Abstract
Conversion to glycogen is a major fate of ingested glucose in the body. A rate-limiting enzyme in the synthesis of glycogen is glycogen synthase encoded by two genes, GYS1, expressed in muscle and other tissues, and GYS2, primarily expressed in liver (liver glycogen synthase). Defects in GYS2 cause the inherited monogenic disease glycogen storage disease 0. We have generated mice with a liver-specific disruption of the Gys2 gene (liver glycogen synthase knock-out (LGSKO) mice), using Lox-P/Cre technology. Conditional mice carrying floxed Gys2 were crossed with mice expressing Cre recombinase under the albumin promoter. The resulting LGSKO mice are viable, develop liver glycogen synthase deficiency, and have a 95% reduction in fed liver glycogen content. They have mild hypoglycemia but dispose glucose less well in a glucose tolerance test. Fed, LGSKO mice also have a reduced capacity for exhaustive exercise compared with mice carrying floxed alleles, but the difference disappears after an overnight fast. Upon fasting, LGSKO mice reach within 4 h decreased blood glucose levels attained by control floxed mice only after 24 h of food deprivation. The LGSKO mice maintain this low blood glucose for at least 24 h. Basal gluconeogenesis is increased in LGSKO mice, and insulin suppression of endogenous glucose production is impaired as assessed by euglycemic-hyperinsulinemic clamp. This observation correlates with an increase in the liver gluconeogenic enzyme phosphoenolpyruvate carboxykinase expression and activity. This mouse model mimics the pathophysiology of glycogen storage disease 0 patients and highlights the importance of liver glycogen stores in whole body glucose homeostasis.
Keywords: Gene Knock-out, Gluconeogenesis, Glycogen, Glycogen Storage Disease, Glycogen Synthase, GYS2
Introduction
After ingestion of a meal, glucose is cleared from the bloodstream primarily by conversion to glycogen in skeletal muscle and liver. Muscle is often considered the major site of insulin-stimulated glucose disposal, especially in humans, accounting for as much as 70–90% of the total body glucose uptake in some studies (1). However, the liver also contributes significantly to glucose disposal (2), and when glucose is given via the oral route, the liver may dispose of as much as one-third of the glucose load (3, 4). A key glycogen biosynthetic enzyme is glycogen synthase (GS),2 which is controlled by glucose-6-phosphate, an allosteric activator, and by phosphorylation, which inactivates the enzyme (5). There are two GS isoforms in mammals encoded by separate genes. GYS1, encoding the muscle isoform (MGS), is expressed in muscle and many other tissues including kidney, heart, and brain, whereas GYS2, encoding the liver isoform (liver glycogen synthase (LGS)), is known to date to be expressed only in liver. To assess the significance of muscle glycogen stores for overall glucose homeostasis, the Gys1 gene was disrupted in a mouse model, MGSKO mice (6, 7). These mice are totally unable to synthesize muscle glycogen, but surprisingly glucose tolerance was actually improved (8, 9). Moreover, MGSKO mice were no different from wild type littermates in their ability to perform exhaustive exercise. One conclusion from studies of this (9) and other genetically engineered murine models (10, 11) was that in rodents, muscle glycogen represents a much smaller fraction of the total body glycogen stores than in humans and therefore may be less critical to glucose homeostasis, as long as insulin action is preserved in other tissues (12). Although muscle glycogen serves as a local source of energy, liver glycogen supplies glucose to the bloodstream to avoid hypoglycemia during the early stages of fasting (2–6 h in humans) and is also an important fuel for long term exercise. There is a genetic disorder known as glycogen storage disease 0 (GSD0) caused by loss-of-function mutations in the GYS2 gene, one of few glycogen storage diseases characterized by reduced levels of the polysaccharide (13, 14). The patients have postprandial hyperglycemia, hyperlactatemia, and hyperlipidemia, suggestive of a role for liver glycogen synthesis in normal glucose disposal. During fasting, the patients are susceptible to ketotic hypoglycemia, but protection from more extreme fasting hypoglycemia is likely due to the fact that gluconeogenesis is intact (15). The rate of gluconeogenesis, the main source of liver glucose output upon long term fasting (16), is determined by a number of factors, including the activities of pyruvate carboxylase, phosphoenolpyruvate carboxykinase (PEPCK), fructose-1,6-bisphosphatase, and glucose-6-phophatase that are subjected to short term allosteric and/or covalent control, and long term transcriptional control (16, 17). In particular, expression of gluconeogenic genes is inhibited by insulin and activated by glucagon and glucocorticoids. This hormonal control is mediated by several transcription factors and coactivators, including peroxisome proliferator-activated protein γ coactivator 1α (PGC-1α) (18) and forkhead box O1 transcription factor (FoxO1) (19). Liver PGC-1α expression is rapidly inducible in fasting and is reversed by refeeding (20).
To assess the contribution of liver glycogen to whole body glucose metabolism, we developed a mouse with liver-specific disruption of the Gys2 gene. A floxed allele of Gys2 was inactivated in liver by crossing with animals expressing the CRE recombinase under the control of the albumin promoter. These LGSKO mice are viable, despite a severe decrease in the ability of the liver to accumulate glycogen and display most of the symptoms of GSD0 patients. They are glucose-intolerant and prone to hypoglycemia upon fasting. The animals are hypersensitive to fasting and have enhanced basal gluconeogenesis. Also, when compared with fed controls, the LGSKO mice exhibit an impaired capacity for exercise.
MATERIALS AND METHODS
Generation of Gys2 Liver-specific Knock-out Mice
The Gys2 targeting vector was assembled by PCR-amplified and restriction enzyme-digested fragments from an ES 129Sv mouse BAC clone (Research Genetics. Inc) utilizing three Triple-lox vectors provided by Dr. Richard Premont (Duke University). The final construct contained a 6.2-kb-long recombination arm, comprising the region between introns 2 and 4, upstream of the 5′-most LoxP site; the 1.5-kb floxed region, flanked by two LoxP sites, consisting of the region between introns 4 and 6; the herpes simplex virus thymidine kinase and the phosphoglycerate kinase neomycin phosphotransferase selection marker genes also flanked by loxP sites; the 1.2-kb short recombination arm spanning introns 6 and 7; and the phosphoglycerate kinase diphtheria toxin negative selection marker gene (see Fig. 1A). All of the junctions in the final construct were confirmed by sequencing and restriction enzyme digestion. The NotI-linearized vector was electroporated into TC1 ES cells, and initial selection of ES cells used diphtheria toxin as a negative marker and the neo gene as positive marker. Cells surviving in the presence of G418 were screened by PCR for targeted integration. Positive clones were confirmed by additional PCR and Southern analyses to contain the three LoxP sites and to be correctly targeted at both the 5′- and 3′-ends. A phosphoglycerate kinase-NLS/Cre plasmid was electroporated into two independent clones, A7 and D11, and the cells were grown in the presence of ganciclovir, which selects against the presence of thymidine kinase. Therefore surviving cells would be either complete knock-out, i.e. the region between the far left and far right LoxP sites deleted, or conditional, i.e. removal of the marker genes with retention of two loxP sites flanking the floxed region of the gene (see Fig. 1A). Screening with appropriate oligonucleotides identified a large number of complete and conditional knock-out cells from both original independent clones. After karyotyping, two independent conditional clones were injected into C57Bl/6J blastocysts for generation of chimeric mice. High percentage male chimeric mice, as judged by the agouti coat color, were mated with C57Bl/6J mice to determine germ line transmission. F1 mice were backcrossed two more times into the C57Bl/6J background before breeding with albumin-Cre mice (Jackson Laboratory) to delete the Gys2 region flanked by the LoxP sites. Gys2Lox/+/Cre were then crossed with Gys2Lox/Lox to generate animals used in the study. Gys2Lox/+/Cre mice were also crossed with C57Bl/6J to generate Cre and wild type animals to be used as controls. Gys2Lox/+ were also crossed for an additional three generations into the C57Bl/6J background, and some experiments were repeated with animals that were backcrossed for a total of six times. Deletion of exons 5 and 6 was confirmed by PCR with a pair of primers that straddle the residual loxP site. The absence of protein was confirmed by Western blotting analyses (see Fig. 1C).
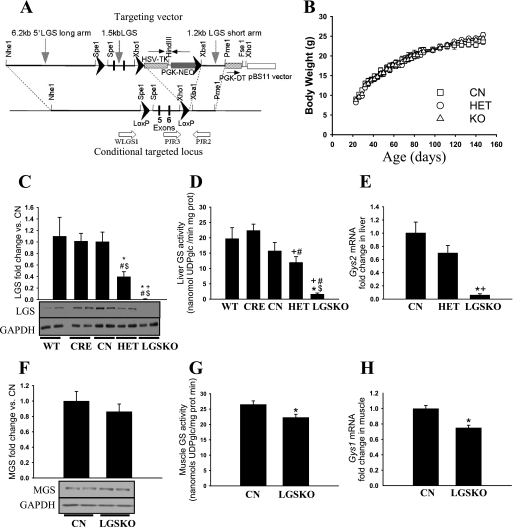
FIGURE 1.
Gene targeting and basic characterization of genetically modified mice. A, partial map of the targeting vector and Gys2 locus containing the LoxP sites flanking the exons 5 and 6 (LGSLox) introduced in ES cells by homologous recombination. ES cells containing this modified locus were used to create the transgenic animals. Crossing LGS(Lox/Lox) mice with alb-CRE(+/−) mice would excise exons 5 and 6 from the two alleles of Gys2 gene via the action of the CRE recombinase, creating a Gys2 knock-out mouse. B, growth rates of CN (squares), HET (circles), and LGSKO (triangles) male mice (n = 22, 21, and 30, respectively). The mice were weighed weekly, at 8–9 a.m. under random fed conditions from 20 to 150 weeks after birth. There were no statistical differences. C and F, fold change, compared with CN mice, of liver GS protein in liver (C) and muscle GS in muscle (F), normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as judged by Western blotting (n = 8/genotype). The lower panels show representative examples of the LGS, MGS, and glyceraldehyde-3-phosphate dehydrogenase Western blots (two for each genotype). D and G, total glycogen synthase activity measured in the presence of 7.2 mm glucose-6-P in liver (D) and skeletal muscle (G) total homogenates (n = 8/genotype). E and H, fold change in Gys2 mRNA content in liver (n = 8 per genotype) (E) and Gys1 mRNA in muscle (n = 4 per genotype) (H) compared with CN mice measured with real time PCR. All of the measurements were for 4-month-old male mice. The data are expressed as the means ± S.E. *, p < 0.05 versus CN; #, p < 0.05 versus wild type (WT); $, p < 0.05 versus CRE; +, p < 0.05 versus HET.
The Gys2 conditional mice were generated by Cheryl Bock (Comprehensive Cancer Center Transgenic Facility, Duke University, Durham, NC). All of the mice were maintained in temperature- and humidity-controlled conditions with a 12:12 h light-dark cycle and were allowed food and water ad libitum. The standard chow was composed of a mixture of 19% protein, 9% fat, and 55% carbohydrate (Harlan Teklad global diet #2019S). All of the mouse studies were conducted in 4-month-old males, unless otherwise indicated. All of the studies were performed in accordance with federal guidelines and were approved by the Institutional Animal Use and Care Committees of Indiana and Duke Universities.
Exercise Protocol
Exercise to exhaustion studies were performed in 7-month-old male mice on a treadmill (Exer6M; Columbus Instruments) following the procedure described previously (8) with minor modifications. Immediately after exhaustion, the mice were sacrificed by cervical dislocation, and the tissues immediately collected in liquid nitrogen and stored at −80 °C for further analyses.
Food Consumption
Food consumption was measured in 3–4-month-old male mice for 5 days. Total food consumed during a 24-h period as well as daytime consumption was calculated.
Glucose and Insulin Tolerance Tests, Fasting, and Blood Metabolite and Hormone Measurements
Glucose tolerance tests (GTT) were performed after an overnight fast as previously described (9) by intraperitoneal injection or by oral administration of 2 mg glucose·g−1 body weight utilizing a Breeze2 glucometer (Bayer). Blood lactate was measured before and after 20 min of intraperitoneal GTT, with a LactatePro test meter (Arkay). For insulin tolerance tests, male mice were fasted for 6 h before intraperitoneal administration of 0.75 unit/kg of body weight of insulin. To monitor the effects of fasting, blood glucose was measured in male mice before and during 24 h of food withdrawal. All of the determinations for random fed conditions were taken between 8 and 10 a.m. Blood ketone bodies (β-hydroxybutyrate), serum glycerol, triacylglycerides, and serum nonesterified fatty acids were measured at the indicated times using a CardioCheck ST analyzer (CardioCheck), a triglyceride determination kit (#TR0100; Sigma), and a nonesterified fatty acid HR kit (Wako), respectively. Insulin, glucagon, adiponectin, resistin, and leptin were measured in plasma samples by the Vanderbilt University School of Medicine Mouse Metabolic Phenotyping Center using the corresponding hormone radioimmunoassay kit.
Preparation of Samples for Biochemical Analyses
Four-month old male mice were weighed and sacrificed by cervical dislocation under random fed conditions, overnight fasting, or 6 h fasting. The tissues were immediately frozen in liquid nitrogen and stored at −80 °C for further analyses. Glycogen synthase was assessed in total homogenates (9) by monitoring the incorporation of glucose from UDP-[U-14C]glucose into glycogen as previously described (21). PEPCK activity was assayed as reported (22). Glycogen content in tissues was determined by measuring amyloglucosidase-released glucose from glycogen as previously described (9). Liver triglycerides were measured enzymatically using a triglyceride and free glycerol determination kit (Sigma) following the manufacturer's instructions.
Western Blotting
Total tissue homogenates used for glycogen synthase activity determination were also analyzed by Western blotting for the presence of muscle (anti-MGS; Novus Biologicals NB110–57010) and liver (anti-LGS, kindly provided by Dr. J. Brozinick, Eli Lilly Co.) glycogen synthase. Tissue homogenates prepared as described in Ref. 23 were used for immunoblots with the antibodies anti-FoxO1; anti-FoxO1-P (Thr24); anti-AMPKα; anti-AMPKα-P (Thr172) (Cell Signaling antibodies 9454, 9464, 2532, and 2535, respectively) and anti-glyceraldehyde-3-phosphate dehydrogenase (Biodesign H86504M). The immunoblots were performed as described in Ref. 9.
Tissue Staining and Immunohistochemistry
The liver sections were prepared from 4-month-old mice, fixed in 10% formalin, and embedded in paraffin. Slices of 5 μm were deparaffinized, oxidized with 0.5% periodic acid for 5 min, stained with Schiff reagent for 15 min, and then counterstained in hematoxylin and eosin for 15 min. Negative controls without periodic acid ensured glycogen staining specificity. Deparaffinized and rehydrated sections were also subjected to CRE recombinase immunohistochemistry following then manufacturer's instructions (Abcam 24608).
RNA Isolation and Quantitative PCR
RNA was isolated from 100 mg of tissue with TRIzol reagent (Invitrogen). Four μg of RNA were subjected to reverse transcription using the Superscript III-RT kit (Invitrogen). Primers and probes for the different genes were designed using the Universal probe library tool from Roche Applied Science. Information on the amplicons used will be available upon request. The real time PCRs were performed using the LightCycler 480 Probes Master reagent (Roche Applied Science). The fold change in the target mRNA contents with respect to the base line and normalized by the reference gene (18 S) was calculated as described (24) using a relative standard curve.
Euglycemic-Hyperinsulinemic Clamp and Body Composition
A euglycemic-hyperinsulinemic (2 milliunits·kg−1·min−1 insulin) clamp was performed in 4-month-old 5-h fasted chronically catheterized conscious mice at the Vanderbilt University School of Medicine Mouse Metabolic Phenotyping Center as described previously (25). Carotid artery and jugular vein catheters were implanted for blood sampling and infusing, respectively, 5 days prior to the study. Only mice that returned to within 10% of pre-surgical body weight were studied. Whole body glucose flux and tissue-specific glucose uptake were assessed using [3-3H] glucose and [2-14C]deoxyglucose, respectively, as described in Ref. 26. Gluconeogenesis was estimated during the study by the infusion of [3-13C]lactate (25 μmol·kg−1·min−1). Washed red blood cells were infused from donor animals to maintain a constant hematocrit. The percent contribution of gluconeogenesis to total glucose flux (f) was calculated after assessing the enrichment (Mol%) of plasma glucose (27). f = M1/(2p(1 − p)) where p is the labeling in the triose pool (p = 2R/(1 + 2R) and r = M2/M1. Body composition was measured with a mq10 NMR analyzer (Bruker Optics, The Woodlands, TX).
Statistical Analyses
The results were analyzed using Statgraphics plus 5.0 (Statistical Graphics Corporation, Herndon, VA). For each parameter, the kurtosis and skewness were calculated to test the normal distribution of the data. When normality was reached, one-way analysis of variance, followed by the least significant difference post-hoc test, was performed to compare the data.
RESULTS
Generation of LGSKO Mice
To investigate the physiological role of liver glycogen in glucose homeostasis, we generated a mouse line with a hepatocyte-specific disruption of the Gys2 gene that encodes the liver isoform of glycogen synthase. LoxP sites flanked exons 5 and 6 of the gene (Fig. 1A), which code for amino acids 227–314 that are in the conserved region of the catalytic domain. Gys2(Lox/+) mice were bred with transgenic mice that express the CRE recombinase under control of the rat albumin promoter (alb-CRE) (28), and various intercrosses were made as described under “Materials and Methods” to generate the experimental animals. Deletion of exons 5 and 6 would cause a frameshift that produces a premature termination codon 4 amino acids into exon 7. This should result in mRNA degradation by non-sense-mediated mRNA decay (29). Furthermore, it is known that a premature stop codon in exon 5, mimicking a GSD0 mutation (13), leads to no activity when expressed in COS cells. The animals carrying the homozygous deletion, LGSKO mice, were viable, were born at the expected frequency for Mendelian inheritance, and were indistinguishable from their littermates of different genotypes.
Glycogen Synthase Expression and Activity in Liver and Other Tissues
Western blotting with antibodies specific for the liver glycogen synthase isoform showed that the protein is essentially absent in the liver of LGSKO mice (Fig. 1C), although overexposure revealed a faint signal in some samples (not shown). Heterozygous (HET) mice had ∼40% of the control levels. These results were consistent with the Gys2 transcript level analyzed by real time PCR (Fig. 1E) and measurements of liver glycogen synthase activity, although it is notable that a low residual activity, ∼10% of control, was detected in LGSKO liver (Fig. 1D). Analysis of glycogen synthase activity in skeletal muscle indicated an ∼15% decrease (Fig. 1G) that correlated with a similar decrease in Gys1 transcript (Fig. 1H) and protein, even though it did not reach statistical significance (Fig. 1F). Comparable results (not shown) were obtained with mice that had been back-crossed into the C57Bl/6J background (see under “Materials and Methods”). However, the main conclusion is that a severe disruption of liver glycogen synthetic capacity did not evoke a major alteration in the glycogen synthetic pathway of muscle.
Glycogen Accumulation in Liver and Other Tissues
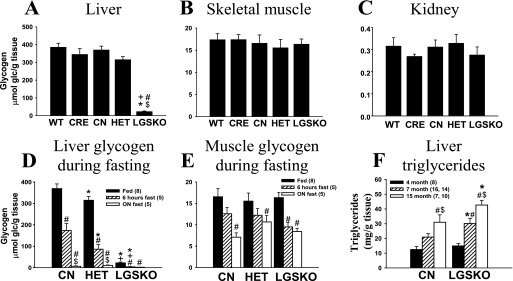
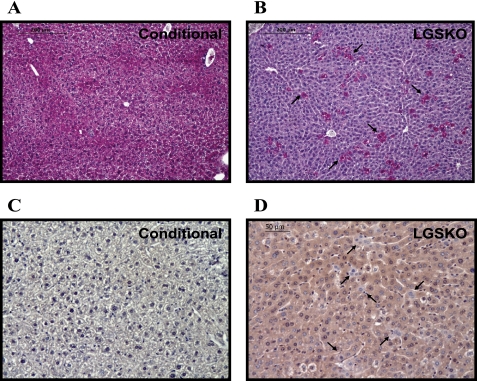
Under random fed conditions, there was a 94% reduction in the liver glycogen content of LGSKO mice (Fig. 2A). Interestingly, the reduced liver glycogen synthase in the HET mice led to no significant reduction in the liver glycogen content (Fig. 2A). Under the same nutritional conditions, periodic acid Schiff staining of the liver sections showed that Gys2lox/lox (CN) hepatocytes contained glycogen (Fig. 3A), as indicated by the pink color, but only ∼7% of the LGSKO hepatocytes were positive for glycogen (Fig. 3B). Analysis of CRE recombinase by immunostaining revealed that CN livers, as expected, lack CRE recombinase (Fig. 3C), but most cells of the LGSKO liver sections were stained (Fig. 3D). However, a small number of hepatocytes in LGSKO liver were negative. Glycogen content in skeletal muscle and kidney (Fig. 2, B and C, respectively), along with other tissues such as heart, epididymal fat, or brain (not shown), presented no substantial differences among genotypes under random fed conditions. Overall, genetic depletion of liver glycogen did not elicit major compensatory glycogen accumulation in the other tissues analyzed. Upon fasting, CN mice3 lost about 50% of their starting hepatic glycogen stores after 6 h and were essentially depleted of glycogen overnight (Fig. 2D). In contrast, LGSKO liver glycogen was totally depleted by 6 h. Muscle glycogen was also diminished up to ∼50% because of overnight fasting, as has been shown in some other studies (30, 31), but there was no major difference between genotypes (Fig. 2E).
FIGURE 2.
Glycogen and triglyceride content in tissues of LGSKO mice. A–C, glycogen content measured in liver (A), skeletal muscle (B), and kidney (C) (n = 8/genotype) under fed conditions of 4-month-old mice. The data are expressed as the means ± S.E. *, p < 0.05 versus CN; #, p < 0.05 versus wild type (WT); $, p < 0.05 versus CRE; +, p < 0.05 versus HET. D and E, glycogen content under random fed (black bars), 6-h fasted (hatched bars), and overnight fasted (white bars) in liver (D) and skeletal muscle (E) of 4-month old mice. *, p < 0.05 versus CN; +, p < 0.05 versus HET; #, p < 0.05 versus fed condition; $, p < 0.05 versus 6 h fasting. F, triglyceride content in liver under random fed conditions of 4-month-old (black bars, n = 8), 7-month-old (hatched bars, n = 14 for CN and n = 16 for LGSKO) and 15-month-old (white bars, n = 8 for CN and n = 10 for LGSKO) mice. *, p < 0.05 versus CN; #, p < 0.05 versus 4-month-old mice; $, p < 0.05 versus 7-month-old mice. The numbers in parentheses indicate the n for the given group. The data are expressed as the means ± S.E.
FIGURE 3.
Glycogen in CN and LGSKO mouse liver sections. A and B, periodic acid-Schiff reagent staining of paraffin-embedded liver sections from random fed CN (A) and LGSKO (B) mice. The sections were counterstained for hematoxylin and eosin. All of the hepatocytes were stained pink in the liver sections from CN. Conversely, LGSKO mice sections only a small amount of cells (about 7%) were stained. The arrows point to those hepatocytes containing glycogen in the LGSKO mice sections. C and D, immunohistochemistry of paraffin-embedded liver sections of CN (C) and LGSKO (D) mice for CRE recombinase and counterstained with hematoxylin. CN mice sections were negative for the staining, whereas LGSKO mice sections were positively stained light brown in almost all the cells. However, some cells, marked by arrows, were not stained, indicating a lack of CRE recombinase protein.
Body Composition and Blood Metabolites
The body weight of LGSKO mice was no different from that of CN or HET littermates, from weaning till adulthood (Fig. 1B), consistent with no changes in either total daily food consumption or food consumption during the light period (Table 1). There was also no alteration in overall body composition as judged by nuclear magnetic imaging, the only statistically significant differences being in liver and heart weights (Table 1). The lower absolute heart weight correlated with a significant lowering of diastolic blood pressure in LGSKO mice. Reduction in liver size has been associated with fasting of normal mice (32) and rats (31), and liver mass is recovered upon refeeding in CN mice. This phenomenon has been attributed to changes in glycogen levels and importantly the associated water volume (33), which would explain the lower liver weight in the LGSKO mice. Three-month-old LGSKO mice had a tendency toward higher liver triglyceride content as compared with CN mice, but at seven and fifteen months there was a statistically significant increase in liver fat (Fig. 2F). The circulating levels of a number of metabolites and hormones were also analyzed in fed and fasted animals (Table 2). Blood glucose was lower in LGSKO mice under fed or fasted conditions. Lactate levels were invariant between genotypes. Fasting led to expected increases in ketone bodies, glycerol, and nonesterified fatty acids in both mouse lines. However, the LGSKO mice responded more acutely, with increases apparent already at 6 h. Triglycerides decreased after 6 h of fasting and were elevated overnight, but there were no differences in the LGSKO mice. Under fed conditions, LGSKO mice had significantly lower serum insulin. Overnight fasting reduced insulin levels in both genotypes, with the level in the LGSKO mice still trending lower (p = 0.087). Glucagon, leptin, adiponectin, and resistin levels were no different between genotypes.
TABLE 1.
Body composition and food consumption of CN and LGSKO mice
Tissue weight ratios are the ratio between wet weight in mg taken immediately prior to freezing in liquid nitrogen and the body weight prior to sacrificing. n = 6–8 for all measurements. Daily food consumption corresponded to a 24-h period (from 8 a.m. to 8 a.m. of the next day). The results are reported as the means ± S.E.
| Parameter | CN | LGSKO |
|---|---|---|
| Body weight (g) | 24.9 ± 0.8 | 22.8 ± 0.8 |
| Total adipose mass (g) | 2.76 ± 0.33 | 2.45 ± 0.19 |
| Total lean mass (g) | 23.9 ± 0.7 | 24.3 ± 0.7 |
| Free fluid (g) | 0.62 ± 0.04 | 0.66 ± 0.06 |
| Liver/body weight ratio (mg/g) | 45 ± 2 | 34 ± 2a |
| Liver weight (mg) | 1475 ± 87 | 1060 ± 71a |
| Epididimal fat/body weight ratio (mg/g) | 31 ± 5 | 34 ± 4 |
| Epididimal fat pads weight (mg) | 1025 ± 206 | 1083 ± 155 |
| Heart/body weight ratio (mg/g) | 4.30 ± 0.16 | 3.71 ± 0.24 |
| Heart weight (mg) | 139 ± 2 | 115 ± 7a |
| Systolic blood pressure (mm/Hg) | 93 ± 1 | 87 ± 3 |
| Diastolic blood pressure (mm/Hg) | 68 ± 4 | 56 ± 4a |
| Daily food consumption (kCal/g of body weight/day) | 0.48 ± 0.01 | 0.45 ± 0.02 |
| Food consumption (8 a.m. to 6 p.m.) (kCal/g of body weight/day) | 0.081 ± 0.010 | 0.069 ± 0.008 |
a p < 0.05 vs. conditional mice.
TABLE 2.
Blood parameters of CN and LGSKO mice under different feeding conditions
All of the results are from 4-month-old males or from 7-month-old mice for the leptin, adiponectin, and resistin hormone levels. β-Hydroxybutyrate levels were measured as total ketone bodies. The numbers in parentheses indicate the n values for the given groups. The results are expressed as the means ± S.E. ND, not determined.
| Conditional |
LGSKO |
|||||
|---|---|---|---|---|---|---|
| Fed | 6-h fast | Overnight fast | Fed | 6-h fast | Overnight fast | |
| Glucose (mg/dl) | 123.6 ± 4.5 (8) | 134.5 ± 10.7 (8) | 93.2 ± 6.1 (8)a,b | 92.0 ± 4.5 (8)c | 70.6 ± 5.0 (8)a,c | 74.2 ± 5.8 (8)a,c |
| Lactate (mm) | 2.83 ± 0.31 (7) | 3.16 ± 0.17 (5) | 2.04 ± 0.20 (5)b | 2.42 ± 0.22 (12) | 2.50 ± 0.13 (5) | 2.54 ± 0.36 (5) |
| Ketone bodies (mg/dl) | 5.77 ± 0.48 (15) | 4.53 ± 0.76 (6) | 17.9 ± 1.6 (13)a | 6.84 ± 0.46 (14) | 7.43 ± 0.94 (7) | 24.4 ± 1.6 (14)a,c |
| Glycerol (mg/dl) | 4.76 ± 0.47 (8) | 5.18 ± 0.80 (5) | 7.53 ± 0.58 (5)a,b | 5.70 ± 0.39 (10) | 7.31 ± 0.61 (5)a,c | 7.78 ± 0.39 (5)a |
| Nonesterified fatty acids (mm) | 0.86 ± 0.08 (7) | 1.02 ± 0.09 (10) | 1.86 ± 0.06 (11)a,b | 1.04 ± 0.07 (12) | 1.60 ± 0.14 (13)a,c | 1.82 ± 0.10 (17)a |
| Triglycerides (mg/dl) | 75.0 ± 11.9 (8) | 22.0 ± 4.2 (5)a | 94.9 ± 17.4 (5)b | 65.3 ± 4.5 (10) | 36.5 ± 7.3 (5)a | 106.3 ± 24.3 (5)a,b |
| Insulin (ng/ml) | 1.59 ± 0.29 (6) | ND | 0.56 ± 0.18 (8)a | 0.73 ± 0.15 (7)c | ND | 0.21 ± 0.04 (8)a |
| Glucagon (pg/ml) | 97.4 ± 6.0 (8) | ND | 77.6 ± 10.8 (8) | 102.2 ± 8.4 (10) | ND | 76.0 ± 10.3 (8) |
| Leptin (ng/ml) | ND | ND | 7.34 ± 3.71 (8) | ND | ND | 7.77 ± 3.26 (8) |
| Adiponectin (μg/ml) | ND | ND | 18.1 ± 2.1 (8) | ND | ND | 15.0 ± 2.1 (8) |
| Resistin (pg/ml) | ND | ND | 677 ± 45 (8) | ND | ND | 519 ± 32 (8) |
a p < 0.05 vs. fed conditions.
b p < 0.05 vs. 6-h fasted conditions.
c p < 0.05 vs. conditional mice.
Impaired Liver Glycogen Accumulation in LGSKO Mice Is Associated with Glucose Intolerance and Increased Sensitivity to Fasting
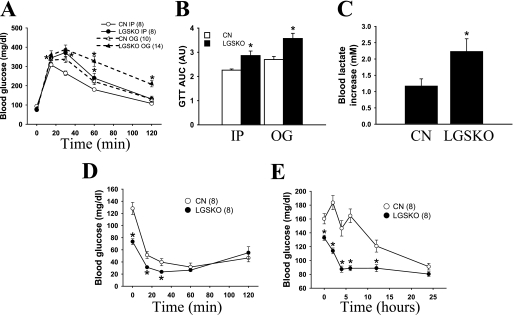
LGSKO mice displayed glucose intolerance as judged by either intraperitoneal or oral GTT (Fig. 4A), correlating with a 30% higher area under the curve of the GTT (Fig. 4B). Blood glucose elevation was more prolonged after oral administration, consistent with a more prominent role for the liver in disposing of glucose administered orally and hence delivered via the portal vein. Blood lactate increased significantly at 20 min during the GTT in the LGSKO mice (Fig. 4C). Intraperitoneal insulin administration lowered blood glucose in both CN and LGSKO mice, reaching similar absolute values after 60 min (Fig. 4D). However, insulin was injected after 6 h of fasting, when the LGSKO mice already have significantly lower blood glucose compared with the CN controls (73 ± 5 versus 128 ± 10 mg/dl), complicating the comparison of the insulin tolerance test with control mice. Similar results for intraperitoneal GTT and insulin tolerance tests were obtained with mice that had been backcrossed into C57Bl/6J (not shown).
FIGURE 4.
Glucose and insulin tolerance tests and time course of fasting in LGSKO mice. A, GTTs were performed on CN (open symbols) and LGSKO (filled symbols) mice. Glucose (2 mg/g of body weight) was administrated by intraperitoneal injection (circles) or oral gavage (triangles) after overnight (15 h) fasting. B, area under the curve (AUC) of the intraperitoneal injection (IP) or oral gavage (OG) GTT displayed A, expressed as arbitrary units. *, p < 0.05 versus CN with same glucose administration. C, increase in blood lactate (mm) 20 min after intraperitoneal injection of glucose in the GTT of A. D, insulin tolerance test displaying the blood glucose levels of CN (open circles) and LGSKO (filled circles) mice. E, long term fasting of CN (open symbols) and LGSKO (filled symbols) mice. Food was withdrawn between 8 and 9 a.m. Blood glucose levels were measured immediately before and at 2, 4, 6, 12, and 24 h after removing the food. The numbers in parentheses indicates the n for the given group. The data are expressed as the means ± S.E. *, p < 0.05 versus CN.
Because the LGSKO mice had generally lower blood glucose (Table 2), we systematically monitored this parameter over a 24-h period after food deprivation (Fig. 4E). After only 4 h of fasting, the blood glucose level of the LGSKO mice dropped to a low value that was maintained for the rest of the fasting period. The control animals reached similar low blood glucose only after 24 h of fasting. Thus, the ability to maintain blood glucose concentration correlates with the starting liver glycogen stores, and the LGSKO mice are more prone to enter into a fasted condition.
Fed LGSKO Mice Have Reduced Exercise Capacity
To determine the role of liver glycogen in exercise performance, LGSKO mice were exercised to exhaustion on a treadmill under random fed and overnight fasting conditions (Table 3). The key finding was that fed LGSKO mice became exhausted earlier compared with CN mice when performing high intensity exercise, doing 55% less work. Although the blood glucose was unchanged by exercise in the control mice, its already lower value was further reduced in the LGSKO animals. The exercise-induced increase in lactate was somewhat blunted in the LGSKO mice, likely reflecting the depleted liver glycogen stores. The β-hydroxybutyrate level post-exercise was also higher in LGSKO animals, but there were no statistically significant changes in nonesterified fatty acids. Muscle glycogen was reduced by exercise in both animal groups, but there was significantly greater depletion in the LGKSO animals. When the experiment was repeated with overnight fasted animals, the exercise capacity of the LGSKO mice was unchanged, whereas that of the CN mice was decreased to the level of the knock-out animals. Now both groups had significant reductions in blood glucose because of exercise. Other blood metabolites were essentially indistinguishable between LGSKO and CN mice. Thus, depletion of liver glycogen by fasting control mice to levels comparable with those of LGSKO animals leads to similar exercise capacity, arguing that the liver glycogen level indeed affects endurance.
TABLE 3.
Exercise performance parameters of CN and LGSKO mice under fed and fasting conditions
All of the reported results are from 7–8-month-old males. For fed conditions, n = 7–8, and for overnight fast conditions, n = 8–10 for all parameters, except muscle and liver glycogen under overnight fast rest condition where n = 5. β-Hydroxybutyrate levels were measured as total ketone bodies. Rest is before exercise, and exercise is immediately after completion of exercise to exhaustion. The results are reported as the means ± S.E. NA, nonapplicable.
| Conditional |
LGSKO |
|||||||
|---|---|---|---|---|---|---|---|---|
| Fed |
Overnight fasted |
Fed |
Overnight fasted |
|||||
| Rest | Exercise | Rest | Exercise | Rest | Exercise | Rest | Exercise | |
| Run time (min) | NA | 31.1 ± 3.5 | NA | 21.1 ± 2.1b | NA | 15.8 ± 1.7c | NA | 17.6 ± 1.8 |
| Work (KJ) | NA | 5.1 ± 0.7 | NA | 2.3 ± 0.2b | NA | 2.9 ± 0.3c | NA | 2.4 ± 0.3 |
| Glucose (mg/dl) | 148 ± 6 | 151 ± 12 | 96.4 ± 4.6b | 72.5 ± 7.9a,b | 127 ± 8c | 51.9 ± 3.4a,b,c | 90.0 ± 4.3 | 65.4 ± 6.0a |
| Lactate (mm) | 2.2 ± 0.3 | 6.3 ± 0.7a | 1.2 ± 0.1 | 3.4 ± 0.4a,b | 2.1 ± 0.4 | 4.5 ± 0.6a,c | 1.3 ± 0.1 | 4.0 ± 0.3a |
| Ketone bodies (mg/dl) | 4.9 ± 0.5 | 6.6 ± 0.6 | 12.4 ± 1.4b | 10.9 ± 1.0b | 5.8 ± 0.7 | 9.5 ± 0.9a,c | 13.1 ± 0.9b | 11.4 ± 0.9 |
| Nonesterified fatty acids (mm) | 1.0 ± 0.1 | 1.4 ± 0.1a | 1.9 ± 0.1b | 1.5 ± 0.1a | 0.85 ± 0.11 | 1.5 ± 0.1a | 1.9 ± 0.1b | 1.4 ± 0.1a |
| Muscle glycogen (μmol of glucose/g of tissue) | 16.8 ± 0.9 | 5.2 ± 0.6a | 7.1 ± 1.0b | 2.0 ± 0.8a,b | 15.9 ± 1.0 | 2.4 ± 0.5a,c | 8.4 ± 0.8b | 2.4 ± 0.5a |
| Liver glycogen (μmol of glucose/g of tissue) | 440 ± 63 | 68.5 ± 18.8a | 6.3 ± 2.5b | 1.0 ± 0.1b | 7.5 ± 1.4c | 2.7 ± 0.3c | 2.0 ± 0.4 | 1.3 ± 0.2 |
a p < 0.05 vs. nonexercised conditions.
b p < 0.05 vs. fed conditions.
c p < 0.05 vs. conditional mice.
LGSKO Mice Have Enhanced Gluconeogenesis
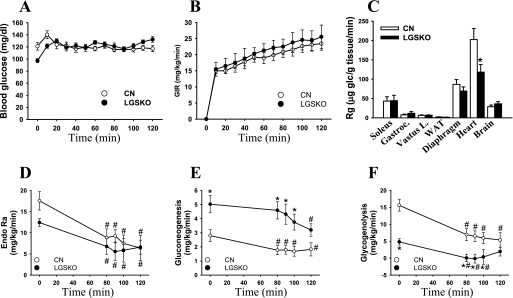
To gain further insight into whole body glucose metabolism, the mice were subjected to a euglycemic-hyperinsulinemic clamp (25). Arterial plasma insulin concentration (ng/ml) increased similarly in CN (0.8 ± 0.1 to 1.7 ± 0.3) and LGSKO mice (0.6 ± 0.1 to 1.6 ± 0.2). The glucose infusion rate (Fig. 5B) to maintain euglycemia (Fig. 5A) during the clamp was no different between CN and LGSKO mice, indicating that there was no gross difference in insulin responsiveness. Consistent with this observation, there was no significant difference in glucose uptake in different tissues, with the notable exception of the heart (Fig. 5C). Heart glucose uptake was lower in LGSKO mice, which is consistent with the lower diastolic blood pressure and the smaller heart size (Table 1). The heart presumably does not need to work as hard in the face of the lower blood pressure. As shown in (Fig. 4E), fasted LGSKO mice maintain constant, but low, blood glucose levels from 4 to 24 h without displaying any symptoms of pathological hypoglycemia such as seizures. We assessed glucose flux using [3-3H]glucose and gluconeogenesis, by assessing the incorporation of [2-13C]lactate into glucose before (−10 min) and during the euglycemic-hyperinsulinemic clamp (0–120 min) (27). After a 5-h fast, LGSKO mice had the elevated basal rate of gluconeogenesis (Fig. 5E), but a lower rate of glycogenolysis (Fig. 5F) correlating with the lack of liver glycogen and the correspondingly only modestly lower total glucose output. During the clamp, endogenous glucose output was decreased to the same extent in both CN and LGSKO mice (Fig. 5D). In CN mice, reduction in glucose output was accounted for a reduction in net gluconeogenesis (Fig. 5E) and an almost complete suppression of glycogenolysis (Fig. 5F). However, suppression of glycogenolysis in LGSKO mice was negligible, and net gluconeogenesis from lactate only began to fall from 80 to 120 min of the clamp. The initial decrease in endogenous glucose production in insulin-infused LGSKO mice without further changes in net gluconeogenesis from lactate may be caused by a decrease in gluconeogenesis from substrates other than lactate, such as glycerol, not accounted for in the present tracer method.
FIGURE 5.
Whole body glucose metabolism during euglycemic-hyperinsulinemic clamp in LGSKO mice. A and B, blood glucose levels (A) and glucose infusion rate (B) before and during a 120-min euglycemic-hyperinsulinemic clamp of CN (open symbols) and LGSKO (filled symbols) mice. There were no statistical differences at any time between genotypes. C, glucose uptake rate (Rg) in soleus, gastrocnemius, vastus lateralis, diaphragm, heart, white adipose tissue, and brain during the euglycemic-hyperinsulinemic clamp. D–F, endogenous glucose appearance (D, Endo Ra, as total glucose appearance minus glucose infusion), net gluconeogenesis rate (E), and glycogenolysis (as endogenous glucose appearance minus net gluconoegenesis) measured as mg of glucose/kg of mouse/min (F). n = 9–10/genotype for all of the parameters. The data are expressed as the means ± S.E. *, p < 0.05 versus CN; #, p < 0.05 versus time 0.
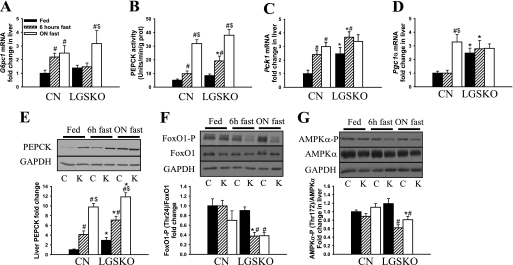
To explore the mechanism of enhanced gluconeogenesis, we analyzed PEPCK and glucose-6-phosphatase (catalytic subunit 1; G6pc1). Expression of the G6pc1 gene was induced in both genotypes but in the LGSKO mice was only observed after overnight fasting (Fig. 6A). The basal fed PEPCK level was elevated, and its induction was more sensitive to fasting in the LGSKO mice. This pattern was evident in Western blots (Fig. 6E), measurements of enzyme activity (Fig. 6B), and real time PCR analysis of gene (PCK1) expression (Fig. 6C). We also analyzed transcription factors involved in PCK1 gene expression (19, 20). PGC-1α transcript was elevated in the livers of fed LGSKO mice and not further induced by fasting, whereas in control animals PGC-1α was only increased upon fasting (Fig. 6D). FoxO1 is regulated by phosphorylation, which leads to inactivation by promoting cytosolic localization (19). FoxO1 phosphorylation (Fig. 6F) was decreased in the LGSKO mice earlier under fasting conditions. Together these results suggest that PEPCK activity and protein content is increased because of enhanced transcriptional activity. The activity state of the AMPK, proposed to be a negative regulator of gluconeogenesis (34, 35), was analyzed using phospho-specific antibodies to Thr172 of the α subunit (Fig. 6G). Fasting up to 18 h had relatively little effect on AMPK phosphorylation in control livers. In the LGSKO animals, AMPK phosphorylation was significantly suppressed during fasting. This result correlates with enhanced FoxO1 and PGC-1α activity during fasting in LGSKO mice compared with CN animals.
FIGURE 6.
Gluconeogenesis in LGSKO mice. A, C, and D, fold change of g6pc1 (A), pck1 (C), and PGC-1α (D) mRNA content in liver measured with real time PCR. The fold change is compared with random fed CN mice. B, PEPCK activity measured in 100 kg of liver supernatants. E–G, change of PEPCK1 protein level normalized by glyceraldehyde-3-phosphate dehydrogenase (n = 8) (E), FoxO1 phosphorylated (Thr24) normalized by total FoxO1 content (F), and phosphorylated (Thr172) AMPKα normalized by total AMPKα content (G) in liver homogenates. The upper panels show representative Western blot data. The measurements in the lower panels were done in liver of fed (black bars), 6-h fasted (hatched bars), and overnight fasted (white bars) mice. n = 8 for fed mice and n = 5 for 6-h and ON fasted mice. The data are expressed as the means ± S.E. *, p < 0.05 versus CN; +, p < 0.05 versus HET; #, p < 0.05 versus fed; $, p < 0.05 versus 6-h fasted.
DISCUSSION
Liver-specific disruption of the Gys2 gene generated viable mice in which liver glycogen storage and glycogen synthase activity are severely impaired. It has been suggested that liver glycogen stores increase during fetal development (36) and are important for neonatal survival until gluconeogenesis is fully established in the newborn liver (37). Other studies, targeting the glucokinase gene for disruption in liver using CRE recombinase under albumin promoter control, showed that CRE expression and targeted gene recombination develop after birth (38). Indeed, our newborn LGSKO pups are less deficient in liver glycogen (33% of control after 1 day, data not shown) than when they reach adulthood (∼5% of control). Therefore, the gradual onset of CRE expression from the albumin promoter may have avoided any harmful effects of lacking liver glycogen stores at birth. Low levels of glycogen were still detectable in the LGSKO liver. There are several possible explanations for this residual glycogen storage. One formal possibility would be compensatory expression of the Gys1 gene in the liver. However, we did not detect muscle glycogen synthase by Western blotting in the livers of LGSKO mice (data not shown). Another possibility is that the glycogen is associated with nonhepatocyte cells of the liver that do not express the albumin gene and that can account for up to 35% of the total cells in the liver (39). However, periodic acid Schiff staining of LGSKO liver sections indicated the presence of glycogen in about 7% of cells that by morphological criteria look like hepatocytes. This observation correlates with a similar proportion of cells that are negative for CRE expression. Postic et al. (28), in their study of liver glucokinase disruption noted above, also did not see 100% recombination of the targeted gene in liver even in mature mice, resulting in some residual glucokinase activity. We favor the explanation that incomplete disruption of the Gys2 gene in hepatocytes accounts for the low levels of glycogen present in the livers of LGSKO mice.
Liver and muscle house the major glycogen stores in mammals, and genetic manipulation of these deposits would be expected to have an impact on whole body glucose metabolism. Disruption of the Gys1 gene in MGSKO mice, in our previous studies (6, 8, 9), totally eliminated glycogen in skeletal muscle and several other tissues and resulted in a complex phenotype. The simplistic expectation that the loss of the muscle glycogen repository would cause glucose intolerance was not realized. However, from our analyses of MGSKO mice, we arrived at the conclusion that skeletal muscle glycogen plays a much lesser role in glucose homeostasis in mice than in humans, as has been suggested previously (40). Furthermore, fed mice contain five to ten times more total glycogen in liver than in skeletal muscle, and the hepatic energy reserve is likely of much greater importance in mice. Our analyses of LGSKO mice support this hypothesis.
In many respects, the LGSKO phenotype resembles that of patients with glycogen storage disease 0 (14), with fasting hypoglycemia, impaired glucose disposal, and elevated lactate during a GTT, and may be a good animal model for the disease. In the basal, random fed state, LGSKO mice have reduced blood insulin and glucose levels compared with controls. When glucose is supplied acutely to the bloodstream, as after a meal or during a GTT, two of its main short term fates in the liver are conversion to glycogen and conversion to lactate by glycolysis. In LGSKO mice, formation of liver glycogen is essentially eliminated and correlates with a reduced ability to dispose of glucose during a GTT. The increased lactate after the GTT would be consistent with an increase in glucose flux through glycolysis, whether in liver or muscle. These results suggest that, in normal mice, liver glycogen synthesis is important for glucose disposal. In the fasted state, insulin levels are reduced, whereas glucagon remains unchanged in our experiments, thereby tipping the hormonal balance to favor glucose production by the liver, acutely by glycogenolysis and in the longer term by gluconeogenesis. Because the LGSKO mice are incapable of liver glycogenolysis, they rely on de novo glucose synthesis. Our interpretation of the metabolic status of the LGSKO mice is that, even when fed, they are already partially programmed toward the fasting state, which they achieve more rapidly than control animals. Many of our observations with LGSKO mice support this hypothesis. Blood glucose, already lower than control, reaches a minimum only 4 h after withdrawal of food, and this level is sustained, presumably by gluconeogenesis, for the subsequent 20 h. By 6 h of fasting, blood ketone bodies produced by the liver and blood glycerol and free fatty acids, presumably generated by lipolysis in adipose tissue, are already elevated compared with control animals. Hepatic PEPCK, already elevated in the fed liver, responds more acutely than in control animals. In the euglycemic-hyperinsulinemic clamp experiment, basal hepatic glucose output from lactate is significantly increased in LGSKO mice and is relatively insensitive to suppression by insulin infusion. The suppression in both genotypes is consistent with previous studies in healthy and type II diabetic humans showing a modest decrease (about 20%) in the gluconeogenesis rate under clamp conditions, whereas glycogenolysis was fully suppressed (41). To explain the reduced and delayed suppression of gluconeogenesis in LGSKO mice, we may consider two possible mechanistic explanations. First, we cannot rule out the possibility that under the clamp conditions CN mice store the de novo synthesized glucose as liver glycogen. This phenomenon may cause underestimation of the gluconeogenic rate and could support the idea that the rate of liver gluconeogenesis is relatively constant under any physiological conditions and that glycogenolysis is the main contributor to glucose output (16). However, in the LGSKO mouse liver, de novo synthesized glucose cannot be stored as glycogen and presumably is exported into the bloodstream, correlating with the observed increase in gluconeogenesis. Second, the LGSKO liver may be more resistant to insulin action. Another fate of glucose in the postprandial state is fat synthesis (42), and we found elevated triglycerides in the LGSKO liver. This may confer insulin resistance, resulting in the inability to suppress gluconeogenesis (41, 43). Although magnetic resonance imaging indicated no change in total fat in LGSKO mice, this technique primarily monitors changes in adipose tissue mass and potentially underestimates changes in intracellular fat content in tissues such as liver.
Our results provide some possible mechanistic insight into the control of metabolism in the LGSKO animals. PGC-1α expression is already elevated in the fed LGSKO liver, and FoxO1 phosphorylation, little affected by short term fasting in the control mice, was significantly decreased upon only 6 h of fasting of the LGSKO mice, consistent with the lower insulin levels and with the elevated basal cytosolic isoform of PEPCK observed in these animals. AMPK is considered a key regulator of energy homeostasis in many cell types (44). In liver, a number of studies have shown that AMPK activation, by AICAR or metformin or physiologically by long term starvation (48 h) (45), reduces lipogenesis and hepatic glucose production by suppressing gluconeogenesis (34, 35). In our hands, there was no change in AMPK protein or phosphorylation state through 18 h of fasting of wild type mice. However, there was a clear decrease in the phosphorylation state in the LGSKO mice upon fasting. Altogether these results are consistent with the greater propensity of these animals to enter the fasted condition.
Liver glycogen is also used as an energy source during short duration exercise (20–30 min) in an intensity-dependent fashion, whereas in exercise lasting for hours there is an initial rise in glucose output and then a gradual decrease (46). Fed MGSKO mice (8) or mice overaccumulating muscle glycogen because of transgenic overexpression of glycogen synthase (47) had no differences in their ability to undergo exhaustive exercise in a treadmill compared with wild type mice. However, after fasting, we saw a reduction in exercise endurance for all three mouse strains. Of the several consequences of fasting, depletion of liver glycogen is of course a strong candidate to explain decreased exercise capacity. Our analysis of the treadmill performance of fed LGSKO mice supports this hypothesis because they exercised less well than wild type controls, but the difference in performance was lost after fasting, conditions in which both genotypes have little liver glycogen.
In summary, disruption of liver glycogen synthesis generates a mouse that essentially mimics the phenotype of patients with GSD0 and is a model for the study of this disease. The mice have basally decreased blood glucose and insulin levels and increased basal gluconeogenesis. They seem metabolically poised to enter more rapidly into a full fasted state to protect their blood glucose levels in the absence of short term glucose production that would normally come from liver glycogenolysis.
This work was supported, in whole or in part, by National Institutes of Health Grants DK27221 (to P. J. R.), DK59637 (to the Vanderbilt Mouse Metabolic Phenotyping Center), and DK082376 (to the Case Western Reserve University Mouse Metabolic Phenotyping Center).
- GS
- glycogen synthase
- MSGKO
- muscle glycogen synthase knock-out
- LGSKO
- liver glycogen synthase knock-out
- CN
- conditional mice with floxed Gys2
- HET
- mice heterozygous for disrupted Gys2
- CRE
- mice carrying the albumin-Cre gene
- GSD0
- glycogen storage disease type 0
- PEPCK
- phosphoenolpyruvate carboxykinase
- PGC-1α
- peroxisome proliferator-activated protein γ coactivator 1α
- FoxO1
- forkhead box O1 transcription factor
- GTT
- glucose tolerance test
- AMPK
- AMP-activated protein kinase
- ES
- embryonic stem.
REFERENCES
- 1.Shulman G. I., Rothman D. L., Jue T., Stein P., DeFronzo R. A., Shulman R. G. (1990) N. Engl. J. Med. 322, 223–228 [DOI] [PubMed] [Google Scholar]
- 2.Taylor R., Magnusson I., Rothman D. L., Cline G. W., Caumo A., Cobelli C., Shulman G. I. (1996) J. Clin. Invest. 97, 126–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ludvik B., Nolan J. J., Roberts A., Baloga J., Joyce M., Bell J. M., Olefsky J. M. (1995) J. Clin. Invest. 95, 2232–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capaldo B., Gastaldelli A., Antoniello S., Auletta M., Pardo F., Ciociaro D., Guida R., Ferrannini E., Saccà L. (1999) Diabetes 48, 958–966 [DOI] [PubMed] [Google Scholar]
- 5.Roach P. J. (2002) Curr. Mol. Med. 2, 101–120 [DOI] [PubMed] [Google Scholar]
- 6.Pederson B. A., Chen H., Schroeder J. M., Shou W., DePaoli-Roach A. A., Roach P. J. (2004) Mol. Cell Biol. 24, 7179–7187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker G. E., Pederson B. A., Obayashi M., Schroeder J. M., Harris R. A., Roach P. J. (2006) Biochem. J. 395, 137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pederson B. A., Cope C. R., Schroeder J. M., Smith M. W., Irimia J. M., Thurberg B. L., DePaoli-Roach A. A., Roach P. J. (2005) J. Biol. Chem. 280, 17260–17265 [DOI] [PubMed] [Google Scholar]
- 9.Pederson B. A., Schroeder J. M., Parker G. E., Smith M. W., DePaoli-Roach A. A., Roach P. J. (2005) Diabetes 54, 3466–3473 [DOI] [PubMed] [Google Scholar]
- 10.Brüning J. C., Michael M. D., Winnay J. N., Hayashi T., Hörsch D., Accili D., Goodyear L. J., Kahn C. R. (1998) Mol. Cell 2, 559–569 [DOI] [PubMed] [Google Scholar]
- 11.Suzuki Y., Lanner C., Kim J. H., Vilardo P. G., Zhang H., Yang J., Cooper L. D., Steele M., Kennedy A., Bock C. B., Scrimgeour A., Lawrence J. C., Jr., DePaoli-Roach A. A. (2001) Mol. Cell Biol. 21, 2683–2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Accili D. (2004) Diabetes 53, 1633–1642 [DOI] [PubMed] [Google Scholar]
- 13.Orho M., Bosshard N. U., Buist N. R., Gitzelmann R., Aynsley-Green A., Blümel P., Gannon M. C., Nuttall F. Q., Groop L. C. (1998) J. Clin. Invest. 102, 507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinstein D. A., Correia C. E., Saunders A. C., Wolfsdorf J. I. (2006) Mol. Genet. Metab. 87, 284–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozen H. (2007) World J. Gastroenterol. 13, 2541–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nuttall F. Q., Ngo A., Gannon M. C. (2008) Diabetes Metab. Res. Rev. 24, 438–458 [DOI] [PubMed] [Google Scholar]
- 17.Postic C., Dentin R., Girard J. (2004) Diabetes Metab. 30, 398–408 [DOI] [PubMed] [Google Scholar]
- 18.Finck B. N., Kelly D. P. (2006) J. Clin. Invest. 116, 615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puigserver P., Rhee J., Donovan J., Walkey C. J., Yoon J. C., Oriente F., Kitamura Y., Altomonte J., Dong H., Accili D., Spiegelman B. M. (2003) Nature 423, 550–555 [DOI] [PubMed] [Google Scholar]
- 20.Yoon J. C., Puigserver P., Chen G., Donovan J., Wu Z., Rhee J., Adelmant G., Stafford J., Kahn C. R., Granner D. K., Newgard C. B., Spiegelman B. M. (2001) Nature 413, 131–138 [DOI] [PubMed] [Google Scholar]
- 21.Thomas J. A., Schlender K. K., Larner J. (1968) Anal. Biochem. 25, 486–499 [DOI] [PubMed] [Google Scholar]
- 22.Rajas F., Croset M., Zitoun C., Montano S., Mithieux G. (2000) Diabetes 49, 1165–1168 [DOI] [PubMed] [Google Scholar]
- 23.Jørgensen S. B., Viollet B., Andreelli F., Frøsig C., Birk J. B., Schjerling P., Vaulont S., Richter E. A., Wojtaszewski J. F. (2004) J. Biol. Chem. 279, 1070–1079 [DOI] [PubMed] [Google Scholar]
- 24.Pfaffl M. W. (2001) Nucleic Acids Res. 29, 2002–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ayala J. E., Bracy D. P., McGuinness O. P., Wasserman D. H. (2006) Diabetes 55, 390–397 [DOI] [PubMed] [Google Scholar]
- 26.Fueger P. T., Shearer J., Bracy D. P., Posey K. A., Pencek R. R., McGuinness O. P., Wasserman D. H. (2005) J. Physiol. 562, 925–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Previs S. F., Fernandez C. A., Yang D., Soloviev M. V., David F., Brunengraber H. (1995) J. Biol. Chem. 270, 19806–19815 [DOI] [PubMed] [Google Scholar]
- 28.Postic C., Shiota M., Niswender K. D., Jetton T. L., Chen Y., Moates J. M., Shelton K. D., Lindner J., Cherrington A. D., Magnuson M. A. (1999) J. Biol. Chem. 274, 305–315 [DOI] [PubMed] [Google Scholar]
- 29.Isken O., Maquat L. E. (2007) Genes Dev. 21, 1833–1856 [DOI] [PubMed] [Google Scholar]
- 30.Antwi D., Youn J. H., Shargill N. S., Lesikar D. D., Kaslow H. R. (1988) Am. J. Physiol. 254, E720–E725 [DOI] [PubMed] [Google Scholar]
- 31.Calder P. C., Geddes R. (1992) Int. J. Biochem. 24, 71–77 [DOI] [PubMed] [Google Scholar]
- 32.Chen C., Williams P. F., Cooney G. J., Caterson I. D., Turtle J. R. (1992) Horm. Metab. Res. 24, 161–166 [DOI] [PubMed] [Google Scholar]
- 33.Marliss E. B., Cuendet G., Balant L., Wolheim C. B., Stauffacher W. (1974) Horm. Metab. Res. (Suppl. 4) 93–102 [PubMed] [Google Scholar]
- 34.Rutter G. A., Da Silva Xavier G., Leclerc I. (2003) Biochem. J. 375, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viollet B., Foretz M., Guigas B., Horman S., Dentin R., Bertrand L., Hue L., Andreelli F. (2006) J. Physiol. 574, 41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhavnani B. R. (1983) Can. J. Biochem. Cell Biol. 61, 191–197 [DOI] [PubMed] [Google Scholar]
- 37.Girard J., Ferré P., Pégorier J. P., Duée P. H. (1992) Physiol. Rev. 72, 507–562 [DOI] [PubMed] [Google Scholar]
- 38.Postic C., Magnuson M. A. (2000) Genesis. 26, 149–150 [DOI] [PubMed] [Google Scholar]
- 39.Ballet F. (1990) Pharmacol. Ther. 47, 281–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hribal M. L., Oriente F., Accili D. (2002) Am. J. Physiol. Endocrinol. Metab. 282, E977–E981 [DOI] [PubMed] [Google Scholar]
- 41.Gastaldelli A., Toschi E., Pettiti M., Frascerra S., Quiñones-Galvan A., Sironi A. M., Natali A., Ferrannini E. (2001) Diabetes 50, 1807–1812 [DOI] [PubMed] [Google Scholar]
- 42.Dentin R., Girard J., Postic C. (2005) Biochimie 87, 81–86 [DOI] [PubMed] [Google Scholar]
- 43.Nagle C. A., Klett E. L., Coleman R. A. (2009) J. Lipid Res. 50, S74–S79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hardie D. G., Sakamoto K. (2006) Physiology 21, 48–60 [DOI] [PubMed] [Google Scholar]
- 45.Assifi M. M., Suchankova G., Constant S., Prentki M., Saha A. K., Ruderman N. B. (2005) Am. J. Physiol. Endocrinol. Metab. 289, E794–E800 [DOI] [PubMed] [Google Scholar]
- 46.Wahren J., Ekberg K. (2007) Annu. Rev. Nutr. 27, 329–345 [DOI] [PubMed] [Google Scholar]
- 47.Pederson B. A., Cope C. R., Irimia J. M., Schroeder J. M., Thurberg B. L., Depaoli-Roach A. A., Roach P. J. (2005) Biochem. Biophys. Res. Commun. 331, 491–496 [DOI] [PubMed] [Google Scholar]