Abstract
In Drosophila, segmentation genes partition the early embryo into reiterative segments along the anterior-posterior axis, while Hox genes assign segments their identities. Each segment is also subdivided into distinct anterior (A) and posterior (P) compartments based on the expression of the engrailed (en) segmentation gene. Differences in Hox expression often correlate with compartmental boundaries, but the genetic basis for these differences is not well understood. In this study, we extend previous results to describe a genetic circuit that controls the differential expression of two Hox genes, Ultrabithorax (Ubx) and abdominal-A (abd-A), within the A and P compartments of the abdominal ectoderm. Consistent with earlier findings, we show that en is essential for high Abd-A levels and low Ubx levels in the P compartment, whereas sloppy-paired (slp) is required for high Ubx levels in the A compartment. Overall, these results demonstrate that the compartmental expression of Ubx and abd-A is established through a repressive regulatory network between en, slp, Ubx and abd-A. We also show that abd-A expression in the P compartment is important for the formation of abdominal-specific cell types, suggesting that en and slp modulation of Hox expression within the A and P compartments is essential for embryonic patterning.
Keywords: Segmentation, Hox, compartment
INTRODUCTION
Drosophila embryogenesis provides one of the best-characterized models for understanding how a fertilized egg develops into a complex organism. Early in development the fly embryo is subdivided into segments that give rise to specialized head, thoracic and abdominal structures in the larva and adult fly. Genetic studies have revealed that the processes of dividing the embryo into repetitive segments (segmentation) and the assignment of each segment its unique fate (segment identity) are controlled by two distinct groups of genes (Carroll et al., 2001; DiNardo et al., 1994; Hatini and DiNardo, 2001; Lawrence, 1992; Lawrence and Struhl, 1996; Manak et al., 1994; McGinnis and Krumlauf, 1992; Sanson, 2001; St Johnston and Nusslein-Volhard, 1992). The segmentation genes, which encode both transcription factors and cell signaling molecules, subdivide the embryo into segments, while the Hox genes encode a family of homeodomain transcription factors that specify segment identity.
The segmentation genes further subdivide each segment of the Drosophila embryo into distinct anterior (A) and posterior (P) compartments. A compartment consists of groups of cells that share a common ancestry and identity (Blair, 1995; Garcia-Bellido et al., 1973; Lawrence, 1992; Lawrence and Struhl, 1996; Martinez-Arias and Lawrence, 1985). Compartments are given their identities by the expression, or lack of expression, of selector genes. For example, the P compartments in Drosophila express engrailed (en) while the A compartments do not express this gene (DiNardo et al., 1985; Fjose et al., 1985; Kornberg, 1981; Kornberg et al., 1985; Lawrence and Morata, 1976; Morata and Lawrence, 1975; Vincent and O’Farrell, 1992). en encodes a homeodomain-containing transcription factor that regulates target genes that encode transcription factors, cell signaling molecules, and adhesion proteins in P cells, giving them a unique identity (Desplan et al., 1985; Gibert, 2002; Jaynes and O’Farrell, 1991; Solano et al., 2003). The lack of P cells in en mutant embryos results in the mis-regulation of other segment polarity genes such as the wingless and hedgehog signaling genes, and a subsequent failure in segmentation and patterning of the fly embryo (Lawrence, 1992; Lawrence et al., 1999; Lawrence et al., 1996; Lawrence and Struhl, 1996).
Compartments play important roles in regulating the expression of many key developmental genes, including the Hox genes. It has long been recognized, for example, that the expression domains of Hox genes respect compartment boundaries (Carroll et al., 1988; Karch et al., 1990; Lawrence, 1992; Macias et al., 1990). The anterior expression boundaries of many Hox genes, including Ultrabithorax (Ubx), Antennapedia (Antp), abdominal-A (abd-A), Sex combs reduced (Scr), and Deformed (Dfd) all coincide with an A/P compartment boundary. In addition, Hox expression patterns in Drosophila are typically modulated in a segmentally reiterated pattern suggesting that segmentation genes are likely to fine-tune Hox expression patterns. For example, abd-A is expressed highly in P compartments of the abdomen, and in a lower, more graded fashion in A compartments (Macias et al., 1994). This abd-A pattern is partially complementary to the expression of Ubx, which is low in P compartments and high in A compartments (Mann, 1994). Moreover, the en segmentation gene has been shown to modulate different expression levels of abd-A and Ubx within the P compartment (Macias et al., 1994; Mann, 1994). However, while we have some understanding of how Hox expression patterns are established, many aspects, including how the segmentally reiterated patterns of Ubx and Abd-A are established, are not well understood.
There are several indications in the literature suggesting that segmentally modulated Hox expression patterns are functionally important for development. One such example is how the two Hox genes, Ubx and abd-A, repress their target gene Distalless (Dll) in the abdomen (Cohen et al., 1989; Mann, 1994; Vachon et al., 1992). Dll is a leg selector gene that is also required for the formation of the Keilin’s organ (KO), a thoracic-specific larval sensory structure (Cohen et al., 1991; Cohen et al., 1989). Both the leg and the KO are derived from cells that straddle the A/P compartment boundary (Struhl, 1984). In wild type embryos, repression of Dll by Ubx and abd-A blocks leg and KO formation in the abdomen (Vachon et al., 1992). In Ubx mutant larvae, Dll is de-repressed in the A compartment of the first abdominal segment resulting in the partial formation of a KO (Mann, 1994). Conversely, in abd-A mutant larvae, Dll is de-repressed in abdominal P compartments resulting in the partial formation of KO in abdominal segments A1 to A7 (Mann, 1994). Thus, Abd-A represses Dll in the P compartments of A1 to A7 while Ubx represses Dll in the A compartment of A1. Recently, a dissection of a cis-regulatory element responsible for Dll repression in the abdomen has provided molecular insights underlying Hox-mediated repression (Gebelein et al., 2002; Gebelein et al., 2004). In the P compartment, Abd-A binds this element directly with En to repress Dll, while in the A compartment, Ubx binds this element with an anterior-specific segmentation gene, sloppy paired (slp), to repress Dll. Thus, abdominal repression of Dll and the suppression of KOs, is mediated by the collaboration of the compartment-specific factors En and Slp with the compartmentally-modulated Hox proteins Abd-A and Ubx, respectively. These findings suggest that interactions between segmentation and segment identity genes regulate gene expression and thereby cell fate in a compartment-specific manner.
In this study, we characterize the regulatory relationships between the slp and en segmentation genes and the abd-A and Ubx Hox genes. Previous results suggested that en regulates the expression of abd-A and Ubx in the P compartment (Macias et al., 1994; Mann, 1994). Here, we extend these observations to describe a repressive genetic circuit between en, slp, abd-A and Ubx by using mutations that also removed engrailed’s sister gene (invected) as well as through a number of loss- and gain-of-function assays with en and slp. Our findings confirm that en regulation of abdominal Hox genes results in high levels of Abd-A and low levels of Ubx in the P compartment (Macias et al., 1994; Mann, 1994), and show that slp is required for the high levels of Ubx in the A compartment. Moreover, we found that abd-A expression in the P compartment correlates with the formation of abdominal-specific sensory organ structures (the lateral chordotonal organs, lch5) and secretory cells (oenocytes) (Brodu et al., 2002; Heuer and Kaufman, 1992; Wong and Merritt, 2002). Thus, the En and Slp transcription factors not only function as abdominal Hox co-factors in regulating key target genes like Dll, but en and slp modulation of Hox expression levels within the A and P compartments is critical for patterning the embryo.
Materials and Methods
Fly stocks and antibody stainings
Immunocytochemistry was performed using the following antibodies: mouse anti-En (1:10, mAb4D9, the Developmental Studies Hybridoma Bank (DSHB) Univ of Iowa), mouse anti-Ubx (1:20, FP3.38) (White and Wilcox, 1985), rabbit anti-Ubx (1:500, a gift from Kevin White), mouse anti-Abd-A (1:400, a gift from Ian Duncan), rat anti-Abd-A (1:500) (Karch et al., 1990), guinea pig anti-Slp1 (1:500, a gift from John Reinitz) (Kosman et al., 1998), rabbit anti-β-gal (Cappell) and mouse anti-22C10 (1:20, DSHB). Mis-expression of UAS-Ubx, UAS-Abd-A, UAS-En, UAS-VP16En, and UAS-Slp1 was driven by prd-Gal4. The ato-lacZ fly line (ato7.2kb-lacZ) was a gift from Yuh Nung Jan (Sun et al., 1998). The fly mutations used were as follows: Df(2R)en-E (removes en and invected, from Gary Struhl), ubxMX12, abd-AM1, slpΔ34 (Cadigan et al., 1994b), and slp/en (Df(2L)edSZ1, Df(2R)en-E) double mutations (Cadigan et al., 1994b). Embryos were harvested, de-chorionated, fixed and immunostained using standard techniques. Images were taken using a Bio-Rad confocal microscope or a Zeiss Apotome fluorescent microscope.
RESULTS
Compartment-specific expression patterns of Ubx and Abd-A in the Drosophila abdomen
By stage 11 of embryogenesis, Ubx and Abd-A exhibit complex and segmentally reiterated expression patterns in the ectoderm (Fig. 1A). The primary anterior limits for Ubx and Abd-A are PS6 and PS7, respectively, although low levels of Ubx are observed in PS5 (Macias et al., 1990; White and Wilcox, 1985). At this stage, the highest levels of Ubx and Abd-A are in adjacent anterior and posterior domains, respectively. Within each segment, cells within the ectoderm are assigned either an anterior (A) or posterior (P) compartment fate based on the expression of en. en encodes a transcription factor that specifies P cell identity and thus, is a useful marker for the P compartment (Kornberg, 1981; Kornberg et al., 1985; Lawrence and Morata, 1976; Morata and Lawrence, 1975). Analysis of Hox expression patterns with En revealed that, consistent with previous results (Carroll et al., 1988; Macias et al., 1990; Macias et al., 1994; Mann, 1994), Abd-A levels are high and Ubx levels are low within the P compartment (Fig. 1D and 1E). In the A compartment, high levels of Ubx expression are observed, but only in the half immediately anterior to En-positive P cells. As development progresses Abd-A levels increase in cells of the A compartment that lack significant Ubx expression (Fig. 1B and 1C), and Ubx expression in the P compartment weakly increases within PS6 but not within parasegments that express abd-A (Fig. 1E and F). Thus, throughout most of embryogenesis, the abdominal ectoderm from PS6 through PS12 express Ubx and Abd-A in an alternating pattern that largely correlates with compartmental identity.
Figure 1. Compartmental expression pattern of Ubx and abd-A.
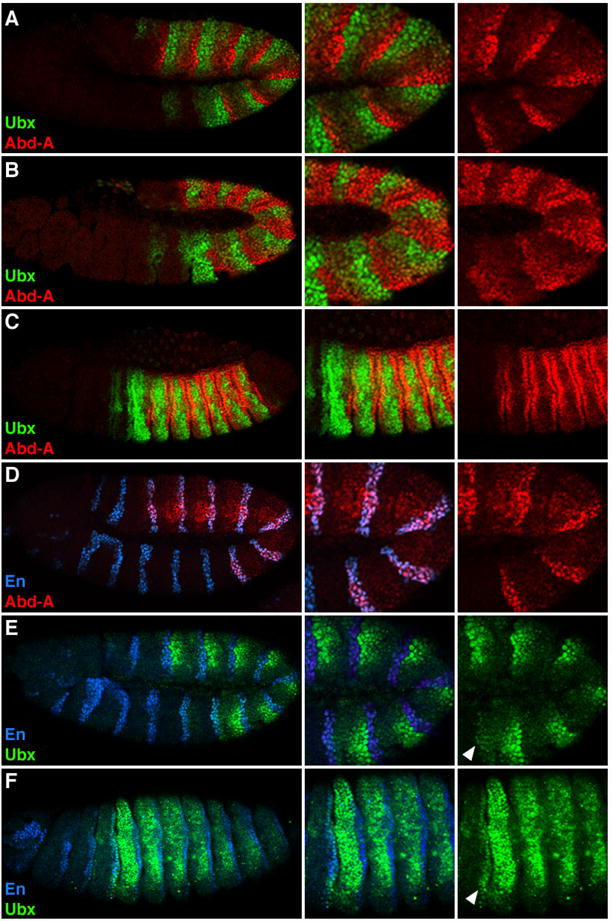
Lateral views of Drosophila embryos immunostained for Ubx (green), Abd-A (red) and En (blue). A, B and C. Wild type embryos showing the expression patterns of Ubx and Abd-A at stage 11 (A), stage 12 (B), and stage 14 (C). D and E. Wild type stage 11 embryos have high levels of Abd-A and low levels of Ubx in En-positive cells. F. Stage 16 wild type embryo showing increased levels of Ubx in En-positive cells within ps7 (arrowhead).
Compartmental modulation of abdominal Hox expression is important for patterning the embryonic ectoderm
The expression patterns described above raise the question of why the levels of Ubx and Abd-A are modulated in the abdominal ectoderm. In Drosophila, the ectoderm differentiates to form the epidermis and the nervous system. The distinct expression of Ubx and abd-A in the A and P compartments suggest that these Hox factors may play different roles in patterning these tissues. In support of this idea, previous studies have shown that abd-A, but not Ubx, regulates the formation of specific abdominal cell types and sensory organs. For example, abd-A is required for the induction of secretory cells known as oenocytes (Brodu et al., 2002). To determine if oenocytes form within the P compartment, we immuno-stained svp-lacZ (an early marker of oenocytes (Elstob et al., 2001)) embryos for En, Abd-A and β-gal. As shown in Fig 2, svp-lacZ is expressed in En-positive cells that express high levels of Abd-A, demonstrating the origin of these cells is the P compartment. abd-A is also known to modulate the development of stretch receptors known as chordotonal (ch) organs (Heuer and Kaufman, 1992; Wong and Merritt, 2002). A ch organ consists of from one to 80 closely associated sensory structures called scolopodia, each of which develops from a single sensory organ precursor (SOP) cell (Lai and Orgogozo, 2004). In the fly embryo, a set of ch organs develops within the P compartment of each body segment (neurons visualized using mAb22C10 and the P compartment marked by En-lacZ, Fig 2b). However, the number and position of the scolopodia differ between the thorax and abdomen. The T2 and T3 thoracic ch organs contain three scolopodia with neurons located in a dorsal position (dch3), whereas the A1 through A7 ch organs contain five scolopodia with neurons located in a lateral position (lch5) (Fig. 2C). Previous studies have shown that the difference between thoracic and abdominal ch organs is dependent upon abd-A, as the ch organs within the A1 through A7 segments of abd-A− embryos are transformed into a dch3 fate (Heuer and Kaufman, 1992; Wong and Merritt, 2002). In contrast, the lch5 organs in the abdomen form normally in the P compartment of each abdominal segment in Ubx− embryos.
Figure 2. Developmental role of abd-A expression in the P compartment .
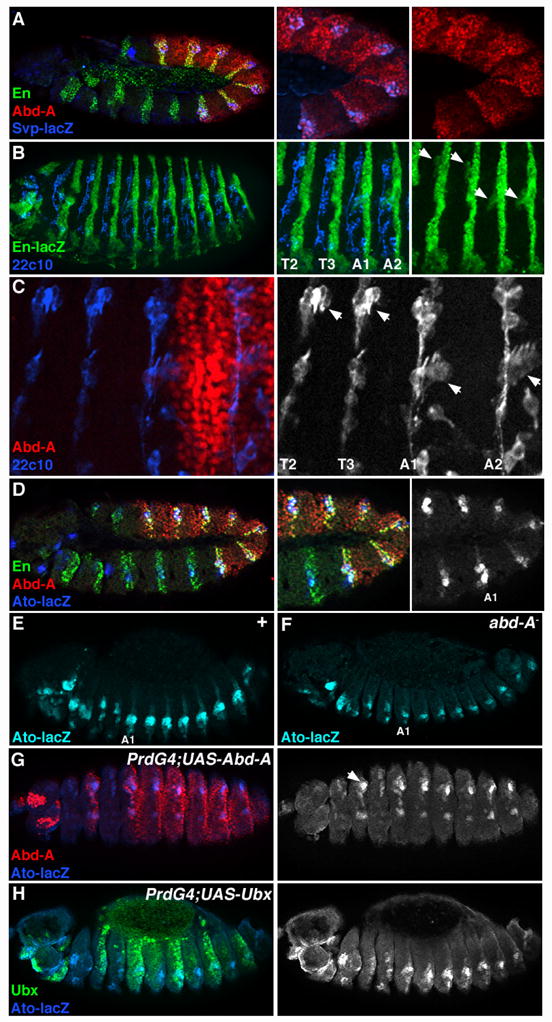
A. Lateral view of a stage 11 svp-lacZ Drosophila embryo immunostained for Abd-A (red), En (green), and β-gal (blue). svp-lacZ expression serves as an early marker for oenocytes. B. Stage 16 en-lacZ Drosophila embryo immunostained for β-gal (green) and with a PNS-specific neuronal marker (mAb22C10, blue). Arrows (in right panel) point to where the dch3 (in T2/T3) and lch5 (A1/A2) sensory organs form within the P compartment. C. Close up view of the T2 and T3 thoracic and A1 and A2 abdominal segments immuostained with Abd-A (red) and mAb22C10 (blue, black and white at right). D. Lateral view of a stage 11 ato-lacZ embryo immunostained for Abd-A (red), En (green) and β-gal (blue, black and white at right). Note that the majority of ato-lacZ expression in the thorax and abdomen is within the P compartment. E. Wild type ato-lacZ embryo (stage 14) immunostained for β-gal (blue). Note the higher levels of ato-lacZ within the abdomen than the thorax. F. ato-lacZ expression is the same in all body segments of abd-A− embryos. G. PrdG4;UAS-Abd-A embryos show that Abd-A (red) expression within the thorax stimulates ato-lacZ activity (blue, black and white at right). H. PrdG4;UAS-Ubx embryos show that ectopic Ubx (green) does not alter ato-lacZ levels within the thorax.
Each ch organ SOP cell is specified by the atonal proneural gene (Jarman et al., 1993). To determine if ato expression is modulated between the thoracic and abdominal segments, we co-stained ato-lacZ embryos for β-gal, Abd-A, and En (Sun et al., 1998). As expected most ato-lacZ expression is observed within the P compartment of each thoracic and abdominal segment (Fig. 2D). In early embryos (stage 11, Fig 2D), ato-lacZ levels are slightly higher in the abdominal than the thoracic segments, and this difference becomes more pronounced in older embryos (stage 14, Fig 2E). The high levels of ato-lacZ expression in the abdomen are lost in abd-A− embryos (Fig 2F). Moreover, ectopic abd-A, but not Ubx, expression in the thorax is sufficient to induce higher levels of ato-lacZ in the thorax (Fig 2G, H). Overall, these results are consistent with previous studies demonstrating that abd-A induces oenocyte formation and modulates sensory organ development in the P compartment. In contrast, Ubx is expressed at low levels in this compartment and even when mis-expressed in P cells, it fails to perform these functions (Brodu et al., 2002). Thus, the compartmental modulation of abdominal Hox factor expression is important for the formation of distinct cell and organ types in the fly ectoderm. In the experiments described below, we address how this modulation of Ubx and abd-A expression arises.
Ubx and abd-A cross-regulation in the Drosophila abdomen
In general, Ubx and Abd-A are not expressed at high levels in the same cells of the ectoderm, suggesting they may repress each other. Consistent with this idea, Ubx is de-repressed in abd-A mutant embryos (Fig. 3A) (Struhl and White, 1985). However, Ubx de-repression is mainly observed in the A compartment and Ubx levels remain relatively low within En-positive cells of the P compartment (data not shown and (Mann, 1994)). In Ubx− embryos, abd-A expression appears unaltered, indicating that Ubx is not required for abd-A’s wild type expression pattern (Fig. 3B). However, Castelli-Gair et al found that Ubx is able to transiently repress abd-A (Castelli-Gair et al., 1994). We addressed this question by mis-expressing Ubx using the Gal4-UAS system with the Paired Gal4 (PrdG4) driver. PrdG4 is ideal for this experiment as it is expressed in every other segment when Ubx and abd-A expression are being initiated, allowing for comparisons with wild type segments in the same embryo. Using this assay, we found that Abd-A represses Ubx in a cell autonomous manner (Fig. 3D), and ectopic Ubx was also able to partially repress abd-A (Fig. 3C) (Castelli-Gair et al., 1994). These results support two conclusions, first that Abd-A restricts Ubx expression, and second that Ubx has the potential to repress abd-A but is not required to perform this function.
Figure 3. Cross-regulation between Ubx and abd-A.
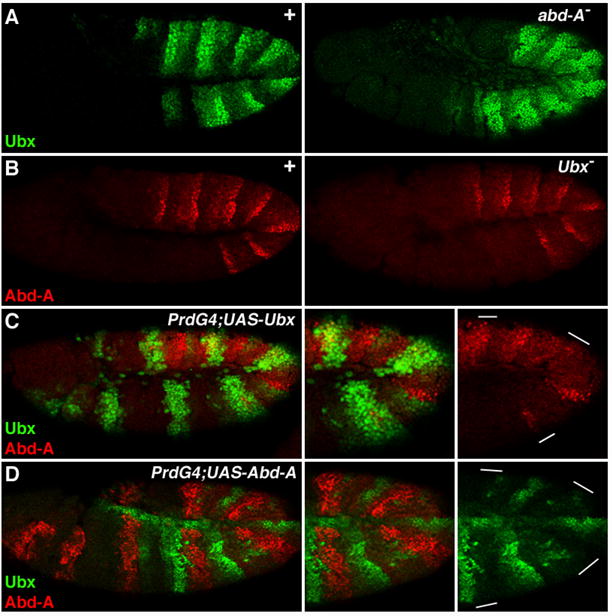
Lateral views of stage 11 Drosophila embryos immunostained for Ubx (green) and Abd-A (red). A. Pattern of Ubx expression in wild type (left) and abd-A− embryos. Note that Ubx is de-repressed in abd-A mutants. B. abd-A expression is the same in wild type (left) and Ubx mutant (right) embryos. C. PrdG4;UAS-Ubx embryo showing that ectopic Ubx represses abd-A. D. PrdG4;UAS-Abd-A embryo showing that ectopic Abd-A represses Ubx.
en modulates Ubx and abd-A expression in the P compartment
Ubx and Abd-A cross-regulation may contribute to the stabilization of their expression patterns, but it does not reveal how these patterns are initiated. The Ubx and abd-A segmentally reiterated expression patterns suggest that segmentation genes may regulate abdominal Hox expression. The high levels of Abd-A and low levels of Ubx in the P compartment indicate that En regulates their expression (Macias et al., 1994; Mann, 1994). Although previous experiments suggested that en positively regulates abd-A, en’s sister gene invected was not mutant in these experiments, raising the possibility that incomplete phenotypes were being measured (Macias et al., 1994). We therefore analyzed stage 11 en inv double mutants, hereafter referred to as en−. At stage 11, high levels of Abd-A are not observed in en− embryos except within the lateral regions of PS12 and 13 (Fig. 4B). As this expression is also observed in A cells of wild type embryos, it is not dependent on En (Fig. 4A and 4B). In older en− embryos (stage 14), Ubx expression weakly expands posteriorly, which is most clearly seen in PS7 and PS8 (Fig. 4D). Compared to wild type, the Ubx and abd-A patterns are less organized in en− embryos with most cells in PS7 and PS8 expressing Ubx and most cells in PS9 through PS12 expressing abd-A (Fig. 4D). However, even in these embryos most cells express either high levels of Ubx or Abd-A, but not both Hox factors.
Figure 4. En regulates abdominal Hox expression in the P compartment.
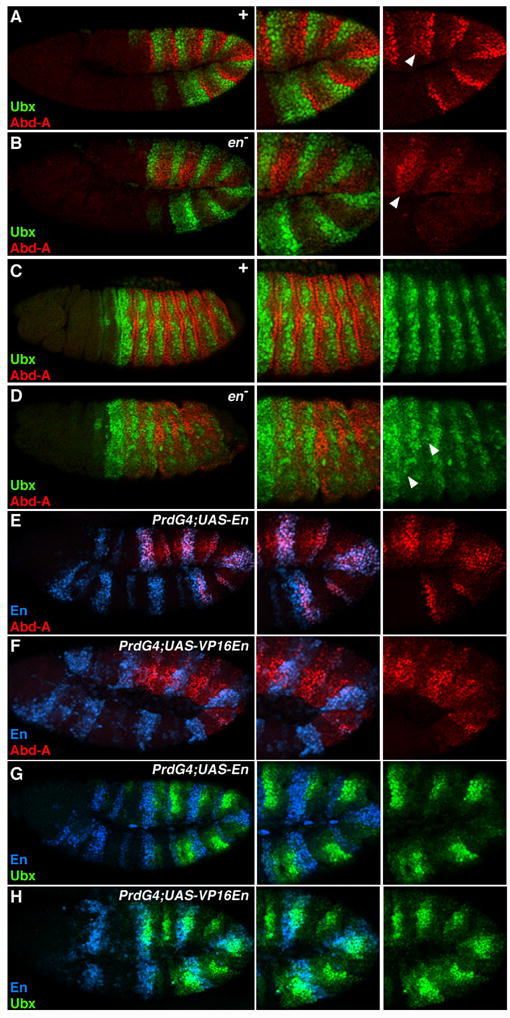
Lateral views of Drosophila embryos immunostained for Ubx (green), Abd-A (red), and En (blue). A and B. Ubx and abd-A expression in wild type (A) and en mutant (B) stage 11 embryos. Note that Ubx levels are relatively normal whereas Abd-A levels are decreased. Arrowhead points to lateral cells that express Abd-A independent of En. C and D. Ubx and abd-A expression in wild type (A) and en mutant (B) stage 14 embryos. Note that in older en− embryos Ubx is de-repressed in the anterior abdominal parasegments (arrowhead), whereas posterior abdominal parasegments show increased abd-A expression. E and G. PrdG4;UAS-En embryos illustrating that ectopic En stimulates abd-A (E) and represses Ubx (G). F and H. PrdG4;UAS-VP16En embryos demonstrate that VP16En represses abd-A (F), and weakly activates Ubx (H).
If En activates abd-A then ectopic En should stimulate abd-A. Using PrdG4 to mis-express En, we determined that En induces abd-A expression in a cell autonomous manner (Fig. 4E). Consistent with a repressive effect of En on Ubx, ectopic En repressed Ubx (Fig. 4G). En is most effective at repressing Ubx in parasegments that express abd-A, suggesting that at least some of its effects on Ubx levels may be mediated indirectly, through regulation of abd-A. Overall these data demonstrate that En modulates abdominal Hox expression within the P compartment. Further, abd-A has at least two phases of expression in the embryo, first it is activated within P cells and second its expression increases in A cells that have low Ubx levels.
slp regulates Ubx and abd-A in the A compartment
Abdominal Hox expression within the A compartment is not uniform, suggesting that factors within this compartment regulate Ubx and abd-A. Previous studies have shown that the partially redundant slp genes (slp1 and slp2, referred to here as slp) are expressed in approximately the posterior half of the A compartment (Fig. 5A) (Cadigan et al., 1994b; Grossniklaus et al., 1992). Much like en, slp is required for proper segmentation of the embryo, and the slp genes encode transcriptional regulatory proteins. Analysis of Slp1 and Ubx protein levels in stage 11 embryos reveals that where slp1 expression is high so is Ubx (Fig. 5B). Consistently, abd-A expression is mutually exclusive with Slp1 (Fig. 5C). Later in embryogenesis (stage 12 and older) slp1 expression is restricted ventrally (Fig. 5D). Interestingly, at the same time Abd-A levels increase in A compartment cells, but only within those that lack Slp1. This is most clearly seen in ventral views of a stage 12 embryo, where high levels of Abd-A surround Slp-positive cells (Fig. 5D). The correlation between low Abd-A levels and high Slp levels is maintained throughout embryogenesis. Thus, slp is a good candidate for an A compartment regulator of abdominal Hox expression.
Figure 5. Slp regulates abdominal Hox expression in the A compartment.
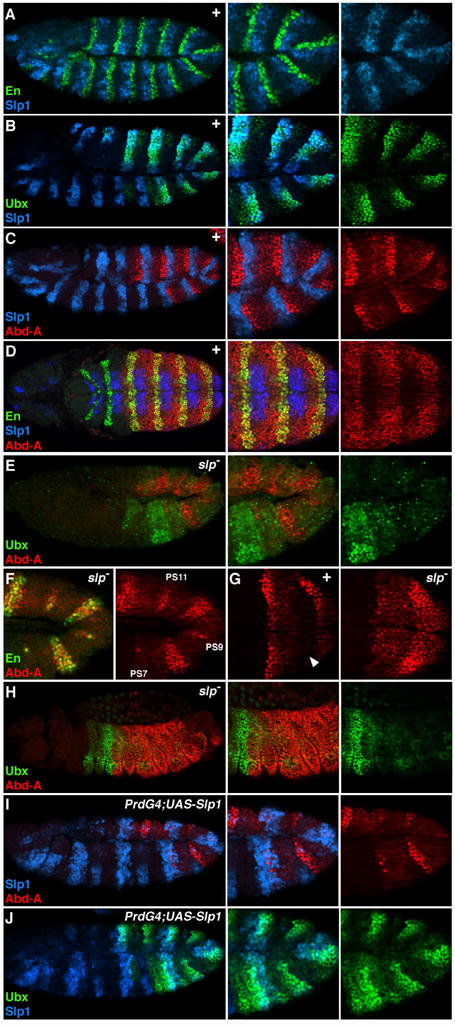
A, B, C, E, F, I, and J are lateral views of stage 11 Drosophila embryos. D and G are ventral views of the abdomen, and H is a lateral view of a stage 14 embryo. All embryos were immunostained for Slp1 (blue), Ubx or En (green), and Abd-A (red) as indicated. A. Slp1 is expressed in cells anterior to En-positive cells within each segment of the embryo. B, C, and D. Wild type embryos have high levels of Ubx (B) and low levels of Abd-A (C) in Slp1-positive cells. In stage 12 embryos Abd-A protein expression increases in Slp1-negative cells of the A compartment to similar levels as the P compartment (marked by En, green in D). E-H. ubx and abd-A expression in slp− embryos. E. Ubx levels are decreased in slp− embryos except in PS6 cells that lack abd-A expression. F. Close up view of Abd-A and En expression in slp mutant embryos shows that the odd-numbered stripes (labeled) of En are lost and even-numbered stripes are broadened. As in wild type embryos, abd-A expression correlates with en expression. G. Close up ventral view of an slp− embryo (right) showing that Abd-A levels increase in cells immediately preceding the posterior compartment compared to wild type embryos (left). In wild type embryos, these cells express Slp1 (arrowhead). H. Ubx expression remains restricted to PS6 in a stage 14 slp− embryo and Abd-A levels are uniform in the ectoderm of PS7 through PS12. I and J. PrdG4;UAS-Slp1 embryos show that ectopic Slp1 represses abd-A (I) and has little affect on Ubx (J).
To test this idea, we used both loss- and gain-of-function approaches to manipulate slp activity. As shown in Fig. 5E, Ubx and abd-A expression are altered in embryos lacking both slp genes (Δ34B is a deletion that removes slp1 and slp2). As in wild type stage 11 embryos, abd-A is expressed in stripes within the abdomen of slp mutant embryos (Fig. 5E). However, these stripes tend to be wider and there are fewer of them. Previous studies have shown that in the absence of slp the odd-numbered stripes of en are lost and the even-numbered stripes are broadened (Cadigan et al., 1994b; Grossniklaus et al., 1992; Jaynes and Fujioka, 2004). Consistently, the slp− embryo in Fig. 5F shows that the odd-numbered en stripes (PS7, 9, and 11 are shown) have mostly disappeared and that Abd-A is greatly reduced in these regions. In the even parasegments, en expression broadens and Abd-A levels are high. In addition, Abd-A levels increase in A cells immediately anterior to En-positive cells in slp− embryos, which in wild type embryos is where slp1 is expressed (Fig. 5G). The increase in abd-A expression is accompanied by a decrease in Ubx (Fig. 5E). Only in PS6, which lacks Abd-A, are Ubx levels normal. Strikingly, in older slp− embryos (stage 14), abd-A is expressed uniformly from PS7 to PS12 at the expense of Ubx expression (Fig. 5H). Consistent with these findings, mis-expression of Slp1 using PrdG4 repressed abd-A and led to a moderate increase in Ubx levels (Fig. 5I and 5J). Taken together these results suggest that slp modulates Hox expression in the A compartment of the abdomen by repressing abd-A, which allows Ubx levels to increase.
Cross-regulation between En and Slp
The combined results of en and slp gain- and loss-of-function provide a possible explanation for how compartment-specific expression patterns of the abdominal Hox factors arise. In the P compartment, En stimulates abd-A and both En and Abd-A repress Ubx. In A compartment cells that express Slp, abd-A is repressed allowing Ubx expression. In A compartment cells that do not express Slp, Abd-A levels increase, which represses Ubx. Complicating our ability to determine how en and slp regulate the Hox factors is that Slp represses en and En represses slp (Suppl Fig. 1 and (Alexandre and Vincent, 2003; Cadigan et al., 1994a; Kobayashi et al., 2003)). Thus, it is possible that either En and/or Slp modulate Hox expression indirectly through mutual cross-repression. We address this question by assaying Hox expression in cells that 1) express an activator form of En (VP16En (Alexandre and Vincent, 2003)), 2) express both En and Slp, and 3) express either En or Slp in en− slp− double mutant embryos.
En indirectly regulates abd-A and Ubx
To determine if En directly activates abd-A and/or directly represses Ubx, we used UAS-VP16En flies, in which the repression domain of En was replaced with the VP16 activation domain (Alexandre and Vincent, 2003). VP16En has previously been shown to activate other en targets, including slp1 (Suppl Fig. 1C (Alexandre and Vincent, 2003)). If En directly activates abd-A then VP16En should also stimulate its expression. However, as shown in Fig. 4F, VP16En represses abd-A. This result is consistent with En stimulating abd-A indirectly, by repressing a repressor of abd-A. One obvious candidate that we test below is slp, as it is highly induced by VP16En and is capable of repressing abd-A. We also determined Vp16En’s affect on Ubx. If En directly represses Ubx, then VP16En should activate Ubx. In stage 11 embryos, VP16En weakly stimulated Ubx in some cells (Fig. 4H). However, the dynamics of this increase are much slower for Ubx than for slp1, a known direct En-target gene, suggesting that En indirectly regulates Ubx. Because VP16En represses abd-A, the gradual increase in Ubx may be due to reduced Abd-A levels in these cells. In summary, the VP16En data suggests that En indirectly regulates the expression of both abdominal Hox proteins, perhaps through the repression of slp1.
En regulates abdominal Hox expression independently of Slp
Cross-repression between en and slp complicates the analysis of how they regulate Hox expression. To determine if En activates abd-A and represses Ubx in the presence of Slp1, we co-expressed both factors using PrdG4 and analyzed abd-A and Ubx levels. As shown in Fig. 6, En failed to activate abd-A but repressed Ubx in the presence of Slp1 (Fig. 6A and 6B). This finding suggests that En stimulates abd-A, at least in part, by repressing slp. These data also demonstrate that Slp1 is unable to stimulate Ubx if En is present, indicating that Slp stimulates Ubx, at least in part, by repressing en.
Figure 6. En and Slp regulate Ubx and abd-A independently.
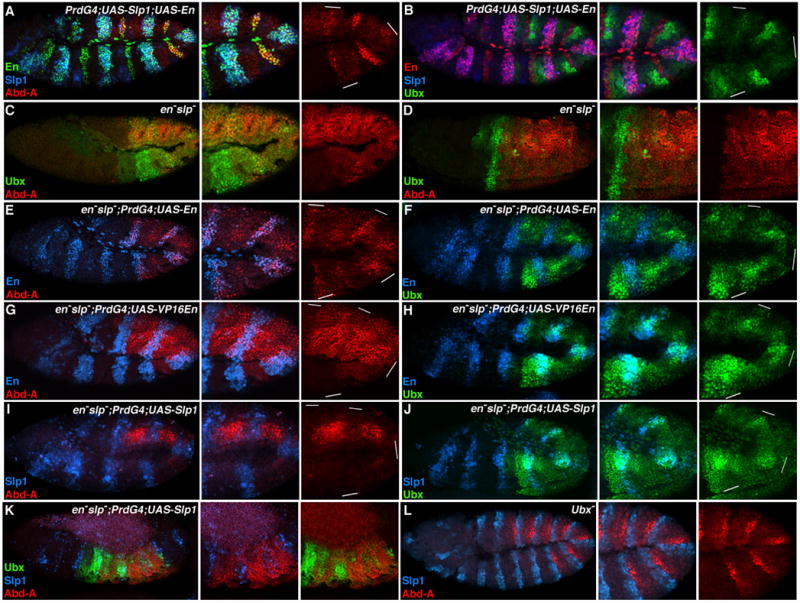
Lateral views of stage 11 (A, B, C, E, F, G, H, I, J, and L) and stage 14 Drosophila embryos (D and K). A. PrdG4;UAS-Slp1;UAS-En embryos show that ectopic Slp1 (blue) represses abd-A (red) in the presence of En (green). B. PrdG4;UAS-Slp1;UAS-En embryos. Ectopic En (red) represses Ubx (green) in the presence of Slp1(blue). C and D. Ubx (green) and Abd-A (red) expression in en− slp− embryos. Note the loss in patterned abdominal Hox expression within en− slp− embryos compared to wild type embryos (see Fig 1C). E and F. en− slp−;PrdG4;UAS-En embryos demonstrates that ectopic En (blue) induces Abd-A (red in E) and represses Ubx (green in F). G and H. en− slp−;PrdG4;UAS-VP16En embryos. VP16En (blue) represses Abd-A (red in G) and stimulates Ubx (green in H) levels. I and J. en− slp−;PrdG4;UAS-Slp1 embryos show that ectopic Slp1 (blue) represses abd-A (red in I) and increases Ubx (green in J). K. Stage 14 en− slp−;PrdG4;UAS-Slp1 embryo. Where Slp1 represses abd-A, Ubx expression increases. L. Ubx− embryos have normal abd-A and slp1 expression, indicating that Slp1 does not require Ubx to repress abd-A.
To further test the role of en and slp in regulating abdominal Hox factors we analyzed Ubx and abd-A expression in en− slp− mutant embryos. Fig. 6C shows that abd-A and Ubx expression in early en− slp− embryos is relatively unpatterned with Ubx levels highest anterior to Abd-A expressing cells. In older en− slp− embryos, Abd-A increases throughout the abdominal ectoderm while Ubx levels decrease (Fig. 6D). This is in stark contrast to wild type embryos, which alternate high levels of Ubx and Abd-A throughout the abdomen (compare Fig. 6D to Fig. 4C). This finding further supports the idea that en and slp are essential for proper abdominal Hox gene expression.
The relatively uniform expression of Ubx and abd-A in en− slp− embryos provides an ideal genetic background to ectopically provide either En or Slp and analyze Hox expression. Mis-expression of En using PrdG4 in en− slp− mutants stimulated abd-A and repressed Ubx (Fig. 6E and 6F). These results suggest that En regulates these two Hox factors independently of its affect on slp. If En directly activates abd-A in en− slp− embryos, then VP16En should also stimulate abd-A in these embryos. However, Abd-A levels do not increase in response to VP16En, and in most, but not all, cells Abd-A levels decrease (Fig. 6G). This result indicates that En is repressing an additional repressor (R) of abd-A. According to this idea, VP16En activates R, which results in decreased Abd-A levels. We also examined Ubx expression in en− slp− embryos that express VP16En. In most stage 11 embryos, VP16En stimulated Ubx expression (Fig. 6H). In older embryos expressing VP16En, the increase in Ubx levels was correlated with a decrease in Abd-A (data not shown). Because the ability of VP16En to increase Ubx expression was slow, we propose the following model: En indirectly regulates abdominal Hox patterns by directly repressing slp and another intermediary repressor (R) of abd-A (Figure 7). In P cells, En repression of slp and R allows Abd-A levels to increase. The combination of En and Abd-A in P cells represses Ubx.
Figure 7. Model for the establishment of abdominal Hox expression patterns.
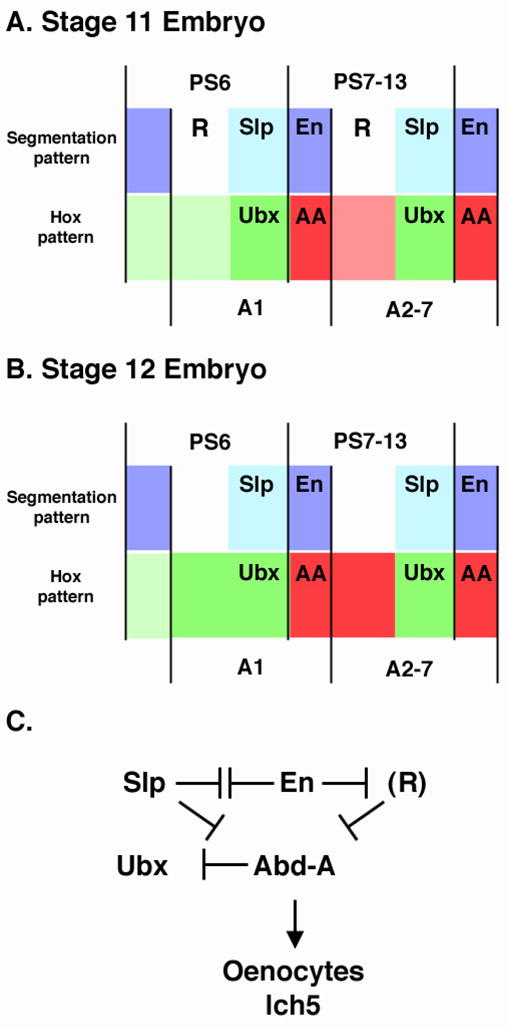
A and B. Representation of En (purple), Slp (blue), Abd-A (red) and Ubx (green) expression levels in stage 11 (A) and stage 12 (B) within two parasegments/segments of an embryo. The segment and parasegment boundaries are denoted. The color intensities represent the relative expression levels of both Hox factors. Note that at stage 11 high levels of Abd-A are observed only within En-positive cells and that high levels of Ubx are observed within Slp-positive cells. We predict that an unknown repressor (R) keeps Abd-A levels low in cells anterior to slp expression. By stage 12, however, the expression of R decreases allowing Abd-A levels to increase in these A compartment cells. In the ventral ectoderm slp expression represses abd-A allowing Ubx levels to be maintained. C. A genetic diagram for how the expression patterns of Ubx and abd-A are established by en and slp. R is shown in parentheses as we predict it is expressed transiently during stage 11 and fades by stage 12. The modulation of abdominal Hox gene expression in the P compartment correlates with the formation of abdominal specific cell fates, oenocytes and the lateral chordotanal organs (lch5).
Slp represses abd-A independently of En
The experiments described above demonstrate that Slp can repress en and abd-A (Fig. 5 and Suppl Fig. 1). The co-expression of Slp with En using PrdG4 indicates that Slp does so even in the presence of En (Fig. 6A). To determine if Slp1 represses abd-A in the absence of En, we ectopically expressed Slp1 in en− slp− embryos. Using this assay, we found that Slp1 represses abd-A in these embryos (Fig. 6I), and that Slp expressing cells have increased Ubx levels (Fig. 6J). This effect on abdominal Hox expression was also seen in older embryos (Fig. 6K) and is consistent with our previous results that suggest Slp1 represses abd-A, which thereby allows Ubx levels to increase. One possibility is that both Slp and Ubx are required to repress abd-A, as recent studies have shown that both are required for the repression of a common target gene (Gebelein et al., 2004). However, Slp does not require Ubx to repress abd-A, as Ubx− embryos show wild type slp1 and abd-A expression in the absence of Ubx function (Fig. 6L).
DISCUSSION
Regulation of Hox gene expression by segmentation genes
The Hox genes comprise a family of transcription factors that specify cell identities along the A-P axis in both vertebrates and invertebrates (Carroll et al., 2001). The precise regulation of Hox gene expression is therefore essential for the development of different cell types and morphological structures within the head, thorax, and abdomen of each organism. In Drosophila, the expression of the eight Hox genes during embryonic development is controlled by several types of transcriptional regulators. First, early in the fly embryo, the Gap genes demarcate the A-P limits of Hox gene expression. Hunchback (Hb), for example, represses the expression of the abdominal Hox genes to establish the anterior expression limits of both Ubx and abd-A (Shimell et al., 2000; White and Lehmann, 1986). Additional Gap genes expressed in distinct regions of the early embryo help establish the A-P limits for each Hox factor (Casares and Sanchez-Herrero, 1995; Qian et al., 1991; Reinitz and Levine, 1990). Once the Gap genes establish broad Hox expression domains, the Hox factors refine their own expression patterns. In general, the posterior Hox factors repress the expression of more anterior Hox factors and thereby establish distinct regions of Hox gene expression along the A-P axis (Capovilla and Botas, 1998; Struhl and White, 1985). Lastly, the Polycomb (Pc) and trithorax (trx) Group genes are required for the long-term repression (Pc-G) and activation (trx-G) of the Hox genes (Gould, 1997). Taken together, these transcriptional regulatory mechanisms provide a broad outline for how Hox gene expression patterns are established and maintained along the A-P axis.
In this study, we show that en and slp, which are expressed in cells of the P or A compartments, respectively, are also required to pattern abdominal Hox expression. Consistent with previous reports, we found that En is required for the high levels of Abd-A and low levels of Ubx observed in P compartment cells (Macias et al., 1994; Mann, 1994). We extend these observations by showing that En performs these functions not by directly activating abd-A but by repressing two repressors of abd-A (Fig. 7). First, En represses slp (Alexandre and Vincent, 2003; Cadigan et al., 1994a; Kobayashi et al., 2003), which we show is a potent repressor of abd-A. Second, we determined that even in the absence of slp, En induces abd-A expression whereas Vp16En represses abd-A expression. These results are consistent with En repressing an additional abd-A repressor, which we have called R (Fig. 7). Although the identity of R is currently unknown, we predict that R is repressed by En and thus will not be expressed in the P compartment. Moreover, we predict R is expressed transiently in the A compartment and begins to fade by embryonic stage 12, allowing abd-A expression in these cells (Fig. 7). We have tested two likely candidates for R: Odd-skipped (Odd), which, like Slp, is expressed only in the A compartment (Mullen and DiNardo, 1995), and Cubitus-interruptis (Ci), which is repressed by En in the P compartment (Eaton and Kornberg, 1990). Both Odd and Ci are known to function as transcriptional repressors. However, mis-expression of either Odd or a constitutive repressor form of Ci in the P compartment using PrdG4 did not dramatically alter abd-A expression (data not shown). These results suggest that Odd and Ci do not repress abd-A, and that an as yet unidentified factor functions in this capacity.
While En expression in the P compartment explains how Abd-A levels become high and Ubx levels become low, it does not reveal how Ubx expression is maintained in the A compartment of abdominal segments that express abd-A. In this study, we provide the following data demonstrating that Slp is required to establish alternating stripes of Ubx and Abd-A in the fly embryo. 1) Slp is highly co-expressed with Ubx, and Abd-A levels are low in Slp-positive cells. 2) In the absence of slp function, abd-A is de-repressed, resulting in a loss of Ubx expression within the abdominal ectoderm. 3) Ectopic Slp expression represses abd-A and allows for an expansion in Ubx expression. Based on these findings, we propose that Slp represses the expression of both en and abd-A to allow for the continued expression of Ubx in the A compartment (Fig. 7). In conclusion, these experiments reveal a symmetry between En and Slp in the establishment of abdominal Hox expression patterns in the A and P compartments: First, en and slp cross-repress each other to establish a sharp boundary between A and P cells, and second, en and slp repress either Ubx (En) or abd-A (Slp) to modulate abdominal Hox expression in a compartment-specific manner.
Compartment-specific Hox expression patterns and the development of the fly ectoderm
Our findings that en and slp modulate Ubx and Abd-A expression in the A and P compartments suggest that the abdominal Hox factors perform compartment-specific functions to pattern the ectoderm. The fly ectoderm gives rise to epidermal cells that secrete a patterned cuticle and neuronal cells that comprise the peripheral and central nervous system. Here we analyzed the development of two abdominal-specific cell and organ subtypes, the formation of secretory cells known as oenocytes and the formation of a specific sensory organ (the lateral chordotonal organ consisting of 5 scolopodia, lch5) in the PNS. Previous studies have shown that both oenocytes and the lch5 organs require abd-A but not Ubx function and that the lch5 organs form within the P compartment of the abdominal segments (Brodu et al., 2002; Heuer and Kaufman, 1992; Wong and Merritt, 2002). We determined that oenocytes also form in the P compartment of abdominal segments, and that the expression of a proneural reporter gene (ato-lacZ) that marks the formation of chordotonal organs is stimulated by Abd-A. Moreover, even the forced expression of Ubx within P compartment cells fails to induce oenocyte formation (Brodu et al., 2002), lch5 formation (Heuer and Kaufman, 1992; Wong and Merritt, 2002), or enhanced ato-lacZ expression (Fig. 2), revealing that these processes can only be regulated by Abd-A.
Overall, the en and slp genes are best known for their ability to regulate segmentation and the expression of signaling molecules that pattern the embryo (Cadigan et al., 1994b; Lawrence et al., 1999; Lawrence et al., 1996). In this study, we have demonstrated that the En and Slp factors also pattern the abdomen by differentially regulating the expression of Hox genes in the ectoderm. Moreover, our previous studies have revealed that En and Abd-A within the P compartment and Slp and Ubx within the A compartment work in concert to repress the expression of the leg selector gene, Dll, in the abdomen (Gebelein et al., 2004). Together with oenocyte and ch organ formation, these results suggest that the modulation of Hox expression in the A and P compartments by En and Slp is essential for the compartment-specific control of gene expression during embryonic development.
Supplementary Material
Lateral views of stage 11 Drosophila embryos immunostained for En (green) and Slp1 (blue). A. PrdG4;UAS-Slp1 embryos. Slp1 is a potent repressor of en. B. PrdG4;UAS-En embryos. En is a repressor of slp1. C. PrdG4;UAS-VP16En embryos. VP16En strongly activates slp1.
Acknowledgments
We thank K. Cadigan, J. Jaynes, J. Reinitz, G. Struhl, and the Developmental Studies Hybridoma Bank, University of Iowa for reagents. We thank T. Cook, M. Crickmore, and J. Jaynes for suggestions on the manuscript. This work was supported by an NIH grant to R.S.M., and a March of Dimes Basil O’Connor Award and a CHRF Trustee Award to B.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- Alexandre C, Vincent JP. Requirements for transcriptional repression and activation by Engrailed in Drosophila embryos. Development. 2003;130:729–39. doi: 10.1242/dev.00286. [DOI] [PubMed] [Google Scholar]
- Blair SS. Compartments and appendage development in Drosophila. Bioessays. 1995;17:299–309. doi: 10.1002/bies.950170406. [DOI] [PubMed] [Google Scholar]
- Brodu V, Elstob PR, Gould AP. abdominal A specifies one cell type in Drosophila by regulating one principal target gene. Development. 2002;129:2957–63. doi: 10.1242/dev.129.12.2957. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Grossniklaus U, Gehring WJ. Functional redundancy: the respective roles of the two sloppy paired genes in Drosophila segmentation. Proc Natl Acad Sci U S A. 1994a;91:6324–8. doi: 10.1073/pnas.91.14.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan KM, Grossniklaus U, Gehring WJ. Localized expression of sloppy paired protein maintains the polarity of Drosophila parasegments. Genes Dev. 1994b;8:899–913. doi: 10.1101/gad.8.8.899. [DOI] [PubMed] [Google Scholar]
- Capovilla M, Botas J. Functional dominance among Hox genes: repression dominates activation in the regulation of Dpp. Development. 1998;125:4949–57. doi: 10.1242/dev.125.24.4949. [DOI] [PubMed] [Google Scholar]
- Carroll SB, DiNardo S, O’Farrell PH, White RA, Scott MP. Temporal and spatial relationships between segmentation and homeotic gene expression in Drosophila embryos: distributions of the fushi tarazu, engrailed, Sex combs reduced, Antennapedia, and Ultrabithorax proteins. Genes Dev. 1988;2:350–60. doi: 10.1101/gad.2.3.350. [DOI] [PubMed] [Google Scholar]
- Carroll SB, Grenier JK, Weatherbee SD. From DNA to Diversity. Malden, MA: Blackwell Science; 2001. [Google Scholar]
- Casares F, Sanchez-Herrero E. Regulation of the infraabdominal regions of the bithorax complex of Drosophila by gap genes. Development. 1995;121:1855–66. doi: 10.1242/dev.121.6.1855. [DOI] [PubMed] [Google Scholar]
- Castelli-Gair J, Greig S, Micklem G, Akam M. Dissecting the temporal requirements for homeotic gene function. Development. 1994;120:1983–95. doi: 10.1242/dev.120.7.1983. [DOI] [PubMed] [Google Scholar]
- Cohen B, Wimmer EA, Cohen SM. Early development of leg and wing primordia in the Drosophila embryo. Mech Dev. 1991;33:229–40. doi: 10.1016/0925-4773(91)90030-a. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Bronner G, Kuttner F, Jurgens G, Jackle H. Distal-less encodes a homoeodomain protein required for limb development in Drosophila. Nature. 1989;338:432–4. doi: 10.1038/338432a0. [DOI] [PubMed] [Google Scholar]
- Desplan C, Theis J, O’Farrell PH. The Drosophila developmental gene, engrailed, encodes a sequence-specific DNA binding activity. Nature. 1985;318:630–5. doi: 10.1038/318630a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo S, Heemskerk J, Dougan S, O’Farrell PH. The making of a maggot: patterning the Drosophila embryonic epidermis. Curr Opin Genet Dev. 1994;4:529–34. doi: 10.1016/0959-437x(94)90068-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo S, Kuner JM, Theis J, O’Farrell PH. Development of embryonic pattern in D. melanogaster as revealed by accumulation of the nuclear engrailed protein. Cell. 1985;43:59–69. doi: 10.1016/0092-8674(85)90012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton S, Kornberg TB. Repression of ci-D in posterior compartments of Drosophila by engrailed. Genes Dev. 1990;4:1068–77. doi: 10.1101/gad.4.6.1068. [DOI] [PubMed] [Google Scholar]
- Elstob PR, Brodu V, Gould AP. spalt-dependent switching between two cell fates that are induced by the Drosophila EGF receptor. Development. 2001;128:723–32. doi: 10.1242/dev.128.5.723. [DOI] [PubMed] [Google Scholar]
- Fjose A, McGinnis WJ, Gehring WJ. Isolation of a homoeo box-containing gene from the engrailed region of Drosophila and the spatial distribution of its transcripts. Nature. 1985;313:284–9. doi: 10.1038/313284a0. [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido A, Ripoll P, Morata G. Developmental compartmentalisation of the wing disk of Drosophila. Nat New Biol. 1973;245:251–3. doi: 10.1038/newbio245251a0. [DOI] [PubMed] [Google Scholar]
- Gebelein B, Culi J, Ryoo HD, Zhang W, Mann RS. Specificity of Distalless repression and limb primordia development by abdominal Hox proteins. Dev Cell. 2002;3:487–98. doi: 10.1016/s1534-5807(02)00257-5. [DOI] [PubMed] [Google Scholar]
- Gebelein B, McKay DJ, Mann RS. Direct integration of Hox and segmentation gene inputs during Drosophila development. Nature. 2004;431:653–9. doi: 10.1038/nature02946. [DOI] [PubMed] [Google Scholar]
- Gibert JM. The evolution of engrailed genes after duplication and speciation events. Dev Genes Evol. 2002;212:307–18. doi: 10.1007/s00427-002-0243-2. [DOI] [PubMed] [Google Scholar]
- Gould A. Functions of mammalian Polycomb group and trithorax group related genes. Curr Opin Genet Dev. 1997;7:488–94. doi: 10.1016/s0959-437x(97)80075-5. [DOI] [PubMed] [Google Scholar]
- Grossniklaus U, Pearson RK, Gehring WJ. The Drosophila sloppy paired locus encodes two proteins involved in segmentation that show homology to mammalian transcription factors. Genes Dev. 1992;6:1030–51. doi: 10.1101/gad.6.6.1030. [DOI] [PubMed] [Google Scholar]
- Hatini V, DiNardo S. Divide and conquer: pattern formation in Drosophila embryonic epidermis. Trends Genet. 2001;17:574–9. doi: 10.1016/s0168-9525(01)02448-9. [DOI] [PubMed] [Google Scholar]
- Heuer JG, Kaufman TC. Homeotic genes have specific functional roles in the establishment of the Drosophila embryonic peripheral nervous system. Development. 1992;115:35–47. doi: 10.1242/dev.115.1.35. [DOI] [PubMed] [Google Scholar]
- Jarman AP, Grau Y, Jan LY, Jan YN. atonal is a proneural gene that directs chordotonal organ formation in the Drosophila peripheral nervous system. Cell. 1993;73:1307–21. doi: 10.1016/0092-8674(93)90358-w. [DOI] [PubMed] [Google Scholar]
- Jaynes JB, Fujioka M. Drawing lines in the sand: even skipped et al. and parasegment boundaries. Dev Biol. 2004;269:609–22. doi: 10.1016/j.ydbio.2004.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaynes JB, O’Farrell PH. Active repression of transcription by the engrailed homeodomain protein. Embo J. 1991;10:1427–33. doi: 10.1002/j.1460-2075.1991.tb07663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch F, Bender W, Weiffenbach B. abdA expression in Drosophila embryos. Genes Dev. 1990;4:1573–87. doi: 10.1101/gad.4.9.1573. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Fujioka M, Tolkunova EN, Deka D, Abu-Shaar M, Mann RS, Jaynes JB. Engrailed cooperates with extradenticle and homothorax to repress target genes in Drosophila. Development. 2003;130:741–51. doi: 10.1242/dev.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg T. Engrailed: a gene controlling compartment and segment formation in Drosophila. Proc Natl Acad Sci U S A. 1981;78:1095–9. doi: 10.1073/pnas.78.2.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg T, Siden I, O’Farrell P, Simon M. The engrailed locus of Drosophila: in situ localization of transcripts reveals compartment-specific expression. Cell. 1985;40:45–53. doi: 10.1016/0092-8674(85)90307-1. [DOI] [PubMed] [Google Scholar]
- Kosman D, Small S, Reinitz J. Rapid preparation of a panel of polyclonal antibodies to Drosophila segmentation proteins. Dev Genes Evol. 1998;208:290–4. doi: 10.1007/s004270050184. [DOI] [PubMed] [Google Scholar]
- Lai EC, Orgogozo V. A hidden program in Drosophila peripheral neurogenesis revealed: fundamental principles underlying sensory organ diversity. Dev Biol. 2004;269:1–17. doi: 10.1016/j.ydbio.2004.01.032. [DOI] [PubMed] [Google Scholar]
- Lawrence PA. The Making of a Fly: The Genetics of Animal Design. Oxford: Blackwell Scientific Publications; 1992. [Google Scholar]
- Lawrence PA, Casal J, Struhl G. hedgehog and engrailed: pattern formation and polarity in the Drosophila abdomen. Development. 1999;126:2431–9. doi: 10.1242/dev.126.11.2431. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Morata G. Compartments in the wing of Drosophila: a study of the engrailed gene. Dev Biol. 1976;50:321–37. doi: 10.1016/0012-1606(76)90155-x. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Sanson B, Vincent JP. Compartments, wingless and engrailed: patterning the ventral epidermis of Drosophila embryos. Development. 1996;122:4095–103. doi: 10.1242/dev.122.12.4095. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Struhl G. Morphogens, compartments, and pattern: lessons from drosophila? Cell. 1996;85:951–61. doi: 10.1016/s0092-8674(00)81297-0. [DOI] [PubMed] [Google Scholar]
- Macias A, Casanova J, Morata G. Expression and regulation of the abd-A gene of Drosophila. Development. 1990;110:1197–207. doi: 10.1242/dev.110.4.1197. [DOI] [PubMed] [Google Scholar]
- Macias A, Pelaz S, Morata G. Genetic factors controlling the expression of the abdominal-A gene of Drosophila within its domain. Mech Dev. 1994;46:15–25. doi: 10.1016/0925-4773(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Manak JR, Mathies LD, Scott MP. Regulation of a decapentaplegic midgut enhancer by homeotic proteins. Development. 1994;120:3605–19. doi: 10.1242/dev.120.12.3605. [DOI] [PubMed] [Google Scholar]
- Mann RS. Engrailed-mediated repression of Ultrabithorax is necessary for the parasegment 6 identity in Drosophila. Development. 1994;120:3205–12. doi: 10.1242/dev.120.11.3205. [DOI] [PubMed] [Google Scholar]
- Martinez-Arias A, Lawrence PA. Parasegments and compartments in the Drosophila embryo. Nature. 1985;313:639–42. doi: 10.1038/313639a0. [DOI] [PubMed] [Google Scholar]
- McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- Morata G, Lawrence PA. Control of compartment development by the engrailed gene in Drosophila. Nature. 1975;255:614–7. doi: 10.1038/255614a0. [DOI] [PubMed] [Google Scholar]
- Mullen JR, DiNardo S. Establishing parasegments in Drosophila embryos: roles of the odd-skipped and naked genes. Dev Biol. 1995;169:295–308. doi: 10.1006/dbio.1995.1145. [DOI] [PubMed] [Google Scholar]
- Qian S, Capovilla M, Pirrotta V. The bx region enhancer, a distant cis-control element of the Drosophila Ubx gene and its regulation by hunchback and other segmentation genes. Embo J. 1991;10:1415–25. doi: 10.1002/j.1460-2075.1991.tb07662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinitz J, Levine M. Control of the initiation of homeotic gene expression by the gap genes giant and tailless in Drosophila. Dev Biol. 1990;140:57–72. doi: 10.1016/0012-1606(90)90053-l. [DOI] [PubMed] [Google Scholar]
- Sanson B. Generating patterns from fields of cells. Examples from Drosophila segmentation. EMBO Rep. 2001;2:1083–8. doi: 10.1093/embo-reports/kve255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimell MJ, Peterson AJ, Burr J, Simon JA, O’Connor MB. Functional analysis of repressor binding sites in the iab-2 regulatory region of the abdominal-A homeotic gene. Dev Biol. 2000;218:38–52. doi: 10.1006/dbio.1999.9576. [DOI] [PubMed] [Google Scholar]
- Solano PJ, Mugat B, Martin D, Girard F, Huibant JM, Ferraz C, Jacq B, Demaille J, Maschat F. Genome-wide identification of in vivo Drosophila Engrailed-binding DNA fragments and related target genes. Development. 2003;130:1243–54. doi: 10.1242/dev.00348. [DOI] [PubMed] [Google Scholar]
- St Johnston D, Nusslein-Volhard C. The origin of pattern and polarity in the Drosophila embryo. Cell. 1992;68:201–19. doi: 10.1016/0092-8674(92)90466-p. [DOI] [PubMed] [Google Scholar]
- Struhl G. Splitting the bithorax complex of Drosophila. Nature. 1984;308:454–457. [Google Scholar]
- Struhl G, White RA. Regulation of the Ultrabithorax gene of Drosophila by other bithorax complex genes. Cell. 1985;43:507–19. doi: 10.1016/0092-8674(85)90180-1. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jan LY, Jan YN. Transcriptional regulation of atonal during development of the Drosophila peripheral nervous system. Development. 1998;125:3731–40. doi: 10.1242/dev.125.18.3731. [DOI] [PubMed] [Google Scholar]
- Vachon G, Cohen B, Pfeifle C, McGuffin ME, Botas J, Cohen SM. Homeotic genes of the Bithorax complex repress limb development in the abdomen of the Drosophila embryo through the target gene Distal-less. Cell. 1992;71:437–50. doi: 10.1016/0092-8674(92)90513-c. [DOI] [PubMed] [Google Scholar]
- Vincent JP, O’Farrell PH. The state of engrailed expression is not clonally transmitted during early Drosophila development. Cell. 1992;68:923–31. doi: 10.1016/0092-8674(92)90035-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RA, Lehmann R. A gap gene, hunchback, regulates the spatial expression of Ultrabithorax. Cell. 1986;47:311–21. doi: 10.1016/0092-8674(86)90453-8. [DOI] [PubMed] [Google Scholar]
- White RA, Wilcox M. Distribution of Ultrabithorax proteins in Drosophila. Embo J. 1985;4:2035–2043. doi: 10.1002/j.1460-2075.1985.tb03889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DC, Merritt DJ. The role of homeotic genes in determining the segmental pattern of chordotonal organs in Drosophila. Int J Dev Biol. 2002;46:475–81. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lateral views of stage 11 Drosophila embryos immunostained for En (green) and Slp1 (blue). A. PrdG4;UAS-Slp1 embryos. Slp1 is a potent repressor of en. B. PrdG4;UAS-En embryos. En is a repressor of slp1. C. PrdG4;UAS-VP16En embryos. VP16En strongly activates slp1.


