Abstract
In HEK cells stably expressing type 1 receptors for parathyroid hormone (PTH), PTH causes a sensitization of inositol 1,4,5-trisphosphate receptors (IP3R) to IP3 that is entirely mediated by cAMP and requires cAMP to pass directly from type 6 adenylyl cyclase (AC6) to IP3R2. Using DT40 cells expressing single subtypes of mammalian IP3R, we demonstrate that high concentrations of cAMP similarly sensitize all IP3R isoforms to IP3 by a mechanism that does not require cAMP-dependent protein kinase (PKA). IP3 binding to IP3R2 is unaffected by cAMP, and sensitization is not mediated by the site through which ATP potentiates responses to IP3. In single channel recordings from excised nuclear patches of cells expressing IP3R2, cAMP alone had no effect, but it increased the open probability of IP3R2 activated by a submaximal concentration of IP3 alone or in combination with a maximally effective concentration of ATP. These results establish that cAMP itself increases the sensitivity of all IP3R subtypes to IP3. For IP3R2, this sensitization results from cAMP binding to a novel site that increases the efficacy of IP3. Using stably expressed short hairpin RNA to reduce expression of the G-protein, Gαs, we demonstrate that attenuation of AC activity by loss of Gαs more substantially reduces sensitization of IP3R by PTH than does comparable direct inhibition of AC. This suggests that Gαs may also specifically associate with each AC·IP3R complex. We conclude that all three subtypes of IP3R are regulated by cAMP independent of PKA. In HEK cells, where IP3R2 selectively associates with AC6, Gαs also associates with the AC·IP3R signaling junction.
Keywords: Adenylate Cyclase (Adenylyl Cyclase), Calcium, Calcium Channels, Calcium Intracellular Release, Cyclic AMP (cAMP), Signal Transduction
Introduction
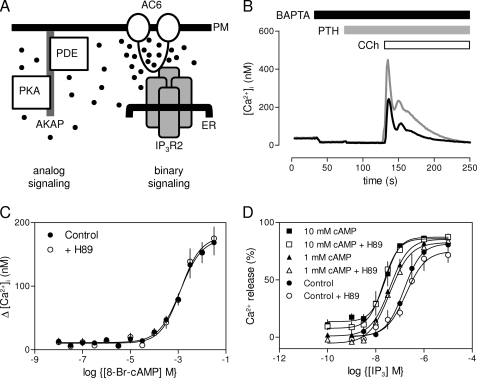
Ca2+ and cAMP are two of a limited number of intracellular messengers used by cells to regulate a diverse array of cellular events in response to many different extracellular stimuli. The specificity of these messengers is provided by the spatio-temporal organization of their concentration changes within cells (1–4) and by complex interactions between them (2, 5, 6). In addressing the means whereby parathyroid hormone (PTH)3 regulates release of Ca2+ from intracellular stores, we have shown that PTH acts entirely via cAMP to increase the sensitivity of IP3 receptors (IP3R) to IP3, thereby potentiating the Ca2+ signals evoked by other receptors that stimulate IP3 formation (7). This effect of PTH is not mediated by the common targets of cAMP, PKA, and exchange proteins activated by cAMP (epac), but results instead from cAMP binding directly to a low affinity site on either the IP3R itself or a protein tightly associated with it (7). These results identify the IP3R as a new target for regulation by cAMP and so reveal another site at which cAMP and Ca2+ signaling pathways interact. The results also highlighted the importance of the precise spatial relationship between adenylyl cyclase (AC) and IP3R, because cAMP appears to pass directly and selectively from type 6 AC (AC6) to the type 2 IP3R (IP3R2) via an association that we termed an AC·IP3R junction (7) (Fig. 1A).
FIGURE 1.
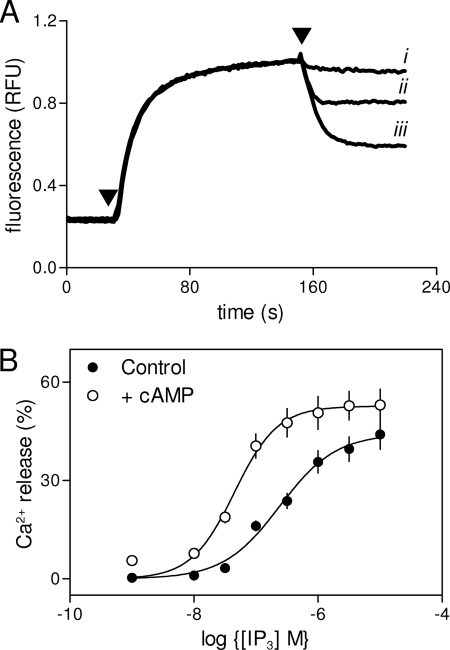
PTH potentiates CCh-evoked Ca2+ release via a direct effect of cAMP on IP3R. A, binary and analog modes of cAMP signaling (7). AKAP, protein kinase A-anchoring protein; PM, plasma membrane. Further details are in text. B, populations of HEK-PR1 cells in Ca2+-free HBS were stimulated with CCh alone (1 mm, black line) or with PTH (100 nm, 60 s) followed by CCh (gray line). Results are typical of at least three similar experiments. C, HEK-PR1 cells were preincubated (20 min) with the indicated concentrations of 8-Br-cAMP alone or with H89 (10 μm) (7) before stimulation with a submaximal concentration of CCh (1 μm). Results show the increase in [Ca2+]i evoked by the CCh addition. D, IP3-evoked Ca2+ release from permeabilized HEK-PR1 cells in the presence of submaximal (1 mm) or maximal (10 mm) concentrations of cAMP after preincubation with or without H89 (10 μm; 20 min). Results (C and D) show means ± S.E., n = 3 independent experiments.
These observations led us to define two forms of local cAMP signaling (Fig. 1A). The first mode, represented by the AC·IP3R junction, is binary signaling. Here, cAMP is delivered directly to its target at very high concentrations by a closely associated AC; cAMP then switches the target on in all-or-nothing fashion. The second mode is analog signaling, where targets further away from AC, like PKA and exchange proteins activated by cAMP, respond to graded changes in cAMP concentration. The low affinity of the IP3R for cAMP ensures that diffusion of cAMP is probably sufficient to terminate a response and insulate one junction from its neighbors. For analog signaling, local degradation of cAMP by associated cyclic nucleotide phosphodiesterases is required to terminate the response and maintain the spatial organization of cAMP signaling (Fig. 1A) (7, 8).
Here, we address two further questions related to cAMP signaling to IP3R. First, although we have shown that, in intact HEK cells the effects of PTH are selectively mediated by IP3R2, it is unclear whether this results solely from a selective association of IP3R2 with AC6, or whether IP3R2 is also unique among the three IP3R subtypes in responding to cAMP. We demonstrate, using IP3R1–3 expressed in DT40 cells lacking native IP3R, that all IP3R subtypes are regulated by cAMP itself. Second, although AC6 and IP3R2 are associated in a signaling complex (the AC6·IP3R2 junction, Fig. 1A), it is unclear whether other components of the signaling pathway linking PTH receptors to sensitization of IP3R are also uniquely associated with individual AC6·IP3R junctions. By selectively inhibiting Gαs expression using stably expressed shRNA, we demonstrate that Gαs is probably also specifically associated with AC·IP3R junctions.
EXPERIMENTAL PROCEDURES
Cells and Vectors
HEK 293 cells stably transfected with human type 1 PTH receptor (HEK-PR1 cells) were cultured as described (9). A modified pSUPER vector (10) was used for shRNA-mediated knockdown of Gαs in HEK-PR1 cells. An expression cassette for blasticidin resistance from pcDNA6/TR (Invitrogen) was cloned upstream of the polymerase-III H1-RNA promoter of pSUPER to generate a pSUPERBlank vector. Primers 1 and 2 for human Gαs (see supplemental Table S1 for primer sequences) were annealed, phosphorylated by T4 polynucleotide kinase (New England Biolabs), and ligated into the BglII-HindIII sites of pSUPERBlank to create the pSUPERGαs vector. The plasmids, pSUPERBlank and pSUPERGαs, were linearized and transfected into HEK-PR1 cells (5 μg of DNA/106 cells) using TransfastTM Reagent (Promega). Transfected cells were selected using blasticidin (10 μg/ml) and G418 (800 μg/ml). Clonally isolated cells were screened for Gαs by immunoblotting using a Gαs antiserum (RM/1, 1:1000, New England Nuclear). Appropriate clones were further propagated for use in assays of cAMP and [Ca2+]i.
DT40 cells in which each IP3R gene had been disrupted (DT40KO cells) (11) were cultured as described (12). The ORF of rat IP3R1 (GenBankTM accession number GQ233032.1) was amplified by PCR from the expression vector pCMVI-9-IP3R1 (13) using primers 3 and 4 (supplemental Table S1), and cloned as an EcoRI fragment into pENTR1a vector (Invitrogen). The ORF of mouse IP3R2 (GenBankTM accession number AB182290) was amplified by PCR as two fragments from the expression vector pcDNA3-IP3R2 (14). The 5′ fragment (1–3297) was amplified by PCR using primers 5 and 6 and cloned as a SalI-XhoI fragment into pENTR1a. Then the entire ORF (1–8196) was amplified by PCR using primers 5 and 7, digested with KpnI and XhoI, and the resulting fragment (2230–8196) was cloned into pENTR1a. Finally, a SalI-BstBI fragment (1–2711) from the first construct was cloned into the second construct (replacing the smaller SalI-BstBI fragment) to give the entire ORF (1–8196) of mouse IP3R2 in pENTR1a. Supplemental Experimental Procedures provide further details of the cloning and expression of IP3R2. The ORF of rat IP3R3 (GenBankTM accession number GQ233031.1) was amplified by PCR from the expression vector pCB6-IP3R3 (15) using primers 8 and 9, and cloned as an EcoRI fragment into pENTR1a vector (Invitrogen). Each pENTR1a-IP3R construct was recombined with pcDNA3.2/V5-DEST (Invitrogen) to generate the Gateway-compatible expression vectors pcDNA3.2-IP3R1 (2 and 3). DT40KO cells were transfected by nucleofection with linearized constructs of pcDNA3.2-IP3R1 (2 or 3) using solution T and program B23 (Amaxa) using 5 μg of DNA/106 cells. G418 (2 mg/ml) was used to select and amplify clones of cells. Expression of IP3R in each cell line was quantified by immunoblotting using an anti-peptide antiserum (AbC (16)) that interacts equally with all three IP3R subtypes. GeneTools (Syngene) was used to quantify band intensities on immunoblots. To estimate numbers of IP3R in each DT40 cell line, immunoblots were calibrated using membranes prepared from rat cerebellum in which the receptor density (Bmax) had been measured by equilibrium competition [3H]IP3 binding.
For expression of mouse IP3R2 in Sf9 cells, pENTR1a-IP3R2 was recombined with Gateway-compatible BaculoDirect C-Term linear DNA (Invitrogen) to generate a recombinant baculovirus using the BaculoDirectTM C-Term expression kit (Invitrogen). Sf9 cells were cultured as previously described (17), they were then infected with the recombinant virus DNA, and the virus was isolated according to the manufacturer's instructions (Invitrogen). For IP3R2 expression, cells were infected at a multiplicity of infection of 10.
Measurements of IP3-evoked Ca2+ Release from Permeabilized Cells
The intracellular stores of DT40 cells stably expressing mammalian IP3R were loaded with Mag-fluo-4 to allow measurement of the luminal-free [Ca2+] (12). Cells were then permeabilized by incubation with saponin (10 μg/ml) and immobilized in 96-well plates to allow continuous monitoring of the Ca2+ content of the intracellular stores using a FlexStation fluorescence plate reader (MDS Analytical Devices) (12). All experiments were performed at 20 °C in cytosol-like medium with a free [Ca2+] of 220 nm (CLM: 140 mm KCl, 20 mm NaCl, 1 mm EGTA, 375 μm CaCl2, 20 mm Pipes, pH 7.0). MgATP (1.5 mm) was added to allow Ca2+ uptake into the stores, and when steady-state loading had been achieved, IP3 was added with thapsigargin (1 μm) to allow Ca2+ release to be measured without further re-uptake. IP3-evoked Ca2+ release is expressed as a fraction of the Ca2+ released by addition of ionomycin (1 μm) (12). Similar methods were used to measure IP3-evoked Ca2+ release from Sf9 cells expressing IP3R2.
Binding of 3H-IP3 to IP3R2
Membranes were prepared from Sf9 cells expressing mouse IP3R2 as described (17). Briefly, cells were harvested 60 h after infection, washed, and resuspended in Ca2+-free CLM supplemented with complete protease inhibitor mixture (Roche Applied Science, 1 tablet/25 ml). The suspension (3 × 107 cells/ml) was homogenized and centrifuged (130,000 × g, 60 min), and the pellet was resuspended in Ca2+-containing CLM and stored at −80 °C. Membranes (72 μg of protein/ml) were resuspended in CLM (0.5 ml, supplemented with 2 mm MgCl2) containing [3H]IP3 (1.5 nm, 18 Ci/mmol) and appropriate concentrations of unlabeled IP3. Variations in the composition of CLM at different steps were dictated by the need to minimize proteolysis during lysis (bivalent cation-free CLM) and then to include both Ca2+ and Mg2+ in the [3H]binding assay more effectively to mimic the composition of cytosol and for comparison with our previous analyses of [3H]IP3 binding to IP3R1 and IP3R3 (16).
After 5 min at 2 °C, during which equilibrium was attained, the incubations were stopped by addition of γ-globulin (30 μl, 25 mg/ml) and polyethylene glycol 8000 (500 μl, 30%). After centrifugation (20,000 × g for 5 min at 2 °C), pellets were dissolved in Triton X-100 (200 μl, 2%), and 3H activity was determined by liquid scintillation counting after addition of Ecoscint A scintillation mixture (1 ml, National Diagnostics). Total [3H]IP3 binding was typically 2300 dpm, of which ∼90% was specific binding. Results were fitted to Hill equations (GraphPad Prism, version 5) from which the half-maximal inhibitory concentration, and thereby the KD, were determined.
Measurements of cAMP and [Ca2+]i in Intact Cells
HEK-PR1 cells in 96-well plates were cultured for 2–3 days until almost confluent, washed, loaded with fluo-4, and [Ca2+]i was then measured using a FlexStation fluorescence plate reader as described before (7). All experiments were performed at 20 °C in HBS or Ca2+-free HBS. HBS had the following composition: 135 mm NaCl, 5.9 mm KCl, 1.2 mm MgCl2, 1.5 mm CaCl2, 11.6 mm HEPES, 11.5 mm glucose, pH 7.3; Ca2+-free HBS was supplemented with 10 mm BAPTA.
Single cell analyses of [Ca2+]i in fura-2-loaded HEK-PR1 cells were performed as previously reported, with fluorescence ratios calibrated, after correction for background fluorescence, to [Ca2+]i by reference to Ca2+-calibration solutions (18).
For assays of cAMP, HEK-PR1 cells in 24-well plates were cultured for 2–3 days (as above) until near confluence and then stimulated under identical conditions to those used for measurements of [Ca2+]i; the only difference was the omission of fluo-4-AM and Pluronic F-127 from the 1-h loading incubation. Cell extracts were prepared, and their cAMP content was determined by radioimmunoassay using acetylated standards prepared from a cAMP stock calibrated by its UV absorption (ϵ258 = 14,100) as described (7, 19). It is important to note that single cell measurements of [Ca2+]i established that 98% of cells responded to CCh, and 99% of those responded to PTH (7). This observation justifies our use of cell populations for comparisons of the effects of stimuli on [Ca2+]i and cAMP levels.
Nuclear Patch Clamp Recording
Currents were recorded from patches excised from the outer nuclear envelope of DT40-KO cells stably expressing mouse IP3R2, using Cs+ as the charge carrier. Bath and pipette (PS) solutions had the following composition: 200 mm CsCH3SO3, 500 μm BAPTA, 211 μm CaCl2 (free [Ca2+] = 200 nm), 10 mm HEPES, pH 7.2 (with CsOH). Where appropriate, IP3, cAMP, and/or ATP were included in PS. Data were collected and analyzed exactly as described previously (20).
Analysis
Concentration-effect relationships for each experiment were individually fitted to Hill equations using non-linear curve-fitting (GraphPad Prism, version 5), and the results obtained from each (EC50, Hill coefficient h, and maximal response) were pooled for analysis and presentation. For simplicity, EC50 values are reported as means ± S.E., although log EC50 values were used for statistical analysis. Student's t test or one-way analysis of variance followed by post hoc Bonferroni test were used as appropriate.
Materials
Sources of materials not specified herein are provided in a previous study (7).
RESULTS AND DISCUSSION
Potentiation of IP3-evoked Ca2+ Release by cAMP
CCh, which activates phospholipase C and thus IP3 formation via endogenous muscarinic acetylcholine receptors, stimulated Ca2+ release from the intracellular stores of HEK-PR1 cells (Fig. 1B). The response was potentiated by forskolin, which activates AC directly (7), or by PTH, which activates AC via heterologously expressed type 1 PTH receptors and the G-protein, Gs (Fig. 1B). The effect of PTH on CCh-evoked Ca2+ release was mimicked by a membrane-permeant analogue of cAMP, 8-Br-cAMP (EC50 = 324 μm) (7), and the sensitivity to 8-Br-cAMP was unaffected by H89 at a concentration (10 μm) sufficient to inhibit fully PKA-mediated protein phosphorylation (Fig. 1C) (7). Neither forskolin nor 8-Br-cAMP alone evoked Ca2+ release (7).
In permeabilized HEK-PR1 cells, IP3 stimulated release of Ca2+ from the intracellular stores, cAMP increased the sensitivity to IP3, and the effects of cAMP were again unaffected by inhibition of PKA (Fig. 1D). These results confirm our earlier conclusion that in HEK-PR1 cells, PTH potentiates IP3-evoked Ca2+ release via cAMP and with no requirement for PKA (7). The earlier work established that IP3R2 and AC6 are selectively associated and both are required for PTH to potentiate CCh-evoked Ca2+ signals (7) (Fig. 1A). But it is not yet clear whether the specific requirement for IP3R2 derives entirely from its association with AC6 or is IP3R2 also the only subtype of IP3R to respond directly to cAMP?
Regulation of All IP3R Subtypes by cAMP
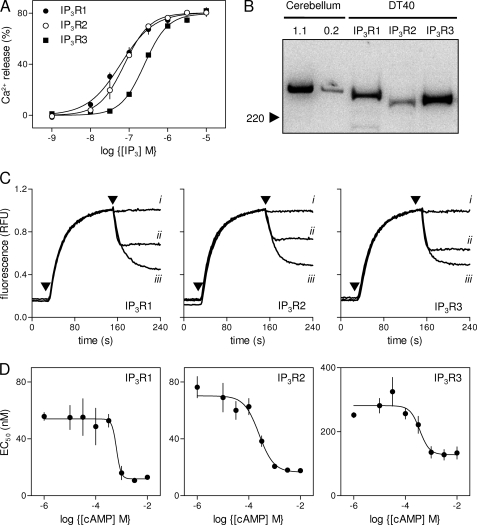
IP3 stimulated release of Ca2+ from the intracellular stores of permeabilized DT40 cells stably expressing mammalian IP3R1, IP3R2, or IP3R3 (Fig. 2A), but not from the parental cells lacking endogenous IP3R (not shown) (21, 22). Because the three IP3R subtypes are not expressed at identical levels (Fig. 2B and Table 1), we cannot assume that the different fractions of the intracellular Ca2+ stores released by a maximal concentration of IP3 or the different EC50 values (Table 1) directly reflect specific properties of the three IP3R subtypes. It is generally suggested that IP3R2 is more sensitive than IP3R1 to IP3, and both are considerably more sensitive than IP3R3 (23, 24). However, in our assays of DT40 cells stably expressing a single IP3R subtype we found the order of sensitivity to be: IP3R1 > IP3R2 ≫ IP3R3 (Table 1). The slightly lower than anticipated sensitivity of cells expressing IP3R2 probably results from IP3R2 being expressed at a lower level (∼30–40%) than the other subtypes (Table 1) (21). The ∼5-fold difference in IP3 sensitivity between cells expressing IP3R1 and IP3R3 does not detract from our ability to resolve the effects of cAMP on IP3-evoked Ca2+ release via each IP3R subtype.
FIGURE 2.
cAMP potentiates IP3-evoked Ca2+ release by the three subtypes of IP3R. A, IP3-evoked Ca2+ release from the intracellular stores of DT40 cells expressing only IP3R1, IP3R2, or IP3R3. B, immunoblots (with AbC) show levels of IP3R expression in each of the three cell lines (95 μg of protein/lane) compared with membranes from rat cerebellum (1.1 and 0.2 μg/lane); the position of the 220-kDa Mr marker is shown. The blot is typical of six experiments from two different preparations. Summary results are shown in Table 1. C, typical results from permeabilized DT40 cells expressing the indicated IP3R incubated with ATP (added at the first arrow) to allow loading of the intracellular Ca2+ stores, before addition (second arrow) of cAMP alone (i, 1 mm), IP3 alone (ii, 30 nm for IP3R1 and IP3R2; 300 nm for IP3R3), or IP3 with cAMP (iii). Each trace is the average of three to four wells from a single experiment and is representative of results from three to four independent experiments. D, from experiments similar to those shown in C, the concentration-dependent effects of cAMP on the EC50 for IP3-evoked Ca2+ release are shown for each IP3R subtype. Results are means ± S.E., n ≥ 3.
TABLE 1.
Responses of IP3R subtypes to IP3 and cAMP
Permeabilized DT40 cells expressing only a single mammalian IP3R subtype were used to examine the sensitivity of the intracellular Ca2+ stores to IP3 (EC50) and the fraction of the stores released by a maximally effective concentration of IP3. For each cell line, IP3R expression was quantified using immunoblotting with AbC (Fig. 2B), which interacts equally with all three IP3R subtypes (16) and calibrated against a stock of rat cerebellar membranes for which Bmax (tetrameric IP3R/106 cells) had been determined from [3H]IP3 binding. The effects of a maximally effective concentration of cAMP on the EC50 for IP3-evoked Ca2+ release are shown, derived from experiments similar to those shown in Figs. 1D and 2. The sensitivity to cAMP is shown by the EC50 for the effects of cAMP on IP3-evoked Ca2+ release. Results are means ± S.E. (n ≥ 3).
| IP3R1 | IP3R2 | IP3R3 | IP3R2-ΔATPB | |
|---|---|---|---|---|
| IP3R expression (fmol/106 cells) | 38 ± 2 | 16 ± 1 | 56 ± 5 | NDa |
| EC50 for IP3 (nm) | 56 ± 3 | 74 ± 10 | 255 ± 9 | 246 ± 17 |
| EC50 for IP3 after cAMP (nm) | 13 ± 1 | 22 ± 1 | 134 ± 19 | 44 ± 3 |
| Fold stimulation by cAMP | 4.2 ± 0.1 | 3.3 ± 0.3 | 2.0 ± 0.3 | 5.7 ± 0.6 |
| Maximum response to IP3 (%) | 83 ± 10 | 80 ± 1 | 80 ± 1 | 45 ± 4 |
| Maximum response to IP3 after cAMP (%) | 79 ± 2 | 81 ± 1 | 82 ± 1 | 53 ± 4 |
| EC50 for cAMP (μm) | 642 ± 158 | 267 ± 80 | 361 ± 74 | ND |
a ND, not determined.
We used the three DT40 cell lines to examine the effects of cAMP on IP3-evoked Ca2+ release. The results demonstrate that cAMP alone had no effect on the intracellular Ca2+ stores (Fig. 2C), but it significantly increased the sensitivity of each IP3R subtype to IP3 (Fig. 2, C and D). For all subtypes, a maximally effective concentration of cAMP caused the EC50 for IP3-evoked Ca2+ release to decrease by between 2- and 4-fold (Table 1). This increase in sensitivity is similar to the ∼3-fold increased sensitivity to CCh of HEK-PR1 cells treated with PTH (7), but less than the ∼7-fold increase in IP3 sensitivity evoked by a maximal concentration of cAMP in permeabilized HEK cells (Fig. 1D). There was no statistical difference (p < 0.05) between either the sensitivities of the three IP3R subtypes to cAMP (EC50) or the Hill coefficients of the concentration-effect relationships. We conclude that the three subtypes of IP3R are similarly sensitive to cAMP, with EC50 values between ∼300 and 650 μm (Table 1).
Interactions of cAMP with IP3R2
Our earlier work established the importance of IP3R2 in mediating responses to PTH (7) and responses of homomeric IP3R2 are potentiated by cAMP (Fig. 2, C and D), we therefore focused on this IP3R subtype for our further analyses of the interactions of cAMP with IP3R.
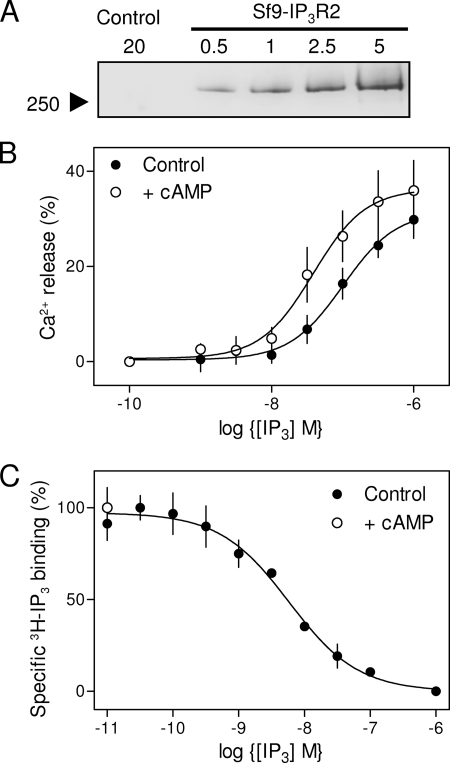
It proved impracticable to measure [3H]IP3 binding in CLM to membranes prepared from DT40-IP3R2 cells; we therefore used membranes from Sf9 cells expressing the same mouse IP3R2 (Fig. 3A). We first confirmed that IP3R2 expressed in Sf9 cells are regulated by cAMP. Whereas IP3 (10 μm) stimulated release of only 7 ± 1% (EC50 = 500 ± 50 nm) of the Ca2+ stores of uninfected Sf9 cells, it released 30 ± 4% (EC50 = 122 ± 35 nm) of the stores from permeabilized Sf9 cells expressing mouse IP3R2 (Fig. 3B). This confirms that most IP3-evoked Ca2+ release from the infected Sf9 cells is mediated by heterologously expressed IP3R2. Treatment with cAMP (1 mm), which itself caused no Ca2+ release, increased the sensitivity of the expressed IP3R2 to IP3: the EC50 was reduced from 122 ± 35 nm to 41 ± 12 nm (Fig. 3B). These results establish that IP3R2 expressed in Sf9 cells, like those expressed in HEK (7) or DT40 cells (Fig. 2), are sensitized to IP3 by cAMP.
FIGURE 3.
Interaction of cAMP with IP3R2 expressed in Sf9 cells. A, immunoblot (with AbC) showing expression of IP3R2 in membranes from infected Sf9 cells, but not from membranes of uninfected cells (control). Protein loadings are shown in micrograms. B, concentration-dependent release by IP3 of Ca2+ from permeabilized Sf9 cells expressing mouse IP3R2 with or without addition of cAMP (1 mm). C, specific binding of [3H]IP3 (1.5 nm) to membranes from Sf9 cells expressing mouse IP3R2 in the presence of the indicated concentrations of unlabeled IP3. There was no detectable specific binding of [3H]IP3 to membranes from uninfected cells. Because the concentration of [3H]IP3 (1.5 nm) was less than the KD for IP3 (4.3 nm), a change in KD or Bmax would be reflected in the specific [3H]IP3 binding. The open circle shows that cAMP (10 mm) had no effect on specific [3H]IP3 binding. Results (B and C) show means ± S.E., n ≥ 3.
IP3 bound to IP3R2 expressed in Sf9 cells with high affinity (KD = 4.30 ± 0.04 nm, Bmax = 2.55 pmol/mg), but specific binding of [3H]IP3 (1.5 nm) was unaffected by cAMP (10 mm) (Fig. 3C). This demonstrates that the effect of cAMP on IP3R2 is not mediated by an increase in its affinity for IP3, but must instead result from an increase in the effectiveness with which IP3 binding is coupled to channel opening; cAMP increases the efficacy of IP3.
Effects of cAMP on Single IP3R2
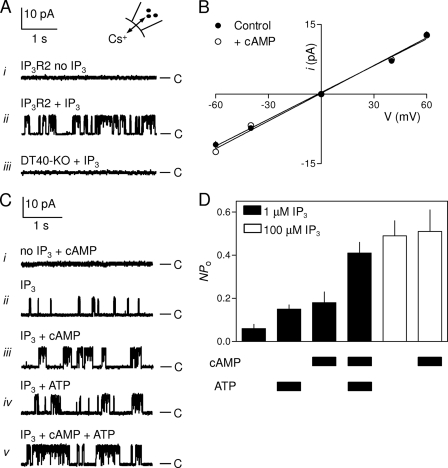
In patch clamp recordings from excised nuclear patches of DT40-IP3R2 cells, IP3 (100 μm) in the absence of ATP (which is not required for high concentrations of IP3 to maximally activate IP3R2 (25)) stimulated opening of large-conductance cation channels (γCs ∼200 pS, Fig. 4, A, i, and ii, and B). These channels were absent from the nuclei of DT40-KO cells (Fig. 4A, iii). The single channel open probability (Po) of these IP3R was similar (Po = 0.49 ± 0.07) (Fig. 4A, ii) to that reported for maximally stimulated IP3R2 expressed in the plasma membrane (25), or nuclear IP3R1 and IP3R3 (20). The latter recordings used K+ as charge-carrier, but Po for IP3R1 is similar whether K+ (Po = 0.45 ± 0.07) or Cs+ (Po = 0.41 ± 0.04) is used as the charge carrier. These results establish that 100 μm IP3 in the absence of ATP maximally activates IP3R2.
FIGURE 4.
Effects of cAMP on single channel activities of IP3R2. A, traces show excised nuclear patch clamp recordings from DT40-IP3R2 (i and ii) or DT40-KO cells (iii) in which IP3 (100 μm) was included in PS as indicated. B, current-voltage (i-V) relationship for single IP3R2 stimulated with IP3 (1 μm), with or without cAMP (1 mm) in PS. The single channel slope conductances (γCs) were 204 ± 6 and 200 ± 12 pS in the absence and presence of cAMP, respectively. C, typical traces from single IP3R2 stimulated with submaximal IP3 (1 μm) in the presence and absence of cAMP (1 mm) and/or ATP (5 mm) in PS as indicated. D, from recordings similar to those shown in D, NPo is shown for IP3R2 stimulated as indicated. Note the use of NPo to report channel activity here because the IP3Rs are less active in some of the recording conditions, making it difficult to determine reliably the number of IP3R in each patch (see text). For all traces, PS contained a free [Ca2+] of 200 nm, the holding potential was +40 mV, C denotes the closed state, and each trace is typical of at least three similar recordings. Results (B and D) are means ± S.E., n ≥ 3.
The effects of cAMP are most evident in Ca2+ release assays after submaximal activation with IP3 (Fig. 1D and Table 1) (7). Our initial examination of the effects of cAMP on single IP3R2 were therefore performed without ATP and with a submaximal concentration of IP3. Because Po is much lower under these conditions, it is impossible to resolve with confidence the number of active IP3R within a patch (20) and so impossible to calculate Po. We therefore use NPo (the overall channel activity) to report the activity of IP3R in these submaximally stimulated patches. A concentration of cAMP (1 mm) sufficient to sensitize maximally IP3-evoked Ca2+ release (Fig. 2D) had no effect alone on channel activity recorded from nuclei of DT40-IP3R2 cells (Fig. 4C, i). But cAMP significantly increased the activity of IP3R2 stimulated with a submaximal concentration of IP3 (1 μm): NPo increased from 0.06 ± 0.02 (n = 11) in the absence of cAMP to 0.18 ± 0.05 (n = 5) in its presence (Fig. 4, C, ii and iii, and D). The single channel Cs+ conductance was unaffected by cAMP (Fig. 4B). Because these experiments were performed in the absence of ATP, the effects of cAMP cannot be attributed to PKA-mediated phosphorylation of IP3R.
In keeping with work from others (25), ATP (5 mm) alone had no effect on IP3R2 activity (not shown), but it potentiated responses to a submaximal concentration of IP3 (1 μm): NPo increased from 0.06 ± 0.02 (n = 11) to 0.15 ± 0.02 (n = 5) in the presence of ATP (Fig. 4, C, ii, and iv, and D). In the presence of this maximally effective concentration of ATP (25), cAMP (1 mm) further potentiated responses to IP3 (1 μm): NPo increased from 0.15 ± 0.02 to 0.41 ± 0.05 (n = 6) in the presence of cAMP (Fig. 4, C, iv and v, and D). In keeping with our results from Ca2+ release assays (Table 1), cAMP did not increase NPo of IP3R2 stimulated with a maximally effective concentration of IP3 (100 μm): NPo was 0.49 ± 0.07 and 0.51 ± 0.1 (n = 3) in the absence and presence of cAMP, respectively (Fig. 4D). We conclude that cAMP, either directly or via a tightly associated accessory protein, increases the activity of IP3R2 stimulated with IP3.
cAMP Potentiation of IP3-evoked Ca2+ Release Is Not Mediated by the Modulatory ATP-binding Site
We further considered the possibility that the effects of cAMP might reflect its interaction with the site(s) through which ATP potentiates IP3R function (25, 26). This seems unlikely from the results of our single channel analyses (Fig. 4) and because cAMP potentiates responses to IP3 in the presence of 1.5 mm ATP (Fig. 2, C and D), which others have shown to be more than sufficient to potentiate maximally responses to IP3 (25). Yule and co-workers have established that an ATP-binding site in IP3R2 (ATPB, residues 1969–1974) mediates modulation of IP3R2 gating by ATP. Mutation of glycine residues (G1969A, G1971A, and G1974A) within this site abolishes the potentiating effect of ATP on IP3-evoked Ca2+ release (25). We used the same cell line (DT40-ΔATPB), kindly provided by David Yule (University of Rochester), to examine the effects of cAMP on IP3-evoked Ca2+ release. Responses to IP3 in these DT40-ΔATPB cells were also potentiated by cAMP (Fig. 5, A and B, and Table 1). These results, together with those from single channel analyses (Fig. 4), establish that the effects of cAMP on IP3R2 are not mediated by its interaction with the site through which ATP potentiates activity.
FIGURE 5.
Potentiation of IP3R2 by cAMP does not require the ATPB-binding site. A, typical results from permeabilized DT40 cells expressing only IP3R2-ΔATPB incubated with ATP (added at the first arrow) before addition (second arrow) of cAMP alone (i, 3 mm), IP3 alone (ii, 300 nm), or IP3 with cAMP (iii). Each trace is the average of three to four wells from one experiment and is representative of results from three independent experiments. B, summary showing the concentration-dependent effects of IP3 on Ca2+ release in the absence and presence of cAMP (3 mm). Results are means ± S.E., n ≥ 3.
PKA-dependent and -independent Regulation of IP3R by cAMP
All three IP3R subtypes respond directly to cAMP (Fig. 2), all three can also be phosphorylated, at multiple sites, by PKA (6, 7, 27–34), and substantial evidence suggests that the phosphorylation can modulate IP3R activity (28, 31, 35–38). Phosphorylation of IP3R1 by PKA increases their activity (27, 31, 32, 39–41). An initial suggestion that PKA inhibits IP3R1 probably resulted from a counteracting stimulatory effect of PKA on Ca2+ re-uptake (30). IP3R2 appears to be a rather poor substrate for PKA (7, 27, 28, 36), although it is phosphorylated at Ser-937 (34), and in cells expressing predominantly IP3R2, PKA typically causes a very modest increase in their sensitivity to IP3 (7, 28, 35, 42). In DT40 cells expressing only IP3R2 forskolin enhances responses to IP3, but the extent to which this requires activation of PKA is unclear. When a PKA-selective analog of cAMP was used, phosphorylation of Ser-937 by PKA was required for potentiation of the Ca2+ signals evoked by threshold concentrations of CCh (34). Clearly, therefore, PKA can both phosphorylate and modestly sensitize IP3R2; the extent to which that underlies the effects of forskolin or cAMP is unresolved. The effects of PKA on IP3R3 are less clear. Analyses from cells in which IP3R3 is the major subtype suggest that PKA either modestly sensitizes them to IP3 (28) or attenuates their responses (33, 43). The only published analysis of homomeric IP3R3 expressed in DT40 cells concluded that PKA had no effect on IP3-evoked Ca2+ release (27).
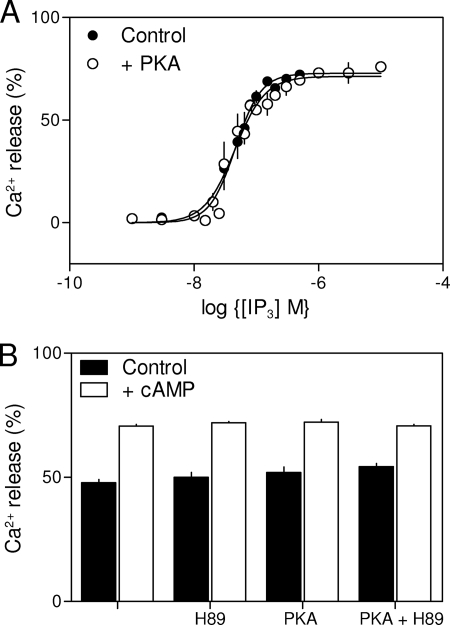
Under conditions where the catalytic subunit of PKA (200 units/ml for 10 min) phosphorylates IP3R in HEK-PR1 cells (7), it had no effect on IP3-evoked Ca2+ release from DT40 cells expressing IP3R2 (Fig. 6A) or IP3R3 (not shown), and it only very modestly increased the sensitivity of IP3R1 to IP3 (the EC50 fell from 36 to 27 nm (supplemental Fig. S1, A and B). We note that, even for IP3R1, where the effects of PKA are relatively uncontentious, phosphorylation by PKA typically increases the IP3 sensitivity by <2-fold (28, 36, 40). In our analyses of HEK-PR1 cells also, the very modest stimulatory effect of PKA on IP3-evoked Ca2+ release (∼30%) is much smaller than that of cAMP (∼450%) (7).
FIGURE 6.
Sensitization of IP3R2 by cAMP does not require activation of PKA. A, IP3-evoked Ca2+ release from the intracellular stores of permeabilized DT40-IP3R2 cells with and without preincubation with the catalytic subunit of PKA (200 units/ml, 10 min). B, Ca2+ release from permeabilized DT40-IP3R2 cells stimulated with IP3 alone (60 nm) or in combination with cAMP (3 mm) after the indicated pretreatments (10 min) with the catalytic subunit of PKA (200 units/ml) and/or H89 (10 μm). Results are means ± S.E., n ≥ 3.
In an attempt to reduce phosphorylation by endogenous PKA (41), we first incubated DT40-IP3R2 cells with H89 (10 μm, 60 min) to inhibit PKA before permeabilizing the cells and assessing their responses to IP3. Here too, there was no detectable effect of the catalytic subunit of PKA on IP3-evoked Ca2+ release via IP3R2 (supplemental Fig. S1C). Under the same conditions, where direct activation of PKA had no effect on IP3-evoked Ca2+ release, cAMP (3 mm) potentiated the response to a submaximal concentration of IP3 (60 nm) to the same extent whether applied alone, or after pretreatment (10 min) with H89 (10 μm), the catalytic subunit of PKA (200 units/ml) or both (Fig. 6B). We conclude that sensitization of IP3R2 by cAMP occurs independently of activation of PKA.
We can only speculate on the negligible (IP3R1) or undetectable effect (IP3R2 and IP3R3) of PKA on IP3-evoked Ca2+ release from DT40 cells expressing homomeric IP3R. Association of IP3R with AKAP facilitates their phosphorylation by endogenous PKA (40, 44, 45), but ineffective targeting of PKA by AKAP is unlikely to prevent exogenous catalytic subunit of PKA from phosphorylating IP3R (Fig. 6 and supplemental Fig. S1). Accessory proteins, perhaps analogous to the IP3R-associated cGMP kinase substrate (IRAG) required for regulation of IP3R by cGMP-dependent protein kinase (46), may be required for PKA to modulate IP3R activity. IP3R may already be phosphorylated in DT40 cells, although the lack of effect of PKA in permeabilized cells after sustained pretreatment of intact cells with an inhibitor of PKA suggests this is unlikely (supplemental Fig. S1C). Furthermore, even when IP3R3 was shown to be phosphorylated by PKA in DT40 cells, there was no change in their response to IP3 (27). A further possibility is that an active protein phosphatase is associated with IP3R (32, 47) and limits their steady-state phosphorylation in DT40 cells.
The essential point for the present discussion is that cAMP directly potentiates IP3-evoked Ca2+ release and the effect is both more substantial than, and independent of, the effects of phosphorylation by PKA.
Loss of Gαs Attenuates Sensitization of IP3 Receptors by PTH
Potentiation of CCh-evoked Ca2+ signals by forskolin or PTH requires an intimate association between IP3R2 and AC6 that allows cAMP to pass directly to the IP3R (7) (Fig. 1A). Subsequent experiments seek to establish whether Gαs, which links PTH receptors to activation of AC, is also specifically associated with these AC·IP3R junctions.
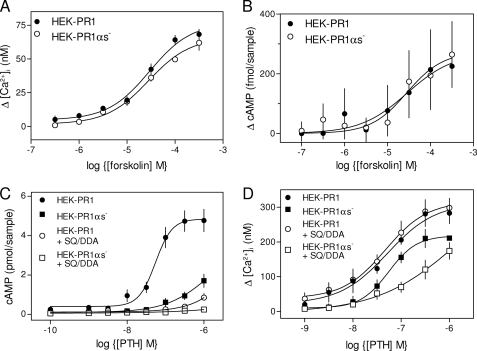
We established seven stable HEK-PR1 cell lines in which shRNA was used to reduce expression of Gαs (HEK-PR1αs− cells) and three mock transfected lines. The responses of the latter to CCh alone or with PTH were indistinguishable from the parental cells (not shown). Results are shown for only one of the HEK-PR1αs− lines, in which Gαs expression was reduced by >95% (7). The concentration-dependent effects of CCh alone or with PTH on Ca2+ signals were similar in each of the other HEK-PR1αs− lines. Neither the Ca2+ signals evoked by CCh alone (7), their potentiation by forskolin (Fig. 7A), nor forskolin-stimulated cAMP production (Fig. 7B) was affected by loss of Gαs. This establishes that selection of the stable HEK-PR1αs− cells had no effect on AC activity, CCh-mediated Ca2+ signaling, or potentiation of IP3R activity by cAMP.
FIGURE 7.
Loss of Gαs attenuates PTH-potentiated Ca2+ signals. A and B, concentration-dependent effects of forskolin on the potentiation of the Ca2+ release evoked by CCh (1 mm) (A) and on the increase in cAMP production measured 30 s after stimulation of HEK-PR1 and HEK-PR1αs− cells (B). C, concentration-dependent effects of PTH on cAMP formation (measured after 30 s) in HEK-PR1 and HEK-PR1αs− cells alone or after pre-incubation (20 min) with SQ 22536 (SQ, 1 mm) and 2′,5′-dideoxyadenosine (DDA, 200 μm) (SQ/DDA). D, cells treated as in C (with the same symbols) but showing the effects of PTH on CCh-evoked Ca2+ signals. Results (A–D) are means ± S.E., n ≥ 3.
To allow direct comparison between PTH-evoked Ca2+ and cAMP signals, we measured both responses under the same conditions, after identical intervals (30 s) and in the absence of 3-isobutylmethylxanthine (see “Experimental Procedures”). Under these conditions, PTH caused a concentration-dependent stimulation of AC activity in both HEK-PR1 and HEK-PR1αs− cells (Fig. 7C). Loss of Gαs caused the EC50 for PTH-evoked cAMP formation to increase by at least 10-fold (Fig. 7C), while the maximal response (to 1 μm PTH) was reduced by 61 ± 7% and then reduced further (by 90 ± 5%) when AC was inhibited by SQ 22536 (1 mm) with DDA (200 μm) (SQ/DDA). In HEK-PR1 cells, inhibition of AC by SQ/DDA more fully inhibited PTH-evoked cAMP formation (81 ± 5%) than did loss of Gαs (61 ± 7%) (Fig. 7C).
Inhibition of AC alone had no effect on PTH-potentiated Ca2+ signals (Fig. 7D) (7), but loss of Gαs reduced their peak amplitude without affecting their sensitivity to PTH (Fig. 7D and Table 2). We confirmed, using single cell analyses of [Ca2+]i, that the same fraction of cells responded to PTH in normal and Gαs-deficient cells, that the two cell lines responded similarly to CCh alone, and that the amplitude of the PTH-potentiated CCh-evoked Ca2+ signal was decreased by 29 ± 4% in HEK-PR1αs− cells (Table 2). This demonstrates that the results with cell populations, where loss of Gαs diminished peak PTH-evoked Ca2+ signals by 34 ± 3% (Fig. 7D), do not arise from an all-or-nothing loss of responsiveness of individual cells. For each cell, therefore, loss of >95% of Gαs caused a ∼30% decrease in the peak Ca2+ signals evoked by PTH.
TABLE 2.
Potentiation of CCh-evoked Ca2+ signals by PTH in Gαs-deficient cells
The EC50 values for potentiation of CCh-evoked Ca2+ release by PTH or forskolin in populations of HEK-PR1 cells are shown. Results are means ± S.E., n ≥ 3. Single cells loaded with fura-2 were stimulated with CCh alone (1 mm) followed by PTH (100 nm) in the continued presence of CCh. Responses from 6–12 independent coverslips, each including 116–233 cells, were analyzed. The results show the increase in [Ca2+]i and the fraction of responsive cells in HEK-PR1 and HEK-PR1αs− cells.
| HEK-PR1 | HEK-PR1αs− | |
|---|---|---|
| Cell populations | ||
| PTH | 52 ± 10 nm | 62 ± 11 nma |
| PTH + H89 | NDb | 46 ± 12 nm |
| PTH + SQ/DDA | NDb | 219 ± 27 nma |
| Forskolin | 30 ± 6 μm | 28 ± 5 μm |
| Single cells | ||
| Initial response to CCh, Δ[Ca2+]i | 585 ± 17 nm | 593 ± 13 nm |
| PTH then CCh, Δ[Ca2+]i | 257 ± 32 nmc | 183 ± 11 nmc |
| Cells responding to PTH then CCh | 95 ± 1% | 91 ± 3% |
a p < 0.05 (between marked values).
b ND, not determined.
c p < 0.05 (between marked values).
These results establish that the ability of all concentrations of PTH to potentiate CCh-evoked Ca2+ signals is insensitive to a uniform ∼80% inhibition of AC activity by low affinity inhibitors of AC (SQ/DDA). But a lesser inhibition of AC arising from loss of Gαs attenuates PTH-mediated Ca2+ signaling (Fig. 7D). Why should inhibition of AC activity by loss of Gαs more effectively inhibit PTH-mediated Ca2+ signaling than direct inhibition of AC by low affinity inhibitors?
Association of Gαs with AC·IP3R Junctions
Earlier experiments comparing the effects of inhibiting AC by reducing its expression with RNA interference or by inhibition of AC with SQ/DDA revealed a similar phenomenon to that observed with loss of Gαs. Complete inhibition of AC within individual AC·IP3R junctions by selective inhibition of AC6 expression attenuated PTH-evoked Ca2+ signaling. However, a greater, but uniformly distributed, inhibition by low affinity inhibitors of AC (SQ/DDA) had no effect on Ca2+ signaling (7). These and related results lead to our conclusion that the AC·IP3R junction (Fig. 1A) is the minimal signaling unit and that within it cAMP is delivered to IP3R at concentrations greater than are needed maximally to sensitize associated IP3R. Each junction operates with a substantial “safety margin.” Removing AC from individual junctions (by inhibition of AC expression) incapacitates individual junctions, whereas SQ/DDA uniformly attenuates cAMP formation at each junction, but the lesser amount of cAMP made within each is still sufficient to sustain effective communication between AC and IP3R (7).
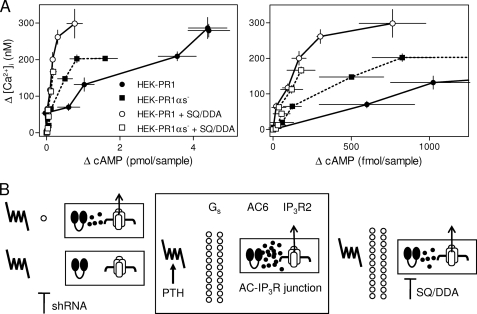
Attenuation of the responses to PTH by loss of Gαs might result entirely from the diminished activity of AC, or it might reflect an additional stimulatory role for Gs in modulating responses of IP3R to cAMP. We previously demonstrated that the maximal effects of 8-Br-cAMP and PTH on the sensitivity of Ca2+ release to CCh were the same, and the two stimuli together had no greater effect (7). This provided persuasive evidence that cAMP alone mediates the effects of PTH on IP3R sensitivity. That conclusion is further supported by the results shown in Fig. 8A, in which we compare the amounts of cAMP associated with comparable Ca2+ signals evoked by PTH under different conditions. The comparison is reasonable, because cAMP and [Ca2+]i are measured at the same time (30 s) under identical conditions, and single cell analyses confirm that all cells respond similarly (Table 2). Comparably potentiated Ca2+ signals are associated with larger amounts of cAMP in normal HEK-PR1 cells than in HEK-PR1αs− cells. These results, where cells with most Gαs appear least sensitive to cAMP, are inconsistent with Gαs potentiating the responses of IP3R to cAMP. These results reinforce our initial conclusion that cAMP alone mediates the effects of PTH on IP3R (7).
FIGURE 8.
Association of Gαs with AC·IP3R junctions. A, from the results shown in Fig. 7, C and D, the relationships between the change in cAMP content and potentiation of CCh-evoked Ca2+ signals by PTH are shown for HEK-PR1 and HEK-PR1αs− cells alone or after pretreatment with SQ/DDA (20 min). The right panel shows an enlarged version of the relationship for the lower ΔcAMP levels. Results are means ± S.E., n ≥ 3. B, proposed arrangement of AC·IP3R junctions. The central panel shows the signaling proteins that regulate the AC·IP3R junction, allowing AC6 to deliver super-saturating concentrations of cAMP to an associated IP3R2. The predicted consequences of incompletely inhibiting AC with low affinity inhibitors (SQ/DDA, right) or diminishing AC activity by removal of Gαs from AC·IP3R junctions (shRNA, left) are shown. As long as some Gαs remains associated with the junction, the cAMP safety margin allows effective signaling to the IP3R (upper left), but complete removal of Gαs from a junction incapacitates signaling (lower left). We speculate that ∼22 Gαs associate with each AC·IP3R junction (see text). Whether PTH receptors associate specifically with individual junctions has not yet been addressed.
Although cAMP is the only signal through which PTH communicates with IP3R, the effective cAMP cannot be uniformly distributed, because otherwise the effects of inhibiting cAMP formation should be identical whatever step in the signaling pathway is targeted for inhibition. We speculate that a small number of Gαs are specifically associated with each AC·IP3R junction (Fig. 8B). Random removal of Gαs from the signaling complex (by inhibition of Gαs expression) inactivates signaling from PTH to a complex without Gαs, whereas complexes with residual Gαs continue to function, albeit with a lesser cAMP “safety margin” (Fig. 8B, upper left) than normal junctions. Hence, for HEK-PR1αs− cells, PTH-potentiated Ca2+ signals are associated with lesser overall increases in cAMP than in HEK-PR1 cells (Fig. 8A). The maximal potentiated Ca2+ signal evoked by PTH is decreased by 30% in HEK-PR1αs− cells, even though lesser amounts of cAMP in normal cells with AC inhibited by SQ/DDA are associated with undiminished Ca2+ signals (Fig. 7D). These results suggest that the ≥95% knockdown of Gαs inactivates signaling by PTH to ∼30% of the AC·IP3R junctions. Assuming that a single Gαs is sufficient to mediate effective transmission to an AC·IP3R junction and that the 95% loss of Gαs is randomly distributed among the junctions, we predict (supplemental text) that there is an average of 1.1 Gαs within each functional synapse of Gαs-deficient cells, and ∼22 Gαs/synapse in normal cells.
Conclusions: Structure and Function of AC·IP3R Junctions
We have shown that all three IP3R subtypes are directly regulated by cAMP binding either directly to the IP3R or to a protein tightly associated with it (Figs. 1–4). The effect of cAMP, which increased the efficacy of IP3 (Fig. 4), required neither PKA (Fig. 6) nor the site through which ATP modulated IP3R2 activity (Figs. 4 and 5). In HEK cells, we have established that a specific association of IP3R2 with AC6 allows cAMP to pass directly from AC to the IP3R within an AC·IP3R junction (Fig. 8B) (7). This “binary mode” of cAMP signaling (Fig. 1A) allows robust communication between cell surface receptors and IP3R, because each AC·IP3R junction operates as an on-off switch with a large safety margin. The similar sensitivity of all IP3R to cAMP (Fig. 2) suggests that the specific requirement for IP3R2 in HEK cells derives from IP3R2 selectively associating with AC6 to form the junctions required to deliver cAMP at sufficient concentration to regulate IP3R. Results from cells with much reduced expression of Gαs (Figs. 7 and 8) suggest that small numbers of Gαs (∼22 αs/AC·IP3R junction) may also associate with the AC·IP3R junction (Fig. 8B).
IP3 and cAMP are ubiquitous intracellular messengers, and interactions between them are common. Here we demonstrate another level of interaction in which cAMP itself sensitizes each IP3R subtype by increasing the efficacy of IP3. The interaction is not mediated by PKA, nor does it involve the ATP-binding site of the IP3R. It remains to be resolved whether cAMP binds directly to IP3R or to a tightly associated accessory protein. Within intact HEK cells, a selective association of IP3R2 with AC6 to form an AC·IP3R junction (Fig. 1A) allows cAMP preferentially to regulate IP3R2. The G-protein, Gs, that couples PTH to stimulation of AC, appears also to selectively associate with the AC·IP3R signaling junction. Aside from defining a novel interaction between cAMP and Ca2+ signaling pathways, our results highlight the importance of the spatial organization of both pathways in mediating effective signal transduction.
Supplementary Material
Acknowledgments
We thank Dr. F. Wolfram (Cambridge) for completing preliminary functional analyses of Sf9 cells, and Drs. D. Yule (Rochester) and K. Mikoshiba (Tokyo) for kind gifts of DT40ΔATPB cells and plasmid encoding mouse IP3R2, respectively.
This work was supported in part by The Wellcome Trust (Grant 085295), the Medical Research Council, UK (Grant G0700843), and the Biotechnology and Biological Sciences Research Council, UK (Grant BB/E004660/1).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1, Table S1 and supplemental Experimental Procedures, Discussion, and an additional reference.
- PTH
- residues 1–34 of human parathyroid hormone
- AC
- adenylyl cyclase
- AC6
- type 6 adenylyl cyclase
- Bmax
- maximal concentration of binding sites
- [Ca2+]i
- intracellular free Ca2+ concentration
- CCh
- carbamylcholine chloride
- CLM
- cytosol-like medium
- DDA
- 2′,5′-dideoxyadenosine
- EC50
- half-maximally effective concentration
- H89
- N-[2-((p-bromocinnamyl)amino)ethyl]-5-isoquinoline sulfonamide
- HBS
- HEPES-buffered saline
- HEK-PR1 (HEK-PR1αs−)
- human embryonic kidney cells stably expressing human type 1 PTH receptor (and also stably expressing shRNA for αs)
- IP3
- inositol 1,4,5-trisphosphate
- IP3R
- IP3 receptor
- ORF
- open reading frame
- PKA
- protein kinase A (cAMP-dependent protein kinase)
- PS
- pipette solution
- shRNA
- short hairpin RNA
- SQ 22536
- 9-(tetrahydro-2′-furyl)adenine
- SQ/DDA
- 1 mm SQ 22536 with 200 μm DDA (used to inhibit AC)
- BAPTA
- 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- ATPB
- B binding site for ATP in IP3R2.
REFERENCES
- 1.Berridge M. J., Lipp P., Bootman M. D. (2000) Nat. Rev. Mol. Cell Biol. 1, 11–21 [DOI] [PubMed] [Google Scholar]
- 2.Willoughby D., Cooper D. M. (2007) Physiol. Rev. 87, 965–1010 [DOI] [PubMed] [Google Scholar]
- 3.Zaccolo M., Di Benedetto G., Lissandron V., Mancuso L., Terrin A., Zamparo I. (2006) Biochem. Soc. Trans. 34, 495–497 [DOI] [PubMed] [Google Scholar]
- 4.Scott J. D., Pawson T. (2009) Science 326, 1220–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Werry T. D., Wilkinson G. F., Willars G. B. (2003) Biochem. J. 374, 281–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruce J. I., Straub S. V., Yule D. I. (2003) Cell Calcium 34, 431–444 [DOI] [PubMed] [Google Scholar]
- 7.Tovey S. C., Dedos S. G., Taylor E. J., Church J. E., Taylor C. W. (2008) J. Cell Biol. 183, 297–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mongillo M., Tocchetti C. G., Terrin A., Lissandron V., Cheung Y. F., Dostmann W. R., Pozzan T., Kass D. A., Paolocci N., Houslay M. D., Zaccolo M. (2006) Circ. Res. 98, 226–234 [DOI] [PubMed] [Google Scholar]
- 9.Short A. D., Taylor C. W. (2000) J. Biol. Chem. 275, 1807–1813 [DOI] [PubMed] [Google Scholar]
- 10.Brummelkamp T. R., Bernards R., Agami R. (2002) Science 296, 550–553 [DOI] [PubMed] [Google Scholar]
- 11.Sugawara H., Kurosaki M., Takata M., Kurosaki T. (1997) EMBO J. 16, 3078–3088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tovey S. C., Sun Y., Taylor C. W. (2006) Nat. Protoc. 1, 259–263 [DOI] [PubMed] [Google Scholar]
- 13.Mignery G. A., Newton C. L., Archer B. T., 3rd, Südhof T. C. (1990) J. Biol. Chem. 265, 12679–12685 [PubMed] [Google Scholar]
- 14.Iwai M., Tateishi Y., Hattori M., Mizutani A., Nakamura T., Futatsugi A., Inoue T., Furuichi T., Michikawa T., Mikoshiba K. (2005) J. Biol. Chem. 280, 10305–10317 [DOI] [PubMed] [Google Scholar]
- 15.Blondel O., Takeda J., Janssen H., Seino S., Bell G. I. (1993) J. Biol. Chem. 268, 11356–11363 [PubMed] [Google Scholar]
- 16.Cardy T. J., Traynor D., Taylor C. W. (1997) Biochem. J. 328, 785–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nerou E. P., Riley A. M., Potter B. V., Taylor C. W. (2001) Biochem. J. 355, 59–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tovey S. C., Goraya T. A., Taylor C. W. (2003) Br. J. Pharmacol. 138, 81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brooker G., Harper J. F., Terasaki W. L., Moylan R. D. (1979) Adv. Cyclic Nucleotide Res. 10, 1–33 [PubMed] [Google Scholar]
- 20.Taufiq-Ur-Rahman, Skupin A., Falcke M., Taylor C. W. (2009) Nature 458, 655–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dellis O., Dedos S. G., Tovey S. C., Taufiq-Ur-Rahman, Dubel S. J., Taylor C. W. (2006) Science 313, 229–233 [DOI] [PubMed] [Google Scholar]
- 22.Sun Y., Taylor C. W. (2008) Biochem. J. 416, 243–253 [DOI] [PubMed] [Google Scholar]
- 23.Tu H., Wang Z., Nosyreva E., De Smedt H., Bezprozvanny I. (2005) Biophys. J. 88, 1046–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwai M., Michikawa T., Bosanac I., Ikura M., Mikoshiba K. (2007) J. Biol. Chem. 282, 12755–12764 [DOI] [PubMed] [Google Scholar]
- 25.Betzenhauser M. J., Wagner L. E., 2nd, Iwai M., Michikawa T., Mikoshiba K., Yule D. I. (2008) J. Biol. Chem. 283, 21579–21587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bezprozvanny I., Ehrlich B. E. (1993) Neuron 10, 1175–1184 [DOI] [PubMed] [Google Scholar]
- 27.Soulsby M. D., Wojcikiewicz R. J. (2007) Cell Calcium 42, 261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wojcikiewicz R. J., Luo S. G. (1998) J. Biol. Chem. 273, 5670–5677 [DOI] [PubMed] [Google Scholar]
- 29.Soulsby M. D., Wojcikiewicz R. J. (2005) Biochem. J. 392, 493–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Supattapone S., Danoff S. K., Theibert A., Joseph S. K., Steiner J., Snyder S. H. (1988) Proc. Natl. Acad. Sci. U.S.A. 85, 8747–8750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakade S., Rhee S. K., Hamanaka H., Mikoshiba K. (1994) J. Biol. Chem. 269, 6735–6742 [PubMed] [Google Scholar]
- 32.Tang T. S., Tu H., Wang Z., Bezprozvanny I. (2003) J. Neurosci. 23, 403–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giovannucci D. R., Groblewski G. E., Sneyd J., Yule D. I. (2000) J. Biol. Chem. 275, 33704–33711 [DOI] [PubMed] [Google Scholar]
- 34.Betzenhauser M. J., Fike J. L., Wagner L. E., 2nd, Yule D. I. (2009) J. Biol. Chem. 284, 25116–25125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burgess G. M., Bird G. S., Obie J. F., Putney J. W., Jr. (1991) J. Biol. Chem. 266, 4772–4781 [PubMed] [Google Scholar]
- 36.Dyer J. L., Mobasheri H., Lea E. J., Dawson A. P., Michelangeli F. (2003) Biochem. Biophys. Res. Commun. 302, 121–126 [DOI] [PubMed] [Google Scholar]
- 37.Quinton T. M., Dean W. L. (1992) Biochem. Biophys. Res. Commun. 184, 893–899 [DOI] [PubMed] [Google Scholar]
- 38.DeSouza N., Reiken S., Ondrias K., Yang Y. M., Matkovich S., Marks A. R. (2002) J. Biol. Chem. 277, 39397–39400 [DOI] [PubMed] [Google Scholar]
- 39.Wagner L. E., 2nd, Li W. H., Joseph S. K., Yule D. I. (2004) J. Biol. Chem. 279, 46242–46252 [DOI] [PubMed] [Google Scholar]
- 40.Wagner L. E., 2nd, Li W. H., Yule D. I. (2003) J. Biol. Chem. 278, 45811–45817 [DOI] [PubMed] [Google Scholar]
- 41.Wagner L. E., 2nd, Joseph S. K., Yule D. I. (2008) J. Physiol. 586, 3577–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bruce J. I., Shuttleworth T. J., Giovannucci D. R., Yule D. I. (2002) J. Biol. Chem. 277, 1340–1348 [DOI] [PubMed] [Google Scholar]
- 43.LeBeau A. P., Yule D. I., Groblewski G. E., Sneyd J. (1999) J. Gen. Physiol. 113, 851–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tu H., Tang T. S., Wang Z., Bezprozvanny I. (2004) J. Biol. Chem. 279, 19375–19382 [DOI] [PubMed] [Google Scholar]
- 45.Collado-Hilly M., Coquil J. F. (2009) Biol. Cell 101, 469–480 [DOI] [PubMed] [Google Scholar]
- 46.Schlossmann J., Ammendola A., Ashman K., Zong X., Huber A., Neubauer G., Wang G. X., Allescher H. D., Korth M., Wilm M., Hofmann F., Ruth P. (2000) Nature 404, 197–201 [DOI] [PubMed] [Google Scholar]
- 47.Cameron A. M., Steiner J. P., Roskams A. J., Ali S. M., Ronnett G. V., Snyder S. H. (1995) Cell 83, 463–472 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.