Abstract
Cyanovirin-N (CV-N) is a two-domain, cyanobacterial protein that inhibits human immunodeficiency virus (HIV) at nanomolar concentrations by binding to high mannose sugars on the HIV envelope glycoprotein gp120. The wild type protein can exist as a monomer or a domain-swapped dimer with the monomer and dimer containing two or four sugar binding sites, respectively, one on each domain. Here we demonstrate that monomeric, single binding site mutants are completely inactive and that a single site, whether located on domain A or B, is insufficient to impart the antiviral activity. Linking inactive, monomeric proteins in a head-to-head fashion by an intermolecular disulfide bond or by creating an exclusively domain-swapped dimer via a hinge residue deletion restored antiviral activity to levels similar to that of wild type CV-N. These findings demonstrate unequivocally that multisite binding by CV-N type lectins is necessary for viral inhibition.
Keywords: Antiviral Agents, Carbohydrate-binding Protein, Glycoprotein, HIV, Lectin
Introduction
It is well appreciated that complex combinations of variables influence the interaction of lectins with their cognate carbohydrates. These include the oligomerization state of the protein, the location of the carbohydrate binding sites on the lectin, the conformational details of the sugar binding epitopes, as well as the disposition of the sugar ligand on its display scaffold. Cyanovirin-N (CV-N)2 is an 11-kDa lectin from Nostoc ellipsosporum (1, 2) that exhibits potent antiviral activity toward the two most well known strains of HIV, HIV-1 and HIV-2, as well as their counterparts in monkeys, the simian immunodeficiency virus, and a number of other enveloped viruses, including influenza and Ebola (3, 4). CV-N exerts its antiviral activity by binding to high mannose sugars on the viral envelope glycoproteins and prevents virus entry into the cell (5, 6). Because of its broad activity, CV-N holds great promise as a potential prophylactic virucide. In solution, CV-N exists as a monomer with a domain-swapped dimeric form observed as a trapped kinetic intermediate (7), whereas in the crystal, the protein is always found as a domain-swapped dimer. The structure of CV-N exhibits pseudo-symmetry with two distinct domains, A and B (see Fig. 1A). One sugar binding site was identified on each domain by NMR titration experiments (5, 8, 9): a shallow cleft on domain A and a somewhat deeper pocket in domain B, with both sites separated by ∼40 Å. The anti-HIV activity of CV-N is mediated via interactions between the protein and α(1–2)-linked di- or trimannose units on the terminal arms of the branched Man-8 and Man-9 structures on gp120 (5, 6, 8, 10).
FIGURE 1.
Sequences and schematic representation of CV-N variants. Domains and respective sequences are color-coded, with domain A in green and domain B in pink. Mutated residues are colored in blue. Disulfide bonds are indicated by brackets. A, amino acid sequence of (P51G)CV-N. In this protein the two binding sites are separated by ∼40 Å. B, amino acid sequence of the disulfide-linked monomer (CVNΔA)ssm. The intramolecular disulfide is shown as a yellow bracket. This protein contains only one binding site in domain B. C, amino acid sequence of the disulfide-linked dimer (CVNΔA)ssd. This head-to-head linked dimer contains binding sites in domains B and B′ only, and they are separated by ∼57 Å. D, amino acid sequence of CVNmutDB. This protein contains only one binding site in domain A. E, amino acid sequence of the domain-swapped dimer (CVNΔB)dsd. This protein contains binding sites on domains A and A′ only, and they are separated by ∼48 Å.
The high mannose-CV-N interaction represents a typical example of a ligand-protein interaction in which multiple recognition epitopes on the ligand can contact more than one binding site on the protein. In such cases, the cost for the loss in translational entropy upon binding is paid partially through the first protein-ligand contact, and subsequent binding interactions can proceed without additional entropic penalties. This type of interaction mode is also called “chelation binding” (11). Unlike monovalent sugars that can only access their primary binding pocket, multivalent sugars gain binding energy from contacting a second site, resulting in binding with a high “functional affinity.”
Several studies have been conducted to dissect the inherent complexity of the carbohydrate interactions of CV-N and elucidate its pertinent features. Previous mutagenesis studies have served to establish the importance of two carbohydrate binding sites on the protein (12, 13) because removal of either binding site renders these mutants devoid of anti-HIV activity (14, 15). We previously characterized the properties of CVNmutDB, in which the binding site on domain B has been removed. High resolution structures were solved by x-ray crystallography and NMR, and thermodynamic parameters of sugar binding were determined (16). This structure was a monomeric form of the protein, and now we have solved the structure of the equivalent domain-swapped dimeric protein, CVNmutDBΔGln-50–Pro-51. Here we show that: (i) a single sugar-protein contact is insufficient for functional high affinity interaction; (ii) the involvement of at least two binding sites is critical for anti-HIV activity of CV-N; (iii) a non-native, disulfide-linked dimer of two single site, inactive monomers exhibits activity approaching that of wild type CV-N; and (iv) enforcing domain swapping of an inactive, single site mutant also restores anti-HIV activity.
EXPERIMENTAL PROCEDURES
Protein Cloning, Expression, and Purification
Proteins were expressed from a synthetic gene using pET26b(+) (Novagen; Madison, WI) and Escherichia coli BL21(DE3) as expression vector and host strain, respectively. The amino acid sequences of all proteins are displayed in Fig. 1. Genes for (CVNΔA)ssm, (CVNΔA)ssd, and (CVNΔB)dsd were created using the QuikChange XL II site-directed mutagenesis (Stratagene) kit. For each mutant, two forward/reverse primers were employed: (CVNΔA)ssm, 5′-CGATGGCCCTTTGCAAATTCTGCGCTGCTTGCT-3′/5′-AGCAAGCAGCGCAGAATTTGCAAAGGGCCATCG-3′; CVNΔA]ssd, 5′-GATGGCCCTTTGCAAATTCTCCGCTGCTTGCTACAACTCCGCTATCCAGG-3′/5′-CCTGGATAGCGGAGTTGTAGCAAGCAGCGGAGAATTTGCAAAGGGCCATC-3′; (CVNΔB)dsd, 5′-CGGTTCCCTGAAATGGCCGTCCAACTTCATCG-3′/5′-CGATGAAGTTGGACGGCCATTTCAGGGAACCG-3′.
For protein expression, E. coli BL21(DE3) cells (Stratagene) were transformed with the respective vectors. Cells were grown at 37 °C and induced with 1 mm isopropyl-1-thio-β-d-galactopyranoside for 3 h. Isotopic labeling was carried out by growing the cultures in modified M9 minimal media containing [15N]H4Cl and/or [13C]glucose (Cambridge Isotope Laboratories, Inc.; Andover, MA) as sole nitrogen and/or carbon sources, respectively. The expressed protein was isolated from the periplasmic fraction of the E. coli cells by twice heating (62 °C) and cooling (0 °C) the cell suspension in phosphate-buffered saline buffer (pH 7.4). After removal of insoluble material by centrifugation, the supernatant containing soluble protein was fractionated by gel filtration on Superdex 75 (HiLoad 2.6 × 60 cm, Amersham Biosciences), equilibrated in 20 mm sodium phosphate buffer (pH 6.0). The protein sample was isolated as monomeric ((CVNΔA)ssm), as a mixture of monomeric and dimeric ((CVNΔA)ssd), or as purely dimeric ((CVNΔB)dsd) folded protein. A pure dimer of (CVNΔA)ssd was obtained by concentrating the protein sample to ∼2 mm under oxidizing conditions. The quaternary state of all proteins was verified by native polyacrylamide and SDS polyacrylamide on 20% gels. The purity and identity of all proteins were assessed and verified by mass spectrometry.
Anti-HIV Assay
HIV-1 infectivity was assayed as described previously (17). For CV-N antiviral assays, recombinant proteins were serially diluted in sterile phosphate-buffered saline, and 5 μl were added to 500 μl of prediluted infectious HIV-1 (produced by transfection of 293T cells with the R9 molecular clone and incubated for 30 min at room temperature). Aliquots of the mixture (125 μl, triplicates) were added to cultures of HeLa-P4 cells (20,000 cells seeded per well the day before in a 48-well format), and after 2 days, cells were fixed and stained with X-gal overnight and counted. Results are expressed as the average number of X-gal-positive cells per well.
NMR Spectroscopy
NMR spectra were recorded at 25 °C on a Bruker AVANCE 600 spectrometer, equipped with 5-mm, triple resonance, three axis gradient probes or z axis gradient cryoprobes. Spectra were processed with NMRPipe (18) and analyzed with NMRview (19). Samples contained 1.5 mm protein in 20 mm sodium phosphate buffer (pH 6.0). For backbone assignments, a series of heteronuclear, multidimensional experiments, routinely used in our laboratory, was employed (20, 21). Complete 1H, 15N, and 13C backbone resonance assignments were obtained using the following heteronuclear two-dimensional and three-dimensional experiments: 1H-15N, HSQC, HNCACB, and CBCA(CO)NH.
Crystallization and X-ray Data Collection
Purified (CVNΔB)dsd protein was crystallized by sitting drop vapor diffusion from a 5.0 mm protein solution in 20 mm sodium phosphate buffer, 0.01 NaN3 (pH 6.0). The best crystals were obtained at room temperature with 20% polyethylene glycol 8000 as the precipitant in 50 mm potassium phosphate. Crystal growth took about 1 day with crystals typically having dimensions of 0.20 × 0.20 × 0.30 mm. X-ray diffraction data were collected from a single flash-cooled crystal (−180 °C) using a Rigaku FR-E generator with a Saturn 944 CCD detector and high flux VariMax optics. To 1.34 Å resolution, 180,912 total observations were reduced to yield 23,076 unique reflections (96.4% complete) with an internal R factor (based on intensities) of 0.09. The data were processed and scaled with the d*TREK package (22).
Crystal Structure Determination and Refinement
The crystal structure of (CVNΔB)dsd was solved by molecular replacement using the monomeric NMR structure of wild type CV-N (Protein Data Bank (PDB) accession code 2EZM) (23) as search model with the program Phaser 1.3.1 (24). The initial model included two independent segments of the chain comprising residues Leu-1–Lys-48 and Glu-56–Glu-101. The hinge-loop region (Trp-49–Ile-55) was omitted from the model. Following rigid body and simulated annealing refinement, the model was used to generate an electron density composite omit map using the program CNS (25). The atomic model for the missing region was built using Coot (26) and further refined by simulated annealing. The final refinement procedure included periodic examinations of composite omit and difference maps, as well as the introduction of water molecules. Analysis of the final structure was performed using WHAT_CHECK (27) and PROCHECK (28). About 95% of all residues are found in the favored region in the Ramachandran plot (29) with no residues in the disallowed regions.
The atomic coordinates and structure factors have been deposited in the Research Collaboratory for Structural Bioinformatics (RCSB) Protein Data Bank under accession code 3LHC. All structural figures were generated with PyMOL.
RESULTS
We designed and produced CV-N variants with single carbohydrate binding sites. The quaternary state of these variants can be controlled such that they are either exclusively monomeric or exclusively dimeric. These molecules provided the tools to investigate questions regarding monovalent versus multivalent sugar binding because the number and spatial disposition of the binding sites could be varied. A schematic illustration of the design as well the amino acid sequences of all variants are summarized in Fig. 1.
Crystal Structure of the Domain-swapped Dimer (CVNΔB)dsd
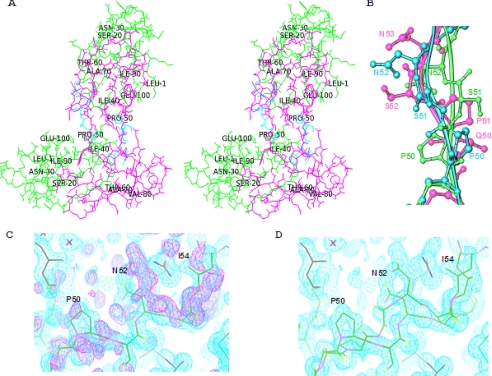
The x-ray crystal structure of the domain-swapped dimer CVNmutDBΔGln-50–Pro-51, designated as (CVNΔB)dsd (see Fig. 1E for the amino acid sequence) was solved at 1.34 Å resolution by molecular replacement using wild type CV-N (PDB accession code 2EZM) (23) as the search model. The current structure of (CVNΔB)dsd is similar to previously determined structures of domain-swapped CV-N variants (7, 30, 31). The space group is P3221 with unit cell dimensions a = 47.14, b = 47.14, c = 78.39 Å, with one molecule in the asymmetric unit. All pertinent crystallographic statistics are provided in Table 1. A stereo view of the overall structure is provided in Fig. 2A. The fold and global conformation of (CVNΔB)dsd are remarkably similar to that of the domain-swapped wild type CV-N x-ray structure (PDB accession code 3EZM) (30), although we did not crystallize the protein at low pH. Thus, our current structure represents another case where changes in hinge residues result in a very similar orientation of the quasi-monomers, with the hinge region adjusting its conformation. This type of rearrangement was previously observed in the P51S/S52P mutant structure (PDB accession code 1LOM) (31). For the Cα backbone atoms, pairwise root mean square deviations of 0.96 and 0.93 Å are observed between the current structure and the 3EZM (30) and 1LOM (31) structures, respectively. In the present structure, the two pseudo-monomers are oriented with an angle of −102.6° between the long axes of the two domains (AB′, A′B), estimated in terms of torsion angle using Sγ atoms of cysteines in the two disulfide bonds (i.e. Cys-8/Cys-57′/Cys-57/Cys-8′). This spatial arrangement is similar to the orientation previously observed between the two halves of the trigonal domain-swapped dimer structures, 3EZM (30) (−100.93°) and 1LOM (31) (−100.5°).
TABLE 1.
Data collection and refinement statistics for the x-ray structure
| (CVNΔB)dsd | |
|---|---|
| Data collection | Space group |
| Space group | P3221 |
| Cell dimensions | |
| a, b, c (Å) | 47.14, 47.14, 78.39 |
| α, β, γ (°) | 90, 90, 120 |
| Resolution (Å) | 36.21 (1.34) |
| Rmerge | 0.09 (0.34) |
| I/σI | 14 (2.1) |
| Completeness (%) | 99.3 (96.4) |
| Redundancy | 7.8 (5.6) |
| Refinement | |
| Resolution (Å) | 1.34 |
| No. of reflections | 21,667 |
| Rwork/Rfree | 19.9/22.1 |
| No. of atoms | 974 |
| Protein | 837 |
| Ions | 17 |
| Water | 120 |
| B factors | 16.5 |
| Protein | 15.6 |
| Ions | 31.1 |
| Water | 22.9 |
| r.m.s.a deviations | |
| Bond lengths (Å) | 0.008 |
| Bond angles (°) | 1.2 |
a r.m.s., root mean square.
FIGURE 2.
Crystal structure of domain-swapped dimer (CVNΔB)dsd. A, stereo view of the structure. The domains corresponding to the two pseudo-monomers are colored green and pink, and the alternative hinge conformation is shown in cyan (Pro-50–Ile-54). B, superposition of different hinge-region conformations shown in ball-and-stick representation (Pro-50–Asn-52). The structure of wild type CV-N (PDB accession code 3EZM) is depicted in green, and the major and minor conformers of (CVNΔB)dsd are colored in pink and cyan, respectively. C, the (2Fo − Fc) density map of the hinge region (Pro-50–Ile-54; cyan) contoured at 1.2 σ as well as the (Fo − Fc) density map prior to taking an alternative conformer into account (magenta). D, the (2Fo − Fc) density map contoured at 1.2 σ and the molecular models for the hinge region (Pro-50–Ile-54) after refinement with two alternative conformations.
Interestingly, in the present structure, two alternative conformations were found for the hinge region, involving both the main chain as well as the side chain atoms. Pairwise root mean square deviations of 0.84 and 0.95 Å (Cα backbone atoms) are observed between the alternative conformer and 3EZM (30) and 1LOM (31) structures, respectively. The polypeptide chain from residues 50–52 for both conformers, superimposed on the wild type structure, is depicted in Fig. 2B. As can be seen from this superposition, the deletion of one residue is readily accommodated in the chain by straightening the backbone when compared with the more undulating wild type chain. Using further refinement, including occupancy refinement, clearly allowed for tracing the alternate conformers for the Pro-50–Ile-54 segment. The resulting two models, placed into the final density, are shown in Fig. 2, C and D.
We previously determined the x-ray structure of CVNmutDB, which comprises identical changes in the domain B sugar binding site as the variant studied here. This protein was monomeric in the crystal, although two monomers were found in the asymmetric unit (16). The sequence of the present dimeric variant, (CVNΔB)dsd, contains two additional changes; Gln-50 was deleted, and in contrast to CVNmutDB, Pro-51 was not changed to Gly. As expected, the deletion caused the protein to become a domain-swapped dimer, both in the crystal and in solution. A comparison between both structures is provided in Fig. 3, depicting the two monomers (Fig. 3A) and the domain-swapped dimer (Fig. 3B) in the same orientation. That indeed this variant also exists as a dimer in solution was verified by NMR (data not shown) and native polyacrylamide gel analysis (Fig. 3C).
FIGURE 3.
Structures of CV-N mutants. A, ribbon representation of the crystal structure of CVNmutDB (PDB accession code 3CZZ). The two domains within each monomer in the asymmetric unit are colored green and pink and labeled by A, B, and A′, B′, respectively. Regions involved in mannose binding are shown in yellow. B, ribbon representation of the crystal structure of the domain-swapped dimer (CVNΔB)dsd. The domains are colored green and pink and are labeled A, B for chain 1, and A′, B′ for chain 2. Regions involved in mannose binding are shown in yellow. C, analysis of monomeric CVNmutDB and domain-swapped dimeric (CVNΔB)dsd on a 20% polyacrylamide native gel. D, ribbon representation of the model structure of the intramolecular disulfide-linked monomeric variant (CVNΔA)ssm. E, ribbon representation of model structure for the intermolecular disulfide-linked dimer (CVNΔA)ssd. F, analysis of monomeric (CVNΔA)ssm and dimeric (CVNΔA)ssd on a 20% polyacrylamide SDS gel. Lane 1 shows the monomeric (CVNΔA)ssm, lane 2 shows the dimeric (CVNΔA)ssd, and lane 3 shows the dimeric (CVNΔA)ssd after reduction with 5 mm dithiothreitol.
Models for the Disulfide-linked Monomer (CVNΔA)ssm and Dimer (CVNΔA)ssd
Cysteine mutagenesis in combination with disulfide bond formation was used to create additional monomeric and dimeric variants of CV-N. An exclusively monomeric variant that has the sugar binding site on domain A removed, (CVNΔA)ssm, was created by introducing two Cys residues at the N terminus of the protein, G2C and S5C, that, upon air oxidation, form an intramolecular disulfide bond (Fig. 1B). Likewise, a head-to-head disulfide-linked dimer devoid of carbohydrate binding sites on domains A, (CVNΔA)ssd, was formed by air oxidation of the G2C mutant (Fig. 1C). The presence of intra- and intermolecular disulfide bonds for these variants was verified by polyacrylamide SDS gel (Fig. 3F).
Structural models for both the intramolecularly linked Cys2–Cys5 variant (Fig. 3D) and the intermolecular Cys2–Cys2′ dimer (Fig. 3E) were built by homology modeling in Modeler (32), using the coordinates of wild type CV-N (PDB accession code 2EZM) (23) as the template. For the dimer, C2M symmetry was imposed, based on the observation of a single set of resonances in the 1H-15N HSQC spectrum. It should be noted, however, that such a disulfide-linked dimer does not necessarily contain a single, static arrangement around the center of the disulfide bond. Indeed, motional flexibility around disulfide bonds has been observed in proteins (33). For the present case, our NMR results strongly suggest the presence of motion around the disulfide link because complete assignments for all amino acids were obtained, except for the first four residues. Resonances for these residues are missing, probably due to broadening caused by a local conformational exchange process around the N-terminal, intermolecular disulfide bond.
15N NMR relaxation measurements were carried out at 25 °C for monomers and dimers. We found average T2 values for the amide nitrogens of monomeric (CVNΔA)ssm and for the dimeric (CVNΔA)ssd of 135 and 78 ms, respectively. These values are consistent with species of molecular masses of ∼11 and ∼22 kDa, respectively.
Carbohydrate Binding
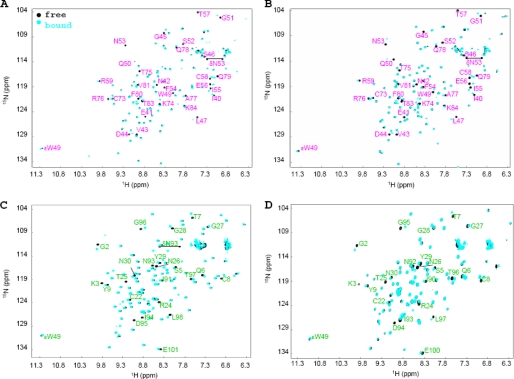
Carbohydrate binding sites on all proteins were mapped by following chemical shift perturbations in the 1H-15N HSQC spectra of 15N uniformly labeled protein as a function of ligand addition. The 1H-15N HSQC spectra of 150 μm (CVNΔA)ssm and 150 μm (CVNΔA)ssd in the absence and presence of 20 molar eq of Manα(1–2)Manα are provided in Fig. 4, A and B. The identity of perturbed amide N-H resonances clearly reveal the presence of a single binding site in domain B for both monomeric (CVNΔA)ssm and dimeric (CVNΔA)ssd. For CVNmutDB, we already previously reported that only domain A interacts with sugars (14, 16). Here we also show for the domain-swapped dimeric version of this mutant, (CVNΔB)dsd, that only amino acids on domain A interact with Manα(1–2)Manα. Representative 1H-15N HSQC spectra for the sugar-free and -bound forms of CVNmutDB and (CVNΔB)dsd are provided in Fig. 4, C and D.
FIGURE 4.
Superposition of 1H-15N HSQC NMR spectra of CV-N variants in the absence and presence of Manα(1–2)Manα. A, (CVNΔA)ssm; B,(CVNΔA)ssd; C, CVNmutDB; and D, (CVNΔB)dsd without (black) and in the presence of 20 molar eq of Manα(1–2)Manα (cyan). For (CVNΔA)ssm and (CVNΔA)ssd, only resonances belonging to domain B are perturbed, demonstrating the presence of a single binding site in domain B (all resonances arising from residues in domain B are labeled in pink). The identity of the perturbed amide N-H resonances in the spectra of CVNmutDB and (CVNΔB)dsd clearly demonstrates the presence of a single binding site in domain A (all resonances arising from residues in domain A are labeled in green).
Anti-HIV Assays
The antiviral activities of the engineered CV-N variants were tested in a single-cycle HIV-1 infection assay, using Hela-P4 cells that encode the LacZ reporter gene under the control of the HIV-1 long terminal repeat. Following viral integration, expression of the HIV-1 Tat protein results in expression of β-galactosidase, thereby allowing identification and facile quantification of the number of infected cells by X-gal staining. This assay scores all early events in HIV-1 infection in a single round, perfectly tailored to investigate effects of entry or membrane fusion inhibitors. We found that monomeric CV-N proteins that contain only one sugar binding site were completely devoid of antiviral activity in this assay up to concentrations of ∼100 nm. Dimerization of these inactive mutant monomers, however, via either intermolecular disulfide cross-linking or domain-swapping, restored anti-HIV activity, albeit to levels somewhat lower than wild type CV-N.
Normalized inhibition curves and IC50 values for the four CV-N variants, (CVNΔA)ssm, (CVNΔA)ssd, CVNmutDB, and (CVNΔB)dsd, as well as wild type CV-N are provided in Fig. 5 and Table 2. No detectable activity was found for either of the monomeric single-site mutants, whereas for the two dimers, (CVNΔA)ssd and (CVNΔB)dsd IC50 values of ∼59 and ∼6 nm were determined, respectively. In the same assay, wild type CV-N yielded an IC50 value of ∼0.7 nm. Thus, the domain-swapped dimer with two binding sites has an ∼10-fold lower activity than wild type CV-N. The disulfide-linked dimer with two binding sites has the lowest activity, and ∼100-fold more protein is needed for inhibition.
FIGURE 5.
Anti-HIV assays for CV-N variants: (P51G)CV-N (black triangles), CVNmutDB (green empty diamonds), (CVNΔB)dsd (green filled diamonds), (CVNΔA)ssm (pink empty circles), and (CVNΔA)ssd (pink filled circles). The symbols represent experimental data, and the lines represent the best fit of the experimental data. Conc, concentration.
TABLE 2.
Anti-viral activity of CV-N variants
| Protein | IC50 |
|---|---|
| (P51G)CV-N | 0.7 ± 0.2 nm |
| CVNmutDB | NDa |
| (CVNΔB)dsd | 6 ± 0.5 nm |
| (CVNΔA)ssm | NDa |
| (CVNΔA)ssd | 59 ± 0.6 nm |
a ND, not detected.
DISCUSSION
Although a large number of studies have been carried out with CV-N, details of the interaction between CV-N and the carbohydrates on the HIV gp120 envelope glycoprotein are still elusive. Retroviral envelope glycoproteins mediate the first step in viral infection. A typical HIV-1 envelope glycoprotein contains many high mannose type oligosaccharides that are clustered together on gp120 trimer to form a unique oligosaccharide microdomain (34). Such clusters were confirmed in the crystal structure of glycosylated gp120 simian immunodeficiency virus (35; PDB accession code 2BF1), and a variety of biochemical studies established that CV-N recognizes the terminal Manα(1–2)Manα units of both the D1 and the D3 arms of Man-8 and Man-9 as the primary viral target (4).
Although it is known that CV-N binds to high mannoses on the heavily glycosylated gp120, the specific binding site is unknown. Like CV-N, a neutralizing monoclonal antibody (monoclonal antibody 2G12) also recognizes a cluster of carbohydrates on the “silent” face of gp120 (36), and binding of CV-N to soluble gp120 or virions inhibits binding of the monoclonal antibody 2G12. However, the reverse is not true; binding of 2G12 monoclonal antibody to gp120 did not block CV-N binding, possibly caused by 2G12 recognizing only a subset of Manα(1–2)Manα termini, whereas CV-N has multiple recognition sites (37–39).
Previous NMR and calorimetric studies revealed that binding sites for Manα(1–2)Manα-containing sugars are located on both CV-N domains, A and B, and that both binding sites are responsible for the interaction with the high mannose oligosaccharides located on gp120. The role of sugar binding by domain B for antiviral activity was established using the CVNmutDB mutant that contains several amino acid changes on domain B, abolishing sugar binding to this domain. The mutant also carries the P51G mutation that substantially increases the thermodynamic stability of the monomer (14, 16). In the present work, we created (CVNΔA)ssm, a variant that comprises the amino acid changes Q6A and T7A to disrupt the binding site on domain A, as well as P51G. In addition, two N-terminal cysteines were introduced via the mutations G2C and S5C that can form an intramolecular disulfide bond. This domain A mutant exists exclusively as a monomer in its oxidized state, does not bind carbohydrate on domain A, and was unable to inhibit HIV-1 infection at concentrations up to 100 nm. Two dimeric proteins were created out of the above monomers. The first is (CVNΔA)ssd, whose sequence contains a single cysteine at the N terminus, G2C, permitting intermolecular disulfide (Cys2–Cys2′) cross-linking of two monomers. The second is an exclusively domain-swapped dimer, CVNmutDBΔGln-50–Pro-51 = (CVNΔB)dsd, created via deletion of Gln-50 in the hinge region of CVNmutDB-Pro-51. Note, that this protein does not contain the P51G change.
Both dimeric proteins possess only one binding site in their monomeric units but become bivalent through dimerization. Both mutants are active, although less potent than wild type CV-N, exhibiting IC50 values ∼10 and ∼100 times higher than CV-N (Fig. 5 and Table 2). Structurally, the two different dimers exhibit quite distinct relative orientations as well as different distances between the two equivalent sugar binding sites. Indeed, the distances between sites range from ∼40 Å in the wild type monomer to over ∼48 Å in the (CVNΔB)dsd domain-swapped dimer structure to ∼57 Å in the disulfide-linked dimer.
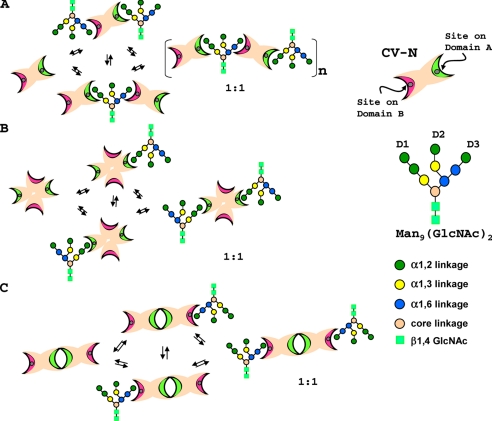
(P51G)CV-N titration with Man-9, even at very low concentration, was accompanied by precipitation of sugar-protein complexes due to multisite/multivalent cross-linking (9, 14), resulting in extreme line broadening and ultimately disappearance of resonances in NMR 15N HSQC spectra. We further investigated the complex formation between CV-N variants and Man-9. In contrast to the findings with wild type CV-N and (P51G)CV-N, no aggregation or precipitation of sugar-protein complexes was observed for dimeric (CVNΔB)dsd and (CVNΔA)ssd variants. This is in perfect accord with the fact that only domain A or domain B is available for the interaction with Man-9 in the case of (CVNΔB)dsd and (CVNΔA)ssd, as evidenced by the 15N HSQC NMR titration results (data not shown). Indeed, 15N HSQC spectra in the presence of Man-9 (∼1:1 molar ratio) exhibit only a slight decrease in resonance intensities, caused by exchange between recognition sites, but not complete loss of NMR signal and precipitation, although both dimeric (CVNΔB)dsd and (CVNΔA)ssd variants contain two binding sites that are potentially available for interaction with Man-9. This is in stark contrast to the situation with wild type CV-N and suggests a different mode of interaction between the engineered dimers with Man-9 when compared with wild type CV-N or (P51G)CV-N. Because domain A exhibits a slight preference for the trimannose units and domain B exhibits a slight preference for the dimannose units, respectively (14), wild type CV-N interacts with Man-9 to create a polymeric, cross-linked aggregate (9) (Fig. 6A). In the case of (CVNΔB)dsd, the D1 arm can interact preferentially with domains A, A′, whereas no recognition site for D3 (on domains B, B′) is available (Fig. 6B). Similarly, (CVNΔA)ssd would bind the D3 preferentially on domains B, B′ with the D1 recognition site (on domains A, A′) missing (Fig. 6C). This type of subtle recognition preference can explain why no such cross-linking was observed in the case of the present two CV-N variants when interacting with Man-9.
FIGURE 6.
Schematic depiction of oligomannose binding by CV-N variants. A, wild type CV-N; B, (CVNΔB)dsd, and C,(CVNΔA)ssd. CV-N domains are represented by two elongated crescents, with or without circles, representing intact or destroyed binding sites, respectively. Site 1 on domain A is colored green, and site 2 on domain B is in pink. The individual sugar units on the oligosaccharide are color-coded according to their linkage pattern.
The distance between the D1 and D3 arms of Man-9 range from ∼15 to ∼17 Å, clearly supporting the notion that CV-N interacts with two different carbohydrate moieties on gp120. The same holds for the interaction between the neutralizing antibody 2G12 and Man-9 (40). Thus, specificity is realized by matching the relative spacing of the binding sites in the protein with the appropriate geometrical arrangement of oligosaccharides epitopes, and avidity is achieved through multivalent binding via clustering of the mannoses (41). The different dispositions of the binding sites on (CVNΔB)dsd and (CVNΔA)ssd together with the loss of the slight preference for D1 versus D3 arms associated with domain A and B, respectively, may explain their lower activity when compared with wild type CV-N.
Our current data clearly demonstrate the critical role of the detailed multivalent nature of the CV-N-gp120 interaction for antiviral activity. Indeed, the present monovalent, monomeric CV-N mutants, ((CVNΔA)ssm and CVNmutDB), are completely inactive against HIV-1, whereas linking them, either via a head-to-head disulfide link or by domain swapping, restores their HIV-inactivating properties. This confirms that multisite/multivalent binding is critically important for the HIV-inactivating properties of CV-N.
Acknowledgments
E. M. gratefully acknowledges useful discussions with Joel Mispelter, and we thank Mike Delk for NMR technical support.
This work was supported, in whole or in part, by National Institutes of Health Grant RO1GM080642 (to A. M. G.).
This paper is dedicated to the memory of Gilles Craescu.
The atomic coordinates and structure factors (code 3LHC) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
- CV-N
- cyanovirin-N
- HIV
- human immunodeficiency virus
- X-gal
- 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside
- HSQC
- heteronuclear single quantum correlation
- ssm
- disulfide-bonded monomer
- ssd
- disulfide-bonded dimer
- dsd
- domain-swapped dimer.
REFERENCES
- 1.Boyd M. R., Gustafson K. R., McMahon J. B., Shoemaker R. H., O'Keefe B. R., Mori T., Gulakowski R. J., Wu L., Rivera M. I., Laurencot C. M., Currens M. J., Cardellina J. H., 2nd, Buckheit R. W., Jr., Nara P. L., Pannell L. K., Sowder R. C., 2nd, Henderson L. E. (1997) Antimicrob. Agents Chemother. 41, 1521–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patterson G. M. L., Baker K. K., Baldwin C. L., Bolis C. M., Caplan F. R., Larsen L. K., Levine I. A., Moore R. E., Moore E., Nelson C. S., Tschappat K. D., Tuang G. D., Boyd M. R., Cardellina J. H., 2nd, Collins R. P., Gustafson K. R., Snader K. M., Weislow O. S., Lewin R. A. (1993) J. Phycol. 29, 125–130 [Google Scholar]
- 3.O'Keefe B. R., Smee D. F., Turpin J. A., Saucedo C. J., Gustafson K. R., Mori T., Blakeslee D., Buckheit R., Boyd M. R. (2003) Antimicrob. Agents Chemother. 47, 2518–2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrientos L. G., Gronenborn A. M. (2005) Mini. Rev. Med. Chem. 5, 21–31 [DOI] [PubMed] [Google Scholar]
- 5.Bewley C. A., Otero-Quintero S. (2001) J. Am. Chem. Soc. 123, 3892–3902 [DOI] [PubMed] [Google Scholar]
- 6.Bolmstedt A. J., O'Keefe B. R., Shenoy S. R., McMahon J. B., Boyd M. R. (2001) Mol. Pharmacol. 59, 949–954 [DOI] [PubMed] [Google Scholar]
- 7.Barrientos L. G., Louis J. M., Botos I., Mori T., Han Z., O'Keefe B. R., Boyd M. R., Wlodawer A., Gronenborn A. M. (2002) Structure 10, 673–686 [DOI] [PubMed] [Google Scholar]
- 8.Barrientos L. G., Gronenborn A. M. (2002) Biochem. Biophys. Res. Commun. 298, 598–602 [DOI] [PubMed] [Google Scholar]
- 9.Shenoy S. R., Barrientos L. G., Ratner D. M., O'Keefe B. R., Seeberger P. H., Gronenborn A. M., Boyd M. R. (2002) Chem. Biol. 9, 1109–1118 [DOI] [PubMed] [Google Scholar]
- 10.Shenoy S. R., O'Keefe B. R., Bolmstedt A. J., Cartner L. K., Boyd M. R. (2001) J. Pharmacol. Exp. Ther. 297, 704–710 [PubMed] [Google Scholar]
- 11.Kiessling L. L., Young T., Gruber T. D., Mortell K. H. (2008) in Glycoscience: Chemistry and Chemical Biology (Fraser-Reid B., Tatsuta K., Thiem J. eds) Vol. 12.4, pp. 2483–2523, Springer Berlin, Heidelberg [Google Scholar]
- 12.Chang L. C., Bewley C. A. (2002) J. Mol. Biol. 318, 1–8 [DOI] [PubMed] [Google Scholar]
- 13.Barrientos L. G., Lasala F., Otero J. R., Sanchez A., Delgado R. (2004) J. Infect. Dis. 189, 1440–1443 [DOI] [PubMed] [Google Scholar]
- 14.Barrientos L. G., Matei E., Lasala F., Delgado R., Gronenborn A. M. (2006) Protein Eng. Des. Sel. 19, 525–535 [DOI] [PubMed] [Google Scholar]
- 15.Fromme R., Katiliene Z., Giomarelli B., Bogani F., Mc Mahon J., Mori T., Fromme P., Ghirlanda G. (2007) Biochemistry 46, 9199–9207 [DOI] [PubMed] [Google Scholar]
- 16.Matei E., Furey W., Gronenborn A. M. (2008) Structure 16, 1183–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang R., Aiken C. (2007) J. Virol. 81, 3749–3756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delaglio F., Grzesiek S., Vuister G. W., Zhu G., Pfeifer J., Bax A. (1995) J. Biomol. NMR 6, 277–293 [DOI] [PubMed] [Google Scholar]
- 19.Johnson B. A. (2004) Methods Mol. Biol. 278, 313–352 [DOI] [PubMed] [Google Scholar]
- 20.Fesik S. W., Zuiderweg E. R. (1990) Q. Rev. Biophys. 23, 97–131 [DOI] [PubMed] [Google Scholar]
- 21.Grzesiek S., Vuister G. W., Bax A. (1993) J. Biomol. NMR 3, 487–493 [DOI] [PubMed] [Google Scholar]
- 22.Pflugrath J. W. (1999) Acta Crystallogr. D Biol. Crystallogr. 55, 1718–1725 [DOI] [PubMed] [Google Scholar]
- 23.Bewley C. A., Gustafson K. R., Boyd M. R., Covell D. G., Bax A., Clore G. M., Gronenborn A. M. (1998) Nat Struct Biol. 5, 571–578 [DOI] [PubMed] [Google Scholar]
- 24.McCoy A. J., Grosse-Kunstleve R. W., Storoni L. C., Read R. J. (2005) Acta Crystallogr. D Biol. Crystallogr. 61, 458–464 [DOI] [PubMed] [Google Scholar]
- 25.Brünger A. T., Adams P. D., Clore G. M., DeLano W. L., Gros P., Grosse-Kunstleve R. W., Jiang J. S., Kuszewski J., Nilges M., Pannu N. S., Read R. J., Rice L. M., Simonson T., Warren G. L. (1998) Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 [DOI] [PubMed] [Google Scholar]
- 26.Emsley P., Cowtan K. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 27.Hooft R. W., Vriend G., Sander C., Abola E. E. (1996) Nature 381, 272. [DOI] [PubMed] [Google Scholar]
- 28.Laskowski R. A., Rullmannn J. A., MacArthur M. W., Kaptein R., Thornton J. M. (1996) J. Biomol. NMR 8, 477–486 [DOI] [PubMed] [Google Scholar]
- 29.Ramachandran G. N., Sasisekharan V. (1968) Adv. Protein Chem. 23, 283–438 [DOI] [PubMed] [Google Scholar]
- 30.Yang F., Bewley C. A., Louis J. M., Gustafson K. R., Boyd M. R., Gronenborn A. M., Clore G. M., Wlodawer A. (1999) J. Mol. Biol. 288, 403–412 [DOI] [PubMed] [Google Scholar]
- 31.Botos I., Mori T., Cartner L. K., Boyd M. R., Wlodawer A. (2002) Biochem. Biophys. Res. Commun. 294, 184–190 [DOI] [PubMed] [Google Scholar]
- 32.Sali A., Blundell T. L. (1993) J. Mol. Biol. 234, 779–815 [DOI] [PubMed] [Google Scholar]
- 33.Grey M. J., Wang C., Palmer A. G., 3rd (2003) J. Am. Chem. Soc. 125, 14324–14335 [DOI] [PubMed] [Google Scholar]
- 34.Leonard C. K., Spellman M. W., Riddle L., Harris R. J., Thomas J. N., Gregory T. J. (1990) J. Biol. Chem. 265, 10373–10382 [PubMed] [Google Scholar]
- 35.Chen B., Vogan E. M., Gong H., Skehel J. J., Wiley D. C., Harrison S. C. (2005) Structure 13, 197–211 [DOI] [PubMed] [Google Scholar]
- 36.Trkola A., Purtscher M., Muster T., Ballaun C., Buchacher A., Sullivan N., Srinivasan K., Sodroski J., Moore J. P., Katinger H. (1996) J. Virol. 70, 1100–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esser M. T., Mori T., Mondor I., Sattentau Q. J., Dey B., Berger E. A., Boyd M. R., Lifson J. D. (1999) J. Virol. 73, 4360–4371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanders R. W., Venturi M., Schiffner L., Kalyanaraman R., Katinger H., Lloyd K. O., Kwong P. D., Moore J. P. (2002) J. Virol. 76, 7293–7305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scanlan C. N., Pantophlet R., Wormald M. R., Ollmann Saphire E., Stanfield R., Wilson I. A., Katinger H., Dwek R. A., Rudd P. M., Burton D. R. (2002) J. Virol. 76, 7306–7321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calarese D. A., Scanlan C. N., Zwick M. B., Deechongkit S., Mimura Y., Kunert R., Zhu P., Wormald M. R., Stanfield R. L., Roux K. H., Kelly J. W., Rudd P. M., Dwek R. A., Katinger H., Burton D. R., Wilson I. A. (2003) Science 300, 2065–2071 [DOI] [PubMed] [Google Scholar]
- 41.Weis W. I., Drickamer K. (1996) Annu. Rev. Biochem. 65, 441–473 [DOI] [PubMed] [Google Scholar]