B) Cells coexpressing cyclin B1–mCherry and IBB-GFP (A) or GFP-M9 (B) were recorded as they entered mitosis (one image/30 s), and the import of cyclin B1 and export of IBB or M9 quantified on optical sections. (A) The mCherry (top) and GFP (bottom) images and mean curves of quantification of nuclear cyclin B1 and cytoplasmic IBB are shown. (B) mCherry (top), GFP (middle), and pseudo-colored GFP (rainbow look up table [LUT]; bottom) images and mean curves of quantification of the nuclear cyclin B1 and cytoplasmic M9 are displayed. Boxed images show the time point when cyclin B1 begins to enter the nucleus and GFP-M9 begins to exit. Error bars show SEM. Bars, 10 µm.
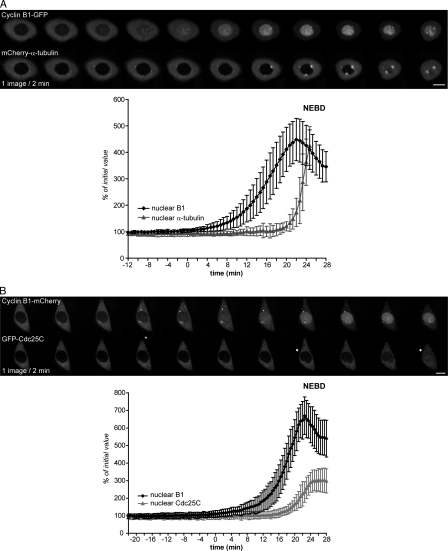
To confirm that the nuclear import of cyclin B1 was not caused by passive diffusion after partial disassembly of nuclear pores or rupture of the nuclear envelope, we coexpressed wild-type cyclin B1–GFP (cyclin B1–Cdk1–GFP complex, molecular weight of ∼100) with mCherry–α-tubulin (mCherry-α–β-tubulin complex, molecular weight of ∼120) and recorded their nuclear accumulation by spinning-disk confocal microscopy. We observed that in a similar manner to IBB-GFP, mCherry–α-tubulin began to move into the nucleus ∼10 min before NEBD, whereas cyclin B1 started to be imported several minutes before this (cyclin B1, mean of 24 ± 4 min before NEBD; α-tubulin, average of 6 ± 2 min before NEBD; n = 10; Fig. 9 A and Video 9). Thus, our results strongly indicate that cyclin B1–Cdk1 activity alters the nuclear transport machinery early in prophase, possibly through the modification of functional properties of nuclear pores, thereby triggering the nuclear import of cyclin B1.
Figure 9.
Cyclin B1 but not Cdc25C moves into the nucleus before the nuclear pores become permeable to tubulin. (A and B) Cells coexpressing cyclin B1–GFP and mCherry–α-tubulin (A) or cyclin B1–mCherry and GFP-Cdc25C (B) were recorded as they entered mitosis (one image/40 s and one image/30 s, respectively), and the import of cyclin B1, α-tubulin, and Cdc25C was quantified on optical sections. (A) The GFP (top) and mCherry (bottom) images and mean curves of quantification of nuclear cyclin B1 and α-tubulin are shown. (B) mCherry (top) and GFP (bottom) images and mean curves of quantification of the nuclear cyclin B1 and Cdc25C are displayed. Error bars show SEM. Bars, 10 µm.
Cdc25C is not imported in the nucleus until late prophase
Because we had evidence that the nuclear transport machinery might be altered when cyclin B1–Cdk1 was activated, we tested whether this might alter the nuclear/cytoplasmic distribution of other mitotic regulators. In particular, we assayed the intracellular localization of Cdc25C because it had previously been reported to move into the nucleus in prophase (Toyoshima-Morimoto et al., 2002). We coexpressed cyclin B1–mCherry with GFP-Cdc25C (Karlsson et al., 1999) and assayed their intracellular localization during prophase. We observed that the behavior of GFP-Cdc25C was indistinguishable from mCherry–α-tubulin or IBB-GFP and that the protein started to be imported in the nucleus in late prophase (Cdc25C, mean of 8.5 ± 2 min before NEBD; compare with cyclin B1, average of 23 ± 4 min before NEBD; n = 9; Fig. 9 B and Video 10). Similar results were obtained with Cdc25C-GFP (unpublished data). Thus, Cdc25C enters the nucleus several minutes after cyclin B1, probably as a consequence of the increase in permeability as nuclear pores disassemble (Lénárt and Ellenberg, 2003).
Discussion
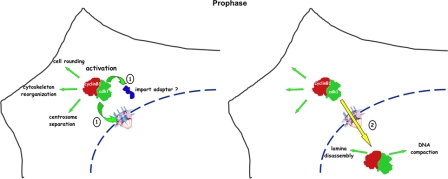
In this study, we have used a biosensor specific for cyclin B1–Cdk1 to show that as soon as it is activated, cyclin B1–Cdk1 immediately triggers its nuclear import, making this an excellent surrogate marker for cyclin B1 activation. Moreover, as an inherent characteristic of its activation, cyclin B1–Cdk1 kinase can coordinate changes in the architecture of the cell in both compartments as cells enter mitosis (Fig. 10). The rapid nuclear accumulation of cyclin B1–Cdk1 in prophase can be achieved exclusively by a change in its rate of import and regardless of whether Plk1 is active. Moreover, import in prophase appears to be caused by a change in the nuclear transport machinery rather than modification of the N terminus of cyclin B1 itself. This change in the nuclear transport machinery eventually changes the equilibrium between nuclear and cytoplasmic cyclin B1 such that it is consistently 2:1 just before NEBD.
Figure 10.
Model showing how activating cyclin B1–Cdk1 synchronizes the cytoplasm with the nucleus. The activation (1) of cyclin B1–Cdk1 kinase synchronizes cytoplasmic (left) and nuclear (right) events through triggering its own nuclear import (2) by modifying nuclear import adapters, the functional properties of nuclear pores, or both.
Cyclin B1–Cdk1 is first activated in the cytoplasm
One strong implication of our work is that cyclin B1–Cdk1 must first be activated in the cytoplasm and not the nucleus in mammalian cells. Were it to be activated only in the nucleus, it is difficult to see how any cyclin B1–Cdk1 activity could reach the cytoplasm until NEBD because activation induces an ∼40-fold increase in the rate of cyclin B1–Cdk1 nuclear import. In agreement with this, we observed that cytoplasmic events such as cell rounding and centrosome separation follow almost immediately after, and remain dependent on, cyclin B1–Cdk1 activation (Gavet and Pines, 2010). Moreover, the nuclear accumulation of cyclin B1 is not affected by inhibiting its export. In further support of this conclusion, antiphospho antibodies that only recognize active cyclin B1–Cdk1 first stain centrosomes in mammalian cells (Jackman et al., 2003), and cyclin B1–Cdk1 can be activated in the absence of nuclei in X. laevis oocytes (Hara et al., 1980; Pérez-Mongiovi et al., 2000). Thus, activation in the cytoplasm allied with the immediate and rapid nuclear import of a proportion but not all of cyclin B1–Cdk1 means that activating cyclin B1–Cdk1 inherently synchronizes mitotic events in the cytoplasm and nucleus.
Our finding that cyclin B1–Cdk1 nuclear import remains dependent on cyclin B1–Cdk1 activity until NEBD also means that any enzyme repressing its activity, such as Chk1, Wee1, or Myt1, would also prevent cyclin B1–Cdk1 import. It has been suggested that inhibiting cyclin B1–Cdk1 specifically in the nucleus or blocking its nuclear import could generate a cell in which cyclin B1–Cdk1 is only active in the cytoplasm. Three ways by which this has been reported to happen are (1) through activating the Chfr tumor suppressor, (2) overexpressing Wee1 in human cells, and (3) when the Grapes/Chk1 kinase is activated in Drosophila melanogaster syncitial embryos (Heald et al., 1993; Summers et al., 2005; Royou et al., 2008). Our results tend to preclude all three mechanisms.
(1) In the study on the effect of Chfr, cyclin B1–Cdk1 itself was not activated (Summers et al., 2005), and our results now show that there is, therefore, no need to postulate an extra effect on cyclin B1 import because activation and import are coupled. (2) We have measured the effect of overexpressing Wee1 using targeted FRET biosensors to monitor cyclin B1–Cdk1 activity in the nucleus and the cytoplasm but find that we cannot uncouple the activities in the two compartments, which is the result predicted by our finding that active cytoplasmic cyclin B1–Cdk1 enhances its nuclear import and that nuclear export is not inhibited during prophase. (3) A similar argument applies to the effect of Chk1, at least in human cells. Thus, at present, we have found no evidence that the nuclear import of cyclin B1 per se is subject to regulation aside from the controls on the activation of cyclin B1–Cdk1, nor is there any need to invoke such regulation.
Plk1 acts upstream of the nuclear import of cyclin B1–Cdk1
A prevailing idea concerning mitotic entry is that by phosphorylating cyclin B1 to block its nuclear export, the Plk1 kinase either triggers or is essential for the accumulation of cyclin B1 in the nucleus. But, in direct contradiction to this and in agreement with our previous conclusions (Jackman et al., 2003) and those of others (Lénárt et al., 2007), we have shown in this study that neither Plk1 activity nor the inhibition of nuclear export is required for the rapid accumulation of cyclin B1–Cdk1 in prophase. Indeed, we find no evidence that altering nuclear export rates contributes to the nuclear accumulation of cyclin B1–Cdk1. Instead, this can be entirely generated through an up to ∼40-fold increase in its import rate. Because the rate of import is the same in the presence or absence of Plk1 activity but fewer cells enter mitosis in the absence of Plk1 (Gavet and Pines, 2010), our experiments indicate that Plk1 acts upstream of cyclin B1–Cdk1 nuclear import.
Phosphorylation of B1 alters its import rate but is only significant in interphase
Our earlier experiments indicated that substituting five conserved Ser in the N terminus of cyclin B1 with Ala appeared to prevent the protein from entering the nucleus until NEBD, whereas substituting them for glutamic acid created a nuclear import signal (Hagting et al., 1999). Since then, however, the development of highly sensitive charge-coupled device cameras and spinning-disk confocal microscopy have improved our ability to assay and quantify the beginning of cyclin B1 nuclear entry, and we now show that both the wild-type and the Ala mutants are imported much earlier (∼20 min before NEBD) than we had previously thought. We have confirmed that glutamic acid substitutions do cause cyclin B1 to become more nuclear in interphase because they increase its rate of import by about fivefold. However, this does not significantly contribute to the ∼40-fold increase in the import rate once cells activate cyclin B1–Cdk1. Thus, the phosphorylation of cyclin B1 might help its nuclear import but is not necessary at prophase.
There is a general change in the nuclear transport machinery at prophase
One clue to the mechanism by which cyclin B1 is imported rapidly in prophase might be that at almost exactly the same time that cyclin B1 begins to accumulate in the nucleus, the M9 domain, which binds to transportin/importin β2, starts to become cytoplasmic. Thus, it is possible that modification of nuclear pore complexes and/or soluble receptors in prophase by active cyclin B1–Cdk1 alters the nuclear/cytoplasmic equilibrium of different proteins at the same time (Onischenko et al., 2005; Mühlhäusser and Kutay, 2007). Although importin β1 has a conserved Cdk consensus site in the region that binds to cyclin B1, overexpression of wild-type importin β1 or phosphorylation site mutants did not alter the nuclear/cytoplasmic distribution of cyclin B1. Unfortunately, the nucleoporins have a plethora of consensus Cdk1 sites that are phosphorylated at mitosis, making the task of identifying the proteins and crucial residues responsible extremely arduous (Blethrow et al., 2008; Dephoure et al., 2008; for review see Kutay and Hetzer, 2008). The driving force behind the rapid import of cyclin B1 is also unclear. One possibility is that the driving force might be generated by the existing nuclear/cytoplasmic gradient allied to cyclin B1–Cdk1 binding with condensed chromatin (Clute and Pines, 1999; Jackman et al., 2003; unpublished data).
In contrast to previous findings, we found that the nuclear accumulation of cyclin B1–Cdk1 kinase is not accompanied by the import of its activator, Cdc25C (Toyoshima-Morimoto et al., 2002). Thus, our experiments do not support the idea that Cdc25C is necessary to keep cyclin B1–Cdk1 active in the nucleus (Toyoshima-Morimoto et al., 2002), which was also somewhat at odds with the finding that Cdc25C is not essential in mice (Chen et al., 2001). We previously observed that Plk1 is imported in the nucleus at almost the same time as cyclin B1–Cdk1 when expressed in G2 phase cells, possibly through a modification of the nuclear transport machinery by active cyclin B1–Cdk1 (Jackman et al., 2003). However, when Plk1 is expressed earlier during the cell cycle, it equilibrates between the cytoplasm and the nucleus, and in these conditions, we do not observe a reproducible and significant import of Plk1 in the nucleus (unpublished data). Thus, at present, we do not have any strong evidence that the activation of cyclin B1–Cdk1 requires a change in the nuclear/cytoplasmic distribution of its upstream regulators.
We conclude that the rapid change in the nuclear import machinery that immediately follows cyclin B1–Cdk1 activation is intrinsic to how cytoplasmic and nuclear events are coordinated at mitosis. Determining the substrates modified by cyclin B1–Cdk1 to cause this change will be a fascinating challenge for the future.
Materials and methods
Reagents
LMB and Cdk1/2 inhibitor III (5-amino-3-((4-(aminosulfonyl) phenyl) amino)-N-(2,6-difluorophenyl)-1H-1,2,4-triazole-1-carbothioamide) were purchased from Merck Biosciences. RO3306 (Vassilev et al., 2006) was provided by L. Vassilev (Roche) and A. Giannis (University of Leipzig, Leipzig, Germany). BI 2536 was purchased from Axon Medchem. ZM 447439 was provided by N. Keen (AstraZeneca).
Cell culture and synchronization
HeLa cells were cultured in advanced DME (Invitrogen) supplemented with 2% FBS (Invitrogen), 200 µM Glutamax-1, 100 U/ml penicillin, 100 µg/ml streptomycin, and 250 ng/ml fungizone at 37°C with 10% CO2. For time-lapse imaging, cells were cultured on glass-bottom dishes (Willco Wells) precoated with fibronectin (Sigma-Aldrich) at 1 µg/cm2 for 2 h before use. Cells were synchronized in S phase by addition of 2.5 mM thymidine for 24 h then released into fresh medium. Cells entered mitosis on average 12 h after release from the thymidine block.
Transfection
HeLa cells were electroporated (3 × 106 cells; 10–20 µg DNA) at 250 V and 1,500 µF in 4-mm cuvettes using an electroporator (Easyject plus; Equibio). We estimated the transfection efficiency to be ∼40–60% depending on the quality of DNA. Cells were immediately plated on coated glass-bottom dishes and synchronized 7 h after transfection by a thymidine block and release. Alternatively, asynchronous cells were recorded 24 h after transfection.
Time-lapse imaging
Time-lapse imaging was performed on cells in L15 medium with 10% serum at 37°C using either an epifluorescence microscope (Deltavision; Applied Precision) controlled by SoftWoRx software and equipped with an inverted microscope (IX70; Olympus), an electron multiplier charge-coupled device camera (Cascade II 512K; Photometrics), and a 40× UApo NA 1.35 objective (Olympus) or a spinning-disk system (Marianas; Intelligent Imaging Innovations) controlled by Slidebook software and comprising a microscope (Axiovert Observer Z1; Carl Zeiss, Inc.) equipped with a disk-scanning unit (CSU-X1 Nipkow; Yokogawa) and a camera (QuantEM 512SC; Yokogawa). FRET imaging was performed using a CFP/YFP filter set with CFPex 436/10, CFPem 470/30, YFPem 535/30, and a CFP/YFP/mCherry dichroic C85363 (Chroma Technology Corp.). The same exposure time was used for CFPex/CFPem and CFPex/YFPem (between 100 and 200 ms). All quantifications were performed using ImageJ software (National Institutes of Health). For emission ratio measurements, we used the following formulas: whole cell signal = sum of the intensity of the pixels for one cell; mean background signal = mean signal per pixel for a region selected just beside the cell; whole cell signal corrected = whole cell signal − (area for the selected cell × mean background); and emission ratio = whole cell YFP signal corrected/whole cell CFP signal corrected.
Intensity-modulated display representations were performed using MetaMorph software according to the manufacturer's recommendations (MDS Analytical Technologies). In brief, for each intensity-modulated display, we used eight color hues and 32 intensities, ranking from dark to bright per hue. The maximum and minimum values were fixed manually and are indicated on each figure. The color intensities displayed for each hue were determined automatically by the software using one of the CFPex/YFPem images as a reference (Tsien and Harootunian, 1990).
For quantifications of cytoplasmic/nuclear ratio, we designed a region of interest corresponding approximately to one third of the nucleus and excluding centrosomes (Fig. 6 A) that could be used to quantify the nuclear signal and the cytoplasmic signal close to the nucleus in the same cell. FRAP experiments were performed using a 488-nm laser at maximum power and an exposure time of 100 ms.
Constructs
FRET biosensor.
Cerulean containing the monomeric mutation A207K (provided by D. Piston, Vanderbilt University, Brentwood, TN; Rizzo et al., 2004) was linked to YPet (provided by P. Daugherty, University of California, Santa Barbara, Santa Barbara, CA; Nguyen and Daugherty, 2005) with the minimal domain of the Polo-like kinase 1 polo box (residues 373–592) that is sufficient for binding to phospho-Ser/Thr residues (Cheng et al., 2003; Elia et al., 2003) plus 16 amino acids from the autophosphorylation site of human cyclin B1 with the Ala at the −1 position (underlined) altered to Ser (PEPILVDT-S-pS126-P-pS128-P-MET).
Homo sapiens cyclin B1–mCherry.
mCherry (NCBI Protein database accession no. ACF75945.1) was amplified by PCR and used to replace the AgeI–NotI fragment containing ECFP in pECFP–cyclin B1. Wild-type and mutated forms of H. sapiens cyclin B1 fused to GFP have been previously described (Hagting et al., 1999). pIBB-GFP and pGFP-M9 (Lénárt et al., 2003) were provided by P. Lénárt (European Molecular Biology Laboratory, Heidelberg, Germany).
pWT–, Ser12A–, or Ser12E–importin β1 IRES2-GFP.
Full-length importin β1 coding sequence was amplified by PCR using mutagenesis primers and subcloned as a SacII–BamHI fragment in pIRES2-GFP (Takara Bio Inc.). GFP-Cdc25C has been previously described (Karlsson et al., 1999).
Online supplemental material
Fig. S1 shows that the nuclear targeted FRET sensor is stably associated with chromatin. Fig. S2 shows that kinetics of cyclin B1 nuclear accumulation is independent of the expression level. Fig. S3 shows that nuclear accumulation of cyclin B1 in prophase is independent of its phosphorylation on Ser126, 128, 133, and 147. Fig. S4 shows that cyclin B1 nuclear import rate increases significantly at mitotic entry. Fig. S5 shows that the nuclear/cytoplasmic distribution of cyclin B1 in interphase is not affected by overexpression of wild-type or phosphorylation mutants of importin β1.
Video 1 shows a HeLa cell expressing FRET sensor and cyclin B1 entering mitosis. Video 2 shows a HeLa cell expressing FRET sensor and cyclin B1 treated with a Cdk inhibitor during prophase. Video 3 shows a HeLa cell expressing FRET sensor targeted to both the nucleus and the cytoplasm undergoing mitosis. Video 4 shows a HeLa cell expressing the 5xA Ala mutant and wild-type cyclin B1 entering mitosis. Video 5 shows a HeLa cell expressing wild-type cyclin B1 entering mitosis and treated with the Plk inhibitor BI 2536. Video 6 shows a HeLa cell expressing the 5xE glutamic acid mutant and wild-type cyclin B1 entering mitosis and treated with a Cdk inhibitor. Video 7 shows a HeLa cell expressing wild-type cyclin B1–mCherry and IBB-GFP entering mitosis. Video 8 shows a HeLa cell expressing wild-type cyclin B1–mCherry and GFP-M9 entering mitosis. Video 9 shows a HeLa cell expressing wild-type cyclin B1–GFP and mCherry–α-tubulin (right) entering mitosis. Video 10 shows a HeLa cell expressing wild-type cyclin B1–mCherry and GFP-Cdc25C entering mitosis. Additional supplemental data shows DNA sequences used in this study. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200909144/DC1.
Supplementary Material
Footnotes
Abbreviations used in this paper:
- DIC
- differential interference contrast
- FRET
- Förster resonance energy transfer
- LMB
- leptomycin B
- NEBD
- nuclear envelope breakdown
- NES
- nuclear export sequence
References
- Allan L.A., Clarke P.R.. 2007. Phosphorylation of caspase-9 by CDK1/cyclin B1 protects mitotic cells against apoptosis. Mol. Cell. 26:301–310. 10.1016/j.molcel.2007.03.019 [DOI] [PubMed] [Google Scholar]
- Andersen S.S., Ashford A.J., Tournebize R., Gavet O., Sobel A., Hyman A.A., Karsenti E.. 1997. Mitotic chromatin regulates phosphorylation of Stathmin/Op18. Nature. 389:640–643. 10.1038/39382 [DOI] [PubMed] [Google Scholar]
- Blethrow J.D., Glavy J.S., Morgan D.O., Shokat K.M.. 2008. Covalent capture of kinase-specific phosphopeptides reveals Cdk1-cyclin B substrates. Proc. Natl. Acad. Sci. USA. 105:1442–1447. 10.1073/pnas.0708966105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkard M.E., Randall C.L., Larochelle S., Zhang C., Shokat K.M., Fisher R.P., Jallepalli P.V.. 2007. Chemical genetics reveals the requirement for Polo-like kinase 1 activity in positioning RhoA and triggering cytokinesis in human cells. Proc. Natl. Acad. Sci. USA. 104:4383–4388. 10.1073/pnas.0701140104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.S., Hurov J., White L.S., Woodford-Thomas T., Piwnica-Worms H.. 2001. Absence of apparent phenotype in mice lacking Cdc25C protein phosphatase. Mol. Cell. Biol. 21:3853–3861. 10.1128/MCB.21.12.3853-3861.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K.Y., Lowe E.D., Sinclair J., Nigg E.A., Johnson L.N.. 2003. The crystal structure of the human polo-like kinase-1 polo box domain and its phospho-peptide complex. EMBO J. 22:5757–5768. 10.1093/emboj/cdg558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou Y.-H., Bischoff J.R., Beach D., Goldman R.D.. 1990. Intermediate filament reorganization during mitosis is mediated by p34cdc2 phosphorylation of vimentin. Cell. 62:1063–1071. 10.1016/0092-8674(90)90384-Q [DOI] [PubMed] [Google Scholar]
- Clute P., Pines J.. 1999. Temporal and spatial control of cyclin B1 destruction in metaphase. Nat. Cell Biol. 1:82–87. 10.1038/10049 [DOI] [PubMed] [Google Scholar]
- Dephoure N., Zhou C., Villén J., Beausoleil S.A., Bakalarski C.E., Elledge S.J., Gygi S.P.. 2008. A quantitative atlas of mitotic phosphorylation. Proc. Natl. Acad. Sci. USA. 105:10762–10767. 10.1073/pnas.0805139105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fiore B., Pines J.. 2008. Defining the role of Emi1 in the DNA replication-segregation cycle. Chromosoma. 117:333–338. 10.1007/s00412-008-0152-x [DOI] [PubMed] [Google Scholar]
- Draviam V.M., Orrechia S., Lowe M., Pardi R., Pines J.. 2001. The localization of human cyclins B1 and B2 determines CDK1 substrate specificity and neither enzyme requires MEK to disassemble the Golgi apparatus. J. Cell Biol. 152:945–958. 10.1083/jcb.152.5.945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia A.E., Cantley L.C., Yaffe M.B.. 2003. Proteomic screen finds pSer/pThr-binding domain localizing Plk1 to mitotic substrates. Science. 299:1228–1231. 10.1126/science.1079079 [DOI] [PubMed] [Google Scholar]
- Gavet O., Pines J.. 2010. Progressive activation of cyclin B1-Cdk1 coordinates entry to mitosis. Dev. Cell. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagting A., Karlsson C., Clute P., Jackman M., Pines J.. 1998. MPF localization is controlled by nuclear export. EMBO J. 17:4127–4138. 10.1093/emboj/17.14.4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagting A., Jackman M., Simpson K., Pines J.. 1999. Translocation of cyclin B1 to the nucleus at prophase requires a phosphorylation-dependent nuclear import signal. Curr. Biol. 9:680–689. 10.1016/S0960-9822(99)80308-X [DOI] [PubMed] [Google Scholar]
- Hara K., Tydeman P., Kirschner M.. 1980. A cytoplasmic clock with the same period as the division cycle in Xenopus eggs. Proc. Natl. Acad. Sci. USA. 77:462–466. 10.1073/pnas.77.1.462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R., McLoughlin M., McKeon F.. 1993. Human wee1 maintains mitotic timing by protecting the nucleus from cytoplasmically activated Cdc2 kinase. Cell. 74:463–474. 10.1016/0092-8674(93)80048-J [DOI] [PubMed] [Google Scholar]
- Jackman M., Lindon C., Nigg E.A., Pines J.. 2003. Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat. Cell Biol. 5:143–148. 10.1038/ncb918 [DOI] [PubMed] [Google Scholar]
- Karlsson C., Katich S., Hagting A., Hoffmann I., Pines J.. 1999. Cdc25B and Cdc25C differ markedly in their properties as initiators of mitosis. J. Cell Biol. 146:573–584. 10.1083/jcb.146.3.573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J., Grummt I.. 1999. Cell cycle-dependent regulation of RNA polymerase I transcription: the nucleolar transcription factor UBF is inactive in mitosis and early G1. Proc. Natl. Acad. Sci. USA. 96:6096–6101. 10.1073/pnas.96.11.6096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft C., Herzog F., Gieffers C., Mechtler K., Hagting A., Pines J., Peters J.M.. 2003. Mitotic regulation of the human anaphase-promoting complex by phosphorylation. EMBO J. 22:6598–6609. 10.1093/emboj/cdg627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutay U., Hetzer M.W.. 2008. Reorganization of the nuclear envelope during open mitosis. Curr. Opin. Cell Biol. 20:669–677. 10.1016/j.ceb.2008.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lénárt P., Ellenberg J.. 2003. Nuclear envelope dynamics in oocytes: from germinal vesicle breakdown to mitosis. Curr. Opin. Cell Biol. 15:88–95. 10.1016/S0955-0674(02)00011-X [DOI] [PubMed] [Google Scholar]
- Lénárt P., Rabut G., Daigle N., Hand A.R., Terasaki M., Ellenberg J.. 2003. Nuclear envelope breakdown in starfish oocytes proceeds by partial NPC disassembly followed by a rapidly spreading fenestration of nuclear membranes. J. Cell Biol. 160:1055–1068. 10.1083/jcb.200211076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lénárt P., Petronczki M., Steegmaier M., Di Fiore B., Lipp J.J., Hoffmann M., Rettig W.J., Kraut N., Peters J.M.. 2007. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr. Biol. 17:304–315. 10.1016/j.cub.2006.12.046 [DOI] [PubMed] [Google Scholar]
- Liakopoulos D., Kusch J., Grava S., Vogel J., Barral Y.. 2003. Asymmetric loading of Kar9 onto spindle poles and microtubules ensures proper spindle alignment. Cell. 112:561–574. 10.1016/S0092-8674(03)00119-3 [DOI] [PubMed] [Google Scholar]
- Lindqvist A., van Zon W., Karlsson Rosenthal C., Wolthuis R.M.. 2007. Cyclin B1-Cdk1 activation continues after centrosome separation to control mitotic progression. PLoS Biol. 5:e123. 10.1371/journal.pbio.0050123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist A., Rodríguez-Bravo V., Medema R.H.. 2009. The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J. Cell Biol. 185:193–202. 10.1083/jcb.200812045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe M., Rabouille C., Nakamura N., Watson R., Jackman M., Jämsä E., Rahman D., Pappin D.J., Warren G.. 1998. Cdc2 kinase directly phosphorylates the cis-Golgi matrix protein GM130 and is required for Golgi fragmentation in mitosis. Cell. 94:783–793. 10.1016/S0092-8674(00)81737-7 [DOI] [PubMed] [Google Scholar]
- Lüscher B., Brizuela L., Beach D., Eisenman R.N.. 1991. A role for the p34cdc2 kinase and phosphatases in the regulation of phosphorylation and disassembly of lamin B2 during the cell cycle. EMBO J. 10:865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusk C.P., Waller D.D., Makhnevych T., Dienemann A., Whiteway M., Thomas D.Y., Wozniak R.W.. 2007. Nup53p is a target of two mitotic kinases, Cdk1p and Hrr25p. Traffic. 8:647–660. 10.1111/j.1600-0854.2007.00559.x [DOI] [PubMed] [Google Scholar]
- Macaulay C., Meier E., Forbes D.J.. 1995. Differential mitotic phosphorylation of proteins of the nuclear pore complex. J. Biol. Chem. 270:254–262. 10.1074/jbc.270.1.254 [DOI] [PubMed] [Google Scholar]
- Moore J.K., Miller R.K.. 2007. The cyclin-dependent kinase Cdc28p regulates multiple aspects of Kar9p function in yeast. Mol. Biol. Cell. 18:1187–1202. 10.1091/mbc.E06-04-0360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J.D., Yang J., Truant R., Kornbluth S.. 1999. Nuclear import of Cdk/cyclin complexes: identification of distinct mechanisms for import of Cdk2/cyclin E and Cdc2/cyclin B1. J. Cell Biol. 144:213–224. 10.1083/jcb.144.2.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlhäusser P., Kutay U.. 2007. An in vitro nuclear disassembly system reveals a role for the RanGTPase system and microtubule-dependent steps in nuclear envelope breakdown. J. Cell Biol. 178:595–610. 10.1083/jcb.200703002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen A.W., Daugherty P.S.. 2005. Evolutionary optimization of fluorescent proteins for intracellular FRET. Nat. Biotechnol. 23:355–360. 10.1038/nbt1066 [DOI] [PubMed] [Google Scholar]
- Nigg E.A. 1991. The substrates of the cdc2 kinase. Semin. Cell Biol. 2:261–270. [PubMed] [Google Scholar]
- Onischenko E.A., Gubanova N.V., Kiseleva E.V., Hallberg E.. 2005. Cdk1 and okadaic acid-sensitive phosphatases control assembly of nuclear pore complexes in Drosophila embryos. Mol. Biol. Cell. 16:5152–5162. 10.1091/mbc.E05-07-0642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ookata K., Hisanaga S., Okano T., Tachibana K., Kishimoto T.. 1992. Relocation and distinct subcellular localization of p34cdc2-cyclin B complex at meiosis reinitiation in starfish oocytes. EMBO J. 11:1763–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Mongiovi D., Beckhelling C., Chang P., Ford C.C., Houliston E.. 2000. Nuclei and microtubule asters stimulate maturation/M phase promoting factor (MPF) activation in Xenopus eggs and egg cytoplasmic extracts. J. Cell Biol. 150:963–974. 10.1083/jcb.150.5.963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter M., Nakagawa J., Dorée M., Labbé J.C., Nigg E.A.. 1990a. Identification of major nucleolar proteins as candidate mitotic substrates of cdc2 kinase. Cell. 60:791–801. 10.1016/0092-8674(90)90093-T [DOI] [PubMed] [Google Scholar]
- Peter M., Nakagawa J., Dorée M., Labbé J.C., Nigg E.A.. 1990b. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell. 61:591–602. 10.1016/0092-8674(90)90471-P [DOI] [PubMed] [Google Scholar]
- Petronczki M., Glotzer M., Kraut N., Peters J.M.. 2007. Polo-like kinase 1 triggers the initiation of cytokinesis in human cells by promoting recruitment of the RhoGEF Ect2 to the central spindle. Dev. Cell. 12:713–725. 10.1016/j.devcel.2007.03.013 [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T.. 1991. Human cyclins A and B1 are differentially located in the cell and undergo cell cycle-dependent nuclear transport. J. Cell Biol. 115:1–17. 10.1083/jcb.115.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisinger C., Körner R., Wind M., Lehmann W.D., Kopajtich R., Barr F.A.. 2005. Plk1 docking to GRASP65 phosphorylated by Cdk1 suggests a mechanism for Golgi checkpoint signalling. EMBO J. 24:753–765. 10.1038/sj.emboj.7600569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo M.A., Springer G.H., Granada B., Piston D.W.. 2004. An improved cyan fluorescent protein variant useful for FRET. Nat. Biotechnol. 22:445–449. 10.1038/nbt945 [DOI] [PubMed] [Google Scholar]
- Royou A., McCusker D., Kellogg D.R., Sullivan W.. 2008. Grapes(Chk1) prevents nuclear CDK1 activation by delaying cyclin B nuclear accumulation. J. Cell Biol. 183:63–75. 10.1083/jcb.200801153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner A.D., Murray A.W.. 2000. Phosphorylation by Cdc28 activates the Cdc20-dependent activity of the anaphase-promoting complex. J. Cell Biol. 149:1377–1390. 10.1083/jcb.149.7.1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirri V., Hernandez-Verdun D., Roussel P.. 2002. Cyclin-dependent kinases govern formation and maintenance of the nucleolus. J. Cell Biol. 156:969–981. 10.1083/jcb.200201024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers M.K., Bothos J., Halazonetis T.D.. 2005. The CHFR mitotic checkpoint protein delays cell cycle progression by excluding Cyclin B1 from the nucleus. Oncogene. 24:2589–2598. 10.1038/sj.onc.1208428 [DOI] [PubMed] [Google Scholar]
- Toyoshima F., Moriguchi T., Wada A., Fukuda M., Nishida E.. 1998. Nuclear export of cyclin B1 and its possible role in the DNA damage-induced G2 checkpoint. EMBO J. 17:2728–2735. 10.1093/emboj/17.10.2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima-Morimoto F., Taniguchi E., Shinya N., Iwamatsu A., Nishida E.. 2001. Polo-like kinase 1 phosphorylates cyclin B1 and targets it to the nucleus during prophase. Nature. 410:215–220. 10.1038/35065617 [DOI] [PubMed] [Google Scholar]
- Toyoshima-Morimoto F., Taniguchi E., Nishida E.. 2002. Plk1 promotes nuclear translocation of human Cdc25C during prophase. EMBO Rep. 3:341–348. 10.1093/embo-reports/kvf069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R.Y., Harootunian A.T.. 1990. Practical design criteria for a dynamic ratio imaging system. Cell Calcium. 11:93–109. 10.1016/0143-4160(90)90063-Z [DOI] [PubMed] [Google Scholar]
- Vasquez R.J., Gard D.L., Cassimeris L.. 1999. Phosphorylation by CDK1 regulates XMAP215 function in vitro. Cell Motil. Cytoskeleton. 43:310–321. [DOI] [PubMed] [Google Scholar]
- Vassilev L.T., Tovar C., Chen S., Knezevic D., Zhao X., Sun H., Heimbrook D.C., Chen L.. 2006. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc. Natl. Acad. Sci. USA. 103:10660–10665. 10.1073/pnas.0600447103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Seemann J., Pypaert M., Shorter J., Warren G.. 2003. A direct role for GRASP65 as a mitotically regulated Golgi stacking factor. EMBO J. 22:3279–3290. 10.1093/emboj/cdg317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T., Goto H., Yokoyama T., Silljé H., Hanisch A., Uldschmid A., Takai Y., Oguri T., Nigg E.A., Inagaki M.. 2005. Phosphorylation by Cdk1 induces Plk1-mediated vimentin phosphorylation during mitosis. J. Cell Biol. 171:431–436. 10.1083/jcb.200504091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro S., Yamakita Y., Hosoya H., Matsumura F.. 1991. Phosphorylation of non-muscle caldesmon by p34cdc2 kinase during mitosis. Nature. 349:169–172. 10.1038/349169a0 [DOI] [PubMed] [Google Scholar]
- Yamashiro S., Chern H., Yamakita Y., Matsumura F.. 2001. Mutant Caldesmon lacking cdc2 phosphorylation sites delays M-phase entry and inhibits cytokinesis. Mol. Biol. Cell. 12:239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Bardes E.S., Moore J.D., Brennan J., Powers M.A., Kornbluth S.. 1998. Control of cyclin B1 localization through regulated binding of the nuclear export factor CRM1. Genes Dev. 12:2131–2143. 10.1101/gad.12.14.2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Song H., Walsh S., Bardes E.S., Kornbluth S.. 2001. Combinatorial control of cyclin B1 nuclear trafficking through phosphorylation at multiple sites. J. Biol. Chem. 276:3604–3609. 10.1074/jbc.M008151200 [DOI] [PubMed] [Google Scholar]
- Yuan J., Eckerdt F., Bereiter-Hahn J., Kurunci-Csacsko E., Kaufmann M., Strebhardt K.. 2002. Cooperative phosphorylation including the activity of polo-like kinase 1 regulates the subcellular localization of cyclin B1. Oncogene. 21:8282–8292. 10.1038/sj.onc.1206011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.