Abstract
Intracellular Ca2+ signaling is fundamental to neuronal physiology and viability. Because of its ubiquitous roles, disruptions in Ca2+ homeostasis are implicated in diverse disease processes and have become a major focus of study in multifactorial neurodegenerative diseases such as Alzheimer disease (AD). A hallmark of AD is the excessive production of β-amyloid (Aβ) and its massive accumulation in amyloid plaques. In this minireview, we highlight the pathogenic interactions between altered cellular Ca2+ signaling and Aβ in its different aggregation states and how these elements coalesce to alter the course of the neurodegenerative disease. Ca2+ and Aβ intersect at several functional levels and temporal stages of AD, thereby altering neurotransmitter receptor properties, disrupting membrane integrity, and initiating apoptotic signaling cascades. Notably, there are reciprocal interactions between Ca2+ pathways and amyloid pathology; altered Ca2+ signaling accelerates Aβ formation, whereas Aβ peptides, particularly in soluble oligomeric forms, induce Ca2+ disruptions. A degenerative feed-forward cycle of toxic Aβ generation and Ca2+ perturbations results, which in turn can spin off to accelerate more global neuropathological cascades, ultimately leading to synaptic breakdown, cell death, and devastating memory loss. Although no cause or cure is currently known, targeting Ca2+ dyshomeostasis as an underlying and integral component of AD pathology may result in novel and effective treatments for AD.
Keywords: Calcium, Calcium/Channels, Cell/Apoptosis, Channels/Calcium, Diseases/Alzheimer Disease, Diseases/Amyloid, Membrane/Channels, Peptides/Conformation
Introduction
Alzheimer disease (AD)2 is an idiopathic neurodegenerative disease, and little is yet understood of its underlying causes or mechanisms. Certain diagnostic features are central to AD (amyloid plaques, neurofibrillary tangles, and elevated levels of soluble amyloids in the brain and cerebrospinal fluid), but their roles in the most devastating aspect of the disease, namely memory loss, are unclear. One common factor that underlies AD pathogenesis is neuronal Ca2+ dysregulation. In this minireview, we focus specifically on the pathogenic interplay between β-amyloid (Aβ) and Ca2+ signaling dysregulation. Ca2+ signaling is fundamental to cellular function, involving a multitude of entry and release channels, clearance mechanisms, and intracellular stores. Among these Ca2+-regulating entities, Aβ may interact with a critical subset as discussed below and enable AD progression by altering Ca2+ homeostasis and triggering downstream pathogenic signaling cascades (1–3).
Implications of Cellular Ca2+ Dysregulation
Sustained disruptions in Ca2+ signaling have significant implications for the health and functionality of neurons over the lifetime of an organism (4) and form the basis of the Ca2+ hypothesis of AD (5). Under resting conditions, cytosolic Ca2+ is maintained at low nanomolar concentrations by an array of pumps, buffers, and transport mechanisms. Ca2+ entry into the cytosol is rigorously regulated and originates from one of two major sources: the extracellular fluid via entry across the plasma membrane (through receptor-, voltage-, and store-operated channels and Ca2+ exchangers) and intracellular stores such as the endoplasmic reticulum (ER) and mitochondria (6).
Interactions between Aβ and intracellular Ca2+ are particularly relevant to AD pathogenesis, as Ca2+ perturbations are a causal factor in excitotoxicity, synaptic degeneration, and cell death, whereas reduced Ca2+ release is neuroprotective (7). Both neuroprotective and pathogenic Ca2+ cascades can be triggered sequentially: the cell attempting to first compensate for metabolic stress by up-regulating protective mechanisms and then succumbing to sustained insults and initiating pathogenic and/or apoptotic pathways. For example, excess Ca2+ release initially activates anti-apoptotic transcription factors such as NFκB (8), which protects cells by inducing genes that promote cell survival and anti-apoptotic proteins (e.g. Bcl-2) and the cAMP response element-binding protein (CREB), which is Ca2+-dependent and plays a critical role in synaptic plasticity and neuronal survival (9). Among pathogenic responses, expression of C/EBP homologous protein (CHOP) inhibits protective proteins such as Bcl-2. Increased Ca2+ levels through Aβ-mediated mechanisms can also lead to mitochondrial Ca2+ overload, generation of superoxide radicals, and pro-apoptotic mitochondrial proteins such as caspases and cytochrome c, which are linked to cell death and neurodegeneration in several AD models (1).
Aβ Physiology and Pathophysiology
Aβ is a 39–42-amino acid peptide produced by the proteolytic cleavage of the amyloid precursor protein (APP), an integral membrane protein involved in signal transduction pathways. Cleavage of APP by α- or β-secretases forms the C-terminal portion of Aβ, and subsequently, the remaining membrane-bound C-terminal fragment is cleaved within its transmembrane domain by the aspartyl protease complex γ-secretase, of which presenilin is a crucial component (10). Mutations in the genes encoding APP and presenilin are associated with familial forms of AD and lead to increased Aβ production, suggesting a causal relationship between Aβ overproduction and AD pathogenesis (11). Moreover, the majority of mutations linked to early-onset AD cause increased production of Aβ42 (Aβ ending at position 42) relative to Aβ40, and Aβ42 appears to be the more toxic form of the peptide and more prone to undergo aggregation (12, 13).
Aβ Aggregation States: Which Is the Toxic Species?
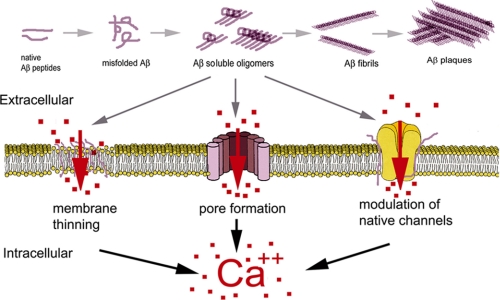
Aβ plaques are the most obvious and characteristic feature of AD. However, increasing evidence suggests that they may not be primarily responsible for the neurological deficits but rather implicates small soluble oligomeric aggregation states of Aβ. Protein aggregation is an aberrant self-associating process that can produce macroscopic entities such as the extracellular aggregates of Aβ peptide found in the brains of many AD patients. This process proceeds, in both in vitro and in vivo settings, through various intermediate aggregation states of Aβ peptides, ranging from small soluble oligomeric species formed by 2–50 peptides to insoluble filamentous aggregates from which plaques are formed (Fig. 1) (14). Several studies have characterized these intermediates, which most likely represent the most toxic forms of Aβ aggregates (15–18).
FIGURE 1.
Schematic model for Aβ monomers in which misfolding triggers self-aggregation into dimers, trimers, oligomers, fibrils, and fibrillar aggregates or plaques. The Aβ aggregates formed by 2–50 monomers are considered the toxic species.
The monomeric form of Aβ (either 1–40 or 1–42) has long been considered to be nontoxic or even protective and fails to evoke Ca2+ influx in in vitro experiments (18, 19, 20). From these monomers, up to 50 Aβ subunits can form intermediate aggregates, termed “small oligomers.” These low molecular weight aggregates are found in the growth medium of Aβ-secreting cells (21) and in extracts from human brain (17, 22). This category also includes Aβ-derived diffusible ligands, a neurotoxic species of Aβ aggregate formed by trimers through 24-mers secreted in in vitro preparations and found in murine and human brain extracts (16, 23–25). Small oligomers are reported to be the most toxic species of Aβ and potently disrupt cellular Ca2+ homeostasis (16, 18, 26). A different approach for classifying Aβ toxicity has been recently proposed by Glabe (27) based on the use of conformation-dependent antibodies that recognize generic epitopes associated with distinct peptide aggregation state of peptides rather than specific amino acid sequence and number of peptides.
The final stage of Aβ peptide aggregation is represented by amyloid plaques in the brains of AD patients. Although plaques are a hallmark of AD, their density does not correlate well with the degree of neuronal or cognitive deficits (28). On the contrary, it has been proposed that plaques may contribute to the removal and inactivation of the smaller soluble toxic species (17, 29), rendering the insoluble plaque deposits as potentially neuroprotective, particularly in the early stage of the disease.
As detailed below, numerous publications studying possible molecular mechanisms of Aβ40/42 oligomers have proposed diverse modalities of action. We believe that many of the apparently contradictory results in the literature may be attributed to different experimental methods and inconsistencies in preparation of Aβ oligomers, resulting in variability in the initial structure and aggregation state of the peptide, the presence of different solvents, heterogeneous nucleation, pH, and starting concentrations of the peptide (30).
Aβ and Membrane Ca2+ Permeability
A major mechanism by which Aβ is believed to alter cellular Ca2+ homeostasis involves disruption of membrane Ca2+ permeability. It is widely accepted that application of Aβ to cultured cells triggers unregulated flux of Ca2+ through the plasma membrane (5, 18, 26). However, the precise molecular mechanism of Aβ toxicity remains to be determined. Here, we outline the three major proposed mechanisms of Aβ interaction with cell membranes, involving interactions with endogenous Ca2+-permeable channels, disruption of membrane lipid integrity, and formation of Ca2+-permeable channels by Aβ.
Actions of Aβ on Endogenous Plasmalemmal Ion Channels
Interactions of Aβ with various Ca2+-permeable channels have been established (31, 32), including voltage-gated Ca2+ channels (N, P, and Q), nicotinic acetylcholine channels (α7 and α4β2), glutamate receptors (AMPA and NMDA), dopamine receptors, serotonin receptors (5-hydroxytryptamine type 3), and intracellular inositol trisphosphate receptors (IP3Rs). Several lines of evidence point to complex dynamics between Aβ and both the cholinergic and glutamatergic neurotransmitter systems during the progression of AD (33, 34). Receptor subtypes within these two receptor families, such as α7-nAChRs and AMPA and NMDA glutamate receptors, are all Ca2+-permeable and expressed in brain regions supporting higher cognitive functions such as the neocortex and hippocampus (35). Moreover, neuronal loss during the course of the disease occurs predominantly in these brain areas (36). These observations, together with the discovery of substantial neocortical deficits in choline acetyltransferase and reduced choline uptake in AD animal models, led to the “cholinergic hypothesis of AD,” wherein the degeneration of cholinergic neurons and loss of cholinergic neurotransmission significantly contribute to cognitive deterioration (37). This hypothesis has been strengthened by positive correlations between nAChR α7- and α4-subunit expression and neurons that accumulate Aβ and by the colocalization of α7-nAChRs with plaques (38). However, Aβ affects nAChR functioning with conflicting results describing Aβ as either an agonist or antagonist of nAChRs (33). Importantly, Aβ has been shown to bind with high affinity to α7- and α4β2-nAChRs (respective Ki of ∼5 pm and 30 nm) in cortical and hippocampal synaptic membrane preparations, suggesting that Aβ peptide accumulation in the synaptic cleft of cholinergic synapses may promote the formation of Aβ·α7-nAChR complexes that seed plaque formation (39).
Similar findings have been reported for the glutamatergic system. The NMDAR is highly Ca2+-permeable (single channel conductance of ∼60 pS, with >10% of the current carried by Ca2+) (40) and is therefore a highly studied target of Aβ-Ca2+ interactions. Aβ peptides affect neuronal function in brain regions where NMDARs are the principal excitotoxic mediators and underlie cell loss during the disease progression (41). Moreover, Aβ oligomers trigger increases in NMDAR-mediated Ca2+ influx, which disrupts neuronal transmission. In critical and vulnerable brain regions such as the hippocampus, impaired neurotransmission could further impact learning and memory mechanisms (17, 42).
Although several studies have examined the effects of amyloid on NMDAR function and Ca2+ influx, the results are not consistent. These differences may partially reflect the distinct effects of different Aβ species on cellular activity, as well as experimental differences in acute versus chronic exposures. For example, short-term incubation of neuronal cultures with Aβ oligomers has been shown to increase Ca2+ influx through NMDA channels. In turn, this is linked to downstream pathogenic effects, such as dynamin 1 degradation, increased reactive oxygen species production, and aberrant calpain activation (43), all of which can impair synaptic integrity. Acute treatment studies applying Aβ1–40 and Aβ25–35 peptides have demonstrated similar patterns of enhanced NMDA currents (44). In contrast, sustained exposure of neurons to Aβ oligomeric peptides reduces NMDA cell-surface expression, Ca2+ influx, and glutamatergic currents (17, 45, 46). Spine density loss, reduced AMPA currents, and impaired synaptic plasticity are resulting consequences and likely involve alterations in calcineurin, a Ca2+-sensitive phosphatase, and cofilin, a cytoskeleton-regulating protein that is activated by calcineurin-mediated dephosphorylation (47).
Another major source of cytosolic Ca2+ entry in neurons is through voltage-gated plasmalemmal Ca2+ channels. Ca2+ entry through the high threshold, low conductance N- and T-type channels (8–13 pS) and high conductance L-type channels (25 pS) (48) is thought to be increased by amyloid peptides (Aβ1–40) (31, 49), resulting in increased postsynaptic Ca2+ responses. In contrast, the high threshold, predominantly presynaptic P/Q-type channels (15–17 pS) are suppressed by Aβ oligomers (50), which serves to reduce synaptic vesicle release, neurotransmission, and plasticity.
Disruption of Membrane Lipid Integrity
Aβ peptides interact with membrane lipids such as phosphoinositides (51), phosphatidylglycerol (52), phosphatidylcholine (53), and gangliosides (54). A direct interaction of Aβ with cell membranes was initially proposed by Cotman and co-workers (55), who showed that d- and l-stereoisomers of a truncated form of Aβ induced similar toxicity levels in cultured hippocampal neurons, suggesting that Aβ toxicity does not involve a specific ligand-receptor interaction. Fluorescence spectroscopy measurements indicate that Aβ interaction with the synaptic plasma membrane causes substantial changes in the membrane fluidity both in the bulk lipid milieu and in proximity to integral membrane proteins. This may account for the effects of Aβ peptides in increasing membrane permeability to Ca2+, Na+, and K+ ions as well as larger molecules such as dyes (56, 57). However, different groups have shown varying results, reporting increases (58), decreases (59), or no effect (60) of Aβ peptides on membrane fluidity.
More recently, using uniform preparations of Aβ peptides (in their monomeric, oligomeric, and fibrillar forms), Sokolov and co-workers (19, 61) reported increases in conductance of lipid bilayer and patch-clamped mammalian cell membranes exclusively by the oligomeric form of Aβ1–42. Because the Aβ-induced conductance showed no selectivity between anionic and cationic probes and was apparent only in membranes formed from soft highly compressible lipids, the authors suggested that Aβ oligomers thin the membrane, thereby lowering the dielectric barrier and increasing its conductance. However, this mechanism has been challenged. Capone et al. (62) proposed that the membrane thinning was due to the residual solvent (hexafluoroisopropanol) used during Aβ oligomer preparation and was independent of the peptide itself.
Aβ Pore Formation
A different mechanism of action posits that Aβ peptides incorporate into the cell membrane and reorganize to form nonselective high conductance cation pores (63–65). Electrophysiological recordings using artificial lipid membranes exposed to Aβ demonstrated cation channels with the permeability sequence PCs > PLi > PCa = PK > PNa (66), which were blocked by Zn2+. These Aβ channels exhibit several different conductances, with spontaneous transition between levels ranging from 400 pS up to 5 nS (63). Channel formation has been proposed as a molecular mechanism for Aβ toxicity because ionic leakages of Na+, K+, and Ca2+ through such high conductance channels could rapidly disrupt cellular homeostasis (63, 67). The pore-forming mechanism for amyloid proteins has been further supported by studies employing atomic force microscopy (64), electron microscopy (68, 69), and theoretical modeling (70, 71). Moreover, high resolution transmission electron microscopy has revealed the presence of Aβ pores distributed in situ in the cell membrane of post-mortem brains of AD patients but not in healthy patients (72).
In a search for a specific blocker, Arispe (73) further strengthened the Aβ channel hypothesis by designing short peptides complementary to the putative mouth of the Aβ channel that potently and selectively blocked Aβ channels and inhibited Aβ cytotoxicity. More recently, Arispe and co-workers (74) also showed that two small enantiomeric molecules, MRS2481 and MRS2485, were both blockers of Aβ channels in the micromolar range and exhibited protective behavior against Aβ neurotoxicity in neurons.
Intracellular Ca2+ Sources and Aβ
In addition to extracellular Ca2+ sources, the ER constitutes a large reservoir of sequestered Ca2+ that is liberated via IP3Rs (whose activation requires binding of the second messenger IP3) and ryanodine receptors (RyRs). Both of these receptor/channel types are activated by Ca2+ itself in a regenerative process termed Ca2+-induced Ca2+ release. Numerous studies have linked up-regulation of ER Ca2+ release with presenilin mutations in early stages of AD progression, prior to the onset of Aβ plaques, neurofibrillary tangles, or cognitive impairment (1, 75), yet evidence exists that Aβ also influences intracellular Ca2+ signaling at later disease stages subsequent to histopathology onset (6). For example, exposing RyRs to Aβ1–42 peptides in lipid bilayers increases the channel open probability and alters gating kinetics, resulting in increased Ca2+ flux (76). Likewise, Aβ exposure enhances the IP3R-evoked Ca2+ response in neurons (77). More subtle interactions of Aβ with Ca2+-regulating G-protein-coupled membrane proteins have also been uncovered. Preincubation with the Aβ1–40 peptide enhances both the expression of Gq-coupled metabotropic glutamate receptor 5, which generates the Ca2+-mobilizing messenger IP3, and the intracellular Ca2+ response to the group I metabotropic glutamate receptor agonist (S)-3,5-dihydroxyphenylglycine (78). Aβ also interferes with the interplay between the APP haloprotein and Go, which leads to G-protein-coupled Ca2+ activation and eventually cell death (79).
New on the neuronal Ca2+ channel list is CALHM1, which is highly permeable to Ca2+ and localized to the ER and plasma membranes. Notably, a CALHM1 polymorphism has been associated with AD and leads to increased Aβ formation by interfering with Ca2+ permeability (80). In addition to being relevant from a Ca2+ signaling/amyloid perspective, the discovery of this polymorphism association with AD adds another possible marker to the short list of genetic risk factors (including ApoE4 allele expression) linked to sporadic AD, thereby permitting early intervention for patients with an otherwise idiopathic neurodegenerative disease.
Functional Evidence for Aβ and Ca2+ Interactions in Brain
Attempts to establish causative links between Aβ histopathology and AD memory deficits have been tenuous, with little direct correlation between plaque load and cognitive decline (81, 82). However, recent evidence demonstrates functional associations between dense core plaques and Ca2+ signaling alterations in AD mouse models. A series of in vivo imaging studies show that Aβ deposits result in intracellular Ca2+ dysregulation in neurons and glia (83, 84) and a structural breakdown of dendritic processes in later stages of AD pathology (85). Utilizing fluorescent live cell imaging techniques in plaque-bearing APP transgenic mice, increased resting Ca2+ levels have been observed in neurites in close proximity (∼20 μm) to dense core plaques (83), suggesting that plaques exert a direct pathogenic effect on steady-state Ca2+ levels in dendrites and spines, regions critical for electrochemical signal transmission. In concert, the compartmentalization of Ca2+ signals between spine heads and the neighboring dendritic branch is lost. These alterations would likely have implications for signal transduction and synaptic transmission, which are reliant on precise spatial and temporal Ca2+ signaling.
Possibly related to the above findings is the observation of increased spontaneous Ca2+ transients in the soma of cells close to plaques, perhaps resulting from reduced inhibitory input through reduced GABAergic tone (86). Alterations in Ca2+ transients may exert global alterations in intracellular function and affect long-range coordination among cells mediated by intercellular Ca2+ waves. This phenomenon is not limited to neurons, as increased Ca2+ activity and synchronized Ca2+ waves are observed across networks of astrocytes (84). Interestingly, astrocytic Ca2+ signals differ from those in neurons in that they are independent of proximity to plaques. Although these in vivo studies detailed above provide some of the most direct evidence for pathogenic Aβ and Ca2+ interactions in intact brains, they are limited in their interpretation because only dense core plaques were visualized, and the role and localization of other Aβ species, notably oligomeric forms, could not be identified in these preparations.
Structural abnormalities in neurites have also been attributed to the activity of calcineurin, a Ca2+-sensitive phosphatase whose many functions include regulation of cofilin, which maintains neuronal cytoarchitecture. These findings may relate to the breakdown of synapses attributed to fibrillar and oligomeric Aβ (87) in that aberrant Ca2+ levels can disrupt glutamate receptor trafficking and Ca2+/calmodulin-dependent protein kinase II and calcineurin activity and alter spine head geometry (88, 89).
Aβ oligomers have also been found to disrupt synaptic function at the circuit level. Associations between naturally produced Aβ oligomers and AD pathology were made by Selkoe and co-workers (90), who identified a naturally secreted species of Aβ aggregates capable of disrupting neuronal plasticity. Soluble Aβ oligomers extracted from AD patients inhibit long-term potentiation, enhance long-term depression, and trigger dendritic spine reduction in rodent hippocampus (17, 91). These pathological effects were shown to be specifically attributable to Aβ1–42 dimers.
A Vicious Spiral in AD: Aβ and Ca2+ Go Round and Round
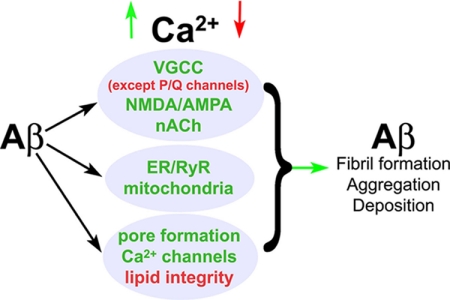
Increased Ca2+ levels are functionally linked to most of the major features and risk factors of AD: presenilin and APP mutations, ApoE4 expression, CALHM1 mutations, Aβ plaques, Tau hyperphosphorylation, apoptosis, and synaptic dysfunction (1). In many of these interactions, a pathogenic feed-forward cascade evolves, wherein Ca2+ facilitates a pathogenic state, which in turn increases Ca2+ levels. For example, Ca2+ can facilitate the formation of pathogenic Aβ fibril formation (92), and in parallel, Aβ can form Ca2+-permeable channels, interfere with existing Ca2+ channels, and increase RyR function (43, 93, 94). Apoptosis can also be triggered by Ca2+-sensitive cell death pathways via caspase and calpain activation and vice versa. Ca2+ dysregulation may then reflect a lifetime of episodic and slowly accumulating insults that favor the aggregation and deposition of pathogenic Aβ peptides, trigger apoptosis via ER and mitochondrial stress responses, and impair synaptic morphology and membrane function. The culmination of these downstream Ca2+-mediated events may ultimately lead to the devastating loss of memory and deteriorating cognitive functions (Fig. 2).
FIGURE 2.
Feed-forward Aβ peptide and Ca2+ signaling interactions. Aβ peptides interact with a variety of Ca2+ channels and sources (center, blue), often serving to up-regulate or aberrantly generate Ca2+ flux within the cell. This increase in Ca2+ from direct calcium channel alteration or through compromised lipid barriers can then serve to accelerate Aβ generation, thereby sustaining a pathogenic cycle. VGCC, voltage-gated Ca2+ channel.
Future Directions
In light of the ubiquity of Ca2+ signaling in neurons and glia and its complex reciprocal interactions with Aβ in the pathogenesis of AD, research is likely to progress in parallel along multiple paths. Below, we highlight just a few areas that we believe most promising.
Consistency of Aβ Preparations
Studies of Aβ toxicity are confounded by inconsistencies in oligomeric state of the peptide, a factor that likely accounts for widely varying and sometimes contradictory reports in the literature. There is therefore a clear need to resolve the effects of the various Aβ species by systematically examining the effect of uniformly prepared and characterized Aβ aggregates.
Mechanisms of Aβ Ca2+ Toxicity
Disruption of membrane integrity and the resulting unregulated Ca2+ flux are now well established as major factors underlying Aβ oligomer toxicity. There is strong evidence that Aβ itself forms cation pores in the membrane, but actions on the lipid bilayer and on endogenous membrane channels may also contribute. Elucidation of the mechanism(s) by which Aβ acts on surface and intracellular membranes is crucial, as this represents a selective and most attractive therapeutic target. Experiments have thus far been limited largely to in vitro systems, but developments in techniques for optical imaging of single channel Ca2+ flux (95) offer considerable potential for extending these studies to intact cell systems (96).
Ca2+ Signaling as a Therapeutic Target
Because Ca2+ signaling impinges upon nearly every characteristic feature, genetic cause, and major risk factor in AD, it is an obvious target for potential therapeutic strategies. Compounds that normalize dysregulated Ca2+ levels or specifically block Ca2+-regulated pathogenic signaling cascades could, in theory, prevent or reduce many of the histopathological and cognitive components of AD. Indeed, the few effective treatments currently available for early-to-mid-stage AD directly or indirectly include some aspect of Ca2+ modification. Memantine is a low affinity NMDAR Ca2+ channel antagonist that prevents excessive Ca2+ influx while maintaining glutamatergic transmission sufficiently to support synaptic transmission and plasticity (97). Another example is dimebon, which was suggested in clinical trials to sustain cognitive function in AD patients. Although the mechanism is unclear, the neuroprotective effects of dimebon may lie in its ability to inhibit L-type Ca2+ channels and NMDAR and protect against mitochondrial stress (98). Another target, though not yet in clinical trials, is the RyR, an intracellular Ca2+ release channel that is up-regulated in an initially neuroprotective manner in response to Aβ1–42 exposure (94) and that shows increased expression and Ca2+ flux in certain familial forms of AD (99, 100). Given the ubiquity of Ca2+ signaling, a caveat with these approaches is the potential to disrupt normal neuronal function. The design of novel compounds to block Ca2+-permeable pores formed by Aβ thus holds particular promise (73, 74).
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants GM48071 (to I. P.), AG30205 (to G. E. S.), and P50-AG16573 (A. D.). This is the twelfth article in the “Thematic Minireview Series on the Molecular Basis of Alzheimer Disease.” This minireview will be reprinted in the 2010 Minireview Compendium, which will be available in January, 2011.
- AD
- Alzheimer disease
- Aβ
- β-amyloid
- ER
- endoplasmic reticulum
- APP
- amyloid precursor protein
- AMPA
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- NMDA
- N-methyl-d-aspartic acid
- IP3R
- inositol trisphosphate receptor
- nAChR
- nicotinic acetylcholine receptor
- NMDAR
- NMDA receptor
- pS
- picosiemens
- RyR
- ryanodine receptor.
REFERENCES
- 1.Stutzmann G. E. (2007) Neuroscientist 13, 546–559 [DOI] [PubMed] [Google Scholar]
- 2.Bezprozvanny I., Mattson M. P. (2008) Trends Neurosci. 31, 454–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green K. N., LaFerla F. M. (2008) Neuron 59, 190–194 [DOI] [PubMed] [Google Scholar]
- 4.Berridge M. J. (1998) Neuron 21, 13–26 [DOI] [PubMed] [Google Scholar]
- 5.Khachaturian Z. S. (1987) Neurobiol. Aging 8, 345–346 [DOI] [PubMed] [Google Scholar]
- 6.Berridge M. J. (2009) Pfluegers Arch. 459, 441–449 [DOI] [PubMed] [Google Scholar]
- 7.Frandsen A., Schousboe A. (1991) J. Neurochem. 56, 1075–1078 [DOI] [PubMed] [Google Scholar]
- 8.Pahl H. L., Baeuerle P. A. (1996) FEBS Lett. 392, 129–136 [DOI] [PubMed] [Google Scholar]
- 9.Mattson M. P., Furukawa K. (1997) Apoptosis 2, 257–264 [DOI] [PubMed] [Google Scholar]
- 10.Thinakaran G., Koo E. H. (2008) J. Biol. Chem. 283, 29615–29619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanzi R. E., Bertram L. (2001) Neuron 32, 181–184 [DOI] [PubMed] [Google Scholar]
- 12.Jarrett J. T., Lansbury P. T., Jr. (1993) Cell 73, 1055–1058 [DOI] [PubMed] [Google Scholar]
- 13.Kim W., Hecht M. H. (2005) J. Biol. Chem. 280, 35069–35076 [DOI] [PubMed] [Google Scholar]
- 14.Roychaudhuri R., Yang M., Hoshi M. M., Teplow D. B. (2009) J. Biol. Chem. 284, 4749–4753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross C. A., Poirier M. A. (2005) Nat. Rev. Mol. Cell Biol. 6, 891–898 [DOI] [PubMed] [Google Scholar]
- 16.Kayed R., Head E., Thompson J. L., McIntire T. M., Milton S. C., Cotman C. W., Glabe C. G. (2003) Science 300, 486–489 [DOI] [PubMed] [Google Scholar]
- 17.Shankar G. M., Li S., Mehta T. H., Garcia-Munoz A., Shepardson N. E., Smith I., Brett F. M., Farrell M. A., Rowan M. J., Lemere C. A., Regan C. M., Walsh D. M., Sabatini B. L., Selkoe D. J. (2008) Nat. Med. 14, 837–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demuro A., Mina E., Kayed R., Milton S. C., Parker I., Glabe C. G. (2005) J. Biol. Chem. 280, 17294–17300 [DOI] [PubMed] [Google Scholar]
- 19.Kayed R., Sokolov Y., Edmonds B., McIntire T. M., Milton S. C., Hall J. E., Glabe C. G. (2004) J. Biol. Chem. 279, 46363–46366 [DOI] [PubMed] [Google Scholar]
- 20.Giuffrida M. L., Caraci F., Pignataro B., Cataldo S., De Bona P., Bruno V., Molinaro G., Pappalardo G., Messina A., Palmigiano A., Garozzo D., Nicoletti F., Rizzarelli E., Copani A. (2009) J. Neurosci. 29, 10582–10587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia W., Zhang J., Kholodenko D., Citron M., Podlisny M. B., Teplow D. B., Haass C., Seubert P., Koo E. H., Selkoe D. J. (1997) J. Biol. Chem. 272, 7977–7982 [DOI] [PubMed] [Google Scholar]
- 22.Kuo Y. M., Emmerling M. R., Vigo-Pelfrey C., Kasunic T. C., Kirkpatrick J. B., Murdoch G. H., Ball M. J., Roher A. E. (1996) J. Biol. Chem. 271, 4077–4081 [DOI] [PubMed] [Google Scholar]
- 23.Gong Y., Chang L., Viola K. L., Lacor P. N., Lambert M. P., Finch C. E., Krafft G. A., Klein W. L. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 10417–10422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haes A. J., Chang L., Klein W. L., Van Duyne R. P. (2005) J. Am. Chem. Soc. 127, 2264–2271 [DOI] [PubMed] [Google Scholar]
- 25.Meyer-Luehmann M., Coomaraswamy J., Bolmont T., Kaeser S., Schaefer C., Kilger E., Neuenschwander A., Abramowski D., Frey P., Jaton A. L., Vigouret J. M., Paganetti P., Walsh D. M., Mathews P. M., Ghiso J., Staufenbiel M., Walker L. C., Jucker M. (2006) Science 313, 1781–1784 [DOI] [PubMed] [Google Scholar]
- 26.Deshpande A., Mina E., Glabe C., Busciglio J. (2006) J. Neurosci. 26, 6011–6018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glabe C. G. (2008) J. Biol. Chem. 283, 29639–29643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldman W. P., Price J. L., Storandt M., Grant E. A., McKeel D. W., Jr., Rubin E. H., Morris J. C. (2001) Neurology 56, 361–367 [DOI] [PubMed] [Google Scholar]
- 29.Lansbury P. T., Jr. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 3342–3344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teplow D. B. (2006) Methods Enzymol. 413, 20–33 [DOI] [PubMed] [Google Scholar]
- 31.Rovira C., Arbez N., Mariani J. (2002) Biochem. Biophys. Res. Commun. 296, 1317–1321 [DOI] [PubMed] [Google Scholar]
- 32.Verdurand M., Berod A., Le Bars D., Zimmer L. (2010) Neurobiol. Aging, in press [DOI] [PubMed] [Google Scholar]
- 33.Buckingham S. D., Jones A. K., Brown L. A., Sattelle D. B. (2009) Pharmacol. Rev. 61, 39–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geerts H., Grossberg G. T. (2006) J. Clin. Pharmacol. 46, 8S–16S [DOI] [PubMed] [Google Scholar]
- 35.Terry A. V., Jr., Buccafusco J. J. (2003) J. Pharmacol. Exp. Ther. 306, 821–827 [DOI] [PubMed] [Google Scholar]
- 36.Kadir A., Almkvist O., Wall A., Långström B., Nordberg A. (2006) Psychopharmacology 188, 509–520 [DOI] [PubMed] [Google Scholar]
- 37.Bartus R. T., Dean R. L., 3rd, Beer B., Lippa A. S. (1982) Science 217, 408–414 [DOI] [PubMed] [Google Scholar]
- 38.Wevers A., Monteggia L., Nowacki S., Bloch W., Schütz U., Lindstrom J., Pereira E. F., Eisenberg H., Giacobini E., de Vos R. A., Steur E. N., Maelicke A., Albuquerque E. X., Schröder H. (1999) Eur. J. Neurosci. 11, 2551–2565 [DOI] [PubMed] [Google Scholar]
- 39.Wang H. Y., Lee D. H., D'Andrea M. R., Peterson P. A., Shank R. P., Reitz A. B. (2000) J. Biol. Chem. 275, 5626–5632 [DOI] [PubMed] [Google Scholar]
- 40.Garashchuk O. M. (1996) J. Physiol. 491, 757–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parameshwaran K., Dhanasekaran M., Suppiramaniam V. (2008) Exp. Neurol. 210, 7–13 [DOI] [PubMed] [Google Scholar]
- 42.Li S., Hong S., Shepardson N. E., Walsh D. M., Shankar G. M., Selkoe D. (2009) Neuron 62, 788–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelly B. L., Ferreira A. (2006) J. Biol. Chem. 281, 28079–28089 [DOI] [PubMed] [Google Scholar]
- 44.Carette B., Poulain P., Delacourte A. (1993) Neurosci. Lett. 151, 111–114 [DOI] [PubMed] [Google Scholar]
- 45.Snyder E. M., Nong Y., Almeida C. G., Paul S., Moran T., Choi E. Y., Nairn A. C., Salter M. W., Lombroso P. J., Gouras G. K., Greengard P. (2005) Nat. Neurosci. 8, 1051–1058 [DOI] [PubMed] [Google Scholar]
- 46.Dewachter I., Filipkowski R. K., Priller C., Ris L., Neyton J., Croes S., Terwel D., Gysemans M., Devijver H., Borghgraef P., Godaux E., Kaczmarek L., Herms J., Van Leuven F. (2009) Neurobiol. Aging 30, 241–256 [DOI] [PubMed] [Google Scholar]
- 47.Wang Y., Shibasaki F., Mizuno K. (2005) J. Biol. Chem. 280, 12683–12689 [DOI] [PubMed] [Google Scholar]
- 48.Fox A. P., Nowycky M. C., Tsien R. W. (1987) J. Physiol. 394, 173–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacManus A., Ramsden M., Murray M., Henderson Z., Pearson H. A., Campbell V. A. (2000) J. Biol. Chem. 275, 4713–4718 [DOI] [PubMed] [Google Scholar]
- 50.Nimmrich V., Grimm C., Draguhn A., Barghorn S., Lehmann A., Schoemaker H., Hillen H., Gross G., Ebert U., Bruehl C. (2008) J. Neurosci. 28, 788–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Decout A., Labeur C., Goethals M., Brasseur R., Vandekerckhove J., Rosseneu M. (1998) Biochim. Biophys. Acta 1372, 102–116 [DOI] [PubMed] [Google Scholar]
- 52.Terzi E., Hölzemann G., Seelig J. (1995) J. Mol. Biol. 252, 633–642 [DOI] [PubMed] [Google Scholar]
- 53.Avdulov N. A., Chochina S. V., Igbavboa U., Warden C. S., Vassiliev A. V., Wood W. G. (1997) J. Neurochem. 69, 1746–1752 [DOI] [PubMed] [Google Scholar]
- 54.McLaurin J., Franklin T., Chakrabartty A., Fraser P. E. (1998) J. Mol. Biol. 278, 183–194 [DOI] [PubMed] [Google Scholar]
- 55.Cribbs D. H., Pike C. J., Weinstein S. L., Velazquez P., Cotman C. W. (1997) J. Biol. Chem. 272, 7431–7436 [DOI] [PubMed] [Google Scholar]
- 56.Müller W. E., Koch S., Eckert A., Hartmann H., Scheuer K. (1995) Brain Res. 674, 133–136 [DOI] [PubMed] [Google Scholar]
- 57.McLaurin J., Chakrabartty A. (1996) J. Biol. Chem. 271, 26482–26489 [DOI] [PubMed] [Google Scholar]
- 58.Avdulov N. A., Chochina S. V., Igbavboa U., O'Hare E. O., Schroeder F., Cleary J. P., Wood W. G. (1997) J. Neurochem. 68, 2086–2091 [DOI] [PubMed] [Google Scholar]
- 59.Kremer J. J., Pallitto M. M., Sklansky D. J., Murphy R. M. (2000) Biochemistry 39, 10309–10318 [DOI] [PubMed] [Google Scholar]
- 60.Mingeot-Leclercq M. P., Lins L., Bensliman M., Van Bambeke F., Van Der Smissen P., Peuvot J., Schanck A., Brasseur R. (2002) Chem. Phys. Lipids 120, 57–74 [DOI] [PubMed] [Google Scholar]
- 61.Sokolov Y., Kozak J. A., Kayed R., Chanturiya A., Glabe C., Hall J. E. (2006) J. Gen. Physiol. 128, 637–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Capone R., Quiroz F. G., Prangkio P., Saluja I., Sauer A. M., Bautista M. R., Turner R. S., Yang J., Mayer M. (2009) Neurotox. Res. 16, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arispe N., Pollard H. B., Rojas E. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 10573–10577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin H., Bhatia R., Lal R. (2001) FASEB J. 15, 2433–2444 [DOI] [PubMed] [Google Scholar]
- 65.Quist A., Doudevski I., Lin H., Azimova R., Ng D., Frangione B., Kagan B., Ghiso J., Lal R. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 10427–10432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arispe N., Rojas E., Pollard H. B. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 567–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pollard H. B., Rojas E., Arispe N. (1993) Ann. N.Y. Acad. Sci. 695, 165–168 [DOI] [PubMed] [Google Scholar]
- 68.Lashuel H. A., Hartley D., Petre B. M., Walz T., Lansbury P. T., Jr. (2002) Nature 418, 291. [DOI] [PubMed] [Google Scholar]
- 69.Lashuel H. A., Hartley D. M., Petre B. M., Wall J. S., Simon M. N., Walz T., Lansbury P. T., Jr. (2003) J. Mol. Biol. 332, 795–808 [DOI] [PubMed] [Google Scholar]
- 70.Durell S. R., Guy H. R., Arispe N., Rojas E., Pollard H. B. (1994) Biophys. J. 67, 2137–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jang H., Ma B., Lal R., Nussinov R. (2008) Biophys. J. 95, 4631–4642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Inoue S. (2008) Amyloid 15, 223–233 [DOI] [PubMed] [Google Scholar]
- 73.Arispe N. (2004) J. Membr. Biol. 197, 33–48 [DOI] [PubMed] [Google Scholar]
- 74.Diaz J. C., Simakova O., Jacobson K. A., Arispe N., Pollard H. B. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 3348–3353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.LaFerla F. M. (2002) Nat. Rev. Neurosci. 3, 862–872 [DOI] [PubMed] [Google Scholar]
- 76.Shtifman A., Ward C. W., Laver D. R., Bannister M. L., Lopez J. R., Kitazawa M., LaFerla F. M., Ikemoto N., Querfurth H. W. (2008) Neurobiol. Aging 10.1016/j.neurobiolaging.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schapansky J., Olson K., Van Der Ploeg R., Glazner G. (2007) Exp. Neurol. 208, 169–176 [DOI] [PubMed] [Google Scholar]
- 78.Casley C. S., Lakics V., Lee H. G., Broad L. M., Day T. A., Cluett T., Smith M. A., O'Neill M. J., Kingston A. E. (2009) Brain Res. 1260, 65–67 [DOI] [PubMed] [Google Scholar]
- 79.Shaked G. M., Chauv S., Ubhi K., Hansen L. A., Masliah E. (2009) FEBS J. 276, 2736–2751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dreses-Werringloer U., Lambert J. C., Vingtdeux V., Zhao H., Vais H., Siebert A., Jain A., Koppel J., Rovelet-Lecrux A., Hannequin D., Pasquier F., Galimberti D., Scarpini E., Mann D., Lendon C., Campion D., Amouyel P., Davies P., Foskett J. K., Campagne F., Marambaud P. (2008) Cell 133, 1149–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Selkoe D. J. (2008) Behav. Brain Res. 192, 106–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blennow K., de Leon M. J., Zetterberg H. (2006) Lancet 368, 387–403 [DOI] [PubMed] [Google Scholar]
- 83.Kuchibhotla K. V., Goldman S. T., Lattarulo C. R., Wu H. Y., Hyman B. T., Bacskai B. J. (2008) Neuron 59, 214–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kuchibhotla K. V., Lattarulo C. R., Hyman B. T., Bacskai B. J. (2009) Science 323, 1211–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsai S. J., Hong C. J., Liu H. C., Liu T. Y., Hsu L. E., Lin C. H. (2004) Neuropsychobiology 49, 10–12 [DOI] [PubMed] [Google Scholar]
- 86.Busche M. A., Eichhoff G., Adelsberger H., Abramowski D., Wiederhold K. H., Haass C., Staufenbiel M., Konnerth A., Garaschuk O. (2008) Science 321, 1686–1689 [DOI] [PubMed] [Google Scholar]
- 87.Kim D., Tsai L. H. (2009) Cell 137, 997–1000 [DOI] [PubMed] [Google Scholar]
- 88.Korkotian E., Segal M. (2007) Cell Calcium 42, 41–57 [DOI] [PubMed] [Google Scholar]
- 89.Reese L. C., Zhang W., Dineley K. T., Kayed R., Taglialatela G. (2008) Aging Cell 7, 824–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Walsh D. M., Klyubin I., Fadeeva J. V., Cullen W. K., Anwyl R., Wolfe M. S., Rowan M. J., Selkoe D. J. (2002) Nature 416, 535–539 [DOI] [PubMed] [Google Scholar]
- 91.Yankner B. A., Lu T. (2009) J. Biol. Chem. 284, 4755–4759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Isaacs A. M., Senn D. B., Yuan M., Shine J. P., Yankner B. A. (2006) J. Biol. Chem. 281, 27916–27923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shemer I., Holmgren C., Min R., Fülöp L., Zilberter M., Sousa K. M., Farkas T., Härtig W., Penke B., Burnashev N., Tanila H., Zilberter Y., Harkany T. (2006) Eur. J. Neurosci. 23, 2035–2047 [DOI] [PubMed] [Google Scholar]
- 94.Supnet C., Grant J., Kong H., Westaway D., Mayne M. (2006) J. Biol. Chem. 281, 38440–38447 [DOI] [PubMed] [Google Scholar]
- 95.Demuro A., Parker I. (2005) J. Gen. Physiol. 126, 179–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Demuro A., Parker I. (2008) Cell Calcium 43, 367–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Molinuevo J. L., Lladó A., Rami L. (2005) Am. J. Alzheimer's Dis. Other Demen. 20, 77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Doody R. S., Gavrilova S. I., Sano M., Thomas R. G., Aisen P. S., Bachurin S. O., Seely L., Hung D. (2008) Lancet 372, 207–215 [DOI] [PubMed] [Google Scholar]
- 99.Stutzmann G. E., Smith I., Caccamo A., Oddo S., LaFerla F. M., Parker I. (2006) J. Neurosci. 26, 5180–5189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chakroborty S., Goussakov I., Miller M. B., Stutzmann G. E. (2009) J. Neurosci. 29, 9458–9470 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.