Abstract
Protocadherin LKC (PLKC) is a member of the heterogeneous subgroup of protocadherins that was identified and described as a potential tumor-suppressor gene involved in contact inhibition (Okazaki, N., Takahashi, N., Kojima, S., Masuho, Y., and Koga, H. (2002) Carcinogenesis 23, 1139–1148 and Ose, R., Yanagawa, T., Ikeda, S., Ohara, O., and Koga, H. (2009) Mol. Oncol. 3, 54–66). Several aspects of the structure, posttranslational processing, targeting, and function of this new protocadherin are still not known. Here, we demonstrate that the expression of PLKC at the apical membrane domain and its concentration at regions of cell-cell contacts occur concomitantly with significant elevation of PLKC-mRNA levels. Furthermore, it can be found within the adherens junctions, but it does not colocalize with tight junctions proteins ZO-1 and occludin, respectively. Additionally, unlike E-cadherin, PLKC is not redistributed upon Ca2+ removal. Biosynthetic labeling revealed N- and O-glycosylation as posttranslational modifications as well as a fast transport to the cell surface and a low turnover rate. During differentiation, PLKC associates with detergent-resistant membranes that trigger its redistribution from intracellular membranes to the cell surface. This association occurs concomitant with alterations in the glycosylation pattern. We propose a role for PLKC in the establishment of a proper epithelial cell polarity that requires O-linked glycosylation and association of the protein with detergent-resistant membranes.
Keywords: Cell/Epithelial, Cell/Differentiation, Cell/Trafficking, Glycoproteins/Biosynthesis, Glycosylation, Lipid/Raft, Protein/Post Translational Modification, Protein/Sorting
Introduction
The establishment and maintenance of cell polarity is one of the most important issues in a variety of tissues within organisms from worms to humans. Achievement of tissue polarity is linked to a distinct state of differentiation of each cell within the monolayer. This differentiation occurs during cell proliferation and development and is facilitated by cell-cell contacts and extracellular stimulation via hormones, cytokines, or other signals.
The molecular mechanisms underlying cell differentiation and formation of epithelial monolayers are highly conserved among animals (3). During cell development, particular proteins are targeted to the sites of cell-cell contacts to establish strong connections with neighboring cells and form a closed monolayer of cells that is capable of accomplishing barrier functions.
In vertebrate cells several connective molecule families are known to form zones of cell-cell contacts. The expression of some of them is up-regulated (like several cadherins (4)), and many proteins are redistributed to the sites of cell-cell contacts (like zonula occludens protein 1 (ZO-1)2) during progression of differentiation (5).
The cadherin superfamily has been long known to mediate Ca2+-dependent cell-cell adhesion in a homophilic manner (6). Cadherins are involved in several pivotal cellular functions, such as recognition, signaling, and communication as well as morphogenesis and angiogenesis (7–9). Various cadherins mediate specific cell-cell adhesion and play a crucial role in the formation and maintenance of tissues (10), particularly by establishment of the adherent junction belt whereby they bridge proteins to the actin cytoskeleton (11, 12).
A striking structural feature of cadherins is the unique extracellular fragment that consists of multiple domains, the cadherin repeats, each comprising around 100 highly conserved amino acid residues. Cadherins, such as E-cadherin, VE-, or N-cadherin exhibit five extracellular repeats and show a high similarity in their cytosolic domains (for review, see Ref. 13) which interact with β-catenins and small GTPases of the Rho family and interfere in receptor-tyrosine kinase signaling (11, 14). Unlike cadherins, protocadherins form a heterogeneous cadherin subgroup with a varying range of extracellular cadherin repeats (15, 16). Well known protocadherins are those expressed in the nervous system (17, 18). However, protocadherins in other tissues have been also characterized, such as μ-protocadherin (19), VE-cadherin-2 (20), or FAT-1 (21). Some protocadherins function in embryonic development (22) or gastrulation (23) and may play a role in the development and function of the nervous system (17, 18, 24, 25). In contrast to cadherins, protocadherins reveal distinct structural features that vary in the number of repeats (e.g. 4 in μ-protocadherin to 34 in FAT-1) and in their cytoplasmic tails, suggesting different types of interactions with cellular components as well as functional diversity.
A more recently described protocadherin is that expressed in the liver, kidney, and colon (PLKC) (1, 2), which was later renamed PCDH24 by the HUGO Nomenclature Committee. Characteristics of PLKC are seven extracellular cadherin repeats with 93–117 amino acids each and a 35-amino acid-long cytoplasmic domain that is terminated by a PDZ binding domain. An interaction with the PDZ domain of hMAST205 has been demonstrated, suggesting a possible role as a switch for intracellular signaling (1). The aim of this study was to investigate the structural features of PLKC, its biosynthesis, intracellular processing, and sorting during differentiation.
EXPERIMENTAL PROCEDURES
Materials and Reagents
Streptomycin, penicillin, glutamine, Dulbecco's modified Eagle's medium, methionine-free Dulbecco's modified Eagle's medium, fetal calf serum, and trypsin were purchased from PAA Laboratories GmbH (Pasching, Austria). Pepstatin, leupeptin, aprotinin, trypsin inhibitor, and molecular mass standards for SDS-PAGE were from Sigma. Soybean trypsin inhibitor was obtained from Roche Diagnostics.
l-[35S]Methionine (1000 Ci/mmol), Alkophos direct labeling of DNA probes kit, Hybond N+ nylon membranes, and protein A-Sepharose were obtained from Amersham Biosciences. Acrylamide, N,N′-methylenebisacrylamide, and N,N,N′,N′-tetramethylethylenediamine were purchased from Carl Roth GmbH (Karlsruhe, Germany). SDS, ammonium persulfate, dithiothreitol, and Triton X-100 were obtained from Merck. The pEYFP-N1 vector was purchased from Clontech Laboratories Inc. (Heidelberg, Germany). Immunoprecipitation and Western blotting of PLKC-YFP were performed with anti-GFP antibody (BD Transduction Laboratories). For immunofluorescence studies, anti-ZO-1 and anti-occludin (Zymed Laboratories Inc., San Francisco) and anti-E-cadherin (BD Transduction Laboratories) were used. Phalloidin-ALEXA Fluor 546 for actin staining and Alexa Fluor 568 anti mouse were purchased from Invitrogen. Commercial monoclonal antibody against flotillin-2 and RhoA were from Santa Cruz Biotechnology (Heidelberg, Germany).
RNA Isolation, Reverse Transcriptase-PCR, and Competitive PCR
RNA was isolated from cells using the RNeasy® mini kit from Qiagen (Hilden, Germany) according to the manufacturer's instructions. The cDNA was synthesized by using the First Strand cDNA Synthesis kit (MBI Fermentas, St. Leon-Rot, Germany) with random hexamer nucleotide primers. PCR was performed on a Biometra Personal Cycler (Biometra, Göttingen, Germany) using the following primers: PLKC forward (5′-CAGCGGGCTCAGCCTCCGTTC-3′) and PLKC reverse (5′-CTGTCCAGGGGCCCCAGGTTTCTG-3′) or β-actin forward (5′-GGCATCGTGATGGACTCCG-3′) and β-actin reverse 5′-GCTGGAAGGTGGACAGCGA-3′, respectively. Intensity of the DNA bands was assessed in a charged coupled device (CCD) camera and quantified using Quantity One 4.5.2 software (both are products of Bio-Rad). The maximum expression of PLKC in fully differentiated and polarized cells was set as 100%, and other samples were quantified accordingly.
Northern blot analysis of RNA from subconfluent and confluent MDCK cells was performed on nylon membranes according to Sambrook et al. (26) using a 32P-labeled DNA probe corresponding to a fragment of the PLKC cDNA (1–1700 bp). The nylon membrane was exposed overnight to x-ray film (Eastman Kodak Co.). The quantitative analysis of the labeling intensities of the RNA transcripts was further determined using Fluor S multiimager specific programs (Bio-Rad).
Expression Vectors
To generate a YFP-tagged version of PLKC, an expression vector was generated using the following primers: PLKC forward (5′-GAATTCGATGGCCCAGCTATGGCTGTC-3′) and PLKC reverse (5′-CCGCGGTGACAGGTCCGTGGT-3′). The resulting expression vector pEYFP-PLKC contained enhanced YFP fused in-frame to the 3′ end of PLKC.
Transfection and Generation of Stable Cell Lines
MDCK-II cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 2 mm glutamine, 50 units/ml penicillin, and 50 mg/ml streptomycin at 37 °C in a 5% CO2 atmosphere. The cells were transfected with 5 μg of the appropriate recombinant DNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Stably transfected cell lines were selected in the presence of 0.25 mg/ml active G418 (Invitrogen), and after 18–23 days, surviving colonies were isolated. Stable cells expressing PLKC-YFP were screened by immunoprecipitation and by fluorescence laser confocal microscopy. The generated clones were indicated MDCK-PLKC-YFP. For co-localization studies MDCK-PLKC-YFP cells were transiently transfected with Golgi-dsRed cDNA (Clontech) using Lipofectamine (Invitrogen) following the manufacturer's instructions.
Biosynthetic Labeling, Immunoprecipitation, and Immunoblotting
Cells were incubated in methionine-free minimum Eagle's medium for 2 h before labeling in the same medium containing 50 μCi of [35S]methionine for the indicated time intervals. In pulse-chase experiments the cells were labeled with [35S]methionine for the indicated times and chased with methionine-containing standard culture medium for different periods of time. Cell solubilization and immunoprecipitation of GFP-tagged PLKC molecules were performed according to established procedures (27) using anti-GFP antibodies. The immunoprecipitated proteins were further analyzed by SDS-PAGE. The radioactive protein bands were visualized using a phosphorimaging device (Bio-Rad. In some experiments N-linked deglycosylation of the precipitated proteins was performed with endoglycosidase H or endoglycosidase F/PNGase F (Roche Diagnostics) according to the manufacturer's instructions. Western blotting was performed using anti-GFP antibody at a dilution of 1:1000. The secondary antibody employed anti-mouse antibody conjugated to horseradish peroxidase (Amersham Biosciences), and the protein bands were visualized by enhanced chemiluminescence using SuperSignal® ELISA Femto Maximum Sensitivity Substrate from Pierce according to the manufacturer's instructions. Detection occurred by the ChemiDoc system together with the QuantityOne software (both from Bio-Rad) and x-ray (x-ray Retina XBA) film sheets (Fotochemische Werke GmbH Berlin, Germany).
Detergent-resistant Membrane (DRM) Extraction
Association of PLKC with DRMs was investigated by a discontinuous sucrose-density gradient as reported previously (28). In brief, cells at a subconfluent stage of differentiation (around 50% optical confluence) and 2 days after confluence were lysed with 1% (w/v) of Triton X-100 in PBS. After low speed centrifugation, each sample was diluted to a final sucrose concentration of 40% and laid onto an 80% sucrose cushion. Then 1 ml of sample was overlaid with 7 ml of 30% sucrose and 1 ml of 5% sucrose on the top and, finally, centrifuged for 18 h at 100,000 × g with a swing-out rotor. Nine fractions of 1 ml each were collected from the top and analyzed for the protein content by Western blotting. The distribution of the marker proteins flotillin-2 and RhoA was tested as the positive and negative control, respectively.
Immunofluorescence and Confocal Laser Scanning Microscopy
Cells grown on coverslips were fixed with 4% paraformaldehyde in PBS for 45 min at room temperature. For imaging of intracellular and surface-localized proteins, the cells were treated with 0.5% saponin in 1% bovine serum albumin-PBS. Primary antibodies were used at the following dilutions in PBS (v/v): anti ZO-1 (1:100), anti-occludin (1:100), and anti-E-cadherin (1:50). The secondary antibodies employed Cy3 anti-rabbit (Sigma) and Alexa Fluor 568 anti-mouse (Invitrogen) at a dilution of 1:1000. Confocal laser scanning microscopy of living or fixed cells was performed in a Leica TCS SP2 microscope with a 63× water planapochromat lens (Leica Microsystems, Mannheim, Germany). Dual color YFP and Cy3 or Alexa Fluor images were obtained by sequential scans with the 468-nm excitation line of an argon laser or the 543-nm excitation line of a He/Ne laser, respectively, and the optimal emission wave length for GFP or Cy3/Alexa.
Treatment of Cell Membrane Proteins with Trypsin
Stably transfected MDCK cells were labeled with 50 μCi of [35S]methionine for 8 h, washed twice with ice-cold PBS, and incubated with 100 μg trypsin/ml in PBS on ice for 45 min. After quenching excess trypsin with 200 μg of soybean trypsin, inhibitor cells were processed for immunoprecipitation as described above.
Inhibition of Glycosylation and Depletion of Cholesterol
To test the role of glycosylation on the sorting of PLKC, inhibitors of glycosylation processing were added to the growth medium during 24 h of cell culture. Benzyl-GalNAc (4 mm) was used as an inhibitor of O-glycosylation and deoxymannojirimycin (DMM) (5 mm) was utilized to interfere with processing of N-glycosylated PLKC. For cholesterol depletion, methyl-β-cyclodextrin was used at 50 mm final concentration. All inhibitors were obtained from Sigma.
RESULTS
Expression and Subcellular Distribution of PLKC
The subcellular distribution and intracellular processing of PLKC were analyzed using PLKC fused at its C-terminal end to YFP. This chimeric protein was stably expressed in MDCK-II cells, which are denoted thereafter as MDCK-PLKC-YFP. We used this cell line rather than wild type MDCK cells to investigate the structure and function of PLKC for two main reasons. First, the limited access to anti-PLKC antibodies hampered these analyses, whereas the recombinant PLKC-YFP protein could be analyzed using commercially available anti-GFP antibodies that recognize the YFP variant of GFP. Second, wild type endogenous PLKC showed a similar cellular distribution as PLKC-YFP as assessed by anti-PLKC antibodies (1).
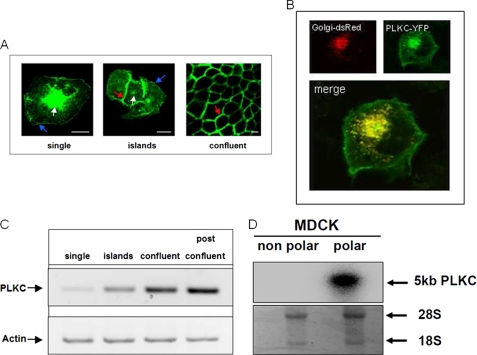
We first analyzed the cellular localization of PLKC-YFP in differentiating MDCK cells. Fig. 1 reveals a striking alteration in the protein distribution pattern of PLKC at the plasma membrane. In single MDCK-II cells (Fig. 1A, single) PLKC accumulates intracellularly, mainly in the Golgi apparatus (white arrow) as confirmed by colocalization of PLKC-YFP with a Golgi-dsRed marker that was transiently co-expressed in these cells (Fig. 1B).
FIGURE 1.
PLKC expression is affected by cell-cell contacts in MDCK-II. A, MDCK-PLKC-YFP cells were plated at low density, and subcellular distribution was analyzed using confocal laser microscopy during formation of a confluent monolayer. Blue arrow, cell surface; white arrow, Golgi apparatus; red arrow, cell-cell contact sites. B, shown is confocal laser microscopy of MDCK-PLKC-YFP cells transiently transfected with cDNA Golgi-dsRed. C, mRNA samples from MDCK-II cells of distinct stages of differentiation were estimated for expression of PLKC-mRNA by semiquantitative PCR. For quantification purposes the β-actin mRNA levels were used. D, Northern blot analysis is shown of polar and non-polar MDCK-II cells including 28 S and 18 S RNA controls. Scale bar, 10 μm.
Furthermore, PLKC-YFP appears also in single cells as filamentous structures at the cell surface (blue arrow). Readily upon first contacts with neighboring cells, PLKC protein molecules are sorted toward the cell-cell contact sites (Fig. 1A, islands). Finally in a fully differentiated monolayer at 5 days postconfluence, nearly the entire protein is directed to the cell-cell contacts or localizes at the apical membrane (Fig. 1A, confluent), whereas the basal cell surface is totally devoid of PLKC (data not shown). Remarkably, all the cells examined exhibited high expression levels of PLKC molecules in cell membrane protrusions, particularly in cell-cell contact areas.
Transcriptional Analysis of PLKC-YFP in MDCK Cells
Concomitant with differentiation, the expression of PLKC mRNA increases substantially as assessed by semiquantitative PCR (Fig. 1C). In fact, the mRNA levels increase steadily and strikingly when comparing cells revealing random membrane and intracellular distribution of PLKC (left two lanes, single and islands) with their fully differentiated epithelial counterparts (right two lanes, confluent and postconfluent). Additionally, RNA from polarized, differentiated (confluent), and non-polarized (20% confluent) MDCK cells were analyzed by Northern blot analysis (Fig. 1D). The quantitative analysis of the labeling intensities in both polar and non-polar cells was evaluated relative to the labeling intensities of the ribosomal RNA before blotting, and the results revealed an almost 7-fold higher expression of the PLKC gene in polarized MDCK cells compared with the non-polarized ones.
PLKC Is Colocalized with E-cadherin in the Adherens Junctions but Is Not Present in Zonula Occludens
We next examined the subcellular distribution of PLKC at the cell-cell contact regions of the lateral side in epithelial cells.
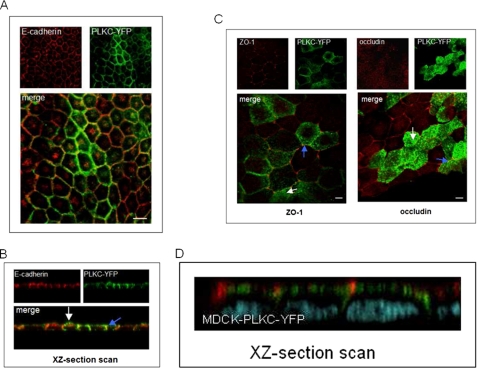
PLKC colocalizes with E-cadherin at sites of lateral cell-cell contacts (Fig. 2, A and B), but not with ZO-1 and occludin (Fig. 2, C and D), two protein markers of the zonula occludens belt complex in polarized epithelial monolayers (5, 29). Both tight junction proteins are expressed in limited regions at the most apical cell-cell contacts (blue arrow), but their localization is clearly distinct from spots of PLKC, which never colocalized with ZO-1 or occludin. Interestingly, the strongest PLKC expression was found at the apical membrane (white arrow).
FIGURE 2.
PLKC is targeted in epithelial cells to the adherens but not to the tight junctions. MDCK-PLKC-YFP cells were grown to confluence and fixed with paraformaldehyde. Cells were permeabilized with 0.5% saponin. A, E-cadherin was stained with anti-E-cadherin antibody. An Alexa Fluor® 568 conjugate was used as secondary antibody. B, shown is an xz-section scan of MDCK-PLKC-YFP cells stained for E-cadherin. C, ZO-1 and occludin were labeled in MDCK-PLKC-YFP cells using a Cy3-conjugated secondary antibody. D, shown is an xz-section scan of MDCK-PLKC-YFP (green) cells stained for occludin (red). Cell integrity was confirmed by DNA staining (blue). Scale bar, 10 μm.
Membrane Localization of PLKC Is Not Affected by Calcium Depletion
Calcium concentration plays a crucial role in establishment and maintenance of epithelial monolayers. In fact, chelation of extracellular calcium ions disrupts the adherens junctions with subsequent dramatic alteration in the distribution of E-cadherin and other cadherins as well as cell detachment (30, 31).
We investigated, therefore, the impact of extracellular calcium on the subcellular distribution and cell surface expression of PLKC by calcium depletion in a time-course experiment utilizing the calcium specific chelating reagent EGTA. The positive control employed E-cadherin that is normally affected by calcium.
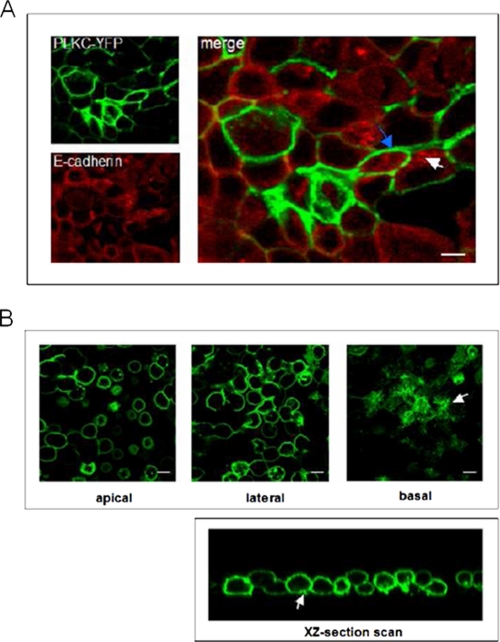
Fig. 3A shows that the addition of EGTA to MDCK-PLKC-YFP cells did not affect the cell surface localization of PLKC, whereas a substantial redistribution of E-cadherin from the cell membrane to submembranous and vesicular structures occurred (compare Fig. 3A (+ EGTA) with Fig. 2A (− EGTA)). No change in the distribution patterns of these two proteins was observed with increasing time until cell detachment occurred after ∼45 min. Here, nearly all E-cadherin molecules accumulated in the cell body, whereas PLKC persisted at the plasma membrane regardless of calcium removal. Interestingly, after 30 min most cells lost their intercellular contacts and adopted a rounded shape but maintained their adherence to the plates via a filamentous network in basal cell protrusions that exhibited a high expression of PLKC-YFP (Fig. 3B). The effect of calcium depletion was reversible.
FIGURE 3.
E-cadherin, but not PLKC, is redistributed upon calcium removal. MDCK-PLKC-YFP cells were grown until 5 days after confluence. Thereafter, calcium was chelated by the addition of EGTA (5 mm final concentration). Samples were fixed at different time points (the 10 min time point is shown in A). For visualization of E-cadherin, cells were processed by immunofluorescence as described above. In B, subcellular localization of PLKC after 30 min of incubation with EGTA was visualized by differential section scanning. A basal cell protrusion is indicated by a white arrow. Scale bar, 10 μm.
Biosynthesis, Processing, and Cell Surface Expression of PLKC
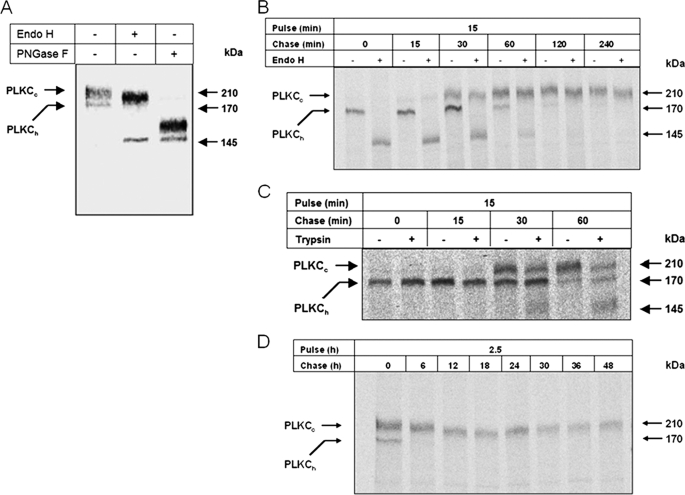
We next investigated the biosynthesis and processing of PLKC in differentiated MDCK-PLKC-YFP as well as its delivery to the cell surface. Fig. 4, A and B, shows that the earliest detectable biosynthetic form of PLKC-YFP is the ER-resident, endo H-sensitive 170-kDa mannose-rich polypeptide (PLKCh) that shifts to 145 kDa after endo H treatment. Further processing in the Golgi apparatus generates the endo H-resistant complex-glycosylated 210-kDa mature form of PLKC (PLKCC), which shifts upon N-deglycosylation with PNGase F generating a 160-kDa protein (Fig. 4A, third lane). Similar to endo H treatment, PNGase F shifted the mannose-rich polypeptide also to a 145-kDa protein. The difference between the two PNGase products could, therefore, be attributed to the presence of O-glycans on PLKC.
FIGURE 4.
Biosynthesis of PLKC. A, MDCK-II cells stably expressing PLKC-YFP were labeled with [35S]methionine for 6 h, and the detergent extracts were processed by immunoprecipitation with anti-GFP antibody. Immunoprecipitates were treated with endo H or PNGase F before SDS-PAGE on 5% slab-gels. In pulse-chase experiments, MDCK-PLKC-YFP cells were labeled for 15 min (B and C) or 2.5 h (D) with [35S]methionine and subsequently chased with nonradioactive culture medium for the indicated time intervals. Thereafter, PLKC-YFP was immunoprecipitated from the detergent extracts of the cells with anti-GFP antibodies. Where indicated, intact cells were treated with 100 μg trypsin/ml in PBS for 45 min on ice before cell lysis.
Another issue we addressed was the rate of maturation and trafficking of PLKC using pulse-chase experiments. Endo H was used to distinguish between early ER mannose-rich and mature processed complex-glycosylated forms of PLKC. Fig. 4B shows that PLKC is transported from the ER to the Golgi at a rapid rate as assessed by the appearance of endo H-resistant PLKC forms already after 15 min of chase.
The trafficking of mature PLKC to the cell surface was probed by treatment of intact cells with trypsin. Trypsin was demonstrated in preliminary experiments to cleave PLKC and is, therefore, expected to cleave cell surface exposed PLKC molecules (not shown). As shown in Fig. 4C this treatment generated a cleaved form of PLKC already at 30 min of chase after complex glycosylation of PLKC in the Golgi occurred. The intensity of the truncated PLKC form increased at 1 h of chase with a concomitant decrease in that of uncleaved PLKCC; the intracellular mannose-rich form PLKCh remained unaffected. This result points to a precursor-product relationship between PLKCC and the cleaved form and indicates that PLKCC is exposed at the cell surface. The data further support the notion that PLKC transport to the cell surface occurs rapidly and at almost similar rates to its transport between the ER and the Golgi. In fact, truncated surface-exposed PLKCC (30 min of chase) occurs 15 min after complex glycosylation, which in turn also occurs after 15 min of chase. Moreover, PLKC has a very slow turnover rate (Fig. 4D).
PLKC Associates with Lipid Rafts during Differentiation of MDCK Cells
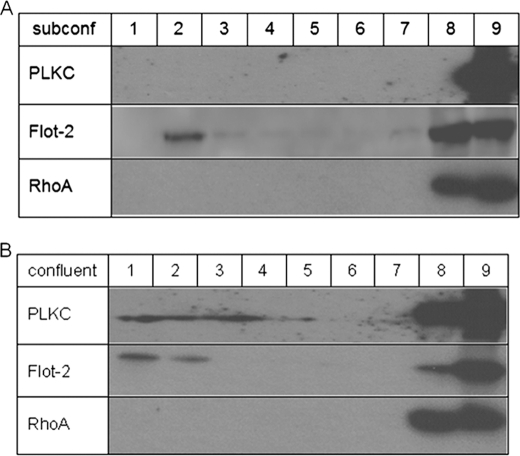
The altered cellular localization of PLKC from intracellular compartments in non-polarized cells to sites of cell-cell contacts and to the apical membrane raises questions regarding potential mechanisms implicated in this redistribution. This is particularly interesting in light of the unique and predominant localization of this protocadherin at the apical membrane. One potential sorting mechanism in epithelial cells implicates the association of proteins with specialized membrane microdomains that are resistant to solubilization in the cold with non-ionic detergents (for review, see Refs. 32 and 33). These microdomains, also referred to as DRMs or lipid rafts, are enriched in cholesterol and sphingolipids when Triton X-100 is used as the discriminating detergent. We, therefore, asked whether PLKC associates with DRMs along the secretory pathway with particular emphasis on the temporal requirements of this association. Triton X-100 cell extracts of MDCK-PLKC-YFP at subconfluent stage of differentiation and 2 days postconfluence were subjected to sucrose gradients, and the presence of PLKC was examined in the floating fractions. Fig. 5 shows that PLKC was entirely recovered from the Triton X-100-soluble fractions in subconfluent MDCK cells (Fig. 5A). By contrast, analysis of confluent MDCK-PLKC-YFP cells, in which PLKC was exclusively expressed at the cell surface, revealed a shift in the pattern of PLKC toward the Triton X-100-insoluble floating fractions (Fig. 5B). The distribution pattern for flotillin, a protein that is often used as a marker of DRMs, differed from that of PLKC. Here, flotillin bands were detected in the floating fractions of the lysates of subconfluent as well as confluent cells. RhoA, a marker of proteins that are not associated with DRMs, was recovered in the soluble fractions regardless of cell confluency. The differing band pattern of PLKC in the floating fractions as compared with that in the soluble fractions suggests at first glance qualitative differences between the two forms concomitant with different subpopulations of PLKC in the floating and soluble fractions. The finding that PLKC redistributes from intracellular sites to the cell surface, particularly to the apical membrane, concomitant with its association with DRMs, is indicative of an essential role of these microdomains in the trafficking and cell surface delivery of PLKC during cellular differentiation.
FIGURE 5.
PLKC associates with DRMs during differentiation. Two-day confluent (A) or subconfluent (∼50%) (B) cells were lysed with 1% (w/v) of Triton X-100 in PBS. After low speed centrifugation, the sample was diluted to a final sucrose concentration of 40% and laid onto an 80% sucrose cushion. The 1-ml sample was then overlaid with 7 ml of 30% sucrose and 1 ml of 5% sucrose on the top and finally centrifuged for 18 h at 100,000 × g with a swing-out rotor. Nine fractions of 1 ml each were collected from the top and analyzed for the protein content by immunoblotting. The distribution of the marker proteins flotillin-2 and RhoA was tested as positive and negative control, respectively. subconf, subconfluent.
Influence of O-Glycosylation on PLKC Sorting
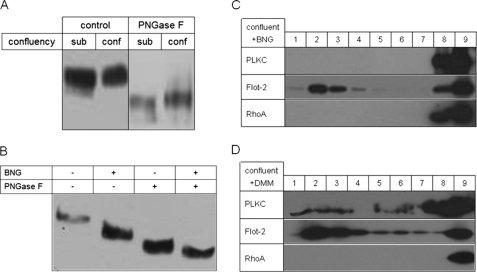
It has been previously shown that a potential sorting mechanism of membrane glycoproteins to the apical membrane utilizes O-glycans as a sorting signal in association with DRMs (34–38). We, therefore, tested if the glycosylation of PLKC is altered in differentiated and undifferentiated MDCK-PLKC-YFP. Fig. 6A shows a Western blot analysis of PLKC in total cell lysates extracted from subconfluent and 2 days confluent cells. Although a difference in the size of PLKC in the subconfluent and confluent and fully differentiated cells is not clearly seen, treatment with PNGase F reveals a slight, but definite increase in the digested form of PLKC in differentiated cells in comparison to the subconfluent cells. Because PNGase F digests N-linked but not O-linked glycans, we propose that the elevated size of PLKC in differentiated cells is due to an increased content of O-glycans in these cells. To assess the role of O-glycosylation in the association of PLKC with DRMs, we utilized benzyl-N-acetylgalactosamine (BNG) that interferes and inhibits O-glycosylation. First, we examined the inhibitory capacity of BNG on the O-glycosylation of PLKC by comparing the electrophoretic mobilities of PLKC before and after treatment with BNG. Fig. 6B shows that BNG has reduced the size of PLKC, supporting the notion that PLKC and PNGase F digestion generates a smaller protein product from this glycoform as compared with PLKC from BNG-non-treated cells. These results support the notion that the O-glycosylation of PLKC has been inhibited or substantially affected in the presence of BNG. We next investigated the relevance of O-glycosylation to the association of PLKC with DRMs by assessment of the presence of BNG-affected PLKC in the floating fractions of Triton X-100 cell extracts of BNG-treated cells. Fig. 6C reveals that PLKC was not detected in the floating fraction, whereas the DRM marker flotillin was clearly found in these fractions. Therefore, O-glycosylation is necessary for the association of PLKC with DRMs, and an alteration in the content of O-glycosylation, for instance during cellular differentiation, redistributes PLKC from the Triton X-100-insoluble DRMs to the soluble fractions. On the other hand, inhibition of N-glycosylation by DMM did not exclude PLKC completely from detergent-resistant membranes (Fig. 6D).
FIGURE 6.
Influence of O-glycosylation on PLKC differentiation. A, 2-day confluent or subconfluent (∼50%) MDCK-PLKC-YFP were lysed, and proteins were precipitated, treated with PNGase F or not treated, separated via SDS-PAGE, and visualized by immunoblotting. B, confluent MDCK-PLKC-YFP cells were treated with BNG for 24 h or not treated. Subsequently, cells were lysed, and proteins were precipitated, treated with PNGase F or not treated, separated via SDS-PAGE, and visualized by immunoblotting. C, confluent cells were treated for 24 h with BNG and thereafter lysed with 1% (w/v) of Triton X-100 in PBS. After low speed centrifugation, the sample was diluted to a final sucrose concentration of 40% and laid onto an 80% sucrose cushion. The 1-ml sample was then overlaid with 7 ml of 30% sucrose and 1 ml of 5% sucrose on the top and finally centrifuged for 18 h at 100,000 × g with a swing-out rotor. Nine fractions of 1 ml each were collected from the top and analyzed for the protein content by immunoblotting. sub, subconfluent; conf, confluent. D, the experimental setup was the same as described in C, but the cells were treated with DMM instead of BNG.
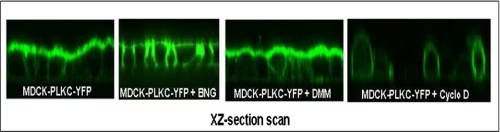
To further investigate the role of O-glycosylation in the trafficking of PLKC, we examined its localization on the cell surface in the presence of BNG, i.e. after inhibition of O-glycosylation. Fig. 7 shows that PLKC is distributed on both sides of the membrane in the presence of BNG, supporting the view that O-glycosylation is implicated in polarized trafficking of PLKC. DMM, by contrast, did not alter the subcellular localization of PLKC in comparison to untreated control. As shown above, PLKC associates with DRMs during cellular differentiation. We examined whether DRMs are required for PLKC-polarized trafficking and treated MDCK-PLKC-YFP cells with cyclodextrin, an inhibitor of cholesterol. Confocal images in Fig. 7 reveal a dramatic change in the pattern of PLKC and a shift to non-polarized cellular distribution.
FIGURE 7.
Subcellular distribution of PLKC changes after cholesterol depletion or BNG treatment but is independent of N-glycosylation. MDCK-PLKC-YFP cells were plated on coverslips, and subcellular distribution was analyzed using confocal laser microscopy after treatment with BNG, DMM, or methyl-β-cyclodextrin (Cyclo D).
DISCUSSION
The subcellular localization of PLKC depends strongly on the differentiation state of epithelial cells as it redistributes from a predominant intracellular localization in non-polarized cells to sites of cell-cell contacts during polarization. In addition to this localization, a substantial proportion of PLKC is also located at the apical domain of polarized MDCK cells, rendering PLKC unique among other cadherins (39, 40). The apical localization is presumably mediated by a putative PDZ binding domain that is present in the cytosolic tail of PLKC. Importantly, the subcellular distribution of PLKC is linked to its association with DRMs during differentiation of MDCK and acquisition of polarized morphology. Thus, in non-confluent or subconfluent cells PLKC accumulates mostly intracellularly and is not associated with DRMs. On the other hand, a major proportion of the protein becomes associated with DRMs and is trafficked to the apical membrane when the cells acquire a polarized morphology. Our data strongly suggest that the putative mechanism responsible for this altered mode of association depends on the pattern of O-glycosylation of PLKC during differentiation. In fact, O-glycosylation of PLKC slightly increases during differentiation of MDCK cells, and inhibition of this type of glycosylation with BNG results in a complete redistribution of PLKC from DRMs to the Triton X-100-soluble fractions. It is, therefore, likely that O-glycans could act as a target signal that interacts with a potential sorting receptor with subsequent recruitment of PLKC to DRMs. It has been previously shown that a potential sorting mechanism of membrane glycoproteins to the apical membrane utilizes O-glycans as a sorting signal in association with DRMs (34–38). The molecular basis of this putative sorting mechanism is still unresolved. Nevertheless, the discrimination between PLKC molecules in MDCK cells based on their association with DRMs and elevated O-glycans provides an invaluable and promising model system that could be utilized to address this mechanism at the molecular level. Another feature of PLKC that is presumably linked to its association with DRMs is its persistence at the cell surface upon depletion of extracellular calcium and subsequent disruption of cell-cell contacts. This feature clearly distinguishes PLKC from cadherins, which are usually redistributed to submembranous compartments under similar conditions (41). Cadherins are predominantly localized at the sites of cell-cell contacts and are not associated with DRMs. It is tempting to speculate that PLKC clustered into DRMs does not require calcium ions for its stability and retains its cellular localization independent of calcium. The data presented here unequivocally demonstrate that the expression of PLKC at the mRNA level is significantly up-regulated when cell-cell contacts occur and reaches its maximum in polarized monolayers several days after confluence.
Finally, DRMs have been shown to serve as platforms for signaling pathways, and it is possible that DRMs-associated PLKC may trigger downstream signaling cascades that ultimately regulate essential components of the tight junctions, such as claudins and occludins. Pece and Gutkind (42) report that E-cadherin is capable of inducing mitogen-activated protein kinase pathway signaling by a ligand-independent activation of the epidermal growth factor receptor. A correlation between overexpression of E-cadherin and its tight junction barrier function has not been yet established. It is, therefore, tempting to speculate that even the permeability of renal cortical ducts can be modulated by different expression levels of cell-adhesion molecules, not only tight junction proteins, but also members of the cadherin family. Because more evidence is provided that the latter are capable of mediating intracellular signaling pathways, a dynamic and rapid regulation of the tightness of the intercellular barrier upon extracellular signal activation can be proposed. One step further is the assumption that PLKC might serve as a potential receptor by virtue of its localization at the apical membrane and, thus, its exposure to intraluminal signals. Altogether, we conclude that PLKC modulates cell polarity and structural properties of epithelial cells presumably upon induction of adhesive cell-cell contacts. This modulation depends on the interaction of PLKC with DRMs that occurs during cellular differentiation in MDCK cells.
Acknowledgments
We thank Gabi Wetzel and Jürgen Eikemeyer for expert technical assistance.
This work was supported by the Deutsche Forschungsgemeinschaft, Bonn, Germany (SFB 621, Project C8 (to H. Y. N.)) and the Lebanese American University Research Council (URC-s-2009-04 (to S. R.)).
- ZO-1
- zonula occludens protein 1
- BNG
- benzyl-N-acetylgalactosamine
- DMM
- deoxymannojirimycin
- endo H
- endo-N-β-acetylglucosaminidase H
- ER
- endoplasmic reticulum
- MDCK
- Madin-Darby canine kidney
- PLKC
- protocadherin liver, kidney, colon
- PLKCh
- high mannose glycoform
- PLKCC
- complex glycosylated, mature form
- PNGase F
- peptide N-glycanase F
- YFP and GFP
- yellow and green fluorescent proteins, respectively
- DRM
- detergent-resistant membrane
- PBS
- phosphate-buffered saline.
REFERENCES
- 1.Okazaki N., Takahashi N., Kojima S., Masuho Y., Koga H. (2002) Carcinogenesis 23, 1139–1148 [DOI] [PubMed] [Google Scholar]
- 2.Ose R., Yanagawa T., Ikeda S., Ohara O., Koga H. (2009) Mol. Oncol. 3, 54–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson W. J. (2003) Nature 422, 766–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuzmenko Y. S., Kern F., Bochkov V. N., Tkachuk V. A., Resink T. J. (1998) FEBS Lett. 434, 183–187 [DOI] [PubMed] [Google Scholar]
- 5.Furuse M., Hirase T., Itoh M., Nagafuchi A., Yonemura S., Tsukita S., Tsukita S. (1993) J. Cell Biol. 123, 1777–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yap A. S., Brieher W. M., Pruschy M., Gumbiner B. M. (1997) Curr. Biol. 7, 308–315 [DOI] [PubMed] [Google Scholar]
- 7.Gory-Fauré S., Prandini M. H., Pointu H., Roullot V., Pignot-Paintrand I., Vernet M., Huber P. (1999) Development 126, 2093–2102 [DOI] [PubMed] [Google Scholar]
- 8.Dejana E., Bazzoni G., Lampugnani M. G. (1999) Exp. Cell Res. 252, 13–19 [DOI] [PubMed] [Google Scholar]
- 9.Carmeliet P., Lampugnani M. G., Moons L., Breviario F., Compernolle V., Bono F., Balconi G., Spagnuolo R., Oosthuyse B., Dewerchin M., Zanetti A., Angellilo A., Mattot V., Nuyens D., Lutgens E., Clotman F., de Ruiter M. C., Gittenberger-de Groot A., Poelmann R., Lupu F., Herbert J. M., Collen D., Dejana E. (1999) Cell 98, 147–157 [DOI] [PubMed] [Google Scholar]
- 10.Takeichi M. (1991) Science 251, 1451–1455 [DOI] [PubMed] [Google Scholar]
- 11.Nelson W. J. (2008) Biochem. Soc. Trans. 36, 149–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasioukhin V., Bauer C., Yin M., Fuchs E. (2000) Cell 100, 209–219 [DOI] [PubMed] [Google Scholar]
- 13.Nollet F., Kools P., van Roy F. (2000) J. Mol. Biol. 299, 551–572 [DOI] [PubMed] [Google Scholar]
- 14.Wheelock M. J., Johnson K. R. (2003) Curr. Opin. Cell Biol. 15, 509–514 [DOI] [PubMed] [Google Scholar]
- 15.Morishita H., Yagi T. (2007) Curr. Opin. Cell Biol. 19, 584–592 [DOI] [PubMed] [Google Scholar]
- 16.Sano K., Tanihara H., Heimark R. L., Obata S., Davidson M., St John T., Taketani S., Suzuki S. (1993) EMBO J. 12, 2249–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirayama T., Yagi T. (2006) Curr. Opin. Neurobiol. 16, 336–342 [DOI] [PubMed] [Google Scholar]
- 18.Kohmura N., Senzaki K., Hamada S., Kai N., Yasuda R., Watanabe M., Ishii H., Yasuda M., Mishina M., Yagi T. (1998) Neuron 20, 1137–1151 [DOI] [PubMed] [Google Scholar]
- 19.Goldberg M., Peshkovsky C., Shifteh A., Al-Awqati Q. (2000) J. Biol. Chem. 275, 24622–24629 [DOI] [PubMed] [Google Scholar]
- 20.Telo' P., Breviario F., Huber P., Panzeri C., Dejana E. (1998) J. Biol. Chem. 273, 17565–17572 [DOI] [PubMed] [Google Scholar]
- 21.Mahoney P. A., Weber U., Onofrechuk P., Biessmann H., Bryant P. J., Goodman C. S. (1991) Cell 67, 853–868 [DOI] [PubMed] [Google Scholar]
- 22.Bradley R. S., Espeseth A., Kintner C. (1998) Curr. Biol. 8, 325–334 [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto A., Amacher S. L., Kim S. H., Geissert D., Kimmel C. B., De Robertis E. M. (1998) Development 125, 3389–3397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirano S., Yan Q., Suzuki S. T. (1999) J. Neurosci. 19, 995–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Senzaki K., Ogawa M., Yagi T. (1999) Cell 99, 635–647 [DOI] [PubMed] [Google Scholar]
- 26.Sambrook J., William D. (2001) Molecular Cloning: A Laboratory Manual, 3rd Ed., Cold Spring Harbor Laboratory, Cold Spring Harbor, New York [Google Scholar]
- 27.Naim H. Y., Sterchi E. E., Lentze M. J. (1987) Biochem. J. 241, 427–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castelletti D., Alfalah M., Heine M., Hein Z., Schmitte R., Fracasso G., Colombatti M., Naim H. Y. (2008) Biochem. J. 409, 149–157 [DOI] [PubMed] [Google Scholar]
- 29.Stevenson B. R., Siliciano J. D., Mooseker M. S., Goodenough D. A. (1986) J. Cell Biol. 103, 755–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volberg T., Geiger B., Kartenbeck J., Franke W. W. (1986) J. Cell Biol. 102, 1832–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeda H. (2004) Biochem. Biophys. Res. Commun. 316, 822–826 [DOI] [PubMed] [Google Scholar]
- 32.Schuck S., Simons K. (2004) J. Cell Sci. 117, 5955–5964 [DOI] [PubMed] [Google Scholar]
- 33.Lindner R., Naim H. Y. (2009) Exp. Cell Res. 315, 2871–2878 [DOI] [PubMed] [Google Scholar]
- 34.Alfalah M., Jacob R., Preuss U., Zimmer K. P., Naim H., Naim H. Y. (1999) Curr. Biol. 9, 593–596 [DOI] [PubMed] [Google Scholar]
- 35.Jacob R., Alfalah M., Grünberg J., Obendorf M., Naim H. Y. (2000) J. Biol. Chem. 275, 6566–6572 [DOI] [PubMed] [Google Scholar]
- 36.Alfalah M., Jacob R., Naim H. Y. (2002) J. Biol. Chem. 277, 10683–10690 [DOI] [PubMed] [Google Scholar]
- 37.Breuza L., Garcia M., Delgrossi M. H., Le Bivic A. (2002) Exp. Cell Res. 273, 178–186 [DOI] [PubMed] [Google Scholar]
- 38.Proszynski T. J., Simons K., Bagnat M. (2004) Mol. Biol. Cell 15, 1533–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shore E. M., Nelson W. J. (1991) J. Biol. Chem. 266, 19672–19680 [PubMed] [Google Scholar]
- 40.Adams C. L., Nelson W. J., Smith S. J. (1996) J. Cell Biol. 135, 1899–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leve F., de Souza W., Morgado-Díaz J. A. (2008) J. Pharmacol. Exp. Ther. 327, 777–788 [DOI] [PubMed] [Google Scholar]
- 42.Pece S., Gutkind J. S. (2000) J. Biol. Chem. 275, 41227–41233 [DOI] [PubMed] [Google Scholar]