Abstract
The compound 5-(4-methoxyarylimino)-2-N-(3,4-dichlorophenyl)-3-oxo-1,2,4-thiadiazolidine (P3-25) is known to possess anti-bacterial, anti-fungal, and anti-tubercular activities. In this report, we provide evidence that P3-25 inhibits NF-κB, known to induce inflammatory and tumorigenic responses. It activates AP-1, another transcription factor. It inhibits TRAF2-mediated NF-κB activation but not TRAF6-mediated NF-κB DNA binding by preventing its association with TANK (TRAF for NF-κB). It facilitates binding of MEKK1 with TRAF2 and thereby activates JNK and AP-1. We provide evidence, for the first time, that suggests that the interaction of P3-25 with TRAF2 leads to inhibition of the NF-κB pathway and activation of AP-1 pathway. These results suggest novel approaches to design of P3-25 as an anti-cancer/inflammatory drug for therapy through regulation of the TRAF2 pathway.
Keywords: AP-1 Transcription Factor, Inflammation, JNK, NF-κB Transcription Factor, Transcription Factors, Tumor Suppressor
Introduction
Regulation of nuclear transcription factor κB (NF-κB)3 has a role in oncogenesis and cancer therapy (1). Various inflammatory stimuli, including cytokines, mitogens, bacterial products, viral proteins, and apoptosis-inducing agents, activate NF-κB via expression of several genes, including adhesion molecules, cox-2 (cyclooxygenase 2), and matrix metalloproteinases (2, 3). AP-1 (activator protein 1), another transcription factor like NF-κB, is involved in inflammation, oncogenesis, lymphoid differentiation, embryonic development, and apoptosis (4–6). AP-1 consists of Fos and Jun families of proteins. The heterodimer of Jun and Fos binds to AP-1 promoter site with high affinity and expresses cyclin D1 as cell proliferator (7). Higher activity of NF-κB is a common characteristic of many tumor cells (1, 2, 8, 9). The phosphoryl and acetyl forms of p65 (RelA) subunit of NF-κB is the important determinant for its multifold gene expression and tumorigenic activity (10, 11). Inhibiting constitutively expressed NF-κB in tumor cells, the cell growth has been shown to be reduced dramatically both in vitro and in vivo (12–14). The growing evidence of the role of NF-κB in tumorigenesis and resistance to chemotherapy prompt a study on the mechanism and regulation of constitutive activity of NF-κB in tumor cells.
TNF superfamily members convey signals that elicit diverse cellular responses. Trimerization of TNF receptors recruits adapter proteins (members of the TNF receptor-associated factor family) to the cytoplasmic domain to initiate signaling pathways that regulate apoptosis, tumorigenesis, viral replication, etc. (15). TNF-mediated signaling thus has become a prominent target for therapeutic intervention. Interaction of TNFR1 with TNF receptor-associated death domain (TRADD) and TRAF2 (TNF receptor-associated factor 2) leads to activation of NF-κB and cell death (16, 17). Although interaction of TRAF2 with TANK (TRAF for NF-κB) leads to activation of IκBα kinases (IKKs) via NF-κB-inducing kinase, followed by NF-κB activation (18). Interaction of TRAF2 with TANK also activates JNK (18). Interaction of TRAF2 with MEKK1 (mitogen-activated protein kinase kinase kinase 1) has been shown to activate JNK and AP-1 (19). Interaction of TRAF2 with MEKK1 activates not only the JNK but also IKKβ via recruitment of receptor-interacting protein (20).
The thiazolidones have drawn considerable attention for their anti-bacterial, anti-fungal, and anti-inflammatory activities. Newer derivatives of 1,2,4-thiadiazolidines, including 3-oxo-1,2,4-thiadiazolidines, were synthesized, and biological effects were studied (21, 22). 5-(4-Methoxyarylimino)-2-N-(3,4-dichlorophenyl)-3-oxo-1,2,4-thiadiazolidine (P3-25) has been shown to inhibit TNF-induced cell signaling (22). We investigated the binding affinities of P3-25 and structurally similar 5-aryl thiazolidine-2,4-diones (5-aryl TZD) with TRADD-TRAF2, TRAF2-TANK, and TRAF2-MEKK1 complexes in silico by an advanced docking program, AutoDock4 (23). The selective inhibition of interacting molecules is becoming a promising approach of this class of anti-tumor or anti-inflammatory agents.
In this report, for the first time, we provide evidence that the dimethyl form of 1,2,4-thiadiazolidine (P3-25) interacts with TRAF2-TANK and thereby prevents NF-κB activity and inhibits downstream signaling. P3-25 facilitated AP-1 activation via TRAF2-MEKK1 interaction, which might explain the regulated cell cycle through AP-1-mediated gene expression. The twin effects of P3-25 would contribute to the therapeutic value of inhibition of TNF-mediated signaling as well as high basal expressed tumor cells. Overall, multiple effects of P3-25 to block NF-κB completely might cut the life line of tumor cells by blocking excessive proliferation and metastasis and thereby regulate proliferation through AP-1 activation. By bypassing several side effects due to complete NF-κB inhibition, P3-25 might prove to be a safe molecule as a chemotherapeutic drug.
EXPERIMENTAL PROCEDURES
Materials
Glycine, 4-methyl umbelliferyl phosphate, Sepharose 4B, cyanogen bromide, and anti-tubulin antibody were obtained from Sigma. 5-aryl TZD was obtained from Merck. Penicillin, streptomycin, neomycin, RPMI 1640 medium, and fetal bovine serum were obtained from Invitrogen. Antibodies against IKKα, IKKβ, JNK, TRADD, TRAF2, MEKK1, and TANK and double-stranded AP-1 oligonucleotides were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antibody against phospho-JNK was obtained from Cell Signaling Technologies (Danvers, MA). Recombinant IL-8 and TNF were purchased from Peprotech. Plasmids for NF-κB-SEAP, AP-1-luciferase, TRAF2, TRADD, TRAF2-DN, TRADD-DN, and GFP were kindly supplied by Prof. B. B. Aggarwal (University of Texas M. D. Anderson Cancer Center (Houston, TX)). The P3-25 was prepared from 1-(4-methoxyaryl)thiocarbamide as described previously (21, 22) (also see supplemental Fig. 1A for a flow chart of the synthesis of thiadiazolidine, supplemental Fig. 1B for modification of side groups, and supplemental Fig. 1C for the structure of P3-25).
Cell Lines and Treatment with P3-25
The cell lines used in this study were Jurkat (T-cell) and HuT-78 (modified T-cell) obtained from the American Type Culture Collection (Manassas, VA). Cells were cultured in RPMI 1640 medium containing 10% fetal bovine serum, penicillin (100 units/ml), and streptomycin (100 μg/ml). P3-25 was chemically synthesized as described previously (21). A stock solution of 10 mm in DMSO was prepared and added to the cell culture medium to give the mentioned concentrations. Culture supernatant from HuT-78 cells, treated with 100 nm P3-25 for 0, 12, and 24 h, when assayed for lactate dehydrogenase activity by incubating with substrate solution (23 mm sodium pyruvate and 0.05 mm NADH in 0.1 m phosphate buffer, pH 7.5), showed 1.22 ± 0.08, 1.18 ± 0.09, and 1.16 ± 0.078 absorbance, respectively, at 340 nm, indicating little cytolysis during incubation with the compound.
NF-κB and AP-1 DNA Binding Assay
DNA binding of AP-1 and NF-κB was determined by gel shift assay (24). Briefly, the cells were subjected to different treatments as described, and cytoplasmic and nuclear extracts were prepared. Nuclear extract (8 μg of protein) was incubated with 32P-end-labeled double-stranded NF-κB oligonucleotide of HIV-LTR, 5′-TTGTTACAAGGGACTTTCCGCTGGGGACTTTCCAGGGAGGCGTGG-3′, for 30 min at 37 °C, and the DNA-protein complex was separated from free oligonucleotide on 6.6% native PAGE. AP-1 DNA binding was assayed similarly using specific double-stranded labeled oligonucleotide. Visualization of radioactive bands was done in a phosphor imager (Fuji, Japan).
Assay of NF-κB-dependent Secretory Alkaline Phosphatase (SEAP) Reporter Gene
Cells were transiently transfected with Qiagen SuperFect transfection reagent (Hilden, Germany) with reporter plasmid containing the NF-κB binding site cloned upstream of heat-stable SEAP, designated as NF-κB-SEAP and GFP (0.5 μg each) constructs, for 3 h and cultured for 12 h. GFP-positive cells were counted and evaluated for transfection efficiency. These cells were treated with P3-25 for different time periods. Cell culture-conditioned medium (25 μl) was analyzed for SEAP activity essentially as per the Clontech (Palo Alto, CA) protocol, and results are reported as -fold activation with respect to vector-transected cells as described previously (25).
Assay of AP-1-dependent Luciferase Gene Transcription
The expression of the AP-1-dependent luciferase reporter gene was carried out as described previously (26). Cells, transiently transfected with 0.5 μg of reporter plasmid containing an AP-1 binding site cloned upstream of luciferase (designated as AP-1-luciferase) and GFP constructs, were washed and cultured for 12 h, followed by treatment with P3-25 for different time periods. The cell pellets were extracted with lysis buffer (part of the luciferase assay kit from Promega), and the extracts were incubated with the firefly luciferin substrate (Promega). Light emission was monitored with a Luminometer, and values were calculated as -fold activation over vector-transfected value.
Immunoprecipitation of MEKK1, TRAF2, and TANK and Detection by Western Blot
Sepharose 4B beads were coupled with anti-TRAF2 or anti-TANK antibody by cyanogen bromide. HuT-78 cells were transfected with vector, TRAF2, or TRAF2-DN constructs for 3 h and then cultured for 12 h. Cells were treated with 100 nm P3-25 for 12 h. Cells extracts were prepared and precleared with IgG, followed by removal by protein A-Sepharose beads and incubation with antibody-coupled beads for 6 h. The column was prepared with these beads and washed and eluted with high salt solution. Elutes were used to measure the amounts of TRAF2, TANK, and MEKK1 by Western blot.
Assay of IKK and JNK
The IKK and JNK assays were performed by a method described previously (25). Briefly, IKK complex or JNK from whole-cell extract (300 μg) was precipitated with anti-IKKα and anti-IKKβ or anti-JNK antibodies (1 μg each), followed by incubation with protein A/G-Sepharose beads (Pierce). After a 2-h incubation, the beads were washed with lysis buffer and assayed for IKK or JNK activity using 2 μg of GST-IκBα (amino acids 1–54) or GST-Jun (amino acids 1–100) as substrate proteins, respectively.
Preparation of Ligands and Docking
For docking experiments with the AutoDock 4 suite of programs, ligand molecules P3-25 and 5-aryl TZD were drawn, optimized, and saved in the mol2 format with the aid of Hyperchem programs. Full hydrogens were added to the ligands, and Gasteiger partial atomic charges were computed using the VEGA program in the required format (27, 28). The x-ray structure of TRADD in the bound state with TRAF2 was taken from the Protein Data Bank (29) (accession number 1FV3) (30). For the building of the complex structure of TRAF2 with TANK or MEKK1, the crystal structures of TANK or MEKK1 co-ordinates were inserted into the experimental model of TRAF2. All bound waters, polar hydrogens, and the Kollman-united charges were added to the proteins. Protein pockets and cavities were located and measured using the program CastP Server (31). AutoDock requires a precalculated electrostatic grid map for each atom type present in the complex molecules. Affinity (grid) maps of 150 × 150 × 200 grid points and 0.375 Å (roughly a quarter of the length of a carbon-carbon single bond) spacing between grid points were utilized. The grids (one for each atom type in the ligand plus one for electrostatic interactions) were generated using the Autogrid program, and maps were centered on the protein center. AutoDock parameter set and distance-dependent dielectric functions were used in the calculation of the van der Waals and the electrostatic terms, respectively. Docking simulations were performed using the Lamarckian genetic algorithm and the Solis and Wets local search method (30). Initial position, orientation, and torsions of the ligand molecules were set randomly. All rotatable torsions were released during docking. Each docking experiment was derived from 100 different runs that were set to terminate after a maximum of 2,500,000 energy evaluations. The population size was set to 250. During the search, a translational step of 0.2 Å, and quaternion and torsion steps of 5 were applied. Binding free energies of each job were collected, and the first rank results were selected. The clusters were ranked by the lowest energy representative of each bunch. All calculations were carried out on PC-based machines running Linux x 64 as the operating system. The resultant structure files were analyzed using PyMOL visualization programs (available on the World Wide Web).
Docking Performance on TRADD-TRAF2, TRAF2-TANK, and TRAF2-MEKK1 Complexes with P3-25 and 5-Aryl TZD
Binding sites of these complexes were identified as described previously (31). The conformation of the binding site was constructed manually to dock P3-25 and 5-aryl TZD. The validation of the docking accuracy was done by docking of the native co-crystallized P3-25 5-(4-methoxyarylimino)-2-N-(3,4-dichlorophenyl)-3-oxo-1,2,4-thiadiazolidine and 5-aryl TZD into its binding site of TRADD-TRAF2, TRAF2-TANK, and TRAF2-MEKK1. The binding affinities of P3-25 and 5-aryl TZD with TRADD-TRAF2, TRAF2-TANK, and TRAF2-MEKK1 complexes were evaluated by the binding free energies (ΔGb, kcal/mol), inhibition constants (Ki), hydrogen bonds, and r.m.s. deviation values.
Cluster Details
Cluster analysis or “structure binning” superimposes each final ligand structure with each other structure and measures the r.m.s. deviation between each atom pair. Structures that have an r.m.s. deviation lower than rmstol (listed in ligand.dpf) are automatically assumed to be similar; structures that have an r.m.s. deviation greater than rmstol are assumed to be different. This determines if different families of structures have been produced from multiple runs.
Statistical Analysis
Data were expressed as mean ± S.D. of three independent experiments. Statistical analyses of the samples were done by Student's t test wherever applicable. p < 0.05 was considered to be significant.
RESULTS
In this study, we examined the effect of P3-25 in regulation of DNA binding activity of NF-κB and AP-1 in the HuT-78 cell line with constitutive activity of NF-κB and AP-1 and Jurkat T-cells.
P3-25 Inhibits NF-κB but Increases AP-1 Activation Pathway
Jurkat and HuT-78 cells were treated with P3-25 (100 nm) for different times, and then DNA binding to NF-κB was measured by a gel shift assay. P3-25 treatment did not alter the amount of DNA bound to NF-κB in these cells. HuT-78 cells showed high basal DNA binding to NF-κB (Fig. 1A1). A decrease in the high basal activity (3.8-fold, p < 0.001) of the NF-κB-dependent SEAP was observed in NF-κB promoter-driven SEAP construct (NF-κB-SEAP)-transfected HuT-78 cells (Fig. 1A2).
FIGURE 1.
Effect of P3-25 on activation of NF-κB, AP-1, IKK, and JNK in Jurkat and HuT-78 cells. Jurkat and HuT-78 cells were treated with 100 nm P3-25 for different times, and nuclear extracts were prepared. NF-κB (A1) and AP-1 (B1) DNA binding was measured by a gel shift assay. Cells were co-transfected with plasmids for NF-κB promoter DNA that had been linked to SEAP (NF-κB-SEAP) or AP-1 promoter DNA that was linked to the luciferase reporter gene (AP-1-luciferase) and GFP. After washing, cells were cultured for 12 h and then treated with P3-25 (100 nm) for different times. The GFP-positive cells were counted, and transfection efficiency was calculated. Culture supernatant was assayed for SEAP activity. The results are represented as -fold activation over the nontransfected control (A2). After treatment, the cell pellet was extracted and assayed for luciferase activity (B2). Cells were treated with 100 nm P3-25 for different times. The whole cell extracts (300 μg of protein) were immunoprecipitated with anti-IKKα and IKKβ (1 μg each) antibodies, and kinase was assayed using GST-IκBα as a substrate (C). The IKKα was assayed from 100 μg of protein extract by Western blot. The amount of phospho-JNK was measured from 100 μg of whole cell extracts by Western blot using anti-phospho-JNK antibody (D, top). The whole cell extracts (300 μg of protein) were immunoprecipitated with 1 μg of anti-JNK antibody, and JNK activity was assayed using GST-Jun as substrate (D, bottom). HuT-78 cells, transfected with the NF-κB-SEAP construct were treated with 100 pm P3-25 or 5-aryl TZD for different times, and activity of SEAP was measured from culture supernatant (E).
The amount of DNA binding to AP-1 was enhanced in Jurkat cells with increasing time of P3-25 treatment, whereas the high basal DNA binding to AP-1 (5.2-fold, p < 0.005) remained unaltered upon P3-25 treatment in HuT-78 cells (Fig. 1B1). The activity of AP-1-dependent luciferase increased upon P3-25 treatment in both Jurkat and HuT-78 cells (Fig. 1B2). These results suggest that P3-25 treatment inhibited NF-κB activity and enhanced AP-1 activation. The basal activity of IKK in Jurkat and HuT-78 cells was unaltered with increasing time of P3-25 treatment (Fig. 1C). The basal activity of JNK in both Jurkat (1-fold, p < 0.001) and HuT-78 (4.2-fold, p < 0.005) cells increased with increasing time of P3-25 treatment as shown by Western blot and in vitro JNK assay data (Fig. 1D), as expected of activation of AP-1 and inhibition of NF-κB.
The high basal SEAP activity in HuT-78 cells (5.8-fold, p < 0.005) decreased in a time-dependent manner of P3-25 treatment (Fig. 1E). The related compound, 5-aryl TZD, was ineffective under these conditions. These results suggest that P3-25 specifically blocks NF-κB-dependent gene expression.
P3-25 Inhibits TNF- and IL-8-induced NF-κB-dependent Reporter Gene Expression but Is Unable to Block IL-8-induced DNA Binding to NF-κB
TNF-induced but not IL-8-induced NF-κB-DNA binding decreased in the nuclear extract with increasing time of P3-25 treatment of the Jurkat cells (Fig. 2A). Surprisingly, activity of SEAP (NF-κB-dependent reporter gene) was inhibited with increasing time of P3-25 treatment under these conditions (Fig. 2B). TNF- or IL-8-induced PKAα activity (4- and 5.8-fold, respectively, with p < 0.005) was inhibited by P3-25 treatment as detected by an in vitro kinase assay using GST-p65 as substrate protein (Fig. 2C). These results suggest that NF-κB activation induced by IL-8 differs from that of TNF. P3-25 inhibits p65 phosphorylation by inhibiting activity of PKAα and thereby inhibits NF-κB-dependent gene transcription as reported earlier (10, 32).
FIGURE 2.
Effect of P3-25 on TNF- or IL-8-induced NF-κB activation and PKAα activity. Jurkat cells were treated with 100 nm P3-25 for different times and then stimulated with 100 pm TNF or 100 ng/ml IL-8 for 2 h. NF-κB DNA binding was measured from nuclear extracts (A). The whole cell extracts (300 μg of protein), prepared from P3-25-treated but TNF- or IL-8-stimulated cells for 1 h, were immunoprecipitated with anti-PKAα (1 μg) antibody. PKAα activity was assayed using GST-p65 as substrate (C). The amount of PKAα was detected in the cell extract by Western blot. Jurkat cells were transfected with NF-κB-SEAP and GFP constructs cultured for 12 h and treated with P3-25 for different times. Cells were then stimulated with TNF (100 pm) or IL-8 (100 ng/ml) for 12 h. Culture supernatants were used to detect SEAP activity, and activity is indicated as -fold activation relative to vector-transfected cells (B).
P3-25 Inhibits TRAF2- but Not TRAF6-mediated DNA Binding of NF-κB and Activity of IKK
The effect of P3-25 on TRAF6-induced NF-κB activation was next studied because IL-8 is known to predominantly activate NF-κB by recruiting TRAF6 (26). Cells transfected with TRAF2 and also TRAF6 showed higher NF-κB-DNA binding (4.4- and 3.6-fold, respectively, with p < 0.001). Treatment of P3-25 showed a decrease in NF-κB DNA binding in TRAF2-transfected but not in TRAF6-transfected or TRAF2- and TRAF6-co-transfected cells (Fig. 3A1). NF-κB-dependent expression of the SEAP gene decreased in all of these transfection conditions upon P3-25 treatment (Fig. 3A2). P3-25 treatment decreased IKK activity in a time-dependent manner in TRAF2-transfected but not in TRAF6-transfected cells (Fig. 3C), suggesting that P3-25 interfered with TRAF2-mediated signaling upstream of IKK.
FIGURE 3.
Effect of P3-25 on TRAF2- and/or TRAF6-induced NF-κB, AP-1, IKK, and JNK activation. Jurkat cells were transfected with plasmids for NF-κB-SEAP or AP-1-luciferase and GFP. After washing, cells were cultured for 12 h and then treated with P3-25 (100 nm) for different times. Nuclear extracts were prepared, and NF-κB (A1) and AP-1 (B1) DNA binding was measured by gel shift assay. Culture supernatant was assayed for SEAP activity (A2). Cell pellet was extracted and assayed for luciferase activity (B2). The results are represented as -fold activation over the nontransfected control. TRAF2- and/or TRAF6-transfected cells were treated with P3-25 for different times. The whole cell extract (300 μg of protein) was immunoprecipitated with anti-IKKα and IKKβ (1 μg of each) antibodies, and IKK was assayed using GST-IκBα as substrate (C). The IKKα was assayed from 100 μg of protein extract by Western blot. The amounts of phospho-JNK were measured from 100 μg of whole cell extracts by Western blot (D, top). The whole cell extracts (300 μg of protein) were immunoprecipitated with anti-JNK antibody (1 μg), and JNK activity was measured using GST-Jun as substrate (D, bottom).
P3-25 Induces TRAF2- or TRAF6-mediated Activation of AP-1 and JNK
P3-25 induced AP-1-DNA binding activity in vector-transfected cells, and this remained unaltered in TRAF2-transfected, TRAF6-transfected, or TRAF2- and TRAF6-transfected cells (Fig. 3B1). However, P3-25 treatment increased AP-1-dependent luciferase activity in vector-transfected, TRAF2-transfected, TRAF6-transfected, or TRAF2- and TRAF6-transfected cells (Fig. 3B2). These results indicate that interaction of P3-25 with TRAF2 leads to activation of AP-1. High basal activity of JNK in TRAF2-transfected (4.2-fold, p < 0.005) or TRAF6-transfected (3.4-fold, p < 0.005) cells was enhanced further by P3-25 treatment in a time-dependent manner, as shown by an in vitro JNK assay, which was further supported by Western blot data using anti-phospho-JNK antibody (Fig. 3D). These data further suggest AP-1 activation upon P3-25 treatment through JNK activation via the TRAF2 and TRAF6 pathway.
P3-25 Does Not Interfere with DNA Binding to NF-κB and AP-1 in Dominant Negative TRAF2- or TRADD-transfected Cells
High basal expression of NF-κB progressively decreased in vector-transfected HuT-78 cells upon P3-25 treatment kinetically. The basal expression of NF-κB decreased in TRAF2-DN- or TRADD-DN-transfected HuT-78 cells, and P3-25 treatment had no further effect on this at any time of treatment (supplemental Fig. 2A1). Similarly, P3-25 treatment had no effect on the changes of the NF-κB-dependent reporter gene SEAP activity in NF-κB-SEAP-transfected cells (supplemental Fig. 2A2). Treatment of P3-25 in TRAF2-DN- or TRADD-DN-transfected cells decreased high basal amount of AP-1 DNA binding (supplemental Fig. 2B1) and AP-1-dependent luciferase activity (supplemental Fig. 2B2). These data suggest that TRAF2 and TRADD are required for P3-25-mediated AP-1 activation as well as NF-κB inhibition.
High basal activity of IKK decreased in TRADD-DN or TRAF2-DN HuT-78 cells, and this remained unaltered with P3-25 treatment with time in both of these transfected cells (supplemental Fig. 2C). The basal activity of JNK, measured by the amount of phospho-JNK, was further increased with increasing time of P3-25 treatment. TRADD-DN or TRAF2-DN transfection decreased the basal amount of phospho-JNK, and P3-25 treatment of these cells had no effect on these values at any time of treatment (supplemental Fig. 2D). These data further support the role of TRADD and TRAF2 in P3-25-mediated cell signaling.
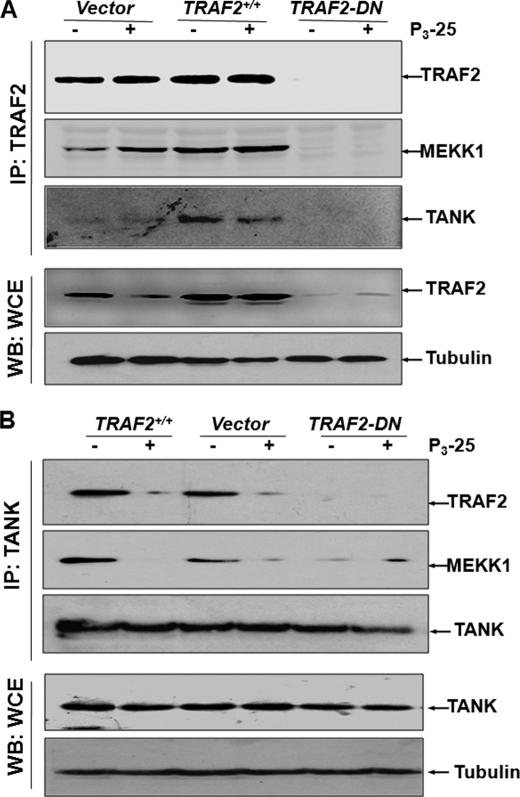
P3-25 Treatment Blocks TRAF2-TANK and Facilitates TRAF2-MEKK1 Interaction
TRAF2 interacts with TANK (TNF receptor-associated factor for NF-κB) and MEKK1 (18). When TRAF2 was immunoprecipitated with anti-TRAF2 antibody from TRAF2-DN-transfected HuT-78 cells, the amount of MEKK1 or TANK was undetected as shown by Western blot. TRAF2 interacted with MEKK1 and TANK as shown by a Western blot from a fraction immunoprecipitated against anti-TRAF2 antibody of TRAF2- or vector-transfected HuT-78 cell extract. P3-25 treatment inhibited interaction of TRAF2 with TANK but not with MEKK1 (Fig. 4A). P3-25 treatment showed the inhibition of TRAF2 and TANK interaction in vector- or TRAF2-transfected HuT-78 cells, as shown by Western blot from the immunoprecipitated fraction by anti-TANK antibody. The amount of MEKK1 decreased in vector- or TRAF2-transfected HuT-78 cell extracts upon P3-25 treatment (Fig. 4B). In TRAF2-DN-transfected cells, the amounts of both MEKK1 and TRAF2 were not detected in immunoprecipitated fractions with anti-TANK antibody. These data further indicate that TRAF2 interacts with TANK and MEKK1 and that P3-25 inhibits TRAF2 and TANK interaction.
FIGURE 4.
Effect of P3-25 on TRAF2, TANK, and MEKK1 interaction. HuT-78 cells were transfected with vector, TRAF2, or TRAF2-DN constructs. Cells were cultured for 12 h and then treated with P3-25 (100 nm) for 12 h. The whole cell extracts were prepared and incubated with Sepharose CL 4B beads coupled with anti-TRAF2 (A) or anti-TANK (B) antibody for 4 h. Beads were packed in a 2-ml column, washed with 0.2 m NaCl solution, and then eluted with 0.5 m NaCl solution. The fractions were run in 12% SDS-PAGE, and TRAF2, MEKK1, and TANK were detected by Western blot. IP, immunoprecipitation; WB, Western blot.
P3-25 Interacts with TRAF2 More Potently Than TRAF6
The binding affinities of P3-25 with TRAF2 and TRAF6 were evaluated by the binding free energies (ΔGb, kcal/mol), inhibition constants (Ki), hydrogen bonds, and r.m.s. deviation values. The predicted binding sites for TRAF2 and TRAF6 with P3-25 are shown in Fig. 5A. P3-25 mostly binds with positive charged basic amino acid residues and the predicted active sites of TRAF2 and TRAF6 (Fig. 5B). The different surface pocket for residues seems to be an important factor in determining the different mode of P3-25 interaction with TRAF2 and TRAF6, where the P3-25 binds with Arg393, Phe447, and Asp399 of TRAF2 and Arg392, Lys469, and Gly470 of TRAF6 (Fig. 5B). The binding free energies of these residues, calculated based on their best docking scores, are −18.43 kcal/mol and −11.21 kcal/mol for TRAF2 and TRAF6, respectively (Table 1). The difference between the lowest binding interaction of TRAF2 and TRAF6 with P3-25 is shown in Fig. 5C. It revealed the energy difference and cluster runs of the TRAFs. From the above results, it is clear that P3-25 interferes with TRAF2 action more efficiently than with that of TRAF6.
FIGURE 5.
Effect of P3-25 on interaction with TRAF2 and TRAF6 in silico. Shown is a surface representation of TRAF2, colored on the basis of electrostatic potential (−57.293kbT/e to +57.293kbT/e, where kb, T, and e are the Boltzmann constant, temperature, and electron charge, respectively) and the bound P3-25. A surface representation of TRAF6 is shown with the bound P3-25 (A). P3-25 bound into the binding site of TRAF2, represented in stick form (light blue), forms hydrogen bonds with Arg393, Asp399, and Phe447 and the binding site of TRAF6 and forms hydrogen bonds with Arg392, Lys469, and Gly470 (B). The lowest energies of different clusters of TRAF2 and TRAF6 that bind with P3-25 have been calculated and are indicated in the graph (C).
TABLE 1.
Energy values of TRAF2 and TRAF6 with P3-25
Docking energies are as determined by AutoDock.
| Protein | Cluster rank | Cluster run | Clustera | r.m.s. deviationb | Lowest energyc | Free energy (ΔG) | Inhibition constant (Ki) |
|---|---|---|---|---|---|---|---|
| kcal/mol | |||||||
| TRAF2 (1LB4) | 1 | 16 | 1 | 0.00 | −18.43 | −18.57 | +1.82e−13 |
| 2 | 2 | 1 | 0.00 | −17.10 | −17.29 | +2.10e−13 | |
| 3 | 4 | 1 | 0.00 | −16.63 | −16.85 | +4.45e−13 | |
| 4 | 27 | 2 | 0.00 | −16.49 | −16.53 | +7.67e−13 | |
| 5 | 22 | 1 | 0.20 | −16.28 | −16.47 | +8.43e−13 | |
| TRAF6 (1CA4) | 1 | 98 | 1 | 0.75 | −11.21 | −11.35 | +3.77e−8 |
| 2 | 82 | 2 | 1.25 | −11.03 | −11.16 | +3.02e−8 | |
| 3 | 45 | 1 | 0.23 | −10.78 | −10.89 | +5.45e−8 | |
| 4 | 15 | 1 | 0.00 | −10.43 | −10.52 | +7.55e−8 | |
| 5 | 3 | 2 | 0.48 | −10.11 | −10.18 | +8.13e−8 |
a The best clusters of a total 100 runs are shown.
b The geometry fit was measured by r.m.s. deviation of angles, r.m.s. deviation = (Σαi − αideal)2/n)1/2, where αi is the measured angle, αideal is the angle in ideal coordination geometry, and n is the total number of angles of the corresponding geometry.
c Value for the optimal structure in the cluster.
P3-25 Interferes Interaction between TRAF2 and TANK but Not between TRADD and TRAF2 or TRAF2 and MEKK1
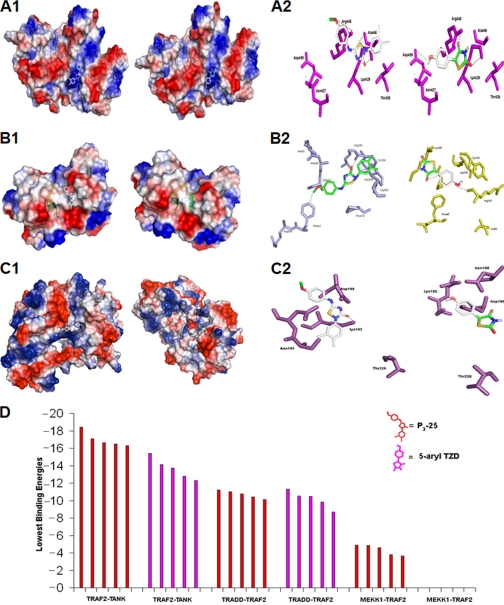
TRADD-TRAF2, TRAF2-TANK, and TRAF2-MEKK1 binding sites were identified as described previously (31), and predicted binding sites of P3-25 and 5-aryl TZD with these complexes were shown by surface binding in Fig. 6, A1, B1, and C1, respectively. The complex of TRADD-TRAF2 formed a hydrogen bond with P3-25 and 5-aryl TZD (Fig. 6A2) with binding free energy of −11.35 and −11.55 kcal/mol, respectively (Table 2, top) suggesting weak interaction for both. The benzene ring of P3-25 is surrounded by hydrophobic residues (Ala466, Ser467, Tyr395, and Gly400), and they formed hydrophic interactions in the TRAF2-TANK complex. In addition, the two oxygen atoms of the benzene ring of P3-25 formed three hydrogen bonds with Arg393, Phe447, and Asp399. The 5-aryl TZD formed hydrogen bonds with Arg392, Lys446, and Gly470 of the TRAF2-TANK complex (Fig. 6B2). The energies of these residues for TRAF2-TANK are −18.43 and −15.43 kcal/mol for P3-25 and 5-aryl TZD, respectively (Table 2, middle) suggesting that P3-25 has higher binding affinity than 5-aryl TZD for forming TRAF2-TANK complex. The TRAF2-MEKK1 electrostatic potential surface showed the possible docking interaction with P3-25 and 5-aryl TZD (Fig. 6C1). P3-25 formed a single hydrogen bond with the active site Lys192 residue of the TRAF2-MEKK1 complex (Fig. 6C2). It showed the lowest binding energy (−4.89 kcal/mol). The 5-aryl TZD showed the lowest binding energy (+157.21 kcal/mol) with residues in the TRAF2-MEKK1 complex (Table 2, bottom) suggesting no interaction. The docking of the complex of TRAF2-MEKK1 with P3-25 showed fewer interactions compared with TRAF2-TANK or TRADD-TRAF2 complex. The above results revealed that synthetic P3-25 molecule more potently complexes with TRAF2-TANK than 5-aryl TZD (Fig. 6D), supporting the experimental studies obtained.
FIGURE 6.
Effect of P3-25 and 5-aryl TZD on interaction with TRADD-TRAF2, TRAF2-TANK, or TRAF2-MEKK complexes. A surface representation of TRADD-TRAF2 complex and binding of P3-25 and 5-aryl TZD at positive regions of the complex in silico is depicted (A1). Interaction of 5-aryl TZD and P3-25 with TRADD-TRAF2 complex is indicated (A2). Shown is a surface representation of the TRAF2-TANK complex, colored on the basis of electrostatic potential (−57.293kbT/e to +57.293kbT/e, where kb, T, and e are the Boltzmann constant, temperature and the electron charge, respectively) and the bound P3-25 and 5-aryl TZD (B1). P3-25 binds into the binding site of the TRAF2-TANK complex, represented in stick form (light blue), forms hydrogen bonds with Arg393, Asp399, and Phe447, and 5-aryl TZD forms hydrogen bonds with Arg393, Lys447, and Asn407 (B2). The electrostatic potential surface area of TRAF2-MEKK1 complex in binding with P3-25 and 5-aryl TZD is represented (C1). P3-25 interacts with the active site amino acid (magenta) Lys192 of MEKK1 and TRAF2 complex (C2, left). Interaction of 5-aryl TZD with MEKK1 active site residues (magenta) and TRAF2 complex is analyzed (C2, right). The graph shows the lowest binding energies of complexes with P3-25 and 5-aryl TZD with different complexes (D).
TABLE 2.
Energy values for the docking interaction of TRADD-TRAF2, TRAF2-TANK, and TRAF2-MEKK1 with P3-25 and 5-aryl TZD
Docking energies are as determined by AutoDock.
| Protein complex | Ligand | Cluster rank | Clustera | r.m.s. deviationb | Lowest energy (kcal/mol)c | Free energy (ΔG) | Inhibition constant (Ki)d |
|---|---|---|---|---|---|---|---|
| TRADD-TRAF2 | P3-25 | 1 | 1 | 0.75 | −11.21 | −11.35 | +3.77e−8 |
| 2 | 1 | 1.25 | −11.03 | −11.16 | +3.02e−8 | ||
| 3 | 1 | 0.23 | −10.78 | −10.89 | +5.45e−8 | ||
| 4 | 2 | 0.00 | −10.43 | −10.52 | +7.55e−8 | ||
| 5 | 1 | 0.48 | −10.11 | −10.18 | +8.13e−8 | ||
| TRADD-TRAF2 | 5-Aryl TZD | 1 | 1 | 0.56 | −11.43 | −11.55 | +7.87e−8 |
| 2 | 1 | 1.00 | −10.53 | −10.66 | +8.07e−8 | ||
| 3 | 1 | 0.00 | −10.48 | −10.59 | +8.55e−8 | ||
| 4 | 2 | 0.00 | 009.83 | −9.92 | +9.45e−8 | ||
| 5 | 1 | 0.02 | −8.71 | −8.98 | +9.83e−8 | ||
| TRAF2-TANK | P3-25 | 1 | 1 | 0.00 | −18.43 | −18.48 | +1.82e−8 |
| 2 | 2 | 0.00 | −17.10 | −17.29 | +2.10e−8 | ||
| 3 | 1 | 0.00 | −16.63 | −16.85 | +4.45e−8 | ||
| 4 | 1 | 0.00 | −16.53 | −16.53 | +7.67e−8 | ||
| 5 | 2 | 0.20 | −16.28 | −16.47 | +8.43e−8 | ||
| TRAF2-TANK | 5-Aryl TZD | 1 | 1 | 0.10 | −15.43 | −15.48 | +1.52e−8 |
| 2 | 2 | 0.20 | −14.10 | −14.39 | +1.98e−8 | ||
| 3 | 1 | 0.32 | −13.73 | −13.95 | +3.45e−8 | ||
| 4 | 1 | 0.01 | 12.80 | −12.93 | +5.67e−8 | ||
| 5 | 2 | 0.20 | −12.28 | −12.57 | +6.03e−8 | ||
| TRAF2-MEKK1 | P3-25 | 1 | 4 | 0.23 | −4.89 | −4.92 | +2.59e−8 |
| 2 | 1 | 1.88 | −4.85 | −4.88 | +2.77e−8 | ||
| 3 | 3 | 1.89 | −4.60 | −4.64 | +4.60e−8 | ||
| 4 | 2 | 2.56 | −4.58 | −4.60 | +4.23e−8 | ||
| 5 | 2 | 0.54 | −4.40 | −4.38 | +4.88e−8 | ||
| TRAF2-MEKK1 | 5-Aryl TZD | 1 | 2 | 0.00 | +157.21 | +159.80 | +0.004e−8 |
| 2 | 1 | 0.24 | +154.78 | +156.78 | +0.004e−8 | ||
| 3 | 3 | 0.80 | +140.58 | +142.56 | +0.003e−8 | ||
| 4 | 2 | 0.00 | +140.22 | +142.30 | +0.003e−8 | ||
| 5 | 3 | 0.06 | +135.26 | +135.87 | +0.002e−8 |
a The best clusters of a total 100 runs are shown.
b The geometry fit was measured by r.m.s. deviation of angles. r.m.s. deviation = (Σai − αideal)2/n)1/2, where αi is the measured angle, αideal is the angle in ideal coordination geometry, and n is the total number of angles of the corresponding geometry.
c Value for the optimal structure in the cluster.
d 5-Aryl TZD lacks binding ability with the TRAF2-MEKK1 complex, which gives a negligible value of the inhibition constant (Ki) compared with other ligands.
DISCUSSION
The present study demonstrated that the dichlorophenyl derivative of 1,2,4-thiadiazolidine (P3-25) decreases NF-κB activation and enhances AP-1 activation by interacting with TRAF2. It did not inhibit IL-8- or TRAF6-induced NF-κB DNA binding but inhibited NF-κB-dependent reporter gene expression. IL-8-mediated NF-κB activation requires recruitment of TRAF6 (26). We did not observe TRAF6- or IL-8-induced inhibition of IKK activity. P3-25 inhibited dependent gene expression of NF-κB in cells irrespective of inducers of NF-κB, overexpression of upstream molecules, such as TRAFs, or having constitutive NF-κB expression. This is in complete agreement with our previous report on P3-25 blocking expression of NF-κB-dependent genes through inhibition of p65 phosphorylation (10). TNF signals through recruitment of TRAF2 and IKKs via TANK. TNF-induced activation of IKKs was not observed upon P3-25 treatment, which was reflected in inhibition of NF-κB, supporting our previous observation (21). This suggests that the effect of P3-25 lies on TRAF2, but not on IKK. TRAF2 interacts with TANK and MEKK1 (18). P3-25 treatment inhibited TANK interaction with TRAF2, which was supported by a pull-down assay as well as AutoDock evidence. P3-25 did not interfere with TRAF2-MEKK1 interaction. TRAF2-TANK interaction has been shown to induce JNK and AP-1 (18). Although we observed JNK activation, which may be independent of TRAF2-TANK interaction, in silico data also suggest that P3-25 interferes with TRAF2-TANK but not TRADD-TRAF2 or TRAF2-MEKK1 interactions.
AutoDock evidence and the values of lowest binding energy suggest that the binding affinity of P3-25 with the TRAF2-TANK complex is stronger than the TRADD-TRAF2 or TRAF2-MEKK1 complex. The 5-aryl thiazolidine-2,4-diones, structurally similar to P3-25, having the same backbone, does not interact with TRAF2-MEKK1 at all and weakly interact with TRADD-TRAF2 or TRAF2-TANK. The strong binding ability of P3-25 with the TRAF2-TANK complex hinders the recruitment and activation of IKKs, the immediate molecules of such interaction. P3-25 is also known to inhibit p65 phosphorylation by blocking upstream kinases, casein kinase 2 and protein kinase A (10). The double sword effect of P3-25 on inhibition of NF-κB might be important for the potential therapeutic value of P3-25 in NF-κB-driven inflammatory and tumorigenic responses. Although P3-25 induces AP-1 by facilitating MEKK1 recruitment with TRAF2 followed by JNK activation, as shown in the schematic diagram in Fig. 7, the AP-1-mediated signaling may be less responsible for such responses than NF-κB.
FIGURE 7.
Schematic diagram of P3-25-mediated interference with cell signaling to inhibit NF-κB but to activate AP-1.
NF-κB and AP-1 regulate cell cycle in embryonic development and body growth. Excessive activation of these transcription factors results in inflammatory response and rapid cell cycle progression, the hallmark of tumorigenesis. Regulation of these transcription factors is required to control inflammatory diseases and cancer metastasis. Predominantly enhanced NF-κB activation through p65 phosphorylation and acetylation via recruitment of several cofactors is a prerequisite for the severalfold NF-κB-mediated transcription of genes required for tumorigenesis and tumor metastasis. Knock-out of NF-κB has several deleterious effects and is even lethal for embryo. Even T-cell-mediated apoptosis of dendritic cells has been shown to be inhibited through JNK-AP-1 activation when signaling mediated by TNF receptor superfamily members was ligated (33). Thus, deregulation of NF-κB function, while allowing AP-1 activation to progress regulated cell division through the interaction of P3-25 with TRAF2, would be ideal for many reasons. AP-1 is a mild transcription factor and is mostly involved in regulated cell cycle progression. Initially, it was considered to be a housekeeping transcription factor. In this report, for the first time we provide evidence that P3-25, a synthetic derivative, interacts with TRAF2 in such a manner that recruitment of TANK is completely inhibited and thereby inhibits recruitment of IKKs. Thus, P3-25 is inhibiting IκBα degradation, thereby arresting NF-κB in cytoplasm. Although TRAF6- or IL-8-mediated IKKs activation is unaffected by P3-25 treatment, it blocks NF-κB-dependent gene transcription by inhibiting p65 phosphorylation. Thus, the double sword mechanisms of P3-25 action are shutting down NF-κB functions completely (Fig. 7). Although P3-25 binding with TRAF2 inhibited TANK binding, it facilitated binding with MEKK1 required for AP-1 activation via JNK. Thus, P3-25 is allowing AP-1 activation. However, P3-25 would be an important drug target by blocking NF-κB action totally but allowing AP-mediated cell signaling to regulate inflammatory and tumorigenic responses without compromising its regulated cell growth. Thus, P3-25 might be a safe molecule as a potential chemotherapeutic drug by avoiding several side effects otherwise obtained upon complete inhibition of NF-κB and anticipating the function of NF-κB by activation of AP-1 in a regulated fashion.
Supplementary Material
Acknowledgments
We thank Drs. T. Ramasarma and H. A. Nagarajaram for critically and carefully reading the manuscript.
This work was supported by the Department of Biotechnology, Government of India. This work was also supported by a University Grants Commission, India, Major Research Project (to B. B.) and a Council for Scientific and Industrial Research, New Delhi, Senior Research Fellowship (to P. B. R.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
- NF-κB
- nuclear transcription factor κB
- IL
- interleukin
- JNK
- c-Jun N-terminal kinase
- TZD
- thiazolidine-2,4-diones
- P3-25
- 5-(4-methoxyarylimino)-2-N-(3,4-dichlorophenyl)-3-oxo-1,2,4-thiadiazolidine
- SEAP
- secretory alkaline phosphatase
- TNF
- tumor necrosis factor
- TANK
- TNF receptor-associated factor for NF-κB
- TRADD
- TNF receptor-associated death domain
- IKK
- IκBα kinase
- GFP
- green fluorescent protein
- r.m.s.
- root mean square.
REFERENCES
- 1.Rayet B., Gelinas C. (1999) Oncogene 18, 6938–6947 [DOI] [PubMed] [Google Scholar]
- 2.Karin M., Greten F. R. (2005) Nat. Rev. Immunol. 5, 749–759 [DOI] [PubMed] [Google Scholar]
- 3.Baeuerle P. A., Baichwal V. R. (1997) Adv. Immunol. 65, 111–137 [PubMed] [Google Scholar]
- 4.Fujioka S., Niu J., Schmidt C., Sclabas G. M., Peng B., Uwagawa T., Li Z., Evans D. B., Abbruzzese J. L., Chiao P. J. (2004) Mol. Cell. Biol. 24, 7806–7819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wisdom R., Johnson R. S., Moore C. (1999) EMBO J. 18, 188–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakiri L., Lallemand D., Bossy-Wetzel E., Yaniv M. (2000) EMBO J. 19, 2056–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaulian E., Karin M. (2001) Oncogene 20, 2390–2400 [DOI] [PubMed] [Google Scholar]
- 8.Wang C. Y., Cusack J. C., Jr., Liu R., Baldwin A. S., Jr. (1999) Nat. Med. 5, 412–417 [DOI] [PubMed] [Google Scholar]
- 9.Barkett M., Gilmore T. D. (1999) Oncogene 18, 6910–6924 [DOI] [PubMed] [Google Scholar]
- 10.Manna S. K., Manna P., Sarkar A. (2007) Cell. Death Differ. 14, 158–170 [DOI] [PubMed] [Google Scholar]
- 11.Dai Y., Rahmani M., Dent P., Grant S. (2005) Mol. Cell. Biol. 25, 5429–5444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manna S. K., Aggarwal B. B. (1999) J. Immunol. 162, 1510–1518 [PubMed] [Google Scholar]
- 13.Sreenivasan Y., Sarkar A., Manna S. K. (2003) Oncogene 22, 4356–4369 [DOI] [PubMed] [Google Scholar]
- 14.Duffey D. C., Chen Z., Dong G., Ondrey F. G., Wolf J. S., Brown K., Siebenlist U., Van Waes C. (1999) Cancer Res. 59, 3468–3474 [PubMed] [Google Scholar]
- 15.McWhirter S. M., Pullen S. S., Holton J. M., Crute J. J., Kehry M. R., Alber T. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 8408–8413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu H., Shu H. B., Pan M. G., Goeddel D. V. (1996) Cell 84, 299–308 [DOI] [PubMed] [Google Scholar]
- 17.Rothe M., Sarma V., Dixit V. M., Goeddel D. V. (1995) Science 269, 1424–1427 [DOI] [PubMed] [Google Scholar]
- 18.Chin A. I., Shu J., Shan Shi C., Yao Z., Kehrl J. H., Cheng G. (1999) Mol. Cell. Biol. 19, 6665–6672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chadee D. N., Yuasa T., Kyriakis J. M. (2002) Mol. Cell. Biol. 22, 737–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J. W., Joe C. O., Choi E. J. (2001) J. Biol. Chem. 276, 27064–27070 [DOI] [PubMed] [Google Scholar]
- 21.Manna P., Singh R., Narang K. K., Manna S. K. (2004) Ind. J. Heterocycl. Chem. 13, 249–252 [Google Scholar]
- 22.Manna P., Narang K. K., Manna S. K. (2005) Int. J. Cancer 113, 549–560 [DOI] [PubMed] [Google Scholar]
- 23.Morris G. M., Goodsell D. S., Halliday R. S., Huey R., Hart W. E., Belew R. K., Olson A. J. (1998) J. Comput. Chem. 19, 1639–1662 [Google Scholar]
- 24.Manna S. K., Zhang H. J., Yan T., Oberley L. W., Aggarwal B. B. (1998) J. Biol. Chem. 273, 13245–13254 [DOI] [PubMed] [Google Scholar]
- 25.Sarkar A., Sreenivasan Y., Ramesh G. T., Manna S. K. (2004) J. Biol. Chem. 279, 33768–33781 [DOI] [PubMed] [Google Scholar]
- 26.Manna S. K., Ramesh G. T. (2005) J. Biol. Chem. 280, 7010–7021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gasteiger J., Marsili M. (1980) Tetrahedron 36, 3219–3228 [Google Scholar]
- 28.Pedretti A., Villa L., Vistoli G. (2002) J. Mol. Graph. Model. 21, 47–49 [DOI] [PubMed] [Google Scholar]
- 29.Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr., Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. (1977) J. Mol. Biol. 112, 535–542 [DOI] [PubMed] [Google Scholar]
- 30.Park Y. C., Ye H., Hsia C., Segal D., Rich R. L., Liou H. C., Myszka D. G., Wu H. (2000) Cell 101, 777–787 [DOI] [PubMed] [Google Scholar]
- 31.Binkowski T. A., Naghibzadeh S., Liang J. (2003) Nucleic Acids Res. 31, 3352–3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manna S. K., Gangadharan C. (2009) Mol. Immunol. 46, 1340–1350 [DOI] [PubMed] [Google Scholar]
- 33.Kriehuber E., Bauer W., Charbonnier A. S., Winter D., Amatschek S., Tamandl D., Schweifer N., Stingl G., Maurer D. (2005) Blood 106, 175–183 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.