Abstract
Whereas the role of adipose tissue in glucose and lipid homeostasis is widely recognized, its role in systemic protein and amino acid metabolism is less well-appreciated. In vitro and ex vivo experiments suggest that adipose tissue can metabolize substantial amounts of branched chain amino acids (BCAAs). However, the role of adipose tissue in regulating BCAA metabolism in vivo is controversial. Interest in the contribution of adipose tissue to BCAA metabolism has been renewed with recent observations demonstrating down-regulation of BCAA oxidation enzymes in adipose tissue in obese and insulin-resistant humans. Using gene set enrichment analysis, we observe alterations in adipose-tissue BCAA enzyme expression caused by adipose-selective genetic alterations in the GLUT4 glucose-transporter expression. We show that the rate of adipose tissue BCAA oxidation per mg of tissue from normal mice is higher than in skeletal muscle. In mice overexpressing GLUT4 specifically in adipose tissue, we observe coordinate down-regulation of BCAA metabolizing enzymes selectively in adipose tissue. This decreases BCAA oxidation rates in adipose tissue, but not in muscle, in association with increased circulating BCAA levels. To confirm the capacity of adipose tissue to modulate circulating BCAA levels in vivo, we demonstrate that transplantation of normal adipose tissue into mice that are globally defective in peripheral BCAA metabolism reduces circulating BCAA levels by 30% (fasting)-50% (fed state). These results demonstrate for the first time the capacity of adipose tissue to catabolize circulating BCAAs in vivo and that coordinate regulation of adipose-tissue BCAA enzymes may modulate circulating BCAA levels.
Keywords: Diseases/Diabetes, Diseases/Metabolic, Metabolism/Amino Acid, Metabolism/Carbohydrate, Adipose tissue metabolism, Fasting, Integrated Physiology, mTOR
Introduction
The branched chain amino acids (BCAAs)2, leucine, isoleucine, and valine, are three of the nine essential amino acids and are relatively abundant in the food supply accounting for ∼20% of total protein intake (1). In contrast to the other 17 amino acids, which are predominantly metabolized in the liver, BCAAs are poorly metabolized during first pass through the liver as the liver expresses only low levels of the mitochondrial branched chain aminotransferase (BCAT2 or BCATm), the first enzyme in the catabolism of BCAAs in most peripheral tissues (2, 3). BCAAs are therefore in a unique position among amino acids to signal to the periphery and the brain the amino acid content of a meal. Circulating BCAAs, acting as nutrient signals, regulate protein synthesis, and degradation, and insulin secretion, and have been implicated in central nervous system control of food intake and energy balance (4–7). Our knowledge of the physiologic mechanisms which regulate circulating BCAA levels remains incomplete. In this study, we provide evidence that adipose tissue contributes to the regulation of circulating BCAAs.
Over the last two decades, adipose tissue has emerged as a key endocrine organ and a regulator of integrated fuel homeostasis. Whereas its role in glucose and lipid homeostasis is widely recognized, its role in systemic protein and amino acid metabolism is less well appreciated. However, considerable in vitro and ex vivo evidence suggests that adipose tissue is capable of metabolizing significant quantities of BCAAs (8, 9). Based upon ex vivo measurements of leucine flux in rat tissues, Rosenthal et al. (9) estimated that adipose tissue is second only to skeletal muscle in its capacity to catabolize BCAAs, and that the capacities of skeletal muscle and adipose tissue are 6–7-fold larger than liver taking into account the relative masses of different tissues. However, a study specifically examining the capacity of the first two enzymes (BCAT2 and the branched chain ketoacid dehydrogenase complex (BCKDHC)) required for BCAA oxidation across different tissues discounted adipose tissue as a quantitatively significant site of BCAA catabolism in rodents, primates, and humans (10). Similarly, no net uptake of BCAAs could be detected across rat inguinal fat by microdialysis sampling or arterio-venous differences (11). Thus the quantitative significance of adipose tissue BCAA catabolism in vivo was unclear. In the present study, we address this issue.
Reports of elevated serum BCAA levels (12, 13) and recent observations showing down-regulation of the expression of adipose tissue BCAA metabolizing enzymes in obesity and insulin-resistant states (7, 14) has renewed interest in the potential significance of adipose tissue BCAA metabolism. Of note, adipose tissue has been hypothesized to be the major site where excess BCAAs are stored in the form of lipid (12). Adipose tissue efficiently converts BCAA carbon skeletons into newly synthesized fatty acids ex vivo (9). Furthermore, insulin increases the rate of leucine conversion to lipid in adipose tissue explants, but not in muscle or liver (9). Elevated circulating BCAA levels and reduced expression of adipose tissue BCAA oxidation enzymes in obese individuals normalize following gastric bypass surgery and weight loss (15). A recent study of monozygotic twins which were discordant for obesity demonstrated down-regulation of the BCAA oxidation enzymes in the obese twin, which correlated with elevated fasting insulin levels and insulin resistance (14). The potential causal relationship between altered BCAA enzyme expression and obesity and insulin resistance is of great interest.
We previously made mice with adipose-specific overexpression (AG4OX) (16) or knock-out (AG4KO) (17) of the insulin-stimulated glucose transporter, GLUT4, because GLUT4 is down-regulated selectively in adipose tissue of obese and type 2 diabetic humans (18). These mice had reciprocal alterations in glucose homeostasis. In the course of studies to investigate the physiologic mechanisms by which GLUT4 expression in adipose tissue regulates systemic fuel homeostasis, we unexpectedly observed coordinate down-regulation and up-regulation of branched chain amino acid metabolizing enzymes selectively in adipose tissue of AG4OX and AG4KO mice, respectively. In the present studies, we have taken advantage of the selective down-regulation of BCAA enzyme expression in AG4OX mice to examine the physiologic significance of adipose tissue BCAA oxidation in vivo. We also utilized a second genetic mouse model globally defective for peripheral BCAA catabolism to investigate whether adipose tissue is capable of modulating circulating BCAA levels. Our data show for the first time that in vivo, adipose tissue can modulate circulating BCAA levels.
EXPERIMENTAL PROCEDURES
Animal Studies
Generation and initial metabolic characterization of the adipose-specific GLUT4-overexpressing mice (AG4OX) and adipose-specific GLUT4 knock-out mice (AG4KO) were previously described (16, 17). Mice were housed at Beth Israel Deaconess Medical Center with a 14/10 light-dark cycle and were fed standard chow (Formulab 5008) ad libitum. All studies were performed on age- and sex-matched littermates. All blood collections were performed by tail vein bleeding. Serial plasma amino acid levels were measured in 4-month-old, female AG4OX, and wild-type controls (n = 10). Bleeds were performed at baseline (8 AM) and 2 h and 6 h following food removal. For assessment of mTOR signaling, rapamycin (10 mg/kg body weight, LC Labs) or vehicle (2% EtOH in phosphate-buffered saline) was injected intraperitoneally in 2-month-old, female AG4OX and wild-type littermates 5 h after food removal. One hour later, insulin (10 units/kg body weight) or normal saline was injected via the tail vein. Mice were sacrificed by decapitation 5 min after insulin injection. Plasma was collected, and tissues were harvested, snap frozen in liquid nitrogen, and stored at −80C for processing.
BCAT2−/− mice were housed at Penn State University College of Medicine. Prior to surgery male BCAT2−/− mice were randomized to a sham-operated control group or an adipose tissue transplant group (n = 6–8 per group). Transplantation of ∼750 mg of perigonadal fat from wild-type male littermates or sham surgery was performed under halothane anesthesia as previously described at 2 months of age (19). Mice were housed singly following surgery and allowed to recover for 2 weeks prior to further experimentation. Both sham and transplanted mice were provided free access to both normal chow (NC, Harlan 2018) and a purified amino acid BCAA-free diet (-BCAA, Dyets 510081). Plasma BCAAs, food intake, and body composition were measured 2 weeks after surgery (∼10 weeks of age). Mouse studies were conducted in accordance with federal guidelines and were approved by the Institutional Animal Care and Use Committee.
Microarray Analysis
Total RNA from epididymal adipose tissue was extracted using the RNeasy Mini Kit from Qiagen from three mice from each of four genotypes: aP2-Cre transgenic littermates (controls for AG4KO mice), AG4KO mice; FVB littermates (controls for AG4OX) and AG4OX. RNA from each mouse was hybridized on an Affymetrix MG-U74-A.v2 Genechip microarray. Affymetrix gene chip hybridization and analysis were performed at the Genomics Core Facility of the Beth Israel Deaconess Medical Center. Array results were analyzed using DChip software (20). Genome-wide expression analysis of the microarray data were performed using gene set enrichment analysis (GSEA) (21).
RNA Extraction and Quantitative Real-time PCR
Tissues were harvested from 5-week-old female mice in the fed state (8 a.m.), snap frozen in liquid nitrogen, and stored at −80C for processing. Total RNA was extracted from frozen tissue with TRI Reagent (Molecular Research Center, Inc.). Real-time PCR was performed using TaqMan One-step RT-PCR Master Mix (Applied Biosystems) in an Mx3000P thermocycler (Stratagene). The Mx3000P software was used to calculate the cycle threshold for each reaction. Relative expression levels were determined using the comparative Ct method with normalization of target gene expression levels to 18s. Assay-on-demand (Applied Biosystems) Primer and probe sets are: Bcat2 Mm00802192_m1; Bckdha Mm00476112_m1; Dbt Mm00501651_m1; Dld Mm00432831_m1; BCKDK Mm00437777_m1.
Body Composition Analysis
Serial body composition was measured in 7-month-old, female AG4OX mice, and wild-type littermates by dual-energy x-ray absorptiometry (DEXA) between 8 and 10 a.m. on three consecutive days using halothane anesthesia. Food was removed following the initial body composition measurement and for the subsequent 48 h. Free access to water was provided throughout the fast. For BCAT2−/− mice, body composition was measured by NMR (Echo Medical Systems).
Valine Oxidation in Tissue Explants
Valine oxidation and αKIV accumulation in tissue explants were measured using a protocol adapted from Joshi et al. (22). Adipose tissue, soleus, or extensor digitorum longus muscle from fed, 7-month-old, female AG4OX and wild-type littermates were excised, weighed, and placed in Erlenmeyer flasks (10 ml) containing 1.5 ml of Krebs-Ringer-phosphate-HEPES buffer (pH 7.4). The buffer was supplemented with 5 mm glucose, 1 mm valine containing 160 μCi/mmol [1-14C]valine. For adipose tissue samples, the buffer was also supplemented with 200 nm adenosine and 2% (w/v) BSA (fatty acid free). For muscle samples, the buffer was supplemented with 0.2% (w/v) BSA. Adipose tissue samples were minced to 1 mm sized pieces. The flasks were sealed with rubber stoppers fitted with hanging center wells (Kontes, Vineland, NJ) and incubated with shaking at 37 °C. The reaction was terminated after 1 h with injection of 100 μl of 60% (w/v) perchloric acid into the reaction mixture and 300 μl of 1 m benzothenium hydroxide into the center wells for collecting the 14CO2 produced. After 20 min, the flasks were resealed with fresh rubber stoppers fitted with hanging center wells. Hydrogen peroxide (350 μl, 30% w/v) was injected into the reaction mixture and 300 μl of 1 m benzothenium hydroxide into the center wells for collecting the 14CO2 generated by the decarboxylation of α-ketoisovalerate.
Western Blotting
Aliquots of frozen tissues were homogenized on ice in radioimmunoprecipitation assay buffer supplemented with phospho-preserving and anti-protease agents (sodium fluoride, sodium pyrophosphate, sodium orthovanadate, PMSF, aprotinin, and leupeptin). Protein concentration was assayed using the BCA assay. Equal amounts of protein were loaded and transferred to nitrocellulose membranes. The membranes were probed with antibodies against BCKDHC (provided by Dr. Robert Harris), phospho-BCKDH E1α (as previously published (23)), p70 S6 kinase (provided by Dr. John Blenis), and PI3 kinase p85 (Upstate). Results were quantified using the GeneGnome chemiluminescent imaging apparatus and software (Syngene).
Ribosomal S6 Kinase Activity Assay
Tissues were homogenized in lysis buffer containing 20 mm Tris. pH 7.4, 1% Nonidet P-40, 10% glycerol, 2 mm EDTA, 10 mm sodium pyrophosphate, 50 mm sodium fluoride, 1 mm DTT, 1 mm sodium orthovanadate, 1 mm PMSF, and sigma protease inhibitor mixture. Protein concentration was determined using the DC protein assay with BSA as a standard (Bio-Rad). Lysate (200 μg) was immunoprecipitated using anti-p70 S6 kinase antibody (provided by Dr. John Blenis) and protein A-Sepharose. Immunoprecipitates were washed with 0.5 ml each of lysis buffer, buffer A (1 m NaCl, 10 mm Tris, pH 7.4, 0.1% Nonidet P-40, 2 mm DTT, 1 mm phenylmethylsulfonyl fluoride (PMSF)), and buffer B (150 mm NaCl, 50 mm Tris (pH 7.4, 2 mm DTT, 1 mm PMSF). Kinase activity toward a recombinant GST-S6 peptide (32 final amino acids of ribosomal S6) in washed immunoprecipitates was assayed in a reaction containing 200 mm Tris, pH 7.4, 100 mm MgCl2, 1 mg/ml BSA, 0.3 μg/μl GST-S6 peptide, 50 μm ATP unlabeled, 0.1 μCi [γ-32P]ATP/μl reaction volume for 10 min at 30 °C. The reaction was stopped by addition of 2× Laemmli buffer containing 200 mm DTT. Reactions were subjected to 18% SDS-PAGE. Gels were stained in Coomassie Blue, destained, dried, and the amount of 32P incorporated into GST-S6 was quantitated by phosphorimaging (Molecular Dynamics Storm 860).
Analytical Procedures
Plasma amino acid levels were measured either by HPLC at the Vanderbilt Mouse Metabolic Phenotyping Center or by enzymatic assay as previously described (15).
Statistical Analyses
All values are given as means ± S.E. Differences between two groups were assessed using unpaired two-tailed Student's t tests unless otherwise indicated in the text and figure legends.
RESULTS
Regulation of BCAA Metabolizing Enzymes in Adipose Tissue
Initially, we sought to gain insight into the mechanisms by which changes in adipose tissue glucose flux affect adipocyte function and systemic fuel metabolism. We performed global gene expression analyses on perigonadal adipose tissue from AG4KO (which are insulin resistant) and AG4OX mice (which have enhanced glucose tolerance) (24). Gene set enrichment analysis revealed that enzymes of BCAA oxidation are coordinately down-regulated (supplemental Fig. S1A) and up-regulated (supplemental Fig. S1B) in AG4OX and AG4KO adipose tissue, respectively (21). In fact, this was the most highly regulated gene set in adipose tissue of AG4OX and AG4KO mice among 522 gene sets in the data base (data not shown).
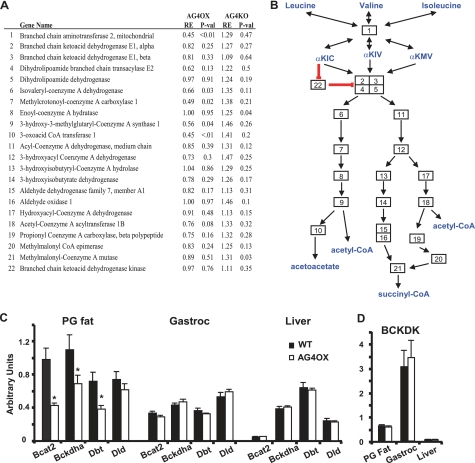
BCAT2 catalyzes the reversible, equilibrium conversion of BCAAs to their respective α-ketoacids (gene 1, Fig. 1A and B). The branched chain ketoacid dehydrogenase complex (BCKDHC) (Fig. 1, A and B, genes 2–5) is a large multisubunit complex that catalyzes the second step, the oxidative decarboxylation of the α-ketoacids to their acyl-CoA esters. Decarboxylation is irreversible and considered rate limiting (25). The changes in expression of BCAA-oxidizing enzymes in adipose tissue of AG4OX mice are individually relatively small, but are distributed throughout the pathway and in aggregate are highly statistically significant by gene set enrichment analysis.
FIGURE 1.
Expression of BCAA-oxidizing enzymes in adipose tissue. A, microarray results from adipose tissue from AG4OX versus control and AGKO versus control female mice at 5 weeks of age that were sacrificed in the fed state (n = 3 per group). RE, relative expression (the expression in the experimental group relative to its control group). B, diagram of the BCAA oxidation pathway indicating genes included in the analysis using the numbering in A. αKIC, α-ketoisocaproic acid; αKIV, α-ketoisovaleric acid; αKMV, α-keto-β-methylvaleric acid. C and D, Q-PCR results for selected enzymes of the BCAA oxidation pathway in perigonadal fat (PG fat), gastrocnemius muscle (Gastroc), and liver from fed, 5-week-old female mice. (*, p < 0.05 versus WT).
Q-PCR (Fig. 1C) demonstrated 40–60% reductions in BCAT2 and the BCKDHC subunits, BCKDHA and Dbt, in AG4OX adipose tissue. Dld, which encodes the dihydrolipoamide dehydrogenase subunit of the BCDKHC and is shared by the pyruvate dehydrogenase and α-ketoglutarate dehydrogenase complexes, was unchanged, demonstrating specificity for enzymes unique to the BCAA-metabolizing pathway. BCAA enzyme expression in gastrocnemius muscle and liver was unchanged (Fig. 1C). We found that BCAA enzyme expression was higher in perigonadal fat compared with gastrocnemius and liver. In liver, BCAT2 expression was very low, consistent with the reported near absence of this enzymatic activity in liver (2, 26).
Branched chain keto acid dehydrogenase kinase (BCKDK) phosphorylates and inhibits the BCKDH complex. Regulation of BCKDK expression is a key mechanism for nutritional and hormonal regulation of BCAA oxidative flux, particularly in muscle and liver (28). Fig. 1D shows that BCKDK expression in gastrocnemius muscle is ∼6-fold higher than in perigonadal fat and is nearly undetectable in liver, consistent with prior studies indicating that BCKDHC, the target of BCKDK, is largely inactivated in muscle and nearly completely active in liver of chow-fed animals (25). Importantly, BCKDK expression was unchanged (Fig. 1D) in AG4OX perigonadal fat, liver, and muscle. These results indicate that regulation of BCAA oxidative flux in adipose tissue (see Fig. 2A) can occur independent of changes in BCKDK expression, which is thought to be the classic mechanism by which nutrients and hormones regulate BCAA oxidative flux in other tissues.
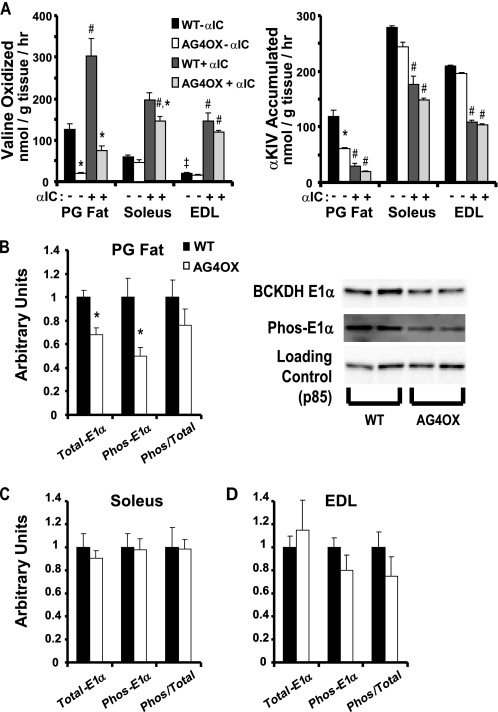
FIGURE 2.
Valine oxidation and E1α phosphorylation in skeletal muscle and adipose tissue. A, valine oxidation and αKIV accumulation were measured with and without addition of α-chloroisocaproic acid (αIC) in adipose tissue (n = 10 per group), soleus (n = 6 per group), and EDL (n = 6 per group) explants from fed, 7-month-old female AG4OX and wild-type control mice. Comparisons within tissues performed by 2-way ANOVA using Tukey's test for post-hoc analysis between groups; *, p < 0.05 comparing effect of genotype within αIC; #, p < 0.05 comparing effect of αIC within genotype; Comparison across tissues in wild-type animals without αIC performed by one-way ANOVA, ‡, p < 0.05. B and C, quantitation of Western blots for total BKDH E1α, phospho-BCKDH, and the ratio of total to phospho-BCKDH E1α for (B) perigonadal fat with representative blot, and for (C) soleus and (D) EDL muscle from tissues harvested in experiment described in A. (n = 5–6 per group, *, p < 0.05 versus WT by t test).
To determine whether the coordinate changes in BCAA enzyme expression result in changes in BCAA oxidation rates, we measured valine oxidation in tissue explants from AG4OX mice and controls. α-Chloroisocaproic acid (αIC) is an irreversible inhibitor of BCKDK leading to rapid dephosphorylation and activation of BCKDHC (29). Valine oxidation in the absence of αIC reflects basal valine oxidation whereas αIC elicits maximal stimulation of valine oxidation. Compared with controls, basal, and maximally stimulated valine oxidation was 6.6- and 4-fold lower, respectively, in perigonadal adipose tissue from AG4OX mice (Fig. 2A). The basal rate of valine oxidation in adipose tissue per mg of wet tissue weight was 2-fold greater than in oxidative muscle (soleus) and 6-fold greater than in glycolytic muscle (EDL). We do not see regulation of valine oxidation in either soleus or EDL muscle of AG4OX mice except for a small decrease in soleus in the presence of αIC. Even taking into account the relative amounts of adipose and muscle mass in vivo, these results suggest that adipose tissue may contribute a quantitatively significant amount to whole body BCAA oxidation.
We sought to determine whether BCAA metabolism undergoes the same regulation in adipose tissue as in muscle and liver. Phosphorylation of the E1α subunit of BCKHDC by BCKDK has been reported to be the major site for nutritional or hormonal regulation of BCKDHC activity and BCAA oxidation (30). Although total E1α protein is lower in AG4OX adipose tissue, the ratio of E1α phosphorylation to total E1α is unaltered (Fig. 2B). No changes in total E1α protein, phosphorylated E1α, or the ratio of E1α phosphorylation to total E1α were observed in soleus or EDL muscle (Fig. 2, C and D). If BCKDHC is rate limiting for flux through the BCAA oxidation pathway, increased accumulation of the BCKDHC substrate would be expected to accompany a decrease in BCKDHC activity. However, αKIV accumulation was reduced 4-fold in AG4OX adipose tissue explants compared with control in the absence of αIC (Fig. 2A). This suggests that steps upstream of BCKDHC are partially rate-determining for valine oxidation in adipose tissue. These results indicate a novel mechanism for regulation of BCAA oxidation in adipose tissue, i.e. alterations in the expression of BCAA enzymes, in contrast to altered BCKDHC phosphorylation, which is the major mechanism regulating BCAA oxidation in muscle and liver.
Adipose Tissue GLUT4 Overexpression Regulates Protein Metabolism, BCAA Levels, and mTOR Signaling
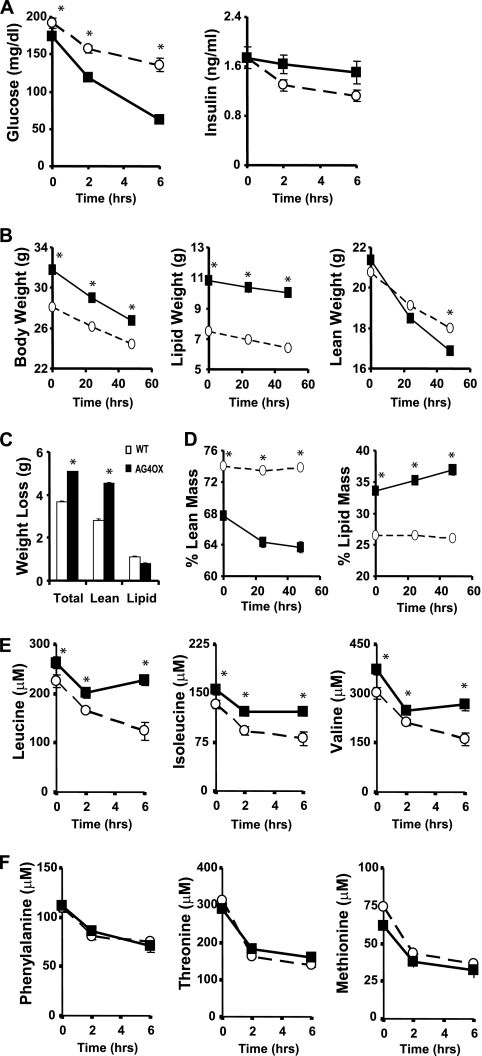
In AGOX mice, the initial fed glucose level is lower and blood glucose falls more rapidly 2 and 6 h after food removal (Fig. 3A). Insulin levels are not different between groups at any time point (Fig. 3A). Given the susceptibility to fasting hypoglycemia in AG4OX mice and the importance of mobilization of fat and protein to defend against hypoglycemia, we investigated whether prolonged fasting leads to changes in whole-body composition in this model. AG4OX mice are modestly obese compared with wild-type littermates (Fig. 3B). AG4OX mice lost significantly more weight than controls during a 48 h fast (Fig. 3C). Loss of lean mass accounted for all of the excess weight loss in AG4OX mice. Control animals maintained their percent lean mass (Fig. 3D) and percent lipid mass at constant levels throughout the fast. In contrast, in AG4OX mice, lean mass decreased (from 68 to 64% (p < 0.001)) and lipid mass increased (from 34% to 37% (p < 0.001)) as percentages of total body mass. Thus, overexpression of GLUT4 specifically in adipose tissue resulted in derangements in systemic fuel metabolism associated with preservation of fat mass at the expense of lean body mass during a prolonged fast.
FIGURE 3.
Changes in glycemia, protein metabolism, and circulating amino acid levels following food removal. A, serial glucose and insulin levels were measured in 4-month-old, female AG4OX and wild-type controls (n = 10 per group). Tail vein bleeds were performed at baseline (8 a.m.) and 2 h and 6 h following food removal (*, p < 0.05 for WT versus AG4OX at each time). B–D, serial body composition measurements were made by DEXA in 7-month-old female AG4OX and wild-type control mice at baseline and after 24 h and 48 h of fasting (n = 9 per group). Comparisons were made for changes in (B) total body weight, lipid weight, lean weight (C) total cumulative weight loss, and (D) % lean mass and % lipid mass (○, WT, ■, AG4OX). Body weight, lipid weight, and lean weight differed between genotypes (*, p < 0.05). Body weight, lipid weight, and lean weight differed within genotypes at 24 and 48 h of fasting compared with time 0 (p < 0.05, paired Student's t test). % lean mass and % lipid mass differed in AG4OX mice after 24 and 48 h of fasting compared with time 0 (p < 0.05, paired Student's t test), but remained unchanged in wild-type mice. E and F, serial plasma amino acid levels were measured under the conditions described in A (*, p < 0.05 for WT versus AG4OX at each time).
We next asked whether the decreases in BCAA enzyme expression in adipose tissue and the increased protein degradation during fasting in AG4OX affect circulating BCAAs. Levels of all three BCAAs were elevated in the fed state and 2 and 6 h following food removal (Fig. 3E). If this were due to increased net protein breakdown as indicated by the greater loss of lean mass in AG4OX, levels of other circulating amino acids should also be elevated (31). We were surprised to find no increase in the circulating levels of other essential amino acids including phenylalanine, threonine, and methionine in AG4OX (Fig. 3F). Because changes in net protein breakdown affect most amino acids proportionately, the specific increases in circulating BCAA levels suggest a selective decrease in BCAA clearance (31). Taken together with the selective decrease in BCAA oxidation in adipose tissue in AG4OX mice and the high rate of BCAA oxidation in adipose tissue explants compared with skeletal muscle, these data suggest that adipose tissue is an important site for whole body BCAA oxidation and thereby affects circulating BCAA levels. Changes in BCAA oxidation in other tissues could also contribute.
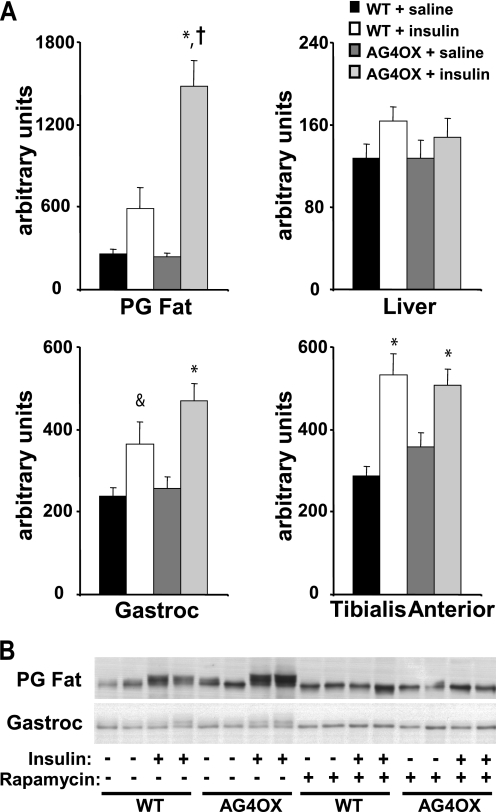
Because circulating BCAAs have been implicated as nutrient signals, we examined the mTOR signaling pathway in the AG4OX model. There was a tendency for a 2-fold stimulation of S6 kinase (S6K) activity by insulin in adipose tissue of WT mice (Fig. 4A). In AG4OX, basal (saline injection) S6K activity was the same as WT. Strikingly, insulin stimulated S6K activity 6.5-fold in WAT of AG4OX mice. There was no increase in S6K activity in liver, gastrocnemius muscle, or tibialis anterior muscle (Fig. 4A) of AG4OX compared with WT. Insulin injection in WT mice induced a gel mobility shift in S6K in adipose tissue and, consistent with the S6K activity, the insulin-stimulated shift in S6K was more pronounced in adipose tissue of AG4OX mice (Fig. 4B). In contrast, insulin stimulated a similar degree of mobility shift in gastrocnemius of AG4OX compared with WT mice. Pretreatment with rapamycin prevented the insulin-stimulated shift in S6 Kinase gel mobility in both WT and AG4OX mice in both tissues. The increase in circulating BCAAs may contribute to the increase in adipose tissue insulin-stimulated S6 kinase activity in AG4OX mice or may be the direct result of increased glucose flux to enhance mTOR signaling. However, these results indicate that the increase in circulating BCAAs in fasted AG4OX mice are not sufficient to increase insulin stimulated mTOR activation in other insulin target tissues.
FIGURE 4.
Changes in mTOR signaling. A, p70 S6 kinase activity in perigonadal adipose tissue, liver, gastrocnemius muscle, and tibialis anterior muscle of 2-month-old female, WT, and AG4OX mice. Awake mice were injected with saline or insulin (10 units/kg ip) and sacrificed 5 min later (n = 7–9 per group). Tissues were frozen for assays. Statistical comparisons in performed by 2-way ANOVA with Tukey's post-hoc testing; *, p < 0.001 for insulin effect within genotype; †, p < 0.001 compared with WT insulin group; &, p = 0.057 compared with WT-saline group. B, representative blot demonstrating p70 S6 kinase gel mobility shift measured by Western blotting in perigonadal fat and gastrocnemius muscle in animals described in A. Mice were pretreated with rapamycin (10 mg/kg ip) or vehicle 1 h before saline or insulin injection.
Fat Transplantation Reduces BCAAs in Mice Defective for Peripheral BCAA Metabolism
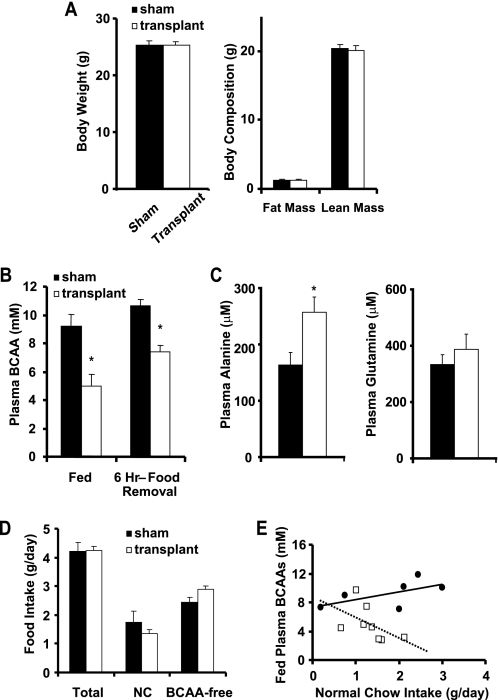
Mice lacking BCAT2 have massively elevated circulating BCAA levels due to the inability to oxidize BCAAs in peripheral tissues, and they are hypermetabolic (7). To determine whether adipose tissue BCAA oxidation is sufficient to affect systemic BCAA homeostasis and circulating BCAA levels, we transplanted 750 mg of adipose tissue from wild-type littermates into BCAT2−/− mice. BCAT2−/− mice were randomized to sham surgery or transplant. Prior to surgery, body weights (sham: 23.5 ± 0.6 g versus transplant: 23.4 ± 0.9; p = 0.92) and fed BCAA levels (sham: 7.0 ± 1.4 mm versus transplant: 6.7 ± 0.9; p = 0.86) were similar between the two groups of BCAT2−/− mice. Two weeks after transplantation or sham surgery (Fig. 5A), body weight and body composition were not different between the two groups. Transplantation of wild-type fat into BCAT2−/− mice lowered fed plasma BCAAs 46% and BCAAs 6 h after food removal 31% compared with sham-operated BCAT2−/− controls (Fig. 5B). Fed plasma alanine levels increased 58% after transplantation of wild-type fat into BCAT2−/− mice (Fig. 5C). Fed plasma glutamine levels tended to increase (16%) in the transplanted mice as well. The increase in circulating alanine and tendency toward increased glutamine may reflect increased BCAA oxidation in the transplanted adipose tissue since adipose tissue can utilize BCAA-derived nitrogen to synthesize alanine and glutamine (8, 32).
FIGURE 5.
BCAT2 fat transplantation. A and B, body weight, body composition, and plasma BCAAs in the fed state and following 6 h of food removal were measured in male BCAT2−/− mice transplanted with 750 mg of wild-type fat versus sham operated controls (n = 6–8 per group; *, p < 0.05). C, plasma alanine and glutamine were measured in the fed mice described above (n = 4–7 per group; *, p < 0.05). Mice had free access to a choice of normal chow (NC) or BCAA-free diet and food intake was measured for 1 week at ∼10 weeks of age, following the 2 week recovery period. D, food intake is presented as average daily values. E, fed plasma BCAA levels for individual mice versus average daily intake of normal chow.
Both sham and transplanted mice were provided free access to normal chow and a BCAA-free diet to prevent toxicity associated with extreme elevations in plasma BCAAs in BCAT2−/− mice (7). Intake of total food, normal chow, or BCAA-free diet (Fig. 5D) was not different though a trend toward increased BCAA-free diet intake was noted in the transplanted group (p = 0.057). Plasma BCAAs in the transplanted group tend to be lower than in the sham-operated group for any amount of normal chow intake (Fig. 5E). The ratio of normal chow (g/day) to plasma BCAA levels tends to be higher in the transplanted group (0.34 ± 0.12) compared with the sham-operated group (0.20 ± 0.04, p = 0.13). Thus, the reduction in circulating BCAAs in the transplant group does not result from reduced BCAA intake but is due to increased BCAA clearance, most likely through catabolism in the transplanted normal fat. The reductions in plasma BCAAs in the transplant group occurred in the absence of changes in fed or fasted glycemia, insulin or leptin levels (Table 1). These results strongly support the conclusion that in vivo, adipose tissue is an important site of BCAA oxidation and is capable of contributing significantly to regulation of circulating BCAA levels.
TABLE 1.
Fed and fasting blood metabolites after fat transplantation
Three weeks after surgery, blood was collected from 8 to 10 a.m. from sham-operated controls and fat-transplanted Bcat2−/− mice in the fed state and after an overnight fast. Values are means ± S.E. n = 6–8 per group. Statistical significance at a p value of 0.05 was not achieved for any comparison between sham control and fat transplant groups.
| Sham control |
Fat transplant |
|||
|---|---|---|---|---|
| Fed | Fasted | Fed | Fasted | |
| Glucose (mg/dl) | 216 ± 16 | 97 ± 9 | 186 ± 4 | 88 ± 2 |
| Insulin (ng/ml) | 0.95 ± 0.02 | 0.21 ± 0.01 | 0.82 ± 0.16 | 0.19 ± 0.03 |
| Leptin (ng/ml) | 1.58 ± 0.32 | 0.37 ± 0.12 | 2.29 ± 0.29 | 0.41 ± 0.06 |
| Glucose-insulin product | 204 ± 15 | 20 ± 2.5 | 151 ± 26 | 16 ± 2.1 |
DISCUSSION
We observed the coordinate and reciprocal regulation of expression of enzymes involved in BCAA oxidation using GSEA of adipose tissue from mice with adipose-selective alterations in Glut4 expression. In AG4OX mice, down-regulation of the BCAA oxidative enzymes caused a significant decrease in BCAA oxidation in adipose tissue explants. This decrease could contribute to the increased circulating BCAA levels in AG4OX mice.
Results from the AG4OX mice provide insights into the mechanisms by which BCAA oxidative flux may be regulated. Activity of BCKDHC in muscle and liver is potently regulated by inhibitory phosphorylation by BCKDK in response to nutritional and hormonal cues (30). The dramatic alterations in BCAA oxidation rates in AG4OX adipose tissue explants occurred independently of changes in BCKDK expression and BCKDHC phosphorylation suggesting that there may be important differences in the mechanisms regulating BCAA metabolism in adipose tissue compared with muscle and liver.
Numerous investigators have considered BCKDHC to be the rate-determining enzyme in BCAA oxidation (33–37). According to metabolic control theory, if BCKDHC were rate-determining, down-regulation of its activity would be expected to result in accumulation of its substrates (38). Despite the significant reductions in BCKDHC expression in AG4OX adipose tissue, the accumulation of αKIV decreased 4-fold in AG4OX adipose tissue explants compared with controls. Thus, in adipose tissue explants, BCKDHC does not appear to be rate-limiting. Consistent with modern metabolic control theory (38) and as has been documented for other metabolic pathways such as the fatty acid synthesis pathway (39), small but coordinate changes in the expression level of enzymes distributed throughout a pathway can translate into substantial changes in the rate of flux through that pathway.
The results from the AG4OX mice provide additional insights into the mechanisms by which BCAA oxidative flux may be regulated. We observe a profound decrease in adipose tissue BCAA oxidation as a result of adipose-specific GLUT4 overexpression. Our results might suggest that the increased glucose flux in AG4OX adipocytes can impair BCAA oxidative flux per se. However, treating adipose tissue ex vivo with glucose either with or without insulin increases rather than decreases BCAA oxidation rates acutely (8, 40). Glucose likely exerts its positive effect on BCAA oxidation by increasing the availability of cofactors required for transamination and/or decarboxylation (41). However, our study now demonstrates that the coordinate down-regulation of BCAA oxidative enzyme expression and resulting decrease in BCAA oxidative flux is dominant over the positive effects of glucose to increase BCAA oxidative flux.
It is of interest to use these ex vivo measurements to estimate the potential contribution of adipose tissue BCAA oxidation to whole body BCAA oxidation. In our wild-type mice, adipose tissue weighs 7.5 g and lean mass weighs 20.8 g. Assuming that skeletal muscle accounts for the majority of lean mass and extrapolating our ex vivo measurements of BCAA flux averaging the soleus and Edl measurements to obtain a representative skeletal muscle flux, adipose tissue could account for oxidation of ∼950 nmol BCAAs/hour comparable to the ∼830 nmol BCAAs/hour in skeletal muscle. Despite the modestly increased adiposity in AG4OX mice (10.8 g), the BCAA oxidation rate would remain 80% lower in AG4OX (∼205 nmol/h) compared with control at the whole adipose tissue organ level. These estimates must be interpreted with the large caveats that other organs such as liver, kidney, and brain are included in the lean mass measurement. Additionally, we assume that the oxidation rate in perigonadal fat is representative of all fat pads and the average of soleus and Edl are representative of all skeletal muscle. Lastly, these calculations are based upon ex vivo measurements in which substrate availability is constant. In vivo, the actual flux rates may be significantly affected by differences in blood flow and delivery of substrate to different tissues.
Although calculated estimates of adipose tissue's capacity for BCAA oxidation indicate that adipose tissue BCAA metabolism may be physiologically significant, experimental evidence confirming this in vivo are lacking. Ex vivo, rat epididymal fat pads release glutamine and alanine in response to increasing BCAA exposure (8, 32) and BCAAs were hypothesized to provide the nitrogen for net alanine and glutamine synthesis. Arteriovenous sampling across rat inguinal fat pads and human subcutaneous fat pads confirmed net alanine and glutamine release from adipose tissue, and Frayn et al. (11, 42) suggested that BCAAs were the most likely source of nitrogen. A study in anesthetized rats failed to detect arteriovenous differences in BCAA concentrations across the inguinal fat pad although it was suggested that BCAAs derived from intracellular proteolysis were catabolized in adipose tissue and contributed to glutamine synthesis and release (11). Our data now demonstrate that in vivo, adipose tissue can avidly metabolize circulating BCAAs at least when BCAA levels are markedly elevated. We show that transplanting only 750 mg of fat (less than 10–20% of total fat mass in a lean mouse) from wild-type mice into BCAT2−/− mice is sufficient to dramatically lower circulating BCAA levels. Furthermore, plasma alanine levels increase after transplantation consistent with the potential role of adipose tissue BCAA oxidation to provide a nitrogenous source for alanine (and/or glutamine) synthesis. Insulin can reduce circulating BCAAs (43), but the reduction in plasma BCAAs in our transplant study occurs in the absence of any changes in circulating insulin. Increased BCAA catabolism in the transplanted fat most likely accounts for the dramatic decrease in circulating BCAAs in the transplanted mice though we cannot exclude indirect effects of fat transplantation to impact BCAA metabolism in other tissues. However, these results conclusively demonstrate for the first time the potential for adipose tissue to alter circulating BCAA levels in vivo.
The marked elevations in circulating BCAAs in BCAT2−/− mice have profound effects on growth, metabolism, and viability (7). It is not surprising that we did not observe normalization of these metabolic parameters in the transplanted BCAT2−/− mice because despite a 30–50% reduction in circulating BCAA levels with transplantation, the circulating BCAA levels remain severalfold higher than in wild-type mice.
Additional putative consequences of elevated circulating BCAAs are through mTOR signaling either as a nutrient signal to increase protein synthesis or by contributing to down-regulation of insulin signaling. Leucine in synergy with insulin activates the mTOR signaling pathway (44). Prolonged mTOR activation negatively feeds back on the insulin signaling pathway via inhibitory serine phosphorylation of IRS1 by S6K (45, 46). In our study, insulin-stimulated S6K activity in AG4OX adipose tissue is markedly increased compared with controls, but activity in muscle and liver is not different from controls. These results agree with other studies that have shown that increased circulating leucine alone is insufficient to increase mTOR signaling in all tissues (47). In AG4OX adipose tissue, either the increased glucose flux or the increased circulating BCAAs may contribute to the increased insulin-stimulated SK6 activity.
Whereas the physiologic role of adipose tissue BCAA oxidation remains uncertain, our observations support the conclusion that the coordinate down-regulation of BCAA oxidative enzymes may dramatically alter adipose tissue BCAA oxidative flux. This is of interest because down-regulation of adipose tissue BCAA oxidative enzymes has also recently been observed in obese humans and the expression inversely correlates with insulin-resistance (14, 15, 48). Adipose tissue BCAA enzyme expression increases with surgically-induced weight loss (15) or thiazolidinedione treatment (48) and parallels improvements in insulin sensitivity. In addition, in an unbiased metabolomics-based profiling approach, an elevated circulating BCAA-related metabolic “signature” best predicted insulin-resistance in human subjects (13).
The down-regulation of BCAA oxidative enzymes in AG4OX mice may provide a new perspective on the physiologic significance of adipose tissue BCAA oxidation. Physiologic states in which the capacity of adipose tissue to catabolize BCAAs falls rapidly are fasting (8, 27, 49) or feeding a protein-deficient diet (8). The rapid decrease in adipose tissue capacity to degrade BCAAs with fasting has been suggested to preserve BCAAs for glucose production or ketogenesis and prevent their irreversible conversion to lipids for storage (27). There is indirect support for this in a study suggesting that the proportion of radiolabeled leucine carbon skeletons stored as lipid decreased with fasting (8). The down-regulation of BCAA oxidation in AG4OX adipose tissue may represent a physiological adaptation to protect against fasting hypoglycemia (Fig. 3) by preserving BCAA carbon for gluconeogenesis and ketogenesis in the liver rather than lipogenesis in adipose tissue.
Goodman and Frick (41) suggested that the down-regulation of BCAA enzymes with fasting or protein deficiency may be signaled by decreased insulin or leucine per se. In the AG4OX mice, coordinate down-regulation of the adipose tissue BCAA oxidation enzymes occurs despite the fact that circulating leucine and insulin levels are increased or normal. Thus alterations in plasma insulin or leucine levels cannot explain the regulation of BCAA oxidative enzymes in this model although this does not exclude the possibility of a novel circulating factor.
In conclusion, the present study demonstrates the potential capacity for adipose tissue to regulate circulating BCAAs in vivo. Further it shows an important relationship in vivo between regulation of adipose glucose and BCAA metabolism. Understanding the mechanistic relationships between adipose tissue BCAA, glucose, and lipid metabolism may provide new avenues for treating conditions associated with altered fuel metabolism.
Supplementary Material
Acknowledgments
We thank J. Villoria for technical assistance, T. Martin for technical instruction, J. Blenis for antibodies and helpful discussion, R. Harris for antibodies, the Vanderbilt Mouse Metabolic Phenotyping Center for measuring BCAA levels, and C. Newgard for initial pilot measurements.
This work was supported, in whole or in part, by National Institutes of Health Grants R01DK43051 and P30DK57521 (to B. B. K.), Grant K08DK076726 (to M. A. H.), and Grant R01DK062880 (to C. J. L.). This work was also supported by a grant from the Picower Foundation (to B. B. K.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- BCAA
- branched chain amino acids
- BSA
- bovine serum albumin
- DTT
- dithiothreitol
- WT
- wild type
- GSEA
- gene set enrichment analysis
- BCKDK
- branched chain keto acid dehydrogenase kinase
- ANOVA
- analysis of variance
- PMSF
- phenylmethylsulfonyl fluoride.
REFERENCES
- 1.Layman D. K. (2003) J. Nutr. 133, 261S–267S [DOI] [PubMed] [Google Scholar]
- 2.Ichihara A., Koyama E. (1966) J. Biochem. 59, 160–169 [DOI] [PubMed] [Google Scholar]
- 3.Wahren J., Felig P., Hagenfeldt L. (1976) J. Clin. Invest. 57, 987–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fajans S. S. (1965) N. Engl. J. Med. 272, 1224–1227 [DOI] [PubMed] [Google Scholar]
- 5.Hay N., Sonenberg N. (2004) Genes Dev. 18, 1926–1945 [DOI] [PubMed] [Google Scholar]
- 6.Cota D., Proulx K., Smith K. A., Kozma S. C., Thomas G., Woods S. C., Seeley R. J. (2006) Science 312, 927–930 [DOI] [PubMed] [Google Scholar]
- 7.She P., Reid T. M., Bronson S. K., Vary T. C., Hajnal A., Lynch C. J., Hutson S. M. (2007) Cell Metab. 6, 181–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tischler M. E., Goldberg A. L. (1980) J. Biol. Chem. 255, 8074–8081 [PubMed] [Google Scholar]
- 9.Rosenthal J., Angel A., Farkas J. (1974) Am. J. Physiol. 226, 411–418 [DOI] [PubMed] [Google Scholar]
- 10.Suryawan A., Hawes J. W., Harris R. A., Shimomura Y., Jenkins A. E., Hutson S. M. (1998) Am. J. Clin. Nutr. 68, 72–81 [DOI] [PubMed] [Google Scholar]
- 11.Kowalski T. J., Wu G., Watford M. (1997) Am. J. Physiol. 273, E613–622 [DOI] [PubMed] [Google Scholar]
- 12.Felig P., Marliss E., Cahill G. F., Jr. (1969) N. Engl. J. Med. 281, 811–816 [DOI] [PubMed] [Google Scholar]
- 13.Newgard C. B., An J., Bain J. R., Muehlbauer M. J., Stevens R. D., Lien L. F., Haqq A. M., Shah S. H., Arlotto M., Slentz C. A., Rochon J., Gallup D., Ilkayeva O., Wenner B. R., Yancy W. S., Jr., Eisenson H., Musante G., Surwit R. S., Millington D. S., Butler M. D., Svetkey L. P. (2009) Cell Metab. 9, 311–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pietiläinen K. H., Naukkarinen J., Rissanen A., Saharinen J., Ellonen P., Keränen H., Suomalainen A., Götz A., Suortti T., Yki-Järvinen H., Oresic M., Kaprio J., Peltonen L. (2008) PLoS Med. 5, e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.She P., Van Horn C., Reid T., Hutson S. M., Cooney R. N., Lynch C. J. ( 2007) Am. J. Physiol. Endocrinol. Metab. 293, E1552– 1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shepherd P. R., Gnudi L., Tozzo E., Yang H., Leach F., Kahn B. B. (1993) J. Biol. Chem. 268, 22243–22246 [PubMed] [Google Scholar]
- 17.Abel E. D., Peroni O., Kim J. K., Kim Y. B., Boss O., Hadro E., Minnemann T., Shulman G. I., Kahn B. B. (2001) Nature 409, 729–733 [DOI] [PubMed] [Google Scholar]
- 18.Shepherd P. R., Kahn B. B. (1999) N. Engl. J. Med. 341, 248–257 [DOI] [PubMed] [Google Scholar]
- 19.Gavrilova O., Marcus-Samuels B., Graham D., Kim J. K., Shulman G. I., Castle A. L., Vinson C., Eckhaus M., Reitman M. L. (2000) J. Clin. Invest. 105, 271–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C., Wong W. H. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramanian A., Tamayo P., Mootha V. K., Mukherjee S., Ebert B. L., Gillette M. A., Paulovich A., Pomeroy S. L., Golub T. R., Lander E. S., Mesirov J. P. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 15545–15550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joshi M. A., Jeoung N. H., Obayashi M., Hattab E. M., Brocken E. G., Liechty E. A., Kubek M. J., Vattem K. M., Wek R. C., Harris R. A. (2006) Biochem. J. 400, 153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lynch C. J., Halle B., Fujii H., Vary T. C., Wallin R., Damuni Z., Hutson S. M. (2003) Am. J. Physiol. Endocrinol. Metab. 285, E854–E863 [DOI] [PubMed] [Google Scholar]
- 24.Yang Q., Graham T. E., Mody N., Preitner F., Peroni O. D., Zabolotny J. M., Kotani K., Quadro L., Kahn B. B. (2005) Nature 436, 356–362 [DOI] [PubMed] [Google Scholar]
- 25.Harper A. E., Miller R. H., Block K. P. (1984) Annu. Rev. Nutr. 4, 409–454 [DOI] [PubMed] [Google Scholar]
- 26.Sweatt A. J., Wood M., Suryawan A., Wallin R., Willingham M. C., Hutson S. M. (2004) Am. J. Physiol. Endocrinol. Metab. 286, E64–E76 [DOI] [PubMed] [Google Scholar]
- 27.Odessey R. (1986) in Problems and Potential of Branched Chain Amino Acids in Physiology and Medicine ( Odessey R. ed) pp.49– 79, Elsevier, New York [Google Scholar]
- 28.Harris R. A., Joshi M., Jeoung N. H. (2004) Biochem. Biophys. Res. Commun. 313, 391–396 [DOI] [PubMed] [Google Scholar]
- 29.Harris R. A., Kuntz M. J., Simpson R. (1988) Methods Enzymol. 166, 114–123 [DOI] [PubMed] [Google Scholar]
- 30.Popov K. M., Zhao Y., Shimomura Y., Jaskiewicz J., Kedishvili N. Y., Irwin J., Goodwin G. W., Harris R. A. (1995) Arch Biochem. Biophys 316, 148–154 [DOI] [PubMed] [Google Scholar]
- 31.Crofford O. B., Felts P. W., Lacy W. W. (1964) Proc. Soc. Exp. Biol. Med. 117, 11–14 [DOI] [PubMed] [Google Scholar]
- 32.Snell K., Duff D. A. (1977) Biochem. Biophys. Res. Commun. 77, 925–931 [DOI] [PubMed] [Google Scholar]
- 33.Hutson S. M. (1987) J. Biol. Chem. 262, 9629–9635 [PubMed] [Google Scholar]
- 34.Block K. P., Richmond W. B., Mehard W. B., Buse M. G. (1987) Am. J. Physiol. 252, E396–E407 [DOI] [PubMed] [Google Scholar]
- 35.Mitsubuchi H., Owada M., Endo F. (2005) J. Nutr. 135, 1565S–1570S [DOI] [PubMed] [Google Scholar]
- 36.Harris R. A., Kobayashi R., Murakami T., Shimomura Y. (2001) J. Nutr. 131, 841S–845S [DOI] [PubMed] [Google Scholar]
- 37.Norton L. E., Layman D. K. (2006) J. Nutr. 136, 533S–537S [DOI] [PubMed] [Google Scholar]
- 38.Fell D. A. (2005) J. Exp. Bot. 56, 267–272 [DOI] [PubMed] [Google Scholar]
- 39.Horton J. D., Goldstein J. L., Brown M. S. (2002) J. Clin. Invest. 109, 1125–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodman H. M. (1977) Am. J. Physiol. 233, E97–E103 [DOI] [PubMed] [Google Scholar]
- 41.Goodman H. M., Frick G. P. (1986) in Problems and Potential of Branched Chain Amino Acids in Physiology and Medicine ( Odessey R. ed) pp.173– 198, Elsevier, New York [Google Scholar]
- 42.Frayn K. N., Khan K., Coppack S. W., Elia M. (1991) Clin. Sci. 80, 471–474 [DOI] [PubMed] [Google Scholar]
- 43.Zinneman H. H., Nuttall F. Q., Goetz F. C. (1966) Diabetes 15, 5–8 [DOI] [PubMed] [Google Scholar]
- 44.Greiwe J. S., Kwon G., McDaniel M. L., Semenkovich C. F. (2001) Am. J. Physiol. Endocrinol. Metab. 281, E466–E471 [DOI] [PubMed] [Google Scholar]
- 45.Tremblay F., Marette A. (2001) J. Biol. Chem. 276, 38052–38060 [DOI] [PubMed] [Google Scholar]
- 46.Um S. H., Frigerio F., Watanabe M., Picard F., Joaquin M., Sticker M., Fumagalli S., Allegrini P. R., Kozma S. C., Auwerx J., Thomas G. (2004) Nature 431, 200–205 [DOI] [PubMed] [Google Scholar]
- 47.Lynch C. J., Hutson S. M., Patson B. J., Vaval A., Vary T. C. (2002) Am. J. Physiol. Endocrinol. Metab. 283, E824–E835 [DOI] [PubMed] [Google Scholar]
- 48.Sears D. D., Hsiao G., Hsiao A., Yu J. G., Courtney C. H., Ofrecio J. M., Chapman J., Subramaniam S. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 18745–18750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meikle A. W., Klain G. J. (1972) Am. J. Physiol. 222, 1246–1250 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.