Abstract
Voltage-dependent anion channels (VDACs) are a family of small pore-forming proteins of the mitochondrial outer membrane found in all eukaryotes. VDACs play an important role in the regulated flux of metabolites between the cytosolic and mitochondrial compartments, and three distinct mammalian isoforms have been identified. Animal and cell culture experiments suggest that the various isoforms act in disparate roles such as apoptosis, synaptic plasticity, learning, muscle bioenergetics, and reproduction. In Drosophila melanogaster, porin is the ubiquitously expressed VDAC isoform. Through imprecise excision of a P element insertion in the porin locus, a series of hypomorphic alleles have been isolated, and analyses of flies homozygous for these mutant alleles reveal phenotypes remarkably reminiscent of mouse VDAC mutants. These include partial lethality, defects of mitochondrial respiration, abnormal muscle mitochondrial morphology, synaptic dysfunction, and male infertility, which are features often observed in human mitochondrial disorders. Furthermore, the observed synaptic dysfunction at the neuromuscular junction in porin mutants is associated with a paucity of mitochondria in presynaptic termini. The similarity of VDAC mutant phenotypes in the fly and mouse clearly indicate a fundamental conservation of VDAC function. The establishment and validation of a new in vivo model for VDAC function in Drosophila should provide a valuable tool for further genetic dissection of VDAC role(s) in mitochondrial biology and disease, and as a model of mitochondrial disorders potentially amenable to the development of treatment strategies.
Keywords: Bioenergetics/Respiratory Chain, Metabolism/Energy, Organisms/Drosophila, Subcellular Organelles/Mitochondria, Neuron, VDAC, Male Infertility, Mitochondrial Disease, Porin
Introduction
The voltage-dependent anion channel (VDAC)2 is an integral membrane protein present in the mitochondrial outer membrane. VDAC is a monomeric, voltage-gated channel that allows passage of molecules up to 5,000 daltons (1), and multiple isoforms have been identified in numerous eukaryotic species (2–5). VDAC provides the predominant pathway for small metabolites such as ATP, ADP, phosphocreatine and small ions across the mitochondrial outer membrane (1, 6). The mechanism of channel permeation appears to reflect large changes in protein conformation and channel charge rather than a physical restriction to ion flow (7). In addition to its role in energy metabolism, VDAC has been implicated in various basic cellular and developmental processes such as cytochrome c-dependent apoptosis (8, 9) and the mitochondrial permeability transition pore (10, 11). These potential roles in fundamental cellular activities underscore the importance of elucidating VDAC biological functions to better understand mitochondrial functions.
Studies in mammalian model systems have detected functional differences in various VDAC isoforms. Mice deficient for Vdac1 and Vdac3 have distinct phenotypes, with Vdac1−/− mice exhibiting partial embryonic lethality (11), as well as abnormal respiratory chain activities and mitochondrial morphology in muscle (12). Vdac3−/− mice demonstrate sperm motility defects and, similarly, abnormal respiratory chain activities and mitochondrial morphology in muscle (13). In addition, Vdac1−/−, Vdac3−/−, and Vdac1/3−/− double mutants all exhibit defects in associative and spatial learning that correlate with electrophysiological deficits in synaptic plasticity (11). Despite these studies implicating VDAC in neuromuscular function and spermatogenesis, the specific cellular and developmental roles of the various VDAC isoforms, as well as the proteins or pathways with which VDACs interact, are not yet well understood. The use of genetic model systems such as Drosophila melanogaster offers the possibility of gaining additional in vivo insights into VDAC functions, its intracellular interactions, and its potential as a therapeutic target.
D. melanogaster contains a cluster of four genes (porin, CG17137 (Porin2), CG17139, and CG17140) that encode proteins that are homologous to known VDACs (14–16). porin exhibits the greatest homology to mammalian VDACs and is ubiquitously expressed in the fruit fly, whereas the other three fly VDACs have a more spatially restricted expression pattern and are predominantly present in the male reproductive tract (17). When expressed in VDAC-deficient yeast, porin and CG17137 can rescue a conditional lethal phenotype, whereas the other two cannot, demonstrating functional complementation for a subset of these genes (7). Given its homology to mammalian VDACs and its ubiquitous expression pattern, it was hypothesized that porin represents the predominant functional ortholog of VDAC in Drosophila. This hypothesis predicts that mutant porin phenotypes should exhibit significant similarities to mammalian mutant VDAC phenotypes. In this study, analysis of mutant porin phenotypes reveals a striking concordance with mammalian mutant VDAC phenotypes. In addition, the demonstration of a secondary respiratory chain deficiency and abnormal distribution of mitochondria within motor neurons of porin mutants provides new insights into the phenotypic consequences of VDAC deficiency, illustrating the potential of Drosophila as a useful model system to study mitochondrial function and disease.
EXPERIMENTAL PROCEDURES
Fly Stocks
Unless mentioned elsewhere, all primary stocks described were obtained from the Bloomington Drosophila Stock Center. All stocks were maintained at room temperature using standard cornmeal-molasses-yeast media. “yw” indicates the genotype y1 w67c23.
P Element Excision
The P element line y w; porinEY2333 (18) was crossed to y w; L/CyO; Ki1 Δ2–3 (provided by Hugo Bellen, Baylor College of Medicine) to induce excisions, and 400 lines were generated. One allele, porinRev8 failed to complement porinl(2)k05123 (19) and was balanced over SM6b. Long range PCR demonstrates that this imprecise excision event results in an insertion of ∼7 kb (supplemental Fig. S1C). Three other alleles (porinEx75, porinEx78, and porinEx365) were identified as deletions from imprecise excisions by PCR analysis, and the breakpoints identified by DNA sequencing of the deletion junction fragments. In addition, two other alleles (porinEx341 and porinExS27) were established as independent precise excision controls and verified by DNA sequencing across the parental P element insertion site (supplemental Fig. S1).
Transgenic Rescue
A cDNA (from Berkley Drosophila Genome Project clone GM13853) (17) containing the wild type full coding sequence of porin was cloned into the P element transformation vector pUAST and injected into yw embryos; transgenic lines were established, and ectopic wild type porin was expressed in porin mutant background using the GAL4-UAS system (20). The GAL4 drivers were the ubiquitous Tubulin-GAL4 (21), the neuronal ELAV-GAL4 (22), and c135-GAL4, which is expressed in the male reproductive tract (23).
Molecular Biology
Whole fly lysates or dissected testes lysates were used for Western blot analyses that were performed as described previously (17).
Lifespan Analysis
For measuring lifespan, flies of indicated genotypes were isolated on the day of eclosion and placed into same-sex cohorts of five flies per vial. Each day the number of surviving flies was recorded until all flies had died. The flies were placed in fresh vials three times per week (Monday, Wednesday, and Friday) during the entire test.
Mitochondrial Polarography and Enzymology
Polarographic experiments were performed as described previously (24) with the following changes. Intact mitochondria were isolated by differential centrifugation from fresh homogenates of three-day-old adult flies, with the homogenates first filtered through cheesecloth to remove residual particulate remains of the exoskeleton. Oxygen consumption of mitochondria was measured in a 650-μl chamber fitted with a Clark microelectrode (YSI Life Sciences), recorded using a PowerLab electronic data recorder, and analyzed using LabChart (AD Instruments).
For enzymologic assays of respiratory chain complexes I–IV, sufficient third instar larval stage larvae were collected to fill a 1.5-ml Eppendorf tube to 50 μl. Potassium phosphate buffer (25 mm, pH 7.5) was added to a final volume of 300 μl, and the larvae were sonicated (5-s pulse ×4, 60% power) using a Microson XL2000 Ultrasonic Cell Disruptor (Misonix). The spectrophotometric kinetic assays were performed at 30 °C in a volume of 100 μl using a monochromator microplate reader (Tecan M200). Complex I activity (NADH:ubiquinone oxidoreductase) was determined by measuring oxidation of NADH at 340 nm (using ferricyanide as the electron acceptor) in a reaction mixture of 25 mm potassium phosphate (pH 7.5), 0.2 mm NADH, and 1.7 mm potassium ferricyanide. Complex II activity (succinate dehydrogenase) was determined by measuring the reduction of the artificial electron acceptor 2,6-dichlorophenol-indophenol at 600 nm in a reaction mixture of 25 mm potassium phosphate (pH 7.5), 20 mm succinate, 0.5 mm 2,6-dichlorophenol-indophenol, 10 μm rotenone, 2 μg/ml antimycin A, and 2 mm potassium cyanide. Complex III activity (ubiquinol:cytochrome c oxidoreductase) was determined by measuring the reduction of cytochrome c at 550 nm in a reaction mixture of 25 mm potassium phosphate (pH 7.5), 35 μm reduced decylubiquinone, 15 μm cytochrome c, 10 μm rotenone, and 2 mm potassium cyanide. Complex IV activity (cytochrome c oxidase) was determined by measuring the oxidation of cytochrome c at 550 nm in a reaction mixture of 10 mm potassium phosphate (pH 7.5) and 0.1 mm reduced cytochrome c. Citrate synthase activity was determined by measuring the reduction of 5,5′-dithiobis(2-nitrobenzoic acid) at 412 nm, which is coupled to the reduction of acetyl-CoA by citrate synthase in the presence of oxaloacetate. The reaction mixture consists of 10 mm potassium phosphate (pH 7.5), 100 μm 5,5′-dithiobis(2-nitrobenzoic acid), 50 μm acetyl-CoA, and 250 μm oxaloacetate. All activities were calculated as nmol/min/mg protein, normalized to citrate synthase activity and expressed as a percentage of wild type activity.
Transmission Electron Microscopy
For transmission electron microscopy of indirect flight muscle, the indirect flight muscle of 7-day-old flies was dissected in ice-cold 4% paraformaldehyde/1% glutaraldehyde/0.1 m cacodylic acid (pH 7.2). Fixation, post-fixation, staining, embedding, sectioning, and transmission electron microscopy were performed as described previously (25).
Fertility and Fecundity Tests
For male fertility tests, individual 3-day-old males of the indicated genotypes were mated with two virgin yw females. After 3 days, each vial was visually examined to ensure eggs had been laid and the parents were discarded. After 17 days (from the initial mating), the total number of adult progeny was recorded. A minimum of 20 males for each genotype was used for testing. For female fecundity and fertility tests, individual virgin females of the indicated genotypes were paired with single virgin yw males. For 21 consecutive days, each mating pair was transferred to a fresh vial. The vial from the previous 24-h period was visually inspected and the total number of eggs laid was recorded and summed over the 21-day period. Each vial was also saved for 17 days, and then the total number of adult progeny was recorded and summed over the 21-day period. From these data, the total number of eggs laid, the total number of adult progeny, and the ratio of total progeny to total number of eggs laid were determined. Ten females of each genotype were tested to obtain mean values for the above parameters.
Measurement of Testis Width
Testes from 3-day-old males of each indicated genotype were dissected in 1× phosphate-buffered saline and photographed using a Leica S8 stereomicroscope fitted with a Leica DC300 digital camera (Meyer Instruments). Images were captured using Leica IM50 image management software. An image of a ruler (1-mm units) at the same magnification as the testes (80×) was photographed to provide reference for measuring the absolute length. The images were analyzed using ImageTool for Windows (version 3.00) (University of Texas Health Science Center in San Antonio), and the maximal width for each testis was determined. A minimum of 14 testes of each genotype was measured.
Bang Sensitivity Assay
For the bang sensitivity assay, flies were anesthetized with CO2 and individually placed into 7-ml borosilicate glass scintillation vials (Thermo Fisher Scientific). Flies were allowed to recover from CO2 for 2–3 h, and then each vial was vortexed (maximal setting) for 10 s, and the time for the fly to right itself and resume normal behavior was recorded. Any trial in which the fly remained stunned for >30 s was terminated and recorded as 30 s. A minimum of 20 flies was tested for any particular genotype.
Locomotor Assay
For each genotype, 20 5-day-old male flies were placed into an empty 10-cm plastic vial. With the top of the vial plugged, the vertical distance from the bottom of the vial to the top was 7 cm. After a 2-h period of recovery from CO2 anesthesia, the vial was gently tapped once on the bench top to displace the flies to the bottom of the vial, and a digital camera recorded the movements of the flies for 6 s. Individual frames at 1-s intervals from 0 to 5 s were digitally captured and analyzed using ImageTool for Windows (version 3.00). The relative distance from the bottom of the vial for each fly at each time point was determined and used to obtain a mean distance for each group of flies at each time point. The assay was repeated for each genotype with a new cohort of male flies at least three times.
Electroretinograms (ERGs)
ERGs from flies immobilized with nail polish (Top Speed, Revlon) were recorded with a fine glass pipette filled with 3 m NaCl placed on the corneal surface of the fly's eye. The reference electrode was inserted into the thorax. Light flashes of 1 s were delivered from a 150-watt halogen lamp (Volpi), and field potential recordings were digitized and stored on a personal computer using Clampex software. At least two flies for each genotype were used to generate the ERG traces, and the amplitudes of the “on” and “off” transients as well as the sustained negative component were analyzed using Clampfit.
Larval NMJ Electrophysiology
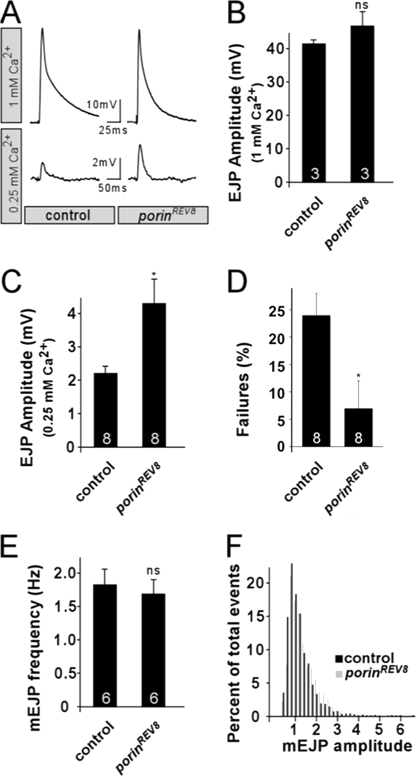
Larval electrophysiological recordings and temperature control were performed as described (26). Modified HL3 contained 110 mm NaCl, 5 mm KCl, 10 mm NaHCO3, 5 mm HEPES, 30 mm sucrose, 5 mm trehalose, and 20 mm MgCl2 (pH 7.2), and CaCl2 (concentrations indicated in Fig. 6). Excitatory junctional potential (EJP) recordings were made from muscles 6 or 7 using 90–110-megohm electrodes, and motor neurons were stimulated several times above threshold to ensure action potential initiation. For each animal, 60 EJPs were recorded, and the amplitudes were averaged to determine the individual EJP amplitude (observed failures at 0.25 mm Ca2+ were excluded). The mean EJP amplitude was determined from several animals (sample sizes indicated in bar graphs of Fig. 6).
FIGURE 6.
Abnormal NMJ neurotransmission in porin mutants. A, representative EJP traces recorded at 1 Hz at the indicated extracellular Ca2+ concentrations for both control (yw) and porinRev8 animals. B and C, mean EJP amplitudes recorded at 1 Hz at 1 mm Ca2+ in B or 0.25 mm Ca2+ in C. D, percentage of stimulations that failed to evoke EJP at 0.25 mm Ca2+. E and F, spontaneous release events (mEJPs) were recorded for both control (yw) and porinRev8 animals and expressed as quantification of mEJP frequency in E and amplitude distribution in F. Statistical significance was evaluated by Student's t test. *, p < 0.05, ns, not significant.
Immunofluorescence Microscopy
Staining protocols were performed as described (27): anti-DLG (mouse; 4F3) was used at a dilution of 1:50. Anti-GluRIII/IIC was a gift from A. Di Antonio (Washington University, St. Louis, MO) and was used at a dilution of 1:1,000. Secondary antibodies tagged with Cy3 (Jackson ImmunoResearch Laboratories) or Alexa 488 (Molecular Probes) were used at 1:250. All fluorescent images were captured using a Zeiss 510 confocal microscope and processed using Amira 2.2 and ImageJ. Z stacks of synapses were acquired as delimited by DLG fluorescence. Using ImageJ, all three fluorescent channels of each Z stack were analyzed as two-dimensional projections of maximal fluorescence and quantitated as percentage of voxels in each Z stack that had a detectable signal above background. GluRIII/IIC and mitoGFP fluorescence were normalized to DLG fluorescence. Two Z stacks each from three individual animals (i.e. six total) for both wild type and porinRev8 were used for the analysis.
RESULTS
Generation of a Hypomorphic Mutant porin Allelic Series
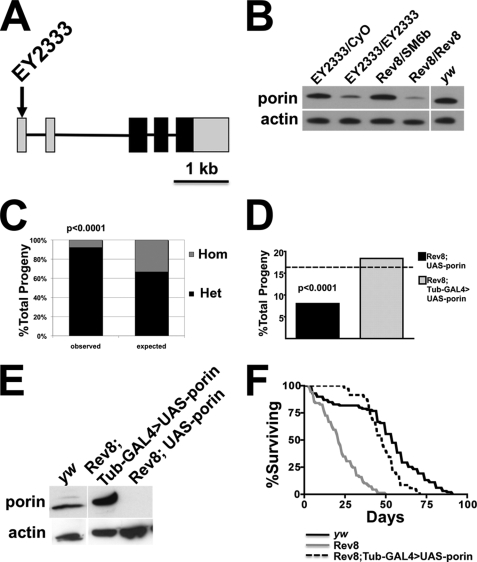
A P element insertion allele of porin (Fig. 1A), porinEY2333, was obtained from the Drosophila Gene Disruption Project (18). A series of hypomorphic alleles were derived from porinEY2333 by imprecise P element excision (28). Long range PCR analysis of one of these excision alleles, porinRev8, revealed an insertion of ∼7 kb in the porin locus (supplemental Fig. S1C), whereas PCR analysis of three other alleles demonstrate deletions involving the alternatively spliced 5′-untranslated exons and the first intron (supplemental Fig. S1, A and B). Sequence analysis of all alleles demonstrated intact coding exons. Western blot analysis of these alleles demonstrated severe reduction in Porin expression levels (Fig. 1B and supplemental Fig. S2). Based on the low residual amount of porin protein in porinRev8 mutants, this allele was chosen for further study.
FIGURE 1.
porin-deficient flies exhibit partial developmental lethality and reduced lifespan. A, the genomic organization of the porin locus is indicated in the schematic. The arrow indicates the insertion point of the P element in the porin gene. The black boxes indicate protein-coding exonic sequences, and the gray boxes indicate untranslated exonic regions (UTR). B, Western blot analysis of whole fly lysates of the indicated genotypes is shown. Blots were sequentially probed with Porin and actin antisera. CyO and SM6b are balancer chromosomes for chromosome 2 present in porin mutant heterozygotes. C, heterozygous porin mutant intercross was performed and the porin genotype of 457 adult F1 progeny was inferred from the presence or absence of the balancer chromosome dominant marker. The stacked bar graph shows observed F1 genotype proportions compared with the expected Mendelian ratio for wild type porin. Hom, porinRev8/porinRev8; Het, porinRev8/SM6b. D, a cross of porin mutant heterozygotes harboring either a wild type UAS-porin or Tubulin-GAL4 (Tub-GAL4) (21) transgenes was performed. The bar graph shows the observed proportions of porinRev8 homozygotes harboring the UAS_porin transgene with or without the Tubulin GAL4 driver (Tub_GAL4) (total number of F1 progeny observed, 685). The dashed line indicates the expected frequency of both genotypes for wild type porin. Statistical significance for C and D was evaluated by Chi square analysis. E, a Western blot of whole-fly lysates of the indicated genotypes is shown to demonstrate restoration of porin levels by ectopic porin expression. F, survival curves for wild type (yw, n = 99), homozygous mutant (porinRev8, n = 69), and rescued homozygous mutant (porinRev8;Tub-GAL4>UAS-porin, n = 35) are shown. Rev8, porinRev8.
porin Mutants Exhibit Partial Lethality and Reduced Viability
porinRev8 homozygous mutants are semilethal because significantly fewer than expected adult homozygotes were observed (Fig. 1C). Homozygotes progress through embryogenesis and early larvogenesis, but ∼75% fail to successfully transition from late larvogenesis to pupation and eclosion (data not shown). This partial lethality is specific to porin deficiency because homozygotes that ectopically express wild type porin are rescued into adulthood (Fig. 1, D and E). Adult porinRev8 homozygotes also exhibit reduced lifespans, and this phenotype is similarly rescued by ectopic porin expression (Fig. 1, E and F).
porin-deficient Mitochondria Exhibit Respiratory Defects
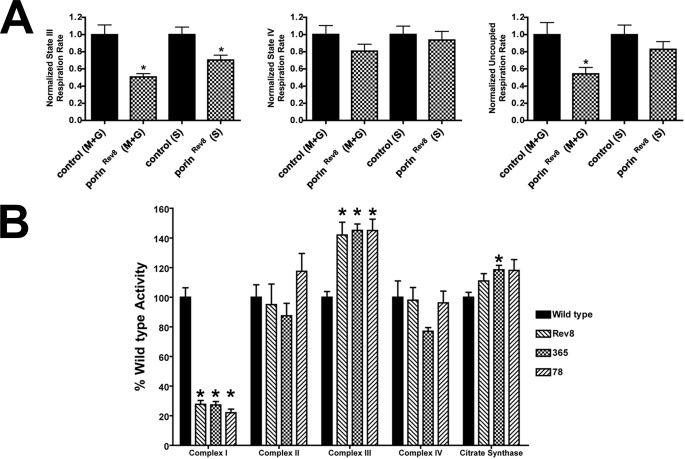
To examine the consequences of Porin deficiency on mitochondrial respiration, oxygen consumption in the presence of oxidizable substrates and ADP was measured from fresh mitochondria isolated from wild type and porinRev8 flies (Fig. 2A and supplemental Table S1). Mitochondria from porinRev8 mutants demonstrated a global respiratory defect, with significantly decreased ADP-stimulated oxygen consumption (“state III” respiration) in the presence of either complex I or II-dependent substrates. When activities of individual respiratory chain complexes were measured, porin mutants exhibited a significant partial deficiency of complex I activity (∼30% of wild type activity) as well as an increase of complex III activity (∼140% of wild type activity) (Fig. 2B and supplemental Table S2). These results demonstrate that in addition to decreased mitochondrial outer membrane permeability, Porin deficiency leads to perturbations in respiratory chain function and thus is valid a model for secondary respiratory chain deficiency.
FIGURE 2.
porin mutants have defects of mitochondrial respiration and complex I activities. A, normalized ADP-stimulated (State III), ADP-limiting (State IV), and uncoupled oxygen consumption rates of isolated mitochondria from control (yw) and porinRev8 flies are presented. The oxidizable substrates used in the assay were either malate and glutamate (M+G) or succinate (S). *, p < 0.05 by Student's t test with Welch correction. B, normalized activities from larval lysates of the individual respiratory chain complexes I–IV as well as citrate synthase are presented. Wild type, porinEx341; Rev8, porinRev8; 365, porinEx365; 78, porinEx78. *, p < 0.001 by Student's t test with Welch correction (versus wild type). Error bars represent S.E.
porin Mutants Exhibit Defects of Fecundity and Fertility
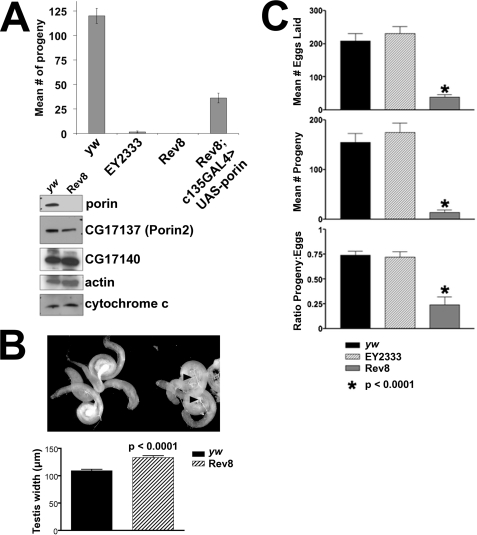
Mutant porin flies have reduced fertility. porinEY2333 male homozygotes are subfertile, whereas porinRev8 male homozygotes are absolutely infertile (no fertile cross has been observed after testing more than 1,000 individual porinRev8 males). This infertility is rescued by ectopic expression of porin cDNA in the testes using the c135-GAL4 driver, which is expressed both in somatic cyst cells and spermatocytes (Fig. 3A) (23). Importantly, expression of other Drosophila VDAC isoforms present in the testis (CG17137 (Porin2) and CG17140) is not significantly altered in porinRev8 mutants (Fig. 3A). The other hypomorphic mutant porin alleles also demonstrate decreased fertility (supplemental Fig. S3B). Similar to the infertile mutant described by Oliva et al. (16), these mutants exhibit normal testes morphology, with normal appearing Nebenkern bodies by differential interference microscopy, yet they produce immotile sperm (data not shown). Further examination of dissected male reproductive tracts revealed that in porinRev8 male homozygotes the seminal vesicles are small and devoid of sperm, whereas the testes themselves are enlarged ∼25% compared with wild type controls (Fig. 3B). In addition, although female porinEY2333 homozygotes have normal fecundity and fertility, female porinRev8 homozygotes have severely reduced fecundity and fertility (Fig. 3C).
FIGURE 3.
porin hypomorphic mutant homozygotes are infertile/subfertile. A, male fertility assay. Bar graph shows mean number of progeny observed from mating individual males of the indicated genotypes with yw virgin females. c135-GAL4 is a GAL4 driver that is expressed throughout the male reproductive tract (23). Error bars represent S.E. Sample sizes: yw (n = 21), porinEY2333 (n = 51), porinRev8 (n = 81), rescued porinRev8 (n = 74). Western blot of testis lysates from males of the indicated genotypes is also shown. B, a representative dissected male reproductive tract is shown for yw (right) and porinRev8 (left). A bar graph indicating the mean greatest width of testes for both genotypes is shown below the picture. Error bars represent S.E. Sample sizes: yw (n = 15), porinRev8 (n = 14). C, Female fecundity/fertility assays. Bar graphs show mean number of eggs laid (top), mean number of adult progeny (middle), and mean ratio of progeny to eggs laid (bottom) observed for females of the indicated genotypes mated with yw males. Statistical significance evaluated by Student's t test with Welch correction (compared with yw). Error bars represent S.E. Sample sizes: yw (n = 10), porinEY2333 (n = 9), and porinRev8 (n = 9). EY2333, porinEY2333; Rev8, porinRev8.
porin Mutants Demonstrate Neurological and Muscular Abnormalities
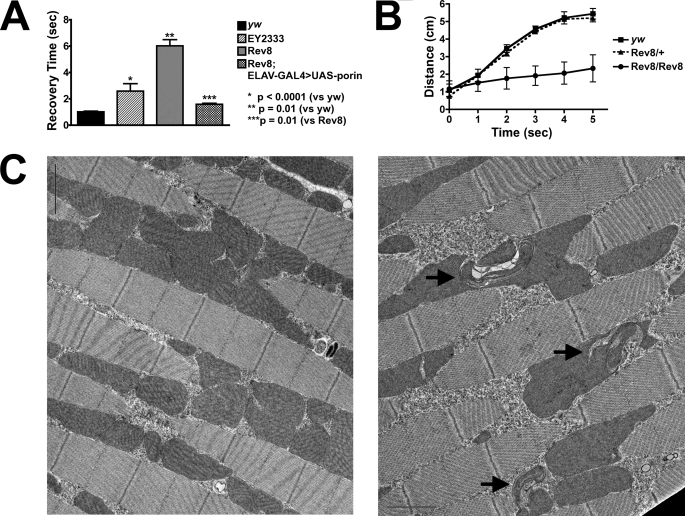
“Bang-sensitive” or stress-sensitive mutants are a phenotypic class of mutants that exhibit temporary paralysis when exposed to mechanical stress (29–32). This phenotype has been associated with mutations in genes involved in neuronal function as well as mitochondrial integrity (33–38). Two stress-sensitive mutants have been identified as nuclear-encoded mitochondrial genes: stress sensitive B (sesB), encoding the fly ortholog of the adenine nucleotide translocase (39), and technical knock-out (tko), encoding a subunit of the mitochondrial ribosome (40). Furthermore, mutations in the gene encoding Drp1, a protein involved in mitochondrial fission, also cause bang sensitivity (41). Analysis of mutant porin homozygotes revealed that these mutants also exhibit enhanced bang sensitivity, with hypomorphic porinEY2333 homozygotes demonstrating a 2–3-fold increase in recovery time and imprecise excision mutant porin homozygotes showing at least a 6–7-fold increase in recovery time compared with wild type. Ectopic expression of wild type porin in the central nervous system (CNS) of mutant homozygotes rescues this abnormal phenotype (Fig. 4A and supplemental Fig. S3A). This defect is consistent with a defect in mitochondrial function.
FIGURE 4.
porin mutants demonstrate neurologic dysfunction and abnormal muscle mitochondria. A, bang sensitivity assay. The mean recovery time in seconds for each of the indicated genotypes is depicted. ELAV-GAL4 is a CNS-specific GAL4 driver (22). The error bars represent S.E. Statistical significance was evaluated by the Student's t test with Welch correction. Sample size for each genotype: yw (n = 50); porinEY2333 homozygotes (n = 20); porinRev8 homozygotes (n = 93); and rescued porinRev8 homozygotes (n = 78). B, locomotion assay. Graph depicts distance traveled from bottom of vial over time for flies of the indicated genotypes. Each data point represents the average median distance for a population of 20 males. The error bars represent one S.D. for three to four replicates. EY2333, porinEY2333; Rev8, porinRev8. C, a representative EM section from indirect flight muscle for wild type (left) and mutant (right) is shown. The black arrows in the mutant EM section indicate mitochondria with abnormal cristae.
In light of the pathological changes in muscle structure and function observed in Vdac1−/− mice (12) as well as in several Drosophila mutants that affect mitochondrial integrity (42–46), the possibility of muscle mitochondrial pathology and dysfunction was also examined. Electron microscopy of indirect flight skeletal muscle from 7-day-old flies revealed that mitochondria in porin mutant homozygotes appear larger than the wild type. Additionally, these mitochondria exhibit abnormal morphology, with regions of striking patterning of cristae (Fig. 4C). This abnormal morphology was not observed in age-matched control fly muscle (3.5 ± 0.4 mitochondrial inclusions per field (mean ± S.E.) for mutant; no inclusions were observed in wild type fields (n = 8 for wild type; n = 15 for mutant)). Interestingly, this abnormal cristae patterning appears very similar to recently described mitochondrial “swirls” that reportedly represent early stages of mitochondrial degeneration. These swirls are observed in flies as a function of aging and accumulate more rapidly when flies are reared in hyperoxic conditions (47). To grossly assess overall muscle function, normal locomotion of young adult control flies was quantified and compared with age-matched mutant flies (Fig. 4B). Homozygous mutant flies clearly exhibit a locomotor defect when compared with heterozygotes or wild type controls.
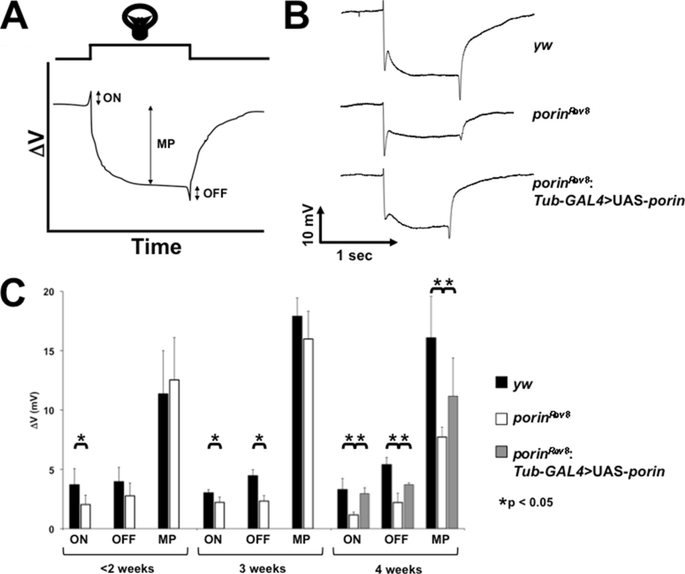
To further investigate potential CNS dysfunction in these mutants, electroretinograms were recorded from individual young and old flies. In a normal fly ERG, three potential changes are observed: the maintained potential associated with photoreceptor depolarization, and the “on” and “off” transients at the light/dark boundaries associated with communication between the retina and second order neurons (Fig. 5A) (48, 49). 3-week-old flies homozygous for porinRev8 demonstrate depression of all three potentials, suggesting a generalized dysfunction of the retina and CNS, an abnormality that is rescued by ectopic wild type porin expression (Fig. 5B). Interestingly, young porinRev8 mutant flies (2–3 days post-eclosion) exhibit normal ERGs, indicating a progressive and age-dependent pathology (Fig. 5C). Taken together, the observed ERG abnormalities, increased bang sensitivity, defective locomotion, and abnormal muscle mitochondrial morphology demonstrate that porin-deficient flies manifest an encephalomyopathy, comprising both primary CNS and primary muscle pathology.
FIGURE 5.
porin homozygous mutants exhibit abnormal ERGs. A, stereotypical “wild type” ERG are depicted. On-transient (ON), off-transient (OFF), and maintained potentials (MP) evidenced during light pulse are indicated. B, representative ERGs for wild type (yw), homozygous mutant (porinRev8), and rescue homozygous mutant (porinRev8; Tub-GAL4>UAS_porin) are shown to the right. C, bar graph depicts mean potentials (+S.E.) for each genotype. Statistical significance was evaluated by the Student's t test with Welch correction. Sample size for each genotype: yw (n = 3); porinRev8 homozygotes (n = 5); and rescued porinRev8 homozygotes (n = 3).
porin Mutants Exhibit Abnormalities of Synaptic Transmission
To examine the consequence of Porin deficiency on synaptic transmission, we evaluated basal neurotransmitter release by recording EJPs at the larval neuromuscular junction (NMJ) (Fig. 6). When stimulated at 1 Hz in a bath containing 1 mm Ca2+, there was no significant difference in mean EJP amplitude between wild type and porinRev8 mutants (Fig. 6B). However, when the experiment was performed in the presence of low extracellular Ca2+ (0.25 mm), a significant increase in EJP amplitude was observed for porinRev8 compared with controls (Fig. 6C). In addition, wild type controls on average failed to evoke EJPs in 24% of the stimulations, whereas porinRev8 larvae only failed at a mean frequency of 7% in 0.25 mm extracellular Ca2+ (Fig. 6D). We also recorded spontaneous synaptic (“mini”) release events (mEJPs) in the presence of tetrodotoxin and 0.5 mm extracellular Ca2+ and analyzed all events with an mEJP amplitude >0.4 mV. Comparing wild type to Porin-deficient mutants, we did not observe any significant difference in mEJP amplitude, frequency, or distribution, indicating no gross difference in synaptic vesicular size or number (Fig. 6, E and F). Taken together, these observations indicate that Porin-deficient mutants exhibit a subtle increase in the release of neurotransmitters at the NMJ under conditions of low release probability.
porin Mutants Exhibit Abnormal Distribution of Mitochondria in Motor Neurons
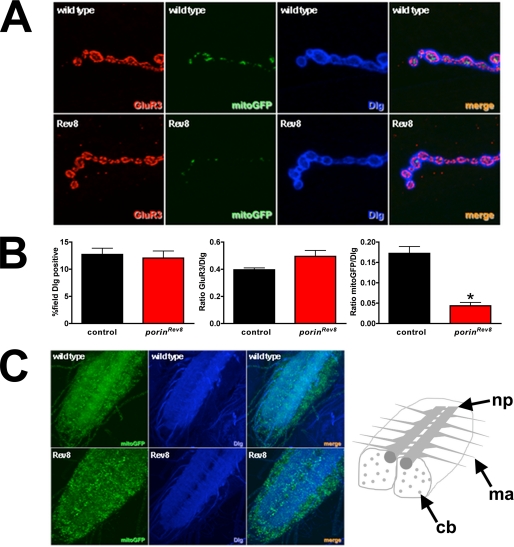
Given the observed mitochondrial and neuromuscular dysfunction, we also examined the distribution of mitochondria in motor neurons by expressing mitochondrial-targeted GFP (mitoGFP) in larval motor neurons using the UAS/GAL4 system with a GAL4 driver that is specifically expressed in motor neurons (50). When examining the NMJ by immunofluorescence, there is no observable difference in staining for the synaptic marker Dlg (51) or for the post-synaptic glutamate receptor (GluRIII/IIC) (52); however, there is an ∼70% reduction in mitoGFP fluorescence when comparing wild type to porinRev8 mutants (Fig. 7, A and B). When mitoGFP fluorescence is examined at the level of the ventral nerve cord in controls, mitoGFP predominantly localizes to the branching motor axons and motor neuron projections in the neuropil (Fig. 7C). In contrast, the porinRev8 mutant appears to have reduced mitoGFP in the neuropil and motor axon, with increased punctate clusters in the motor neuron cell bodies bordering the ventral nerve cord (Fig. 7C). These observations of abnormal mitochondrial distribution suggest that porin deficient mutants exhibit abnormal neuronal mitochondrial trafficking.
FIGURE 7.
porin mutants have reduced mitochondria in NMJ presynaptic termini. A, labeling of third instar larval NMJs with Dlg (blue) to outline boutons, GluR3 (GluRIII/IIC, red) to demarcate postsynaptic receptor field, and mitoGFP (green) expressed in motor neurons. B, quantification of fluorescence per field demonstrates decreased mitochondria in porinRev8. Error bars represent S.E. *, p < 0.05 by Student's t test with Welch correction. C, third instar larval ventral nerve cords labeled with Dlg (blue), which labels the neuropil (np) and motor axons (ma), and with mitoGFP (green). In porinRev8 animals, mitoGFP is more prominent in the cell bodies (cb) and sparser in the neuropil and motor axons compared with wild type (yw). Rev8, porinRev8.
DISCUSSION
These studies demonstrate that deficiency of porin in Drosophila causes a wide range of phenotypes that are remarkably reminiscent of mutant phenotypes seen in VDAC-deficient mice. Flies mutant for porin exhibit a partial developmental lethality, similar to mice deficient for Vdac1 that manifest variable partial embryonic lethality depending on the genetic background (11). Both porin mutant flies and VDAC mutant mouse embryonic stem cells exhibit reductions in coupled mitochondrial respiration associated with secondary partial respiratory chain deficiencies (53). Whereas mice deficient for Vdac1 and/or Vdac3 have learning deficits and electrophysiological abnormalities of synaptic plasticity in the hippocampus (11), mutant flies have CNS dysfunction as demonstrated by enhanced bang sensitivity and electrophysiological abnormalities evident at both the NMJ and the retina. Both porin-deficient flies and Vdac1-null mice have enlarged abnormal mitochondria in skeletal muscle (12). In addition, mutant flies show locomotor problems, whereas mice with severely reduced VDAC (Vdac1/3−/−) have marked exercise intolerance (54). Finally, male hypomorphic porin mutants are infertile with immotile sperm, whereas Vdac3 deficient male mice are infertile from a sperm motility defect (13). The remarkable similarity of mutant phenotypes in the fly and mouse suggest that VDAC function is conserved among higher eukaryotes and validates Drosophila as a genetic model system to study VDAC regulation and function.
To date, no VDAC mutations in humans have been reported. One prior case report described an infant with hypotonia and developmental delay that demonstrated reduced in vitro substrate oxidation in mitochondria isolated from skeletal muscle and reduced VDAC by Western blot analysis of skeletal muscle mitochondria and fibroblasts (55). However, a DNA mutation for this patient has not been reported, and thus, the reduction in VDAC may represent a secondary phenomenon. Therefore, at this time, abnormalities of VDAC have not been established convincingly as a cause of human mitochondrial encephalomyopathy. However, the observed mutant phenotypes of fruit flies and mice deficient for VDAC, including the presence of secondary respiratory chain deficiencies, suggest that VDAC deficiency may be an unrecognized cause of human mitochondrial encephalomyopathy and that the study of VDAC deficiency in model systems should provide insights into the pathophysiology of mitochondrial disease and potential therapeutic approaches.
Although the hypomorphic alleles porinRev8, porinEx365, and porinEx78 demonstrate similar gross expression (supplemental Fig. S2) and biochemical phenotypes (Fig. 2), the porin mutants demonstrate a spectrum of severity for the bang sensitivity and male fertility mutant phenotypes, with porinEx78 showing milder phenotypes compared with porinEx365 and porinRev8 (supplemental Fig. S3). These abnormal phenotypes are caused by deficiency of Porin because ectopic expression of wild type porin in porinRev8, the most severe mutant, is sufficient to rescue the mutant phenotypes (Figs. 3A and 4A). The mechanism for this observed variable expressivity is not currently understood and requires further investigation. One possibility is that because these alleles differentially involve the 5′-untranslated exons and introns upstream from the porin-coding sequence (supplemental Fig. S1), they may have different effects on the cellular expression patterns for porin in the brain and in the testes that fall below the sensitivity of the Western blot analyses employed for this study.
Male infertility in Drosophila caused by mutations in genes affecting mitochondrial function and/or morphology other than porin has been reported previously. These include mutations in components of the respiratory chain, such as bellwether (α subunit of the mitochondrial ATP synthase) (56) and cyt-c-d (cytochrome c) (57), as well as genes that regulate mitochondrial fission/fusion such as fuzzy onion (fzo) (44) and Rhomboid-7 (58). Whether the mechanism of male infertility in porin mutant homozygotes is related to a sperm flagellar structural defect, as seen in Vdac3−/− mice (13), a defect in spermatid individualization, as seen with cyt-c-d mutants (57), and/or a deficiency in mitochondrial ATP production or mitochondrial dynamics contributing to sperm immotility remains to be determined.
A previous report suggested that expression of porin and CG17137 (Porin2) is coordinately regulated based on a failure to detect Porin2 messenger RNA in pupa homozygous for a P element inserted in intron 1 of porin (porinl(2)k08405) downstream of porinEY2333 (59). The authors also hypothesized that the male infertility phenotype could be due to absence of Porin2 expression. In contrast, as demonstrated in this report, Porin2 expression in porinRev8 testes is not significantly altered by Western analysis and the infertility phenotype in porin mutants is rescued by ectopic expression of porin wild type cDNA in mutant testes (Fig. 3A). These data suggest that the male infertility phenotype in porin hypomorphic mutants is due entirely to loss of porin expression. However, the possibility that porinRev8 and porinl(2)k08405 differentially affect expression of Porin2 cannot be formally excluded.
Although the mechanism of abnormal neuronal mitochondrial distribution secondary to Porin deficiency remains to be determined, the electrophysiological and mitochondrial defects observed at the NMJ in porin-deficient mutants is very similar to the phenotype observed in drp1 mutants (41). DRP1, dynamin-related protein, is a GTPase that is a central component of the mitochondrial outer membrane fission machinery involved in regulating mitochondrial dynamics (60). It was also recently reported that pharmacological inhibition of complex I activity in Drosophila larvae significantly reduces presynaptic anterograde trafficking of mitochondria (61). Mitochondria isolated from porin-deficient flies demonstrate a significant reduction in complex I activity (Fig. 2B). In addition, it has recently been demonstrated that the mitochondrial protein Miro inhibits kinesin-mediated anterograde transport of neuronal mitochondria in a Ca2+-dependent manner (62). Given that the NMJ phenotypes of porin and drp1 mutants are similar and that presynaptic resting Ca2+ levels are elevated in drp1 mutants (41), it is possible that presynaptic Ca2+ levels in porin mutants are elevated, resulting in Miro-dependent inhibition of mitochondrial anterograde transport. Further studies are required to address whether disruption of a possible direct or indirect interaction with Drp1, mitochondrial complex I deficiency with mitochondrial depolarization, and/or perturbations in presynaptic Ca2+ homeostasis contribute to abnormal neuronal distribution of mitochondria in porin mutants.
Supplementary Material
Acknowledgments
We acknowledge Hugo Bellen for generous support and insightful comments and suggestions. We thank Yi Zhou for technical assistance in generating transmission electron microscopy images of indirect flight muscle. We acknowledge Yuchun He for performing the embryo injections to generate UAS_porin transgenic flies and Yan Huang for technical molecular biology assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants K08 HD44808 (to B. H. G.) and R01 NS0423193 (to W. J. C.). This work was also supported by March of Dimes FY-99-323 (to W. J. C.), Fund for Scientific Research Flanders (FWO, G.0747.09) (to P. V.), Research Fund Katholieke Universiteit Leuven (P. V.), a Marie Curie Excellence Grant (MEXT-CT-2006-042267) (to P. V.), The Baylor College of Medicine Mental Retardation Developmental Disabilities Research Center (MRDDRC HD024064), and VIB (to P. V.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Experimental Procedures,” Tables S1 and S2, Figs. S1–S3, and additional references.
- VDAC
- voltage-dependent anion channel
- CNS
- central nervous system
- NMJ
- neuromuscular junction
- mitoGFP
- mitochondrial-targeted GFP
- ERG
- electroretinogram
- EJP
- excitatory junctional potential.
REFERENCES
- 1.Sorgato M. C., Moran O. (1993) Crit. Rev. Biochem. Mol. Biol. 28, 127–171 [DOI] [PubMed] [Google Scholar]
- 2.Blachly-Dyson E., Song J., Wolfgang W. J., Colombini M., Forte M. (1997) Mol. Cell Biol. 17, 5727–5738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sampson M. J., Lovell R. S., Craigen W. J. (1997) J. Biol. Chem. 272, 18966–18973 [DOI] [PubMed] [Google Scholar]
- 4.Blachly-Dyson E., Zambronicz E. B., Yu W. H., Adams V., McCabe E. R., Adelman J., Colombini M., Forte M. (1993) J. Biol. Chem. 268, 1835–1841 [PubMed] [Google Scholar]
- 5.Rahmani Z., Maunoury C., Siddiqui A. (1998) Eur. J. Hum. Genet. 6, 337–340 [DOI] [PubMed] [Google Scholar]
- 6.Rostovtseva T., Colombini M. (1996) J. Biol. Chem. 271, 28006–28008 [DOI] [PubMed] [Google Scholar]
- 7.Komarov A. G., Graham B. H., Craigen W. J., Colombini M. (2004) Biophys. J. 86(1 Pt 1), 152–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimizu S., Narita M., Tsujimoto Y. (1999) Nature 399, 483–487 [DOI] [PubMed] [Google Scholar]
- 9.Cheng E. H., Sheiko T. V., Fisher J. K., Craigen W. J., Korsmeyer S. J. (2003) Science 301, 513–517 [DOI] [PubMed] [Google Scholar]
- 10.Gincel D., Zaid H., Shoshan-Barmatz V. (2001) Biochem. J. 358, 147–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weeber E. J., Levy M., Sampson M. J., Anflous K., Armstrong D. L., Brown S. E., Sweatt J. D., Craigen W. J. (2002) J. Biol. Chem. 277, 18891–18897 [DOI] [PubMed] [Google Scholar]
- 12.Anflous K., Armstrong D. D., Craigen W. J. (2001) J. Biol. Chem. 276, 1954–1960 [DOI] [PubMed] [Google Scholar]
- 13.Sampson M. J., Decker W. K., Beaudet A. L., Ruitenbeek W., Armstrong D., Hicks M. J., Craigen W. J. (2001) J. Biol. Chem. 276, 39206–39212 [DOI] [PubMed] [Google Scholar]
- 14.Messina A., Neri M., Perosa F., Caggese C., Marino M., Caizzi R., De Pinto V. (1996) FEBS Lett. 384, 9–13 [DOI] [PubMed] [Google Scholar]
- 15.Ryerse J., Blachly-Dyson E., Forte M., Nagel B. (1997) Biochim. Biophys. Acta 1327, 204–212 [DOI] [PubMed] [Google Scholar]
- 16.Oliva M., De Pinto V., Barsanti P., Caggese C. (2002) Mol. Genet. Genomics 267, 746–756 [DOI] [PubMed] [Google Scholar]
- 17.Graham B. H., Craigen W. J. (2005) Mol. Genet. Metab. 85, 308–317 [DOI] [PubMed] [Google Scholar]
- 18.Bellen H. J., Levis R. W., Liao G., He Y., Carlson J. W., Tsang G., Evans-Holm M., Hiesinger P. R., Schulze K. L., Rubin G. M., Hoskins R. A., Spradling A. C. (2004) Genetics 167, 761–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spradling A. C., Stern D., Beaton A., Rhem E. J., Laverty T., Mozden N., Misra S., Rubin G. M. (1999) Genetics 153, 135–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brand A. H., Perrimon N. (1993) Development 118, 401–415 [DOI] [PubMed] [Google Scholar]
- 21.Lee T., Luo L. (1999) Neuron 22, 451–461 [DOI] [PubMed] [Google Scholar]
- 22.Lin D. M., Goodman C. S. (1994) Neuron 13, 507–523 [DOI] [PubMed] [Google Scholar]
- 23.Hrdlicka L., Gibson M., Kiger A., Micchelli C., Schober M., Schöck F., Perrimon N. (2002) Genesis 34, 51–57 [DOI] [PubMed] [Google Scholar]
- 24.Trounce I. A., Kim Y. L., Jun A. S., Wallace D. C. (1996) Methods Enzymol. 264, 484–509 [DOI] [PubMed] [Google Scholar]
- 25.Verstreken P., Koh T. W., Schulze K. L., Zhai R. G., Hiesinger P. R., Zhou Y., Mehta S. Q., Cao Y., Roos J., Bellen H. J. (2003) Neuron 40, 733–748 [DOI] [PubMed] [Google Scholar]
- 26.Koh T. W., Verstreken P., Bellen H. J. (2004) Neuron 43, 193–205 [DOI] [PubMed] [Google Scholar]
- 27.Bellen H. J., Budnik V. (2000) in Drosophila Protocols (Sullivan M., Ashburner M., Hawley R. S. eds) pp. 175–199, CSHL Press, Cold Spring Harbor, New York [Google Scholar]
- 28.Robertson H. M., Preston C. R., Phillis R. W., Johnson-Schlitz D. M., Benz W. K., Engels W. R. (1988) Genetics 118, 461–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benzer S. (1971) Jama 218, 1015–1022 [PubMed] [Google Scholar]
- 30.Homyk T., Jr. (1977) Genetics 87, 105–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Homyk T., Jr., Szidonya J., Suzuki D. T. (1980) Mol. Gen. Genet. 177, 553–565 [DOI] [PubMed] [Google Scholar]
- 32.Ganetzky B., Wu C. F. (1982) J. Neurophysiol. 47, 501–514 [DOI] [PubMed] [Google Scholar]
- 33.Ramaswami M., Tanouye M. A. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 2079–2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pavlidis P., Ramaswami M., Tanouye M. A. (1994) Cell 79, 23–33 [DOI] [PubMed] [Google Scholar]
- 35.Schubiger M., Feng Y., Fambrough D. M., Palka J. (1994) Neuron 12, 373–381 [DOI] [PubMed] [Google Scholar]
- 36.Kuebler D., Tanouye M. A. (2000) J. Neurophysiol. 83, 998–1009 [DOI] [PubMed] [Google Scholar]
- 37.Lee J., Wu C. F. (2002) J. Neurosci. 22, 11065–11079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trotta N., Rodesch C. K., Fergestad T., Broadie K. (2004) J. Neurobiol. 60, 328–347 [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y. Q., Roote J., Brogna S., Davis A. W., Barbash D. A., Nash D., Ashburner M. (1999) Genetics 153, 891–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Royden C. S., Pirrotta V., Jan L. Y. (1987) Cell 51, 165–173 [DOI] [PubMed] [Google Scholar]
- 41.Verstreken P., Ly C. V., Venken K. J., Koh T. W., Zhou Y., Bellen H. J. (2005) Neuron 47, 365–378 [DOI] [PubMed] [Google Scholar]
- 42.Clark I. E., Dodson M. W., Jiang C., Cao J. H., Huh J. R., Seol J. H., Yoo S. J., Hay B. A., Guo M. (2006) Nature 441, 1162–1166 [DOI] [PubMed] [Google Scholar]
- 43.Greene J. C., Whitworth A. J., Kuo I., Andrews L. A., Feany M. B., Pallanck L. J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 4078–4083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hales K. G., Fuller M. T. (1997) Cell 90, 121–129 [DOI] [PubMed] [Google Scholar]
- 45.Xu Y., Condell M., Plesken H., Edelman-Novemsky I., Ma J., Ren M., Schlame M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 11584–11588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yarosh W., Monserrate J., Tong J. J., Tse S., Le P. K., Nguyen K., Brachmann C. B., Wallace D. C., Huang T. (2008) PLoS Genet. 4, e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walker D. W., Benzer S. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 10290–10295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pak W. L. (1995) Invest. Ophthalmol. Vis. Sci. 36, 2340–2357 [PubMed] [Google Scholar]
- 49.Lo M. V., Pak W. L. (1981) J. Gen. Physiol. 77, 155–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yeh E., Gustafson K., Boulianne G. L. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 7036–7040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parnas D., Haghighi A. P., Fetter R. D., Kim S. W., Goodman C. S. (2001) Neuron 32, 415–424 [DOI] [PubMed] [Google Scholar]
- 52.Marrus S. B., Portman S. L., Allen M. J., Moffat K. G., DiAntonio A. (2004) J. Neurosci. 24, 1406–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu S., Sampson M. J., Decker W. K., Craigen W. J. (1999) Biochim. Biophys. Acta 1452, 68–78 [DOI] [PubMed] [Google Scholar]
- 54.Anflous-Pharayra K., Cai Z. J., Craigen W. J. (2007) Biochim. Biophys. Acta 1767, 136–142 [DOI] [PubMed] [Google Scholar]
- 55.Huizing M., Ruitenbeek W., Thinnes F. P., DePinto V., Wendel U., Trijbels F. J., Smit L. M., ter Laak H. J., van den Heuvel L. P. (1996) Pediatr. Res. 39, 760–765 [DOI] [PubMed] [Google Scholar]
- 56.Jacobs H., Stratmann R., Lehner C. (1998) Mol. Gen. Genet. 259, 383–387 [DOI] [PubMed] [Google Scholar]
- 57.Arama E., Bader M., Srivastava M., Bergmann A., Steller H. (2006) EMBO J. 25, 232–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McQuibban G. A., Lee J. R., Zheng L., Juusola M., Freeman M. (2006) Curr. Biol. 16, 982–989 [DOI] [PubMed] [Google Scholar]
- 59.Guarino F., Specchia V., Zapparoli G., Messina A., Aiello R., Bozzetti M. P., De Pinto V. (2006) Biochem. Biophys. Res. Commun. 346, 665–670 [DOI] [PubMed] [Google Scholar]
- 60.Santel A., Frank S. (2008) IUBMB Life 60, 448–455 [DOI] [PubMed] [Google Scholar]
- 61.Tong J. J. (2007) Biol. Bull 212, 169–175 [DOI] [PubMed] [Google Scholar]
- 62.Wang X., Schwarz T. L. (2009) Cell 136, 163–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.