Abstract
This study explores degree of vinyl conversion (DVC), polymerization shrinkage (PS) and shrinkage stress (PSS) of the experimental amorphous calcium phosphate (ACP) composites intended for use as an endodontic sealer. Light-cure (LC), chemical cure (CC) or dual-cure (DC; combined light and chemical cure) resins comprised urethane dimethacrylate (UDMA), 2-hydroxyethyl methacrylate (HEMA), methacryloyloxyethyl phthalate (MEP) and a high molecular mass oligomeric co-monomer, poly(ethyleneglycol)-extended UDMA (PEG-U) (designated UPHM resin). To fabricate composites, a mass fraction of 60 % UPHM resin was blended with a mass fraction of 40 % as-made (am-ACP) or ground ACP (g-ACP). DVC values of copolymer (unfilled UPHM resin) and composite specimens were determined by infrared spectroscopy. Glass-filled composites were used as controls. PS and PSS of composites were determined by dilatometry and tensometry, respectively. LC copolymers attained extraordinary high DVC values at 24 h post-cure (95.7 %), compared to CC (52 %) and DC (79.3 %) copolymer specimens. While the DVC values of LC and DC am-ACP composites were reduced between 5 and 10 %, DVC values of DC g-ACP composites increased almost 8 % compared to the corresponding copolymers. High DVC attained in LC composites was, expectedly, accompanied with high PS values (on average 7 vol%). However, PSS developed in LC and especially DC composites did not exceed PSS values seen in other UDMA-based composites. Based on this initial evaluation, it is concluded that, DC, g-ACP filled UPHM composite shows promise as an endodontic sealer. However, further physicochemical evaluations, including water sorption, mechanical stability and ion release as well as a leachability studies need to be performed before this experimental material is tested for cellular responses and, eventually recommended for clinical utility.
Keywords: amorphous calcium phosphate, endodontic composite, degree of vinyl conversion, polymerization shrinkage, polymerization stress
Introduction
Dental materials containing amorphous calcium phosphate (ACP) have tremendous appeal due to (a) their ability to arrest demineralization and/or remineralize defective tooth structures and (b) their potential for a high degree of biocompatibility. For the last decade we have systematically investigated structure-composition-property relationships of several ACP fillers with a variety of resins in order to develop strategies that better control ACP’s dispersion in the polymer matrix and learn about the complex interaction(s) occurring at the inorganic filler/organic matrix interface [1-3]. As a result of our research, prototypes of ACP-based composites intended for clinical use as a pit and fissure sealant and as an orthodontic adhesive were formulated [4, 5]. In both cases, solution solubility of ACP enabled the release of supersaturating levels of calcium and phosphate ions intra-lesionally and shifted the solution thermodynamic driving forces toward the formation of apatite [6, 7]. Our in vitro experiments indicated that ACP can sustain these supersaturation conditions over extended periods of time [1, 2].
In this study, we present the research aimed to extend the utility of ACP composites to endodontic application as biocompatible, easy-to-manipulate root canal sealer. Fine-tuning of the resin and filler modification [1-3] were undertaken to formulate the experimental resin and develop ACP composite with satisfactory physicochemical characteristics. The hypothesis was that resin formulated from urethane dimethacrylate (UDMA) as a base monomer can attain high degree of vinyl conversion (DVC), and that blending UDMA-based resin with an ACP filler to form bioactive ACP composite would not adversely affect DVC, polymerization shrinkage (PS) and/or stress developed upon polymerization (PSS). UDMA was chosen as the base monomer because UDMA-based resin composites achieved higher DVC values compared to the binary and ternary 2,2-bis[p-(2′-hydroxy-3′-methacryloxypropoxy)phenyl]propane (Bis-GMA)- and 2, 2-bis[p-(2′-methacryloxypropoxy)phenyl]propane (EBPADMA)-based systems [2, 3]. Inclusion of UDMA into resins also resulted in moderately enhanced mechanical strength of its copolymers [8]. The inclusion of the hydrophilic, monofunctional, highly diffusive 2-hydroxyethyl methacrylate (HEMA) into resin formulations produces an uptake of the environmental water sufficient to attain desirable ion release profiles, which, in turn, gives ACP composites an increased anti-demineralizing/remineralizing capacity [1-5]. Poly(ethyleneglycol)-extended UDMA (PEG-U) was found to further improve DVC of UDMA-based matrices, while not adversely affecting PS and PSS [9]. Methacryloyloxyethyl phthalate (MEP) improved the adhesiveness of the composite to the tooth structures without excessively bonding Ca ions released from the composites once they are exposed to the oral environment [10]. Relevant tasks were to 1) explore the feasibility of formulating light-cure (LC), chemical-cure (CC) and dual-cure (DC; light + chemical cure) UDMA/PEG-U/HEMA/MEP resins (due to the nature of the intended application as endodontic sealer, light cure alone may be clinically inadequate), 2) fabricate ACP-filled composites, 3) measure the DVC attained in copolymers and composites, and 4) determine the accompanying PS and PSS that developed in composites. High DVC values, if attained in the experimental ACP/UPHM composite, would imply low probability for the un-reacted monomers and/or resin degradation products to leach-out from these materials. Consequently, this composite would likely exhibit propitious cellular responses. The results of this initial evaluation will determine if there is a need to further test this material for water sorption, mechanical strength, micro-leakage and adhesion in a simulated oral environment
Materials and Methods
Resin formulation
Resin was formulated from the commercial UDMA, HEMA and MEP monomers and a high molecular mass oligomeric urethane dimethacrylate co-monomer, PEG-U (Table 1).
Table 1.
The composition of the unactivated UPHM resin used in the study
| Monomer | Molecular mass |
Acronym | Mass fraction, % |
|---|---|---|---|
| urethane dimethacrylate | 470 | UDMA | 49.2 |
| poly(ethyleneglycol)-extended UDMA | 870 | PEG-U | 30.3 |
| 2-hydroxyethyl methacrylate | 130 | HEMA | 17.5 |
| methacryloyloxyethyl phthalate | 278 | MEP | 3.0 |
They were hand-blended into UDMA:PEG-U:HEMA:MEP matrix (designated UPHM resin) with 9.6:3.2:12.4:1.0 molar ratio. The proposed molar ratio corresponds to the average ratio for the various base monomers (Bis-GMA, EBPADMA or UDMA), diluent monomers (TEGDMA, HEMA, or hexamethylene dimethacrylate) and adhesive monomer (MEP) in the resin formulations that have been shown to yield the desirable levels of DVC [1, 4]. After combining all the monomers, their mixture was magnetically stirred (38 rad/s; in the absence of blue light). Resins were activated for light-cure (LC), chemical cure (CC) and dual-cure (DC; light plus chemical) as indicated in Table 2. In LC series, besides the conventional camphorquinone (CQ)/tertiary amine system, the acyl phosphine oxide type photo-initiators (1850IRGACURE, 4265 DAROCUR; Ciba Specialty Chemicals Corporation, Tarrytown, NY, USA) were studied as alternatives to eliminate or reduce the need for a tertiary amine such as 4EDMAB, in addition to the conventional CQ/4EDMAB system. As an alternative to benzoyl peroxide (BPO)–based systems, which are known for their relatively low storage stability, Antonucci et al. [11] have systematically studied initiator systems that employed ascorbic acid (AA) and/or ascorbyl palmitate with relatively stable peresters and hydroxyperoxides as oxidants. Their findings were used as guidelines for selecting the concentrations of AA and t-butyl perbenzoate (TBPB) used in CC2 and DC2 formulations. After introducing the appropriate amounts of the components of the initiating systems to the monomer blend, the activated resin mixtures were magnetically stirred until fully homogenized.
Table 2.
Components of the light-cure (LC), chemical-cure (CC) and dual-cure (DC) systems and their content (mass faction %) in the resin
| Type of the polymerization initiation |
Component | Acronym | Mass fraction % |
|---|---|---|---|
| Light-cure (LC) | |||
| LC1 | Camphorquinone Ethyl-4-N,N-dimethylamino benzoate |
CQ 4EDMAB |
0.2 0.8 |
| ---------------------------------------------- | |||
| LC2 | -Bis(2,6-dimethoxybenzoyl)-2,4,4- trimethylpentyl phosphine oxide & 1-hydroxycyclohexyl phenyl ketone |
1850 IRGACURE |
1.0 |
| ---------------------------------------------- | |||
| LC3 | - Camphorquinone Ethyl-4-N,N-dimethylamino benzoate Diphenyl(2,4,6-trimethylbenzoyl) phosphine oxide & 2-hydroxy-2- methyl-1-phenyl-1- propanone 2-Benzyl-2-(dimethylamino)-1-(4- (4-morpholinyl)phenyl)-1-butanone |
CQ 4EDMAB 4265 DAROCUR 369 IRGACURE |
0.4 0.4 0.8 1.5 |
|
| |||
| Chemical-cure (CC) | |||
| CC1 | Benzoyl peroxide 2,2′-Dihydroxyethyl-p-toluidine |
BPO DHEPT |
2.0 1.0 |
| ---------------------------------------------- | |||
| CC2 | - L(+) ascorbic acid t-Butyl perbenzoate |
AA TBPB |
0.5 1.0 |
|
| |||
| Dual-cure (DC) | |||
| DC1 | Bis(2,6-dimethoxybenzoyl)-2,4,4- trimethylpentyl phosphine oxide & 1-hydroxycyclohexyl phenyl ketone Benzoyl peroxide 2,2′-Dihydroxyethyl-p-toluidine |
1850 IRGACURE BPO DHEPT |
1.0 2.0 1.0 |
| ---------------------------------------------- | |||
| DC2 | - Bis(2,6-dimethoxybenzoyl)-2,4,4- trimethylpentyl phosphine oxide & 1-hydroxycyclohexyl phenyl ketone L(+) ascorbic acid t-Butyl perbenzoate |
1850 IRGACURE AA TBPB |
1.0 0.5 1.0 |
Synthesis and characterization of ACP filler
Zirconia-hybridized ACP (assigned as-made or am-ACP) was synthesized as previously described [12]. Zr-ACP was chosen as a standard filler based on the extensive evaluation of its stability in different aqueous environments as well as the mechanical strength and ion-release properties of the composites based on this type of ACP and a variety of resin matrices [1, 2, 9]. To reduce the level of ACP particle agglomeration, approximately one half of the as-made Zr-ACP was dispensed in isopropanol (commonly used as non-aqueous dispersant for particle size analysis of the powders that undergo transformation in aqueous medium) and ground with a porcelain mortar and pestle in its wet state for 1 minute. The isopropanol was then evaporated in a vacuum oven at 75 °C. Ground ACP was assigned g-ACP. The amorphous state of both am- and g-ACP has been verified by X-ray diffraction (XRD; Rigaku DMAX 2000 X-ray diffractometer, Rigaku/USA Inc., Danvers, MA, USA) and Fourier-transform Infrared (FTIR) spectroscopy (Nicolet Magna-IR FTIR 550 spectrophotometer, Nicolet Instrumentations Inc., Madison, WI, USA). The particle size distribution (PSD) of the fillers in dry state was determined using laser obscuration concurrently with a computerized inspection system (CIS-100 Particle Size Analyzer, Ankersmid Ltd., Yokneam, Israel). Their morphology was examined by scanning electron microscopy (SEM; JEOL 35C instrument, JEOL Inc., Peabody, MA, USA) after the specimens were sputter-coated with gold.
Fabrication of copolymers and ACP composites
Copolymer (unfilled resin) and composite specimens alike were placed into Teflon molds, each opening was covered with a Mylar film and glass slide, and the entire assembly was clamped in place by spring clips. Light cure (LC1, LC2 and LC3) copolymers were irradiated for 30 s at an intensity of 450 mW/cm2 with a dental curing unit (Dentsply Spectrum 800, Dentsply Caulk, Milford, DE, USA). In CC series, AA- or BPO-containing resins were combined with TBPB- and 2,2′-dihydroxyethyl-p-toluidine (DHEPT)-containing resins (1: 1 mass ratio) to initiate chemical polymerization. DC formulations were mixed in the same manner as CC formulations and then additionally light-cured in the manner of LC systems. Composite pastes were prepared by hand-mixing UTHM resin (mass fraction 60 %) and either am- or g-ACP (mass fraction 40 %). The paste was mixed until a uniform consistency was achieved, with no remaining visible particulates. Prior to curing, the homogenized pastes were spread thinly on a dental slab (flat glass block) and kept under moderate vacuum (2.7 kPa) to eliminate the air entrained during mixing. Once homogenized, composite pastes were kept under moderate vacuum (2.7 kPa) overnight to reduce the air entrained during mixing. Composites in all three groups were cured in the same manner as the copolymer samples. Composites in the control group were prepared with the unsilanized Sr-glass (Denstly Caulk, Milford, DE, USA). To achieve handling properties comparable to ACP-composites, 70 mass % glass was blended with the resin.
Degree of vinyl conversion (DVC)
To determine the degree of vinyl conversion (DVC) attained after polymerization of the copolymers and composites, a near infrared (NIR) spectroscopic technique was employed [13]. The absorption =CH2 absorption band at 6165 cm−1 in the overtone region was used to assess the DVC in paired unpolymerized and polymer samples. NIR spectra of the specimens were acquired before cure, immediately after cure, 5 h, 24 h and 5 d post-cure. The decrease in integrated peak area following polymerization was used to calculate DVC.
Polymerization shrinkage (PS)
The PS of composite resin samples was measured by a computer-controlled mercury dilatometer (Fig.1; fabricated at the PRC-ADAF, Gaithersburg, MD, USA). Composite pastes were cured using a standard 60s/30s exposure and data acquisition of (60 min + 30 min). PS of a specimen corrected for temperature fluctuations during the measurement was plotted as a function of time. The overall shrinkage (volume fraction, %) was calculated based on the known mass of the sample (50–100 mg) and its density. The latter was determined by means of the Archimedean displacement principle using an attachment to a microbalance (YDK01 Density Determination Kit; Sartorius AG, Goettingen, Germany).
Fig. 1.
Schema of the dilatometer used to measure polymerization shrinkage.
Polymerization stress (PSS) development
A cantilever beam tensometer (Fig. 2; designed and fabricated at the PRC-ADAF, Gaithersburg, MD, USA) was used to assess the PSS of the composites. The corresponding software program was also been developed at the PRC-ADAF. The tensometer, an effective tool for investigating the PSS kinetics as well as for probing various aspects that dictate shrinkage stress development, is based on the cantilever beam deflection theory that a tensile force generated by the bonded shrinking sample causes a cantilever beam to deflect.
Fig. 2.
Drawing of the tensometer used to measure stress developed in composites upon polymerization (light-curing of the specimen is shown in part a).
The design of the sample assembly facilitates convenient sample insertion, experimental reproducibility and a short preparation time between the consecutive measurements. The deflection of the cantilever beam was measured with a linear variable differential transformer. The force was calculated from a beam length (12.5 cm) and a calibration constant (3.9 N/μm). PSS was obtained by dividing the measured force by the cross sectional area of the sample (diameter = 6 mm). A detailed description of the technique is provided in ref. [14]. For a circular quartz rod of diameter 2r and a specimen of height h, cavity design factor (C-factor) was calculated as the ratio of bonded composite area (the silanated ends of the silica rods) to the unbonded area (the compliant plastic enclosure) according to the expression:
| (1) |
A C-factor of 1.33 (h = 2.25 mm) was maintained in all experimental groups.
Statistical analysis
The results were analyzed by the analysis of variance (ANOVA; α = 0.05). Statistical significance of change values was obtained from two-tailed P values using the Holm-Sidak test for paired data. Statistical calculations were done by means of SigmaStat software (version 3.5; SPSS Inc., Chicago, IL, USA). One standard deviation (SD) is identified in this paper for comparative purposes as the estimated uncertainty of the measurements.
Results
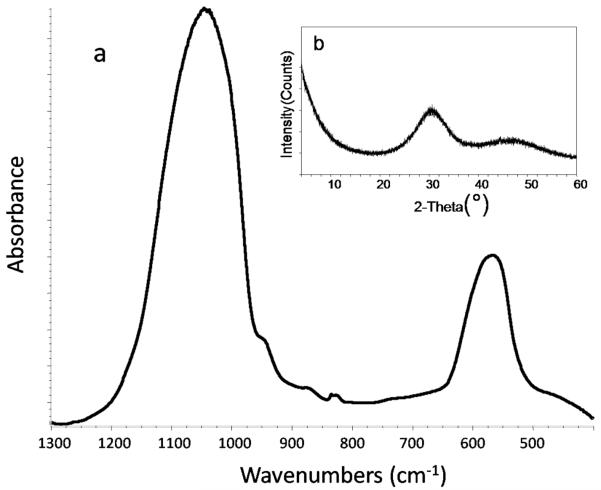
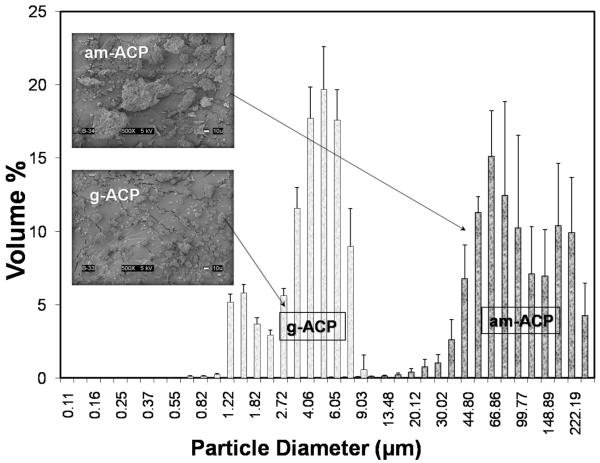
FTIR spectroscopy and XRD analysis revealed no differences in the structural features of am- and g-ACP used to make the experimental ACP composites. A typical FTIR spectrum exhibited two wide phosphate absorbance bands at (1200 to 900) cm−1 and (630 to 550) cm−1 (Fig. 3a), while XRD showed two diffuse broad bands in the 2θ = (4 to 60)° region (Fig. 3b). Am-ACP was highly agglomerated; its particle sizes ranged from 1 μm to 270 μm with a median diameter, dm = (75 ± 5) μm (Fig. 4). The PSD of g-ACP ranged from 0.5 μm to 11 μm with dm = (5 ± 1) μm, indicating a significant reduction in the size of ACP agglomerates after grinding. SEM images complement the results of PSD measurements (Fig. 4). Sr-glass used in the control composite group had dm = (1.3 ± 0.9) μm (size distribution histogram not shown).
Fig. 3.
FTIR spectrum (a) and X-ray diffraction pattern (b) typical of am- and g-ACP filler.
Fig. 4.
Volume particle size distributions and the corresponding SEM images of am-ACP and g-ACP fillers used to formulate UPHM/ACP composites.
Results of DVC screening of the unfilled polymers and their composites are summarized below. LC copolymers attained exceptionally high DVC values ranging from 93.5 % to 97.5 % at 24 h post-cure. The differences between the LC1, LC2 and LC3 groups were not statistically significant. Similarly, there was no statistical difference between the two CC and two DC formulations. The DVC values obtained in CC copolymers at 24 h post cure were, on average, 35 % lower than DVC values achieved in LC formulations. DVC values achieved in DC copolymers ranged between 78.6 % and 83.5 % and were, on average, 14.5 % lower than the LC formulations. Based on these observations, CC composites were excluded from further evaluations. DVC values obtained for LC am- and g-ACP UPHM composites were between 8.7 % and 7.6 % lower than DVC attained in the corresponding copolymer specimens. The apparent reduction in DVC seen in glass-filled control composites compared to copolymers was not statistically significant. In DC composite series, am-ACP composites yielded the DVC values comparable to those attained in corresponding copolymers. DVC values achieved with g-ACP composites were higher than DVC of DC copolymers reaching the same conversion levels as the LC ACP composites. Since there was no discernible difference in DVC between DC1 and DC2, the study was continued with only DC1 due to its slightly more favorable handling properties.
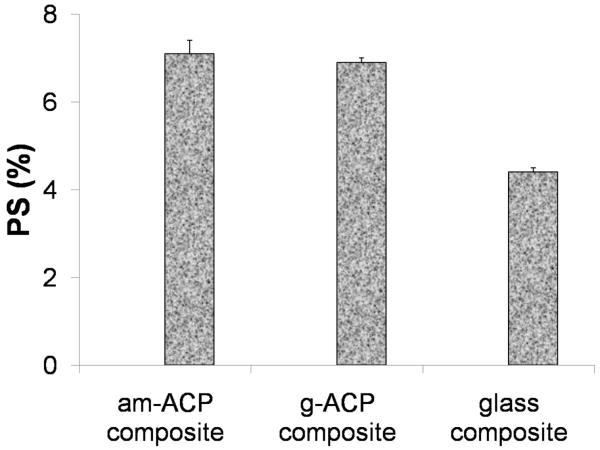
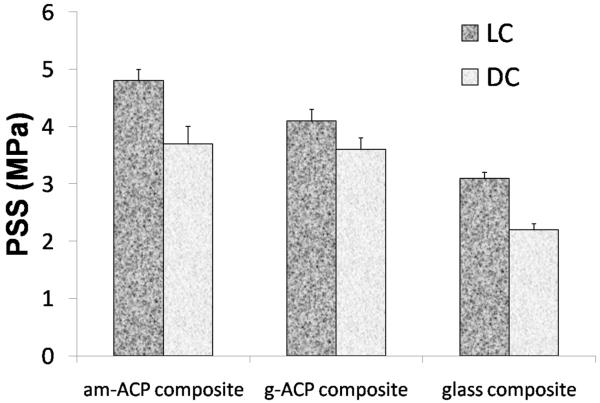
Polymerization shrinkage (PS) was measured successfully only with LC formulations. In DC systems, hardening of the paste occurrs within 10 min of mixing the chemically activated components. Some degree of contraction therefore occurs before the sample is even placed in the instrument, making the material unsuitable for measurement by this method. Relatively high PS values measured in LC am- and g-ACP composites ((7.1 ± 0.3) % and (6.9 ± 0.1) %, respectively) significantly exceeded the PS values of glass-control composite ((4.4 ± 0.1) %; Fig. 5). However, normalizing PS values of the glass control to the resin/filler ratios of ACP composites brought them in line, with an average of 7.7 %. Polymerization stress that developed in LC and DC composites is presented in Fig. 6. A decreasing trend in PSS could be seen in going from am-ACP to g-ACP to glass filler in both LC and DC formulations. Significantly, DC formulations saw a reduction in PSS compared to LC formulations for all three filler groups: 23 %, 12 % and 29 %, for am-ACP, g-ACP and glass-filler, respectively.
Fig. 5.
Polymerization shrinkage of light-cure composites. Indicated are mean values + one standard deviation (n ≥ 3/group).
Fig. 6.
Polymerization stress developed in light-cure (LC) and dual-cure (DC) composites. Indicated are mean values + one standard deviation (n ≥ 3/group).
Discussion
Degree of vinyl conversion (DVC) attained in dental resin systems upon polymerization is affected by a number of factors, among which the chemical structure of the monomers and the glass transition temperature (Tg) appear to be of high importance. At ambient temperatures, the most commonly used base monomer in dental resins, the very viscous dimethacrylate Bis-GMA, reaches the gel point on polymerization very quickly. Consequently, the diffusion of monomer and other reactive species to the radical sites or pendant vinyl groups on the relatively immobilized polymer network is being hindered, yielding resins with relatively low DVC. Introduction of less viscous, diluents monomers such as triethylene glycol dimethacrylate (TEGDMA) typically yields DVC values ranging from 55 % to 75 % in Bis-GMA/TEGDMA copolymers [15]. We have shown that LC copolymers based on Bis-GMA, EBAPDMA or UDMA blended with TEGDMA and HEMA, and the corresponding am-ACP composites could reach DVC values from 82 % to 94 %, and 74 % to 91 %, respectively [3, 16]. The high DVC values attained in these HEMA-containing systems are primarily attributed to the high diffusivity and monofunctionality of HEMA. A comparable effect was observed when a diluent monomethacrylate monomer with a structure similar to HEMA, i.e., hydroxypropyl methacrylate (HPMA), was added to Bis-GMA [17]. However, this low molecular mass monomer may show enhanced leachability of unreacted HEMA from the resin and possibly adversely affect the PS and PSS of the composites. In order to attain a favorable DVC, and presumably, maintain high biocompatibility of our experimental endodontic sealer, a high molecular mass oligomeric urethane dimethacrylate co-monomer PEG-U was introduced into the resin formulation. High DVC values obtained with PEG-U containing matrices are attributed to the long and flexible structure between the vinyl groups of PEG-U oligomer.
Reduction in DVC values in going from UPHM copolymers to am-ACP composites has already been observed with Bis-GMA-, EBPADMA- and/or UDMA-based copolymers and composites [1, 2, 10, 16]. This trend is being attributed to the reduction in exotherm of resin polymerization by the filler phase. It is, however, possible that other factors such as the level of air entrapment and light scattering (affected by profoundly heterogeneous sizes of ACP filler particles) may also reduce the DVC of composites. The more homogeneous distribution of glass filler and g-ACP throughout the UPHM resin and smaller number of voids (entrapped air) in LC glass control and DC g-ACP systems could explain the DVC values attained in composite specimens being equal or even slightly higher compared to DVC values of the corresponding copolymers.
Generally, it is expected that a high DVC would result in elevated PS values [18]. PS is indirectly related to relative molecular mass of the monomers and directly related to DVC [19]. It is not surprising that relatively high levels of contraction upon polymerization (on average 7.0 % by volume) were seen in UPHM composites reaching 88 % double bond conversion. An undesirable effect of such high PS values would be the increased probability of developing strains and gaps in the microstructure of composite and at the composite/dentin interface, which could, in turn, act as potential sites for microleakage. While being significantly higher than the range of PS values reported for the commercial restorative composites ((1.9 to 4.1) %; [20]) and flowable composites (3.6 to 6.0) %; [21]), the PS values of ACP/UPHM composites only slightly exceeded the lower end values seen in adhesive resins ((6.7 to 13.5) %; [21]). Additionally, the PS values of am- and g-ACP/UPHM composites were higher than the PS values of light-cured am-ACP/UDMA and am-ACP/PEG-U composites formulated without HEMA and adhesive comonomer (on average 5.3 vol % and 5.1 vol %, respectively; [19]). The observed relatively high contraction of ACP/UPHM composites may be attributed to the intensified hydrogen bonding that may occur in UPHM resin matrices containing relatively high amount of HEMA (17.5 mass %). This excessive hydrogen bonding could ultimately lead to the densification of polymerization [3].
The PSS measured in LC am- and g-ACP/UPHM specimens ((4.7 ± 0.2) MPa and (4.5 ± 0.2) MPa, respectively) compares well with the PSS that developed in am-ACP composites based on binary UDMA/HEMA composites ((4.5 ± 0.1) MPa; [19]). This finding would suggest that the actual stress developed as a consequence of polymerization shrinkage in UPHM matrices was not elevated by the simultaneous inclusion of PEG-U and HEMA in UDMA-based resin matrix. While the differences in the PSS of binary UDMA/PEG-U and UDMA/HEMA may possibly be related to the higher relative molecular mass and the more flexible character of the PEG-U oligomer (compared to the less flexible poly(HEMA) segments in the matrix) it may be erroneous to apply the same reasoning to explain PSS developing in the more complex UPHM resins and their ACP composites. Therefore, the apparent discrepancy between the PS and PSS in relation to the resin matrix composition has yet to be resolved. It may be prudent to collect data on the water sorption of these materials to establish whether there is a reasonable possibility that the relatively high PS may be offset by the hygroscopic expansion of UPHM resins from water uptake. Preliminary studies on aqueous immersion of ACP/UPHM composites (unpublished data) indeed indicate an increase in the volume of the composite disk specimens of up to 13 % for both LC and DC formulations.
To better assess the suitability of the experimental dual cure ACP/UPHM composites for the intended endodontic application, further evaluations are needed, and should include a systematic exploration of the mass and volume changes of composite upon exposure to a simulated oral environment, their mechanical stability and remineralizing potential seems s necessary. Favorable outcomes of these physicochemical tests would be prerequisite for further studies including dentin barrier measurements, cell viability, membrane integrity and cell necrosis tests.
Summary
Dual cure, urethane dimethacrylate-based ACP polymeric composites formulated for endodontic utility attained high vinyl conversion, which was accompanied with relatively high polymerization shrinkage and moderate levels of polymerization stress development. The high degree of double bond conversion attained in these composites suggests that the likelihood of leaching-out un-reacted monomeric species is minimal. Therefore, a minimal adverse cellular response to these composites is expected. Additional physicochemical evaluations are needed to establish the mechanical stability of these composites, assess whether negative shrinkage effects may be ameliorated by composite’s hygroscopic expansion, and determine their remineralization potential.
Acknowledgements
This investigation was supported by Research grant DE13169 from the National Institute of Dental and Craniofacial Research, the National Institute of Standards and Technology and the American Dental Association Foundation. We acknowledge Esstech, Essington, PA, USA for generously providing the monomers used in this study.
Footnotes
Disclaimer
Certain commercial materials and equipment are identified in this work for adequate definition of the experimental procedures. In no instance does such identification imply recommendation or endorsement by the American Dental Association Foundation or the National Institute of Standards and Technology, or that the material and the equipment identified is necessarily the best available for the purpose.
Literature References
- [1].Skrtic D, Antonucci JM, Eanes ED. J Res. Natl. Inst. Stands. Technol. 2003;108(3):167. doi: 10.6028/jres.108.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Antonucci JM, Skrtic D. In: Polymers for Dental and Orthopedic Applications. Shalaby W, Salz U, editors. CRC Press; Boca Raton, FL: 2007. pp. 217–242. [Google Scholar]
- [3].Skrtic D, Antonucci JM. J. Biomat. Appl. 2007;21:375. doi: 10.1177/0885328206064823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Skrtic D, Antonucci JM, Liu DW. Acta Biomaterialia. 2006;2:85. doi: 10.1016/j.actbio.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].O’Donnell JNR, Langhorst SE, Fow MD, Skrtic D. J. Bioact. Comp. Polym. 2008;23:207. doi: 10.1177/0883911508089932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Skrtic D, Hailer AW, Takagi S, Antonucci JM, Eanes ED. J. Dent. Res. 1996;75(9):1679. doi: 10.1177/00220345960750091001. [DOI] [PubMed] [Google Scholar]
- [7].Langhorst SE, O’Donnell JNR, Skrtic D. Dent. Mater. 2009 doi: 10.1016/j.dental.2009.01.094. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Antonucci JM, Stansbury JW. In: Desk Reference of Functional Polymer Syntheses and Applications. Arshady R, editor. ACS; Washington DC: 1997. pp. 719–738. [Google Scholar]
- [9].Antonucci JM, Skrtic D, Eanes ED. In: Hydrogels and Biodegradable Polymers for Bioapplications. Ottenbrite R, Huang S, Park K, editors. ACS; Washington DC: 1996. pp. 243–254. [Google Scholar]
- [10].Antonucci JM, Skrtic D. J. Bioact. Compat. Polym. 2005;20:29. doi: 10.1177/0883911506064476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Antonucci JM, Grams CL, Termini DJ. J. Dent. Res. 1979;58(9):1887. doi: 10.1177/00220345790580090801. [DOI] [PubMed] [Google Scholar]
- [12].Eanes ED. In: Calcium Phosphates in Biological and Industrial Systems. Amjad Z, editor. Kluwer Academic Publ.; Boston, MA: 1998. pp. 21–39. [Google Scholar]
- [13].Stansbury JW, Dickens SH. Polymer. 2001;42:6363. [Google Scholar]
- [14].Lu H, Stansbury JW, Dickens SH, Eichmiller FC, Bowman CN. J. Mater. Sci: Mater. Med. 2004;15:1097. doi: 10.1023/B:JMSM.0000046391.07274.e6. [DOI] [PubMed] [Google Scholar]
- [15].Lowell LG, Stansbury JW, Syrpes DC, Bowman CN. Macromolecules. 1999;32(12):3913. [Google Scholar]
- [16].Skrtic D, Antonucci JM. Polym. Int. 2007;56:497. doi: 10.1002/pi.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Venhoven BAM, DeGee AJ, Davidson CL. Biomaterials. 1993;14(11):871. doi: 10.1016/0142-9612(93)90010-y. [DOI] [PubMed] [Google Scholar]
- [18].Silikas N, Eliades G, Watts DC. Dent. Mater. 2000;16(4):292. doi: 10.1016/s0109-5641(00)00020-8. [DOI] [PubMed] [Google Scholar]
- [19].Antonucci JM, Regnault WF, Skrtic D. submitted to J. Comp. Mater. 2009 doi: 10.1177/0021998309345180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Price RB, Rizkalla AS, Hall GC. Am. J. Dent. 2000;13(4):176. [PubMed] [Google Scholar]
- [21].Labella R, Lambrechts P, VanMeerbeek B, Vanherle G. Dent. Mater. 1999;15(2):128. doi: 10.1016/s0109-5641(99)00022-6. [DOI] [PubMed] [Google Scholar]