INTRODUCTION
Despite substantial declines over the past two decades in U.S. incidence rates for hepatitis B virus (HBV) infection, chronic HBV infection and its sequelae—chronic liver disease/cirrhosis and primary liver cancer—remain significant public health problems (1–7). These conditions may have a particularly strong impact on correctional health-care systems in the United States, as studies suggest that the prevalence of current or past HBV infection in incarcerated populations may be four to five times greater than that in the U.S. general population (6, 8–30). However, HBV seroprevalence estimates from U.S. incarcerated populations appear to vary widely across studies, with prevalence estimates for HBV surface antigen (HBsAg) (current infection) ranging from 0.9% to 11.4% (12, 28) and HBV core antibody (anti-HBc) (past or current infection) estimates ranging from 6.5% to 42.6% (12, 19). Variation in HBV seroprevalence estimates across incarcerated populations has received limited attention in the literature (12–14), and several authors have suggested that HBV seroprevalence estimates reported in their studies were consistent with previous estimates (15, 17, 19, 21, 27–29). We conducted a systematic review of studies reporting HBV seroprevalence estimates from U.S. adult incarcerated populations to describe variation in these estimates across studies.
METHODS
Data Sources and Search
Medline via Ovid, Web of Science-Science Citation Index and Social Sciences Citation Index, National Criminal Justice Reference Service Abstracts Database, and UMI Proquest Digital Dissertations were searched using the keywords “hepatitis” and “prison*” for reports of HBV seroprevalence estimates in incarcerated adults that were indexed from January 1, 1975 through August 31, 2005. Bibliographies of review articles and initially eligible studies were also searched.
Study Selection
The following eligibility criteria were pre-specified and applied in the order mentioned: (1) indexed in the time period noted above; (2) conducted in the United States; (3) primary study; (4) reporting prevalence estimates of HBV infection; (5) study population sampled from prisons, jails, or other correctional facilities; (6) measurement of one or more standard serologic markers for HBV (HBsAg, total anti-HBc, IgM anti-HBc, anti-HBs); (7) HBV seroprevalence estimates based on screening a defined population (i.e., not mathematical models (31) or surveys of medical directors (32–34)); (8) sample drawn exclusively from a prison, or correctional system, or facility housing primarily adults; (9) sample not drawn from a facility housing primarily non-U.S. residents; and (10) sample not restricted to those with another illness (e.g., HIV-positive prisoners). When unique citations reflected duplicate reports of the same data, HBV seroprevalence estimates and sample characteristics were drawn from the more complete report.
Data Extraction
HBV seroprevalence estimates and study characteristics considered potential sources of variation (35–37) were extracted independently by two authors (correlation coefficient, r = 94.4; percentage agreement [95% confidence interval (CI)] = 89.4% [87.5%–91.1%]). Discrepancies were resolved by A.J.H. and K.J.G.
Statistical Analysis
Data were analyzed using Stata 8.2 (38). Outcomes of interest were prevalence estimates for the most commonly reported seromarkers: HBsAg, total anti-HBc, and any positive HBV marker (i.e., one or more seromarkers positive when more than one seromarker was tested). Wilson 95% CIs (39) were calculated to display prevalence estimates using a common metric for precision.
The Q-statistic was used to test for heterogeneity in prevalence estimates overall and by study level strata for each seromarker. Prevalence estimates and corresponding standard errors were transformed into logits and weighted using the inverse variance method to calculate a Q-statistic [Q=Σwi(L − L̄)2] (meta command) (40–44), for which a small p value indicates heterogeneity beyond what would be expected from random variation (i.e., the smaller the p value the greater, the departure from homogeneity) (40–44). Logit transformation of proportions has been recommended for meta-analysis because logits afford the advantages of a normal distribution and stable variance analysis (40, 43). Statistical outliers among weighted logit prevalence estimates were identified using the method proposed by Hamilton (2003) (iqr command) (45). Analyses were repeated excluding outliers to examine their influence on Q-statistics.
RESULTS
Search and Study Selection
Of 579 unique citations screened, 23 eligible studies were initially identified. Two of these studies estimated HBV seroprevalence for two distinct populations, so these were treated as separate estimates (28, 30). Thus, our systematic review included reported HBV seroprevalence estimates from 25 distinct U.S. incarcerated populations, with 15 estimates of HBsAg prevalence, 11 of anti-HBc prevalence, and 13 of any positive HBV marker prevalence.
Heterogeneity in HBV Seroprevalence Estimates
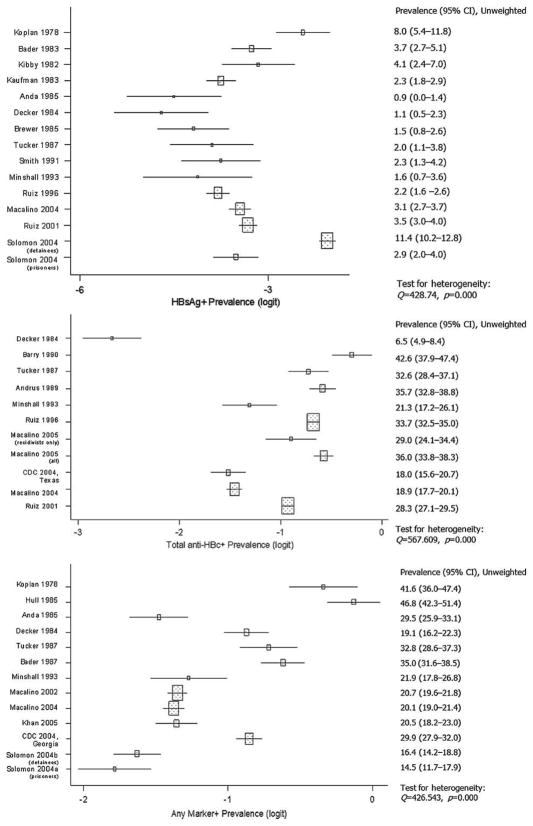
Q-statistics indicated heterogeneity of estimates for each of the 3 outcomes (Fig. 1). Outlier analysis indicated one mild outlier for HBsAg prevalence (Solomon et al., 2004, detainees) (28). After exclusion of the outlier, the p value for the heterogeneity test for HBsAg prevalence estimates remained extremely small (p = 0.000), indicating that substantial heterogeneity persisted. Q-statistics also indicated heterogeneity of seroprevalence estimates for all definable within-study subgroups (e.g., all males, all females, all injection drug users) and for nearly all study-level strata (e.g., all studies with mean age ≥31 years). Prevalence estimates of any positive marker for studies with 30% or less injection drug users appeared to be homogeneous (p = 0.656).
FIGURE 1.
HBV seromarker prevalence estimates with 95% confidence intervals and heterogeneity statistics.
DISCUSSION
Results of this study confirmed that adult incarcerated populations in the United States are heterogeneous with respect to prevalence of 3 commonly reported HBV seromarker outcomes; that is, the dispersion of HBV seroprevalence estimates around their mean was far greater than would be expected from within-study sampling error alone. This heterogeneity and its potential sources should be considered in the future when interpreting study-specific HBV seroprevalence estimates from U.S. adult incarcerated populations, when comparing these estimates across studies, and when assessing studies that rely upon these estimates, such as cost-effectiveness analyses of HBV-related preventive and therapeutic strategies in correctional settings. Potential sources of heterogeneity may include across-study differences in sample demographic characteristics and behavioral risk factors, background disease prevalence, and methodologic factors (35–37). Because of the small number of HBV seroprevalence estimates and differences in reporting across studies, application of meta-regression techniques to HBV seroprevalence estimates from U.S. incarcerated populations provides only limited insight into the sources of heterogeneity across studies (37). A national, population-based study may be required to explore fully the factors affecting HBV seroprevalence estimates from U.S. incarcerated populations.
Acknowledgments
A.J.H. was supported by a Pre-doctoral Cancer Prevention and Control Fellowship during the conduct of this research (R25 CA 57712, granted to P.D.M.).
Assistance in developing the electronic search strategy was provided by Helena VonVille, MLS, Directory of Library Services, University of Texas School of Public Health.
Selected Abbreviations and Acronyms
- HBV
hepatitis B virus
- HBsAg
HBV surface antigen
- anti-HBc
HBV core antibody
References
- 1.Centers for Disease Control and Prevention. [Accessed October 1, 2004.];Disease burden from viral hepatitis A, B, and C in the United States. 2002 Available at: http://www.cdc.gov/ncidod/diseases/hepatitis/resource/PDFs/disease_burden2004.pdf.
- 2.Margolis HS, Coleman PJ, Brown RE, Mast EE, Sheingold SH, Arevalo JA. Prevention of hepatitis B virus transmission by immunization: an economic analysis of current recommendations. JAMA. 1995;274:1201–1208. [PubMed] [Google Scholar]
- 3.McQuillan GM, Coleman PJ, Kruszon-Moran, Moyer LA, Lambert SB, Margolis HS. Prevalence of hepatitis B virus infection in the United Status: the National Health and Nutrition Examination Surveys, 1976 through 1994. Am J Public Health. 1999;89:14–18. doi: 10.2105/ajph.89.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Protection against viral hepatitis: recommendations of the Immunization Practices Advisory Committee (ACIP) MMWR Recomm Rep. 1990;39(RR-2):1–26. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Prevention and control of infections with hepatitis viruses in correctional settings. MMWR Recomm Rep. 2003;52(RR-1):1–44. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP): Part 1: Immunization of Infants, Children, and Adolescents. MMWR Recomm Rep. 2005;54(RR-16):1–39. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Hepatitis Surveillance Report No. 60. Atlanta (GA): US Department of Health and Human Services, Centers for Disease Control and Prevention; 2005. [Google Scholar]
- 8.Koplan JP, Walker JA, Bryan JA, Berquist KR. Prevalence of hepatitis B surface antigen and antibody at a state prison in Kansas. J Infect Dis. 1978;137:505–506. doi: 10.1093/infdis/137.4.505. [DOI] [PubMed] [Google Scholar]
- 9.Kibby T, Devine J, Love C. Prevalence of hepatitis B among men admitted to a federal prison. N Engl J Med. 1982;306:175. doi: 10.1056/NEJM198201213060317. [DOI] [PubMed] [Google Scholar]
- 10.Bader T. Hepatitis B carriers in the prison population. N Engl J Med. 1983;308:281. doi: 10.1056/nejm198302033080516. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman ML, Faiver KL, Harness JK. Hepatitis B markers among Michigan prisoners. Ann Intern Med. 1983;98:558. doi: 10.7326/0003-4819-98-4-558_1. [DOI] [PubMed] [Google Scholar]
- 12.Decker MD, Vaughn WK, Brodie JS, Hutcheson RH, Jr, Schaffner W. Seroepidemiology of hepatitis B in Tennessee prisoners. J Infect Dis. 1984;150:450–459. doi: 10.1093/infdis/150.3.450. [DOI] [PubMed] [Google Scholar]
- 13.Anda RF, Perlman SB, D’Alessio DJ, Davis JP, Dodson VN. Hepatitis B in Wisconsin male prisoners: considerations for serologic screening and vaccination. Am J Public Health. 1985;75:1182–1185. doi: 10.2105/ajph.75.10.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brewer F. Hepatitis B–Similarity of risk among populations. J Pris Jail Health. 1985;5:102–107. [Google Scholar]
- 15.Hull HF, Lyons LH, Mann JM, Hadler SC, Steece R, Skeels MR. Incidence of hepatitis B in the penitentiary of New Mexico. Am J Public Health. 1985;75:1213–1214. doi: 10.2105/ajph.75.10.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bader T, Kibby T, Mueller M, Love C, Khayrallah M, Ham R. Hepatitis-B in United States prisoners. J Med Virol. 1987;21:A4–A4. [Google Scholar]
- 17.Tucker RM, Gaffey MJ, Fisch MJ, Kaiser DL, Guerrant RL, Normansell DE. Seroepidemiology of hepatitis D (delta agent) and hepatitis B among Virginia state prisoners. Clin Ther. 1987;9:622–628. [PubMed] [Google Scholar]
- 18.Andrus JK, Fleming DW, Knox C, McAlister RO, Skeels MR, Conrad RE, et al. HIV testing in prisoners: is mandatory testing mandatory? Am J Public Health. 1989;79:840–842. doi: 10.2105/ajph.79.7.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barry MA, Gleavy D, Herd K, Schwingl PJ, Werner BG. Prevalence of markers for hepatitis B and hepatitis D in a municipal house of correction. Am J Public Health. 1990;80:471–473. doi: 10.2105/ajph.80.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith PF, Mikl J, Truman BI, Lessner L, Lehman JS, Stevens RW, et al. HIV infection among women entering the New York state correctional system. Am J Public Health. 1991;81(Suppl):35–40. doi: 10.2105/ajph.81.suppl.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minshall ME, Dickinson DJ, Fleissner ML. Prevalence of syphilis, hepatitis B virus (HBV), and human immunodeficiency virus (HIV) infection in new arrestees at the Lake County Jail, Crown Point, Indiana. J Pris Jail Health. 1993;12:135–155. [Google Scholar]
- 22.Ruiz JD, Mikanda J. Seroprevalence of HIV, hepatitis B, hepatitis C, and risk behaviors among inmates entering the California correctional system. Sacramento, California: Department of Health Services; 1996. [Accessed August 1, 2004.]. Available at: http://www.dhs.ca.gov/ps/ooa/Reports/PDF/Corrections99.pdf. [Google Scholar]
- 23.Ruiz JD, Chang JS, Bernstein K, Chow JM, Bolan G, Pettifor A, Parikh-Patel A, Moliter F. Prevalence of HIV infection, sexually transmitted diseases, hepatitis, and riskbehaviors amonginmates enteringprison at the California Department of Corrections; 1999. Sacramento: Department of Health Services; 2001. [Accessed August 1, 2004.]. Available at: http://www.dhs.ca.gov/ps/ooa/Reports/PDF/1996SeroofHIV-HepBHepCandRiskAmongInmates.pdf. [Google Scholar]
- 24.Macalino GE, Rich JD, Sandford-Colby S, Salas CM, Vlahov D. Intake prevalence and intraprison incidence of HIV, hepatitis B (HBV), and hepatitis C (HCV) among sentenced inmates in Rhode Island, USA. International Conference on AIDS; Abstract 9208; July 7–12, 2002; Barcelona, Spain. [Google Scholar]
- 25.Centers for Disease Control and Prevention. Hepatitis B vaccination of inmates in correctional facilities–Texas, 2000–2002. MMWR Morb Mortal Wkly Rep. 2004;53:681–683. [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. Transmission of hepatitis B virus in correctional facilities–Georgia, January 1999–June 2002. MMWR Morb Mortal Wkly Rep. 2004;53:678–681. [PubMed] [Google Scholar]
- 27.Macalino GE, Vlahov D, Sanford-Colby S, Patel S, Sabin K, Salas C, et al. Prevalence and incidence of HIV, hepatitis B virus, and hepatitis C virus infections among males in Rhode Island prisons. Am J Public Health. 2004;94:1218–1223. doi: 10.2105/ajph.94.7.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solomon L, Flynn C, Muck K, Vertefeuille J. Prevalence of HIV, syphilis, hepatitis B, and hepatitis C among entrants to Maryland correctional facilities. J Urban Health. 2004;81:25–37. doi: 10.1093/jurban/jth085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan AJ, Simard EP, Bower WA, Wurtzel HL, Khristova M, Wagner KD, et al. Ongoing transmission of hepatitis B virus infection among inmates at a state correctional facility. Am J Public Health. 2005;95:1793–1799. doi: 10.2105/AJPH.2004.047753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macalino GE, Vlahov D, Dickinson BP, Schwartzapfel B, Rich JD. Community incidence of hepatitis B and C among reincarcerated women. Clin Infect Dis. 2005;41:998–1002. doi: 10.1086/432936. [DOI] [PubMed] [Google Scholar]
- 31.Hammett TM, Harmon MP, Rhodes W. The burden of infectious disease among inmates of and releasees from U.S. correctional facilities, 1997. Am J Public Health. 2002;92:1789–1794. doi: 10.2105/ajph.92.11.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harrison PM, Beck AJ. Prisoners in 2002. [Available at Accessed October 15, 2004.];Bur Justice Stat Bull 2003. 2002 48:1–14. NCJ. http://www.ojp.usdoj.gov/bjs/pubalp@.htm#P.
- 33.Harrison PM, Karberg JC. Prison and jail inmates at midyear 2003. [Accessed October 15, 2004.];Bur Justice Stat Bull. 2004 :1–14. NCJ 203947. Available at: http://www.ojp.usdoj.gov/bjs/pubalp@.htm#P.
- 34.Beck AJ, Maruschak LM. Hepatitis testing and treatment in state prisons. [Accessed October 30, 2004.];Bur Justice Stat Bull. 2004 NCJ 199173C. Available at: http://www.ojp.usdoj.gov/bjs/pub/pdf/httsp.pdf.
- 35.Loney PL, Chambers LW, Bennett KJ, Roberts JG, Stratford PW. Critical appraisal of the health research literature: prevalence or incidence of a health problem. Chronic Dis Can. 1998;19:170–176. [PubMed] [Google Scholar]
- 36.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 37.Harzke AJ. Dr. P.H. dissertation. Houston (TX): University of Texas School of Public Health; 2007. Hepatitis B and C infections in U.S. Incarcerated Populations: Prevalence and Related Mortality. [Google Scholar]
- 38.Statistics/Data Analysis, version 8.2. College Station (TX): StataCorp; pp. 1984–2003. [Google Scholar]
- 39.Brown LD, Cai TT, Das Gupta A. Interval estimation for a binomial proportion. Stat Sci. 2001;16:101–133. [Google Scholar]
- 40.Lipsey MW, Wilson DB. Practical meta-analysis. Thousand Oaks (CA): Sage Publications; 2001. [Google Scholar]
- 41.Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Smith GD, Altman DG, editors. Systematic reviews in health care: meta-analysis in context. London: BMJ Publishing Group; 2001. pp. 285–312. [Google Scholar]
- 42.Greenland S. Meta-analysis. In: Rothman KJ, Greenland S, editors. Modern epidemiology. 2. Philadelphia: Lippincott Williams & Wilkins; 1998. pp. 643–673. [Google Scholar]
- 43.Benjamin DK, Poole C, Steinbach WJ, Rowen JL, Walsh TJ. Neonatal candidemia and end-organ damage: a critical appraisal of the literature using meta-analytic techniques. Pediatrics. 2003;112:634–640. doi: 10.1542/peds.112.3.634. [DOI] [PubMed] [Google Scholar]
- 44.Thompson SG. Why and how sources of heterogeneity should be investigated. In: Egger M, Smith GD, Altman DG, editors. Systematic reviews in health care: meta-analysis in context. London: BMJ Publishing Group; 2001. pp. 157–175. [Google Scholar]
- 45.Hamilton LC. Statistics with Stata: Updated for Version 8. Belmont (CA): Brooks/Cole-Thomson Learning; 2004. [Google Scholar]