Abstract
Cells of the monocyte series respond to follicle stimulating hormone (FSH) by poorly characterized mechanisms. We studied FSH-receptors (FSH-R) and FSH response in nontransformed human monocytes and in osteoclasts differentiated from these cells. Western blot and PCR confirmed FSH-R expression on monocytes or osteoclasts, although at low levels relative to ovarian controls. Monocyte and osteoclast FSH-Rs differed from FSH-R from ovarian cells, reflecting variable splicing in exons 8–10. Monocytes produced no cAMP, the major signal in ovarian cells, in response to FSH. However, monocytes or osteoclasts transcribed TNFα in response to the FSH. No relation of expression of osteoclast FSH-R to the sex of cell donors or to exposure to sex hormones was apparent. Controls for FSH purity and endotoxin contamination were negative. Unamplified cRNA screening in adherent CD14 cells after 2 hours in 25 ng/ml FSH showed increased transcription of RANKL signalling proteins. Transcription of key proteins that stimulate bone turnover, TNFα and TSG-6, increased 2–3 fold after FSH treatment. Smaller but significant changes occurred in transcripts of selected signalling, adhesion, and cytoskeletal proteins. We conclude that monocyte and osteoclast FSH response diverges from that of ovarian cells, reflecting, at least in part, varying FSH-R isoforms.
Keywords: Inositol-(1,45)-trisphosphate receptor 1; tumor necrosis factor-stimulated gene sequence-6; cofilin; fibronectin; PLAUR urokinase receptor
Introduction
Receptors for pituitary glycoprotein hormones that mediate endocrine response were once believed to be limited to endocrine glands. This is altered by discoveries including thyrotropin receptors (TSH-R) in mesenchymal stem cells that modulate bone turnover [1,2]. Our studies showed a direct effect of FSH on bone turnover [3]. Studies in murine marrow suggest that this is related to TNFα production by immune cells [4], but the response in human osteoclasts and their precursors to FSH has not been evaluated, beyond that the FSH increases the differentiation of osteoclasts in vitro [3]. Indeed, whether the osteoclast or its precursor are the responsive cells remains uncertain. Precedents for FSH response in macrophages include that FSH-dependent lactate and cAMP production in rat macrophages [5]. Skatchard analysis in these cells was consistent with FSH-R binding [6]. However, the investigators reporting these results were unable to amplify FSH-R by PCR [7].
The FSH receptor is a rhodopsin-like G protein-coupled receptor of ~60 kD. Glycoprotein hormone receptors act including Gαs (cAMP/cAMP-dependent protein kinase) or Gαi/o pathways [8] both of which occur in osteoclasts [9,10]. It has a conserved seven-transmembrane domain structure and an ~40 kDa extracellular hormone binding domain. There are many precedents for splice variants of the FSH-R [11,12,13], which in the ovary has ten exons, nine encoding the extracellular domain and a large tenth exon with the conserved seven-transmembrane domain. FSH-R variants might signal by alternative mechanisms, including FSH-R omitting the last extracellular domain (type 2) that may represent a developmental form of the FSH-R [13], and forms with a single transmembrane domain or missing a portion of the C-terminal [11,12], although physiological functions of these alternative transcripts are not clear.
We used affinity isolated CD14 human monocytes and osteoclasts produced in vitro from monocytes to evaluate the presence and activity of FSH-R under conditions with high sensitivity and specificity. Response of cells to FSH at short time points was studied by unamplified cRNA screening to avoid subjective judgements or observer bias. We find that human monocytes and osteoclasts express the FSH receptor at low levels. Receptor activity caused no detectable cAMP production. Several proteins, including TNFα and TSG-6, were transcribed after FSH stimulus. Small but significant changes were consistent with increased osteoclast differentiation and activity in response to FSH.
Materials and Methods
Human cells and media
Procedures were approved by the Institutional Review Board. Human peripheral blood monocytes by centrifugation on a density to isolate cells with specific gravity <1.077. Subjects were between the ages of 18 and 40. For selected assays where indicated affinity purified CD14+ cells were isolated by anti-CD14 immuno-magnetic selection with verification of purity by flow cytometry [14]. Osteoclast differentiation in vitro used recombinant human CSF-1 (M-CSF) and RANKL [15]. Endotoxin was assayed by ELISA using Limulus amebocyte lysate and a chromogenic substrate, Boc-Leu-Gly-Arg-p-nitroanalide with diazo blue to produce a diazonium-nitroanalide adduct with absorbance at 545 nm [16], standardized using E. coli endotoxin (Genscript, Piscataway NY).
Polymerase chain reaction assays
Messenger RNA was isolated and first strand cDNA synthesis was performed using antisense primers, for FSH-R targets, or random hexamers for other targets. Reverse transcription used MMLV reverse transcriptase (Superscript; Invitrogen). Quantitative PCR used brilliant Sybr green fluorescent DNA intercalating dye as the analyte. It was purchased in a mixture containing nucleotides and buffer (Stratagene, La Jolla, CA); reactions were initiated by adding 2.5 mM Mg, 100 nM of primers, and first strand mixture containing 1–2 μg of RNA. After 10 min at 95 °C, cycles of 30 sec at 95 °C and 1 min at 53–59 °C (depending on primers) were run on MX3000P RT-PCR (Stratagene) or mastercycler Gradient PCR (Epindorf, Hippauge, NY), for 40 cycles unless noted.
Oligonucleotide primers
All primers extend across introns. For FSH-R: sequences (GenBank NM_181446) were: Set 1 (exons 2–3; 120 bp product from transcript variants 1 or 2 [13]) f/CTCACCAAGCTTCGAGTCATCCAA r/AAGGTTGGAGAACACATCTGCCTCT. Set 2 (forward primer across exons 8 and 10, reverse in exon 10; transcript variant 2, 134 bp), f/TGGACCAGTCATTCTCTCTGA r/CTCTGCTGTAGCTGGACTCAT. Set 3 (exons 9–10, transcript variant 1, 124 bp), f/ATCTTAAGAAGCTGAGGGCCAGGT r/CAGTTTGCAAAGGCACAGCAATGG. Set 4 (variants 1 and 2, exons 8 to 10; 328 bp from variant 1; 142 bp from variant 2) f/CGGAGCCTCTGGACCAGTCATTCT r/TCTGCTGTAGCTGGACTCAT. Set 5 (variants 1 and 2, exons 8 to 10; 320 bp for variant 1, 140 bp for variant 2.) f/AGCCTCTGGACCAGTCATTCT r/CTCTGCTGTAGCTGGACTCAT. For glyceraldehyde-3-phosphate dehydrogenase (GAPDH) from NM_002046 f/GAGTCAACGGATTTGGTCGT r/TTGATTTTGGAGGGATCTCG, 237 bp. For TNFα (NM_000594) f/CCCAGGCAGTCAGATCATCTTC r/AGCTGCCCCTCAGCTTGA. Product 85 bp.
Western analysis and cAMP assays
Antibodies to the FSH receptor were rabbit anti-FSH receptor polyclonal AB75200 from AbCam (Cambridge, MA), recognizing an internal peptide of the extracellular domain of human FSH-R, or goat anti FSH-R polyclonal antibody sc7798, from Santa Cruz (Santa Cruz, CA, USA), raised against a 20 amino acid peptide from the N-terminal of human FSH-R. For Western blots, cells were lysed in 0.3% SDS, 50 mM tris, pH 7, with proteinase and phosphatase inhibitors [17]. Proteins were separated on SDS-PAGE, transferred to derivitized nylon, reacted with primary antibodies followed by alkaline phosphatase-coupled secondary antibodies and visualized by chemiluminescence (ECL plus, Amersham, Piscataway, NJ). Cyclic AMP produced by adherent human mononuclear cells was measured by enzyme linked immunoassay (Stressgen, Ann Arbor, MI).
Genome-wide expression screening
Gene-screening was essentially as described [18], using isolated RNA to make double-stranded cDNA, from which biotin labeled cRNA was made an hybridized to the DNA array on glass, the Hu 133.2 probe-set of 54676 assays with 20 replicates per target (Affymetrix, Santa Clara, CA). Targets include segments of most known genes, most with two or more probes per target. Presence of transcripts and differences between treatments were determined from the signal and variation of each assay replicate, with statistical confidence indicated. Analysis included determination of effects on common metabolic pathways by MetaCore (www.genego.com, St Joseph, MI) comparing effects of FSH treatment and control against a library of 121 intracellular pathways. Analysis excluded genes not expressed (p > 0.05) with and without FSH. Only differences with p < 0.002 for different expression between conditions were considered.
Results
Western blots for FSH-R in human monocytes and osteoclasts
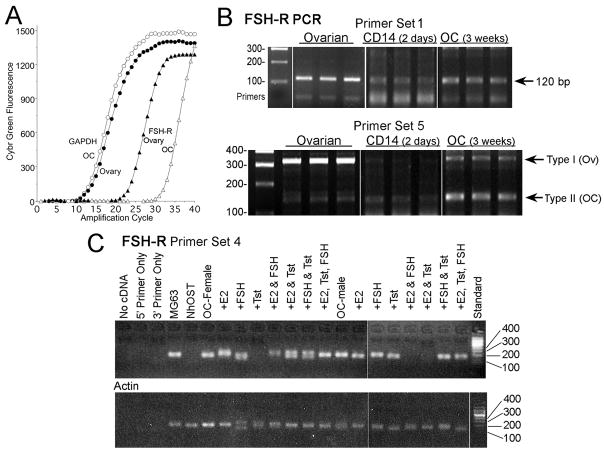
Western blots for FSH-R were performed using antibodies raised against an internal peptide of the extracellular domain (Fig 1A) or an N-terminal FSH-R epitope (Fig 1B). Ovarian controls showed the receptor clearly; much smaller amounts of protein were detected in osteoclasts or monocytic cells. The FSH-R had a lower apparent Mr than receptor in ovarian cells on 7% agarose. The difference was confirmed with improved resolution of the monocyte receptor using larger amounts of protein and by separation on 12% agarose (Fig 1B). Controls including human osteoblasts were negative for FSH-R. The apparent Mr, ~55 kD, was consistent with the reported type 2 receptor transcript [13], the function of which was previously unclear. The difference in receptor could also reflect variation in the processing and technical differences in Western blots, so the differences were confirmed using PCR probes for FSH-R exons 8–10.
Figure 1. Western blots of human peripheral blood monocytes for FSH receptor.
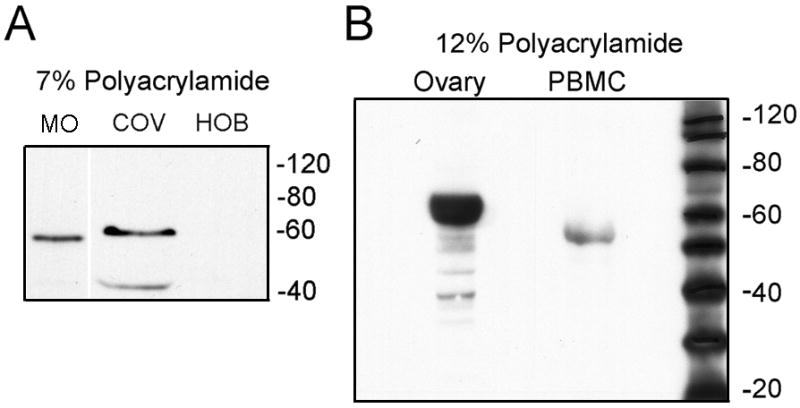
A. Western blot of 20 μg cell extracts separated on 7% acrylamide analyzed using anti FSH-R (AbCam) recognizing an internal peptide of the receptor. Left: human monocytes (MO) in RANKL and CSF-1 3 days. Middle: ovarian cell extract (COV; positive control). Right: human osteoblasts (negative). Irrelevant lanes between MO and COV are deleted for better comparison of Mr.
B. High resolution Western blot. Fifty μg cell extracts separated on 12% polyacrylamide were evaluated using antibody (Santa Cruz) to an N-terminal epitope of the FSH-R. A protein with Mr ~55 kD is again identified in isolated human peripheral blood monocytes (PBMC). The positive control (ovarian extract, left) has much more FSH-R than PBMC, as in (A), with Mr ~ 60 kD.
Human macrophages and osteoclasts contain mRNA for FSH-R
Messenger RNA for the FSH-R was a low level product in human monocytes and osteoclasts, amplifying ~8 cycles later than mRNA for the receptor in ovarian cells (Figure 2A). Probe-set 1 (Fig 2B, top) gave results typical of probes including an intron in regions with no known variability in mRNA, weak reactivity in CD14 cells and more mRNA in osteoclasts. Probe-sets that amplify two known variants of the receptor gave consistent results. The major transcript in ovarian cells was, as expected, type 1, containing exons 8, 9, and 10, with a trace of type 2 omitting exon 9 (Fig 2B, bottom). Both CD14-purified monocytes and osteoclasts produced mainly products of ~ 140 bp, the type 2 isoform. These are consistent with Fig 1; the type 2 isoform is 60 amino acids shorter than type 1. Differences in glycosylation or other post-translational processing may also occur. A product at 190 bp was in some cases seen in CD14 cells; this was an artifact (by sequencing; not shown). Probes for type 1 or type 2 isoforms only (Methods) provided no additional information. Fragments expected to amplify with these probe-sets were seen (not shown). Amplification of segments of exons 8–10 was verified by sequencing (not shown).
Fig 2. FSH-R in human monocytes and osteoclasts by PCR assays.
All FSH-R amplicons cross exons to avoid amplifying trace genomic DNA (see Methods)
A. GAPDH and FSH-R signal indicated as fluorescence of Sybr green (abscissa) as a function of cycle number (ordinate). The data use cDNA samples amplified with FSH-R primer set 1; the amplified FSH-R fragments (right two curves) are shown in the top row of (B), lanes 3 and 9.
B. FSH-R transcripts produced by monocytes and osteoclasts mainly exclude exon 9 of ovarian FSH-R. Probe-set 1 (top panel) shows results typical of probes including an intron but in regions without known variability in mRNA. The product in three independent reactions using CD14 cells was slightly less than in osteoclasts, and much less than in ovarian cells (see A). Probe-sets that amplify variants of the receptor including or excluding exon 9 differentiated FSH-R in the different cell types (bottom). The major amplicon in ovarian cells is the type 1 transcript containing exons 8, 9, and 10 (320 bp band). A trace of the type 2 transcript (140 bp) was also present, in keeping with other reports. In contrast, assays using CD14 macrophages or osteoclasts after three weeks in CSF-1 and RANKL showed major isoforms with sizes of ~140 bp but only traces of the ovarian form containing exon 9. Replicates are all from independent PCR reactions. Products were verified by sequencing (see text).
C. FSH-R expression in osteoclasts does not vary with cell donor sex or with exposure to FSH or sex steroids. No consistent difference in FSH-receptor was seen in cells in osteoclast differentiation medium 10 days using monocytes from male or female donors, with or without sex steroids and FSH. Additions as indicated were: 10 nM estradiol (E2), 100 ng/ml of FSH, or 10 nM Delatestryl (Tst). Amplification of FSH-R (top panel) used primer pair 4. The 142 bp region amplified in all but three osteoclast samples; negatives occurred randomly and probably reflect technical errors. The split bands in a few samples are believed to be artifacts but were not in this case sequenced. Controls shown are actin amplified from the same mRNA samples (bottom panel) and FSH-R reactions from human osteoblast (NhOst) cDNA (negative), and the osteoblast-like cell line MG63, in which detectible FSH-R was present.
Sex and sex hormone status not reflected in FSH-R expression in osteoclasts
Cells from male or female donors in osteoclast differentiation media with estrogen, testosterone, FSH, combinations of these, or no added hormones, were used to determine whether FSH-R transcription reflects the hormonal milieu (Fig 2C). There was no consistent variation with hormonal conditions. PCR showed the receptor in ~85% of cDNA preparations; negative assays, including assays from cell preparations made from the same starting cells probably reflect the technical difficulty of amplification of the low-abundance targets. The primer set 5 is contained within primer set 4. Primer set 5 detected small amounts of 320 bp product when samples from Fig 2C were re-amplified, in keeping with results in Fig 2B (not shown).
FSH increases production of TNFα in adherent human monocytes
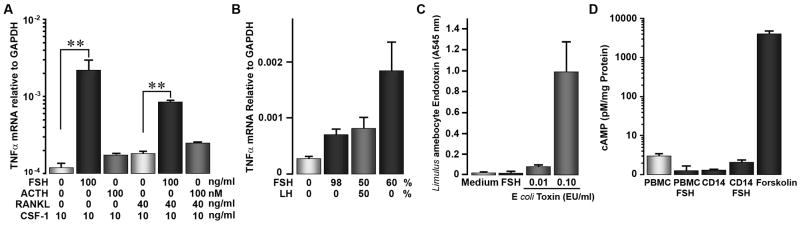
A number of endocrine effects on bone are related to TNFα production, including effects of TSH [19]. Increases in TNFα in murine marrow cells followed FSH treatment [4]. Issues include whether TNFα is a common pathway, whether human cells respond similarly, and whether monocytes are the hormone-responsive cells. We maintained adherent human monocytes ± RANKL for 1 week in 10 ng/ml CSF-1. At seven days, surviving cells were almost entirely adherent monocyte-derived cells. Production of TNFα was increased, with a high degree of confidence, in these cells by 25 ng/ml FSH added for 2 hours (Figure 3A, left bars). An unrelated hormone, ACTH, had no measurable effect. Differences between monocyte and osteoclast TNFα response were small, and it not clear if they are meaningful (Fig 3A, right bars).
Figure 3. FSH increases FSH mRNA in adherent human monocytes.
There was variation in quantitative response between assays and between FSH preparations, but all cell preparations and all FSH sources gave qualitatively similar effects.
A. In 7 day cultures of adherent monocytes and osteoclasts, production of TNFα mRNA by real-time PCR was increased by addition of 25 ng/ml FSH. ACTH, 100 nM, is included as a control. Medium was Dubelco’s MEM with 10% fetal calf serum and 10 ng/ml CSF-1; RANKL for osteoclast differentiation was 40 ng/ml. N=2., mean ± range.
B. Different sources of FSH increase TNFα production; admixture with LH had no effect. TNFα mRNA transcription in response to three preparations of human FSH are shown. Highly pure and partially pure FSH caused significant increases in TNFα mRNA relative to controls without FSH (left bar) A mixture of pure FSH with an equal amount LH (third bar), did not change response relative to FSH alone. N=2, mean ± range.
C. Endotoxin does not contribute to the FSH effect. The Limulus amebocyte assay was used; endotoxin controls at 0.01 and 0.1 EU/ml are shown (right two bars). Osteoclast medium and each FSH sample gave results corresponding to endotoxin levels of 0.001 EU/ml or less. N=2, mean ± range.
D. Cyclic AMP is not produced in response to FSH, 25 ng/ml, 15 minutes, added to mononuclear cell cultures. A standard curve was used to calculate cAMP. Control untreated and FSH treated mononuclear cells and osteoclasts are shown, in addition to a forskolin (1 μM, 15 minutes) positive control cells (right bar). Note the logarithmic scale.
Comparison of different FSH preparations and endotoxin; lack of cAMP response
We compared TNFα mRNA transcription in response to three preparations of human FSH (Fig 3B). Both pure and partially purified FSH both increased in TNFα mRNA. Mixing FSH with an equal amount LH did not affect response. Variation in potency in the range of two fold was seen, but the only consistent effect was that response decreased after freezing. Thus, it is likely that denaturation of FSH reduces response. Because bacterial cell wall contaminants (endotoxin) might stimulate TNFα, Limulus amebocyte assay was used to rule out endotoxin contamination (Fig 3C). Osteoclast medium and each FSH sample contained no detectable endotoxin. Ovarian response to FSH is mediated by cAMP, and reported in testicular macrophages [5]. However, with a strong forskolin control (Fig 3D), no cAMP production response to FSH was seen in human monocyte cultures. The assay detects responses of ~0.1% of maximally stimulated cAMP production; note the logarithmic scale in Fig 3D.
Effects of FSH on genome-wide transcription in human monocytes
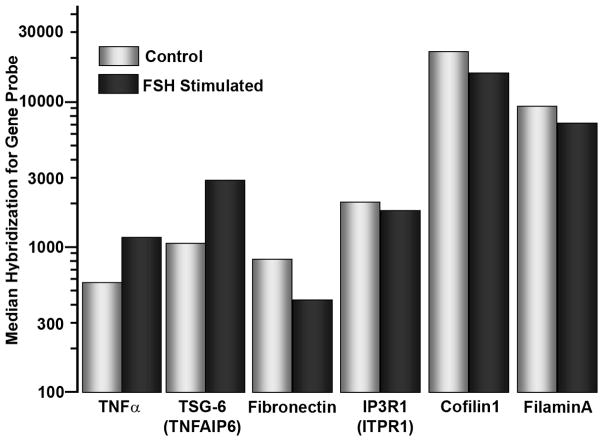
We isolated the CD14 cells by immuno-magnetic bead binding to reduce T or B cell contaminants to ~1% [14]. These cells, after incubation in medium with CSF-1 (10 ng/ml) for two days, were treated, or not, with FSH 25 ng/ml for 2 hours, and the cells were harvested for cRNA-based gene screening. Results were analyzed using Metacore (GeneGo, St Joseph, MI) (Table I), evaluating the effects of FSH treatment against a library of 121 intracellular pathways. Five pathways with the most consistent effects are summarized in Table I. These pathways included overlap, particularly in NF-κB related genes. Other pathways with lower p-values (not shown) suggested effects related to TGFβ, lymphotoxin, and inflammatory response; these will require further analysis for confirmation. Differences in transcription of specific genes of interest in osteoclasts are shown (Fig 4). These include, in addition to TNFα itself, TSG-6, a hyaluronan-binding protein that is a TNF-responsive product that activates bone resorption and decreases bone formation [20]. There were smaller but significant and consistent ~40% negative changes in several attachment and cytoskeletal regulating proteins, the Inositol-(1,4,5)-trisphosphate receptor-1 [17], cofilin, fibronectin, and the urokinase receptor PLAUR (Fig 4, right four bars). There were moderate and less consistent effects, mainly negative and in the ~20–30% range, on selected steroid response genes and chaperone proteins, such as HSP70. These effects, while individual probes showed effects with p < 0.002, were not selected by pathway analysis. More detailed and specific studies will be needed to confirm them and to determine their importance, if any.
Table I. Regulatory pathways modified in CD14 human monocytes by 2 hour FSH treatment.
Genome-wide transcriptional activity without and with 25 ng/ml FSH were compared against 121 consensus pathways, the five most significant shown. Only gene products transcribed above background (p < 0.05) and in different amounts after FSH treatment (p < 0.002) were considered.
| Pathway/P value | Upregulated/Downregulated | Comment |
|---|---|---|
| [a] TLRs and IL1-R associated kinases/2.23 × 10−8 | IRAK2 †, TNFα, NF-κB*, RELB †, IL1β*/TLR4*, CD14, STAT1, IKK †, CathepsinB/S/L* | FSH increases osteoclast differentiation [3] |
| [b] Cell adhesion and immune response/3.45 × 10−8 | ICAM1* †, IL1β*, MEK3, NIK, IκB, CXCL1, SP1, NF-κB*/JAK1, MEK2, ERK1/2, IKKβ†, C/EBPβ | Modified cell adhesion |
| [c] Gαs regulatory pathways/3.46 × 10−8 | MEK3, β-Catenin, NF-κB*/Gαs/q, SOS2, IP3R1, PKRA1A*, MEK2, ERK,C/EBPβ | Downregulate cAMP response |
| [d] Anti-apoptotic TNFs/NF-κB/Bcl-2/3.71 × 10−8 | IRAK2 †, TNFα, TRAF3, NF-κB*, IκB, RELB †/STAT1, IKK † | Promote osteoclast survival |
| [e] TGF, WNT, cytoskeletal remodeling/3.71 × 10−7 | PLAUR (urokinase receptor), β-Catenin, Others in [b]/Fibronectin*, FOX03, CDC42*, SOS2, actinB*/G*, cofilin*, eIF4E | Increased turnover of attachment structures |
Differences shown were > 30% between FSH treated and untreated. Differences confirmed by multiple probes meeting these criteria (*) and differences greater than ten fold (†) are indicated. Titles in the first column describe key genes in osteoclast differentiation. Original pathway designations are [a] Bacterial infections in CF airways; [b] Immune response_IL-17 signaling pathway; [c] Development_A2B receptor: action via G-protein alpha s; [d] Apoptosis and survival_Anti-apoptotic; [e] Cytoskeleton remodeling_TGF, WNT and cytoskeletal remodeling. For detailed algorithms and p value calculation, see http://genego.com.
Figure 4. Changes in selected genes in two day CD14 adherent monocytes after two hour FSH treatment (25 ng/ml).
The ordinate roughly correlates with transcript abundance. The data show 2–3 fold (note log scale) for increases in TNFα and TSG-6 mRNA after FSH treatment, and ~2-fold decrease in fibronectin. Cytoskeletal organizing proteins, including cofilin1 and filaminA, were modestly, ~40%, but consistently decreased. Multiple probes showed similar magnitude effects, except for TSG-6, for which only one probe-set was included in the array. For each measurement, n=20. All differences shown had p < 0.001 between untreated and FSH treated measurements in each pair.
Discussion
While low abundance receptors are sometimes of minimal importance, the G-protein coupled receptors are an exception. One receptor may trigger a cellular response [21]. However, analysis of low-abundance targets requires meticulous technique. The conditions indicated, particularly for PCR analysis represent as accurately as possible the buffers, substrate, cDNA production, and settings on the instruments we used. However, it is recommended that, for replication of our experiments, the annealing temperatures be re-examined using a gradient thermocycler to establish ideal conditions. The temperature at annealing, while indicated to a tenth of a degree in most instruments, may differ significantly from the expected temperature. We obtained the most consistent results when using probe-specific antisense cDNA for reverse transcription. All FSH-R amplicons should cross an exon boundary to avoid genomic DNA artifacts. Sequencing of products is recommended also, since artifacts often contaminate low level PCR products.
Although we considered the possibility of endotoxin contamination and tested several sources of FSH, it remains a concern that the effects of FSH on gene transcription overlap with the expected effects of activating toll-like receptors or other inflammatory pathways. Thus, while our results are mainly consistent with pathways reported [3], it is possible that FSH effects are mixed with nonspecific activation of the cells due to the low abundance of the specific receptors. On the other hand, two hour FSH response in TNF and TSG-6 in CD14 purified cells were similar to the discrete effects on TNF by PCR analysis of long-term monocyte and osteoclast assays, and different sources of FSH produced similar effects (Fig 3), arguing that the results, at least in the main, reflect FSH response accurately.
We conclude that human monocytes and osteoclasts respond to FSH mainly via FSH-R isoforms that occur due to variable splicing of the primary transcript of the receptor. This involves, at least in part, differential splicing between exons 8 and 10, previously described to produce variants of the receptor during development [13], but where the functional importance was unclear. There are many other splice variants of the FSH-R, and it is possible that monocytes and osteoclasts produce additional variants that may affect signalling (e.g., [11,12]) which were not detected. Human monocytic cells did not produce cAMP in response to FSH, but gene screening showed increased transcription of RANKL signalling proteins. Transcription of two key proteins that stimulate bone turnover, TNFα and TSG-6 [4,20], increased 2–3 fold after 2 hour FSH treatment. Additionally, proteins that regulate cellular attachment and motility, including the Inositol (1,4,5)-trisphosphate receptor-1 [17], cofilin, fibronectin, and the urokinase receptor PLAUR were downregulated, although by moderate amounts (Table 1, Fig 4). Thus, monocyte or osteoclast FSH response diverges from that of ovarian cells due to different FSH-R isoforms that occur, at least in part, via alternative processing of the primary FSH-R transcript.
Acknowledgments
Supported by National Institutes of Health grants AR053976, AR055208, AR053566 and by the Department of Veteran’s Affairs (USA). MZ and LS also acknowledge National Institutes of Health grants AG23176, DK70526, and DK70526.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marians RC, Ng L, Blair HC, et al. Defining thyrotropin-dependent and-independent steps of thyroid hormone synthesis by using thyrotropin receptor-null mice. Proc Natl Acad Sci USA. 2002;99:15776–15781. doi: 10.1073/pnas.242322099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abe E, Marians RC, Yu W, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003;115:151–162. doi: 10.1016/s0092-8674(03)00771-2. [DOI] [PubMed] [Google Scholar]
- 3.Sun L, Sharrow AC, Zhang Z, et al. FSH Directly Regulates Bone Mass. Cell. 2006;125:247–260. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 4.Iqbal J, Sun L, Blair HC, Zaidi M. FSH Stimulates TNF Production From Immune Cells. Proc Natl Acad Sci USA. 2006;103:14925–14930. doi: 10.1073/pnas.0606805103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yee JB, Hutson JC. Testicular macrophages: isolation, characterization and hormonal responsiveness. Biol Reprod. 1983;29:1319–1326. doi: 10.1095/biolreprod29.5.1319. [DOI] [PubMed] [Google Scholar]
- 6.Yee JB, Hutson JC. Biochemical consequences of follicle-stimulating hormone binding to testicular macrophages in culture. Biol Reprod. 1985;32:872–879. doi: 10.1095/biolreprod32.4.872. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter AM, Lukyanenko YO, Lee VH, Hutson JC. FSH does not directly influence testicular macrophages. J Androl. 1998;19:420–427. [PubMed] [Google Scholar]
- 8.Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- 9.May LG, Gay CV. G-protein involvement in parathyroid hormone regulation of acid production by osteoclasts. J Cell Biochem. 1997;64:161–170. [PubMed] [Google Scholar]
- 10.Gorn AH, Rudolph SM, Flannery MR, et al. Expression of two human skeletal calcitonin receptor isoforms cloned from a giant cell tumor of bone. J Clin Invest. 1995;95:2680–2691. doi: 10.1172/JCI117970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattiske D, Pask AJ, Shaw JM, Shaw G. Structure and expression of the follicle-stimulating hormone receptor gene in a marsupial, Macropus eugenii. Mol Reprod Dev. 2002;63:24–31. doi: 10.1002/mrd.10161. [DOI] [PubMed] [Google Scholar]
- 12.Sairam MR, Jiang LG, Yarney TA, Kahn H. Alternative splicing converts the G-protein coupled follitropin receptor gene into a growth factor type I receptor. Mol Reprod Dev. 1997;48:471–479. doi: 10.1002/(SICI)1098-2795(199712)48:4<471::AID-MRD7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 13.Rajapaksha WR, Robertson L, O’Shaughnessy PJ. Expression of follicle-stimulating hormone-receptor mRNA alternate transcripts in bovine granulosa cells during luteinization in vivo and in vitro. Mol Cell Endocrinol. 1996;120:25–30. doi: 10.1016/0303-7207(96)03816-6. [DOI] [PubMed] [Google Scholar]
- 14.Robinson LJ, Yaroslavskiy BB, Griswold RD, et al. Estrogen inhibits RANKL-stimulated osteoclastic differentiation of human monocytes through estrogen and RANKL-regulated interaction of estrogen receptor-α with BCAR1 and Traf6. Exp Cell Res. 2009;315:1287–1301. doi: 10.1016/j.yexcr.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yaroslavskiy BB, Zhang Y, Kalla SE, et al. NO-dependent osteoclast motility. J Cell Sci. 2005;118:5479–5487. doi: 10.1242/jcs.02655. [DOI] [PubMed] [Google Scholar]
- 16.Ketcyum PA, Novitsky TJ. Assay of endotoxin by Limulus amebocyte lysate. Meth Molecular Med. 2000;36:3–12. doi: 10.1385/1-59259-216-3:3. [DOI] [PubMed] [Google Scholar]
- 17.Yaroslavskiy BB, Sharrow AC, Wells A, et al. Necessity of inositol-(1,4,5)-trisphosphate receptor 1 and μ-calpain in NO-induced osteoclast motility. J Cell Sci. 2007;120:2884–2894. doi: 10.1242/jcs.004184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bu R, Borysenko CW, Li Y, et al. Expression of TNF-family proteins and Receptors in Human Osteoblasts. Bone. 2003;33:760–770. doi: 10.1016/j.bone.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Wang HC, Dragoo J, Zhou Q, Klein JR. An intrinsic thyrotropin-mediated pathway of TNFα production by bone marrow cells. Blood. 2003;101:229–236. doi: 10.1182/blood-2002-02-0544. [DOI] [PubMed] [Google Scholar]
- 20.Mahoney DJ, Mikeczz K, Ali T, et al. TSG-6 regulates bone remodeling through inhibition of osteoblastogenesis and osteoclast activation. J Biol Chem. 2008;283:25952–25962. doi: 10.1074/jbc.M802138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blair HC, Wells A, Isales CM. Pituitary glycoprotein hormone receptors in non-endocrine organs. Trends Endocrinol Metabolism. 2007;18:227–233. doi: 10.1016/j.tem.2007.06.001. [DOI] [PubMed] [Google Scholar]