Abstract
Transcription factor nuclear factor-κB (NF-κB) is constitutively activated in most pancreatic cancer tissues and cell lines but not in normal pancreas nor in immortalized/nontumorigenic human pancreatic ductal epithelial cells. Inhibition of constitutive NF-κB activation in pancreatic cancer cell lines suppresses tumorigenesis and tumor metastasis. Recently, we identified autocrine secretion of proinflammatory cytokine interleukin (IL)-1α as the mechanism of constitutive NF-κB activation in metastatic pancreatic cancer cell lines. However, the role of IL-1α in determining the metastatic potential of pancreatic tumor remains to be further investigated. In the current study, we stably expressed IL-1α in the nonmetastatic, IL-1α–negative MiaPaCa-2 cell lines. Our results showed that the secretion of IL-1α in MiaPaCa-2 cells constitutively activated NF-κB and increased the expression of NF-κB downstream genes involved in the different steps of the metastatic cascade, such as urokinase-type plasminogen activator, vascular endothelial growth factor, and IL-8. MiaPaCa-2/IL-1α cells showed an enhanced cell invasion in vitro compared with parental MiaPaCa-2 cells and induced liver metastasis in an orthotopic mouse model. The metastatic phenotype induced by IL-1α was inhibited by the expression of phosphorylation-defective IκB (IκB S32, 36A), which blocked NF-κB activation. Consistently, silencing the expression of IL-1α by short hairpin RNA in the highly metastatic L3.6pl pancreatic cancer cells completely suppressed their metastatic spread. In summary, these findings showed that IL-1α plays key roles in pancreatic cancer metastatic behavior through the constitutive activation of NF-κB. Our findings further support the possible link between inflammation and cancer and suggest that IL-1α may be a potential therapeutic target for treating pancreatic adenocarcinoma.
Introduction
Pancreatic adenocarcinoma remains one of the most lethal and poorly understood human malignancies. It ranks fourth in the leading causes of cancer-related mortality among adults in the United States (1). At the time of diagnosis, two thirds of patients present with advanced disease characterized by early local regional spread and distant metastasis (2). Current chemotherapy, radiation therapy, and surgical procedures are largely ineffective in the treatment of this disease (3).
Nuclear factor-κB (NF-κB) is a family of pleiotropic transcription factors that control the expression of numerous genes involved in growth, tumorigenesis, tumor metastasis, apoptosis, and immune and inflammatory responses (4). Many tumors have acquired genetic alterations in the signaling pathways that regulate NF-κB activation. We previously reported that RelA, the p65 subunit of the NF-κB transcription factor, is constitutively activated in most pancreatic cancer tissues and human pancreatic cancer cell lines but not in normal pancreatic tissues or immortalized pancreatic ductal epithelial cells (5). In different models of pancreatic tumor, inhibiting constitutive NF-κB activation by expression of an IκBα phosphorylation mutant suppresses tumorigenicity by reducing expression of antiapoptotic proteins Bcl-x(L) and Bcl-2 and inhibits liver metastasis and angiogenic potential by reducing in vivo expression of vascular endothelial growth factor (VEGF; refs. 6–8).
Interleukin (IL)-1α and IL-1β are between the most potent proinflammatory cytokines (9). They initiate and potentiate the inflammatory response working by themselves, but more importantly, they induce the expression of proinflammatory gene molecules mainly acting as potent activators of NF-κB.
Although only rarely secreted by normal cells other than macrophages, IL-1α was shown to act as an autocrine growth stimulant for several malignant cell types (10, 11). We recently showed that an autocrine stimulation of IL-1α, but not IL-1β, primarily induced by activator protein-1 activity, accounts for the constitutive activation of NF-κB in metastatic human pancreatic cancer cell lines but not in nonmetastatic ones. The constitutive NF-κB activity triggered by IL-1α autocrine stimulation enhances the IL-1α expression, initiating the formation of a positive feedback loop. Neutralization of IL-1α activity suppressed the constitutive activation of NF-κB and the expression of its downstream target gene, urokinase-type plasminogen activator (uPA), in metastatic pancreatic cancer cell lines (12). In an in vitro model of inflammatory breast cancer cells, Streicher et al. (13) more recently showed that amphiregulin, but not epidermal growth factor, induces an increased expression of IL-1, triggering a similar autocrine loop through a prompt activation of NF-κB.
In malignant cells, exogenous recombinant IL-1 induces secretion of growth, invasiveness promoting, and angiogenic factors (9). In an in vitro model of breast cancer cells, secreted IL-1α of tumor origin was shown to induce expression of pro-metastatic genes in cancer as well as in stromal cells (14). In pancreatic cancer in vitro models, expression of IL-1α mRNA and protein was observed only in the highly liver metastatic pancreatic cancer cell lines (15). Several studies showed in vitro that exogenous recombinant IL-1α can favor the metastatic and invasive behavior of human pancreatic cancer cells (16–20). In a series of 30 pancreatic cancer patients, IL-1α was found to be increased, whereas IL-1β decreased when liver metastases were present (21).
To show in an in vivo model that the IL-1α autocrine loop is indeed responsible for the pronounced metastatic behavior of pancreatic cancer mediated by a constitutive activation of NF-κB, we stably expressed IL-1α in the nonmetastatic pancreatic cancer cell line MiaPaCa-2 and used phosphorylation-defective mutant IκBαM (S32, 36A) to completely inhibit the activation of NF-κB. Our results showed that autocrine secretion of IL-1α constitutively activated NF-κB in MiaPaCa-2 cells and endowed the cells with metastatic features. The inhibition of NF-κB by IκBαM (S32, 36A) suppressed the metastatic potential of the MiaPaCa-2/IL-1α cells. Short hairpin RNA (shRNA) to knock down IL-1α completely suppressed the spread of the highly metastatic L3.6pl pancreatic cancer cells.
Results
Expression of IL-1α in Pancreatic Cancer Cell Line MiaPaCa-2 Activated NF-κB for the Transcription of Prometastatic Genes
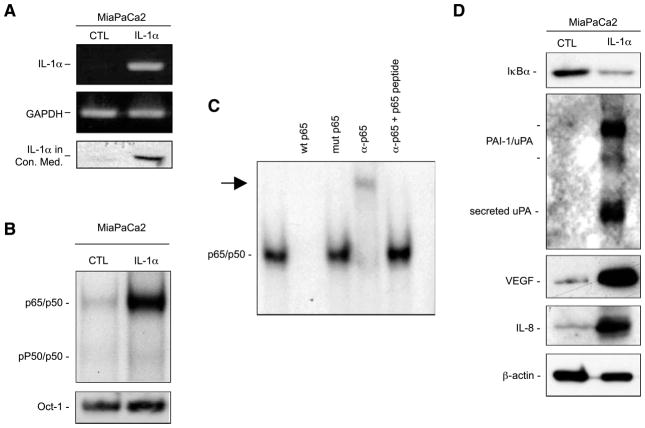
To show our hypothesis, we used the nonmetastatic Mia-PaCa-2 pancreatic cancer cell line, which is characterized by a minimal basal NF-κB activation, and no IL-1α expression. We used retrovirus infection to generate a variant of MiaPa-Ca-2 cell line that stably expressed IL-1α (MiaPaCa-2/IL-1α). As shown in Fig. 1A, MiaPaCa-2/IL-1α cells showed a constitutive expression and secretion of IL-1α in their conditioned medium. IL-1α expression constitutively activated NF-κB, but not Oct-1, as shown by electrophoretic mobility shift assay (EMSA; Fig. 1B). To confirm the specificity of NF-κB binding, we also did a supershift assay. As shown in Fig. 1C, unlabeled wild-type (WT) but not mutant NF-κB oligonucleotides completely blocked NF-κB activation by competition, and p65 antibody further retarded NF-κB shift in the gel, as antibody binding resulted in a larger complex. This supershift was inhibited when p65 peptide was added, which prevented the antibody from binding to the NF-κB complex. Consistent with the NF-κB activation, the IκBα level decreased in MiaPa-Ca-2/IL-1α cells, indicating the degradation of IκBα, whereas the expression of some of the NF-κB downstream genes involved in the metastatic processes, uPA, VEGF, and IL-8, was elevated by the IL-1α expression (Fig. 1D).
FIGURE 1.
Expression of IL-1α in MiaPaCa-2 cells induces constitutive NF-κB activation. A. Reverse transcription-PCR and Western blot analysis were done to determine the expression and secretion of IL-1α in conditioned medium collected in the same volume/cell number ratios and subjected to dialysis and concentration before the analysis. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. B. NF-κB activity was determined by EMSA using nuclear extracts (15 μg) in this analysis with a HIV κB probe. An Oct-1 probe was used as a loading control for quality and quantity of cell nuclear extracts. C. The nuclear extracts (15 μg) from MiaPaCa-2/IL-1α cells were used for the competition and supershift assays as indicated. Arrow, supershift band. D. For Western blot analysis, conditioned medium and cytoplasmic protein extracts were isolated as described and probed with anti-IκBα, anti-uPA, anti–IL-8, anti-VEGF, or anti–β-actin antibodies.
Autocrine Secretion of IL-1α, but not IL-1β, Accounted for the NF-κB Activation
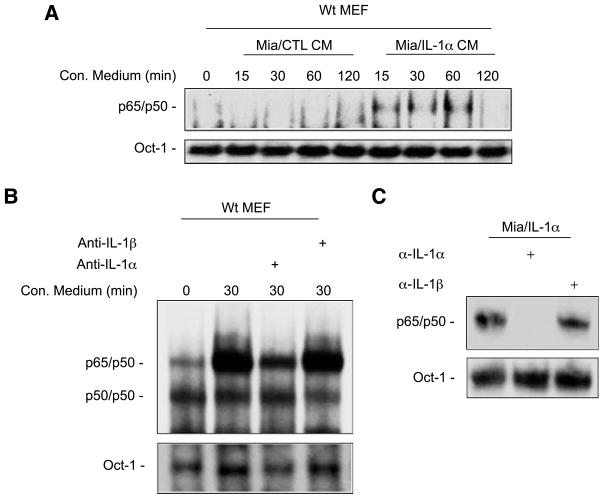
To determine if the constitutive NF-κB activation in MiaPa-Ca-2/IL-1α cells is the result of expression of IL-1α, we collected the conditioned medium from MiaPaCa-2/CTL cells and MiaPaCa-2/IL-1α cells and used them to treat WT mouse embryonic fibroblast (MEF) cells at the time intervals indicated (Fig. 2A). These results showed that the conditioned medium from MiaPaCa-2/IL-1α cells but not MiaPaCa-2/CTL cells was able to activate NF-κB (Fig. 2A). When conditioned medium from MiaPaCa-2/IL-1α cells was pretreated by IL-1α neutralizing antibody, it failed to activate NF-κB in MEF cells, whereas IL-1β neutralizing antibody did not block its NF-κB–inducing activity (Fig. 2B), indicating that IL-1α is the cytokine secreted by MiaPaCa-2/IL-1α cells responsible for the NF-κB activation. We also treated MiaPaCa-2/IL-1α cells directly with neutralizing antibodies against IL-1α and IL-1β for 30 minutes and isolated the nuclear extracts for EMSA. Anti–IL-1α neutralizing antibody completely inhibited constitutive NF-κB activity in these cells, whereas anti–IL-1β neutralizing antibody had no effects on this activity (Fig. 2C). Together, these results suggest that autocrine secretion of IL-1α conferred the induction of constitutive NF-κB activation in MiaPaCa-2/IL-1α cells.
FIGURE 2.
Autocrine secretion of IL-1α is responsible for constitutive NF-κB activation in MiaPaCa-2/IL-1α cells. A. EMSA was done to determine the NF-κB activity in WT MEF cells stimulated by the conditioned medium isolated from MiaPaCa-2/CTL and MiaPaCa-2/IL-1α cells at the time intervals indicated and an Oct-1 probe was used as a loading control for quality and quantity of cell nuclear extracts. B. Conditioned media from MiaPaCa-2/IL-1α cells treated with anti–IL-1α or anti–IL-1β antibodies (2 μg/mL for 2 h) were used to stimulate WT MEF cells for 30 min. EMSA was done to determine the NF-κB activity with Oct-1 probe as a loading control. C. EMSA was done to determine the NF-κB activity in MiaPaCa-2/IL-1α cells treated with anti–IL-1α or anti–IL-1β. Indicated neutralizing antibodies (2 μg/mL) were applied for 1 h and an Oct-1 probe was used as a loading control for cell nuclear extracts.
IL-1α Autocrine Activity Enhanced the Low-Anchorage Colony Formation and Invasion of Pancreatic Cancer Cells
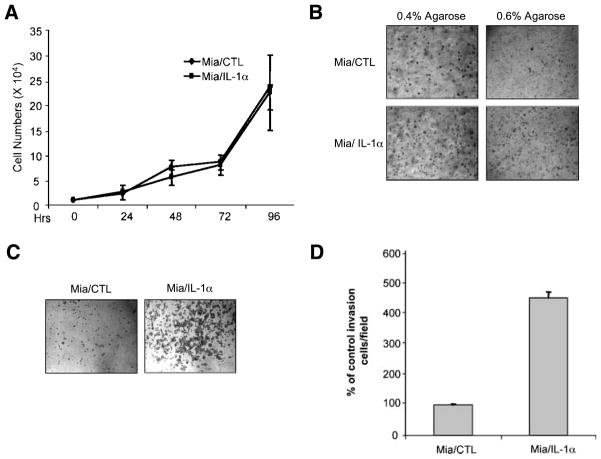
Because low-anchorage growth is one of the most common characteristics of metastatic cancer cells, we evaluated the ability of MiaPaCa-2/CTL and MiaPaCa-2/IL-1α cells to form colonies in soft agar. Although MiaPaCa-2/IL-1α cells did not show a significant growth advantage over the control cell line in normal culture conditions (Fig. 3A), IL-1α expression significantly increased the low-anchorage growth for MiaPaCa-2 cells, especially in the less permissive soft agar higher concentration (Fig. 3B).
FIGURE 3.
IL-1α expression in MiaPaCa-2 cells enhances anchorage-independent cell growth and cell invasion. A. Growth curve. MiaPaCa-2/CTL and MiaPaCa-2/IL-1α cells (2 × 104) were cultured in regular DMEM. Cells were counted in triplicate at the time intervals indicated. Points, mean of three independent experiments; bars, SE. B. Soft agar assay. MiaPaCa-2/CTL and MiaPaCa-2/IL-1α cells (5 × 104) were dispensed in either 0.4% or 0.6% top agarose on top of the 0.8% agarose-containing bottom agar. After 2 wk, the colonies were photographed under light microscopy. Magnification, ×10. C and D. Cell invasion assay. MiaPaCa-2/CTL and MiaPaCa-2/IL-1α cells were added to the top compartment of a Boyden chamber coated with Biocoat growth factor–reduced Matrigel basement membrane. After incubation for 48 h, cells that traversed the Matrigel-coated filters were stained, counted, and photographed. The representative fields are shown in C. Magnification, ×10. The number of cells that traversed Matrigel-covered filters was determined by counting at least three randomized fields per insert. Columns, mean of three independent experiments; bars, SE.
Cell invasion is an initial key step in the development of metastasis. Using a Matrigel-coated Boyden chamber method, MiaPaCa-2/IL-1α cells showed a significantly higher invasive activity than that of control cells (Fig. 3B and C). Thus, their invasive phenotype also seems to be dependent on autocrine IL-1α signaling.
IL-1α Secretion Induced a Metastatic Phenotype in a Pancreatic Cancer Orthotopic In vivo Model
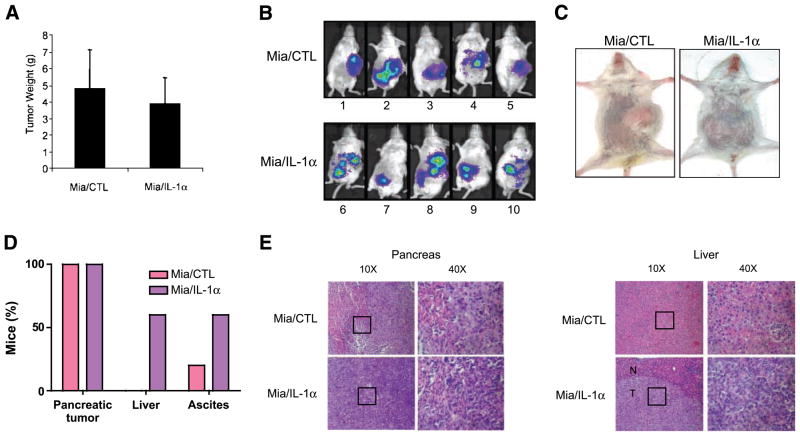
To determine the metastatic potential induced by the secretion of IL-1α in a preclinical in vivo model that could be predictive of similar activity in humans, allowing also to monitor tumor growth and dissemination over time, MiaPaCa-2/CTL cells and MiaPaCa-2/IL-1α cells infected by lentivirus to stably express high levels of luciferase were orthotopically injected into the pancreas of severe combined immunodeficient (SCID) mice. Although the mice in both groups showed a similar primary tumor volume (Fig. 4A), only mice bearing the MiaPa-Ca-2/IL-1α tumors (mice no. 6, 8, and 10) had a diffuse pattern of abdominal metastases and developed ascites (Fig. 4B–D). At necropsy, liver metastases of pancreatic adenocarcinoma were observed only in the livers from the mice injected with MiaPa-Ca-2/IL-1α cells (Fig. 4D and E). Thus, our results indicate that independently of any activity on the orthotopic primary, the secretion of IL-1α induces the development of abdominal metastases from pancreatic cancer cells.
FIGURE 4.
IL-1α secretion induces a metastatic phenotype in a pancreatic cancer orthotopic in vivo model. A. Tumors from mice injected with MiaPaCa-2/CTL or MiaPaCa-2/IL-1α cells were weighed. Columns, mean of all individual tumors in the group; bars, SE. B and C. Tumor development, as indicated by the Lumi-light, was monitored and photographed in real time as shown by the color. D. Percentage of mice that developed pancreatic tumors, liver metastasis, and ascites. E. H&E staining of the pancreas and liver tissues from SCID/NCr mice injected with MiaPaCa-2/CTL and MiaPaCa-2/IL-1α cells, either with ×10 or ×40 magnification. The square in ×10 magnification represents the field in the ×40 magnification. N, normal; T, tumor.
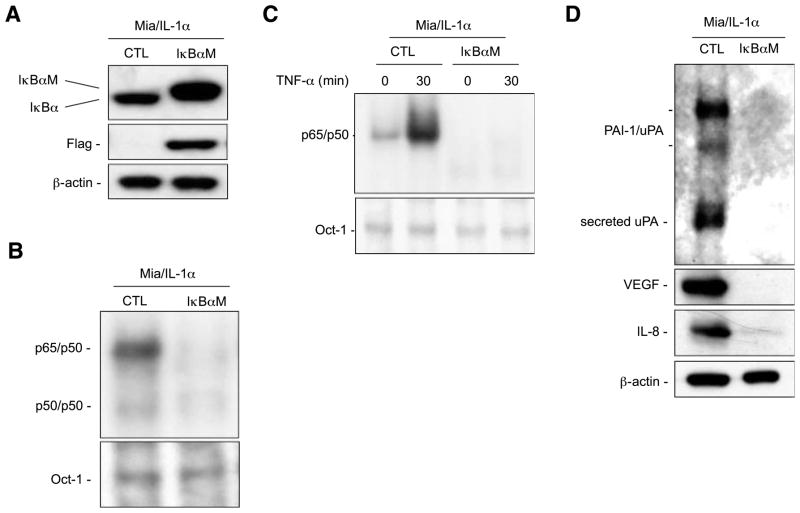
Expression of IκBα (S32, 36A) Phosphorylation Mutant (IκBαM) Inhibited the IL-1α–Induced NF-κB Activity and the Expression of NF-κB–Regulated Genes
To determine the roles of the NF-κB activation in IL-1α–induced pancreatic tumor metastatic processes, we used retroviral infection to generate stable clones (MiaPaCa-2/IL-1α/IκBαM) that expressed IκBαM (IκBα S32, 36A) with mutated PEST domain to increase its stability (22) and these clones were pooled. As shown in Fig. 5A, the expression of IκBαM seemed as a slower migrating band, compared with WT IκBα, as IκBαM is Flag tagged. In the presence of IκBαM in MiaPaCa-2/IL-1α cells, the endogenous IκBα protein was reduced to a minimum level due to the negative feedback regulation of IκBα by NF-κB (Fig. 5A; ref. 23). Constitutive NF-κB activation and tumor necrosis factor-α–mediated NF-κB activation were inhibited completely by I BaM as determined by EMSA (Fig. 5B and C). The expression of the NF-κB downstream target genes uPA, VEGF, and IL-8 was inhibited by I BaM (Fig. 5D).
FIGURE 5.
Phosphorylation-defective IκBα mutant (IκBαM) inhibited constitutive NF-κB activation and expression of NF-κB downstream genes in Mia-PaCa-2/IL-1α cells. A. Western blot analysis for expression of Flag-tagged phosphorylation-defective IκBα mutant (IκBαM) in MiaPaCa-2/IL-1α cells was done using cytoplasmic extracts (20 μg) with anti-Flag and anti-IκBα antibodies. Anti–β-actin antibody was used as a loading control. B. Nuclear extracts (15 μg) isolated from MiaPaCa-2/IL-1α/CTL and MiaPaCa-2/IL-1α/IκBαM cells were used in EMSA analysis with a HIV κB probe to determine NF-κB activity and an Oct-1 probe was used as a loading control for quality and quantity of cell nuclear extracts. C. The IκBαM-mediated inhibition of cytokine-induced NF-κB activation was confirmed by NF-κB EMSA using MiaPaCa-2/IL-1α and MiaPaCa-2/IL-1α/IκBαM cells with and without tumor necrosis factor-α (TNF-α) stimulation as indicated and an Oct-1 probe was used as a loading control. D. The expression levels of NF-κB downstream genes uPA, IL-8, and VEGF were determined by Western blot analysis with the antibodies as indicated. β-Actin protein level was used as a loading control.
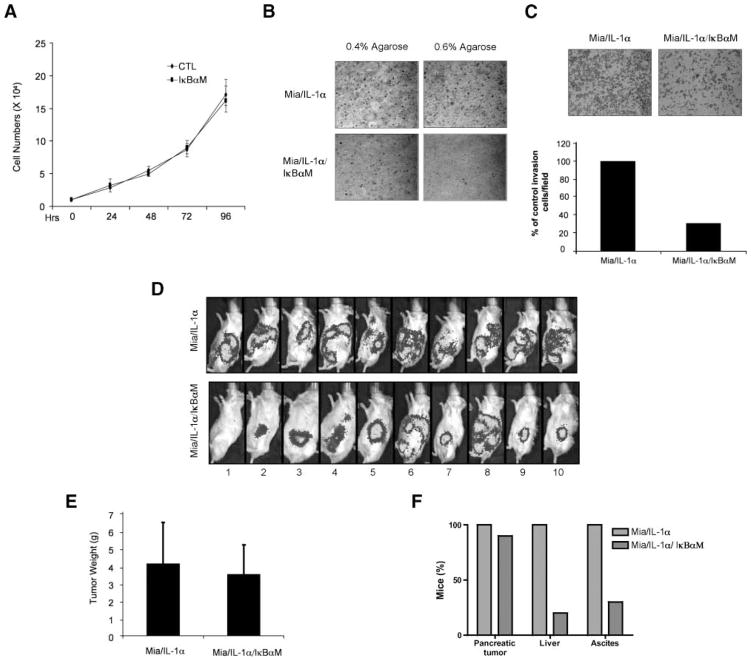
IκBαM Inhibited Tumor Aggressiveness of MiaPaCa-2/IL-1α Cells
To show that the metastatic phenotype induced by IL-1α is indeed mediated through the activation of NF-κB, we evaluated the behavior of MiaPaCa-2/IL-1α and MiaPaCa-2/IL-1α/IκBαM cell lines in vitro and in vivo.
Whereas the inhibition of NF-κB by IκBαM had no significant antiproliferative effects on cells grown as a monolayer in cell culture dishes (Fig. 6A), the low-anchorage growth of MiaPaCa-2/IL-1α cells was significantly inhibited by IκBαM expression, especially at the higher concentration of top layer agarose (Fig. 6B). Likewise, the in vitro cell invasion was also inhibited by the expression of IκBαM, as shown in Fig. 6C.
FIGURE 6.
Inhibition of NF-κB in MiaPaCa-2/IL-1α cells inhibited pancreatic tumor metastatic potential. A. Growth curve. MiaPaCa-2/IL-1α/CTL and MiaPaCa-2/IL-1α/IκBαM cells (2 × 104) were cultured in regular DMEM. Cells were counted in triplicate at indicated time intervals. Points, mean of three independent experiments; bars, SE. B. Soft agar assay. MiaPaCa-2/IL-1α/CTL and MiaPaCa-2/IL-1α/IκBαM cells (5 × 104) were dispensed in either 0.4% or 0.6% top agarose on top of the 0.8% agarose-containing bottom agar. After 2 wk, the colonies were photographed under light microscopy. Magnification, ×10. C. Invasion assay. MiaPaCa-2/IL-1α/CTL and MiaPaCa-2/IL-1α/IκBαM cells were added to the top compartment of a Boyden chamber coated with Biocoat growth factor–reduced Matrigel basement. After incubation for 48 h, cells that traversed the Matrigel-coated filters were stained, counted, and photographed. The representative fields are shown in C. Magnification, ×10. The number of cells that traversed Matrigel-covered filters was determined by counting at least three randomized fields per insert. Columns, mean of three independent experiments; bars, SE. D. SCID/NCr mice injected with MiaPaCa-2/IL-1α/CTL or MiaPaCa-2/IL-1α/IκBαM cells. The tumor development was monitored by Lumi-light display and photographed in real time. E and F. Tumor profiles for SCID/NCr mice injected with MiaPaCa-2/IL-1α/CTL and MiaPaCa-2/IL-1α/IκBαM cells. Tumors were weighed. Columns, mean of all individual tumors in the group; bars, SE. Percentage of mice that developed pancreatic tumors, liver metastases, and ascites.
To test our in vitro findings in an in vivo setting, 20 mice were injected with MiaPaCa-2/IL-1α or MiaPaCa-2/IL-1α/IκBαM cells. Ten weeks after the injection, the mice injected with Mia-PaCa-2/IL-1α cells developed a diffuse pattern of abdominal metastases. Although 9 of the 10 mice injected with MiaPaCa-2/IL-1α/IκBαM cells developed a primary pancreatic tumor, only 2 of them (no. 6 and 8) developed liver metastasis (Fig. 6D). Although the mice in both groups showed a similar primary tumor volume (Fig. 6E), at necropsy significantly fewer mice injected with MiaPaCa-2/IL-1α/IκBαM cells developed liver nodules and ascites (Fig. 6F). Taken together, these results showed that the establishment of an IL-1α autocrine loop in pancreatic cancer is sufficient to elicit an in vivo metastatic phenotype through a constitutive activation of NF-κB.
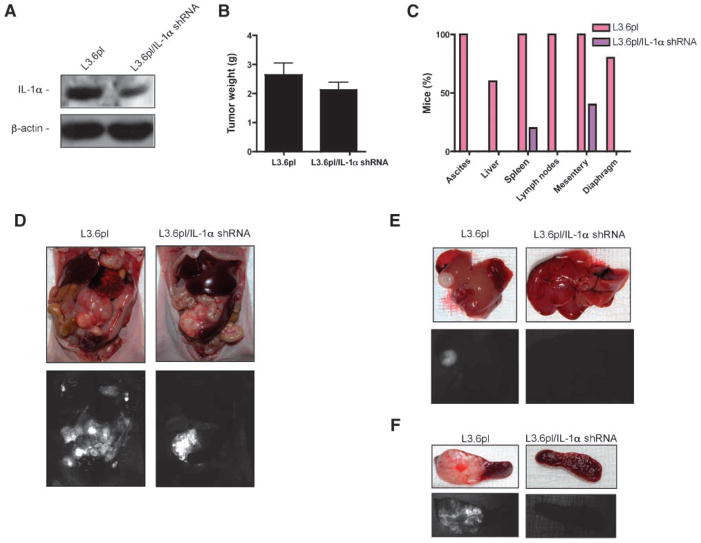
Silencing of IL-1α Suppressed the Dissemination of the Highly Metastatic L3.6pl Pancreatic Cancer Orthotopic In vivo Model
To further prove our hypothesis, we knocked down the expression of IL-1α in the highly metastatic L3.6pl pancreatic cancer cell line by using a lentivirus expressing green fluorescent protein and an IL-1α shRNA (L3.6plIL-1αshRNA) or a scramble sequence (L3.6pl) as control (Fig. 7A). To determine the in vivo antimetastatic effect of silencing IL-1α, a group of 20 mice was orthotopically inoculated with L3.6pl or L3.6plIL-1αshRNA cells. Although the mice in both groups showed a similar primary tumor volume (Fig. 7B), the mice bearing orthotopic L3.6pl pancreatic tumors had a diffuse pattern of abdominal metastasis at necropsy, and the mice bearing orthotopic L3.6plIL-1αshRNA pancreatic tumors developed significantly fewer metastatic lesions, and in some of them, no metastatic lesion as indicated by the green fluorescent protein signal could be identified in the abdomen (Fig. 6C–F). Thus, our results indicate that independently of any activity on the orthotopic primary, silencing of IL-1α significantly reduces metastasis from pancreatic cancer cells.
FIGURE 7.
Silencing IL-1α suppressed the formation of secondary lesions from L3.6pl highly metastatic cells. A. Western blot analysis for the expression of IL-1α was done using cytoplasmic extracts (20 μg) from L3.6pl or L3.6plIL-1αshRNA cells. B. Athymic nude mice (NCI-nu) inoculated with L3.6pl or L3.6plIL-1αshRNA cells. Tumors were weighed. Columns, mean of all individual tumors in the group; bars, SE. C and D. At necropsy, the presence of ascites and fluorescent tumor lesions in the liver (E), spleen (F), intestinal mesentery, diaphragm, lymph nodes (celiac and para-aortic), and other peritoneal organs was confirmed with a stereoscopic dissecting fluorescence microscope.
Discussion
The functional relationship between chronic inflammation and cancer is a widely accepted concept (24). However, many of the molecular mechanisms mediating this relationship remain unresolved. Of special relevance are different proinflammatory cytokine signaling mechanisms that are important mediators of chronic inflammatory responses and can be redirected to promote cancer cells invasion, migration, and metastasis (25).
IL-1α and IL-1β are between the most potent cytokines that primarily affect inflammation, immunity, and hematopoiesis (26). Abundant at tumor site, microenvironment and tumor cell–derived IL-1 affect the process of carcinogenesis, tumor growth, and invasiveness and also the patterns of tumor-host interactions. In particular, IL-1α has been suggested to regulate cancer cell invasion and metastasis, inducing invasiveness-promoting factors (i.e., matrix metalloproteinases) and adhesion molecules (27, 28). In an in vitro model of pancreatic cancer, recombinant IL-1α was shown to enhance the adhesion of metastatic cells to extracellular matrix proteins by inducing the α6-integrin subunit (20).
Whereas IL-1α and IL-1β in their recombinant forms bind to the same receptors and induce the same biological functions in primary cells, the most recent models about the IL-1 system propose a different role for IL-1α and IL-1β based on their compartmentalization within the producing cell or the microenvironment. Whereas IL-1β is solely active as a secreted product, IL-1α is normally secreted only in a limited manner and therefore it is mainly active as an intracellular precursor or in its membrane-associated form (9). On the other hand, tumor cells were largely shown to generate and secrete IL-1 either constitutively or in response to cytokines. In particular, the overexpression of IL-1α by different malignant cells can induce diametrically opposite effects in accordance with its compartmentalization. In a model of fibrosarcoma cells, the transfection of the active form of IL-1α resulted in its cytosolic and membrane-associated expression and increased the immunogenicity of malignant cells in vivo. Regression of IL-1α–positive fibrosarcomas involved their early infiltration by mononuclear cells and replacement by a fibrotic scar tissue (29). However, the exertion of this antitumor immune responses by tumor cell–associated IL-1α is not observed when the overexpression in malignant cells leads to the secretion of the cytokine. Thus, IL-1α–transfected A375 melanoma cells were shown to actively secrete the cytokine and to induce in vivo the expression of vascular cell adhesion molecule-I on lung microvascular endothelial cells, enhancing their adhesiveness for tumor cells and increasing experimental lung metastases in nude mice (30).
NF-κB activation was recently identified as a key modulator in driving inflammation to cancer (31). NF-κB is activated by inflammatory stimuli and its constitutive activation is found in cancer. Our laboratory previously reported that RelA, the p65 subunit of the NF-κB transcription factor, was constitutively activated in most pancreatic cancer tissues and cell lines but not in normal pancreatic tissues and immortalized pancreatic ductal epithelial cells (5, 32). Further studies showed that inhibition of constitutive NF-κB activity by a phosphorylation-defective mutant IκBα (S32, 36A) completely suppressed the liver metastasis and the tumorigenic phenotype of pancreatic cancer cell lines, suggesting that constitutive NF-κB activity plays a key role in pancreatic cancer metastasis and tumor progression (7, 33).
Noticeably, it has been shown that feedback loops exist between some proinflammatory cytokines and NF-κB activation (34). In particular, IL-1α is not only a NF-κB inducer but also a downstream target gene of NF-κB (35, 36), whereas IL-1β production is negatively controlled by IκB kinase β–dependent NF-κB signaling (37).
More recently, we showed that autocrine secretion of IL-1α, but not IL-1β, leads to the activation of NF-κB in metastatic pancreatic cancer cell lines (12). In turn, NF-κB activation induces expression of IL-1α, initiating the formation of a positive feedback loop and establishing a mechanism for the constitutive NF-κB activation in this disease.
However, whether this IL-1α autocrine loop plays a key role in pancreatic cancer metastasis was unclear. In the current study, we closed the circle showing that the autocrine secretion of IL-1α in MiaPaCa-2 pancreatic cancer cell line constitutively activated NF-κB and in turn induced a metastatic behavior in vivo as shown by the higher incidence of liver metastases and ascites in an orthotopic mouse model. In particular, we found that the IL-1α secreted by pancreatic cancer cells enhanced the expression of several NF-κB–regulated genes involved in the metastatic processes and angiogenesis, including uPA, IL-8, and VEGF. The inhibition of NF-κB by phosphorylation-defective IκBαM (IκBα S32A, S36A) inhibited these responses, suggesting that the effects of IL-1α on pancreatic tumor progression are indeed mediated by the activation of NF-κB.
These findings provide us with a better understanding of the role for the secreted IL-1α-NF-κB positive feedback loop as a fundamental molecular mechanism to support the malignant phenotype in pancreatic cancer. In our opinion, IL-1α autocrine secretion remains a main therapeutic target for pancreatic cancer patient treatment.
Materials and Methods
Cell Lines and Reagents
The human pancreatic cancer cell line MiaPaCa-2 was purchased from the American Type Culture Collection. Human pancreatic cancer cell line L3.6pl was previously described (38). The human IL-1α cDNA fragment was cloned from the MDAPanc28 pancreatic cancer cell line with reverse transcription-PCR and cloned into the HpaI site of retrovirus vector pLHCX. The stable MiaPaCa-2/IL-1α and MiaPaCa-2/IL-1α/IκBαM cell lines were established with retrovirus infection as previously described by Dong et al. (32). All MiaPaCa-2 cells were infected with lentivirus containing luciferase, making them suitable for in vivo imaging. WT MEF cells were established in our laboratory according to the report by Friess et al. (39). To silence the expression of IL-1α, two human IL-1α shRNA target sequences were chosen: 5′-gatccccAAGTATAATTCGAGCCAAT-ttcaagagaATTGGCTC-GAATTATACTT3tttttc-3′, 5′-tcgagaaaaaAAGTATAATTC-GAGCCAATtctcttgaaATTGGCTCGAATTATACTT3ggg-3′, 5′-gatccccGGCCAAAGTTCCAGACATGttcaagagaCATG-TCTGGAACTTTGGCCtttttc-3′, and 5′-tcgagaaaaaGGCCAA-AGTTCCAGACATGtctcttgaaCATGTCTGGAA-CTTT-GGCCggg-3′.
These sequences were cloned into FG12 lentivirus vector (gift from Dr. Qin Xiaofeng, The University of Texas M. D. Anderson Cancer Center, Houston, TX) with BamHI and HindIII. The shRNA vector and packaging vectors were co-transfected into 293T cells. The virus-containing supernatants were collected after 72 h of transfection and filtered through a minipore 0.45-μm filter. The L3.6pl cells were transduced by the lentivirus in the presence of the polycation Polybrene. The stably transduced cells were sorted by green fluorescent protein.
All cell lines were grown in the original cell culture medium specified by the American Type Culture Collection and DMEM containing 10% fetal bovine serum. IL-1α and IL-1β neutralizing antibodies were obtained from R&D Systems.
Electrophoretic Mobility Shift Assay
Conditioned media were prepared as previously described (12). The harvested conditioned media were used to treat WT MEFs at different intervals. Neutralizing antibodies (2 mg/mL) were used to block the corresponding cytokines. The nuclear extracts were prepared according to the method of Andrews and Faller (40). DNA binding assays for NF-κB proteins were done with 10 μg of nuclear extracts as described by Sclabas et al. (6). The WT double-stranded oligonucleotides containing the κB site were obtained from Santa Cruz Biotechnology, Inc. and labeled with 32P to be used as probes. The mutant κB site for HIV long terminal repeat (5′-CTCAACAGAGTT-GACTTTTCGAGAGGCCAT-3′) was used for competition studies. The competition was done with a 50-fold excess of unlabeled WT or mutant κB oligonucleotides. Supershift experiments were done with anti-p65 antibody (Santa Cruz Biotechnology). The reactions were analyzed on 4% polyacrylamide gels containing 0.25× Tris/borate/EDTA buffer.
Western Blot Analysis
MiaPaCa-2 cells were washed twice with cold PBS and lysed at 4°C into radioimmunoprecipitation assay buffer [50 mmol/L Tris-HCl (pH 8), 150 mmol/L NaCl, 1% NP40, 0.5% sodium deoxycholate, 0.1% SDS]. The lysates were cleared by centrifugation. To detect secreted IL-1α, uPA, VEGF, and IL-8, conditioned media were collected, centrifuged at 15,000 × g for 5 min to remove cell debris, and then subjected to immunoblotting under nonreducing conditions. Twenty-four–hour conditioned media were dialyzed for 24 h and concentrated using Microcon YM-10 centrifugal filter devices from Millipore. The volumes of conditioned medium loaded on gels were normalized to the protein concentrations of cell lysates. The concentrated media or 50 μg of protein extracts were resolved by SDS-PAGE, transferred to nylon membranes (Immobilon-P, Millipore), and detected with IκBα, IL-1α (Santa Cruz Biotechnology), uPA (American Diagnostica), IL-8, VEGF (BioSource International), or β-actin antibody (Sigma Chemical). Subsequent Western blot analyses were carried out with Lumi-light Western blot substrate (Roche Diagnostics).
Reverse Transcription-PCR
IL-1α mRNA level was detected with reverse transcription-PCR. In brief, total RNA from MiaPaCa-2 cells was extracted using Trizol reagent according to the manufacturer’s protocol (Invitrogen Life Technologies). The RNA was then reverse transcribed into cDNA and amplified at 94°C for 5 min, then 30 cycles at 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min, and finally 72°C for 7 min. The primers used for IL-1α were 5′-GGTAGTAGCAACCAACGGGA-3′ and 5′-TGGGTATCTCAGGCATCTCC-3′. The PCR products were 419 bp long.
Growth Curve
MiaPaCa-2 cells (1 × 104) were seeded in six-well plates and kept in normal DMEM (contains 10% fetal bovine serum). Cells were counted every 24 h in triplicate for 4 d. Each experiment was done independently at least thrice with similar results each time. Results were expressed as the mean ± SE of three independent experiments. All of the statistical analyses were done using StatView 5.0 (Abacus Concepts, Inc.).
Cell Invasion Assay
Invasion assay was done in 24-well plates by using a BD Biocoat growth factor–reduced Matrigel invasion chamber (BD Biosciences) with an 8.0-μm pore size positron emission tomography membrane as previously described (41). In brief, 5 × 104 MiaPaCa-2 cells were seeded in the invasion chambers and incubated at 37°C for 48 h. Noninvading cells from the interior of the inserts were removed by using cotton-tipped swabs. Invading cells on the under side of the membrane were fixed by 10% formaldehyde for 10 min and then stained with 1% crystal violet, and at least three random fields per insert were counted under an Olympus microscope. A field representative of each experiment was photographed on an Olympus DP 10 digital camera under Olympus IMT-2 light microscope with ×4 lens and ×2.5 magnification. Results are expressed as the mean ± SE of three independent experiments.
Soft Agar Assay
A 24-well plate was coated with 0.8% agarose-containing DMEM as bottom agar; 5 × 104 MiaPaCa-2 cells were dispensed in either 0.4% or 0.6% agarose-containing medium and placed on the top of the bottom agar. After 2 wk, the colonies were photographed under an Olympus IMT-2 light microscopy.
Orthotopic Mouse Model
Female SCID/NCI (BALB/c background) or female athymic nude mice (NCI-nu) were purchased from National Cancer Institute (Frederick, MD) at 4 to 6 wk old. Mice were housed in cabinets with laminar flow under specific pathogen-free conditions. Animals were maintained according to institutional regulations and the animal protocol was approved by the Institutional Animal Care and Use Committee at The University of Texas M. D. Anderson Cancer Center. The orthotopic injection of pancreatic cancer cells was done as previously described (42). Briefly, mice are anesthetized with methoxyflurane; a small left abdominal flank incision is made, and the spleen is exposed. One million of cells with >90% viability in trypan blue exclusion assay, suspended in 50 μL PBS, were injected subcapsularly in a region of the pancreas just beneath the spleen. A 30-gauge needle, a 1-mL disposable syringe, and a calibrated, push button–controlled dispensing device are used to inject the cell suspension. A successful subcapsular intrapancreatic injection of tumor cells is confirmed by the appearance of a fluid bleb without i.p. leakage. A cotton swab is held for 1 min over the site of injection to prevent such leakage. One layer of the abdominal wound is closed with wound clips. The animals usually tolerate the surgical procedure well, and no anesthesia-related deaths have occurred. Tumor development was monitored in real time with the IVIS imaging system, equipped with Living Imaging software (Xenogen).
At necropsy, the presence of ascites and fluorescent tumor lesions in the pancreas, spleen, lymph nodes (celiac and para-aortic), liver, diaphragm, and other peritoneal organs was confirmed with a Leica MZ16 stereoscopic dissecting fluorescence microscope equipped with a Hamamatsu Orca ER cooled charge-coupled device digital camera coupled to a data acquisition computer running the image acquisition software Image-Pro version 6.0. The mice were sacrificed when required by our institutional guidelines. Formalin-fixed, paraffin-embedded pancreas or liver tissues were dissected from the mice immediately after sacrifice and processed for H&E staining.
Acknowledgments
We thank Walter J. Pagel (Department of Scientific Publication) for his editorial assistance.
Grant support: National Cancer Institute grants CA097159 and CA109405 and Lockton Fund for Pancreatic Cancer Research. J. Niu’s funding as an Odyssey fellow was supported by the Odyssey Program and The H-E-B Award for Scientific Achievement at M. D. Anderson Cancer Center. D. Melisi was supported by the Program D.R. n. 1112/30.03.2000 from University of Naples (Naples, Italy).
Footnotes
Disclosure of Potential Conflicts of Interest
There are no potential conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035–46. doi: 10.1245/ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Wolff RA. Chemotherapy for pancreatic cancer: from metastatic disease to adjuvant therapy. Cancer J. 2007;13:175–84. doi: 10.1097/PPO.0b013e318074e6c3. [DOI] [PubMed] [Google Scholar]
- 4.Melisi D, Chiao PJ. NF-κB as a target for cancer therapy. Expert Opin Ther Targets. 2007;11:133–44. doi: 10.1517/14728222.11.2.133. [DOI] [PubMed] [Google Scholar]
- 5.Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor-κB RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5:119–27. [PubMed] [Google Scholar]
- 6.Sclabas GM, Fujioka S, Schmidt C, et al. Overexpression of tropomysin-related kinase B in metastatic human pancreatic cancer cells. Clin Cancer Res. 2005;11:440–9. [PubMed] [Google Scholar]
- 7.Fujioka S, Sclabas GM, Schmidt C, et al. Function of nuclear factor κB in pancreatic cancer metastasis. Clin Cancer Res. 2003;9:346–54. [PubMed] [Google Scholar]
- 8.Fujioka S, Sclabas GM, Schmidt C, et al. Inhibition of constitutive NF-κB activity by IκBαM suppresses tumorigenesis. Oncogene. 2003;22:1365–70. doi: 10.1038/sj.onc.1206323. [DOI] [PubMed] [Google Scholar]
- 9.Apte RN, Dotan S, Elkabets M, et al. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006;25:387–408. doi: 10.1007/s10555-006-9004-4. [DOI] [PubMed] [Google Scholar]
- 10.Ito R, Kitadai Y, Kyo E, et al. Interleukin 1α acts as an autocrine growth stimulator for human gastric carcinoma cells. Cancer Res. 1993;53:4102–6. [PubMed] [Google Scholar]
- 11.Kawakami Y, Nagai N, Ota S, Ohama K, Yamashita U. Interleukin-1 as an autocrine stimulator in the growth of human ovarian cancer cells. Hiroshima J Med Sci. 1997;46:51–9. [PubMed] [Google Scholar]
- 12.Niu J, Li Z, Peng B, Chiao PJ. Identification of an autoregulatory feedback pathway involving interleukin-1α in induction of constitutive NF-κB activation in pancreatic cancer cells. J Biol Chem. 2004;279:16452–62. doi: 10.1074/jbc.M309789200. [DOI] [PubMed] [Google Scholar]
- 13.Streicher KL, Willmarth NE, Garcia J, Boerner JL, Dewey TG, Ethier SP. Activation of a nuclear factor κB/interleukin-1 positive feedback loop by amphiregulin in human breast cancer cells. Mol Cancer Res. 2007;5:847–61. doi: 10.1158/1541-7786.MCR-06-0427. [DOI] [PubMed] [Google Scholar]
- 14.Nozaki S, Sledge GW, Jr, Nakshatri H. Cancer cell-derived interleukin 1α contributes to autocrine and paracrine induction of pro-metastatic genes in breast cancer. Biochem Biophys Res Commun. 2000;275:60–2. doi: 10.1006/bbrc.2000.3241. [DOI] [PubMed] [Google Scholar]
- 15.Matsuo Y, Sawai H, Ochi N, et al. Interleukin-1α secreted by pancreatic cancer cells promotes angiogenesis and its therapeutic implications. J Surg Res. 2008 doi: 10.1016/j.jss.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 16.Sawai H, Okada Y, Funahashi H, et al. Interleukin-1α enhances the aggressive behavior of pancreatic cancer cells by regulating the α6β1-integrin and urokinase plasminogen activator receptor expression. BMC Cell Biol. 2006;7:8. doi: 10.1186/1471-2121-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawai H, Funahashi H, Yamamoto M, et al. Interleukin-1α enhances integrin α(6)β(1) expression and metastatic capability of human pancreatic cancer. Oncology. 2003;65:167–73. doi: 10.1159/000072343. [DOI] [PubMed] [Google Scholar]
- 18.Sawai H, Takeyama H, Yamamoto M, et al. Enhancement of integrins by interleukin-1α, and their relationship with metastatic and invasive behavior of human pancreatic ductal adenocarcinoma cells. J Surg Oncol. 2003;82:51–6. doi: 10.1002/jso.10187. [DOI] [PubMed] [Google Scholar]
- 19.Stefani AL, Basso D, Panozzo MP, et al. Cytokines modulate MIA PaCa 2 and CAPAN-1 adhesion to extracellular matrix proteins. Pancreas. 1999;19:362–9. doi: 10.1097/00006676-199911000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Sawai H, Yamamoto M, Okada Y, et al. Alteration of integrins by interleukin-1α in human pancreatic cancer cells. Pancreas. 2001;23:399–405. doi: 10.1097/00006676-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Basso D, Plebani M, Fogar P, et al. Insulin-like growth factor-I, interleukin-1 α and β in pancreatic cancer: role in tumor invasiveness and associated diabetes. Int J Clin Lab Res. 1995;25:40–3. doi: 10.1007/BF02592575. [DOI] [PubMed] [Google Scholar]
- 22.Van Antwerp DJ, Verma IM. Signal-induced degradation of IκBα: association with NF-κB and the PEST sequence in IκBα are not required. Mol Cell Biol. 1996;16:6037–45. doi: 10.1128/mcb.16.11.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiao PJ, Miyamoto S, Verma IM. Autoregulation of IκBα activity. Proc Natl Acad Sci U S A. 1994;91:28–32. doi: 10.1073/pnas.91.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 26.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–147. [PubMed] [Google Scholar]
- 27.Andersen K, Maelandsmo GM, Hovig E, Fodstad O, Loennechen T, Winberg JO. Interleukin-1α and basic fibroblast growth factor induction of matrix metalloproteinases and their inhibitors in osteosarcoma cells is modulated by the metastasis associated protein CAPL. Anticancer Res. 1998;18:3299–303. [PubMed] [Google Scholar]
- 28.Nguyen M, Corless CL, Kraling BM, et al. Vascular expression of E-selectin is increased in estrogen-receptor-negative breast cancer: a role for tumor-cell-secreted interleukin-1α. Am J Pathol. 1997;150:1307–14. [PMC free article] [PubMed] [Google Scholar]
- 29.Dvorkin T, Song X, Argov S, et al. Immune phenomena involved in the in vivo regression of fibrosarcoma cells expressing cell-associated IL-1α. J Leukoc Biol. 2006;80:96–106. doi: 10.1189/jlb.0905509. [DOI] [PubMed] [Google Scholar]
- 30.Chirivi RG, Chiodoni C, Musiani P, et al. IL-1α gene-transfected human melanoma cells increase tumor-cell adhesion to endothelial cells and their retention in the lung of nude mice. Int J Cancer. 1996;67:856–63. doi: 10.1002/(SICI)1097-0215(19960917)67:6<856::AID-IJC16>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 31.Karin M, Greten FR. NF-κB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–59. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 32.Dong QG, Sclabas GM, Fujioka S, et al. The function of multiple IκB: NF-κB complexes in the resistance of cancer cells to Taxol-induced apoptosis. Oncogene. 2002;21:6510–9. doi: 10.1038/sj.onc.1205848. [DOI] [PubMed] [Google Scholar]
- 33.Fujioka S, Sclabas GM, Schmidt C, et al. Inhibition of constitutive NF-κB activity by IκBαM suppresses tumorigenesis. Oncogene. 2003;22:1365–70. doi: 10.1038/sj.onc.1206323. [DOI] [PubMed] [Google Scholar]
- 34.Perkins ND. The Rel/NF-κB family: friend and foe. Trends Biochem Sci. 2000;25:434–40. doi: 10.1016/s0968-0004(00)01617-0. [DOI] [PubMed] [Google Scholar]
- 35.Mori N, Prager D. Transactivation of the interleukin-1α promoter by human T-cell leukemia virus type I and type II Tax proteins. Blood. 1996;87:3410–7. [PubMed] [Google Scholar]
- 36.Osborn L, Kunkel S, Nabel GJ. Tumor necrosis factor α and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor κB. Proc Natl Acad Sci U S A. 1989;86:2336–40. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greten FR, Arkan MC, Bollrath J, et al. NF-κB is a negative regulator of IL-1β secretion as revealed by genetic and pharmacological inhibition of IKKβ. Cell. 2007;130:918–31. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruns CJ, Harbison MT, Kuniyasu H, Eue I, Fidler IJ. In vivo selection and characterization of metastatic variants from human pancreatic adenocarcinoma by using orthotopic implantation in nude mice. Neoplasia. 1999;1:50–62. doi: 10.1038/sj.neo.7900005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friess H, Yamanaka Y, Buchler M, et al. Enhanced expression of transforming growth factor β isoforms in pancreatic cancer correlates with decreased survival. Gastroenterology. 1993;105:1846–56. doi: 10.1016/0016-5085(93)91084-u. [DOI] [PubMed] [Google Scholar]
- 40.Andrews NC, Faller DV. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991;19:2499. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niu J, Chang Z, Peng B, et al. Keratinocyte growth factor/fibroblast growth factor-7-regulated cell migration and invasion through activation of NF-κB transcription factors. J Biol Chem. 2007;282:6001–11. doi: 10.1074/jbc.M606878200. [DOI] [PubMed] [Google Scholar]
- 42.Melisi D, Ishiyama S, Sclabas GM, et al. LY2109761, a novel transforming growth factor β receptor type I and type II dual inhibitor, as a therapeutic approach to suppressing pancreatic cancer metastasis. Mol Cancer Ther. 2008;7:829–40. doi: 10.1158/1535-7163.MCT-07-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]