Abstract
In the mammalian brain, acetylcholinesterase (AChE) is anchored in cell membranes by a transmembrane protein PRiMA (proline-rich membrane anchor). We present evidence that at least part of the PRiMA-linked AChE is integrated in membrane microdomains called rafts. A significant proportion of PRiMA-linked AChE tetramers from rat brain was recovered in raft fractions; this proportion was markedly higher at low rather than at high concentrations of cold Triton X-100. The detergent-resistant fraction increased during brain development. In NG108-15 neuroblastoma cells transfected with cDNAs encoding AChET and PRiMA, PRiMA-linked G4 AChE was found in membrane rafts and showed the same sensitivity to cold Triton X-100 extraction as in the brain. The association of PRiMA-linked AChE with rafts was weaker than that of glycosylphosphatidylinositol-anchored G2 AChE or G4 QN-HC-linked AChE. It was found to depend on the presence of a cholesterol-binding motif, called CRAC (cholesterol recognition/interaction amino acid consensus), located at the junction of transmembrane and cytoplasmic domains of both PRiMA I and II isoforms. The cytoplasmic domain of PRiMA, which differs between PRiMA I and PRiMA II, appeared to play some role in stabilizing the raft localization of G4 AChE, because the Triton X-100-resistant fraction was smaller with the shorter PRiMA II isoform than that with the longer PRiMA I isoform.
Keywords: Lipid/Raft, Membrane/Proteins, Acetylcholinesterase, Membrane Trafficking, Protein Targeting, Cholesterol Recognition/Interaction Consensus, Detergent Resistance, Heterogeneity, PRiMA, Tetrameric Acetylcholinesterase
Introduction
Acetylcholinesterase (AChE;2 EC 3.1.1.7) controls synaptic and neurohumoral cholinergic activity by hydrolyzing acetylcholine. The function of AChE relies on precise regulations on its expression and localization. Alternative splicing in the 3′ region of the primary transcript generates subunits of AChE that contain the same catalytic domain but distinct C-terminal peptides, which determine their post-translational maturation and oligomeric assembly (1, 2). In mammals, the AChER variant produces a soluble monomer that is reported to be up-regulated in the brain during stress (3, 4); the AChEH variant produces a glycosylphosphatidylinositol (GPI)-anchored dimer that is mainly expressed in blood cells, and the AChET variant, characterized by a 40-residue C-terminal “t” peptide, forms a number of oligomeric assemblies (5). AChET subunits are predominant in all cholinergic tissues and represent the physiologically active variant; these subunits form tetramers associated with the collagen ColQ and with the transmembrane protein PRiMA (proline-rich membrane anchor), which allow their functional localization. ColQ-associated AChE is attached to the basal lamina at vertebrate neuromuscular junctions, and PRiMA-linked AChET tetramers (G4) are anchored in cell membranes. The association of AChET subunits with ColQ and PRiMA proteins is mostly based on a tight interaction between the four C-terminal t peptides of AChET subunits and a “proline-rich attachment domain” (PRAD) that exists in both ColQ and PRiMA (6–8). In mammals, PRiMA-linked G4 AChE exists in the brain and muscle and is the predominant AChE species in the adult brain (9–12).
PRiMA is a type I transmembrane protein of ∼20 kDa, consisting of a secretory signal peptide, an N-terminal extracellular domain containing the PRAD and an N-glycosylation site, a transmembrane domain, and a C-terminal cytoplasmic domain. Two splice variants, PRiMA I and II, differ only by their C-terminal domains; the first seven cytoplasmic residues are common and include a cysteine that may represent a palmitoylation site. The cytoplasmic domain of PRiMA I (40 residues) contains several serines that are predicted to be constitutive phosphorylation sites (5, 13). The cytoplasmic domain of PRiMA II has only 11 residues and does not contain these serines. Both PRiMA I and PRiMA II can anchor AChET tetramers in cell membranes (13, 14). The expression of PRiMA I is up-regulated during development and is the major form being expressed in the brain. In contrast, PRiMA II is only detected as a minor component at the adult stage in the brain (12, 13). This regulation during brain maturation suggests that the difference between the two splice variants could have functional implications. The structure of PRiMA, and in particular its possible fatty acylation, suggests that it may interact with the cholesterol-sphingolipid-rich membrane microdomains or rafts (15, 16), which have been shown to be involved in synaptic signaling and plasticity (17, 18).
Here, we demonstrate that part of the PRiMA-linked G4 AChE is integrated in membrane rafts in the rat brain. The fraction of membrane-bound AChE recovered in raft fractions was lower in the presence of a high rather than a low detergent concentration (0.05 and 0.5% Triton X-100 in the cold). The proportion of high detergent-resistant AChE in rafts increased during brain development, suggesting that it may fulfill a possible physiological function. In cultured neuroblastoma cells, we demonstrated a critical role of PRiMA in directing the association of AChE with membrane rafts and its heterogeneity in detergent resistance. PRiMA contains a cholesterol-binding motif, or CRAC (cholesterol recognition/interaction amino acid consensus). A CRAC motif is defined as a sequence pattern (L/V)X1–5YX1–5(R/K), where X1–5 represents between one and five residues of any amino acid (19, 20). We found that this motif is absolutely required for raft association. The longer intracellular domain of PRiMA I seems to participate in the stability of this association, because the proportion of raft-associated AChE at high detergent concentration was higher with PRiMA I than with PRiMA II.
EXPERIMENTAL PROCEDURES
Cell Culture
Neuroblastoma × glioma NG108-15 hybrid cells were maintained in Dulbecco's modified Eagle's medium supplemented with 5% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin and incubated at 37 °C in a water-saturated 5% CO2 incubator. All reagents for cell cultures were from Invitrogen.
DNA Construction and Transfection
All constructs were expressed in the pEF-Bos vector (21), except rat alkaline phosphatase (ALP) tagged with a C-terminal t peptide (ALP-t), which was in pcDNA3 vector (22). Mutagenesis was performed as described previously (23). The cDNA encoding full-length mouse PRiMA isoform I was tagged with an HA epitope (YPY DVP DYA) inserted before the stop codon at the C terminus. The cDNAs encoding full-length mouse PRiMA isoform II, mouse PRiMA isoform I with C-terminal cysteine or serines mutations, and with CRAC motif mutations were tagged with the same HA epitope inserted between the signal peptide and the N terminus of the mature protein. AChET was also tagged similarly at its C terminus. NG108-15 cells were transfected using jetPEITM reagent (Polyplus transfection, Illkirch, France) according to the manufacturer's instructions. The transfection efficiency was consistently 50–60%. The cells were collected for analysis 48 h after the transfection.
Preparation of Membrane Raft Fractions
Brains were obtained from 7- (P7), 14- (P14), and 100-day-old (P100) male Sprague-Dawley rats. The preparation of raft membrane fractions was carried out at 4 °C, with different concentrations of detergent, as described previously with modifications (24, 25). In brief, fresh tissues were homogenized (1 g/10 ml) in buffer A (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 5 mm benzamidine HCl, 10 μg/ml aprotinin, 10 μg/ml leupeptin) for 10 s six times at 9,500 rpm (IKA, Staufen, Germany). The cells were also suspended in buffer A. The tissues or cell suspensions were then sonicated at low intensity three times for 0.5-s periods, with 30-s intervals to avoid heating. The preparation was centrifuged at 500 × g for 5 min to remove cell debris and nuclei, and the post-nuclear extracts of tissues or cells were centrifuged at 35,000 rpm for 30 min in a Sorvall TST 60.4 rotor. The resulting pellet was washed and resuspended by sonication in 600 μl of buffer A containing 5% glycerol. This was used as the total membrane fraction. For analysis of membrane rafts, a 500-μl sample was incubated with Triton X-100 at the indicated concentrations on ice for 1 h and applied to a discontinuous flotation gradient; it was mixed with an equal volume of 80% sucrose in buffer A, placed at the bottom of a 4-ml ultracentrifugation tube, and overlaid with 2.4 ml of buffer A containing 30% sucrose and with 0.6 ml of buffer A containing 5% sucrose. The resulting discontinuous gradient was spun at 50,000 rpm in a Sorvall TST 60.4 rotor for 18 h at 4 °C. Twenty fractions of ∼190 μl were collected from the top and used for various assays.
Cholesterol Depletion Assay
Three hours after transfer of the cells to serum-free medium, the cultures were treated with 10 mm cyclodextrin, MβCD (Sigma), for 1 h at 37 °C and analyzed as described above. The effectiveness of MβCD was determined by a cholesterol assay kit (Thermo Electron Co., Australia) and mass spectrometry (supplemental material).
Western Blots
Aliquots in 300 μl of fractions from discontinuous flotation gradients were incubated with 20% trichloroacetic acid (final concentration) on ice, and a protein pellet was obtained by high speed centrifugation at 4 °C for 15 min. The pellet was washed with ice-cold acetone twice and air-dried, then dissolved at 100 °C for 5 min in a denaturing buffer containing 1% SDS and 1% dithiothreitol, and analyzed by electrophoresis in 8 or 12% SDS-polyacrylamide gels. For Western blot analysis, we used anti-PSD 95 antibody (1:2000; Millipore, Billerica, MA), anti-flotillin-2 antibody (1:5000; BD Biosciences), anti-transferrin receptor antibody (H-300; 1:2000; Santa Cruz Biotechnology, Santa Cruz, CA), and anti-HA antibody (1:2000; Sigma) as primary antibodies; secondary anti-IgG antibodies against different species were conjugated with horseradish peroxidase. The immune complexes were visualized using the enhanced chemiluminescence method (Supersignal West Pico kit, Thermo Fisher Scientific, Waltham, MA). The exposure time was 1–10 min.
Sucrose Density Gradients
For sedimentation analyses, 100–300-μl samples were mixed with Escherichia coli β-galactosidase and ALP (Sigma), as internal sedimentation markers, layered onto 5–20% sucrose gradients (containing 50 mm Tris-HCl, pH 7.5, 20 mm MgCl2, in the presence of 0.2% Brij-97 or 0.2% Triton X-100), and centrifuged in a Beckman SW41 rotor at 38,000 rpm, for 16 h at 10 °C. Fractions of ∼200 μl were collected and assayed for AChE, β-galactosidase, and ALP activities, as described previously (7). The fraction of AChE activity corresponding to the PRiMA-linked or QN-HC-linked G4 forms was determined from the sedimentation profiles.
Enzyme Assay and Statistical Tests
AChE enzymatic activity was assayed according to a modified method of Ellman et al. (11, 26). The cell lysates were incubated with 0.1 mm tetraisopropylpyrophosphoramide (Sigma) for 10 min to inhibit the butyrylcholinesterase activity; 5–20-μl samples were then added to the reaction mixture containing 0.625 mm acetylthiocholine iodide (Sigma) and 0.5 mm 5,5′-dithiobis-2-nitrobenzoic acid (Sigma) in 80 mm Na2HPO4, pH 7.4. The increase in absorbance at 410 nm was recorded, and the specific enzyme activity was expressed as absorbance units/min/μg of protein. For the measurement of ALP activity, 50-μl samples from the gradient fractions were mixed with 50 μl of 5 mm p-nitrophenyl phosphate (Sigma) in a buffer containing 0.1 m glycine, 1 mm MgCl2, and 1 mm ZnCl2, pH 10.4. After incubation at 37 °C for 1–5 h, 50 μl of 2 m NaOH was added to stop the reaction, and the absorbance was measured at 405 nm. The statistical significance of differences between experimental data was evaluated by the PRIMER program, version 1.
RESULTS
Membrane Rafts Isolated from Adult Rat Brain Contain PRiMA-linked G4 AChE
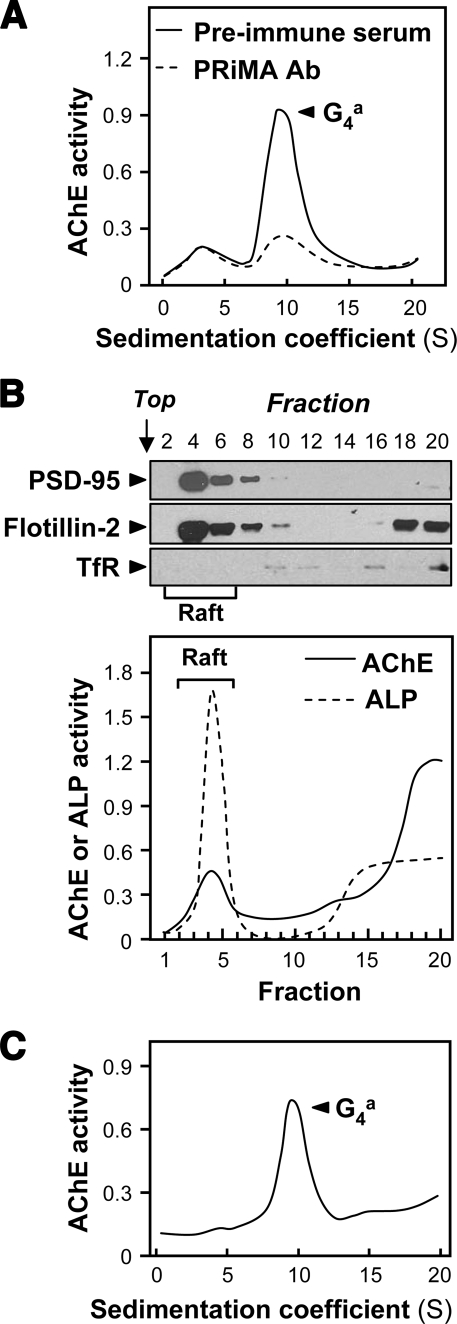
Total membrane preparations were obtained from fresh cerebra of adult rats, as described under “Experimental Procedures.” Sedimentation analysis after solubilization with 0.5% Triton X-100 showed that they contained mostly AChE tetramers (G4), with a small proportion of dimers (G2) and/or monomers (G1) (Fig. 1A). To establish the association of PRiMA with G4 AChE in the cerebrum, the protein extract was immunoprecipitated by anti-PRiMA antibody, as reported previously (11, 15, 27). As shown in Fig. 1A, the antibody treatment depleted the peak of G4 AChE, but not the G1 or G2 forms. This result confirmed that G4 AChE in the cerebrum is mostly associated with PRiMA.
FIGURE 1.
Adult rat brain contains PRiMA-linked G4 AChE in membrane rafts. A, total membrane fraction was isolated from 400 mg of fresh tissue samples of adult rat cerebrum; AChE was solubilized with 0.5% Triton X-100 and analyzed by sedimentation in sucrose gradients containing 0.2% Brij-97. To verify the presence of PRiMA, an aliquot of the extract was incubated with anti-PRiMA antibody (Ab) (10 μg/ml). After the precipitation by protein G-agarose, the supernatant was subjected to sucrose density gradient analysis. Preimmune serum was used in the control. In the sedimentation profiles, AChE activity (arbitrary units) is plotted as a function of the sedimentation coefficients, determined with the internal standards β-galactosidase (16 S) and ALP (6.1 S). The major AChE component 9.1 S corresponds to amphiphilic tetramers (G4a). B, AChE activity from cerebrum membranes in detergent-resistant (raft; fractions 2–6) and detergent-soluble (non-raft) fractions was determined after flotation in discontinuous sucrose gradients with 0.5% cold Triton X-100. Aliquots of each even fraction were analyzed by 8% SDS-PAGE, and the expressions of PSD-95 (∼95 kDa), flotillin-2 (∼55 kDa), and transferrin receptor (∼70 kDa) are shown in Western blots as controls (upper panel). Enzymatic activities of AChE and ALP are expressed in arbitrary units (lower panel). ALP serves as control to identify raft-enriched fractions. TfR, transferrin receptor. C, sedimentation profile of AChE solubilized from the raft-enriched fractions of cerebrum from B was determined. The sedimentation was performed as in A. The profile shows a single molecular form at 9.1 S corresponding to amphiphilic tetramers. For all sedimentation analyses, one representative profile is shown, n = 3.
To prepare raft-enriched fractions, the membrane pellets were treated with 0.5% Triton X-100 in the cold and subjected to flotation in discontinuous sucrose gradients. About 20% of the total membrane-bound G4 AChE activity in the cerebrum was recovered in the raft-enriched fractions at 5–30% sucrose interface. These fractions contained 60–70% of total membrane-bound ALP, which served as a membrane raft marker (Fig. 1B) (28). In addition, Western blots analyses of these raft-enriched fractions showed the presence of the raft-associated proteins flotillin-2 (29) and PSD-95 (30). The transferrin receptor, which is not associated with rafts (31), was not detected in raft-enriched fractions, but only at the bottom of the gradient, in fractions containing proteins that had been solubilized by the detergent. The distribution of all these marker proteins indicated that the method used for the isolation of membrane rafts was reliable. To analyze the molecular forms of AChE that were associated with rafts, a sample of the raft fractions was solubilized with 1% Triton X-100 and subjected to sucrose density sedimentation, as illustrated in Fig. 1C, and it contained only the PRiMA-linked G4 form.
Association of PRiMA-linked AChE with Membrane Rafts Is Developmentally Regulated
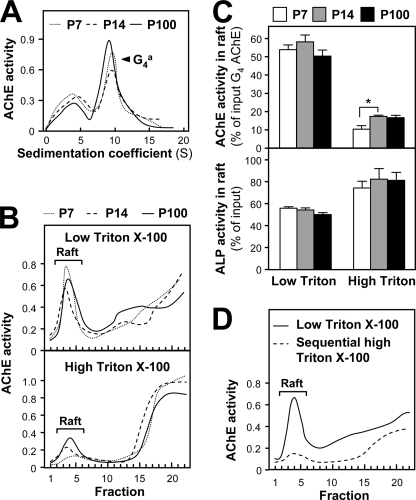
The association of PRiMA-linked G4 AChE with membrane rafts in the brain may be relevant to its physiological function. To address the significance of this association, we analyzed this raft association during maturation of the brain. Because proteins differ in the detergent sensitivity of their attachment to rafts (32, 33), we used low (0.05%) and high (0.5%) concentrations of cold Triton X-100 to evaluate the association of AChE activity with rafts in the cerebrums of P7, P14, and P100 rats. The membrane-bound amphiphilic G4 AChE was the major form of AChE at the three chosen stages (Fig. 2A). In all cases, a higher proportion of membrane-bound AChE was found to be associated with the rafts isolated with low Triton X-100 than with high Triton X-100 (Fig. 2B), which was opposite to the case of ALP (Fig. 2, B and C). The fraction of AChE activity recovered in low detergent-resistant rafts remained essentially constant (50–60% of total membrane G4 AChE activity), although the fraction in high detergent-resistant rafts increased during postnatal development, from ∼10% at P7 to more than 15% at P14 and P100 (Fig. 2C).
FIGURE 2.
Developmental increase of the fraction of the raft-associated AChE resistant to cold detergent. A, distribution of AChE molecular forms at different stages (P7, P14, and P100) of rat cerebrum and analyzed by sedimentation analysis as in Fig. 1A. One representative profile is shown, n = 3. B, AChE activity in detergent-soluble fractions (bottom) and detergent-resistant fractions (Raft) after flotation of the membrane extracts of the cerebra from different stages (P7, P14, and P100) was analyzed in discontinuous sucrose gradients, in the presence of 0.05% (low, upper panel) or 0.5% (high, lower panel) Triton X-100 in the cold. Enzymatic activities are expressed in arbitrary units. One representative profile is shown, n = 4. C, proportions of AChE and ALP activity recovered in the raft fractions, isolated as in B. D, low Triton-resistant raft fraction from P100 rat cerebrum as in C was further subjected to 0.5% Triton extraction in the cold for 1 h. AChE activities were determined after discontinuous sucrose gradients. The values, expressed as the proportion of the membrane-bound G4 AChE activity and total ALP activity input in the discontinuous gradient, respectively, are means ± S.E. (n = 4). The total G4 AChE activity was calculated according to the total activity input in the gradient and the proportion of G4 AChE from A. *, p < 0.05.
The fact that most of the AChE was weakly associated with rafts at low detergent was demonstrated by subjecting a raft-enriched fraction (from 0.05% Triton X-100) to a second flotation in the presence of 0.5% Triton X-100 (Fig. 2D). The resulting high detergent-resistant raft fraction only contained 10 ± 0.5% (mean ± S.E., n = 3) of the AChE activity associated with the original low detergent-resistant raft fraction. This result suggests the presence of two populations of G4 AChE in the rafts. The AChE fraction remaining associated with rafts at high detergent concentration could represent the functional enzyme in the adult brain.
PRiMA Directs G4 AChE to Membrane Rafts via Its Cholesterol-binding Motif CRAC
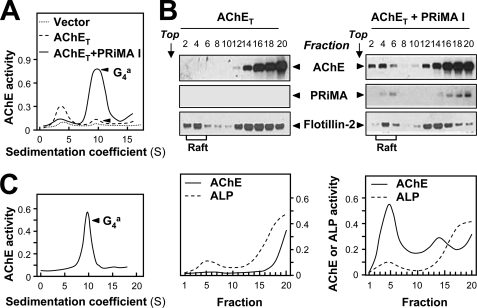
Because the membrane rafts from the cerebrum only contain PRiMA-linked amphiphilic G4 AChE form, we examined the role of PRiMA in recruiting AChE into rafts. Mouse neuroblastoma × rat glioma NG108-15 cells spontaneously express very little PRiMA-linked AChE (34). They were transfected with various cDNAs encoding HA-tagged PRiMA constructs together with HA-tagged AChET cDNA. Transient transfection with AChET cDNA markedly increased the level of AChE monomers, but only slightly that of membrane-bound tetramers, indicating that the expression of PRiMA limited the formation of PRiMA-linked G4 AChE in this system (Fig. 3A). This was confirmed by the fact that co-expression of AChET with the mouse PRiMA I isoform did produce a high level of membrane-bound G4 tetramers (Fig. 3A). Because the recruitment of AChE in the raft fraction of cerebrum membranes was more obvious at a low detergent concentration, we analyzed its distribution in NG108-15 cell membrane fractions under similar conditions. Considering the lower total protein concentration of the cell preparations, we used 0.02% instead of 0.05% cold Triton X-100.
FIGURE 3.
PRiMA directs amphiphilic G4 AChE to the membrane raft in transfected cells. A, NG108-15 cells were transfected with an empty control vector as a control and with a vector encoding AChET-HA without or with a vector encoding PRiMA I-HA. The sedimentation profiles of AChE from cell extracts showed that co-transfection with AChET and PRiMA I produced an amphiphilic tetramer at 9.1 S. Sedimentation was performed as in Fig. 1A. B, AChE activity in detergent-soluble (bottom) and detergent-resistant (Raft) fractions was revealed after flotation in discontinuous sucrose gradients in the presence of 0.02% Triton X-100 (low) in the cold. Enzymatic activities are expressed in arbitrary units (lower panel). Aliquots of even fractions were analyzed by 12% SDS-PAGE and Western blotting with an anti-HA antibody to detect AChE (∼68 kDa) and PRiMA (∼20 kDa), and with an antibody against flotillin-2 (∼55 kDa), which served as a membrane raft marker (upper panel). C, sedimentation profile of AChE, solubilized from the low density membrane raft fractions, from cells expressing AChET and PRiMA I (B). The sedimentation was performed as in A. The profile shows a single molecular form at 9.1 S, corresponding to amphiphilic tetramers. For all sedimentation analyses, one representative profile is shown, n = 3.
When AChET subunits, tagged with HA, were expressed alone in cultured NG108-15 cells, AChE enzymatic activity and protein were only present in the soluble fractions, i.e. at the bottom of the gradients (Fig. 3B, left panel). When they were co-expressed with HA-tagged PRiMA I, a significant fraction of both proteins was detected in the rafts (Fig. 3B, right panels). However, the soluble fractions appeared to contain a higher proportion of AChE protein than the raft fractions, as shown in the Western blots, but a lower proportion of AChE activity. This discrepancy between AChE protein and enzymatic activity suggests that the non-raft-soluble fractions contained inactive AChE protein, probably not associated with PRiMA. The existence of inactive AChE has been reported previously (34–36). As shown in the brain, the raft-associated AChE in NG108-15 cells expressing AChET and PRiMA was exclusively amphiphilic G4 AChE (Fig. 3C).
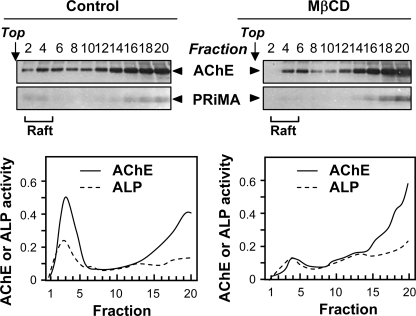
To verify that PRiMA-linked AChE was associated with cholesterol-sphingolipid membrane rafts, we treated the cultured NG108-15 cells expressing AChET and PRiMA with 10 mm MβCD to deplete cholesterol. After 1 h, the cells started to become spherical. This observation indicates that they could not tolerate any longer treatments. However, they were not detached from the plates, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay showed that the cell viability was not significantly changed after the treatment (data not shown). The efficiency of the cholesterol depletion by MβCD was shown by the fact that the cholesterol content of the total membrane fraction, determined by a cholesterol detection kit and mass spectrometry, was decreased by about 70 ± 2.5% (mean ± S.E., n = 3). Cholesterol depletion reduced the ALP content of membrane raft fractions by about half and the proportion of AChE activity in raft-enriched fractions even more markedly (Fig. 4, lower panel). This was confirmed by Western blots of AChET and PRiMA proteins (Fig. 4, upper panel). These results indicated that the association of PRiMA-linked AChE with membrane rafts depends on cholesterol.
FIGURE 4.
Treatment with MβCD reduces the association of PRiMA-linked G4 AChE with rafts. NG108-15 cells were transfected with cDNAs encoding AChET-HA and PRiMA I-HA. Two days later, the cultures were treated without (control; left panel) or with the cholesterol-depleting reagent, MβCD (10 mm; right panel) for 1 h at 37 °C. Detergent-soluble (bottom) and detergent-resistant (Raft) fractions were obtained after flotation in discontinuous sucrose gradients in the presence of 0.02% Triton X-100 (low) in the cold. Aliquots of each fraction were analyzed by 12% SDS-PAGE and Western blotting with an anti-HA antibody to detect AChET (∼68 kDa) and PRiMA (∼20 kDa) (upper panel). AChE activity in each fraction was determined (lower panel). The reduction of ALP association with rafts indicates the efficiency of cholesterol depletion. One representative profile is shown, n = 3.
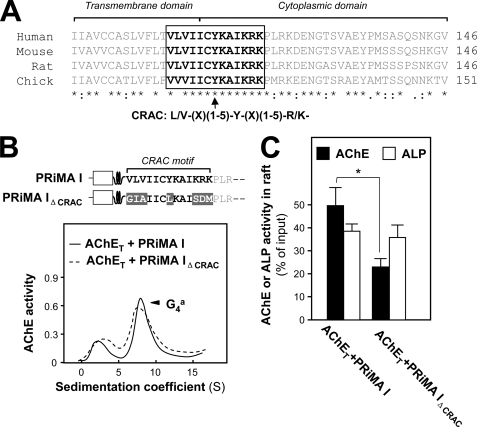
There are several hypotheses on the partitioning of proteins between raft and non-raft domains (19, 37). A peptidic motif of integral membrane proteins, named CRAC, located at the membrane interface, is thought to interact with cholesterol; its sequence is defined as (L/V)X1–5YX1–5(R/K), where X1–5 represents between one and five residues of any amino acid (19, 20). The 109–120 peptidic sequence overlapping the junction between the transmembrane and the cytoplasmic domains, which is common to both PRiMA I and PRiMA II (VLV IIC YKA IKRK) (13, 15), corresponds to a CRAC motif and has the appropriate location. This motif is conserved among human, mouse, rat, and chick PRiMA sequences (Fig. 5A). Therefore, the CRAC motif of PRiMA may be responsible for the association with cholesterol and targeting PRiMA-linked AChE to the rafts.
FIGURE 5.
Identification of CRAC in PRiMA. A, alignment of peptidic sequence overlapping the junction between the transmembrane and cytoplasmic domains of PRiMA I among human, mouse, rat, and chick. The amino acid sequences of human, mouse, and rat PRiMA I were deduced from the nucleotide sequences accessed from GenBankTM accession numbers AY225516 and AY043275 and Ensembl ENSRNOT00000011906, respectively. The sequence of chick PRiMA was determined in a previous study (14). The putative CRAC motif defined as (L/V)X1–5YX1–5(R/K) is shown in the open box, which is almost 100% conserved among the different species. Identical and similar amino acids among all the peptides are indicated by asterisks and dots, respectively. B, upper panel, schematic representation of the difference between PRiMA I and PRiMA IΔCRAC; the major part of the transmembrane domain is represented as a coiled shape. Lower panel, NG108-15 cells were transfected with cDNAs encoding PRiMA I or PRiMA IΔCRAC (VLVIICYKAIKRK→GIAIICLKAISDM) for 2 days. The sedimentation profiles of AChE from cell extracts showed that co-transfection with AChET and PRiMA constructs produced similar proportions of amphiphilic G4 tetramers, sedimenting at 9.1 S. Sedimentation was performed as in Fig. 1A. C, detergent-soluble (bottom) and detergent-resistant (Raft) fractions from the cultures in B were analyzed by flotation in discontinuous sucrose gradients in the presence of 0.02% Triton X-100 (low) in the cold. The histograms show the proportions of AChE and ALP activities recovered in the raft fractions. The values, expressed as proportion of the membrane-bound G4 AChE activity and total ALP activity input in the discontinuous gradient, respectively, are means ± S.E. (n = 4). The total G4 AChE activity was calculated according to the total activity input in the gradient and the proportion of G4 AChE from B. *, p < 0.05.
To assess the role of the CRAC motif, we constructed a mutant called PRiMA IΔCRAC in which key residues were modified as underlined, GIA IIC LKA ISDM. The amounts of PRiMA-linked G4 AChE in the transfected NG108-15 cells expressing the wild type and mutated PRiMA were similar (Fig. 5B). This is in agreement with the fact that the mutation does not affect the PRAD motif, which is responsible for the assembly with AChET subunits. However, the proportion of membrane G4 AChE activity associated with the raft fraction, isolated with 0.02% Triton X-100, was only about 20% with PRiMA IΔCRAC as compared with 50% with wild type PRiMA I; the amount of raft-associated ALP, used as a control, was the same in both cultures (Fig. 5C). Thus, the CRAC motif of PRiMA plays an important role in the recruitment of AChE in the rafts, in cultured neurons.
PRiMA Is Responsible for the Lower Resistance to Detergent for the Membrane Raft-associated G4 AChE
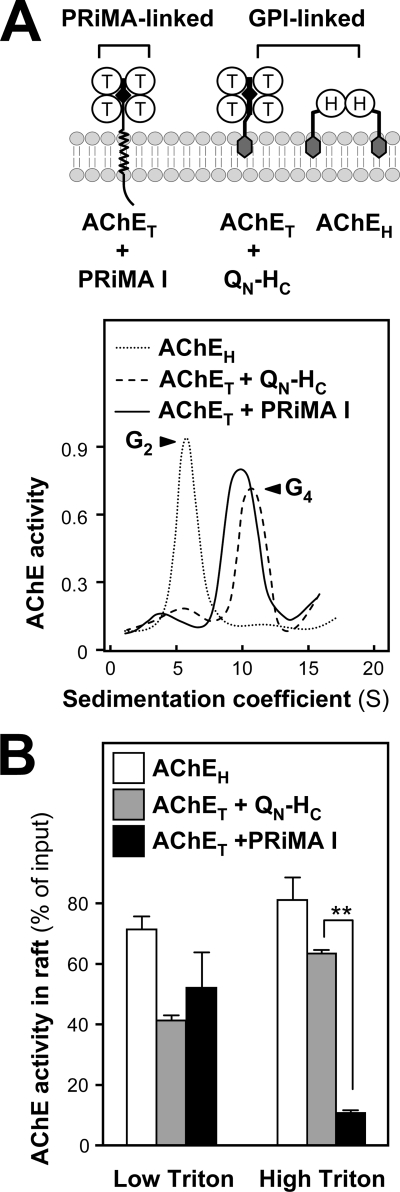
Because GPI-linked proteins are known to be associated with raft microdomains, we wished to compare PRiMA-linked G4 AChE with GPI-linked AChE (G2) forms. In mammals, AChEH subunits possess a C-terminal GPI addition signal; they are mostly expressed in blood cells and produce GPI-anchored dimers, exposed at the cell surface (1). AChET tetramers could also be assembled and anchored in cell membranes through a GPI glycolipid when co-expressed with a fusion protein, QN-HC, composed of the N terminus of Torpedo ColQ containing the PRAD, followed by the C-terminal GPI addition signal of Torpedo AChEH (8, 38). In cultured NG108-15 cells, AChEH, as well as AChET + QN-HC and AChET + PRiMA were overexpressed (Fig. 6A), and their distributions in membrane fractions with PRiMA-linked AChE were compared.
FIGURE 6.
Comparison between the detergent resistance of PRiMA-linked and GPI-anchored AChE in membrane rafts. A, AChET may be associated as tetramers with the transmembrane protein PRiMA or with the chimeric GPI-anchored protein QN-HC, the AChEH variant forms GPI-anchored dimers. Sedimentation profiles of AChE activity obtained from transfected NG108-15 cells expressing the different proteins, as indicated. Sedimentation was performed as in Fig. 1A. B, proportions of AChE activities from total membrane preparations recovered in raft fractions obtained from the cells in A after flotation in discontinuous sucrose gradients (data not shown) in the presence of 0.02% (low) or 0.2% (high) cold Triton X-100 are shown. The values, expressed as % of the membrane-bound G2 or G4 AChE activity input in the discontinuous gradient, are means ± S.E. (n = 4). The total G4 AChE activity was calculated from the total activity input in the gradient and the proportion of G4 AChE from (A). **, p < 0.01.
The production of membrane-bound AChE in transfected cells was demonstrated by sedimentation in sucrose density gradients. As expected, G4 AChE was produced by cells expressing AChET with either PRiMA or QN-HC, whereas G2 AChE was produced by cells expressing AChEH (Fig. 6A). The proportion of AChE activity recovered in the raft fractions of transfected NG108-15 cells was determined under low (0.02%) and high detergent (0.2%) conditions. The GPI-anchored molecules were found to be associated with the membrane raft fraction at similar levels under low and high detergent conditions. In contrast, more than 50% of the membrane-bound AChET-PRiMA complex was associated with rafts at low detergent concentration, although only ∼10% was in the raft fraction at high detergent concentration (Fig. 6B), as previously observed for membranes from rat cerebrum (Fig. 2C). Therefore, the association of PRiMA with membrane rafts is much less resistant to detergent than that of GPI-anchored AChE.
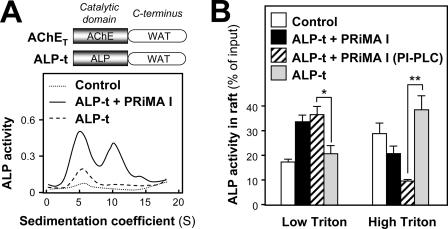
To confirm that anchoring through PRiMA is responsible for the reduced association of AChE with rafts at high detergent concentration, we analyzed a complex made from a mutant of human ALP-t, in which the C-terminal GPI-addition signal was replaced by a t peptide derived from AChET (39). The co-expression of ALP-t and PRiMA I in transfected NG108-15 cells formed amphiphilic, membrane-anchored tetramers (Fig. 7A). Thus, the ALP-t protein could associate with PRiMA I, like AChET. We analyzed the distribution of ALP-t in raft and non-raft fractions, when expressed with or without PRiMA. When ALP-t was expressed alone, the proportion of membrane-bound ALP activity in the raft fraction was less than 20% at low detergent concentrations (Fig. 7B); this proportion was similar to that of control without any transfection and corresponded to the endogenous GPI-anchored ALP. The exogenous ALP activity derived from ALP-t cDNA was recovered at the bottom of the gradient (data not shown). When ALP-t was co-expressed with PRiMA I, the proportion of membrane-bound ALP activity recovered in the raft fraction reached ∼35% at low detergent concentrations but ∼20% at high detergent concentrations (Fig. 7B). Thus, the ALP-t-PRiMA complex is efficiently targeted to the raft microdomains, and its association with rafts is sensitive to detergent, like the AChE-PRiMA complex. The relatively high proportion of ALP activity in the raft under high Triton X-100 conditions, as compared with that of PRiMA-linked AChE, may be accounted for by the presence of endogenous GPI-anchored ALP (Fig. 7B).
FIGURE 7.
PRiMA directs associated proteins to membrane rafts. A, NG108-15 cells were transfected with an empty vector or with a vector encoding the ALP-t chimera with or without a vector encoding PRiMA I-HA. A schematic graph of ALP-t chimera is shown in the upper panel, which has the same tryptophan amphiphilic tetramerization (WAT) domain with that of AChET at the C terminus. Sedimentation profiles of ALP activity obtained from transfected NG108-15 cells are shown. Sedimentation was performed as in Fig. 1A. One representative profile is shown, n = 3. B, proportions of ALP activity from total membrane preparations recovered in raft fractions after flotation in discontinuous sucrose gradients (data not shown), in the presence of 0.02% (low) or 0.2% (high) Triton X-100 in the cold from the cells in A with or without phosphatidylinositol-phospholipase C (PI-PLC) treatment; there is no significant difference between the proportions from ALP-t transfected cultures and the control. The values, expressed as percent of the membrane-bound ALP activity input in the discontinuous gradient, are means ± S.E. (n = 4). *, p < 0.05; **, p < 0.01.
To eliminate the influence of the endogenous GPI-anchored ALP, the ALP-t and PRiMA co-transfected cells were pretreated with 0.4 unit/ml phosphatidylinositol-phospholipase C at 30 °C for 1 h before the raft extraction (38). By eliminating the GPI-anchored ALP (background), the proportions of PRiMA-linked ALP activity being recruited to the raft were around 35 and 10% under low and high detergent conditions, respectively (Fig. 7B). These results support the notion that the low affinity of the raft association of PRiMA-linked G4 AChE is due to PRiMA but not AChET.
Role of the C Terminus of PRiMA in Its Association with Membrane Rafts
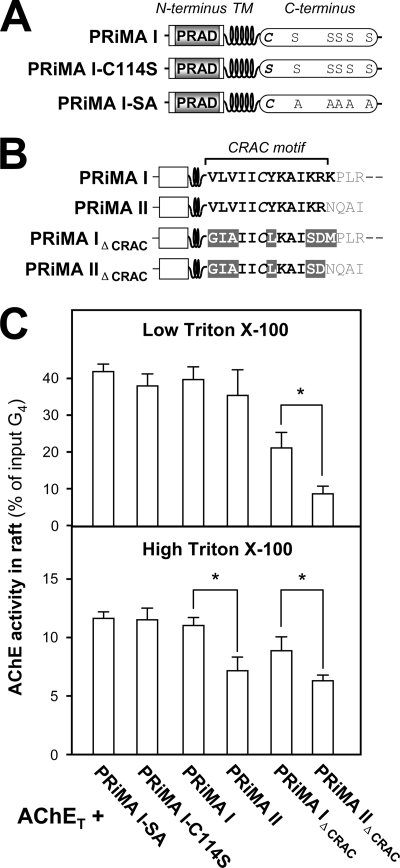
We examined the possible role of the intracellular cytoplasmic domain of PRiMA I in raft association. The cytoplasmic domain of PRiMA I contains a putative palmitoylation site (Cys-114), close to the transmembrane domain, and potential serine phosphorylation sites (Ser-131, Ser-138 to Ser-140, and Ser-142) (Fig. 8A). The corresponding post-translational modifications may be involved in intracellular protein interactions and signaling (15). The minor splice variant PRiMA II also possesses Cys-114 but not the potential phosphorylation sites (Fig. 8B) (13, 14). Both PRiMA variants could anchor AChET tetramers in cell membranes but might differ in their localization and interactions, in particular in their recruitment into the membrane rafts.
FIGURE 8.
Role of the C-terminal cytoplasmic domain of PRiMA in the association of PRiMA-linked AChE with membrane rafts. A, schematic representation for the difference among wild type PRiMA I, mutant in which the putative palmitoylation site was suppressed (PRiMA I-C114S) and mutant in which all potentially phosphorylated serines were replaced by alanines (PRiMA I-SA). B, schematic representation for the difference among wild type PRMA I, PRiMA II in which the C-terminal residues (NQAI) differ from those of PRiMA I, PRiMA IΔCRAC (VLVIICYKAIKRK→GIAIICLKAISDM) as in Fig. 5, and PRiMA IIΔCRAC (VLVIICYKAIKR→GIAIICLKAISD). C, proportions of AChE activity recovered in the raft fractions after flotation in discontinuous sucrose gradients, in the presence of low (0.02%) or high (0.2%) Triton X-100 in the cold. The raft-associated AChE activity was determined. The values, expressed as % of the total G4 AChE activity input in the discontinuous gradient, are means ± S.E. (n = 4). *, p < 0.05.
To analyze the possible role of post-translational modifications on the insertion of PRiMA-linked AChE complexes in the rafts, we mutated the corresponding sites in the intracellular C terminus, as shown in Fig. 8A. We compared the recruitment of AChET in rafts, when co-expressed with PRiMA I, the mutants PRiMA I-C114S and PRiMA I-SA under low and high detergent conditions (Fig. 8C). Similar to what was already observed for the wild type PRiMA I, the proportion of membrane-bound AChE-PRiMA complexes associated with rafts for all those mutants on the potential functional residues of the C terminus was ∼40% at low detergent concentration (0.02% Triton X-100) and ∼10% at high concentration (0.5% Triton X-100) (Fig. 8C). The distribution of endogenous ALP in raft and non-raft fractions was analyzed for comparison; it was similar among all the transfected cells but varied under different detergent conditions, i.e. ∼20% of total activity input with low detergent and ∼60% with high detergent, similar with the result shown in Fig. 2C (lower panel).
In addition, we also compare the difference between the shorter C terminus in PRiMA II and the longer one in PRiMA I for raft association (Fig. 8B). We found that both PRiMA I and PRiMA II could recruit AChE complexes into rafts to the same degree under low detergent conditions. Under high detergent conditions, however, the recruitment of the complexes was significantly lower with PRiMA II (∼5%) than with PRiMA I (∼10%), suggesting that the intracellular domain of PRiMA I might participate in the stabilization of AChE in rafts (Fig. 8C). This might involve an interaction with some scaffold proteins, associated with the rafts. Because PRiMA II and PRiMA I possess the same CRAC motif, except for the final lysine residue (Fig. 8B), the role of the C terminus in recruiting AChE to the raft needs further investigation in the absence of a CRAC motif. Therefore, we compared a PRiMA II mutant in which the CRAC motif was suppressed (PRiMA IIΔCRAC) with PRiMA IΔCRAC. PRiMA IIΔCRAC recruited significantly less AChE than PRiMA IΔCRAC to the rafts, in either high or low detergent conditions (Fig. 8C). This result strengthens the notion that the C terminus of PRiMA plays a role in raft targeting.
DISCUSSION
Here, we demonstrate a partial association of PRiMA-linked G4 AChE with membrane rafts in the rat cerebrum. This association was considerably reduced when the rafts were isolated in the presence of cold 0.5% Triton X-100, rather than cold 0.05% Triton X-100, and therefore appeared weaker than that of GPI-anchored proteins, including the AChE G2 form possessing a GPI anchor or a G4 AChE form associated with a PRAD-containing GPI-anchored construct (QN-HC), indicating that PRiMA determines the detergent sensitivity of raft-associated AChE in the brain.
Although the resistance to extraction in cold Triton X-100 was initially considered to define membrane rafts, the situation is in fact quite complex (33, 40, 41), because of the heterogeneity of low density detergent-resistant membrane domains (41, 42). These domains differ in their lipid and protein composition, as well as in the detergent solubility of individual raft proteins (32, 41). The association of PRiMA-linked G4 AChE with rafts is clearly heterogeneous in its sensitivity to the cold detergent, both in brain membranes and in transfected cells. Under low detergent conditions (0.05% Triton X-100 for tissue and 0.02% Triton X-100 for cultured cells), around 50% of membrane-bound G4 AChE was found to be associated with membrane rafts (Fig. 2C and Fig. 3B). A large part of this raft-associated AChE population, anchored through PRiMA I or PRiMA II, could be dissociated by 0.5% cold Triton X-100, perhaps reflecting a high mobility between raft and non-raft domains. A fraction of PRiMA-linked AChE remained to be associated with rafts at high detergent concentration, perhaps because of interactions with intracellular raft-associated proteins (Fig. 2D and Fig. 8C).
The proportion of membrane-bound AChE associated with rafts under high detergent conditions increased from 10% at P7 up to 15% at P14 (Fig. 2C), suggesting a maturation process during the brain development and therefore a physiological role of this raft localization. Thus, two compartments of raft-associated PRiMA-linked AChE may be physiologically distinct.
It has long been known that the interaction between nicotinic acetylcholine receptors and scaffold proteins in muscle membranes reduces their mobility and detergent extractability (43, 44). This notion leads us to propose here that the interaction of PRiMA with intracellular scaffold proteins may stabilize its association with the rafts, and it consequently affects the mobility of PRiMA-linked AChE in neuronal membranes. Two mechanisms may be involved in the stabilization for the raft association. First, the developmental increase of the proportion of PRiMA I in the brain or in cell cultures (12) may serve as a direct reason to explain in part that AChE is more stably associated with rafts in the adult brain. Besides the relative abundant expression of PRiMA I in adult stage, an increased expression, or maturation, of scaffold proteins associated with PRiMA I may also help to strengthen the association of AChE with rafts in the adult brain. PSD-95 is one of the possible proteins for this function; the expression level of PSD-95 is increased during neuronal differentiation, which is in line with the expression of AChE and PRiMA (12, 45).
Both PRiMA I and PRiMA II possess a cholesterol-binding CRAC motif at the interface of their transmembrane and intracellular domains. We found that the depletion of cholesterol (Fig. 4) or mutation of this associated motif (Fig. 5) strongly reduced the association of PRiMA-linked AChE with rafts; these results suggest a direct interaction of PRiMA with cholesterol in the liquid-ordered rafts. Apart from the CRAC motif, the role of possible post-translational modification(s) of PRiMA in the raft association was also considered. Based on our preliminary data (supplemental material), PRiMA I could be at least phosphorylated via the potential functional residue(s) at its C terminus (15). Although such phosphorylation and potential palmitoylation sites in the cytoplasmic domain of PRiMA I might interact with intracellular binding proteins (15), we did not find any evidence that they contribute to the targeting or stabilization of raft-associated AChE (Fig. 8C). However, a comparison between PRiMA I and PRiMA II (Fig. 8C) suggests that the C terminus of PRiMA plays some role in raft targeting and resistance to detergent. Thus, we speculate that the raft association of PRiMA-linked AChE may depend on other functional residues for the interaction with intracellular binding partners. The mechanism for the association of PRiMA with the raft is still not fully understood; the possible function of the putative palmitoylation and phosphorylation sites has to be determined.
The developmental increase of stable raft-associated AChE suggests that it may be physiologically significant in the adult brain. Several lines of evidence show that membrane rafts exist at neuron-neuron synapses and that some synaptic molecules are concentrated in the rafts, so that the rafts probably contribute to synaptic structure, function, maintenance, and plasticity (18, 30). The co-localization of AChE with PSD-95 in cultured cortical neurons and the fact that PSD-95 is associated with membrane rafts from the brain (supplemental material) (45) suggest that PSD-95, or other raft-associated proteins, may interact with PRiMA in post-synaptic rafts. This association might correspond to the population of PRiMA-linked AChE that is more strongly associated with rafts. However, there is no PDZ-binding site on the C terminus of PRiMA, which would imply an indirect interaction, if any (15). In addition, AChE may also be included in rafts at presynaptic sites, because PRiMA-linked AChE is expressed in presynaptic cholinergic neurons (supplemental material) (13, 27).
A major proportion of AChE was found to be associated with rafts only under low detergent conditions, indicating a loose, probably highly dynamic interaction with these membrane microdomains. A high mobility of AChE, anchored by PRiMA I or PRiMA II, in the neuronal membrane may be required for central cholinergic activity, because acetylcholine does not act only as a synaptic neurotransmitter but also in a more diffuse neurohumoral manner (46). Apart from the inactivation of acetylcholine, AChE has been proposed to participate in many aspects of the brain functions, such as in neurite outgrowth (47), cell adhesion (48), synaptogenesis (49), neuronal tumors, and Alzheimer disease (50, 51). These functions may be based on the catalytic activity of AChE or on protein-protein interactions, as suggested by the fact that this enzyme has sequence and putative structural homology to a group of neuronal adhesion proteins, such as gliotactin and neurotactin (52). Therefore, whether PRiMA-linked AChE localizing in the membrane raft could play a role in these noncholinergic functions may be an interesting direction for revealing the physiological functions of the raft localization.
Here, we have not determined the raft association of PRiMA-linked AChE in muscle, even though muscle is another major source of this form of enzyme. However, we predict that AChE is also raft-associated in muscle membranes. Muscles, in particular the fast-twitch muscle in rat (11) or the slow twitch muscle in chicken (14), express PRiMA I in a tightly controlled manner during maturation. PRiMA-linked AChE was found to be located both pre- and post-synaptically at the vertebrate neuromuscular junctions (27, 53) and may be associated with scaffold proteins.
Moreover, expression of PRiMA and AChE in COS-7 cells or in osteoblast cells produced a PRiMA-linked G4 AChE that was partially associated with membrane rafts, as in neuroblastoma cells.3 This suggests that the ability of PRiMA to direct G4 AChE to membrane rafts is not tissue- or cell type-specific, in agreement with its capacity to interact with cholesterol via its CRAC motif. However, variations of the strength of PRiMA association with rafts may reflect heterogeneity of the rafts among different cell types. This issue clearly deserves further investigation.
Supplementary Material
Acknowledgment
We thank Dr. Jean Cartaud (Biologie Cellulaire des Membranes, Institut Jacques Monod, UMR 7592 CNRS, Universités Paris 6 et Paris 7, Paris) for valuable discussions on the CRAC motif.
This work was supported by Research Grants Council of Hong Kong Grants N_HKUST629/07, 662407, 662608, and F-HK21/06T, Croucher Foundation Grant CAS-CF07/08.SC03 (to K. W. K. T.), CNRS, Ecole Normale Supérieure, Association Français Contre les Myopathies, and French Ministry of Foreign Affairs (to J. M.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Methods and Figs. 1 and 2.
H. Q. Xie et al., unpublished data.
- AChE
- acetylcholinesterase
- PRiMA
- proline-rich membrane anchor
- CRAC
- cholesterol recognition/interaction amino acid consensus
- GPI
- glycosylphosphatidylinositol
- PRAD
- proline-rich attachment domain
- ALP
- alkaline phosphatase
- HA
- hemagglutinin
- MβCD
- methyl-β-cyclodextrin.
REFERENCES
- 1.Massoulié J., Pezzementi L., Bon S., Krejci E., Vallette F. M. (1993) Prog. Neurobiol. 41, 31–91 [DOI] [PubMed] [Google Scholar]
- 2.Massoulié J., Bon S. (2006) J. Mol. Neurosci. 30, 233–236 [DOI] [PubMed] [Google Scholar]
- 3.Pick M., Flores-Flores C., Soreq H. (2004) Ann. N.Y. Acad. Sci. 1018, 85–98 [DOI] [PubMed] [Google Scholar]
- 4.Perrier N.A., Salani M., Falasca C., Bon S., Augusti-Tocco G., Massoulié J. (2005) J. Neurochem. 94, 629–638 [DOI] [PubMed] [Google Scholar]
- 5.Massoulié J. (2002) Neurosignals 11, 130–143 [DOI] [PubMed] [Google Scholar]
- 6.Falasca C., Perrier N., Massoulié J., Bon S. (2005) J. Biol. Chem. 280, 878–886 [DOI] [PubMed] [Google Scholar]
- 7.Noureddine H., Schmitt C., Liu W., Garbay C., Massoulié J., Bon S. (2007) J. Biol. Chem. 282, 3487–3497 [DOI] [PubMed] [Google Scholar]
- 8.Noureddine H., Carvalho S., Schmitt C., Massoulié J., Bon S. (2008) J. Biol. Chem. 283, 20722–20732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jasmin B. J., Gisiger V. (1990) J. Neurosci. 10, 1444–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inestrosa N. C., Moreno R. D., Fuentes M. E. (1994) Neurosci. Lett. 173, 155–158 [DOI] [PubMed] [Google Scholar]
- 11.Xie H. Q., Choi R. C., Leung K. W., Siow N. L., Kong L. W., Lau F. T., Peng H. B., Tsim K. W. (2007) J. Biol. Chem. 282, 11765–11775 [DOI] [PubMed] [Google Scholar]
- 12.Xie H. Q., Choi R. C., Leung K. W., Chen V. P., Chu G. K., Tsim K. W. (2009) Brain Res. 1265, 13–23 [DOI] [PubMed] [Google Scholar]
- 13.Perrier N. A., Khérif S., Perrier A. L., Dumas S., Mallet J., Massoulié J. (2003) Eur. J. Neurosci. 18, 1837–1847 [DOI] [PubMed] [Google Scholar]
- 14.Mok M. K., Leung K. W., Xie H. Q., Guo A. J., Chen V. P., Zhu J. T., Choi R. C., Tsim K. W. (2009) Neurosci. Lett. 461, 202–206 [DOI] [PubMed] [Google Scholar]
- 15.Perrier A. L., Massoulié J., Krejci E. (2002) Neuron 33, 275–285 [DOI] [PubMed] [Google Scholar]
- 16.Chakrabandhu K., Hérincs Z., Huault S., Dost B., Peng L., Conchonaud F., Marguet D., He H. T., Hueber A. O. (2007) EMBO J. 26, 209–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma L., Huang Y. Z., Pitcher G. M., Valtschanoff J. G., Ma Y. H., Feng L. Y., Lu B., Xiong W. C., Salter M. W., Weinberg R. J., Mei L. (2003) J. Neurosci. 23, 3164–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki T., Du F., Tian Q. B., Zhang J., Endo S. (2008) J. Neurochem. 104, 596–610 [DOI] [PubMed] [Google Scholar]
- 19.Li H., Papadopoulos V. (1998) Endocrinology 139, 4991–4997 [DOI] [PubMed] [Google Scholar]
- 20.Epand R. M. (2006) Prog. Lipid Res. 45, 279–294 [DOI] [PubMed] [Google Scholar]
- 21.Legay C., Bon S., Vernier P., Coussen F., Massoulié J. (1993) J. Neurochem. 60, 337–346 [DOI] [PubMed] [Google Scholar]
- 22.Belbeoc'h S., Massoulié J., Bon S. (2003) EMBO J. 22, 3536–3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunkel T. A., Roberts J. D., Zakour R. A. (1987) Methods Enzymol. 154, 367–382 [DOI] [PubMed] [Google Scholar]
- 24.Smart E. J., Ying Y. S., Mineo C., Anderson R. G. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 10104–10108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukherjee A., Arnaud L., Cooper J. A. (2003) J. Biol. Chem. 278, 40806–40814 [DOI] [PubMed] [Google Scholar]
- 26.Ellman G. L., Courtney K. D., Andres V. J., Feather-Stone R. M. (1961) Biochem. Pharmacol. 7, 88–95 [DOI] [PubMed] [Google Scholar]
- 27.Leung K. W., Xie H. Q., Chen V. P., Mok M. K., Chu G. K., Choi R. C., Tsim K. W. (2009) FEBS J. 276, 3031–3042 [DOI] [PubMed] [Google Scholar]
- 28.Saslowsky D. E., Lawrence J., Ren X., Brown D. A., Henderson R. M., Edwardson J. M. (2002) J. Biol. Chem. 277, 26966–26970 [DOI] [PubMed] [Google Scholar]
- 29.Neumann-Giesen C., Falkenbach B., Beicht P., Claasen S., Lüers G., Stuermer C. A., Herzog V., Tikkanen R. (2004) Biochem. J. 378, 509–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hering H., Lin C. C., Sheng M. (2003) J. Neurosci. 23, 3262–3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harder T., Simons K. (1999) Eur. J. Immunol. 29, 556–562 [DOI] [PubMed] [Google Scholar]
- 32.Brügger B., Graham C., Leibrecht I., Mombelli E., Jen A., Wieland F., Morris R. (2004) J. Biol. Chem. 279, 7530–7536 [DOI] [PubMed] [Google Scholar]
- 33.Shogomori H., Brown D. A. (2003) Biol. Chem. 384, 1259–1263 [DOI] [PubMed] [Google Scholar]
- 34.Choi R. C., Pun S., Dong T. T., Wan D. C., Tsim K. W. (1997) Neurosci. Lett. 236, 167–170 [DOI] [PubMed] [Google Scholar]
- 35.Rotundo R. L. (1988) J. Biol. Chem. 263, 19398–19406 [PubMed] [Google Scholar]
- 36.Chatel J. M., Grassi J., Frobert Y., Massoulié J., Vallette F. M. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 2476–2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parton R. G., Hanzal-Bayer M., Hancock J. F. (2006) J. Cell Sci. 119, 787–796 [DOI] [PubMed] [Google Scholar]
- 38.Duval N., Krejci E., Grassi J., Coussen F., Massoulié J., Bon S. (1992) EMBO J. 11, 3255–3261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simon S., Krejci E., Massoulié J. (1998) EMBO J. 17, 6178–6187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schuck S., Honsho M., Ekroos K., Shevchenko A., Simons K. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 5795–5800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pike L. J. (2004) Biochem. J. 378, 281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schnitzer J. E., McIntosh D. P., Dvorak A. M., Liu J., Oh P. (1995) Science 269, 1435–1439 [DOI] [PubMed] [Google Scholar]
- 43.Stya M., Axelrod D. (1983) J. Cell Biol. 97, 48–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wallace B. G. (1995) J. Cell Biol. 128, 1121–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siow N. L., Xie H. Q., Choi R. C., Tsim K. W. (2005) Chem. Biol. Interact. 157–158, 423–426 [DOI] [PubMed] [Google Scholar]
- 46.Descarries L., Gisiger V., Steriade M. (1997) Prog. Neurobiol. 53, 603–625 [DOI] [PubMed] [Google Scholar]
- 47.Sternfeld M., Ming G., Song H., Sela K., Timberg R., Poo M., Soreq H. (1998) J. Neurosci. 18, 1240–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grifman M., Galyam N., Seidman S., Soreq H. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 13935–13940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brimijoin S., Hammond P. (1996) Neuroscience 71, 555–565 [DOI] [PubMed] [Google Scholar]
- 50.Inestrosa N., Alarcón R. (1998) J. Physiol. Paris. 92, 341–344 [DOI] [PubMed] [Google Scholar]
- 51.Perry C., Sklan E. H., Birikh K., Shapira M., Trejo L., Eldor A., Soreq H. (2002) Oncogene 21, 8428–8441 [DOI] [PubMed] [Google Scholar]
- 52.Silman I., Sussman J. L. (2005) Curr. Opin. Pharmacol. 5, 293–302 [DOI] [PubMed] [Google Scholar]
- 53.Gisiger V., Stephens H. R. (1988) J. Neurosci. Res. 19, 62–78 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.