Abstract
The dopamine transporter locus (DAT1) has been studied as a risk factor for attention-deficit/hyperactivity disorder (ADHD) and in pharmacogenetic studies of stimulant response. Several prospective studies have reported an association between the homozygous 9 repeat allele of the DAT1 3′ untranslated region (UTR) variable number tandem repeat (VNTR) (DAT1 3′) and decreased efficacy of methylphenidate (MPH). We hypothesized that children with the 9/9 genotype would display higher rates of specific stimulant side effects. Data on adverse events and DAT1 3′ genotypes were combined from two, double-blind, placebo-controlled, crossover studies of MPH conducted in child psychiatric outpatient clinics in Montreal and Washington, D.C. There were 177 participants, 5–16 years old (mean age = 8.99, standard deviation [SD] = 2), with ADHD. Parents completed the Stimulant Side Effect Scale (SERS) after a week of placebo and a week of MPH treatment. Principal components analysis of the SERS resulted in three factors: Emotionality, Somatic Complaints, and Over-focused. Children with the 9/9 genotype displayed higher scores on the Emotionality factor during placebo than children with the 9/10 and the 10/10 genotype, and their Emotionality scores increased further during MPH treatment (F[2,151] = 3.24, p < 0.05). Children with the 10/10 genotype displayed a significant increase in Somatic Complaint factor scores during MPH treatment relative to the other genotype groups (F[2,150] = 3.4, p < 0.05). These data provide suggestive evidence that DAT1 variants are differentially associated with specific stimulant side effects. Children with the 9/10 genotype displayed less severe stimulant side-effect ratings than either of the homozygous groups, who each displayed increased susceptibility to different types of adverse events. Preliminary evidence suggests that pharmacogenetic analysis using DAT1 variants shows promise for identifying individuals at increased or decreased risk for poor tolerability.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a highly familial neurobehavioral disorder that is frequently treated with stimulant medications, such as methylphenidate (MPH) or amphetamine (Goldman et al. 1998). Despite robust short-term efficacy in the majority of ADHD efficacy studies, many children and adolescents with ADHD discontinue treatment prematurely (Thiruchelvan et al. 2001; Schachar et al. 2002). This is presumably due to adverse effects contributing to poor tolerability (Efron et al. 1998; Charach et al. 2004). Mild stimulant side effects are common, especially insomnia, decreased appetite, and irritability (Barkley et al. 1990; Efron et al. 1997). Insomnia and decreased appetite are often dose dependent or transitory (Douglas et al. 1986; Greenhill et al. 2001; Rapport and Moffitt 2002; Stein et al. 2003). For example, Barkley et al. reported that decreased appetite, insomnia, stomachaches, and headaches increased significantly in frequency and severity during the two active medication periods as compared to the placebo treatment (Barkley et al. 1990). Irritability is common at baseline and often improves during treatment, although it can also be exacerbated with stimulants or occur as part of a rebound phenomenon as stimulant concentrations decline. More severe, but far less common, stimulant side effects have also been reported, including psychotic symptoms and severe emotionality (Cherland and Fitzpatrick 1999; Sarampote et al. 2002). It is unclear how prevalent severe dysphoria and other psychiatric side effects are. In one study, 6 of 192 patients reviewed displayed severe behaviors, including several who displayed psychotic symptoms during stimulant treatment.
At present, pharmacological treatment for ADHD is determined empirically (American Academy of Child and Adolescent Psychiatry 2002), as there are few predictors of response to stimulants (Owens et al. 2003), to type of stimulant (i.e., MPH or amphetamine) (Pelham et al. 1990; Barbaresi et al. 2006) or of optimal dose. The strong heritability of ADHD and wide individual variability in dosing and tolerability has increased interest in pharmacogenetic studies of stimulant response (McGough 2005; Stein and McGough 2008). Not surprisingly, the majority of pharmacogenetic studies have examined logical candidates, such as the dopamine transporter gene (DAT1) and other genes related to catecholamine metabolism (Vandenbergh et al. 2000). This is because the primary mechanism of action of stimulants is thought to be through blocking reuptake of synaptic dopamine by binding to the dopamine transporter (Swanson et al. 2000; Volkow et al. 2002; Volkow et al. 2007). The dopamine transporter locus has been investigated in murine knockout models (Heiser et al. 2004), as candidate genes in studies of ADHD susceptibility (Maher et al. 2002; Lee et al. 2007), and more recently pharmacogenetic studies (Greenhill et al. 2001; Rohde et al. 2003; Stein and McGough 2008).
In the first pharmacogenetic study of MPH in ADHD, Winsburg and Comings reported decreased efficacy in African-American children homozygous for the 10-repeat allele as compared to other DAT1 3′ genotype groups (Winsberg and Comings 1999). Subsequently, a handful of studies with markedly different methods and study populations and predominantly small samples have yielded conflicting results in terms of risk genotype and direction of effect (Roman et al. 2004; McGough et al. 2005). However, three recent prospective studies have examined the 9/9 genotype separately from the 9/10 genotype and found decreased efficacy for the 9/9 genotype group relative to other DAT1 genotype groups on parent-report measures of ADHD symptoms (Stein et al. 2005; Joober et at. 2006; McGough et al. 2006).
Genetic variation in DAT1 may be associated with other response characteristics besides efficacy. As described by Roses (Roses 2004), pharmacogenetic analysis can be used to differentiate phenotypic heterogeneity, to segment populations that are responsive or unresponsive to a medication, and to identify accurately individuals who are at increased risk of an adverse event. For example, Zeni et al. (2005) (examined the effects of DAT1 on insomnia and decreased appetite in a naturalistic study of 111 children and did not find any association. However, in the Preschool ADHD study (McGough et al. 2006), McGough et al. reported that preschoolers with the SNAP25 G allele at T1065 had two to three times the risk of irritability and sleep problems than preschoolers homozygous for the more common T variant. In addition, preschool children with the C allele at SNAP25 1069 were two to four times more likely to develop tics than those homozygous for the T allele. The DAT1 3′ genotype was not associated with adverse events in this sample, although there were only 8 subjects with the 9/9 genotype. In contrast, Stein et al. (2005) reported DAT1 3′ genotype differences on total stimulant side effects in a sample that included 6 children with the 9/9 genotype.
These preliminary efforts at detecting the relationship between DAT 3′ genotypes and susceptibility for specific adverse events have been hampered by the small number of individuals with the 9/9 genotype and the wide range of potential stimulant side effects to examine, necessitating multiple comparisons within small samples. Consequently, the purpose of the present study is to examine more closely the relationship between DAT1 3′ genotypes and statistically derived factor scores rather than individual side effects in children treated with MPH and placebo.
Methods
Participants
Data were combined from two previous studies conducted in North America with clinic-referred children with ADHD. A total of 177 children between 5 and 16 years of age (mean age = 8.99, standard deviation [SD] = 2) with a Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (American Psychiatric Association 1994), diagnosis of ADHD were included in the study. A total of 73.4% (130) were male and 26.6% (47) were female; 87.6% (155) of the subjects were white, 4.5% (8) were African American, and 7.9% (11) reported other ethnicities; 114 children (64.4%) were stimulant naïve.
The study was approved by the Research Ethics Board of Douglas Hospital and by the Institutional Review Boards of Children's National Medical Center and The University of Chicago. Informed consent was obtained from parents, and all children assented to participation in the study.
Medication, side effects, and DAT1 genotyping
At each site, parents blinded to study medication completed the Side Effects Rating Scale (SERS) after a week of a low to moderate dose of MPH and a week of placebo in randomized order. For the Montreal sample, children were administered 0.5 mg/kg per day (2.5 mg/kg dose), whereas children in the Washington, D.C., sample were administered 36 mg of OROS MPH. Barkley developed the SERS, which is a 17-item, parent-rated scale of potential stimulant side effects, where each item is rated from 0 “absent” to 9 “serious” (Barkely et al. 1990). Following the recommendations of Barkley et al., ratings of 7 and higher were considered “severe.”
DNA was extracted from blood and the 3′-untranslated region (UTR) variable number tandem repeat (VNTR) polymorphism of the SLC6A3 gene was genotyped using PCR amplification. The genotyping procedure is described in detail in our previous manuscripts (Stein et al. 2005; Joober et al. 2006).
Statistical analysis
Subjects were divided into three genotype groups (9/9, 9/10, 10/10) on the basis of their DAT1 3′ polymorphism. Differences in demographics and intellectual and psychiatric characteristics were considered as dependent variables and were compared across the genotypes using either one-way analysis of variance (ANOVA) or chi-squared analysis, depending on the nature of the data.
Analysis of variance and the Pearson correlation were used to examine the relationships between SERS factor scores and descriptive and demographic variables; chi-squared analysis was used to compare the frequency of severe side effects between genotype groups.
Principal component analysis (PCA) with varimax rotation was used to reduce the number of variables and to aggregate side effects into reliable indices reflecting the main domains affected by MPH. SERS Items with a mean rating ≥1 were included in the PCA. Items that loaded at 0.5 or greater were included on each factor. The relationship between polymorphism in the DAT1 gene and changes in SERS following administration of MPH of children with ADHD was examined using multicovariate analysis of variance (MANCOVAs), where: The DAT1 3′ genotype group (9/9, 9/10, 10/10) (3) was the between-subject independent factor; Condition (MPH, placebo) (2) was the repeated, within-subject, independent factor; Side Effects Factor Scores: Somatic, Emotionality, Overfocused (3) were the dependent variables; and the child's age and sex were covariates. Significant interaction of the DAT1 3′ genotype group with medication would indicate that the effects of MPH were moderated by genotype. To adjust for pretreatment internalizing scores on measures that could potentially confound the results, the baseline score on the CBCL Anxious/Depressed Scale was added as a covariate to the analyses for the Emotionality factor.
SPSS 12.0 for Windows (SPSS, Inc., Chicago, Ill) was used for all statistical analyses. p values <0.05 were considered to indicate statistical significance.
Results
Demographic and clinical characteristics of DAT1 genotype groups
Genotype frequencies for the 177 children were as follows: 16 (9%) had 9/9, 68 (38.4%) had 9/10, and 93 (52.5%) had 10/10 genotypes. DAT1 genotype distribution was in Hardy–Weinberg equilibrium for both sites (χ2 = 0.009, not significant [NS], Washington, D.C.; χ2 = 3.25, NS, Montreal). In Table 1, we present the means and standard deviations of the demographic and clinical characteristics of children with ADHD grouped according to their DAT1 genotype.
Table 1.
Demographic and Clinical Characteristics of Children with Attention-Deficit/Hyperactivity Disorder Stratified by DAT1 Group
| Item | 9, 9 (n = 16) | 9, 10 (n = 68) | 10, 10 (n = 93) | p value |
|---|---|---|---|---|
| Gender, male/female | 14/2 | 49/19 | 67/26 | χ2 = 1.78, df = 1, p = 0.42 |
| Mean age (SD) | 8.59 (2.14) | 8.93 (2.04) | 9.11 (2.00) | F(2, 174) = 0.53, p = 0.59 |
| WISC-III | ||||
| Full-Scale IQ | 103.88 (18.36) | 104.85 (14.40) | 99.82 (15.32) | F(2, 159) = 2.04, p = 13 |
| CBCL (T scores) | ||||
| Total | 69.93 (8.68) | 67.04 (8.86) | 67.02 (9.99) | F(2, 169) = 0.64, p = 53 |
| Internalizing | 66.87 (8.72) | 60.84 (11.89) | 61.94 (12.16) | F(2, 169) = 1.60, p = 0.21 |
| Externalizing | 68.67 (13.03) | 66.18 (10.48) | 66.28 (11.56) | F(2, 169) = 0.32, p = 0.73 |
Abbreviations: DAT = dopamine transporter gene; SD = standard deviation; WISC = Wechsler Intelligence Scale for Children, third edition; IQ = intelligence quotient; CBCL = Child Behavior Checklist.
No significant differences were found between the DAT1 genotype groups and age, intelligence quotient (IQ), gender ratio, or externalizing psychopathology. Of note, however, the mean CBCL Internalizing T score of 65 for the 9/9 genotype group was within the clinical range for this measure.
PCA of SERS items
PCA resulted in a three-factor solution, which accounted for 57.7% of the variance (Table 2). The first factor, Emotionality (eigenvalue 3.25), accounted for 32.6% of the variance in stimulant SERS scores and was composed of items reflecting irritability, sadness, proneness to cry, and anxiety. The second factor, Somatic Complaints (eigenvalue 1.5), accounted for 15% of the variance, and was characterized by three common stimulant side effects: Decreased appetite, stomachache, and insomnia. Finally, the third factor, Over-focused, was made up of two items often associated with high stimulant dosage, staring, and nail biting. This factor accounted for 10.1% of the variance (eigenvalue 1.1).
Table 2.
Rotated Factor Loadings for Side Effects While on Methylphenidate
| |
Factor loading |
||
|---|---|---|---|
| Side effects while on MPH | Emotionality | Somatic | Over-focused |
| Staring | 0.17 | 0.22 | 0.54 |
| Decreased appetite | 0.02 | 0.75 | 0.13 |
| Irritability | 0.61 | 0.13 | 0.47 |
| Stomach ache | 0.24 | 0.79 | −0.16 |
| Head ache | 0.40 | 0.45 | −0.35 |
| Sadness | 0.82 | 0.21 | 0.10 |
| Prone to cry | 0.84 | 0.08 | 0.17 |
| Anxious | 0.79 | 0.01 | 0.07 |
| Biting nails | 0.11 | −0.05 | 0.68 |
| Insomnia | 0.03 | 0.62 | 0.22 |
Abbreviations: MPH = methylphenidate.
To examine the criterion-oriented validity of the SES factors, we examined the association of factor scores with demographic characteristics of age and gender, and with dimensional measures of psychopathology obtained from the Child Behavior Checklist (CBCL) prior to medication treatment. Emotionality was positively correlated with CBCL Internalizing T score (r = 0.16, p < 0.05). This suggests that children who displayed more emotionality during stimulant treatment were also likely to display more internalizing symptoms prior to beginning stimulant treatment.
No significant sex or age differences were found on any of the factors, and there was no relationship between previous stimulant treatment and SERS factor scores.
SERS factor scores and DAT1 genotype
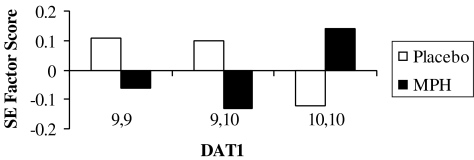
ANCOVAs were conducted to detect DAT1 genotype effects on SERS factors, while controlling for age, gender, and site. These analyses revealed significant differences between the DAT1 genotype groups on the Emotionality factor, F(2,151) = 3.09, p < 0.038, with the 9/9 displaying a higher rated level of negative emotion compared to the 9/10 and the 10/10 genotype groups during placebo. Significant genotype by factor effect was found, with the 9/9`s score increasing dramatically following treatment with MPH F(2,151) = 3.33, p < 0.048 (Fig. 1).
FIG. 1.
Emotionality factor scores for DAT1 genotypes groups during placebo and MPH Treatment. DAT1= Dopamine transporter gene; MPH = methylphenidate; SE =standard error.
In contrast, children with the 10/10 genotype displayed an increase in Somatic Complaint scores on the SERS with MPH treatment, F(2,150) = 3.4, p < 0.037, which was not displayed by the other genotype groups (Fig. 2). No significant genotype group differences or interactions were detected for the Over-focused factor.
FIG. 2.
Somatic complaint scores for DAT1 genotype groups during placebo and MPH treatment. DAT1 =Dopamine transporter gene; MPH = methylphenidate; SE =standard error.
Comment
For the most part, studies of ADHD treatments and pharmacogenetic studies have emphasized the efficacy of stimulant medications and have focused little on adverse events and tolerability. Not surprisingly, the frequency and severity of adverse events differs markedly among studies depending on sample characteristics, informant, method of ascertainment, and when in the trial adverse events are assessed (Efron et al. 1997; Efron et al. 1998). A common measure of adverse events in ADHD treatment, the SERS, is composed of items that represent a heterogeneous collection of symptoms or behaviors that are considered to be side effects of stimulant treatment. However, individual items on the SERS may also reflect symptoms of co-morbid psychopathology (e.g., irritability, nightmares), or even the beneficial effects of treatment (e.g., talks less). Using multivariate statistical techniques, we have reduced the number of variables examined and identified factors representing different stimulant side effects.
Although there is general agreement that safety and tolerability of stimulant medications is a high research priority based upon the public health significance of ADHD and the increasing prevalence of stimulant treatment (Vitiello et al. 2003), at present there are few specific or reliable predictors of adverse events in children with ADHD. We report a specific association between the DAT1 3′ 9/9 genotype and increased dysphoria and emotionality that worsens during MPH treatment. While several studies, including the Multimodal Treatment Study of Children with ADHD (MTA) (March et al. 2000; Hinshaw 2007), did not find a significant moderating effect of emotional symptoms on efficacy (Abikoff et al. 2005), other studies suggest that youths with “emotional” symptoms are most likely to display an adverse response to stimulants (Taylor et al. 1987; DuPaul et al. 1994; Goez et al. 2007). The present study opens the possibility that increased emotionality and dysphoria, which occur in a small but significant subgroup of MPH-treated children with ADHD, may be partially moderated by variations in DAT1.
An alternative explanation for the foregoing phenomenon is that elevated Emotionality factor scores may be associated with stimulant rebound, or a worsening of symptom and emotionality as stimulant concentrations decrease. In a recent study of stimulant rebound, one third of children displayed rebound on at least one dose, and the symptoms were severe enough to cause discontinuation of treatment in 8% of the sample (Carlson and Kelly 2003). Prospective studies are needed to identify the specific relationship of elevations on this factor to psychiatric co-morbidity and rebound effects. Moreover studies of gene expression, pharmacokinetics, and pharmacodynamics are needed to determine potential mechanisms of differential response (Swanson and Volkow 2002).
Insomnia and decreased appetite, side effects commonly associated with ADHD treatment with stimulants, are components of the Somatic Complaints factor. While the present study design does not evaluate which adverse effects are dose dependent, previous studies suggest that insomnia and decreased appetite increase with higher stimulant dosages (Stein et al. 2003). For example, in a previous crossover study of 234 children with ADHD who were treated with placebo and two MPH doses, 0.3 mg/kg, and 0.5 mg/kg/, insomnia, appetite disturbance, stomachache, headache, and dizziness increased relative to placebo with the increasing MPH dose (Ahmann et al. 1993). In contrast, staring, daydreaming, irritability anxiety, and nail biting decreased with MPH treatment. These items are related to the SERS Emotionality and Over-focused factors.
The results of this study suggest several hypotheses of potential clinical relevance. First, individuals with the 9/9 genotype may be candidates for expanded psychosocial treatment to target mood symptoms or pharmacotherapy with a nonstimulant (e.g., atomoxetine). Further study of the mechanisms related to increased emotionality, per se, is needed; these specific symptoms may represent differential effects of MPH on pathways other than the dopaminergic frontal-striatal networks that are frequently associated with ADHD (Pliszka et al. 1996; Taylor 1999; Solanto 2002). Second, individuals with the 10/10 genotype appear to be more susceptible to somatic and sleep problems, which are frequently reported in children taking stimulant medications. Perhaps individuals with this genotype may respond to lower dosages or a slower titration schedule. Finally, the present study suggests that DAT1 3′ heterozygotes are less likely to experience emotional or somatic side effects during stimulant treatment. The majority of previous pharmacogenetic studies of efficacy have reported improved response rates for this genotype group relative to the homozygous DAT1 3′ genotypes (Winsberg et al. 1999; Rohde et al. 2003). Because longitudinal studies suggest increased severity of impairment in youths with the 9/10 genotype (Barkley et al. 2006), being able to optimize their dose quickly given the decreased risk of adverse events could potentially result in improved effectiveness of stimulant medication for children and adolescents with this common genotype.
There are several limitations, however, which should be considered when interpreting the results of this study. The primary limitation is the small number of individuals with the 9/9 genotype. However, given the relatively small sample size of individuals with the 9/9 genotype, failing to combine samples would have made the analysis impossible and resulted in a missed opportunity for a first look at these effects. Future studies with larger samples are needed to confirm the associations between DAT1 genotypes and stimulant side effects, and to examine gene × environment and gene × gene interactions, which may account for a greater percentage of the variation in response.
Second, the present findings are based on studies of a fixed, low to moderate MPH dose and a very brief, 1-week study period. Thus, the findings may not generalize to wider dose ranges or more subtle side effects that develop over time.
Finally, due to the exploratory nature of this study, multiple statistical tests were conducted, increasing the possibility of a false-positive finding. This is particularly salient given the previous history of false-positive findings in candidate gene studies (McGough 2005).
Furthermore, future genetic analyses in clinical trials may assist in distinguishing symptoms of co-morbidity from ADHD symptoms or stimulant side effects. However, if, as the present study suggests, the types and severity of adverse events during stimulant treatment are related to specific DAT polymorphisms, eventually it may be possible to identify those at greater risk of a specific adverse event, thereby increasing the efficiency of treatment planning and ultimately reducing adverse events that contribute to the poor tolerability and adherence of ADHD treatments in older children and adolescents. Each of these elements has important promise for safer, more prompt, and more effective treatment of ADHD.
Footnotes
This work was supported in part by grants from the Fonds de la Recherche du Santé au Québec and the Canadian Institute of Health Research (R.J.), National Institute of Mental Health K24MHO1823 (M.S.), the Jean Young and Walden W. Shaw Foundation (B.L., E.C.), and the Children's Brain Research Foundation (B.L., E.C., M.S.).
Disclosures
Dr. Stein receives research support from McNeil Pediatrics, Novartis Pharamaceuticals, Eli Lilly and Co., and Organon; has participated as an advisor to Abbott, Novartis, and Shire; and serves on the speakers bureau for Novartis. Dr. Joober provides consultation to Johnson and Johnson and Pfizer. Dr. Leventhal receives research support from BMS and Pfizer, and has previously participated in the speakers bureau for BMS and Pfizer; he has also received travel support from Eli Lilly. Drs. Gruber, Grizenko, and Cook have no conflicts of interest or financial ties to disclose.
References
- Abikoff H. McGough J. Vitiello B. McCracken J. Davies M. Walkup J. Riddle M. Oatis M. Greenhill L. Skrobala A. March J. Gammon P. Robinson J. Lazell R. McMahon DJ. Ritz L. Sequential pharmacotherapy for children with comorbid attention-deficit/hyperactivity and anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2005;44:418–427. doi: 10.1097/01.chi.0000155320.52322.37. [DOI] [PubMed] [Google Scholar]
- Ahmann PA. Waltonen SJ. Olson KA. Theye FW. Van Erem AJ. LaPlant RJ. Placebo-controlled evaluation of Ritalin side effects. Pediatrics. 1993;91:1101–1106. [PubMed] [Google Scholar]
- American Academy of Child and Adolescent Psychiatry. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry. 2002;41(2s):26s–48s. doi: 10.1097/00004583-200202001-00003. [DOI] [PubMed] [Google Scholar]
- American Pyschiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) Washington (DC): American Psychiatric Association; 1994. [Google Scholar]
- Barbaresi WJ. Katusic SK. Colligan RC. Weaver AL. Leibson CL. Jacobsen SJ. Long-term stimulant medication treatment of attention-deficithyperactivity disorder: Results from a population-based study. J Dev Behav Pediatr. 2006;27:1–10. doi: 10.1097/00004703-200602000-00001. [DOI] [PubMed] [Google Scholar]
- Barkley RA. McMurray MB. Edelbrock CS. Robbins K. Side effects of methylphenidate in children with attention deficit hyperactivity disorder: A systemic, placebo-controlled evaluation. Pediatrics. 1990;86:184–192. [PubMed] [Google Scholar]
- Barkley RA. Smith KM. Fischer M. Navia B. An examination of the behavioral and neuropsychological correlates of three ADHD candidate gene polymorphisms (DRD4 7+, DBH TaqI A2, and DAT1 40 bp VNTR) in hyperactive and normal children followed to adulthood. Am J Med Genet B Neuropsychiatr Genet. 2006;141:487–498. doi: 10.1002/ajmg.b.30326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson GA. Kelly KL. Stimulant rebound: How common is it and what does it mean? J Child Adolesc Psychopharmacol. 2003;13:137–142. doi: 10.1089/104454603322163853. [DOI] [PubMed] [Google Scholar]
- Charach A. Ickowicz A. Schachar R. Stimulant treatment over five years: Adherence, effectiveness, and adverse effects. J Am Acad Child Adolesc Psychiatry. 2004;43:559–567. doi: 10.1097/00004583-200405000-00009. [DOI] [PubMed] [Google Scholar]
- Cherland E. Fitzpatrick R. Psychotic side effects of psychostimulants: A 5-year review. Can J Psychiatry. 1999;44:811–813. doi: 10.1177/070674379904400810. [DOI] [PubMed] [Google Scholar]
- Douglas V. Barr R. ONeill M. Britton B. Short term effects of methylphenidate on the cognitive, learning, and academic performance of children with attention deficit disorder in the laboratory and the classroom. J Child Psychol Psychiat. 1986;27:191–211. doi: 10.1111/j.1469-7610.1986.tb02330.x. [DOI] [PubMed] [Google Scholar]
- DuPaul G. Barkley R. McMurray M. Response of children with ADHD to methylphenidate: Interaction with internalizing symptoms. J Am Acad Child Adolesc Psychiatry. 1994;33:894–903. doi: 10.1097/00004583-199407000-00016. [DOI] [PubMed] [Google Scholar]
- Efron D. Jarman F. Barker M. Side effects of methylphenidate and dexamphetamine in children with attention deficit hyperactivity disorder: A double-blind, crossover trial. Pediatrics. 1997;100:662–666. doi: 10.1542/peds.100.4.662. [DOI] [PubMed] [Google Scholar]
- Efron D. Jarman FC. Barker MJ. Child and parent perceptions of stimulant medication treatment in attention deficit hyperactivity disorder. J Paediatr Child Health. 1998;34:288–292. doi: 10.1046/j.1440-1754.1998.00224.x. [DOI] [PubMed] [Google Scholar]
- Goez H. Back-Bennet O. Zelnik N. Differential stimulant response on attention in children with comorbid anxiety and oppositional defiant disorder. J Child Neurol. 2007;22:538–542. doi: 10.1177/0883073807303221. [DOI] [PubMed] [Google Scholar]
- Goldman L. Gemel M. Bezman R. Slanetz P. Diagnosis and treatment of attention deficit hyperactivity disorder. JAMA. 1998;279:1100–1107. doi: 10.1001/jama.279.14.1100. [DOI] [PubMed] [Google Scholar]
- Greenhill LL. Swanson JM. Vitiello B. Davies M. Clevenger W. Wu M. Arnold LE. Abikoff HB. Bukstein OG. Conners CK. Elliott GR. Hechtman L. Hinshaw SP. Hoza B. Jensen PS. Kraemer HC. March JS. Newcorn JH. Severe JB. Wells K. Wigal T. Impairment and deportment responses to different methylphenidate doses in children with ADHD: The MTA titration trial. J Am Acad Child Adolesc Psychiatry. 2001;40:180–187. doi: 10.1097/00004583-200102000-00012. [DOI] [PubMed] [Google Scholar]
- Heiser P. Friedel S. Dempfle A. Konrad K. Smidt J. Grabarkiewicz J. Herpertz-Dahlmann B. Remschmidt H. Hebebrand J. Molecular genetic aspects of attention-deficit/hyperactivity disorder. Neurosci Biobehav Rev. 2004;28:625–641. doi: 10.1016/j.neubiorev.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP. Moderators and mediators of treatment outcome for youth with ADHD: Understanding for whom and how interventions work. Ambul Pediatr. 2007;7(1 Suppl):91–100. doi: 10.1016/j.ambp.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Joober R. Grizenko N. Sengupta S. Amor LB. Schmitz N. Schwartz G. Karama S. Lageix P. Fathalli F. Torkaman-Zehi A. Stepanian MT. Dopamine transporter 3′-UTR VNTR genotype and ADHD: A pharmaco-behavioural genetic study with methylphenidate. Neuropsychopharmacology. 2006;32:1370–1376. doi: 10.1038/sj.npp.1301240. [DOI] [PubMed] [Google Scholar]
- Lee SS. Lahey BB. Waldman I. Van Hulle CA. Rathouz P. Pelham WE. Loney J. Cook EH. Association of dopamine transporter genotype with disruptive behavior disorders in an eight-year longitudinal study of children and adolescents. Am J Med Genet B Neuropsychiatr Genet. 2007;144:310–317. doi: 10.1002/ajmg.b.30447. [DOI] [PubMed] [Google Scholar]
- Maher BS. Marazita ML. Ferrell RE. Vanyukov MM. Dopamine system genes and attention deficit hyperactivity disorder: A meta-analysis. Psychiatry Genet. 2002;12:207–215. doi: 10.1097/00041444-200212000-00003. [DOI] [PubMed] [Google Scholar]
- March JS. Swanson JM. Arnold LE. Hoza B. Conners CK. Hinshaw SP. Hechtman L. Kraemer HC. Greenhill LL. Abikoff HB. Elliott LG. Jensen PS. Newcorn JH. Vitiello B. Severe J. Wells KC. Pelham WE. Anxiety as a predictor and outcome variable in the multimodal treatment study of children with ADHD (MTA) J Abnorm Child Psychol. 2000;28:527–541. doi: 10.1023/a:1005179014321. [DOI] [PubMed] [Google Scholar]
- McGough JJ. Attention-deficit/hyperactivity disorder pharmacogenomics. Biol Psychiatry. 2005;57:1367–1373. doi: 10.1016/j.biopsych.2004.10.021. [DOI] [PubMed] [Google Scholar]
- McGough J. McCracken J. Swanson J. Riddle M. Kollins S. Greenhill L. Abikoff H. Davies M. Chuang S. Wigal T. Wigal S. Posner K. Skrobala A. Kastelic E. Ghuman J. Cunningham C. Shigawa S. Moyzis R. Vitiello B. Pharmacogenetics of methylphenidate response in preschoolers with ADHD. J Am Acad Child Adolesc Psychiatry. 2006;45:1314–1322. doi: 10.1097/01.chi.0000235083.40285.08. [DOI] [PubMed] [Google Scholar]
- Owens EB. Hinshaw SP. Kraemer HC. Arnold LE. Abikoff HB. Cantwell DP. Conners CK. Elliott G. Greenhill LL. Hechtman L. Hoza B. Jensen PS. March JS. Newcorn JH. Pelham WE. Severe JB. Swanson JM. Vitiello B. Wells KC. Wigal T. Which treatment for whom for ADHD? Moderators of treatment response in the MTA. J Consult Clin Psychol. 2003;71:540–552. doi: 10.1037/0022-006x.71.3.540. [DOI] [PubMed] [Google Scholar]
- Pelham WE., Jr. Greenslade KE. Vodde-Hamilton M. Murphy DA. Greenstein JJ. Gnagy EM. Guthrie KJ. Hoover MD. Dahl RE. Relative efficacy of long-acting stimulants on children with attention deficit-hyperactivity disorder: A comparison of standard methylphenidate, sustained-release methylphenidate, sustained-release dextroamphetamine, pemoline. Pediatrics. 1990;86:226–237. [PubMed] [Google Scholar]
- Pliszka SR. McCracken JT. Maas JW. Catecholamines in attention-deficit hyperactivity disorder: Current perspectives. J Am Acad Child Adolesc Psychiatry. 1996;35:264–272. doi: 10.1097/00004583-199603000-00006. [DOI] [PubMed] [Google Scholar]
- Rapport MD. Moffitt C. Attention deficit/hyperactivity disorder and methylphenidate. A review of height/weight, cardiovascular, and somatic complaint side effects. Clin Psychol Rev. 2002;22:1107–1131. doi: 10.1016/s0272-7358(02)00129-0. [DOI] [PubMed] [Google Scholar]
- Rohde LA. Roman T. Hutz MH. Attention-deficit/hyperactivity disorder: Current aspects on pharmacogenetics. Pharmacogenomics J. 2003;3:11–13. doi: 10.1038/sj.tpj.6500158. [DOI] [PubMed] [Google Scholar]
- Roman T. Rohde LA. Hutz MH. Polymorphisms of the dopamine transporter gene: Influence on response to methylphenidate in attention deficit-hyperactivity disorder. Am J Pharmacogenomics. 2004;4:83–92. doi: 10.2165/00129785-200404020-00003. [DOI] [PubMed] [Google Scholar]
- Roses AD. Pharmacogenetics and drug development: The path to safer and more effective drugs. Nature Rev. 2004;5:645–656. doi: 10.1038/nrg1432. [DOI] [PubMed] [Google Scholar]
- Sarampote CS. Efron LA. Robb AS. Pearl PL. Stein MA. Can stimulant rebound mimic pediatric bipolar disorder? J Child Adolesc Psychopharmacol. 2002;12:63–67. doi: 10.1089/10445460252943588. [DOI] [PubMed] [Google Scholar]
- Schachar R. Jadad AR. Gauld M. Boyle M. Booker L. Snider A. Kim M. Cunningham C. Attention-deficit hyperactivity disorder: Critical appraisal of extended treatment studies. Can J Psychiatry. 2002;47:337–348. doi: 10.1177/070674370204700404. [DOI] [PubMed] [Google Scholar]
- Solanto MV. Dopamine dysfunction in ADHD: Integrating clinical and basic neuroscience research. Behav Brain Res. 2002;130:65–71. doi: 10.1016/s0166-4328(01)00431-4. [DOI] [PubMed] [Google Scholar]
- Stein M. McGough J. The pharamacoenomic era: Promise for personalizing ADHD treatment. Child Adolesc Psychiatry Clin N Am. 2008;17:475–490. doi: 10.1016/j.chc.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MA. Sarampote CS. Waldman ID. Robb AS. Conlon C. Pearl PL. Black DO. Seymour KE. Newcorn JH. A dose-response study of OROS methylphenidate in children with attention-deficit hyperactivity disorder. Pediatrics. 2003:112–e404. doi: 10.1542/peds.112.5.e404. [DOI] [PubMed] [Google Scholar]
- Stein MA. Waldman ID. Sarampote CS. Seymour KE. Robb AS. Conlon C. Kim SJ. Cook EH. Dopamine transporter genotype and methylphenidate dose response in children with ADHD. Neuropsychopharmacology. 2005;30:1374–1382. doi: 10.1038/sj.npp.1300718. [DOI] [PubMed] [Google Scholar]
- Swanson JM. Flodman P. Kennedy J. Spence MA. Moyzis R. Schuck S. Murias M. Moriarity J. Barr C. Smith M. Posner M. Dopamine genes and ADHD. Neurosci Biobehav Rev. 2000;24:21–25. doi: 10.1016/s0149-7634(99)00062-7. [DOI] [PubMed] [Google Scholar]
- Swanson JM. Volkow ND. Pharmacokinetic and pharmacodynamic properties of stimulants: Implications for the design of new treatments for ADHD. Behav Brain Res. 2002;130:73–78. doi: 10.1016/s0166-4328(01)00433-8. [DOI] [PubMed] [Google Scholar]
- Taylor E. Developmental neuropsychopathology of attention deficit and impulsiveness. Dev Psychopathol. 1999;11:607–628. doi: 10.1017/s0954579499002230. [DOI] [PubMed] [Google Scholar]
- Taylor E. Schachar R. Thorley G. Wieselberg H. Everitt B. Rutter M. Which boys respond to stimulant medication? A controlled trial of methylphenidate in boys with disruptive behavior. Psychol Med. 1987;17:121–143. doi: 10.1017/s0033291700013039. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam D. Charach A. Schachar RJ. Moderators and mediators of long-term adherence to stimulant treatment in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2001;40:922–928. doi: 10.1097/00004583-200108000-00014. [DOI] [PubMed] [Google Scholar]
- Vandenbergh DJ. Thompson MD. Cook EH. Bendahhou E. Nguyen T. Krasowski MD. Zarrabian D. Comings D. Sellers EM. Tyndale RF. George SR. O'Dowd BF. Uhl GR. Human dopamine transporter gene: Coding region conservation among normal, Tourette's disorder, alcohol dependence and attention-deficit hyperactivity disorder populations. Mol Psychiatry. 2000;5:283–292. doi: 10.1038/sj.mp.4000701. [DOI] [PubMed] [Google Scholar]
- Vitiello B. Riddle MA. Greenhill LL. March JS. Levine J. Schachar RJ. Abikoff H. Zito JM. McCracken JT. Walkup JT. Findling RL. Robinson J. Cooper TB. Davies M. Varipatis E. Labellarte MJ. Scahill L. Capasso L. How can we improve the assessment of safety in child and adolescent psychopharmacology? J Am Acad Child Adolesc Psychiatry. 2003;42:634–641. doi: 10.1097/01.CHI.0000046840.90931.36. [DOI] [PubMed] [Google Scholar]
- Volkow ND. Fowler JS. Wang GJ. Ding YS. Gatley SJ. Role of dopamine in the therapeutic and reinforcing effects of methylphenidate in humans: Results from imaging studies. Eur Neuropsychopharmacol. 2002;12:557–566. doi: 10.1016/s0924-977x(02)00104-9. [DOI] [PubMed] [Google Scholar]
- Volkow ND. Wang GJ. Newcorn J. Telang F. Solanto MV. Fowler JS. Logan J. Ma Y. Schulz K. Pradhan K. Wong C. Swanson JM. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit hyperactivity disorder. Arch Gen Psychiatry. 2007;64:932–940. doi: 10.1001/archpsyc.64.8.932. [DOI] [PubMed] [Google Scholar]
- Winsberg BG. Comings DE. Association of the dopamine transporter gene (DAT1) with poor methylphenidate response. J Am Acad Child Adolesc Psychiatry. 1999;38:1474–1477. doi: 10.1097/00004583-199912000-00006. [DOI] [PubMed] [Google Scholar]
- Zeni CP. Guimaraes AP. Polanczyk GV. Genro GP. Roman T. Hutz MH. Rohde LA. No significant association between response to methylphenidate, genes of the dopaminergic, serotonergic systems in a sample of Brazilian children with attention deficit hyperactivity disorder. Am J Med Genet Part B. 2005;144B:391–394. doi: 10.1002/ajmg.b.30474. [DOI] [PubMed] [Google Scholar]