It is now almost 60 years since a Scottish pediatrician, Dr. James Farquhar, noticed the familial recurrence of a disease affecting male and female siblings aged 2 months, causing fever, cytopenia, hepatosplenomegaly, and rapidly leading to death despite treatment with antibiotics and steroids.1 Based on the familial recurrence and absence of bone lesions and granulomas, he recognized that this disease, characterized by ubiquitous infiltration by lymphocytes and histiocytes, often engulfed by blood cells, was clearly different from Abt-Letterer-Siwe disease, which had been described only a few years previously and which we now know as the most aggressive, disseminated clinical picture of Langerhans’ cell histiocytosis.
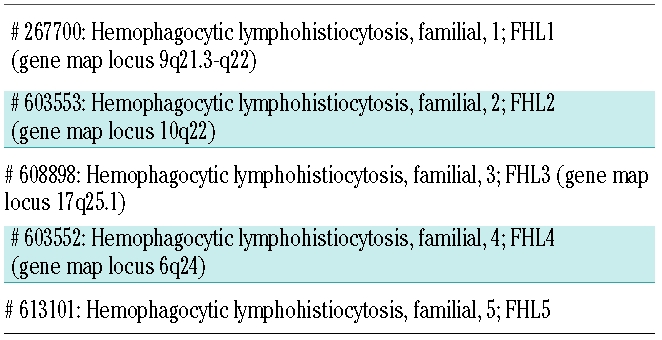
The road to the identification of the gene(s) causing familial hemophagocytic lymphohistiocytosis (FHL) was still long.2 Only at the end of the past century, in December 1999, did a group from Paris announce the identification of the first FHL-related gene. This started a new age for researchers and clinicians involved in the diagnosis and treatment of FHL. About 10 years later, we have learned that FHL is a very heterogeneous disorder. Five types of familial hemophagocytic lymphohistiocytosis are currently included in the OMIM database as reported in Table 1.
Table 1.
OMIM classification of familial hemophagocytic lymphohistiocytosis.
Familial hemophagocytic lymphohistiocytosis, type 1
A first gene mapping approach applied to four consanguineous families of Pakistani origin identified a 7.8 cM region on chromosome 9q21.3–22. Unfortunately, the underlying defect in this subset of the disease remains unknown.3
Familial hemophagocytic lymphohistiocytosis, type 2
Another report, published simultaneously to that describing FHL-1, documented linkage to region on chromosome 10q21–22 in 10/17 investigated families, indicating genetic heterogeneity.4 Mutations in the perforin gene (PRF1) were soon described at this locus.5
One of the main pathways of lymphocyte-mediated cytolysis involves secretion of cytolytic granules, contained in T-cell or NK-cell cytolytic effector lymphocytes, onto target membranes. Perforin is a pore-forming protein with a mechanism of trans-membrane channel formation similar to that of complement component 9 (C9), with which it shows extensive structural similarity. In patients with FHL-2, PRF1 mutations induce a complete or partial reduction of the synthesis of the perforin protein, resulting in impairment of the granule-mediated cytotoxic machinery of NK and T cells.5–7 As result, patients are unable to cope with infections caused by common pathogens because of insufficient killing of the infected cells and also of antigen-presenting cells. Overproduction of activating cytokines, especially tumor necrosis factor and interferon, drives the clinical manifestations of FHL.8
The ethnic origin of patients with FHL-2 is varied, with documented cases from all the continents. The age at disease onset is usually very young, with the median being 3 months, but the range is wide, reaching the third decade.9
Over 70 different mutations have been identified so far in the two coding exons, with more than half being missense mutations. Some associations have been noted between ethnic origin and the disease-causative mutations. The most common single mutation is c.1122G>A, resulting in p.W374X, found in 74% of Turkish patients with FHL-2 but not restricted to this ethnic subgroup, probably as a result of migration in the distant past.10 Only 10% of African (and African-American) patients with FHL do not harbor the c.50delT (p.L17fsX) mutation, which is thus confirmed as the founder mutation in this ethnic group.11 The c.1090-91delCT mutation, resulting in p.L364fsX, has never been identified in non-Japanese patients and thus seems to be confined to the Japanese ethnic group, in which it accounts for one-third of all the mutations. In this issue of the Journal, Kim et al. report the discovery of this mutation in a single Korean patient, likely as the result of migration in the past.12
Familial hemophagocytic lymphohistiocytosis, type 3
Since 2003, mutations of UNC13D have been associated with FHL.13 The UNC13D gene encodes for the Munc13-4 protein, a critical effector of the exocytosis of cytotoxic granules priming cytotoxic granule fusion. Munc13-4 deficiency impairs the delivery of the effector proteins, perforin and granzymes, into the target cells resulting in defective cellular cytotoxicity and a clinical picture which appears very similar to that of FHL-2.
UNC13D is a large gene with 32 coding exons, 4,389 base pairs and a translation length of 1,090 residues. Over 50 different mutations of this gene have been reported so far in patients with FHL-3. The mutations are scattered throughout the entire gene sequence, making mutation analysis far more complicated and time-consuming than for FHL-2. It was recently clarified that up to 15% of disease-related mutations in mammalian genes affect mRNA splicing. Yet, for some genes, such as NF1 and ATM, nucleotide substitutions affecting the splicing process may account for up to 50% of all pathogenic mutations. The splicing process involves many different proteins, including those of the spliceosome, which usually binds to the classical splice sequences located at exon-intron borders (donor and acceptor sites). However, auxiliary sequences, scattered throughout the gene, may serve as exonic and intronic splicing enhancers that help in the recognition of exons.
A few years ago, we identified several patients with FHL-3 and documented Munc13-4 protein deficiency,14 in whom only monoallelic mutations were identified by mutation analysis of the coding exons. This prompted us to investigate alternative defects of UNC13D. This approach showed that about one half of FHL-3-related mutations affect the splicing machinery.15 The 753+1G>T is the most frequently observed single mutation in patients from different countries.16 It falls at the consensus site and causes skipping of the adjacent exon. However, splice errors may also result from deep intronic mutations involving intronic splicing enhancer motifs which promote exon recognition. Deep intronic mutations might either create a new intronic splicing enhancer (IVS1+59C>T and +394C>T) or disrupt a pre-existing one (IVS1+525G>T). In particular, mutation IVS1+394C>T seems to promote activation of nearby splicing and insertion of a cryptic exon. These findings are in keeping with observations made in other human disease such as Alport syndrome (MIM 301050) and familial retinoblastoma (MIM 180200) caused by the COL4A5 and RB1 genes, respectively.
Data on correlations between genotype and phenotype in a large cohort of patients have not yet been reported. Based on the data that are available, the clinical picture of patients with FHL-3 does not appear to be different from that of patients with FHL-2, and associations of specific mutations with ethnic groups have not been identified (M. Aricò, U zur Stadt; unpublished data).
Familial hemophagocytic lymphohistiocytosis, type 4
Genome-wide homozygosity mapping in Turkish/Kurdish FHL kindreds found linkage to a 10 cM region on chromosome 6q24, in which the syntaxin 11 gene (STX11) maps.17
Syntaxin 11 is a member of the family of soluble N-ethylmaleimide sensitive factor attachment protein receptors present on target membranes (t-SNARE). SNARE proteins play a role in regulating intracellular protein transport between donor and target membranes. This docking and fusion process involves the interaction of specific vesicle-SNARE with specific cognate target-SNARE. Whereas it is understood how mutations leading to loss of perforin and Munc13-4 function can cause the manifestations of FHL, it is unclear how syntaxin-11 loss-of-function mutations contribute to the disease. It has been shown that resting NK and CD8+ T cells express syntaxin-11. NK cells from patients with FHL-4 fail to degranulate when encountering susceptible target cells. Unexpectedly, interleukin-2 stimulation partially restores NK-cell degranulation and cytotoxicity, which could explain why disease progression is observed to be less severe in FHL-4 patients than in FHL-2 and FHL-3 patients.18
Very few patients with FHL-4 have been reported so far, and almost all are of Turkish/Kurdish descent. Reported mutations include a 5 bp deletion, one large 19.2 kb genomic deletion spanning the entire coding region of STX11 (exon 2), and a nonsense mutation causing a premature stop codon in the C-terminal end of the protein.
Familial hemophagocytic lymphohistiocytosis, type 5
Very recently, zur Stadt et al. reported the identification of a novel FHL-related site at chromosome 19p in consanguineous families of Saudi Arabian or Turkish origin. Based on this finding, nine different mutations, either truncating or missense, were found in the syntaxin-binding protein-2 gene (STXBP2) in 12 patients from Turkey, Saudi Arabia, and Central Europe.19
STXBP2 encodes for syntaxin-binding protein-2 or Munc18-2 protein, involved in the regulation of vesicle transport to the plasma membrane. Syntaxin 11, the FHL-4 related protein, is an interaction partner of STXBP2. This interaction is eliminated by the missense mutations found in patients with FHL-5, which leads to decreased stability of both proteins. Patients homozygous for missense mutations or a 3-bp deletion had early-onset disease, diagnosed before 1 year of age, whereas the disease in the remaining five patients, who were homozygous for a splice site mutation or compound heterozygous for the splice site mutation and another mutation, manifested their disease after 1 year of age.
Soon thereafter, an independent study by Cote et al. from the Paris group confirmed these findings and also showed that defective exocytosis of NK-cell cytotoxic granules could be overcome by ectopic expression of wild-type STXBP2.20 Cases of FHL-5 have also been diagnosed in Italy and the United Kingdom.21 Given that the activity of NK and cytotoxic T cells is markedly reduced or absent in FHL-5, it appears that STXBP2 is required at a late step of the secretory pathway for the release of cytotoxic granules by binding syntaxin 11.
Diagnosing familial hemophagocytic lymphohistiocytosis: work in progress and future challenges
The diagnostic approach to FHL has changed over the years. Initially the diagnosis was based on the identification of a constellation of clinical signs and symptoms and biochemical alterations, supported by a very young age at presentation and a family history.2 The identification of impaired NK activity as a marker of FHL was the first diagnostic milestone, allowing the disease to be attributed to a functional defect.22 Nevertheless, the NK-cell cytotoxicity assay is laborious and not readily accessible to most clinicians, thus remaining a confirmatory assay restricted to reference laboratories. The identification of the genetic defects not only set the diagnostic standard, but also indicated the way forward for developing new tools for rapid screening of FHL.
In 2002, Kogawa et al. reported that NK, CD8+, and CD56+ cells from patients with PRF1 mutations lacked intracellular perforin, as detectable by flow-cytometry, thus providing a very reliable and rapid way of identifying patients with FHL-2.23 Based on the assumption that the cytotoxic machinery leads to surface expression of CD-107a (LAMP-1), our group demonstrated in 2006 that surface CD107a expression represents a rapid tool for identification of patients with a degranulation defect. On target interaction and degranulation, FHL-3 NK cells displayed low levels of surface CD107a staining, in contrast to cells from healthy control subjects or perforin-deficient NK cells.24 Thus, this assay became the standard for identifying patients with FHL-3. As initially hypothesized, patients with different genetic defects hampering cytotoxic cell degranulation, such as syntaxin 11 (FHL-4) and Munc 18-2 (FHL-5) can also be detected by this granule release assay.18–20
The diagnostic criteria proposed by the Histiocyte Society have been of great help in clinical practice and for therapeutic trials.25,26 Nevertheless, researchers are aware that they must be used as guidelines; the combination of fever, splenomegaly and elevated serum ferritin represents the starting point for the clinical suspicion of FHL. Flow-cytometry tests can provide an initial confirmation and direct the mutation analysis.27 The use of terms such as “primary” and “secondary” hemophagocytic lymphohistiocytosis turned out to be not very fortunate; although cases with a documented genetic defect are definitely “primary”, those in which the clinical syndrome is associated with evidence of a triggering infection, in the absence of a family history, might be erroneously defined as “secondary”, especially when initial treatment produces a marked improvement. The patient with FHL-3 described by Kim et al. in this issue of the Journal had a “waxing-and-waning course and eventually died from progressive disease”;12 other cases of infants with recurrent bouts of the disease and prolonged “remissions” lasting more than 1 year have been observed (M. Aricò; unpublished data). In such cases, the indication for hematopoietic stem cell transplantation, which is currently the only curative therapeutic approach,26,28 may be questioned, unless a clear genetic defect has been documented. Indeed, a few patients have been addressed to hematopoietic stem cell transplantation before being discovered to have a parasitic infection. We, therefore, recommend that complete functional and genetic studies are performed, especially in patients with an atypical phenotype (acute liver failure, acute encephalopathy) or late onset disease.
Current knowledge allows a genetic defect to be identified in variable proportions of patients, depending on their geographic area of provenience and ethnic origin. PRF1 mutations have been detected in 6–58% of non-Asian FHL patients, with clear differences in the distribution of mutations; for example, mutations in PRF1 were found in 44% of patients from Turkey, but in only 13% of patients from Germany.16 UNC13D mutations have not been found in Nordic patients, but were identified in 8% of Turkish patients, and 17% of German and Middle East patients. STX11 mutations (FHL-4) were identified in approximately 20% of FHL patients from Turkish/Kurdish families. Among Asian countries, data on the molecular genetics of FHL are available only from Japan; FHL-2 and FHL-3 accounted for approximately 19% and 20% to 25% cases of FHL in Japan, respectively.
The relation between diseases and monoalleic mutations should be interpreted with care. Patients with documented functional and/or protein defects likely deserve deeper investigation of the gene sequence, maybe including an RNA-approach. Otherwise, the hypothesis that the monoallelic mutation is not the pathogenic defect should be considered for patients for whom functional data are not available, given the occurrence of patients who are carriers of a heterozygous mutation (or polymorphism) in another of the FHL-related genes (M. Aricò; unpublished data).
X-linked lymphoproliferative disease (XLP1, MIM 308240) is a disorder that may present with symptoms resembling hemophagocytic lymphohistiocytosis; this disease has been associated with mutations in SH2D1A, encoding for an adaptor molecule called signaling lymphocyte activation molecule-associated protein (SAP) which is involved in signaling pathways that trigger cytotoxic granule release, and, more recently, X-linked inhibitor of apoptosis (XIAP, MIM 300635). There are three other rare genetic disorders that can present hemophagocytic lymphohistiocytosis-like symptoms together with distinctive additional features: Chédiak-Higashi syndrome (CHS, MIM 214500), Griscelli syndrome type 2 (GS2, MIM 607624) and Hermansky-Pudlak syndrome type 2 (HPS2, MIM 608233). The affected proteins in these diseases regulate release of cytotoxic granules, but also secretory granules in other cells (e.g. melanin granules in melanocytes resulting in partial albinism).
Although FHL is an autosomal recessive disorder, a possible detrimental role of monoallelic mutations in the FHL-related genes has been proposed. It is possible that moderately reduced NK activity associated with monoallelic mutations may reduce the efficiency of host defenses against tumor- and immune-mediated disorders. Association studies have shown a significantly increased frequency of monoallelic mutations of PRF1 or UNC13D in patients with lymphoma, leukemia, diabetes mellitus, multiple sclerosis, and juvenile arthritis.
Based on current knowledge, it seems that there are at least two additional FHL-related genes to be identified: one (or more) could explain the subset of patients with defective NK activity and an unassigned defect, while at least one more gene is expected to underlie FHL in a subset of families with normal NK activity (M. Aricò; unpublished data). In this regard, functional tools, such as the degranulation assay, confocal microscopy and protein studies, appear to be of paramount importance for pinpointing novel defects. Beyond providing a confirmation of the diagnosis and a genetic marker for family studies, the information gained during the investigation of patients with FHL has turned out to be of great value in improving our knowledge of some aspects of the immune system.
Footnotes
No potential conflicts of interests relevant to this article were reported.
References
- 1.Farquhar J, Claireaux A. Familial haemophagocytic reticulosis. Arch Dis Child. 1952;27(136):519–25. doi: 10.1136/adc.27.136.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aricò M, Janka G, Fischer A, Henter JI, Blanche S, Elinder G, et al. Hemophagocytic lymphohistiocytosis. Report of 122 children from the International Registry. FHL Study Group of the Histiocyte Society. Leukemia. 1996;10(2):197–203. [PubMed] [Google Scholar]
- 3.Ohadi M, Lalloz MR, Sham P, Zhao J, Dearlove AM, Shiach C, et al. Localization of a gene for familial hemophagocytic lymphohistiocytosis at chromosome 9q21.3–22 by homozygosity mapping. Am J Hum Genet. 1999;64(1):165–71. doi: 10.1086/302187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dufourcq-Lagelouse R, Jabado N, Le Deist F, Stephan JL, Souillet G, Bruin M, et al. Linkage of familial hemophagocytic lymphohistiocytosis to 10q21–22 and evidence for heterogeneity. Am J Hum Genet. 1999;64(1):172–9. doi: 10.1086/302194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stepp SE, Dufourcq-Lagelouse R, Le Deist F, Bhawan S, Certain S, Mathew PA, et al. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286(5446):1957–9. doi: 10.1126/science.286.5446.1957. [DOI] [PubMed] [Google Scholar]
- 6.Clementi R, zur Stadt U, Savoldi G, Varotto S, Conter V, De Fusco C, et al. Six novel mutations in the PRF1 gene in children with haemophagocytic lymphohistiocytosis. J Med Genet. 2001;38(9):643–6. doi: 10.1136/jmg.38.9.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trambas C, Gallo F, Pende D, Marcenaro S, Moretta L, De Fusco C, et al. A single amino acid change, A91V, leads to conformational changes that can impair processing to the active form of perforin. Blood. 2005;106(3):932–7. doi: 10.1182/blood-2004-09-3713. [DOI] [PubMed] [Google Scholar]
- 8.Aricò M, Danesino C, Pende D, Moretta L. Pathogenesis of haemophagocytic lymphohistiocytosis. Br J Haematol. 2001;114(4):761–9. doi: 10.1046/j.1365-2141.2001.02936.x. [DOI] [PubMed] [Google Scholar]
- 9.Clementi R, Emmi L, Maccario R, Liotta F, Moretta L, Danesino C, Aricó M. Adult onset and atypical presentation of hemophagocytic lymphohistiocytosis in siblings carrying PRF1 mutations. Blood. 2002;100(6):2266–7. doi: 10.1182/blood-2002-04-1030. [DOI] [PubMed] [Google Scholar]
- 10.Trizzino A, zur Stadt U, Ueda I, Risma K, Janka G, Ishii E, et al. Histiocyte Society HLH Study group. Genotype-phenotype study of familial haemophagocytic lymphohistiocytosis due to perforin mutations. J Med Genet. 2008;45(1):15–21. doi: 10.1136/jmg.2007.052670. [DOI] [PubMed] [Google Scholar]
- 11.Lee SM, Sumegi J, Villanueva J, Tabata Y, Zhang K, Chakraborty R, et al. Patients of African ancestry with hemophagocytic lymphohistiocytosis share a common haplotype of PRF1 with a 50delT mutation. J Pediatr. 2006;149(1):134–7. doi: 10.1016/j.jpeds.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Hoi-Soo Yoon, Hee-Jin Kim, Keon-Hee Yoo, Ki-Woong Sung, Hong-Hoe Koo, Hyoung-Jin Kang, et al. The UNC13D gene is the predominant causative gene with recurrent splicing mutations in Korean patients with familial hemophagocytic lymphohistiocytosis. Haematologica. 2010;95(4):622–6. doi: 10.3324/haematol.2009.016949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, Dumont C, et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Cell. 2003;115(4):461–73. doi: 10.1016/s0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- 14.Santoro A, Cannella S, Bossi G, Gallo F, Trizzino A, Pende D, et al. Novel Munc13-4 mutations in children and young adult patients with haemophagocytic lymphohistiocytosis. J Med Genet. 2006;43(12):953–60. doi: 10.1136/jmg.2006.041863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santoro A, Cannella S, Trizzino A, Bruno G, De Fusco C, Notarangelo LD, et al. Mutations affecting mRNA splicing are the most common molecular defect in patients with familial hemophagocytic lymphohistiocytosis type 3. Haematologica. 2008;93(7):1086–90. doi: 10.3324/haematol.12622. [DOI] [PubMed] [Google Scholar]
- 16.Zur Stadt U, Beutel K, Kolberg S, Schneppenheim R, Kabisch H, Janka G, Hennies HC. Mutation spectrum in children with primary hemophagocytic lymphohistiocytosis: molecular and functional analyses of PRF1, UNC13D,STX11,and RAB27A. Hum Mutat. 2006;27(1):62–8. doi: 10.1002/humu.20274. [DOI] [PubMed] [Google Scholar]
- 17.zur Stadt U, Schmidt S, Kasper B, Beutel K, Diler AS, Henter JI, et al. Linkage of familial hemophagocytic lymphohistiocytosis (FHL) type-4 to chromosome 6q24 and identification of mutations in syntaxin 11. Hum Mol Genet. 2005;14(6):827–34. doi: 10.1093/hmg/ddi076. [DOI] [PubMed] [Google Scholar]
- 18.Bryceson YT, Rudd E, Zheng C, Edner J, Ma D, Wood SM, et al. Defective cytotoxic lymphocyte degranulation in syntaxin-11 deficient familial hemophagocytic lymphohistiocytosis 4 (FHL4) patients. Blood. 2007;110(6):1906–15. doi: 10.1182/blood-2007-02-074468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.zur Stadt U, Rohr J, Seifert W, Koch F, Grieve S, Pagel J, et al. Familial hemophagocytic lymphohistiocytosis type 5 (FHL-5) is caused by mutations in munc18-2 and impaired binding to syntaxin 11. Am J Hum Genet. 2009;85(4):482–92. doi: 10.1016/j.ajhg.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Côte M, Ménager MM, Burgess A, Mahlaoui N, Picard C, Schaffner C, et al. Munc18-2 deficiency causes familial hemophagocytic lymphohistiocytosis type 5 and impairs cytotoxic granule exocytosis in patient NK cells. J Clin Invest. 2009;119(12):3765–73. doi: 10.1172/JCI40732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cetica V, Santoro A, Gilmour KG, Sieni E, Pende D, Marcenaro S, et al. STXBP2 mutations in children with familial hemophagocytic lymphohistiocytosis type 5. J Med Genet. doi: 10.1136/jmg.2009.075341. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez N, Virelizier JL, Arenzana-Seisdedos F, Fischer A, Griscelli C. Impaired natural killer activity in lymphohistiocytosis syndrome. J Pediatr. 1984;104(4):569–73. doi: 10.1016/s0022-3476(84)80549-1. [DOI] [PubMed] [Google Scholar]
- 23.Kogawa K, Lee SM, Villanueva J, Marmer D, Sumegi J, Filipovich AH. Perforin expression in cytotoxic lymphocytes from patients with hemophagocytic lymphohistiocytosis and their family members. Blood. 2002;99(1):61–6. doi: 10.1182/blood.v99.1.61. [DOI] [PubMed] [Google Scholar]
- 24.Marcenaro S, Gallo F, Martini S, Santoro A, Griffiths GM, Aricò M, et al. Analysis of natural killer-cell function in familial hemophagocytic lymphohistiocytosis (FHL): defective CD107a surface expression heralds Munc13-4 defect and discriminates between genetic subtypes of the disease. Blood. 2006;108(7):2316–23. doi: 10.1182/blood-2006-04-015693. [DOI] [PubMed] [Google Scholar]
- 25.Henter JI, Horne A, Arico M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124–31. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 26.Henter JI, Samuelsson-Horne A, Aricò M, Egeler RM, Elinder G, Filipovich AH, et al. Histocyte Society. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood. 2002;100(7):2367–73. doi: 10.1182/blood-2002-01-0172. [DOI] [PubMed] [Google Scholar]
- 27.Aricò M, Allen M, Brusa S, Clementi R, Pende D, Maccario R, et al. Haemophagocytic lymphohistiocytosis: proposal of a diagnostic algorithm based on perforin expression. Br J Haematol. 2002;119(1):180–8. doi: 10.1046/j.1365-2141.2002.03773.x. [DOI] [PubMed] [Google Scholar]
- 28.Cesaro S, Locatelli F, Lanino E, Porta F, Di Maio L, Messina C, et al. Hematopoietic stem cell transplantation for hemophagocytic lymphohistiocytosis: a retrospective analysis of data from the Italian Association of Pediatric Hematology Oncology (AIEOP) Haematologica. 2008;93(11):1694–701. doi: 10.3324/haematol.13142. [DOI] [PubMed] [Google Scholar]


