Abstract
Methamphetamine (Meth) use and human immunodeficiency virus (HIV) infection are major public health problems in the world today. Ample evidence indicates that HIV transfection risk is greatly enhanced with Meth use. Studies have shown that both HIV infection and Meth abuse can cause neuronal injury leading to neurodegeneration. While many studies have focused on the individual effects of Meth and HIV on the brain, few investigations have been carried out on their co-morbid effect in the nervous system. In this review, we try to summarize recent progress on individual effects of Meth and HIV on neurodegeneration and their potential underlying mechanisms, in addition to exploring their co-morbid effect on the brain.
Keywords: HIV-1, AIDS, methamphetamine, neurotoxicity, neurodegeneration
Introduction
Methamphetamine (Meth) use represents a major public health concern with greater than 35 million users worldwide. In the United States, 10-15% of human immunodeficiency virus-1 (HIV-1) positive individuals acknowledge Meth use [1] with greater than 7% of American high school students having tried Meth [2,3]. Due to its ability to be synthesized in small clandestine laboratories [4], this highly addictive drug [5] is difficult to combat. Meth is very desirable to illicit drug users due to its inexpensive cost of manufacturing, low cost to purchase and its long duration of action [6]. With an elimination half-life between 10-12 hours [7], its pharmacokinetics allow it to produce effects lasting 10-times longer than that of cocaine [8]. Meth abuse has been associated with many health disorders, such as stroke, increased blood pressure, cardiac arrhythmia, hyperthermia, central nervous system (CNS) abnormalities and most notably HIV-1 infection [9, 10]. As the most widely used recreational drug among men who have sex with men (MSM) [11-14], Meth is associated with a doubling of the risk of HIV-1 acquisition [15], higher blood viral loads, alterations in anti-retroviral medication concentrations, and greater high-risk sexual behaviors, which may lead to HIV super infection [16-18]. Current estimates of overall HIV-1 prevalence among young injection drug users is about 2.8 percent [19], with transmission through injection drug use representing 13 percent of all new cases of HIV-1 in 2006 [20].
HIV-1 infection of the brain, or NeuroAIDS, results in a chronic neurological disorder termed HIV-1 associated dementia (HAD) in approximately 20-30% of patients infected with HIV-1 [21, 22]. HAD is characterized by deficits in attention, impairments of short-term memory, compromised fine motor skills, tremors, slowness of movements, and abnormal gait [22, 23]. While the prevalence of HAD has been greatly reduced in the modern era of highly active anti-retroviral therapy (HAART), other complexes of milder symptoms, specifically the minor cognitive motor disorder (MCMD), have been growing in prevalence [24]. This is generally believed to be due to a chronic low level persistent viral infection of mononuclear phagocytes (MP; blood-borne tissue and perivascular macrophages and microglia) having supplanted high-level productive HIV replication as the pivotal mechanism for the pathogenesis of HIV-associated neurocognitive disorders (HAND). Evidence suggests that HIV-infected individuals who use illicit stimulants, in particular Meth, are more likely to have HAND compared with non-drug abusing HIV-infected individuals. This may be because stimulants compound the effects of the neurotoxic substances released during HIV infection, with dopa-minergic and glutamatergic systems being particularly vulnerable [25-28].
Meth use and HIV-1 infection, two major public health problems worldwide today, have been associated with detrimental changes in neuropsychology. Using a variety of psychological tests, it has been shown that chronic Meth users show significant deficits in attention, spatial learning and memory, and executive functions [29-31]. HIV-1 infected individuals usually manifest changes in personality, apathy, and depression levels, as well as social withdraw and psychotic symptoms [32, 33]. Asymptomatic HIV-1 positive individuals often display minor deficits in attention [34], cognitive speed [35], and fine motor skills [36]. Individuals with co-morbid HIV-1 infection and Meth use have been demonstrated to show greater reductions in cognitive abilities compared to either individuals with HIV-1 infection or Meth use alone [37, 38].
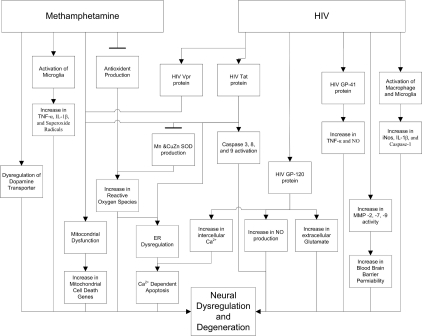
While many studies have focused on the individual effects of Meth and HIV-1 on the brain, limited investigations have been done on their intersecting influence. In this review, we attempt to summarize the individual effects of both Meth use and HIV-1 infection on neurodegeneration, and to evaluate their intersecting effects on neurodegeneration as well. A summary chart diagram illustrating the effects of Meth and HIV-1 on neurodegeneration is shown Figure 1.
Figure 1.
Effects of Meth and HIV-1 on neural dysregulation and degeneration
Meth-associated neurotoxic activity
Neuroimaging studies have documented extensive changes in the brains of Meth abusing individuals. Various studies using positron emission tomography (PET) on the brains of Meth abusers have found remarkably consistent decreases in dopamine (DA) transporter (DAT) levels in the caudate nucleus, putamen, nucleus accumbens, orbito-frontal cortex, and the dorsolateral prefrontal cortex [39-44], as well as reductions in post-synaptic D2 dopamine receptors in the caudate and putamen [39, 45]. While this represents major physiological changes, it may also reflect a neuroadaptive change in response to repeated exposure to Meth [46].
Morphological changes in the brains of Meth users have been characterized using structural magnetic resonance imaging (MRI) technique. These changes include decreased volumes of the medial cingulate gyms, limbic and para-limbic cortices, and the bilateral hippocampus [47], and an increase in the volumes of the putamen and the globus pallidus [48].
A significant number of proton magnetic resonance spectroscopy (MRS) studies have been conducted on Meth abusers who have recently abstained from Meth use. These studies showed decreased levels of N-acetylaspartate (NAA), a marker for neuronal integrity and density, in the basal ganglia, frontal white and gray matter and anterior cingulated cortex [49-52]. Increased levels of choline-containing compounds (CHO), a measure of cell membrane degradation and lipid changes, have been found in the frontal gray and white matter and the anterior cingulated cortex, while decreased levels of CHO have been found in the basal ganglia of recently abstained Meth users [50-53]. Increased levels of myo-inositol (Ml), a putative marker of glial content, were seen in frontal white and gray matter [49], and decreased levels of creatine (CR) and phosphocreatine, a marker of energy stores and energy metabolites, have been shown in the basal ganglia and the anterior cingulated cortex [50, 52].
Potential mechanisms underlying Meth-associated neurotoxic activity
Oxidative Stress
Meth is known to cause persistent damage to DA and serotonin (5HT) nerve terminals in animal models of drug abuse [54-56], as well as to human abusers of Meth [39, 47]. While not fully understood, oxidative stress, the cytotoxic consequences of reactive oxygen species (ROS) (e.g. H2O2, O2, OH), is believed to play a major role in the neurodegeneration associated with the use of Meth. Yamamoto and Zhu demonstrated this by showing an increase in the extracellular concentrations of the hydroxylated products of salicylate and d-phenylalanine in the brain after administration of a four-injection regimen of Meth [57]. This increase in ROS has also been shown to be present in a time dependent manner with respect to the last dose of Meth in a neurotoxic regiment [58]. With ROS also being produced as a byproduct of normal aerobic metabolism, cells contain elaborate anti-oxidant systems for dealing with ROS [59]. Cumulative evidence suggests that Meth is able to cause oxidative stress by affecting the balance between ROS production and enzymatic and non-enzymatic antioxidant systems [60-62]. Increases in ROS concentration can affect DNA resulting in nucleotide oxidation [63], lipids resulting in lipid peroxide-tion [57, 60, 64, 65], and cellular proteins resulting in protein nitration [60].
The role of oxidative stress in Meth-associated neurotoxicity is also confirmed by the observations that antioxidants such as ascorbic acid, ethanol, mannitol, vitamin E, N-acetyl-L-cysteine, and selenium [66-68] are able to attenuate striatal DA and 5-HT depletions, while inhibition of superoxide dismutase (SOD), an antioxidant enzyme, by diethyldithio-carbamate exacerbates both DA and 5-HT depletions [66]. Similarly, transgenic mice with over-expression of copper/zinc SOD show resistance to Meth induced neurotoxicity [69].
Another possible mechanism associated with Meth-induced neurotoxicity may be the formation of hydroxyl radicals in the synaptic cleft. Upon administration, Meth causes the release of DA from synaptic vesicles inside of synaptic monoaminergic terminals. Following its release, inter-terminal DA is transported into the synaptic space by reverse transport by DAT [70]. This reverse transport of DA by DAT is encouraged by Meth ability to enhance the phosphorylation of the DAT via protein kinase C [71]. The reverse transport of DA by DAT is required for neurodegeneration, because DAT knockout mice don't exhibit Meth-induced terminal degeneration [72]. Once in the synaptic cleft, DA is metabolized by monoamine oxidase (MAO) to produce hydrogen peroxide. Interaction of the hydrogen peroxide with metal ions can produce toxic hydroxyl radicals [73].
Neurotoxicity resulting from oxidative stress may also be related to changes in the functionality and concentration of vesicular monoamine transporter 2 (VMAT-2), an integral membrane protein that acts to transport DA from cellular cytosol in synaptic vesicles. Exposure to Meth leads to a reduction in the binding of tetrabenazine to VMAT-2 [74], resulting in a reduction of DA transport into vesicles [75]. Thus, changes in functionality of VMAT-2 can lead to increases in the amount of cytoplasmic DA available to form ROS and DA quinones [58, 76, 77]. DA quinones can form protein-bound cysteinyl catechols, which are selectively toxic to DA terminals [77], however this effect can be attenuated by exposure to antioxidants (ascorbic acid and glutathione) [78]. Further, it has been shown that repeated swim stress is able to down-regulate the concentration of VMAT-2 in the striatum and nucleus accumbens [79]. With the likelihood of stress being associated with Meth addiction, it seems reasonable to conclude that stress-related changes in VMAT-2 concentration accompanied by the effects of Meth on VMAT-2 concentration may represent an enhanced pathway to neurotoxicity due to oxidative damage.
Activation of Mitochondria cell death genes
The mitochondrial Bcl-2 family of genes plays an important role in regulating cellular apoptosis. The Bcl-2 gene family regulates mitochondrial outer membrane permeability, and can be divided into either pro-apoptotic (BAX, BAD, BAK, and BID among others) or anti-apoptotic (Bcl-2, Bcl-w, and BCI-XL among others) splice variants. Several studies have documented the role of the Bcl-2 gene family in Meth-induced neurodegeneration [62, 80, 81]. While over-expression of Bcl-2 in immortalized neural cells offers significant protection from Meth-induced apoptosis [82], treatment of primary cortical neurons with Meth results in changes in the regulation of Bcl-2 splice variants [81]. Specifically, Meth has been shown to cause an increase in the levels of the pro-apoptotic genes BAX, BAD, BAK and BID, and a reduction in the anti-apoptotic genes Bcl-2 and BCI-XL [83], with the peak of pro-death gene expression at approximately eight hours after exposure to Meth and peak cell death at three days post-exposure [84]. These changes in the intrinsic ratios of cell death promoters to cell death repressors are consistent with the finding that Meth exposure results in the release of mitochondrial cytochrome C, Smac/DIABLO, endonuclease G and AIF into the cytosol [85, 86]. Following this release, increases in caspase-3 activity and cleavage of PARP, DFF-45 and lamin A can be observed [87]. When taken together with the in vitro demonstration that Meth can cause release of cytochrome C from mitochondria, activation of caspases 3 and 9, as well as activation of DFF40, and its transit to the nucleus [88], the in vivo data strongly support the role of mitochondria in Meth-induced neuronal degeneration [89, 90].
The Endopiasmic Reticuium and Neural Apoptosis
In addition to the mitochondria, oxidative stress is also able to cause neuronal dysfunction in the Endopiasmic Reticuium (ER) [91, 92]. In its regular state, the ER is responsible for the synthesis, folding and transport of proteins, as well as functioning as the main store for intracellular Ca2+ [91, 93]. Under normal conditions, the ER releases Ca2+ for use by the mitochondria to enhance metabolite flow on the outer mitochondrial membrane and to increase ATP production; however sustained release of Ca2+ from the ER can initiate calcium-dependent apoptosis via the permeabilization of the mitochondrial membrane [94]. Changes in calcium homeostasis have been implicated with Meth-induced cellular demise, because Meth has been shown to activate calpain [85, 95], a Ca2+ responsive cytosolic cysteine protease that is an important mediator of ER-dependent cell death [96]. Further evidence for the participation of the ER in Meth-related cell death is demonstrated in the finding that apoptotic doses of Meth influence the expression of the proteins caspase-12, GRP78/Bip, and CH0P/GADD153 [85], proteins known to participate in ER-induced apoptosis and the ER-mediated unfolded protein response [97, 98]. Despite this evidence, Meth-induced ER dysfunction may play a secondary role to Meth-related oxidative stress [62, 73, 99] and to increases in the BAX/Bcl-2 ratio [83].
Reactive Microgliosis
Microglia, the resident immune cells within the central nervous system, function in immune surveillance in the intact brain and are activated during neurodegenerative processes [100]. It is believed that activated microglia might contribute to the progressive course of neurodegenerative disorders, including Parkinson's [101], Alzheimer's disease [102] and HAD [103]. More recently, it has also been shown that abstinent Meth abusers show significant increases in the levels of activated microglia in the midbrain, striatum, thalamus, orbitofrontal cortex, and the insular cortex in comparison to control (i.e. individuals with no self-reported history of methamphetamine use) [104]. These data are also consistent with increases in activated microglia that have been observed in mice following Meth injections designed to mimic a recreational dosing regimen in humans [105].
Current evidence indicates that over activation of microglia can result in neuronal damage through proinflammatory processes, including, but not limited to, the production of tumor necrosis factor-α, interleukin-β, and inter-leukin-6 or through oxidative mechanisms via the generation of superoxide radicals [106-109]. When combined with the observation that Meth-induced neurotoxicity is attenuated in interleukin-6-null mice [110] and that activation of microglia appears to precede Meth-induced damage to striatal dopaminergic terminals in rodents [105, 111-113], it seems reasonable to suggest that reactive microgliosis is indeed associated with Meth-induced neurodegeneration.
HIV-induced neurotoxic activity
HIV brain infection produces progressive neural damage. Studied using MRI techniques, patients suffering from HAD have shown greater losses of white matter than non-demented HIV positive individuals [114]. Specifically, decreases in the volume of the cerebellum, caudate nucleus and the hippocampus have been shown [115]. Perfusion MRI (pMRI) has been shown to be sensitive to the changes in cerebral blood flow (CBF) that have been associated with reductions in motor functioning in HIV positive individuals; including decreases in CBF in the lateral frontal and medial parietal lobes and increased CBF in the posterior parietal white matter [116]. In addition, a single-photon emission computed tomography (SPECT) study also showed changes in CBF, specifically a decrease in the temporoparietal white matter [117]. It has also been shown that CD4 counts in HIV positive individuals correlate with changes in CBF detected by pMRI [116] and accelerated ventricular volume enlargement and reduction in the volume of white matter and of the caudate nucleus seen using MRI [118].
Computer tomography (CT) studies have demonstrated diffuse cerebral atrophy, and enlarged ventricles, which progress with the evolution of HIV infection [119, 120]. PET has shown the differences in glucose uptake between HIV positive individuals and HIV negative controls [121], and the time course of glucose metabolism levels in the basal ganglia (BG) [122]. Briefly, initial changes in motor performance are associated with diverse hypermetabolism in the BG. A change from hypermetabolism to hypometabolism is associated with moderate changes in motor performance. Later severe deficits in motor performance are associated with widespread hypometabolism in the BG.
MRS studies in HIV positive individuals have shown reductions in NAA in the frontal white matter and basal ganglia [123-130], and reduced NAA/CR and NAA/CR ratios in the centrum semiovale, frontal white matter and thalamus [125, 129, 130]. It should be noted that a partial reversal of the decreased NAA levels in the frontal white matter has been shown with HAART therapy [128]. Reduced CR levels have been shown in the basal ganglia [117, 124], while increased levels of CR have been shown in the frontal lobe [131]. Increased levels of Ml have been shown to be present in the basal ganglia, frontal lobe, and temporoparietal white matter [117, 131], as well as an increase in the MI/CR ratio in the basal ganglia and the frontal white matter [125]. Ml levels have also been shown to correlate with lower CD4 counts and higher viral loads [131]. Despite improvements in CD4 count and viral load with HAART, increased Ml and CHO remain in the basal ganglia after treatment [132]. It was also demonstrated that HIV positive individuals show age related changes in metabolic composition with respect to the normal variations seen. Individuals showed greater than expected levels of glial markers, cholines, and Ml in the frontal white matter, while simultaneously showing further depressed levels of NAA and CR and phosphocreatine in the basal ganglia [124].
Understanding HIV-induced neurotoxic activity
Roles of macrophages and glial cells
HIV-infected macrophages and glial cells produce a variety of neurotoxins, including cytokines, chemokines, ROS, nitric oxide and excitatory amino acids. These toxins, alone or together, can influence the various types of cells in the brain [133-140]. Examination of brain sections from HIV demented and control brains with antibodies against iNOS, IL-ip, and caspase-1 revealed that the levels of all three markers of inflammation and oxidative stress are elevated in HIV demented brains [141]. Increases in markers of oxidative stress were also seen in microglia and astrocytes, suggesting that these cells may represent a site for the production of ROS [141]. Increased levels of macrophage inflammatory protein- 1α and 1β were found in the CSF of demented HIV-1 patients when compared with non-demented HIV patients [142]. In a study of rat cerebrocortical cultures containing neurons, astrocytes, and microglia, gpl20 toxicity was blocked by tuftsin-derived tripeptide (TKP), an inhibitor of reactive microgliosis [143]. It has also been shown that gpl20-related toxicity in hippocampal cultures is dependent on the presence of glial cells [144], and that activation of the p53 pathway appears to be necessary for the induction of gpl20-related neurotoxicity in both neurons and microglia [145].
Secreted by neurons and glial cells [146], matrix metalloproteinases (MMPs), the Zn-containing endopeptidases that enzymatically degrade the extracellular matrix proteins of the blood-brain barrier (BBB) and neuronal synapses [147, 148], have moreover been associated with the pathogenesis of HIV infection [149-151]. A study has shown that levels of MMP-2, -7, and -9 activity, are markedly increased in individuals with HAD when compared to both HIV-1 seronegative controls and HIV-positive, non-demented individuals [152]. This study has also shown that human fetal brain-derived cells can release MMP-2, -7, and -9, and that stimulation with TNF-α can augment the release of both MMP-7 and -9. Other studies have revealed that HIV-1 gp41 and gpl20 are able to induce MMP-2 [153, 154]. Taken together, these studies, with the fact that these particular MMPs are known to target critical components of the BBB, may suggest a possible mechanism for disruption of the BBB in HAD. In addition, MMPs can also cleave chemokines whose cleavage products can cause neurotoxicity [151].
HIV Tat-associated neurotoxicity
HIV Tat, the HIV trans-activator of transcription, vastly increases the amount of transcription of the HIV genome by phosphorylating other cellular factors, leading to explosive replication during infection [155]. High concentrations of Tat can be secreted by infected monocytes, resulting in altering function or killing of uninfected cells [156]. Moreover, the Tat protein causes neuronal loss, despite the inability of HIV in the infection of neurons [155, 157].
In vivo studies using direct stereotaxic injecttion of Tat have described the likelihood of a role for Tat in HIV-1-associated neurodegeneration. Following a single microinjection of Tat 1-72 into the striatum of rats, an increased level of protein oxidation and neuronal degeneration was produced, as well as an observation of the presence of reactive macrophages/microglia and reactive astrocytes near the lesion from injection [158]. In addition to this, stereotactic injections of Tat into the striatum of rats has been shown to produce significant cell loss and an increase in the number of reactive astrocytes [159, 160]. It has also been demonstrated that injection of Tat into the cerebral ventricles of rats can induce infiltration of neutrophils, macrophages, and lymphocytes, reactive astrocytosis, neuronal apoptosis and ventricular enlargement [161]. The consequences of long term exposure to Tat have also been examined. Rat C6 glioma cells that were genetically engineered to stably produce Tat were stereotaxically injected into the striatum or hippocampus of rats. It was demonstrated that Tat was able to be transported via normal anatomical pathways from the dentate gyms to the CA 3/4 region and from the striatum to the substantia nigra, leading to reactive microgliosis, neurotoxicity and behavioral abnormalities [162].
In vitro studies have helped to show possible pathways for Tat-associated neurodegeneration by demonstrating that Tat is able to cause neuronal apoptosis in embryonic rat hippo-campal neurons by a mechanism involving the disruption of calcium homeostasis, mitochondrial calcium uptake, caspase activation and the generation of ROS [163, 164]. It has been shown that Tat-associated neurotoxicity is mediated by activation of caspase-3 and caspase-8, as well as activation of the mitochondrial-related cell death genes [165, 166]. The increase in ROS levels, at least in part, can be attributed with the ability of Tat to suppress Mn-superoxide dismutase (SOD) expression and CuZn-SOD activity, and is dependent on superoxide radicals and hydrogen peroxide [167, 168].
Similarly, it has also been shown that Tat is able to cause neuronal apoptosis in cultured human fetal neurons [169, 170]. The Tat-induced neuronal apoptosis was prevented by NMDA receptor antagonists in both cultured human fetal neurons [169] and rat mixed cortical cells [171]. More recently, Tat-induced neuronal apoptosis has been associated with ER-dependent cell death pathways [172], an observation that is consistent with the idea that changes in ROS levels can induce ER stress [91].
HIV gp 120 and neural injury
During HIV reproduction gpl60, the HIV envelope protein, is cleaved to form both the gpl20 and gp41 viral proteins [173]. Exposure to HIV-gpl20 protein has been shown to be able to induce cell death in human neurons [174], as well as primary rodent cultures, including cortical, hippocampal, cerebral, and retinal cells [175-177]. It has also been demonstrated that overexpression of gpl20 in astrocytes of transgenic mice produces severe neuronal loss, astrogliosis, and an increase in the number of microglial cells present [178]. Behavioral studies in transgenic mice that overexpress gpl20 in glial cells exhibit an age-dependent impairment in open-field and reduced spatial memory, similar to the cognitive and motor deficits seen in patients with HAD [179]. Injections of gpl20 into the striatum of adult male rats resulted in significant areas of tissue loss and an increase in reactive astrocytosis [159], while injection of gpl20 protein into neonatal rats caused dystrophic changes in pyramidal neurons of the cerebral cortex and the pups showed significant signs of retardation in developmental milestones that are associated with complex motor behaviors [180]. Exposure of cultures of hippocampal neurons to gpl20 produced increases in the level of intracellular free calcium [177], an observation that is in agreement with the fact that NMDA antagonists are able to inhibit gpl20-induced changes in intracellular calcium levels and subsequent neuronal injury [138]. Studies have shown that gpl20-induced neuronal injury requires the presence of extracellular glutamate and calcium and the production of nitric oxide (NO). These results are supported by the ability of glutamate receptor antagonists and inhibitors of NO synthetase in the prevention of neurotoxicity [181]. Similarly, gpl20-induced neuronal toxicity in human neurons was able to be attenuated by glutamate antagonists and the blockade of calcium channels [174]. In addition, gpl20 exposure has also been associated with the activation of caspases 3 and 9 and the release of mitochondrial cytochrome c [175, 182]. Also of interest is the fact that inhibitors of both the Fas/TNF-α/death receptor and the mitochondrial death pathways can block gpl20 neuronal apoptosis [182].
gp41 has been shown to be able to induce the expression of interleukin 1, tumor necrosis factor alpha, and NO via iNOS-mediated synthesis in both human and rodent glial cultures [183-185]. The detectable levels of gp41 in HIV-1 infected individuals [186-188] directly correlate with the severity and progression of HAD in humans [189].
HIV Vpr and Nef-induced Neurotoxicity
The viral protein R (Vpr) of HIV-1 regulates the import of the HIV-1 pre-integration complex, induces cell cycle arrest in replicating cells, stimulates viral transcription, and regulates activation of apoptotic pathways in infected cells [190]. In vitro studies using cultured neurons derived from rat hippocampal, cortical and striatal neurons [191, 192] and an in vivo study using Vpr transgenic mice [193] have shown that Vpr has the ability to induce neuronal apoptosis and the Vpr-induced neuronal apoptosis requires the binding of Vpr to the adenine nucleotide translocator (ANT) in the inner membrane of the mitochondria [194-196]. These results are consistent with the findings that Vpr-related neuronal apoptosis involves increased production of ROS and the activation of caspases-3 [192] and caspases 8 [197]. Further, it has been shown that Vpr-induced apoptosis can be prevented by ectopic expression of caspases-inhibiting anti-apoptotic viral proteins [198] and the broad spectrum, irreversible caspases inhibitor Boc-D-FMK [199]. Taken together, the observation that higher levels of Vpr are found in the cerebrospinal fluid of AIDS patients with neurological disorders [200] suggest an important role for Vpr in the progressive neurodegeneration seen in HIV-1 positive individuals.
Nef (Negative Regulatory Factor) is a HIV-1 viral protein that plays both offense and defense in the battle between the AIDS viruses and the body's immune system. On one hand, Nef is associated with promoting the survival of HIV-1 infected cells and downregulating the surface level expression of major histocompatibility complex- I (MHC I) and II (MHC II) on antigen-presenting cells and the expression of CD4 and CD28 on T helper cells [201]. On the other hand, Nef is able to recruit leukocytes into the brains of rodents [202] and enhances the viral infection of primary human astrocytes [203]. It has been shown that Nef is necessary and sufficient for the progression of HIV-1 [204] and to promote AIDS development [205]. In vitro studies have shown that Nef is able to cause apoptosis in primary cultures of brain microvascular endothelial cells [206], and the death of primary human neurons and glia [207, 208]. However, grafting Nef-transduced macrophages in the hippocampus of a rat lead to no detectable apoptotic events [209], although Nef transgenic mice have been shown to develop a severe AIDS-like disease that effects all organ systems, including the brain [209]. It has also been shown that Nef is necessary and sufficient for the progression of HIV-1 [204] and to promote AIDS development [205].
Interaction of HIV-1 and Meth on brain
The amount of literature evaluating neuro-imaging changes on co-morbid HIV-1-positive Meth abusers is much more limited than that of either of the individual groups. Using MRS, significantly lower levels of NAA where demonstrated in co-morbid individuals, than the reductions seen in either of the individual conditions when compared to HIV-1 negative controls [210]. Chang et al. (2005) were able to show greater cumulative reductions of NAA in co-morbid individuals, although their results were not statistically significant. They were also able to show an increase in the levels of Ml in the frontal cortices and basal ganglia.
Research has shown that the consequences of co-morbid Meth use and HIV-1 infection can be especially deleterious. It has been speculated that the use of Meth has contributed to the emergence of distinct neurological variants of HIV. Administration of Meth to rats after intrastriatal HIV-1 Tat injection leads to a synergistic reduction in levels of dopamine and its metabolites [211]. These changes are also associated with neurodegeneration that specifically involves the loss of dopamine terminals and/or macrophage recruitment and microglial activation in both rodent and non-human primate models [212-214]. The synergistic toxic effects of Tat and Meth were able to be attenuated in human fetal neurons with the use of antioxidants [211]. Similar results were also seen in the survival of the HT22 hippocampal cell line and of human primary neurons. Langfor et al. showed that co-administration of Tat and Meth leads to the appearance of earlier cellular demise and extensive cell death, and was associated with mitochondrial damage, disruption of mito-chondrial calcium potential, and increased oxidative stress [215]. The synergistic effects of Tat and Meth-induced neuron degeneration have been further solidified by experimental results showing that animals treated with both Tat and Meth, both in subtoxic doses, showed significant reduction in striatal DA levels and DAT binding [211, 216]. It has also been demonstrated that intra-hippocampal Tat and Meth injection caused oxidative stress and activation of redox-regulated transcription factors in the cortical, striatal and hippocampal regions of the mouse brain [213].
Nevertheless, the mechanisms underlying Tat and Meth co-morbid effects have attracted a great deal of research interest. Flora et al. showed that hippocampal-stereotactic injecttion of Tat and intraperitoneal (ip) injection of Meth produced a marked increase in the levels of TNF-a mRNA in the mouse striatum [213]. The involvement of TNF-a in the potentiation of co-morbid effects of Tat and Meth in neurodegeneration is supported by the observations that the detrimental effects associated with Tat and Meth were attenuated in mice lacking TNF-a receptors, and thatTNF-a synthesis inhibitors can reduce Tat and Meth-mediated neurodegeneration in hippo-campal neuronal cultures [217].
In addition to TNF-α, monocyte chemotactic protein (MCP-1) has also been shown to be involved in Tat and Meth-induced neurotoxicity. Rats treated with Tat and Meth exhibit an increase in the levels of MCP-1 in the striatum in comparison to those treated with either Tat or Meth alone[214]. Theodore et al. also demonstrated that MCP-1 knockout mice were protected against Tat and Meth-induced neurotoxicity.
Moreover, a recent study reported that Tat and Meth together induce an increase in the activity of MMPs [218], suggesting that astroglia may play a role in Meth and HIV-1 interactions [219]. More specifically, treatment with Tat and Meth increased the release of MMP-1 and MMP activator in human neuron/astrocytes cultures [218].
In closing, although a great deal of research has been invested in elucidating the effects behind HIV-1-and Meth-induced neurodegeneration, a great deal still stands to be understood about the co-morbid neurodegeneration seen in HIV-1 infected Meth abusers. With the popularity of Meth abuse and the substantial HIV-1 infection risk among Meth abusers, these co-morbid individuals represent a subset population requiring special considerations for future research, prognosis and clinical treatment.
Acknowledgments
The authors thank Mr. Matthew Beaver for his critical reading of this manuscript. Supported by NIH grant R01 NS041862.
References
- 1.Purcell DW, Moss S, Remien RH, Woods WJ, Parsons JT. Illicit substance use, sexual risk, and HIV-positive gay and bisexual men: differences by serostatus of casual partners. Aids. 2005;19(Suppl 1):S37–47. doi: 10.1097/01.aids.0000167350.00503.db. [DOI] [PubMed] [Google Scholar]
- 2.Romanelli F, Smith KM. Clinical effects and management of methamphetamine abuse. Pharmacotherapy. 2006;26:1148–1156. doi: 10.1592/phco.26.8.1148. [DOI] [PubMed] [Google Scholar]
- 3.Andrew E, Springer RJP, Ross Shegog, Donna L White, Steven H Kelder. Methamphetamine Use and Sexual Risk Behaviors in U.S. High School Students: Findings from a National Risk Behavior Survey. Prevention Science. 2007;8:103–113. doi: 10.1007/s11121-007-0065-6. [DOI] [PubMed] [Google Scholar]
- 4.0'Dea PJ, Murphy B, Balzer C. Traffic and illegal production of drugs in rural America. NIDA Res Monogr. 1997;168:79–89. [PubMed] [Google Scholar]
- 5.Woolverton WL, Cervo L, Johanson CE. Effects of repeated methamphetamine administration on methamphetamine self-administration in rhesus monkeys. Pharmacol Biochem Behav. 1984;21:737–741. doi: 10.1016/s0091-3057(84)80012-x. [DOI] [PubMed] [Google Scholar]
- 6.Cadet JL, Krasnova IN. Interactions of HIV and Methamphetamine: Cellular and Molecular Mechanisms of Toxicity Potentiation. Neurotoxicity Research. 2007;12:181–204. doi: 10.1007/BF03033915. [DOI] [PubMed] [Google Scholar]
- 7.Schepers RJ, Oyler JM, Joseph RE, Jr, Cone EJ, Moolchan ET, Huestis MA. Methamphetamine and amphetamine pharmacokinetics in oral fluid and plasma after controlled oral methamphetamine administration to human volunteers. Clin Chem. 2003;49:121–132. doi: 10.1373/49.1.121. [DOI] [PubMed] [Google Scholar]
- 8.Glittenberg J, Anderson C. Methamphetamines: use and trafficking in the Tucson-Nogales area. Subst Use Misuse. 1999;34:1977–1989. doi: 10.3109/10826089909039435. [DOI] [PubMed] [Google Scholar]
- 9.Ricaurte GA, Guillery RW, Seiden LS, Schuster CR, Moore RY. Dopamine nerve terminal degeneration produced by high doses of methylamphetamine in the rat brain. Brain Res. 1982;235:93–103. doi: 10.1016/0006-8993(82)90198-6. [DOI] [PubMed] [Google Scholar]
- 10.Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res Brain Res Rev. 2001;36:1–22. doi: 10.1016/s0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- 11.Halkitis PN, Parsons JT, Stirratt MJ. A double epidemic: crystal methamphetamine drug use in relation to HIV transmission among gay men. J Homosex. 2001;41:17–35. doi: 10.1300/J082v41n02_02. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell SJ, Morris SR, Kent CK, Stansell J, Klausner JD. Methamphetamine use and sexual activity among HIV-infected patients in care-San Francisco, 2004. AIDS Patient Care STDS. 2006;20:502–510. doi: 10.1089/apc.2006.20.502. [DOI] [PubMed] [Google Scholar]
- 13.Semple SJ, Patterson TL, Grant I. Motivations associated with methamphetamine use among HIV+ men who have sex with men. J Subst Abuse Treat. 2002;22:149–156. doi: 10.1016/s0740-5472(02)00223-4. [DOI] [PubMed] [Google Scholar]
- 14.Thiede H, Valleroy LA, MacKellar DA, Celentano DD, Ford WL, Hagan H, Koblin BA, LaLota M, McFarland W, Shehan DA, Torian LV. Regional patterns and correlates of substance use among young men who have sex with men in 7 US urban areas. Am J Public Health. 2003;93:1915–1921. doi: 10.2105/ajph.93.11.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drumright LN, Little SJ, Strathdee SA, Slymen DJ, Araneta MR, Malcarne VL, Daar ES, Gorbach PM. Unprotected anal intercourse and substance use among men who have sex with men with recent HIV infection. J Acquir Immune Defic Syndr. 2006;43:344–350. doi: 10.1097/01.qai.0000230530.02212.86. [DOI] [PubMed] [Google Scholar]
- 16.Investigation of a new diagnosis of multidrug-resistant, dual-tropic HIV-1 infection-New York City, 2005. MMWR Morb Mortal Wkly Rep. 2006;55:793–796. [PubMed] [Google Scholar]
- 17.Ellis RJ, Childers ME, Cherner M, Lazzaretto D, Letendre S, Grant I. Increased human immunodeficiency virus loads in active methamphetamine users are explained by reduced effectiveness of antiretroviral therapy. J Infect Dis. 2003;188:1820–1826. doi: 10.1086/379894. [DOI] [PubMed] [Google Scholar]
- 18.Smith DM, Wong JK, Hightower GK, Ignacio CC, Koelsch KK, Petropoulos CJ, Richman DD, Little SJ. HIV drug resistance acquired through superinfection. Aids. 2005;19:1251–1256. doi: 10.1097/01.aids.0000180095.12276.ac. [DOI] [PubMed] [Google Scholar]
- 19.Rondinelli AJ, Ouellet LJ, Strathdee SA, Latka MH, Hudson SM, Hagan H, Garfein RS. Young adult injection drug users in the United States continue to practice HIV risk behaviors. Drug Alcohol Depend. 2009 doi: 10.1016/j.drugalcdep.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention, editor. Vol. 18. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. HIV/AIDS Surveillance Report, 2006. http://www.cdc.gov/hiv /topics/surveillance/resources/reports/ [Google Scholar]
- 21.McArthur JC, Hoover DR, Bacellar H, Miller EN, Cohen BA, Becker JT, Graham NM, McArthur JH, Seines OA, Jacobson LP, et al. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology. 1993;43:2245–2252. doi: 10.1212/wnl.43.11.2245. [DOI] [PubMed] [Google Scholar]
- 22.Navia BA, Jordan BD, Price RW. The AIDS dementia complex: I. Clinical features. Ann Neurol. 1986;19:517–524. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- 23.Power C, Johnson RT. HIV-1 associated dementia: clinical features and pathogenesis. Can J Neurol Sci. 1995;22:92–100. doi: 10.1017/s0317167100040154. [DOI] [PubMed] [Google Scholar]
- 24.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koutsilieri E, Gotz ME, Sopper S, Sauer U, Demuth M, ter Meulen V, Riederer P. Regulation of glutathione and cell toxicity following exposure to neurotropic substances and human immunodeficiency virus-1 in vitro. J Neurovirol. 1997;3:342–349. doi: 10.3109/13550289709030748. [DOI] [PubMed] [Google Scholar]
- 26.Nath A, Maragos WF, Avison MJ, Schmitt FA, Berger JR. Acceleration of HIV dementia with methamphetamine and cocaine. J Neurovirol. 2001;7:66–71. doi: 10.1080/135502801300069737. [DOI] [PubMed] [Google Scholar]
- 27.Theodore S, Cass WA, Nath A, Maragos WF. Progress in understanding basal ganglia dysfunction as a common target for methamphetamine abuse and HIV-1 neurodegeneration. Curr HIV Res. 2007;5:301–313. doi: 10.2174/157016207780636515. [DOI] [PubMed] [Google Scholar]
- 28.Tse W, Cersosimo MG, Grades JM, Morgello S, Olanow CW, Koller W. Movement disorders and AIDS: a review. Parkinsonism Relat Disord. 2004;10:323–334. doi: 10.1016/j.parkreldis.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Daberkow DP, Kesner RP, Keefe KA. Relation between methamphetamine-induced monoamine depletions in the striatum and sequential motor learning. Pharmacol Biochem Behav. 2005;81:198–204. doi: 10.1016/j.pbb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez R, Bechara A, Martin EM. Executive functions among individuals with methamphetamine or alcohol as drugs of choice: preliminary observations. J Clin Exp Neuropsychol. 2007;29:155–159. doi: 10.1080/13803390600582446. [DOI] [PubMed] [Google Scholar]
- 31.Sim T, Simon SL, Domier CP, Richardson K, Rawson RA, Ling W. Cognitive deficits among methamphetamine users with attention deficit hyperactivity disorder symptomatology. J Addict Dis. 2002;21:75–89. doi: 10.1300/j069v21n01_07. [DOI] [PubMed] [Google Scholar]
- 32.Upton SA, Gendelman HE. Seminars in medicine of the Beth Israel Hospital, Boston. Dementia associated with the acquired immunodeficiency syndrome. N Engl J Med. 1995;332:934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- 33.McArthur JC, Sacktor N, Seines 0. Human immunodeficiency virus-associated dementia. Semin Neurol. 1999;19:129–150. doi: 10.1055/s-2008-1040831. [DOI] [PubMed] [Google Scholar]
- 34.Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, McCutchan JA, Taylor MJ, Kelly MD, Ellis RJ, et al. The HNRC 500-neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. J Int Neuropsychol Soc. 1995;1:231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- 35.Poutiainen E, Elovaara I. Subjective complaints of cognitive symptoms are related to psychometric findings of memory deficits in patients with HIV-1 infection. J Int Neuropsychol Soc. 1996;2:219–225. doi: 10.1017/s1355617700001156. [DOI] [PubMed] [Google Scholar]
- 36.Baldewicz TT, Leserman J, Silva SG, Petitto JM, Golden RN, Perkins DO, Barroso J, Evans DL. Changes in neuropsychological functioning with progression of HIV-1 infection: results of an 8-year longitudinal investigation. AIDS Behav. 2004;8:345–355. doi: 10.1023/B:AIBE.0000044081.42034.54. [DOI] [PubMed] [Google Scholar]
- 37.Carey CL, Woods SP, Rippeth JD, Gonzalez R, Heaton RK, Grant I. Additive deleterious effects of methamphetamine dependence and immunosuppression on neuropsychological functioning in HIV infection. AIDS Behav. 2006;10:185–190. doi: 10.1007/s10461-005-9056-4. [DOI] [PubMed] [Google Scholar]
- 38.Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, Wolfson T, Grant I. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10:1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- 39.Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102(Suppl 1):16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- 40.McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sekine Y, lyo M, Ouchi Y, Matsunaga T, Tsukada H, Okada H, Yoshikawa E, Futatsubashi M, Takei N, Mori N. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am J Psychiatry. 2001;158:1206–1214. doi: 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- 42.Sekine Y, Minabe Y, Ouchi Y, Takei N, lyo M, Nakamura K, Suzuki K, Tsukada H, Okada H, Yoshikawa E, Futatsubashi M, Mori N. Association of dopamine transporter loss in the orbitofrontal and dorsolateral prefrontal cortices with methamphetamine-related psychiatric symptoms. Am J Psychiatry. 2003;160:1699–1701. doi: 10.1176/appi.ajp.160.9.1699. [DOI] [PubMed] [Google Scholar]
- 43.Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Wong C, Logan J. Higher cortical and lower subcortical metabolism in detoxified methamphetamine abusers. Am J Psychiatry. 2001;158:383–389. doi: 10.1176/appi.ajp.158.3.383. [DOI] [PubMed] [Google Scholar]
- 45.Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 46.Moszczynska A, Fitzmaurice P, Ang L, Kalasinsky KS, Schmunk GA, Peretti FJ, Aiken SS, Wickham DJ, Kish SJ. Why is parkinsonism not a feature of human methamphetamine users? Brain. 2004;127:363–370. doi: 10.1093/brain/awh046. [DOI] [PubMed] [Google Scholar]
- 47.Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T. Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol Psychiatry. 2005;57:967–974. doi: 10.1016/j.biopsych.2005.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang L, Ernst T, Speck 0, Grob CS. Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. Am J Psychiatry. 2005;162:361–369. doi: 10.1176/appi.ajp.162.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ernst T, Chang L, Leonido-Yee M, Speck 0. Evidence for long-term neurotoxicity associated with methamphetamine abuse: A 1H MRS study. Neurology. 2000;54:1344–1349. doi: 10.1212/wnl.54.6.1344. [DOI] [PubMed] [Google Scholar]
- 51.Nordahl TE, Salo R, Natsuaki Y, Galloway GP, Waters C, Moore CD, Kile S, Buonocore MH. Methamphetamine users in sustained abstinence: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2005;62:444–452. doi: 10.1001/archpsyc.62.4.444. [DOI] [PubMed] [Google Scholar]
- 52.Nordahl TE, Salo R, Possin K, Gibson DR, Flynn N, Leamon M, Galloway GP, Pfefferbaum A, Spielman DM, Adalsteinsson E, Sullivan EV. Low N-acetyl-aspartate and high choline in the anterior cingulum of recently abstinent methamphetamine-dependent subjects: a preliminary proton MRS study. Magnetic resonance spectroscopy. Psychiatry Res. 2002;116:43–52. doi: 10.1016/s0925-4927(02)00088-4. [DOI] [PubMed] [Google Scholar]
- 53.Sekine Y, Minabe Y, Kawai M, Suzuki K, lyo M, Isoda H, Sakahara H, Ashby CR, Jr, Takei N, Mori N. Metabolite alterations in basal ganglia associated with methamphetamine-related psychiatric symptoms. A proton MRS study. Neuropsychopharmacology. 2002;27:453–461. doi: 10.1016/S0893-133X(02)00321-4. [DOI] [PubMed] [Google Scholar]
- 54.Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- 55.Lyles J, Cadet JL. Methylenedioxymethamphetamine (MDMA, Ecstasy) neurotoxicity: cellular and molecular mechanisms. Brain Res Brain Res Rev. 2003;42:155–168. doi: 10.1016/s0165-0173(03)00173-5. [DOI] [PubMed] [Google Scholar]
- 56.O'Callaghan JP, Miller DB. Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther. 1994;270:741–751. [PubMed] [Google Scholar]
- 57.Yamamoto BK, Zhu W. The effects of methamphetamine on the production of free radicals and oxidative stress. J Pharmacol Exp Ther. 1998;287:107–114. [PubMed] [Google Scholar]
- 58.Giovanni A, Liang LP, Hastings TG, Zigmond MJ. Estimating hydroxyl radical content in rat brain using systemic and intraventricular salicylate: impact of methamphetamine. J Neurochem. 1995;64:1819–1825. doi: 10.1046/j.1471-4159.1995.64041819.x. [DOI] [PubMed] [Google Scholar]
- 59.Fridovich I. Fundamental aspects of reactive oxygen species, or what's the matter with oxygen? Ann N Y Acad Sci. 1999;893:13–18. doi: 10.1111/j.1749-6632.1999.tb07814.x. [DOI] [PubMed] [Google Scholar]
- 60.Gluck MR, Moy LY, Jayatilleke E, Hogan KA, Manzino L, Sonsalla PK. Parallel increases in lipid and protein oxidative markers in several mouse brain regions after methamphetamine treatment. J Neurochem. 2001;79:152–160. doi: 10.1046/j.1471-4159.2001.00549.x. [DOI] [PubMed] [Google Scholar]
- 61.Harold C, Wallace T, Friedman R, Gudelsky G, Yamamoto B. Methamphetamine selectively alters brain glutathione. Eur J Pharmacol. 2000;400:99–102. doi: 10.1016/s0014-2999(00)00392-7. [DOI] [PubMed] [Google Scholar]
- 62.Jayanthi S, Ladenheim B, Cadet JL. Methamphetamine-induced changes in antioxidant enzymes and lipid peroxidation in copper/zinc-superoxide dismutase transgenic mice. Ann N Y Acad Sci. 1998;844:92–102. [PubMed] [Google Scholar]
- 63.Potashkin JA, Meredith GE. The role of oxidative stress in the dysregulation of gene expression and protein metabolism in neurodegenerative disease. Antioxid Redox Signal. 2006;8:144–151. doi: 10.1089/ars.2006.8.144. [DOI] [PubMed] [Google Scholar]
- 64.Acikgoz 0, Gonenc S, Kayatekin BM, Uysal N, Pekcetin C, Semin I, Gure A. Methamphetamine causes lipid peroxidation and an increase in superoxide dismutase activity in the rat striatum. Brain Res. 1998;813:200–202. doi: 10.1016/s0006-8993(98)01020-8. [DOI] [PubMed] [Google Scholar]
- 65.Iwashita A, Mihara K, Yamazaki S, Matsuura S, Ishida J, Yamamoto H, Hattori K, Matsuoka N, Mutoh S. A new poly(ADP-ribose) polymerase inhibitor, FR261529 [2-(4-chlorophenyl)-5-quinoxalinecarboxamide], ameliorates methamphetamine-induced dopaminergic neurotoxicity in mice. J Pharmacol Exp Ther. 2004;310:1114–1124. doi: 10.1124/jpet.104.068932. [DOI] [PubMed] [Google Scholar]
- 66.De Vito MJ, Wagner GC. Methamphetamine-induced neuronal damage: a possible role for free radicals. Neuropharmacology. 1989;28:1145–1150. doi: 10.1016/0028-3908(89)90130-5. [DOI] [PubMed] [Google Scholar]
- 67.Fukami G, Hashimoto K, Koike K, Okamura N, Shimizu E, lyo M. Effect of antioxidant N-acetyl-L-cysteine on behavioral changes and neurotoxicity in rats after administration of methamphetamine. Brain Res. 2004;1016:90–95. doi: 10.1016/j.brainres.2004.04.072. [DOI] [PubMed] [Google Scholar]
- 68.Imam SZ, Ali SF. Selenium, an antioxidant, attenuates methamphetamine-induced dopaminergic toxicity and peroxynitrite generation. Brain Res. 2000;855:186–191. doi: 10.1016/s0006-8993(99)02249-0. [DOI] [PubMed] [Google Scholar]
- 69.Hirata H, Ladenheim B, Carlson E, Epstein C, Cadet JL. Autoradiographic evidence for methamphetamine-induced striatal dopaminergic loss in mouse braattenuation in CuZn-superoxide dismutase transgenic mice. Brain Res. 1996;714:95–103. doi: 10.1016/0006-8993(95)01502-7. [DOI] [PubMed] [Google Scholar]
- 70.Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 71.Cervinski MA, Foster JD, Vaughan RA. Psychoactive substrates stimulate dopamine transporter phosphorylation and down-regulation by cocaine-sensitive and protein kinase C-dependent mechanisms. J Biol Chem. 2005;280:40442–40449. doi: 10.1074/jbc.M501969200. [DOI] [PubMed] [Google Scholar]
- 72.Fumagalli F, Gainetdinov RR, Valenzano KJ, Caron MG. Role of dopamine transporter in methamphetamine-induced neurotoxicity: evidence from mice lacking the transporter. J Neurosci. 1998;18:4861–4869. doi: 10.1523/JNEUROSCI.18-13-04861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cadet JL, Brannock C. Free radicals and the pathobiology of brain dopamine systems. Neurochem Int. 1998;32:117–131. doi: 10.1016/s0197-0186(97)00031-4. [DOI] [PubMed] [Google Scholar]
- 74.Hogan KA, Staal RG, Sonsalla PK. Analysis of VMAT2 binding after methamphetamine or MPTP treatment: disparity between homogenates and vesicle preparations. J Neurochem. 2000;74:2217–2220. doi: 10.1046/j.1471-4159.2000.0742217.x. [DOI] [PubMed] [Google Scholar]
- 75.Brown JM, Hanson GR, Fleckenstein AE. Methamphetamine rapidly decreases vesicular dopamine uptake. J Neurochem. 2000;74:2221–2223. doi: 10.1046/j.1471-4159.2000.0742221.x. [DOI] [PubMed] [Google Scholar]
- 76.LaVoie MJ, Hastings TG. Peroxynitrite- and nitrite-induced oxidation of dopamine: implications for nitric oxide in dopaminergic cell loss. J Neurochem. 1999;73:2546–2554. doi: 10.1046/j.1471-4159.1999.0732546.x. [DOI] [PubMed] [Google Scholar]
- 77.LaVoie MJ, Hastings TG. Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: evidence against a role for extracellular dopamine. J Neurosci. 1999;19:1484–1491. doi: 10.1523/JNEUROSCI.19-04-01484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hastings TG, Lewis DA, Zigmond MJ. Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proc Natl Acad Sci U S A. 1996;93:1956–1961. doi: 10.1073/pnas.93.5.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zucker M, Weizman A, Rehavi M. Repeated swim stress leads to down-regulation of vesicular monoamine transporter 2 in rat brain nucleus accumbens and striatum. Eur Neuropsychopharmacol. 2005;15:199–201. doi: 10.1016/j.euroneuro.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 80.Cadet JL, Jayanthi S, McCoy MT, Vawter M, Ladenheim B. Temporal profiling of methamphetamine-induced changes in gene expression in the mouse brain: evidence from cDNA array. Synapse. 2001;41:40–48. doi: 10.1002/syn.1058. [DOI] [PubMed] [Google Scholar]
- 81.Stumm G, Schlegel J, Schafer T, Wurz C, Mennel HD, Krieg JC, Vedder H. Amphetamines induce apoptosis and regulation of bcl-x splice variants in neocortical neurons. Faseb J. 1999;13:1065–1072. doi: 10.1096/fasebj.13.9.1065. [DOI] [PubMed] [Google Scholar]
- 82.Cadet JL, Ordonez SV, Ordonez JV. Methamphetamine induces apoptosis in immortalized neural cells: protection by the proto-oncogene, bcl-2. Synapse. 1997;25:176–184. doi: 10.1002/(SICI)1098-2396(199702)25:2<176::AID-SYN8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 83.Jayanthi S, Deng X, Bordelon M, McCoy MT, Cadet JL. Methamphetamine causes differential regulation of pro-death and anti-death Bcl-2 genes in the mouse neocortex. Faseb J. 2001;15:1745–1752. doi: 10.1096/fj.01-0025com. [DOI] [PubMed] [Google Scholar]
- 84.Deng X, Ladenheim B, Tsao LI, Cadet JL. Null mutation of c-fos causes exacerbation of methamphetamine-induced neurotoxicity. J Neurosci. 1999;19:10107–10115. doi: 10.1523/JNEUROSCI.19-22-10107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jayanthi S, Deng X, Noailles PA, Ladenheim B, Cadet JL. Methamphetamine induces neuronal apoptosis via cross-talks between endoplasmic reticulum and mitochondria-dependent death cascades. Faseb J. 2004;18:238–251. doi: 10.1096/fj.03-0295com. [DOI] [PubMed] [Google Scholar]
- 86.Ravagnan L, Roumier T, Kroemer G. Mitochondria, the killer organelles and their weapons. J Cell Physiol. 2002;192:131–137. doi: 10.1002/jcp.10111. [DOI] [PubMed] [Google Scholar]
- 87.Deng X, Cadet JL. Methamphetamine-induced apoptosis is attenuated in the striata of copper-zinc superoxide dismutase transgenic mice. Brain Res Mol Brain Res. 2000;83:121–124. doi: 10.1016/s0169-328x(00)00169-8. [DOI] [PubMed] [Google Scholar]
- 88.Deng X, Cai NS, McCoy MT, Chen W, Trush MA, Cadet JL. Methamphetamine induces apoptosis in an immortalized rat striatal cell line by activating the mitochondrial cell death pathway. Neuropharmacology. 2002;42:837–845. doi: 10.1016/s0028-3908(02)00034-5. [DOI] [PubMed] [Google Scholar]
- 89.Cadet JL, Jayanthi S, Deng X. Methamphetamine-induced neuronal apoptosis involves the activation of multiple death pathways. Review. Neurotox Res. 2005;8:199–206. doi: 10.1007/BF03033973. [DOI] [PubMed] [Google Scholar]
- 90.Cadet JL, Krasnova IN, Jayanthi S, Lyles J. Neurotoxicity of substituted amphetamines: molecular and cellular mechanisms. Neurotox Res. 2007;11:183–202. doi: 10.1007/BF03033567. [DOI] [PubMed] [Google Scholar]
- 91.Gorlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal. 2006;8:1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- 92.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gaddl53 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nat Cell Biol. 2001;3:E255–263. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- 94.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 95.Murachi T, Tanaka K, Hatanaka M, Murakami T. Intracellular Ca2+-dependent protease (calpain) and its high-molecular-weight endogenous inhibitor (calpastatin) Adv Enzyme Regul. 1980;19:407–424. doi: 10.1016/0065-2571(81)90026-1. [DOI] [PubMed] [Google Scholar]
- 96.Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000;150:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev. 2006;86:1133–1149. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- 98.Patil C, Walter P. Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr Opin Cell Biol. 2001;13:349–355. doi: 10.1016/s0955-0674(00)00219-2. [DOI] [PubMed] [Google Scholar]
- 99.Cadet JL, Ali S, Epstein C. Involvement of oxygen-based radicals in methamphetamine-induced neurotoxicity: evidence from the use of CuZnSOD transgenic mice. Ann N Y Acad Sci. 1994;738:388–391. doi: 10.1111/j.1749-6632.1994.tb21827.x. [DOI] [PubMed] [Google Scholar]
- 100.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 101.Kim YS, Joh TH. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson's disease. Exp Mol Med. 2006;38:333–347. doi: 10.1038/emm.2006.40. [DOI] [PubMed] [Google Scholar]
- 102.Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- 103.Garden GA. Microglia in human immunodeficiency virus-associated neurodegeneration. Glia. 2002;40:240–251. doi: 10.1002/glia.10155. [DOI] [PubMed] [Google Scholar]
- 104.Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, Iwata Y, Tsuchiya KJ, Suda S, Suzuki K, Kawai M, Takebayashi K, Yamamoto S, Matsuzaki H, Ueki T, Mori N, Gold MS, Cadet JL. Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci. 2008;28:5756–5761. doi: 10.1523/JNEUROSCI.1179-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thomas DM, Kuhn DM. Attenuated microglial activation mediates tolerance to the neurotoxic effects of methamphetamine. J Neurochem. 2005;92:790–797. doi: 10.1111/j.1471-4159.2004.02906.x. [DOI] [PubMed] [Google Scholar]
- 106.Boje KM, Arora PK. Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res. 1992;587:250–256. doi: 10.1016/0006-8993(92)91004-x. [DOI] [PubMed] [Google Scholar]
- 107.Ehrlich LC, Hu S, Sheng WS, Sutton RL, Rockswold GL, Peterson PK, Chao CC. Cytokine regulation of human microglial cell IL-8 production. J Immunol. 1998;160:1944–1948. [PubMed] [Google Scholar]
- 108.Gruol DL, Nelson TE. Physiological and pathological roles of interleukin-6 in the central nervous system. Mol Neurobiol. 1997;15:307–339. doi: 10.1007/BF02740665. [DOI] [PubMed] [Google Scholar]
- 109.McGuire SO, Ling ZD, Upton JW, Sortwell CE, Collier TJ, Carvey PM. Tumor necrosis factor alpha is toxic to embryonic mesencephalic dopamine neurons. Exp Neurol. 2001;169:219–230. doi: 10.1006/exnr.2001.7688. [DOI] [PubMed] [Google Scholar]
- 110.Ladenheim B, Krasnova IN, Deng X, Oyler JM, Polettini A, Moran TH, Huestis MA, Cadet JL. Methamphetamine-induced neurotoxicity is attenuated in transgenic mice with a null mutation for interleukin-6. Mol Pharmacol. 2000;58:1247–1256. doi: 10.1124/mol.58.6.1247. [DOI] [PubMed] [Google Scholar]
- 111.LaVoie MJ, Card JP, Hastings TG. Microglial activation precedes dopamine terminal pathology in methamphetamine-induced neurotoxicity. Exp Neurol. 2004;187:47–57. doi: 10.1016/j.expneurol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 112.Thomas DM, Dowgiert J, Geddes TJ, Francescutti-Verbeem D, Liu X, Kuhn DM. Microglial activation is a pharmacologically specific marker for the neurotoxic amphetamines. Neurosci Lett. 2004;367:349–354. doi: 10.1016/j.neulet.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 113.Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J Pharmacol Exp Ther. 2004;311:1–7. doi: 10.1124/jpet.104.070961. [DOI] [PubMed] [Google Scholar]
- 114.Aylward EH, Brettschneider PD, McArthur JC, Harris GJ, Schlaepfer TE, Henderer JD, Barta PE, Tien AY, Pearlson GD. Magnetic resonance imaging measurement of gray matter volume reductions in HIV dementia. Am J Psychiatry. 1995;152:987–994. doi: 10.1176/ajp.152.7.987. [DOI] [PubMed] [Google Scholar]
- 115.Archibald SL, Masliah E, Fennema-Notestine C, Marcotte TD, Ellis RJ, McCutchan JA, Heaton RK, Grant I, Mallory M, Miller A, Jernigan TL. Correlation of in vivo neuroimaging abnormalities with postmortem human immunodeficiency virus encephalitis and dendritic loss. Arch Neurol. 2004;61:369–376. doi: 10.1001/archneur.61.3.369. [DOI] [PubMed] [Google Scholar]
- 116.Chang L, Ernst T, Leonido-Yee M, Speck 0. Perfusion MRI detects rCBF abnormalities in early stages of HIV-cognitive motor complex. Neurology. 2000;54:389–396. doi: 10.1212/wnl.54.2.389. [DOI] [PubMed] [Google Scholar]
- 117.Ernst T, Itti E, Itti L, Chang L. Changes in cerebral metabolism are detected prior to perfusion changes in early HIV-CMC: A coregistered (1)H MRS and SPECT study. J Magn Reson Imaging. 2000;12:859–865. doi: 10.1002/1522-2586(200012)12:6<859::aid-jmri8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 118.Stout JC, Ellis RJ, Jernigan TL, Archibald SL, Abramson I, Wolfson T, McCutchan JA, Wallace MR, Atkinson JH, Grant I. Progressive cerebral volume loss in human immunodeficiency virus infection: a longitudinal volumetric magnetic resonance imaging study. HIV Neurobehavioral Research Center Group. Arch Neurol. 1998;55:161–168. doi: 10.1001/archneur.55.2.161. [DOI] [PubMed] [Google Scholar]
- 119.Bursztyn EM, Lee BC, Bauman J. CT of acquired immunodeficiency syndrome. AJNR Am J Neuroradiol. 1984;5:711–714. [PMC free article] [PubMed] [Google Scholar]
- 120.Levy RM, Rosenbloom S, Perrett LV. Neuroradiologic findings in AIDS: a review of 200 cases. AJR Am J Roentgenol. 1986;147:977–983. doi: 10.2214/ajr.147.5.977. [DOI] [PubMed] [Google Scholar]
- 121.Liow JS, Rehm K, Strother SC, Anderson JR, Morch N, Hansen LK, Schaper KA, Rottenberg DA. Comparison of voxel- and volume-of-interest-based analyses in FDG PET scans of HIV positive and healthy individuals. J Nucl Med. 2000;41:612–621. [PubMed] [Google Scholar]
- 122.von Giesen HJ, Antke C, Hefter H, Wenserski F, Seitz RJ, Arendt G. Potential time course of human immunodeficiency virus type 1-associated minor motor deficits: electrophysiologic and positron emission tomography findings. Arch Neurol. 2000;57:1601–1607. doi: 10.1001/archneur.57.11.1601. [DOI] [PubMed] [Google Scholar]
- 123.Chang L, Lee PL, Yiannoutsos CT, Ernst T, Marra CM, Richards T, Kolson D, Schifitto G, Jarvik JG, Miller EN, Lenkinski R, Gonzalez G, Navia BA. A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. Neuroimage. 2004;23:1336–1347. doi: 10.1016/j.neuroimage.2004.07.067. [DOI] [PubMed] [Google Scholar]
- 124.Ernst T, Chang L. Effect of aging on brain metabolism in antiretroviral-naive HIV patients. Aids. 2004;18(Suppl 1):S61–67. [PubMed] [Google Scholar]
- 125.Lee PL, Yiannoutsos CT, Ernst T, Chang L, Marra CM, Jarvik JG, Richards TL, Kwok EW, Kolson DL, Simpson D, Tang CY, Schifitto G, Ketonen LM, Meyerhoff DJ, Lenkinski RE, Gonzalez RG, Navia BA. A multi-center 1H MRS study of the AIDS dementia complex: validation and preliminary analysis. J Magn Reson Imaging. 2003;17:625–633. doi: 10.1002/jmri.10295. [DOI] [PubMed] [Google Scholar]
- 126.Meyerhoff DJ, Weiner MW, Fein G. Deep gray matter structures in HIV infection: a proton MR spectroscopic study. AJNR Am J Neuroradiol. 1996;17:973–978. [PMC free article] [PubMed] [Google Scholar]
- 127.Moller HE, Vermathen P, Lentschig MG, Schuierer G, Schwarz S, Wiedermann D, Evers S, Husstedt IW. Metabolic characterization of AIDS dementia complex by spectroscopic imaging. J Magn Reson Imaging. 1999;9:10–18. doi: 10.1002/(sici)1522-2586(199901)9:1<10::aid-jmri2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 128.Stankoff B, Tourbah A, Suarez S, Turell E, Stievenart JL, Payan C, Coutellier A, Herson S, Baril L, Bricaire F, Calvez V, Cabanis EA, Lacomblez L, Lubetzki C. Clinical and spectroscopic improvement in HIV-associated cognitive impairment. Neurology. 2001;56:112–115. doi: 10.1212/wnl.56.1.112. [DOI] [PubMed] [Google Scholar]
- 129.Suwanwelaa N, Phanuphak P, Phanthumchinda K, Suwanwela NC, Tantivatana J, Ruxrungtham K, Suttipan J, Wangsuphachart S, Hanvanich M. Magnetic resonance spectroscopy of the brain in neurologically asymptomatic HIV-infected patients. Magn Reson Imaging. 2000;18:859–865. doi: 10.1016/s0730-725x(00)00173-9. [DOI] [PubMed] [Google Scholar]
- 130.Tarasow E, Wiercinska-Drapalo A, Kubas B, Dzienis W, Orzechowska-Bobkiewicz A, Prokopowicz D, Walecki J. Cerebral MR spectroscopy in neurologically asymptomatic HIV-infected patients. Acta Radiol. 2003;44:206–212. doi: 10.1080/j.1600-0455.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 131.Chang L, Ernst T, Witt MD, Ames N, Gaiefsky M, Miller E. Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-naive HIV patients. Neuroimage. 2002;17:1638–1648. doi: 10.1006/nimg.2002.1254. [DOI] [PubMed] [Google Scholar]
- 132.Chang L, Ernst T, Witt MD, Ames N, Walot I, Jovicich J, DeSilva M, Trivedi N, Speck 0, Miller EN. Persistent brain abnormalities in antiretroviral-naive HIV patients 3 months after HAART. Antivir Ther. 2003;8:17–26. [PubMed] [Google Scholar]
- 133.Brew BJ, Corbeil J, Pemberton L, Evans L, Saito K, Penny R, Cooper DA, Heyes MP. Quinolinic acid production is related to macrophage tropic isolates of HIV-1. J Neurovirol. 1995;1:369–374. doi: 10.3109/13550289509111026. [DOI] [PubMed] [Google Scholar]
- 134.Gartner S. HIV infection and dementia. Science. 2000;287:602–604. doi: 10.1126/science.287.5453.602. [DOI] [PubMed] [Google Scholar]
- 135.Giulian D, Vaca K, Noonan CA. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990;250:1593–1596. doi: 10.1126/science.2148832. [DOI] [PubMed] [Google Scholar]
- 136.Giulian D, Wendt E, Vaca K, Noonan CA. The envelope glycoprotein of human immunodeficiency virus type 1 stimulates release of neurotoxins from monocytes. Proc Natl Acad Sci U S A. 1993;90:2769–2773. doi: 10.1073/pnas.90.7.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jiang ZG, Piggee C, Heyes MP, Murphy C, Quearry B, Bauer M, Zheng J, Gendelman HE, Markey SP. Glutamate is a mediator of neurotoxicity in secretions of activated HIV-1-infected macrophages. J Neuroimmunol. 2001;117:97–107. doi: 10.1016/s0165-5728(01)00315-0. [DOI] [PubMed] [Google Scholar]
- 138.Lipton SA, Sucher NJ, Kaiser PK, Dreyer EB. Synergistic effects of HIV coat protein and NMDA receptor-mediated neurotoxicity. Neuron. 1991;7:111–118. doi: 10.1016/0896-6273(91)90079-f. [DOI] [PubMed] [Google Scholar]
- 139.Viviani B, Corsini E, Binaglia M, Galli CL, Marinovich M. Reactive oxygen species generated by glia are responsible for neuron death induced by human immunodeficiency virus-glycoprotein 120 in vitro. Neuroscience. 2001;107:51–58. doi: 10.1016/s0306-4522(01)00332-3. [DOI] [PubMed] [Google Scholar]
- 140.Zhao J, Lopez AL, Erichsen D, Herek S, Cotter RL, Curthoys NP, Zheng J. Mitochondrial glutaminase enhances extracellular glutamate production in HIV-1-infected macrophages: linkage to HIV-1 associated dementia. J Neurochem. 2004;88:169–180. doi: 10.1046/j.1471-4159.2003.02146.x. [DOI] [PubMed] [Google Scholar]
- 141.Zhao ML, Kim MO, Morgello S, Lee SC. Expression of inducible nitric oxide synthase, interleukin-1 and caspase-1 in HIV-1 encephalitis. J Neuroimmunol. 2001;115:182–19. doi: 10.1016/s0165-5728(00)00463-x. [DOI] [PubMed] [Google Scholar]
- 142.Letendre SL, Lanier ER, McCutchan JA. Cerebrospinal fluid beta chemokine concentrations in neurocognitively impaired individuals infected with human immunodeficiency virus type 1. J Infect Dis. 1999;180:310–319. doi: 10.1086/314866. [DOI] [PubMed] [Google Scholar]
- 143.Kaul M, Lipton SA. Chemokines and activated macrophages in HIV gpl20-induced neuronal apoptosis. Proc Natl Acad Sci U S A. 1999;96:8212–8216. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Meucci 0, Miller RJ. gpl20-induced neurotoxicity in hippocampal pyramidal neuron cultures: protective action of TGF-betal. J Neurosci. 1996;16:4080–4088. doi: 10.1523/JNEUROSCI.16-13-04080.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Garden GA, Guo W, Jayadev S, Tun C, Balcaitis S, Choi J, Montine TJ, Moller T, Morrison RS. HIV associated neurodegeneration requires p53 in neurons and microglia. Faseb J. 2004;18:1141–1143. doi: 10.1096/fj.04-1676fje. [DOI] [PubMed] [Google Scholar]
- 146.Milward EA, Fitzsimmons C, Szklarczyk A, Conant K. The matrix metalloproteinases and CNS plasticity: an overview. J Neuroimmunol. 2007;187:9–19. doi: 10.1016/j.jneuroim.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 147.Gasche Y, Soccal PM, Kanemitsu M, Copin JC. Matrix metalloproteinases and diseases of the central nervous system with a special emphasis on ischemic brain. Front Biosci. 2006;11:1289–1301. doi: 10.2741/1883. [DOI] [PubMed] [Google Scholar]
- 148.Lo EH, Wang X, Cuzner ML. Extracellular proteolysis in brain injury and inflammation: role for plasminogen activators and matrix metalloproteinases. J Neurosci Res. 2002;69:1–9. doi: 10.1002/jnr.10270. [DOI] [PubMed] [Google Scholar]
- 149.Johnston JB, Zhang K, Silva C, Shalinsky DR, Conant K, Ni W, Corbett D, Yong VW, Power C. HIV-1 Tat neurotoxicity is prevented by matrix metalloproteinase inhibitors. Ann Neurol. 2001;49:230–241. doi: 10.1002/1531-8249(20010201)49:2<230::aid-ana43>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 150.Webster NL, Crowe SM. Matrix metalloproteinases, their production by monocytes and macrophages and their potential role in HIV-related diseases. J Leukoc Biol. 2006;80:1052–1066. doi: 10.1189/jlb.0306152. [DOI] [PubMed] [Google Scholar]
- 151.Zhang K, McQuibban GA, Silva C, Butler GS, Johnston JB, Holden J, Clark-Lewis I, Overall CM, Power C. HIV-induced metalloproteinase processing of the chemokine stromal cell derived factor-1 causes neurodegeneration. Nat Neurosci. 2003;6:1064–1071. doi: 10.1038/nn1127. [DOI] [PubMed] [Google Scholar]
- 152.Conant K, McArthur JC, Griffin DE, Sjulson L, Wahl LM, Irani DN. Cerebrospinal fluid levels of MMP-2, 7, and 9 are elevated in association with human immunodeficiency virus dementia. Ann Neurol. 1999;46:391–398. doi: 10.1002/1531-8249(199909)46:3<391::aid-ana15>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 153.Chong YH, Seoh JY, Park HK. Increased activity of matrix metalloproteinase-2 in human glial and neuronal cell lines treated with HIV-1 gp41 peptides. J Mol Neurosci. 1998;10:129–141. doi: 10.1007/BF02737124. [DOI] [PubMed] [Google Scholar]
- 154.Marshall DC, Wyss-Coray T, Abraham CR. Induction of matrix metalloproteinase-2 in human immunodeficiency virus-1 glycoprotein 120 transgenic mouse brains. Neurosci Lett. 1998;254:97–100. doi: 10.1016/s0304-3940(98)00674-0. [DOI] [PubMed] [Google Scholar]
- 155.Peruzzi F. The multiple functions of HIV-1 Tat: proliferation versus apoptosis. Front Biosci. 2006;11:708–717. doi: 10.2741/1829. [DOI] [PubMed] [Google Scholar]
- 156.Tardieu M, Hery C, Peudenier S, Boespflug 0, Montagnier L. Human immunodeficiency virus type 1-infected monocytic cells can destroy human neural cells after cell-to-cell adhesion. Ann Neurol. 1992;32:11–17. doi: 10.1002/ana.410320104. [DOI] [PubMed] [Google Scholar]
- 157.Li W, Galey D, Mattson MP, Nath A. Molecular and cellular mechanisms of neuronal cell death in HIV dementia. Neurotox Res. 2005;8:119–134. doi: 10.1007/BF03033824. [DOI] [PubMed] [Google Scholar]
- 158.Aksenov MY, Hasselrot U, Wu G, Nath A, Anderson C, Mactutus CF, Booze RM. Temporal relationships between HIV-1 Tat-induced neuronal degeneration, OX-42 immunoreactivity, reactive astrocytosis, and protein oxidation in the rat striatum. Brain Res. 2003;987:1–9. doi: 10.1016/s0006-8993(03)03194-9. [DOI] [PubMed] [Google Scholar]
- 159.Bansal AK, Mactutus CF, Nath A, Maragos W, Hauser KF, Booze RM. Neurotoxicity of HIV-1 proteins gpl20 and Tat in the rat striatum. Brain Res. 2000;879:42–49. doi: 10.1016/s0006-8993(00)02725-6. [DOI] [PubMed] [Google Scholar]
- 160.Gavriil ES, Cooney R, Weeks BS. Tat mediates apoptosis in vivo in the rat central nervous system. Biochem Biophys Res Commun. 2000;267:252–256. doi: 10.1006/bbrc.1999.1894. [DOI] [PubMed] [Google Scholar]
- 161.Jones M, Olafson K, Del Bigio MR, Peeling J, Nath A. Intraventricular injection of human immunodeficiency virus type 1 (HIV-1) tat protein causes inflammation, gliosis, apoptosis, and ventricular enlargement. J Neuropathol Exp Neurol. 1998;57:563–570. doi: 10.1097/00005072-199806000-00004. [DOI] [PubMed] [Google Scholar]
- 162.Bruce-Keller AJ, Chauhan A, Dimayuga FO, Gee J, Keller JN, Nath A. Synaptic transport of human immunodeficiency virus-Tat protein causes neurotoxicity and gliosis in rat brain. J Neurosci. 2003;23:8417–8422. doi: 10.1523/JNEUROSCI.23-23-08417.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bonavia R, Bajetto A, Barbero S, Albini A, Noonan DM, Schettini G. HIV-1 Tat causes apoptotic death and calcium homeostasis alterations in rat neurons. Biochem Biophys Res Commun. 2001;288:301–308. doi: 10.1006/bbrc.2001.5743. [DOI] [PubMed] [Google Scholar]
- 164.Kruman II, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol. 1998;154:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- 165.Bartz SR, Emerman M. Human immunodeficiency virus type 1 Tat induces apoptosis and increases sensitivity to apoptotic signals by up-regulating FLICE/caspase-8. J Virol. 1999;73:1956–1963. doi: 10.1128/jvi.73.3.1956-1963.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Perry SW, Norman JP, Litzburg A, Zhang D, Dewhurst S, Gelbard HA. HIV-1 transactivator of transcription protein induces mitochondrial hyperpolarization and synaptic stress leading to apoptosis. J Immunol. 2005;174:4333–4344. doi: 10.4049/jimmunol.174.7.4333. [DOI] [PubMed] [Google Scholar]
- 167.Agrawal L, Louboutin JP, Strayer DS. Preventing HIV-1 Tat-induced neuronal apoptosis using antioxidant enzymes: mechanistic and therapeutic implications. Virology. 2007;363:462–472. doi: 10.1016/j.virol.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 168.Westendorp MO, Shatrov VA, Schulze-Osthoff K, Frank R, Kraft M, Los M, Krammer PH, Droge W, Lehmann V. HIV-1 Tat potentiates TNF-induced NF-kappa B activation and cytotoxicity by altering the cellular redox state. Embo J. 1995;14:546–554. doi: 10.1002/j.1460-2075.1995.tb07030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Magnuson DS, Knudsen BE, Geiger JD, Brownstone RM, Nath A. Human immunodeficiency virus type 1 tat activates non-N-methyl-D-aspartate excitatory amino acid receptors and causes neurotoxicity. Ann Neurol. 1995;37:373–380. doi: 10.1002/ana.410370314. [DOI] [PubMed] [Google Scholar]
- 170.New DR, Ma M, Epstein LG, Nath A, Gelbard HA. Human immunodeficiency virus type 1 Tat protein induces death by apoptosis in primary human neuron cultures. J Neurovirol. 1997;3:168–173. doi: 10.3109/13550289709015806. [DOI] [PubMed] [Google Scholar]
- 171.Perez A, Probert AW, Wang KK, Sharmeen L. Evaluation of HIV-1 Tat induced neurotoxicity in rat cortical cell culture. J Neurovirol. 2001;7:1–10. doi: 10.1080/135502801300069575. [DOI] [PubMed] [Google Scholar]
- 172.Caporello E, Nath A, Slevin J, Galey D, Hamilton G, Williams L, Steiner JP, Haughey NJ. The immunophilin ligand GPI1046 protects neurons from the lethal effects of the HIV-1 proteins gpl20 and Tat by modulating endoplasmic reticulum calcium load. J Neurochem. 2006;98:146–155. doi: 10.1111/j.1471-4159.2006.03863.x. [DOI] [PubMed] [Google Scholar]
- 173.Willey RL, Bonifacino JS, Potts BJ, Martin MA, Klausner RD. Biosynthesis, cleavage, and degradation of the human immunodeficiency virus 1 envelope glycoprotein gpl60. Proc Natl Acad Sci U S A. 1988;85:9580–9584. doi: 10.1073/pnas.85.24.9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wu P, Price P, Du B, Hatch WC, Terwilliger EF. Direct cytotoxicity of HIV-1 envelope protein gpl20 on human NT neurons. Neuroreport. 1996;7:1045–1049. doi: 10.1097/00001756-199604100-00018. [DOI] [PubMed] [Google Scholar]
- 175.Bachis A, Major EO, Mocchetti I. Brain-derived neurotrophic factor inhibits human immunodeficiency virus-l/gpl20-mediated cerebellar granule cell death by preventing gpl20 internalization. J Neurosci. 2003;23:5715–5722. doi: 10.1523/JNEUROSCI.23-13-05715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Brenneman DE, Westbrook GL, Fitzgerald SP, Ennist DL, Elkins KL, Ruff MR, Pert CB. Neuronal cell killing by the envelope protein of HIV and its prevention by vasoactive intestinal peptide. Nature. 1988;335:639–642. doi: 10.1038/335639a0. [DOI] [PubMed] [Google Scholar]
- 177.Dreyer EB, Kaiser PK, Offermann JT, Upton SA. HIV-1 coat protein neurotoxicity prevented by calcium channel antagonists. Science. 1990;248:364–367. doi: 10.1126/science.2326646. [DOI] [PubMed] [Google Scholar]
- 178.Toggas SM, Masliah E, Rockenstein EM, Rail GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gpl20 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- 179.D'Hooge R, Franck F, Mucke L, De Deyn PP. Age-related behavioural deficits in transgenic mice expressing the HIV-1 coat protein gpl20. Eur J Neurosci. 1999;11:4398–4402. doi: 10.1046/j.1460-9568.1999.00857.x. [DOI] [PubMed] [Google Scholar]
- 180.Hill JM, Mervis RF, Avidor R, Moody TW, Brenneman DE. HIV envelope protein-induced neuronal damage and retardation of behavioral development in rat neonates. Brain Res. 1993;603:222–233. doi: 10.1016/0006-8993(93)91241-j. [DOI] [PubMed] [Google Scholar]
- 181.Dawson VL, Dawson TM, Uhl GR, Snyder SH. Human immunodeficiency virus type 1 coat protein neurotoxicity mediated by nitric oxide in primary cortical cultures. Proc Natl Acad Sci U S A. 1993;90:3256–3259. doi: 10.1073/pnas.90.8.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Garden GA, Budd SL, Tsai E, Hanson L, Kaul M, D'Emilia DM, Friedlander RM, Yuan J, Masliah E, Upton SA. Caspase cascades in human immunodeficiency virus-associated neurodegeneration. J Neurosci. 2002;22:4015–4024. doi: 10.1523/JNEUROSCI.22-10-04015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Koka P, He K, Camerini D, Tran T, Yashar SS, Merrill JE. The mapping of HIV-1 gpl60 epitopes required for interleukin-1 and tumor necrosis factor alpha production in glial cells. J Neuroimmunol. 1995;57:179–191. doi: 10.1016/0165-5728(94)00184-p. [DOI] [PubMed] [Google Scholar]
- 184.Koka P, He K, Zack JA, Kitchen S, Peacock W, Fried I, Tran T, Yashar SS, Merrill JE. Human immunodeficiency virus 1 envelope proteins induce interleukin 1, tumor necrosis factor alpha, and nitric oxide in glial cultures derived from fetal, neonatal, and adult human brain. J Exp Med. 1995;182:941–951. doi: 10.1084/jem.182.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Merrill JE, Koyanagi Y, Zack J, Thomas L, Martin F, Chen IS. Induction of interleukin-1 and tumor necrosis factor alpha in brain cultures by human immunodeficiency virus type 1. J Virol. 1992;66:2217–2225. doi: 10.1128/jvi.66.4.2217-2225.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Dickson DW, Lee SC, Mattiace LA, Yen SH, Brosnan C. Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer's disease. Glia. 1993;7:75–83. doi: 10.1002/glia.440070113. [DOI] [PubMed] [Google Scholar]
- 187.Kure K, Lyman WD, Weidenheim KM, Dickson DW. Cellular localization of an HIV-1 antigen in subacute AIDS encephalitis using an improved double-labeling immunohistochemical method. Am J Pathol. 1990;136:1085–1092. [PMC free article] [PubMed] [Google Scholar]
- 188.Kure K, Weidenheim KM, Lyman WD, Dickson DW. Morphology and distribution of HIV-1 gp41-positive microglia in subacute AIDS encephalitis. Pattern of involvement resembling a multisystem degeneration. Acta Neuropathol. 1990;80:393–400. doi: 10.1007/BF00307693. [DOI] [PubMed] [Google Scholar]
- 189.Adamson DC, McArthur JC, Dawson TM, Dawson VL. Rate and severity of HIV-associated dementia (HAD): correlations with Gp41 and iNOS. Mol Med. 1999;5:98–109. [PMC free article] [PubMed] [Google Scholar]
- 190.Bukrinsky M, Adzhubei A. Viral protein R of HIV-1. Rev Med Virol. 1999;9:39–49. doi: 10.1002/(sici)1099-1654(199901/03)9:1<39::aid-rmv235>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 191.Piller SC, Jans P, Gage PW, Jans DA. Extracellular HIV-1 virus protein R causes a large inward current and cell death in cultured hippocampal neurons: implications for AIDS pathology. Proc Natl Acad Sci U S A. 1998;95:4595–4600. doi: 10.1073/pnas.95.8.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sabbah EN, Roques BP. Critical implication of the (70-96) domain of human immunodeficiency virus type 1 Vpr protein in apoptosis of primary rat cortical and striatal neurons. J Neurovirol. 2005;11:489–502. doi: 10.1080/13550280500384941. [DOI] [PubMed] [Google Scholar]
- 193.Jones GJ, Barsby NL, Cohen EA, Holden J, Harris K, Dickie P, Jhamandas J, Power C. HIV-1 Vpr causes neuronal apoptosis and in vivo neurodegeneration. J Neurosci. 2007;27:3703–3711. doi: 10.1523/JNEUROSCI.5522-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jacotot E, Ferri KF, El Hamel C, Brenner C, Druillennec S, Hoebeke J, Rustin P, Metivier D, Lenoir C, Geuskens M, Vieira HL, Loeffler M, Belzacq AS, Briand JP, Zamzami N, Edelman L, Xie ZH, Reed JC, Roques BP, Kroemer G. Control of mitochondrial membrane permeabilization by adenine nucleotide translocator interacting with HIV-1 viral protein rR and Bcl-2. J Exp Med. 2001;193:509–519. doi: 10.1084/jem.193.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Jacotot E, Ravagnan L, Loeffler M, Ferri KF, Vieira HL, Zamzami N, Costantini P, Druillennec S, Hoebeke J, Briand JP, Irinopoulou T, Daugas E, Susin SA, Cointe D, Xie ZH, Reed JC, Roques BP, Kroemer G. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J Exp Med. 2000;191:33–46. doi: 10.1084/jem.191.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sabbah EN, Druillennec S, Morellet N, Bouaziz S, Kroemer G, Roques BP. Interaction between the HIV-1 protein Vpr and the adenine nucleotide translocator. Chem Biol Drug Des. 2006;67:145–154. doi: 10.1111/j.1747-0285.2006.00340.x. [DOI] [PubMed] [Google Scholar]
- 197.Patel CA, Mukhtar M, Pomerantz RJ. Human immunodeficiency virus type 1 Vpr induces apoptosis in human neuronal cells. J Virol. 2000;74:9717–9726. doi: 10.1128/jvi.74.20.9717-9726.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Stewart SA, Poon B, Song JY, Chen IS. Human immunodeficiency virus type 1 vpr induces apoptosis through caspase activation. J Virol. 2000;74:3105–3111. doi: 10.1128/jvi.74.7.3105-3111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Shostak LD, Ludlow J, Fisk J, Pursell S, Rimel BJ, Nguyen D, Rosenblatt JD, Planelles V. Roles of p53 and caspases in the induction of cell cycle arrest and apoptosis by HIV-1 vpr. Exp Cell Res. 1999;251:156–165. doi: 10.1006/excr.1999.4568. [DOI] [PubMed] [Google Scholar]
- 200.Tungaturthi PK, Sawaya BE, Singh SP, Tomkowicz B, Ayyavoo V, Khalili K, Collman RG, Amini S, Srinivasan A. Role of HIV-1 Vpr in AIDS pathogenesis: relevance and implications of intravirion, intracellular and free Vpr. Biomed Pharmacother. 2003;57:20–24. doi: 10.1016/s0753-3322(02)00328-1. [DOI] [PubMed] [Google Scholar]
- 201.Das SR, Jameel S. Biology of the HIV Nef protein. Indian J Med Res. 2005;121:315–332. [PubMed] [Google Scholar]
- 202.Koedel U, Kohleisen B, Sporer B, Lahrtz F, Ovod V, Fontana A, Erfle V, Pfister HW. HIV type 1 Nef protein is a viral factor for leukocyte recruitment into the central nervous system. J Immunol. 1999;163:1237–1245. [PubMed] [Google Scholar]
- 203.Bencheikh M, Bentsman G, Sarkissian N, Canki M, Volsky DJ. Replication of different clones of human immunodeficiency virus type 1 in primary fetal human astrocytes: enhancement of viral gene expression by Nef. J Neurovirol. 1999;5:115–124. doi: 10.3109/13550289909021993. [DOI] [PubMed] [Google Scholar]
- 204.Harris M. HIV: a new role for Nef in the spread of HIV. Curr Biol. 1999;9:R459–461. doi: 10.1016/s0960-9822(99)80282-6. [DOI] [PubMed] [Google Scholar]
- 205.Kestler HW, 3rd, Ringler DJ, Mori K, Panicali DL, Sehgal PK, Daniel MD, Desrosiers RC. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 206.Acheampong E, Mukhtar M, Parveen Z, Ngoubilly N, Ahmad N, Patel C, Pomerantz RJ. Ethanol strongly potentiates apoptosis induced by HIV-1 proteins in primary human brain microvascular endothelial cells. Virology. 2002;304:222–234. doi: 10.1006/viro.2002.1666. [DOI] [PubMed] [Google Scholar]
- 207.He J, deCastro CM, Vandenbark GR, Busciglio J, Gabuzda D. Astrocyte apoptosis induced by HIV-1 transactivation of the c-kit protooncogene. Proc Natl Acad Sci U S A. 1997;94:3954–3959. doi: 10.1073/pnas.94.8.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Trillo-Pazos G, McFarlane-Abdulla E, Campbell IC, Pilkington GJ, Everall IP. Recombinant nef HIV-IIIB protein is toxic to human neurons in culture. Brain Res. 2000;864:315–326. doi: 10.1016/s0006-8993(00)02213-7. [DOI] [PubMed] [Google Scholar]
- 209.Mordelet E, Kissa K, Cressant A, Gray F, Ozden S, Vidal C, Charneau P, Granon S. Histopathological and cognitive defects induced by Nef in the brain. Faseb J. 2004;18:1851–1861. doi: 10.1096/fj.04-2308com. [DOI] [PubMed] [Google Scholar]
- 210.Taylor MJ, Alhassoon OM, Schweinsburg BC, Videen JS, Grant I. MR spectroscopy in HIV and stimulant dependence HNRC Group. HIV Neurobehavioral Research Center. J Int Neuropsychol Soc. 2000;6:83–85. doi: 10.1017/s1355617700611104. [DOI] [PubMed] [Google Scholar]
- 211.Maragos WF, Young KL, Turchan JT, Guseva M, Pauly JR, Nath A, Cass WA. Human immunodeficiency virus-1 Tat protein and methamphetamine interact synergistically to impair striatal dopaminergic function. J Neurochem. 2002;83:955–963. doi: 10.1046/j.1471-4159.2002.01212.x. [DOI] [PubMed] [Google Scholar]
- 212.Czub S, Koutsilieri E, Sopper S, Czub M, Stahl-Hennig C, Muller JG, Pedersen V, Gsell W, Heeney JL, Gerlach M, Gosztonyi G, Riederer P, ter Meulen V. Enhancement of central nervous system pathology in early simian immunodeficiency virus infection by dopaminergic drugs. Acta Neuropathol. 2001;101:85–91. doi: 10.1007/s004010000313. [DOI] [PubMed] [Google Scholar]
- 213.Flora G, Lee YW, Nath A, Hennig B, Maragos W, Toborek M. Methamphetamine potentiates HIV-1 Tat protein-mediated activation of redox-sensitive pathways in discrete regions of the brain. Exp Neurol. 2003;179:60–70. doi: 10.1006/exnr.2002.8048. [DOI] [PubMed] [Google Scholar]
- 214.Theodore S, Cass WA, Maragos WF. Involvement of cytokines in human immunodeficiency virus-1 protein Tat and methamphetamine interactions in the striatum. Exp Neurol. 2006;199:490–498. doi: 10.1016/j.expneurol.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 215.Langford D, Grigorian A, Hurford R, Adame A, Crews L, Masliah E. The role of mitochondrial alterations in the combined toxic effects of human immunodeficiency virus Tat protein and methamphetamine on calbindin positive-neurons. J Neurovirol. 2004;10:327–337. doi: 10.1080/13550280490520961. [DOI] [PubMed] [Google Scholar]
- 216.Cass WA, Harned ME, Peters LE, Nath A, Maragos WF. HIV-1 protein Tat potentiation of methamphetamine-induced decreases in evoked overflow of dopamine in the striatum of the rat. Brain Res. 2003;984:133–142. doi: 10.1016/s0006-8993(03)03122-6. [DOI] [PubMed] [Google Scholar]
- 217.Theodore S, Cass WA, Nath A, Steiner J, Young K, Maragos WF. Inhibition of tumor necrosis factor-alpha signaling prevents human immunodeficiency virus-1 protein Tat and methamphetamine interaction. Neurobiol Dis. 2006;23:663–668. doi: 10.1016/j.nbd.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 218.Conant K, St Hillaire C, Anderson C, Galey D, Wang J, Nath A. Human immunodeficiency virus type 1 Tat and methamphetamine affect the release and activation of matrix-degrading proteinases. J Neurovirol. 2004;10:21–28. doi: 10.1080/13550280490261699. [DOI] [PubMed] [Google Scholar]
- 219.Hauser KF, El-Hage N, Stiene-Martin A, Maragos WF, Nath A, Persidsky Y, Volsky DJ, Knapp PE. HIV-1 neuropathogenesis: glial mechanisms revealed through substance abuse. J Neurochem. 2007;100:567–586. doi: 10.1111/j.1471-4159.2006.04227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]