Abstract
FREQUENCY (FRQ) is the central component of the Neurospora circadian clock. All FRQ proteins form the FFC complex with FRH (FRQ-interacting RNA helicase) that acts as the negative element in the circadian negative feedback loop by repressing frq mRNA levels. To understand the function of the FRQ-FRH interaction, we mapped and identified the minimal FRQ region that is required for FRQ-FRH interaction. We demonstrated that the FRQ-FRH complex formation is required for the interaction between FRQ and the White Collar Complex (WCC) and clock function. On the other hand, in the FRQ-FRH complex, FRQ is also required for the FRH-WCC interaction. Disruption of FRQ-FRH interaction or down-regulation of FRH results in hypophosphorylation, rapid degradation of FRQ, as well as low levels of WHITE COLLAR-1 and WHITE COLLAR-2. Furthermore, we showed that the rapid FRQ degradation in the absence of FRH is independent of FWD-1, the ubiquitin E3 ligase of FRQ under normal conditions, thus uncovering an alternative pathway for FRQ degradation.
Keywords: Organisms/Fungi, Phosphorylation/Kinases/Serine-Threonine, Phosphorylation/Phosphatases/Serine-Threonine, Phosphorylation/Transcription Factors, Proteases/Ubiquitination, Protein, Protein/Turnover, Circadian Clock
Introduction
The circadian clocks allow organisms to adjust their molecular, cellular, and physiological activities to the daily environmental changes. The eukaryotic circadian oscillators consist of positive elements and autoregulatory negative-feedback loops, which control the rhythmic expression of the negative elements and clock-controlled genes (1–3). In the filamentous fungus Neurospora, one of the best understood circadian model systems (4, 5), WHITE COLLAR-1 (WC-1) and WC-2 form the WC complex (WCC),3 which binds to frequency (frq) promoter and activates its transcription, thus serving as the positive elements in the circadian OSCILLATOR (6–12). FRQ is a core regulator modulating Neurospora circadian clock in a negative feedback loop (13, 14). FRQ forms homodimers, and all the FRQ protein is in complex with FRH (FRQ-interacting RNA helicase) and forms the FFC complex (FFC) (15). FFC represses the binding of WCC to frq gene promoter and frq transcription as a consequence (9, 10, 14–16). FRH is the homolog of Dob1p/Mtr4p in budding yeast, which is a cofactor of the exosome complex (15, 17). The exosome complex is involved in processing, maturation, and turnover of various RNA species (18, 19).
To close the circadian negative feedback loop, FFC represses frq mRNA levels by two mechanisms. On the one hand, FFC represses WCC activity on frq transcription by promoting WC phosphorylation, a process that requires the FFC-WCC interaction (11, 12, 14, 15, 20, 21). On the other hand, FFC promotes the degradation of frq RNA through the exosome complex (22). The combination of these transcriptional and post-transcriptional regulations on frq results in the increase and decrease in frq levels and a robust frq rhythm during a circadian cycle (23).
After its synthesis, FRQ protein is progressively phosphorylated and becomes extensively phosphorylated before its degradation, resulting in a robust rhythm of its phosphorylation profile (23–25). FRQ phosphorylation is known to promote FRQ degradation through the ubiquitin-proteasome pathway mediated by ubiquitin E3 ligase SCFFWD-1 (26, 27). FRQ is phosphorylated by several kinases, including CK-1a, CKII, protein kinase A, and CAMK-1 (12, 23, 28–31). FWD-1, the Neurospora homolog of the mammalian β-transducin repeat-containing proteins, is the substrate-recruiting subunit of the SCF E3 complex that recognizes and binds phosphorylated FRQ to mediate its ubiquitylation and degradation (26, 27). In the fwd-1 mutant, FRQ became hyperphosphorylated, and its normal degradation pathway is blocked, resulting in high FRQ levels and arrhythmic conidiation. It is not clear whether other alternative degradation pathways are involved in FRQ degradation.
Here, we investigated the functional importance of the FRQ-FRH interaction. By mapping and identifying the minimal FRQ region that is required for FFC formation, we demonstrated that the FRQ-FRH interaction is required for FRQ-WC interaction and clock function. In contrast to the previously known mechanism for FRQ degradation, we showed that the disruption of FRQ-FRH interaction or down-regulation of FRH results in hypophosphorylation and rapid degradation of FRQ, indicating that the FRH is required for maintaining the steady state level of FRQ. Furthermore, we showed that the degradation of FRQ in the absence of FRH is mediated by unknown pathway that is independent of FWD-1. Together, these results demonstrate multiple roles of FRH in the Neurospora circadian clock.
EXPERIMENTAL PROCEDURES
Constructs, Strains, and Culture Conditions
The bd, a strain was used as the wild-type strain in this work. 303-3 (bd, his3, frq10), the frq null strain, is the host strain for all FRQ mutant constructs (13). The FRH knockdown strain (dsfrh) was described previously (15). In the dsfrh strain, addition of quinic acid (QA) triggers the silencing of the frh gene. frq10, qa-FRQ is a frq null strain transformed with a construct expressing frq under the control of the qa-2 promoter (8).
pkaj120 plasmids bearing different mutations (Fig. 1A) or deletions were transformed into frq10 host strain at the his-3 locus to generate FRQ6A, FRQ6B1, FRQ6B2, FRQ6B3, FRQ6B4, and FRQ6B5 strains, respectively. The deletions or mutations were generated by site-directed mutagenesis using QuikChange site-directed mutagenesis kit. The deleted sequences and the mutated amino acids are indicated in Fig. 1. Wild-type pkaj120 was also transformed to frq10 strain to generate the frq10, kaj120 strain, which was used as a control strain.
FIGURE 1.
Mapping of the minimal region for FFC association. A, sequence alignments showing the sequence consensus of FRQ proteins from various fungi as well as respective mutated regions or residues of sFRQ6 mutants. Residues labeled with “A” above were mutated to alanine. B–D, immunoprecipitation results using FRH antiserum showing the interaction between FRH and FRQ in different FRQ mutants. PI, preimmune serum used in IP. wt, wild type. N. crassa, Neurospora crassa; Sordaria fimicola, S. fimicola; Lyreidus australiensis, L. australiensis; Chaunus spinulosus, C. spinulosus; Gibberella zeae, G. zeae; Magnaporthe grisea, M. grisea; Pred. sec. struc., predicted secondary structure.
The liquid culture experiments were performed in 2% LCM media containing 1× Vogel's medium and 0.17% arginine, with 2% glucose. Conidia were used to seed mycelial mats in 75-mm Petri dishes, and 0.5-cm disks were cut from these and used for 50-ml cultures. Mats were harvested by filtration, frozen in liquid nitrogen, and ground with liquid nitrogen. For liquid cultures containing QA, 0.01 m QA (pH 5.8) was added into liquid culture medium containing 1× Vogel's medium, 0.1% glucose, and 0.17% arginine (15).
Plasmids
To construct the pkaj120 plasmids comprising different mutations, the mutations were first generated in partial frq sequence cloned in PUC19 vector, by site-directed mutagenesis strategy through overlap extension using PCR with primers harboring respective mutations. Next, the fragments were subcloned into pkaj120 through several specific restriction enzyme sites.
Generation of fwd-1RIP Knockdown Strain
To generate the fwd-1RIP, dsfrh strain, the pdsfrh plasmid was transformed into the 315-13 (fwd-1RIP, his-3) strain at the his-3 locus. 315-13 (fwd-1RIP, his-3) is a strain in which the fwd-1 was disrupted. For the silencing of frh in fwd-1RIP, dsfrh is inducibly expressed by the addition of QA.
Medium for race tube assays contained 1× Vogel's medium, 2% glucose, 50 ng of biotin/ml, and 1.5% agar. After transfer to dark, the growth fronts were marked on the race tubes at certain times every 24 h. The QA concentration for the race tube assays was 10−3 m.
Northern Blot Analysis
RNA extraction and Northern blot analyses were performed as described previously (13). Equal amounts of total RNA (20 μg) were loaded onto agarose gels for electrophoresis, and the gels were blotted and probed with frq probes.
Protein Extraction, Western Analysis, and Co-immunoprecipitation Analyses
Protein extraction, quantification, Western blot analysis, and immunoprecipitation assays were performed as described previously (30). Equal amounts of total protein (40 μg) were loaded in each protein lane of SDS-PAGE, and after electrophoresis, proteins were transferred onto polyvinylidene difluoride membrane, and Western blot analyses were performed. The immunoprecipitation assays were conducted by using specific antisera (IP) or preimmune sera (PI). Membranes stained with Amido Black (Sigma) were used as loading control for some Western blot analyses. Data are mean ± S.D., based on independent experiments.
Protein Stability Assay
To measure FRQ stability, the liquid cultures of Neurospora were treated with 10 μg/ml CHX and harvested at 0, 3, 6, and 9 h, respectively. Proteins were extracted and loaded (40 μg) for SDS-polyacrylamide gel running, followed by Western blots analyses of FRQ protein. The QA concentration for the protein stability assay in dsfrh and fwd-1RIP, dsfrh strains was 0.01 m.
RESULTS
Mapping of the Minimal Region Required for FRQ-FRH Interaction
We have previously shown that the region between amino acids 695 and 778 of FRQ (the region deleted in sFRQ6 mutant) is necessary and sufficient for the interaction between FRQ and FRH (Fig. 1A) (15). To map the minimal FRQ-FRH interacting region, we created two additional FRQ internal deletion mutants (frq10, FRQ6A and frq10, FRQ6B) with amino acids 695–727 and 728–778 deleted, respectively. Immunoprecipitation assays using FRH antibody showed that deletion of the FRQ6B region, but not FRQ6A, completely abolished the interaction between FRQ and FRH, indicating that a region within FRQ6B is responsible for the FFC formation (Fig. 1B).
The comparison of amino acid sequences of different FRQ homologs revealed several clusters of conserved amino acids within the FRQ6B region (Fig. 1A). We reasoned that one or some of these conserved clusters constitute the minimal region that mediates the FRQ-FRH interaction. Thus, we generated five alanine scan mutants (frq10, FRQ6B1–5), in which the conserved clusters were mutated to alanine residues (Fig. 1A). Immunoprecipitation assays showed that that the FRQ-FRH interaction was abolished in FRQ6B2 and FRQ6B5 mutants but not in FRQ6B1, FRQ6B3, and FRQ6B4 strains (Fig. 1, C and D).
FRQ levels in the FRQ6B2 and FRQ6B5 are very low. The comparison of frq mRNA levels by Northern blot analysis showed that the frq levels were comparable in these two mutants and the wild-type strains (supplemental Fig. 1, A and B), suggesting that that the FRQ-FRH interaction is important for maintaining the steady state level of FRQ at a post-translational level.
To examine whether the elimination of the FRQ-FRH interaction in the FRQ6B2 and FRQ6B5 mutants was due to low FRQ levels in these strains, we performed FRH immunoprecipitation assay in a frq null strain (frq10, qa-FRQ) with a construct that can inducibly express FRQ in the presence of QA. The level of FRQ in this strain is dependent on the concentration of QA. As shown in supplemental Fig. 1C, the levels of FRQ in frq10, qa-FRQ strain were comparable with those in the FRQ6B2 and FRQ6B5 mutants. However, the FRH immunoprecipitation assay showed that FRH was associated with FRQ in the frq10, qa-FRQ strain but not in the FRQ6B2 and FRQ6B5 mutants, indicating that the abolishment of FRQ-FRH interaction in these two mutants was not due to their low FRQ levels. Together, these results strongly suggest that the region from 773 to 782 amino acids mediates the association between FRH and FRQ. This region of FRQ consists of mostly hydrophobic residues and is predicted to form a β-strand structure. The conservation of this region in other fungal FRQ sequences suggests it is a functionally important domain for FRQ.
FRH Mediates the Association between FRQ and WCC Complex
FFC acts as negative element in the circadian negative feedback loop that represses the activity of the WCC. To understand the role of FRQ-FRH interaction in this process, we examined the association between FRQ and WCC in the FRQ deletion strains (FRQ6B1–5). Immunoprecipitation assays using WC-2 antibody showed that the FRQ-WCC interaction was abolished in the FRQ6B2 and FRQ6B5 mutants but was maintained in FRQ6B1, FRQ6B3, and FRQ6B4 strains (Fig. 2, A and B). These results are consistent with our previous results (15) and indicate that FRH mediates the interaction between FRQ and WCC.
FIGURE 2.
FRH regulates the association between FRQ and WCC. A and B, IP results show that WC-2 fails to interact with FRQ or FRH in the FRQ6B2 and FRQ6B5 mutants, by using WC-2 antiserum or preimmune serum (PI). wt, wild type. B, longer exposure of FRH is also presented. C, race tube assays of FRQ6B mutants. The strains were grown in constant darkness (DD) in race tubes, and the growth fronts were labeled every 24 h. The race tubes shown here are representative samples from six replicate tubes.
Consistent with the molecular results, race tube assays showed that the circadian conidiation rhythms were completely abolished in the FRQ6B2 and FRQ6B5 strains. In contrast, clear but low amplitude circadian conidiation rhythms could be observed in the FRQ6B3 and FRQ6B4 mutants (Fig. 2C). These results indicate that the FRQ-FRH interaction is required for clock function. Interestingly, the FRQ6B1 strain also exhibited arrhythmic conidiation, suggesting that the five conserved amino acids immediately upstream of the minimal FRQ-FRH interaction domain may contribute to FRQ function although not FFC formation. Interestingly, FRQ6A also exhibited arrhythmic conidiation on race tube, indicating that another functionally important domain within the region deleted in FRQ6A.
Both FRQ and FRH Contribute to the FFC-WCC Interaction
We have previously shown that FRH associates with WCC in the frq null strain (frq10), indicating that FRH can interact with WCC independent of FRQ (15). However, it is unclear whether FRQ also contributes to FFC-WCC interaction. To understand the role of FRQ in this interaction, we conducted immunoprecipitation assays using WC-2 antibody in a series of FRQ internal deletion mutants (sFRQ1–8) (11), which covers most of the FRQ open reading frame (Fig. 3A). As expected, FRH-WCC was abolished in the sFRQ6 mutant. Although FRH associated with WCC in the sFRQ3, sFRQ5a, and sFRQ8 strains, surprisingly, the interaction was completely eliminated in the sFRQ1, sFRQ2, sFRQ4, sFRQ5b, and sFRQ7 mutants. These results demonstrate that although FRH can interact with WCC in the total absence of FRQ, FRQ also contributes to the interaction between FFC and WCC. Thus, in a wild-type strain, FFC interacts with WCC as a complex but not as individual proteins. It is likely that the presence of FRQ can interfere or block the interaction surface of FRH that can associate with WCC in the frq null strain.
FIGURE 3.
FRQ participates in regulation of the FRH-WCC interaction. A, IP results show that WC-2 binds to FRQ, FRH, and WC-1 in the FRQ6B1, FRQ6B3, and FRQ6B4 mutants. WC-2 antibody was used in the IP assays and the preimmune serum for the PI sample. WT, wild type. B, IP results showing FRH binds to FRQ in both frq9 Myc-FRQ165RR strain and frq9 Myc-FRQ strains. FRH antibody was used for the IP assays and preimmune serum for the PI sample. FRH antiserum and c-Myc antibody were used for the Western blot analyses.
Consistent with our previous results (15), we found that in FRQ mutants lacking the FRH-WCC interaction (sFRQ1, sFRQ2, sFRQ4, sFRQ5b, sFRQ6 and sFRQ7), the association between FRQ and WCC was also abolished. In contrast, the FRQ-WCC interaction was maintained in the sFRQ3, sFRQ5a, and sFRQ8 strains (Fig. 3A). These results provide further evidence supporting the importance of both FRQ and FRH in mediating the FFC-WCC interaction and strongly suggest that both FRQ and FRH are required for the FFC-WCC interaction, and multiple domains of FRQ are involved in this process.
We previously showed that FRQ forms homodimer through its coiled-coil domain (32). To determine whether FRQ interacts with FRH independent of its homodimer formation, we examined the FRQ-FRH interaction in the frq9, Myc-FRQ165RR strain. In this strain, two point-mutations in the coiled-coil domain abolished the FRQ-FRQ interaction (32). The immunoprecipitation assay showed that FRQ-FRH was maintained in this strain (Fig. 3B), indicating that the FRQ-FRH interaction is not dependent on FRQ dimerization.
Disruption of FRQ-FRH Interaction or Down-regulation of FRH Results in Hypophosphorylated FRQ
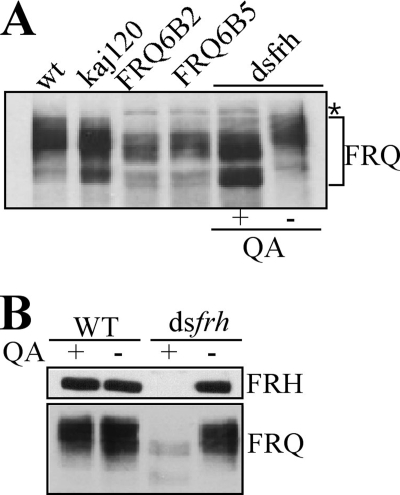
FRQ levels are low in strains that either have the FRQ-FRH interaction abolished or FRH expression silenced (Fig. 1D) (15). Because FRQ phosphorylation promotes FRQ degradation, we examined the FRQ phosphorylation profiles in these strains (Fig. 4A). Because of low levels of FRQ in these mutants, the total proteins for the Western blot analysis were adjusted so that similar amounts of FRQ were present in each sample. Surprisingly, we found that FRQ was hypophosphorylated in the FRQ6B2 and FRQ6B5 strains. Similarly, hypophosphorylated FRQ was found in the presence of quinic acid in the dsfrh strain (Fig. 4, A and B). The dsfrh strain contains a construct that can inducibly express a hairpin RNA specific for frh mRNA with the addition of QA (15). As control, QA had no effect on FRQ level or FRQ phosphorylation profile in a wild-type strain. Note that for the gel in Fig. 4A, more total protein was loaded for the dsfrh sample with QA so that the FRQ levels were similar. These results indicate that the normal FRQ phosphorylation process requires FRH and the FRQ-FRH interaction.
FIGURE 4.
Hypophosphorylation of FRQ in the FRQ6 mutants and dsfrh strain. A, hypophosphorylation of FRQ in FRQ6B2, FRQ6B5, mutants, and dsfrh strain. For comparison, total proteins of the mutants were loaded more than wild type (wt) in the Western blot analyses due to the low FRQ protein levels in the mutants. B, hypophosphorylation of FRQ in wild type (WT) and dsfrh strains by QA treatment. Wild-type strain treated with QA served as control. Asterisk denotes nonspecific bands due to long exposure.
Disruption of FRQ-FRH Interaction or Down-regulation of FRH Results in Rapid FRQ Degradation
The low FRQ levels in strains lacking either the FRQ-FRH interaction or FRH expression suggest that FRQ may be unstable in these strains. To test this hypothesis, we compared the FRQ stability after the addition of the protein synthesis inhibitor CHX. Upon addition of CHX, FRQ was degraded much faster in the FRQ6B2 and FRQ6B5 strains than the strain carrying a wild-type frq construct (kaj120) (Fig. 5A). Despite the low levels of the hypophosphorylated FRQ in the mutant strains at time 0, FRQ was rapidly degraded after the CHX treatment. Interestingly, unlike the KAJ120 strain, in which FRQ first became hyperphosphorylated before its degradation, most of FRQ in the mutant strains was degraded within the first 3 h after the addition of CHX. This result suggests that the wild-type and mutant FRQ were degraded by different mechanisms.
FIGURE 5.
Rapid degradation of FRQ protein in the sFRQ6 mutants and dsfrh strain. A, degradation of FRQ in the FRQ6B2 and FRQ6B5 strains. The frq10,kaj120 strain was used as the control. B, degradation of FRQ in the dsfrh strain with/without QA treatment. The stability of FRQ was also compared in wild type (wt) strain with/without addition of QA, which indicated that QA is not the cause for the change of FRQ degradation rate. Membranes (mem) stained with Amido Black were used as loading control. Cycloheximide was added to inhibit the protein translation (10 μg/ml final concentration). Data are means ± S.D., n = 3. Asterisks indicate p < 0.05.
Similarly, rapid degradation of FRQ was also observed in the dsfrh strain when frh was silenced by the QA treatment (Fig. 5B). In contrast, the FRQ stability was not affected by the QA treatment in the wild-type strain. Together, these results demonstrate that FRH and the FRQ-FRH interaction stabilize FRQ. The rapid degradation of FRQ in the absence of FRH provides a molecular explanation to the low FRQ protein levels when FRH is silenced.
Disruption of FRQ-FRH Interaction or Down-regulation of FRH Results in Reduction of WC Proteins
We also compared the WC protein levels between the wild-type and the FRQ6B2 and FRQ6B5 strains. As shown in Fig. 6, A and B, both WC-1 and WC-2 levels were significantly reduced in FRQ6B2 and FRQ6B5 strains. In the wt,dsfrh strain, the silencing of FRH also resulted in the reduction of WC levels (Fig. 6, C and D). The comparison of WC stability between the wild-type and the mutant strains showed that WC stability was not significantly changed in these mutant strains (supplemental Fig. 2). Thus, the low levels of WCs in these strains indicate the loss of positive feedback loops that maintain the WC levels (8, 33). Therefore, as FRQ and FRH also plays an important role in the positive feedback loops of the clock (33).
FIGURE 6.
Abolishment of FRH-FRQ association resulted in decrease of WC proteins. A–F, Western blot analysis results showing the levels of WCs in the indicated strains. Amido Black-stained membranes were used as loading control. WT, wild type.
FRH Mediates the Degradation of FRQ Protein Independent of SCFFWD-1
FWD-1, the substrate-recruiting subunit of an SCF complex, mediates the phosphorylation-dependent ubiquitination and degradation of FRQ (26, 27, 34). The hypophosphorylation and rapid degradation of FRQ in the mutants shown above suggest an alternative FRQ degradation pathway. To test this hypothesis and to examine the role of FWD-1 in the degradation of FRQ in the absence of FRH, we transformed the dsfrh construct into a fwd-1RIP strain (fwd-1RIP,dsfrh), so that the expression of frh in the fwd-1RIP strain can be silenced by the addition of QA. As shown by the race tube assays in Fig. 7A, in the absence of QA, the fwd-1RIP,dsfrh strain exhibited arrhythmic conidiation after the 1st day, consistent with our previous results (26). The addition of QA resulted in dramatic inhibition of its growth on race tube, indicating that frh expression was silenced. In agreement with this result, FRH level was dramatically reduced in the presence of QA (Fig. 7B).
FIGURE 7.
FRH regulates FRQ stability independent of FWD-1. A, race tube tests of fwd-1RIP,dsfrh. The growth was inhibited in the presence of QA (10−3 m). B, Western blot analysis showing that FRH was silenced in fwd-1RIP,dsfrh strain by QA treatment (10−2 m). Nonspecific bands served as control. C, FRQ protein was hypophosphorylated and dramatically reduced in fwd-1RIP,dsfrh strain in the presence of QA (10−2 m). D, FRQ protein exhibited rapid degradation in the fwd-1RIP,dsfrh strain after QA treatment (10−2 m). Membranes stained with Amido Black were used as loading control. Cycloheximide was added to inhibit the protein translation (10 μg/ml final concentration). Data are mean ± S.D., n = 3. Asterisks indicate p < 0.05. WT, wild type; mem, membrane.
Consistent with our previous results (26), FRQ was hyperphosphorylated in this strain in the absence of QA (Fig. 7C). In the presence of QA, however, FRQ level was dramatically reduced, and FRQ became hypophosphorylated. The comparison of FRQ stability showed that similar to the FRQ6B2 and FRQ6B5 strains, FRQ was rapidly degraded when frh was silenced (Fig. 7D). Together, these results demonstrate that in the absence of FRH, FRQ was degraded independent of FWD-1 through an alternative pathway.
DISCUSSION
In addition to FRQ and the WCs, FRH is a core component of the Neurospora circadian oscillator. In Neurospora, all FRQ protein binds to FRH to form FFC, which acts as negative elements in the circadian negative feedback loops. In addition to its role as a transcriptional inhibitor repressing frq transcription, FFC promotes the degradation of frq mRNA through the exosome complex and is part of the post-transcriptional negative feedback loop critical for clock function (22). In this study, we established a new function of FRH in the Neurospora circadian clock, provided molecular details on how FFC complexes are formed and interact with WCC and uncovered a new pathway for FRQ degradation.
By identifying the minimal domain on FRQ that is required for the FFC formation and by creating point mutation FRQ mutants, we showed that the FRQ-FRH interaction is required for the interaction between FRQ and WCC and is essential for clock function as a consequence. Although FRH is able to interact with WCC independent of FRQ, we showed here that in the FFC, FRQ is also required for the FFC-WCC interaction. In several FRQ deletion mutants, the association between FRH and WCC is abolished although the interaction between FRQ and FRH is maintained. These results indicate that in a wild-type strain, FRQ and FRH interact with WCC as a complex, and both FRQ and FRH contribute to such an interaction.
Silencing of FRH expression results in high levels of frq mRNA (15, 22). We have shown that the high levels of frq mRNA are due to the loss of inhibition of WCC activity by the FFC and the impaired frq mRNA degradation through the exosome (22). Contrary to the high mRNA levels, FRQ protein levels are low when FRH is silenced or when the FRQ-FRH interaction was abolished. We now showed that FRQ protein is hypophosphorylated and rapidly degraded in the absence of FRH. Furthermore, we showed that FRH binds to FRQ independent of the FRQ-FRQ interaction. Together, these data suggest that FRH is critical for the maintenance of FRQ phosphorylation profile and stability. In addition, we showed that FRH and the FRH-FRQ interactions are also important for the maintenance of the levels of WC proteins in the positive feedback loops.
In contrast to the known FRQ degradation pathway, which is promoted by phosphorylation and mediated by the ubiquitin E3 ligase FWD-1, we found that FRQ is hypophosphorylated when the FRQ-FRH interaction is disrupted or when FRH is silenced. In addition, the rapid degradation of FRQ in the absence of FRH is independent of FWD-1. These results reveal an alternative FRQ degradation pathway (26, 34). It is also possible that this alternative FRQ degradation process also contributes to the overall FRQ stability under normal conditions. Consistent with this hypothesis, significant amounts of FRQ degradation were previously observed in the fwd-1 mutant strain after a light-dark transition (26). Although the molecular nature of this degradation process is currently not known, it is likely that in the absence FRH, FRQ may be adopt an alternative conformation that can be rapidly degraded in the cell (31, 32).
Supplementary Material
Acknowledgments
We thank Haiyan Yuan for technical help and thank Dr. Audrey Chang and Dr. Chi-Tai Tang for critical comments regarding the manuscript.
Note Added in Proof
Since the submission of this manuscript, a study was published describing the roles of FRH in both negative and positive feedback loops (Shi, M., Collett, M., Loros, J. J., and Dunlap, J. C. (2010) Genetics 184, 351–361).
This work was supported, in whole or in part, by National Institutes of Health Grants GM068496 and GM062591 (to Y. L.). This work was also supported by The Welch Foundation (to Y. L.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- WCC
- White Collar Complex
- FRH
- FRQ-interaction RNA helicase
- FFC
- FRQ-FRH complex
- FWD-1
- F-box/WD-40 repeat-containing protein 1
- SCFs
- SKP1-cullin-F-box
- CHX
- cycloheximide
- QA
- quinic acid
- IP
- immunoprecipitation
- PI
- preimmune.
REFERENCES
- 1.Bell-Pedersen D., Cassone V. M., Earnest D. J., Golden S. S., Hardin P. E., Thomas T. L., Zoran M. J. (2005) Nat. Rev. Genet. 6, 544–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunlap J. C., Loros J. J., Liu Y., Crosthwaite S. K. (1999) Genes Cells 4, 1–10 [DOI] [PubMed] [Google Scholar]
- 3.Young M. W., Kay S. A. (2001) Nat. Rev. Genet. 2, 702–715 [DOI] [PubMed] [Google Scholar]
- 4.Dunlap J. C., Loros J. J. (2004) J. Biol. Rhythms 19, 414–424 [DOI] [PubMed] [Google Scholar]
- 5.Heintzen C., Liu Y. (2007) Adv. Genet. 58, 25–66 [DOI] [PubMed] [Google Scholar]
- 6.Belden W. J., Loros J. J., Dunlap J. C. (2007) Mol. Cell 25, 587–600 [DOI] [PubMed] [Google Scholar]
- 7.Cheng P., He Q., Yang Y., Wang L., Liu Y. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 5938–5943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng P., Yang Y., Liu Y. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 7408–7413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crosthwaite S. K., Dunlap J. C., Loros J. J. (1997) Science 276, 763–769 [DOI] [PubMed] [Google Scholar]
- 10.Froehlich A. C., Loros J. J., Dunlap J. C. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 5914–5919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He Q., Liu Y. (2005) Genes Dev. 19, 2888–2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He Q., Cha J., He Q., Lee H. C., Yang Y., Liu Y. (2006) Genes Dev. 20, 2552–2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aronson B. D., Johnson K. A., Dunlap J. C. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 7683–7687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aronson B. D., Johnson K. A., Loros J. J., Dunlap J. C. (1994) Science 263, 1578–1584 [DOI] [PubMed] [Google Scholar]
- 15.Cheng P., He Q., He Q., Wang L., Liu Y. (2005) Genes Dev. 19, 234–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arpaia G., Loros J. J., Dunlap J. C., Morelli G., Macino G. (1993) Plant Physiol. 102, 1299–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaCava J., Houseley J., Saveanu C., Petfalski E., Thompson E., Jacquier A., Tollervey D. (2005) Cell 121, 713–724 [DOI] [PubMed] [Google Scholar]
- 18.Schmid M., Jensen T. H. (2008) Trends Biochem. Sci. 33, 501–510 [DOI] [PubMed] [Google Scholar]
- 19.Mitchell P., Petfalski E., Shevchenko A., Mann M., Tollervey D. (1997) Cell 91, 457–466 [DOI] [PubMed] [Google Scholar]
- 20.Cha J., Huang G., Guo J., Liu Y. (2007) Cold Spring Harb. Symp. Quant. Biol. 72, 185–191 [DOI] [PubMed] [Google Scholar]
- 21.He Q., Shu H., Cheng P., Chen S., Wang L., Liu Y. (2005) J. Biol. Chem. 280, 17526–17532 [DOI] [PubMed] [Google Scholar]
- 22.Guo J., Cheng P., Yuan H., Liu Y. (2009) Cell 138, 1236–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garceau N. Y., Liu Y., Loros J. J., Dunlap J. C. (1997) Cell 89, 469–476 [DOI] [PubMed] [Google Scholar]
- 24.Baker C. L., Kettenbach A. N., Loros J. J., Gerber S. A., Dunlap J. C. (2009) Mol. Cell 34, 354–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang C. T., Li S., Long C., Cha J., Huang G., Li L., Chen S., Liu Y. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 10722–10727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He Q., Cheng P., Yang Y., He Q., Yu H., Liu Y. (2003) EMBO J. 22, 4421–4430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He Q., Liu Y. (2005) Biochem. Soc. Trans. 33, 953–956 [DOI] [PubMed] [Google Scholar]
- 28.Yang Y., Cheng P., He Q., Wang L., Liu Y. (2003) Mol. Cell. Biol. 23, 6221–6228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y., Cheng P., Zhi G., Liu Y. (2001) J. Biol. Chem. 276, 41064–41072 [DOI] [PubMed] [Google Scholar]
- 30.Görl M., Merrow M., Huttner B., Johnson J., Roenneberg T., Brunner M. (2001) EMBO J. 20, 7074–7084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang G., Chen S., Li S., Cha J., Long C., Li L., He Q., Liu Y. (2007) Genes Dev. 21, 3283–3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng P., Yang Y., Heintzen C., Liu Y. (2001) EMBO J. 20, 101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee K., Loros J. J., Dunlap J. C. (2000) Science 289, 107–110 [DOI] [PubMed] [Google Scholar]
- 34.He Q., Cheng P., He Q., Liu Y. (2005) Genes Dev. 19, 1518–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.