Abstract
NF-κB activation following engagement of the antigen-specific T cell receptor involves protein kinase C-θ-dependent assembly of the CARMA1-BCL10-MALT1 (CBM) signalosome, which coordinates downstream activation of IκB kinase (IKK). We previously identified a novel role for the adhesion- and degranulation-promoting adapter protein (ADAP) in regulating the assembly of the CBM complex via an interaction of ADAP with CARMA1. In this study, we identify a novel site in ADAP that is critical for association with the TAK1 kinase. ADAP is critical for recruitment of TAK1 and the CBM complex, but not IKK, to protein kinase C-θ. ADAP is not required for TAK1 activation. Although both the TAK1 and the CARMA1 binding sites in ADAP are essential for IκBα phosphorylation and degradation and NF-κB nuclear translocation, only the TAK1 binding site in ADAP is necessary for IKK phosphorylation. In contrast, only the CARMA1 binding site in ADAP is required for ubiquitination of IKKγ. Thus, distinct sites within ADAP control two key activation responses that are required for NF-κB activation in T cells.
Keywords: Gene/Knockout, Immunology, Immunology/T-cell receptor, Phosphorylation/Transcription Factors, Protein/Protein-Protein Interactions, Receptors/Leukocyte/Lymphocyte, Signal Transduction/Adapter Proteins, Transcription/NF-kB
Introduction
In the immune system, the NF-κB transcription factor pathway plays a central role in T cell activation and survival (1, 2). The canonical NF-κB pathway involves activation of the IκB-kinase (IKK)3 complex, which consists of the catalytic IKKα and IKKβ subunits and the regulatory IKKγ (NF-κB essential modulator (NEMO)) subunit. Activated IKK mediates phosphorylation of IκBα, resulting in IκBα degradation and translocation of NF-κB to the nucleus. In T cells, stimulation of the T cell receptor and the CD28 co-stimulatory receptor results in activation of the PKCθ isoform and association of the IKK complex with PKCθ (3). PKCθ phosphorylates the membrane-associated guanylate kinase (MAGUK) family member adapter CARMA1 (4, 5), which then associates with the adapters BCL-10 and mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1) (6, 7). Activation of the IKK complex is proposed to require both Lys-63-linked ubiquitination of IKKγ (8, 9) and IKKα/β phosphorylation (10). Recent studies suggest that IKKγ ubiquitination is dependent on efficient CBM complex formation, whereas phosphorylation of IKKα/β is mediated by TGFβ-activated kinase (TAK1) (11). Both of these regulatory events appear to be required for efficient NF-κB activation as loss of expression of CARMA1, BCL-10, MALT1, or TAK1 results in impaired NF-κB signaling in T cells (7, 12–15). The mechanism by which TAK1 is recruited to the PKCθ signalosome is unclear. Although CARMA1 and TAK1 have been reported to associate with each other (11, 16, 17), TAK1-mediated IKKα/β phosphorylation is intact in CARMA1-deficient Jurkat T cells (11). This suggests that IKKγ ubiquitination and IKKα/β phosphorylation are independently controlled.
Adhesion- and degranulation-promoting adapter protein (ADAP) is a hematopoietic-specific adapter protein that regulates “inside-out” signaling from the T cell receptor to integrins (18, 19). We previously identified a novel function for ADAP in controlling NF-κB activation via regulation of CBM complex assembly (20). A region of ADAP between amino acids 426 and 541 mediates the association of ADAP with CARMA1, and a mutant of ADAP lacking this site is unable to restore NF-κB activation in ADAP−/− T cells (20). This mutant can restore integrin function in ADAP−/− T cells (21), indicating that ADAP independently controls integrin function and NF-κB activation. However, the precise mechanism by which ADAP controls IKK complex activation has not been elucidated. In this study, we identify a novel site in ADAP that is critical for TAK1 association and TAK1-dependent IKKα/β phosphorylation and demonstrate that distinct sites in ADAP are required for IKKα/β phosphorylation and CARMA1-dependent IKKγ ubiquitination.
EXPERIMENTAL PROCEDURES
Mice
ADAP−/− mice and ADAP−/− mice crossed to hCAR transgenic mice (22) on the BALB/c background have been previously described (20). Mice were housed in specific pathogen-free facilities at the University of Minnesota and were used between 8 and 10 weeks of age. All protocols involving use of mice were approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
Cells, Antibodies, and Reagents
The Jurkat E6-1 human T cell line was obtained from American Type Culture Collection and maintained in RPMI 1640 supplemented with 10% fetal calf serum, penicillin/streptomycin, and l-glutamine. Jurkat T cells were transfected by electroporation as described previously (23).
Antibodies used for T cell stimulation were anti-mouse CD3 antibody 2C11, anti-mouse CD28 antibody, and anti-human CD3 antibody OKT3 (all from eBioscience). Additional antibodies used include mouse anti-IκBα, rabbit anti-phospho-IκB, rabbit anti-Erk, rabbit anti-lamin A/C, and rabbit anti-phospho-Erk (all from Cell Signaling); rabbit anti-IKK, goat anti-phospho-IKK, mouse anti-TAK1 (C-9), rabbit anti-PKCθ (C-18), rabbit anti-IKKγ, mouse anti-ubiquitin (P4D1), rabbit anti-p65 (C-20), and mouse anti-BCL10 (331.3) (all from Santa Cruz Biotechnology); and rabbit anti-CARMA1 (Alexis Biochemicals) and allophycocyanin (APC)-conjugated anti-mouse Thy1.1 (H1S51) (eBioscience). Sheep anti-murine and anti-human ADAP antibodies (24) were kindly provided by Dr. E. Peterson (University of Minnesota). For immunoblotting, Alexa Fluor 680-conjugated goat anti-mouse IgG (Invitrogen), IRDye 800-conjugated goat anti-rabbit IgG (Rockland Immunochemicals), and IRDye 800 donkey anti-sheep IgG (Rockland Immunochemicals) were used.
Adenovirus Production and Transduction
Adenovirus expression plasmids and recombinant adenovirus were generated as described previously (20, 21). ADAP mutants were generated using the QuikChange® II XL site-directed mutagenesis kit (Stratagene) via the introduction of a stop codon at amino acid position 604 and 719 or by using primers flanking both sides of the deleted site (mutants Δ673–686, Δ621–672, Δ691–708, and Δ426–541). Freshly isolated resting hCAR+ control and ADAP−/− lymph node T cells were transduced with control virus or ADAP virus and incubated at 37 °C for 3 days in complete T cell medium containing 10 ng/ml mouse interleukin-7 (IL-7) (R&D Systems). All recombinant adenoviruses express the Thy1.1 cell surface antigen. Flow cytometric analysis indicated that >80% of the cells in these cultures were transduced and expressing Thy1.1 and ADAP. Cell lysates were also analyzed by Western blotting with an anti-ADAP antibody to verify comparable expression of ADAP in different cell samples.
Immunoprecipitation and Immunoblotting
Immunoprecipitation and immunoblotting was performed as described previously (20). Briefly, T cells (10 × 106) were resuspended in phosphate-buffered saline containing 0.5% bovine serum albumin and stimulated with 20 μg/ml anti-CD3 (2C11) and 2 μg/ml anti-CD28 or phorbol 12-myristate 13-acetate (50 ng/ml). Stimulated cells were lysed with an equal volume of 2× lysis buffer (2% Nonidet P-40, 100 mm Tris (pH 7.6), 300 mm NaCl, 4 mm EDTA, 2 mm sodium orthovanadate, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 2 mm phenylmethylsulfonyl fluoride) and cleared by centrifugation at 12,000 × g. HA-tagged ADAP expressed in Jurkat cells was immunoprecipitated with anti-HA-agarose (Bethyl Laboratories). Other primary antibodies were absorbed to GammaBind Plus Sepharose beads (GE Healthcare). Immunoprecipitates (IPs) were washed with 1× lysis buffer prior to analysis by Western blotting as described previously (20, 21). Membranes were imaged with an Odyssey infrared imager (LI-COR Biosciences).
To assess NF-κB nuclear translocation, hCAR+ control and ADAP−/− lymph node T cells were transduced with adenoviruses and either left unstimulated or stimulated with anti-CD3 and anti-CD28 antibodies as described above. Nuclear and cytoplasmic extracts were isolated as described (Panomics) and analyzed by immunoblotting with an anti-p65 antibody and an anti-lamin A/C antibody.
TAK1 Kinase Assay
TAK1 immunoprecipitates from unstimulated and CD3/CD28-stimulated cell lysates were analyzed for TAK1 kinase activity. Assays were carried out in a final volume of 50 μl containing 50 mm Tris (pH 7.6), 10 mm MgCl2, 0.25 mm EGTA, 0.1 mm orthovanadate, 100 μm ATP, and 50 ng of GST·IKK. The reaction was initiated with the addition of ATP and incubated at 37 °C for 30 min. Phosphorylation of GST·IKK was assessed by Western blotting using an anti-phospho-IKK antibody and an anti-IKK antibody.
RESULTS
ADAP Regulates IKKα/β Phosphorylation and Recruitment of TAK1 to the PKCθ Signalosome
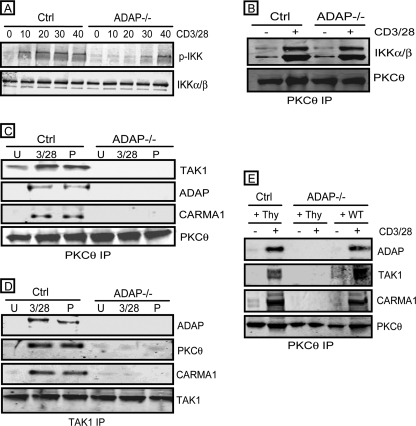
We analyzed TAK1-mediated regulation of NF-κB by first examining ADAP-dependent IKKα/β phosphorylation following stimulation of naive mouse T cells with anti-CD3 and anti-CD28 antibodies that engage the T cell receptor and the CD28 co-stimulatory receptor. Although CD3/CD28 stimulation of control T cells resulted in IKKα/β phosphorylation observed within 10 min of stimulation, IKKα/β phosphorylation was only detectable at late time points (30–40 min) after stimulation of ADAP−/− T cells (Fig. 1A). This was not due to impaired IKK recruitment of the PKCθ signalosome in the absence of ADAP as CD3/CD28 stimulation resulted in increased IKK in PKCθ IPs from control and ADAP−/− T cells (Fig. 1B). CD3/CD28 stimulation has also been reported to induce the recruitment of TAK1 and the CBM complex to PKCθ (11). Thus, we also analyzed PKCθ IPs and found that recruitment of TAK1, as well as CARMA1 and BCL-10, was dramatically impaired following CD3/CD28 stimulation of ADAP−/− T cells (Fig. 1C). Similar results were observed when we specifically immunoprecipitated TAK1 from control and ADAP−/− T cells (Fig. 1D). Expression of wild-type ADAP in ADAP−/− T cells restored CD3/CD28-mediated recruitment of TAK1, CARMA1, and BCL-10 to the PKCθ signalosome (Fig. 1E). Together, these results show that ADAP is critical for CD3/CD28-mediated IKKα/β phosphorylation and recruitment of TAK1 and the CBM complex to the PKCθ signalosome.
FIGURE 1.
ADAP regulates TAK1 recruitment to the PKCθ signalosome. A, T cells from control ADAP+/− mice (Ctrl) and ADAP−/− mice were left unstimulated or CD3/CD28-stimulated for the indicated times (in min). Cells were lysed and probed for phospho-IKK (p-IKK) and IKKα/β. B–E, control and ADAP−/− T cells were left unstimulated (U or −) or stimulated with anti-CD3/CD28 (3/28 or +) or phorbol 12-myristate 13-acetate (P) for 15 min and lysed. B, PKCθ IPs were probed for IKKα/β and PKCθ. C, PKCθ IPs were probed for TAK1, ADAP, CARMA1, and PKCθ. D, TAK1 IPs were probed for ADAP, PKCθ, CARMA1, and TAK1. E, control hCAR+ T cells (Ctrl) and hCAR+ ADAP−/− T cells were first transduced with control adenovirus encoding Thy1.1 alone (Thy) or wild-type ADAP (WT) and cultured for 3 days prior to CD3/CD28 stimulation. PKCθ IPs were probed for ADAP, TAK1, CARMA1, and PKCθ.
A Novel Site in the C-terminal End of ADAP Is Critical for TAK1 Association
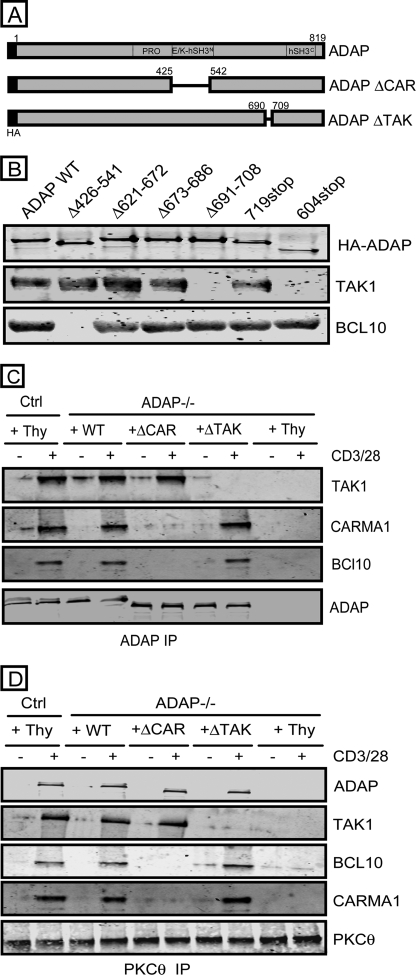
To define the region of ADAP critical for CD3/CD28-mediated association with TAK1, we expressed a series of HA-tagged ADAP truncation mutants in Jurkat T cells and tested their ability to co-IP TAK1 following CD3/CD28 stimulation. Both wild-type ADAP and mutant ADAP containing amino acids 1–719 were able to co-IP TAK1 in CD3/CD28-stimulated T cells (Fig. 2B). In contrast, an ADAP mutant containing amino acids 1–604 was not able to co-IP TAK1. This suggests that the region between amino acids 604 and 719 in ADAP is critical for TAK1 association. The region of ADAP critical for TAK1 association was further mapped to amino acids 691–708 within ADAP (amino acid sequence DASDFPPPPAEMSQGMSV) as a mutant lacking this region (ADAP ΔTAK) was unable to co-IP TAK1 following CD3/CD28 stimulation of Jurkat T cells (Fig. 2B).
FIGURE 2.
A site in the C-terminal end of ADAP is critical for TAK1 association. A, schematic representation of the CARMA1 binding ADAP mutant (ADAP ΔCAR) and TAK1 binding ADAP mutant (ADAP ΔTAK). PRO, proline-rich region, E/K, glutamic and lysine-rich region; hSH3, non-canonical helical SH3 domain. B, Jurkat T cells were transfected with the indicated HA-tagged ADAP constructs and stimulated with anti-CD3 monoclonal antibody OKT3 for 15 min. Anti-HA IPs were probed for HA-ADAP, TAK1, and BCL10. C, control hCAR+ T cells (Ctrl) and hCAR+ ADAP−/− T cells were transduced with adenovirus encoding Thy1.1 alone (Thy) or wild-type ADAP (WT), the ADAP ΔCAR mutant, or the ADAP ΔTAK mutant prior to CD3/CD28 stimulation for 15 min. ADAP IPs were probed for TAK1, CARMA1, BCL-10, and ADAP. D, control hCAR+ T cells (Ctrl) and hCAR+ ADAP−/− T cells were transduced and stimulated as in C. PKCθ IPs were probed for ADAP, TAK1, BCL10, CARMA1, and PKCθ.
We previously showed that a distinct ADAP mutant (ADAP ΔCAR lacking amino acids 426–541) is critical for ADAP association with CARMA1 and CD3/CD28-mediated assembly of the CBM complex in primary naive mouse T cells (20). In contrast to ADAP ΔTAK, immunoprecipitation of ADAP ΔCAR was able to co-IP TAK1 in both Jurkat T cells (Fig. 2B) and ADAP−/− T cells expressing the ADAP ΔCAR mutant (Fig. 2C). Conversely, only the ADAP ΔTAK mutant was able to co-IP BCL-10. Similar results were observed when we examined anti-ADAP IPs of ADAP−/− T cells expressing these mutants (Fig. 2C). Furthermore, expression of the ADAP ΔTAK mutant restored the recruitment of CARMA1 and BCL-10, but not TAK1, to PKCθ, whereas the ADAP ΔCAR mutant restored only the recruitment of TAK1 to PKCθ (Fig. 2D).
ADAP Mediates NF-κB Activation via IKKα/β Phosphorylation and IKKγ Ubiquitination
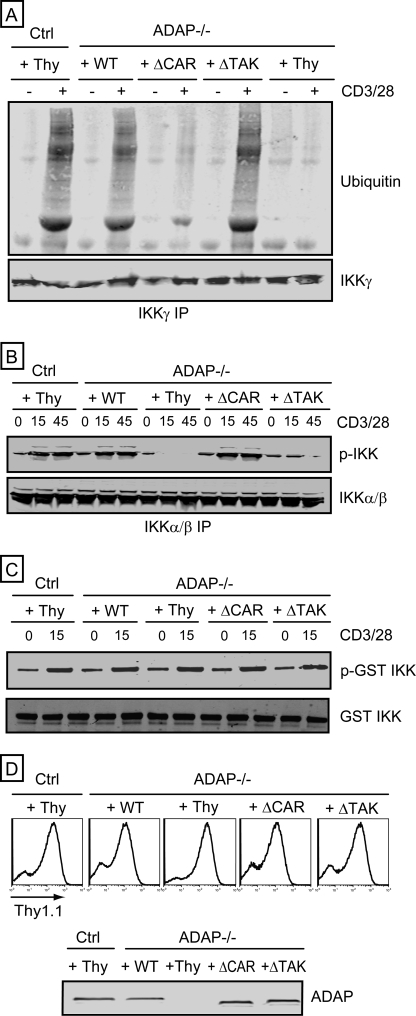
Activation of the IKK complex involves CARMA1-dependent ubiquitination of IKKγ and TAK1-dependent phosphorylation of IKKα/β (8–10). We found that CD3/CD28-mediated ubiquitination of IKKγ was dramatically impaired in ADAP−/− T cells (Fig. 3A). Expression of wild-type ADAP or ADAP ΔTAK in ADAP−/− T cells restored CD3/CD28-mediated ubiquitination of IKKγ to levels observed with control T cells, whereas ADAP ΔCAR was unable to restore IKKγ ubiquitination (Fig. 3A). Expression of wild-type ADAP in ADAP−/− T cells also restored CD3/CD28-mediated IKKα/β phosphorylation (Fig. 3B). Although ADAP ΔTAK was able to restore IKKγ ubiquitination, this mutant was unable to restore IKKα/β phosphorylation (Fig. 3B). In contrast, expression of ADAP ΔCAR in ADAP−/− T cells restored CD3/CD28-mediated phosphorylation of IKKα/β (Fig. 3B). Thus, distinct sites within ADAP independently control CARMA1-dependent IKKγ ubiquitination and TAK1-dependent IKKα/β phosphorylation.
FIGURE 3.
Independent control of IKKγ ubiquitination and IKKα/β phosphorylation by ADAP. Control hCAR+ T cells (Ctrl) and hCAR+ ADAP−/− T cells were transduced with adenovirus encoding Thy1.1 alone (Thy) or wild-type ADAP (WT), the ADAP ΔCAR mutant, or the ADAP ΔTAK mutant prior to CD3/CD28 stimulation for 15 min (A and C) or for 15 or 45 min (B). A, IKKγ IPs were probed for ubiquitin and IKKγ. B, IKKα/β IPs were probed for phosphorylated IKK and IKK. C, in vitro kinase assays were performed with TAK1 IPs. Phosphorylation of GST·IKK was assessed by Western blotting with an anti-phospho-IKK antibody (p-GST IKK). Samples were also probed with an anti-IKK antibody (GST IKK). D, flow cytometry analysis of T cells infected with adenovirus with an anti-Thy1.1 antibody, which detects the Thy1.1 cell surface protein expressed by all recombinant adenoviruses used in this study. Cell lysates were also immunoblotted with an anti-ADAP antibody to confirm ADAP expression (bottom).
We also assessed the role of ADAP in CD3/CD28-mediated increases in TAK1 enzymatic activity. CD3/CD28 stimulation of both wild-type and ADAP−/− T cells resulted in similar increases in TAK1 activity in TAK1 IPs, as assessed by in vitro phosphorylation of a GST·IKK fusion protein (Fig. 3C). Expression of wild-type ADAP, the ADAP ΔTAK mutant, or the ADAP ΔCAR mutant did not alter CD3/CD28-mediated activation of TAK1. These results suggest that CD3/CD28-mediated activation of TAK1 in T cells does not require ADAP expression. Fig. 3D demonstrates that all cell samples analyzed were infected with recombinant adenovirus, as assessed by flow cytometric analysis of expression of the Thy1.1 expression marker encoded by our recombinant adenovirus. In addition, Western blotting analysis demonstrates expression of wild-type ADAP and ADAP mutants at levels comparable with ADAP expression in control wild-type T cells.
Both the TAK1 Binding Site and the CARMA1 Binding Site in ADAP Are Required for IκB Phosphorylation and Degradation and NF-κB Nuclear Translocation
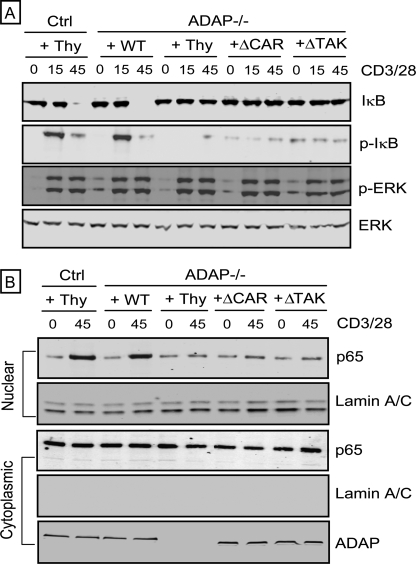
To determine whether both the TAK1 binding site and the CARMA1 binding site in ADAP are important for NF-κB activation, we analyzed IκBα phosphorylation and degradation, as well as nuclear translocation of NF-κB. Each of these sites is independently critical for full activation of NF-κB as expression of either the ADAP ΔTAK or the ADAP ΔCAR mutant in ADAP−/− T cells did not restore CD3/CD28-mediated IκBα phosphorylation and degradation (Fig. 4A). Impaired nuclear translocation of p65 in ADAP−/− T cells was also not restored by expression of either the ADAP ΔTAK or the ADAP ΔCAR mutant (Fig. 4B). CD3/CD28-mediated phosphorylation of Erk was comparable in all samples analyzed, demonstrating that CD3/CD28-mediated signaling was not globally impaired (Fig. 4A).
FIGURE 4.
The ADAP ΔCAR and ADAP ΔTAK mutants are each unable to restore CD3/CD28-mediated IkBα phosphorylation and degradation and p65 nuclear translocation. Control hCAR+ T cells (Ctrl) and hCAR+ ADAP−/− T cells were transduced with adenovirus encoding Thy1.1 alone (Thy) or wild-type ADAP (WT), the ADAP ΔCAR mutant, or the ADAP ΔTAK mutant prior to CD3/CD28 stimulation for 15 or 45 min. A, lysates were immunoblotted with antibodies specific for IkBα, phospho-IκB (p-IκB), phospho-Erk (p-ERK), and Erk. B, nuclear and cytoplasmic extracts were analyzed by immunoblotting with antibodies specific for p65 and the nuclear marker lamin A/C. Cytoplasmic extracts were also probed with an anti-ADAP antibody to verify expression of ADAP.
DISCUSSION
In this study, we have defined the mechanism by which the adapter protein ADAP regulates NF-κB activation in T cells. Because our previous work showed that ADAP associates with CARMA1 and regulates CD3/CD28-mediated assembly of the CBM complex (20), we analyzed signaling responses that are required for IKK complex activation. Ubiquitination of the IKKγ regulatory subunit has been shown to be dependent on CARMA1 expression (11). Consistent with this earlier work, we demonstrate here that ADAP−/− T cells exhibit impaired IKKγ ubiquitination following CD3/CD28 stimulation. In addition, expression of the ADAP ΔCAR mutant, which does not interact with CARMA1 (20), in ADAP−/− T cells cannot restore CD3/CD28-mediated IKKγ ubiquitination. These results highlight the critical role that ADAP plays in CARMA1-dependent activation of IKK and are consistent with other evidence linking CARMA1 to IKKγ ubiquitination (11).
We also revealed that CD3/CD28-mediated recruitment of TAK1 to the PKCθ signalosome is dependent on ADAP, and TAK1 can also be co-immunoprecipitated with ADAP. We traced this activity to a small region in the C-terminal end of murine ADAP between amino acids 691 and 708 (amino acid sequence DASDFPPPPAEMSQGMSV) that is highly conserved in the human, monkey, and chicken orthologs of ADAP. TAK1 has been proposed to play a key role in activating IKK by phosphorylating IKKα/β (9, 10). However, a requirement for TAK1 in T cell receptor-mediated activation of NF-κB appears to be dependent at least in part on the differentiation state of the T cell as NF-κB activation is impaired in TAK1-deficient thymocytes and mature single-positive thymocytes but not in TAK1-deficient effector T cells (14, 15). The expression system in this study utilized recombinant adenovirus and transgenic ADAP−/− mice expressing the hCAR receptor (20, 21). Because this system allows for efficient transduction of primary, non-cycling naive T cells (22), our findings are consistent with a requirement for TAK1 in NF-κB activation in naive T cells.
Regulated recruitment of the IKK complex and proteins that regulate IKK complex activation to PKCθ is a critical early step in CD3/CD28-mediated activation of NF-κB (1, 3, 25). 3-Phosphoinositide-dependent kinase 1 (PDK1) has been proposed to play a dual role in this recruitment by regulating PKCθ-mediated recruitment of the IKK complex and the recruitment of CARMA1 to the PKCθ signalosome (26). ADAP also appears to play a dual role in regulating recruitment of NF-κB signaling proteins to the PKCθ signalosome as distinct sites in ADAP are critical for the recruitment of TAK1 and CARMA1 to PKCθ. Although TAK1 and CARMA1 have been proposed to interact with each other, TAK1 recruitment to PKCθ and IKKα/β phosphorylation is not altered in CARMA1-deficient T cells (11, 16, 17). We suggest that ADAP plays a central role in this CARMA1-independent recruitment of TAK1 to PKCθ. PDK1 is also critical for the phosphorylation and activation of PKCθ (26). In contrast, our earlier studies demonstrated that CD3/CD28-mediated phosphorylation of PKCθ is not dependent on ADAP (20). This is consistent with our findings in this study of the normal recruitment of IKK to PKCθ in ADAP−/− T cells. Similarly, loss of ADAP does not alter CD3/CD28-mediated activation of TAK1. Thus, these results suggest that ADAP functions to recruit active TAK1 to the PKCθ signalosome, where it can then promote the activation of the IKK complex by phosphorylating IKKα/β.
Our identification of sites within ADAP that independently control association with CARMA1 and TAK1 provide a biochemical basis for the independent control of CARMA1-mediated IKKγ ubiquitination and TAK1-dependent IKKα/β phosphorylation in T cells (1, 11, 25). Both of these sites are critical for NF-κB signaling as expression of either the ADAP ΔTAK or the ADAP ΔCAR mutant was not able to restore CD3/CD28-mediated phosphorylation and degradation of IκBα, as well as subsequent nuclear translocation of p65, in ADAP−/− T cells. Thus, our results support a central role for ADAP in regulating both CARMA1-dependent IKKγ ubiquitination and TAK1-mediated IKKα/β phosphorylation, two key events that lead to IKK complex activation and NF-κB activation in T cells.
Acknowledgments
We thank Dr. E. Peterson for providing reagents and for thoughtful discussions, Dr. J. S. Mitchell for technical assistance and intellectual input, T. Lee, M. Schwartz, N. Rahman, and D. Loughran for mouse genotype and colony maintenance, and Dr. C. Weaver for the hCAR transgenic mice.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 AI038474 (to Y. S.).
- IKK
- IκB kinase
- ADAP
- adhesion- and degranulation-promoting adapter protein
- PKC
- protein kinase C
- CARMA1
- caspase recruitment domain membrane-associated guanylate kinase protein 1
- BCL-10
- B-cell lymphoma 10
- CBM
- CARMA1-BCL10-MALT1
- TAK1
- TGF-β-activated kinase 1
- TGF
- transforming growth factor
- hCAR
- human coxsackie-adenovirus receptor
- Erk
- extracellular signal-regulated kinase
- HA
- hemagglutinin
- IP
- immunoprecipitate.
REFERENCES
- 1.Vallabhapurapu S., Karin M. (2009) Annu. Rev. Immunol. 27, 693–733 [DOI] [PubMed] [Google Scholar]
- 2.Hayden M. S., West A. P., Ghosh S. (2006) Oncogene 25, 6758–6780 [DOI] [PubMed] [Google Scholar]
- 3.Khoshnan A., Bae D., Tindell C. A., Nel A. E. (2000) J. Immunol. 165, 6933–6940 [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto R., Wang D., Blonska M., Li H., Kobayashi M., Pappu B., Chen Y., Wang D., Lin X. (2005) Immunity 23, 575–585 [DOI] [PubMed] [Google Scholar]
- 5.Sommer K., Guo B., Pomerantz J. L., Bandaranayake A. D., Moreno-García M. E., Ovechkina Y. L., Rawlings D. J. (2005) Immunity 23, 561–574 [DOI] [PubMed] [Google Scholar]
- 6.Thome M. (2004) Nat. Rev. Immunol. 4, 348–359 [DOI] [PubMed] [Google Scholar]
- 7.Ruland J., Duncan G. S., Elia A., del Barco Barrantes I, Nguyen L., Plyte S., Millar D. G., Bouchard D., Wakeham A., Ohashi P. S., Mak T. W. (2001) Cell 104, 33–42 [DOI] [PubMed] [Google Scholar]
- 8.Zhou H., Wertz I., O'Rourke K., Ultsch M., Seshagiri S., Eby M., Xiao W., Dixit V. M. (2004) Nature 427, 167–171 [DOI] [PubMed] [Google Scholar]
- 9.Sun L., Deng L., Ea C. K., Xia Z. P., Chen Z. J. (2004) Mol. Cell 14, 289–301 [DOI] [PubMed] [Google Scholar]
- 10.Wang C., Deng L., Hong M., Akkaraju G. R., Inoue J., Chen Z. J. (2001) Nature 412, 346–351 [DOI] [PubMed] [Google Scholar]
- 11.Shambharkar P. B., Blonska M., Pappu B. P., Li H., You Y., Sakurai H., Darnay B. G., Hara H., Penninger J., Lin X. (2007) EMBO J. 26, 1794–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruland J., Duncan G. S., Wakeham A., Mak T. W. (2003) Immunity 19, 749–758 [DOI] [PubMed] [Google Scholar]
- 13.Wang D., You Y., Case S. M., McAllister-Lucas L. M., Wang L., DiStefano P. S., Nuñez G., Bertin J., Lin X. (2002) Nat. Immunol. 3, 830–835 [DOI] [PubMed] [Google Scholar]
- 14.Liu H. H., Xie M., Schneider M. D., Chen Z. J. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 11677–11682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wan Y. Y., Chi H., Xie M., Schneider M. D., Flavell R. A. (2006) Nat. Immunol. 7, 851–858 [DOI] [PubMed] [Google Scholar]
- 16.Shinohara H., Yasuda T., Aiba Y., Sanjo H., Hamadate M., Watarai H., Sakurai H., Kurosaki T. (2005) J. Exp. Med. 202, 1423–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCully R. R., Pomerantz J. L. (2008) Mol. Cell. Biol. 28, 5668–5686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson E. J., Woods M. L., Dmowski S. A., Derimanov G., Jordan M. S., Wu J. N., Myung P. S., Liu Q. H., Pribila J. T., Freedman B. D., Shimizu Y., Koretzky G. A. (2001) Science 293, 2263–2265 [DOI] [PubMed] [Google Scholar]
- 19.Griffiths E. K., Krawczyk C., Kong Y. Y., Raab M., Hyduk S. J., Bouchard D., Chan V. S., Kozieradzki I., Oliveira-Dos-Santos A. J., Wakeham A., Ohashi P. S., Cybulsky M. I., Rudd C. E., Penninger J. M. (2001) Science 293, 2260–2263 [DOI] [PubMed] [Google Scholar]
- 20.Medeiros R. B., Burbach B. J., Mueller K. L., Srivastava R., Moon J. J., Highfill S., Peterson E. J., Shimizu Y. (2007) Science 316, 754–758 [DOI] [PubMed] [Google Scholar]
- 21.Burbach B. J., Srivastava R., Medeiros R. B., O'Gorman W. E., Peterson E. J., Shimizu Y. (2008) J. Immunol. 181, 4840–4851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurez V., Dzialo-Hatton R., Oliver J., Matthews R. J., Weaver C. T. (2002) BMC. Immunol. 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medeiros R. B., Dickey D. M., Chung H., Quale A. C., Nagarajan L. R., Billadeau D. D., Shimizu Y. (2005) Immunity 23, 213–226 [DOI] [PubMed] [Google Scholar]
- 24.Fostel L. V., Dluzniewska J., Shimizu Y., Burbach B. J., Peterson E. J. (2006) Int. Immunol. 18, 1305–1314 [DOI] [PubMed] [Google Scholar]
- 25.Schulze-Luehrmann J., Ghosh S. (2006) Immunity 25, 701–715 [DOI] [PubMed] [Google Scholar]
- 26.Lee K. Y., D'Acquisto F., Hayden M. S., Shim J. H., Ghosh S. (2005) Science 308, 114–118 [DOI] [PubMed] [Google Scholar]