Abstract
Background
Hematopoietic stem cells located in the bone marrow interact with a specific microenvironment referred to as the stem cell niche. Data derived from ex vivo co-culture systems using mesenchymal stromal cells as a feeder cell layer suggest that cell-to-cell contact has a significant impact on the expansion, migratory potential and ‘stemness’ of hematopoietic stem cells. Here we investigated in detail the spatial relationship between hematopoietic stem cells and mesenchymal stromal cells during ex vivo expansion.
Design and Methods
In the co-culture system, we defined three distinct localizations of hematopoietic stem cells relative to the mesenchymal stromal cell layer: (i) those in supernatant (non-adherent cells); (ii) those adhering to the surface of mesenchymal stromal cells (phase-bright cells) and (iii) those beneath the mesenchymal stromal cells (phase-dim cells). Cell cycle, proliferation, cell division and immunophenotype of these three cell fractions were evaluated from day 1 to 7.
Results
Phase-bright cells contained the highest proportion of cycling progenitors during co-culture. In contrast, phase-dim cells divided much more slowly and retained a more immature phenotype compared to the other cell fractions. The phase-dim compartment was soon enriched for CD34+/CD38− cells. Migration beneath the mesenchymal stromal cell layer could be hampered by inhibiting integrin β1 or CXCR4.
Conclusions
Our data suggest that the mesenchymal stromal cell surface is the predominant site of proliferation of hematopoietic stem cells, whereas the compartment beneath the mesenchymal stromal cell layer seems to mimic the stem cell niche for more immature cells. The SDF-1/CXCR4 interaction and integrin-mediated cell adhesion play important roles in the distribution of hematopoietic stem cells in the co-culture system.
Keywords: hematopoietic stem cells, CXCR4, integrin β1
Introduction
Hematopoietic stem cells (HSC) are defined by their ability to give rise to all types of blood cells.1–3 During the last three decades hematopoietic stem cell transplantation has become a well established treatment for hematologic malignancies and non-malignant disorders. Lately HSC have been attracting ever increasing attention for their potential use in regenerative medicine and tissue engineering.4 HSC can be harvested from healthy donors by bone marrow aspiration, by peripheral stem cell mobilization or from cord blood. To improve the clinical outcome of autologous and allogeneic HSC transplantation many groups are focusing on ex vivo expansion of HSC particularly for those cases in which the graft is of limited size. Unfortunately, the expansion of HSC in vitro is difficult to achieve because as the cells proliferate they tend to differentiate.5 This is presumably caused by a lack of appropriate cues that are provided in vivo by the microenvironment. In nature HSC are located mainly in the bone marrow where they interact within a specific microenvironment called the stem cell niche, which regulates their fate in terms of quiescence, self-renewal and differentiation.6–8 Recent data suggest that quiescent HSC are primarily located in the trabecular endosteum (i.e. the osteoblastic niche) whereas dividing ones reside in sinusoidal perivascular areas (i.e. the vascular niche) of the bone marrow.9,10 HSC can be released from the vascular niche into the circulation in response to stress or injury.11 An orchestra of signals mediated by soluble factors and/or cell-to-cell contact regulates the balance and homeostasis of self-renewal, proliferation and differentiation in vivo.12 Though important regulatory components of the stem cell niche in vivo have been identified, this has not been translated into improved ex vivo expansion protocols for clinical applications. The best defined culture medium for HSC expansion is supplemented with cytokines such as fetal liver tyrosine kinase-3 ligand (FLT3-L), stem cell factor (SCF), interleukin-3 (IL-3) and thrombopoietin (TPO).13,14 Interestingly, mesenchymal stromal cells (MSC), which are characterized by multidifferentiation potential,15,16 are important components of the bone marrow HSC niche.17 In recent years, MSC have been shown to support HSC maintenance and engraftment.18,19 In addition, MSC have been described to have immuno-modulatory activity.20
According to several recent studies, including those from our laboratory, MSC facilitate HSC maintenance in an in vitro co-culture system through the secretion of soluble factors and cell-cell contact.21–24 In addition evidence is emerging that a three-dimensional architecture is important to mimic physiological conditions ex vivo.11,25 This prompted us to investigate how HSC interact with MSC in different spatial compartments over time in vitro. Usually HSC in co-culture are considered as a single population, and their localization relative to the MSC layer has not been investigated intensively. In this study, MSC served as a physical boundary of distinct compartments, and the properties and features of HSC in different sites were evaluated at various times in order to gain insight into the construction and function of a three-dimensional HSC–MSC co-culture microenvironment in vitro.
Design and Methods
Purification of CD34+ hematopoietic stem cells from mobilized peripheral blood
Mobilized peripheral blood was collected from healthy donors after treatment with 7.5 μg/kg granulocyte colony-stimulating factor for 5 days. CD34+ HSC were purified from leukapheresis samples using CD34 antibody-conjugated magnetic beads according to the manufacturer’s instructions (Miltenyi Biotec, Germany). The purity of CD34+ cells was evaluated by flow cytometry analysis using phycoerythrin (PE)-conjugated CD34 antibody (Miltenyi Biotec, Germany).
Isolation of mesenchymal stromal cells
MSC were isolated from bone marrow aspirates from healthy donors, after informed consent and approval by the local ethics committee, and cultured as described elsewhere.26 The phenotype of all MSC batches was tested by fluorescence-activated cell sorting (FACS): CDw90, CD105, CD166, and CD73 had to be present, whereas CD34 and CD45 had to be absent. MSC batches used in the co-cultures had been tested for their potential to differentiate along the osteogenic and adipogenic lineages using standard commercial differentiation media. MSC of passage two were then seeded in 12- or 24-well plates at a density of 1×104 cells/cm2 with MSC medium. The medium was changed every 3 days until the MSC feeder layer reached confluence.
Co-culture of hematopoietic stem cells with the mesenchymal stromal cell layer
CD34+ HSC were suspended in CellGro® SCGM medium (CellGenix, Freiburg, Germany) containing 10% fetal calf serum (Biochrom, Cambridge, UK), 150 ng/mL FLT3-L, 150 ng/mL SCF (both from Biosource, Camarillo, CA, USA) and 50 ng/mL IL-3 (Miltenyi Biotec, Germany). HSC suspensions were plated at a density of 1×104 cells/cm2 on a confluent MSC layer at 37°C in 5% CO2. In some experiments HSC were cultured without MSC in a cytokinedriven assay. After 4 days the cell suspension containing around 50% CD34+CD38− cells was seeded on an MSC layer for 5 h for further analysis.
Scanning electron microscopy
Samples for the scanning electron microscopy analysis were prepared as previously described.27 Briefly, the co-cultured cells growing on fibronectin-coated coverslips were fixed in 2% glutaraldehyde for 1 h at room temperature and then overnight at 4°C. After being subjected to dehydration in an acetone gradient (30–100%), cells were critical point-dried in a CO2 system (Critical Point Dryer CPD 030, BAL-TEC GmbH, Germany). Samples were then sputter-coated with gold (Sputter Coating Device SCD 050, BAL-TEC GmbH) and examined at 10 kV accelerating voltage in an environmental scanning electron microscope (PHILIPS XL 30 ESEM FEG, Philips, The Netherlands).
Time-lapse video microscopy
Freshly isolated CD34+ HSC were cultured on MSC growing on 24 x 60 mm fibronectin-coated coverslips attached to a silicone reusable chamber. During the time-lapse recording, cells were kept in a chamber at 37°C in a 5% CO2 atmosphere. Serial phase contrast images were captured with an inverted microscope (Zeiss Axiovert 200M; 20X objective) at 30 s intervals. The images were built into a movie using Metamorph software.
Immunofluorescence microscopy
After co-culture the supernatant was discarded and the MSC layer was washed twice gently. Next the remaining cells, including phase- bright and phase-dim cells along with the MSC layer, were fixed using 3.8% formaldehyde in phosphate-buffered saline (PBS) for 15 min and permeabilized using 0.1% Triton X-100 in PBS for 3 to 4 min. After blocking, cells were incubated with CD45-PE (1:10; Miltenyi Biotec, Germany) for 1 h and then incubated with antimouse- TRITC (1:400; Sigma, USA) and phaloidin-ALEXA488 (1:500; Invitrogen, UK) for 1 h at room temperature. The nuclei were then labeled with 4',6-diamidino-2-phenylindole (DAPI; Sigma, USA). Finally, confocal microscopy (Zeiss LSM 510, Germany) was used to determine fluorescence.
Cell collection from three distinct localizations
HSC/MSC co-cultures were prepared as described above. On each day during the first week HSC from three distinct localizations in the co-culture were collected separately. Briefly, the supernatant of the co-culture was aspirated and the cells in the supernatant (non-adherent cells) were collected. The MSC layer was gently washed twice with PBS to remove the remaining non-adherent cells. After washing, the cells remaining on the MSC layer (i.e. phase-bright cells) were collected by further intensive washing steps with PBS. When no phase-bright cells could be observed under phase-contrast microscopy, the MSC layer with the cells underneath the layer (phase-dim cells) was trypsinized and collected as well. To rule out any side effect of the trypsin treatment, non-adherent cells and phase-bright cells were also incubated with this enzyme for 5 min. Finally, the three cell fractions were counted using trypan blue (vitality more than 95%) and measured as described below.
Cell cycle analysis using propidium iodide
After collection, the cells were fixed overnight at 4°C with cold 70% ethanol. They were then washed and incubated in PBS with 50 μg/mL propidium iodide and 1 mg/mL RNase A (both from Sigma, USA) for 30 min at room temperature. After washing, the cells were measured by flow cytometry (FACScalibur; BD, Germany). Finally, the FACS data were analyzed using Modifit software (Becton Dickinson), and the proportions of cells in the G0/G1 phase, S phase and G2/M phase of the cell cycle were calculated automatically.
Polymerase chain reaction analysis
After 5 days of co-culture, cells from the three compartments were labeled with antihuman CD45-fluorescein isothiocyanate and CD166-PE. The cells were then sorted and MSC were discarded based on their forward-scatter and side-scatter properties, and CD45+CD166− staining using BD FACSAria and B&D FACSDiva software. Total RNA isolated by Trizol reagent (Invitrogen, USA) were reverse transcribed to total cDNA with oligo dT. Quantitative TaqMan real-time polymerase chain reaction (RT-PCR) was performed for cyclin-dependent kinase inhibitor 1A (CDKN1A, p21) according to the Taqman manufacturer’s instructions (Applied Biosystems, USA). The housekeeping gene GAPDH was used as a control. All primers were purchased from Applied Biosystems (USA). The relative gene expression of p21 was calculated by normalization to the expression level in phase-bright cells which was considered as one.
Immunophenotype analysis by flow activated cell sorting
After harvesting, each cell fraction was labeled with CD34-allophycocyanin, CD38-fluorescein isothiocyanate and CD45-PE (1:50; Miltenyi Biotec, Germany) at 4°C for 45 min. Appropriate murine antibodies served as a negative isotype control. After staining the cells were acquired using a FACScalibur (BD, Germany) and analyzed by CellQuest software (BD, Germany). Hematopoietic cells were gated according to their forward and side scatter properties and CD45 expression.
Cell generation tracking with carboxyfluorescein succinimidyl ester staining
Generations of HSC were identified using a CellTraceTM CFSE Cell Proliferation Kit (Invitrogen, UK). Briefly, CD34+ HSC were labeled by carboxyfluorescein succinimidyl ester (CFSE) according to the manufacturer’s instructions and placed in co-culture with MSC as described above. At fixed time points, the cells from the various compartments were collected and analyzed using FACS. The number of cell divisions was quantified according to CFSE signal intensity using CellQuest software. As a control CD34+ HSC were labeled with CFSE and treated with 50 μg/mL mitomycin (Santa Cruz) to arrest the cell population at generation 0. The CD34 expression pattern throughout subsequent cell generations was evaluated by CD34-APC and CFSE double-staining.
Colony-forming cell assay
The colony-forming assay was performed as described previously.24 Briefly, 1×103 cells from phase-bright and phase-dim fractions at day 5 were cultured for 14 days in complete methylcellulose medium with recombinant cytokines (MethoCult GF+ H4435; Stem Cell Technology, Vancouver, BC, Canada). Colony-forming units in culture (CFU-C) were subsequently scored with an inverted microscope. Experiments were performed in triplicate. Colonies were defined as clusters consisting of 40 or more cells.
Inhibition of hematopoietic stem cell migration under mesenchymal stromal cells by blocking integrin β1 and CXCR4
HSC were cultured in suspension without a MSC layer for 2 days. These primed HSC started to migrate under the MSC layer within 5 h. In addition, the primed HSC were pre-incubated for 1 h in normal culture medium supplemented with either 10 μg/mL integrin β1 antibody (BD, Germany)28 or 500 ng/mL AMD3100 (a non-peptide antagonist of CXCR4; Sigma, USA)24 or both 500 ng/mL AMD3100 and 10 μg/mL integrin β1 antibody. After blocking, cells (1×105/mL) were plated on the MSC layer for 5 h. Finally HSC from distinct localizations were counted using trypan blue, as mentioned before. P-selectin antibody (10 μg/mL; BD, Germany) was used as a control.
Statistical analysis
All data were derived from at least three independent experiments. Data are represented as the mean ± standard error of the mean (SEM) and analyzed with the paired Student’s test. Observed differences were regarded as statistically significant if the calculated two-sided P value was below 0.05.
Results
Identification of hematopoietic stem cell localization
To localize HSC co-cultured with MSC, we performed scanning electron microscopy. Interestingly, two distinct fractions of cells were observed (Figure 1A, B). HSC were located either on the surface of the MSC layer or beneath (Figure 1A). HSC were also detected migrating underneath the MSC (Figure 1A, B). The time-lapse video microscopy analysis depicted the movement of individual HSC migrating above or below the MSC layer (Figure 1C). By phase contrast microscopy, the HSC population underneath the MSC layer appeared as phase-dim cells whereas the HSC located above appeared as phase-bright cells (Figure 1D). The hematopoietic origin of these two cell fractions was confirmed by immunolabeling for CD45 (Figure 1E). Indeed, three optical confocal x-y–sections showed that both phase-bright and phase-dim cells were positive for CD45 (Figure 1F, H, respectively) whereas MSC were not (Figure 1G). Upon gently washing (for technical details see the Design and Methods section), the only remaining cells were those underneath the MSC layer, i.e the phase-dim cells (Figure 1I, J).
Figure 1.

Distinct compartmentalization of HSC cultured on MSC. (A, B) Scanning electron microscopy analysis revealed that HSC are either above the MSC layer (black asterisks) or beneath (white arrow). HSC migrating underneath the MSC layer were also observed (outlined white asterisks). (C) Individual frames taken from a time-lapse video depict an individual HSC moving above (black arrow) and below (white arrow) the MSC layer for a period of 54 min. M indicates MSC. (D) Phase-contrast microscopy analysis shows that HSC located above or beneath the MSC layer appeared as phase-bright and - dim cells, respectively. (E) Confocal laser scanning microscopy after immunolabeling for CD45. (F) Phase-bright cells on the surface of the MSC layer. (G) MSC layer. (H) Phase-dim cells beneath the MSC layer. (I) Phase contrast microscopy of the cells beneath the MSC layer (phase-dim cells), after removal of the phase-bright cells. (J) A cross-section is shown using immunofluorescence imaging (actin, green; nucleus, blue; CD45, red). Scale bar: 20 μm.
The mesenchymal stromal cell surface is the predominant site of hematopoietic stem cell proliferation
To determine the influence of cellular localization on HSC expansion, we counted cells in their separated environments. Until day 5 of co-culture, the numbers of non-adherent and phase-bright cells increased similarly and much faster than that of the phase-dim cells (Figure 2A). After day 5 the number of non-adherent cells increased further, while the number of phase-bright cells and phase-dim cells remained constant (Figure 2A). Interestingly although the cell count was highest for non-adherent cells, these cells were not in the G2/M phase as demonstrated by propidium iodide staining. In fact, as shown in Figure 2 B and C, the proportion of cells in the G2/M phase of the cell cycle was higher for phase-bright cells than for either non-adherent cells or phase-dim cells throughout the whole week of co-culture: for example, on day 3: phase-bright cells 11.9±2.7% versus non-adherent cells 0±0% (P<0.001) and phase-dim cells 3.6 ± 2.4% (P<0.001) and on day 7: phase-bright cells 13.9±1.0% versus non-adherent cells 1.3±1.2% (P<0.001) and phase-dim cells 2.7±2.0% (P<0.001). This is consistent with the expression of p21 which is an essential regulator for the quiescence of HSC.29 p21 expression was significantly diminished in phasebright cells at day 5 (Figure 2D). These data indicate that, in vitro, the MSC surface is enriched in proliferating progeny and that phase-bright cells may detach from the MSC surface on reaching a non-adherent status.
Figure 2.
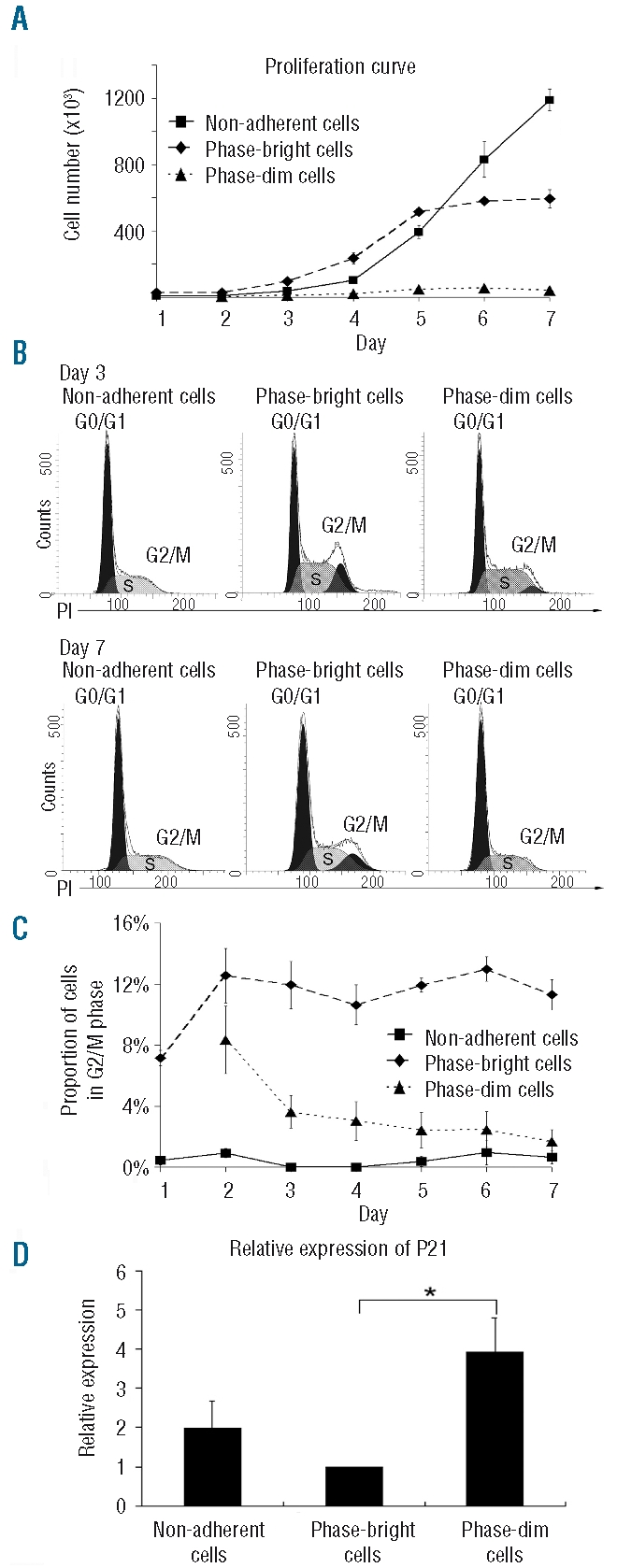
Cell proliferation and cell cycle status. (A) Proliferation kinetics of the three cell fractions (N=4). (B) Representative propidium iodide (PI) staining of cells from the three distinct compartments at day 3 and 7. (C) Dynamics of the three cell fractions in G2/M phase during 1 week of co-culture (N=3). (D) Relative expression of p21 in the three compartments at day 5 (N=3, *P<0.05). The data were normalized to the p21 expression in phase-bright cells, which was arbitrarily set at one.
Hematopoietic stem and progenitor cells beneath the mesenchymal stromal cell layer remain immature
To investigate the impact of the localization on HSC differentiation, HSC phenotypes were determined by FACS analysis. CD34 is a classical HSC marker,30 and CD34+CD38− cells are usually considered as a more primitive HSC population.31 After apheresis we performed MACS purification of CD34+ cells. We found that both CD34+ and CD34+CD38− cells were enriched in the phasedim fraction in comparison to in the phase-bright cells and the non-adherent cells (Figure 3). At day 2, almost 100% of phase-dim cells were CD34+CD38−, while the percentage of CD34+CD38− cells within the non-adherent and phasebright fractions was below 75%. Over the following days, the proportion of CD34+ and CD34+CD38− cells in the phase-dim fraction decreased as well, but the drop was significantly delayed in comparison to that in the other cell fractions. As shown in Figure 3A, CD38 was more highly expressed in non-adherent cells and phase-bright cells than in phase-dim cells at day 4. In addition, we found a higher concentration of clonogenic CFU-C within phase-dim cells than within non-adherent cells or phase-bright cells (phase-dim cells 150±20 colonies versus non-adherent cells 119±11 colonies (N = 6, P=0.07) and phase-bright cells 125±20 colonies (N = 6, P<0.05).
Figure 3.
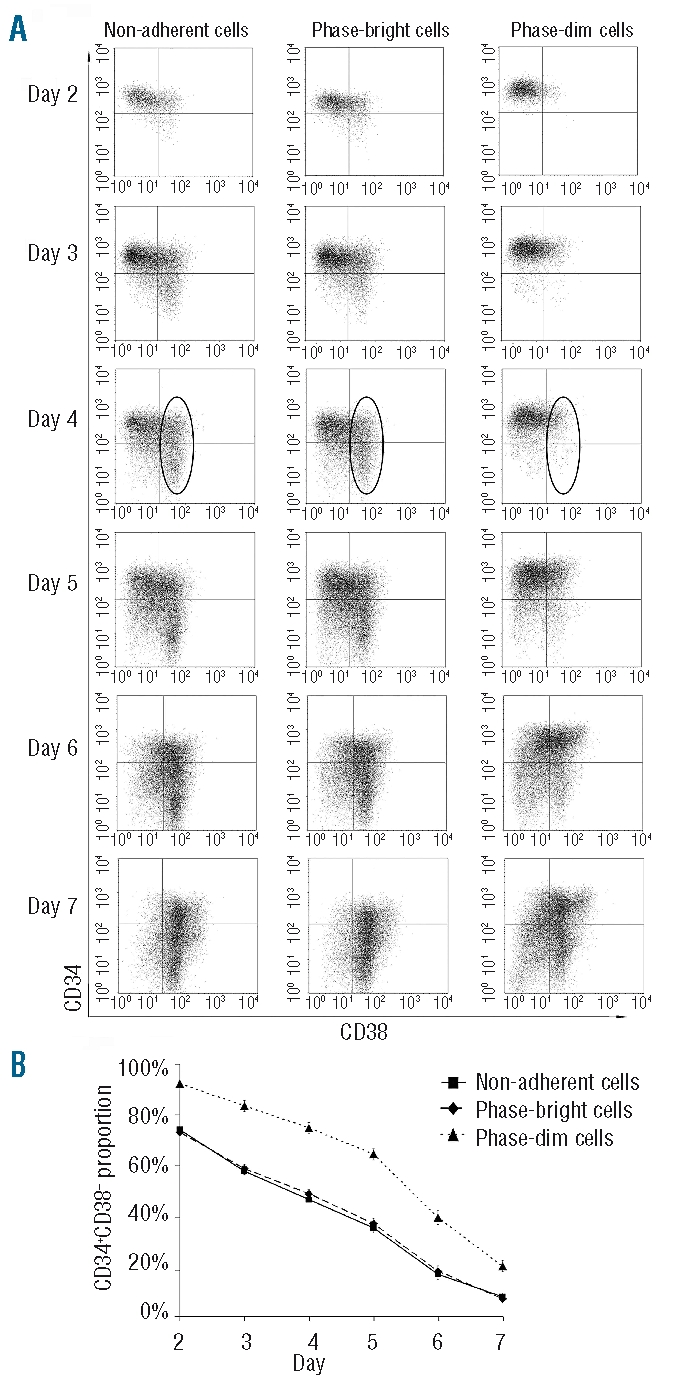
Immunophenotype of the cells in the three compartments. (A) Representative FACS analysis of the three cell fractions from day 2 to 7. The CD38+ cell fraction at day 4 is shown within the circle. (B) Proportion of CD34+CD38− cells in the three cell fractions during 1 week of co-culture (N=7).
Cell divisions in distinct localizations
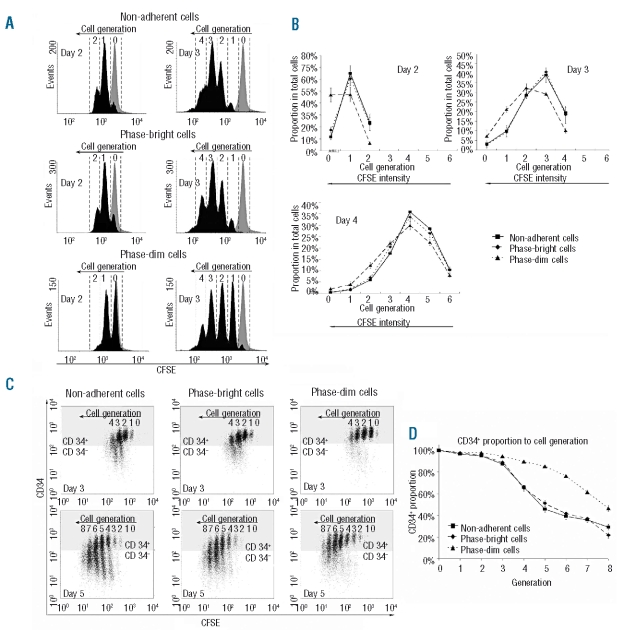
To determine the impact of localization on cell division, HSC generations were tracked by CFSE staining. Generation 0 was represented by the control, which was treated with mitomycin. The results of two representative experiments are shown in Figure 4A. At day 2, approximately 50% of phase-dim cells had not divided (generation 0), while less than 20% of the other two cell fractions were generation 0 cells. Over the following days, the proportion of cells that had undergone few divisions was higher among phase-dim cells than among the other two cell fractions. Comparing the distribution of cell generations in the three compartments at days 2, 3 and 4, the data indicate the down-regulating impact of the compartment beneath the MSC on cell division (Figure 4B).
Figure 4.
Tracking cell division in the three distinct compartments using CFSE staining. (A) Representative FACS analysis of CFSE staining at days 2 and 3. Cell generations were identified according to the control cells which were treated with mitomycin at day 0 (gray peak). (B) Distributions of cell generations of the three cell fractions at days 2, 3 and 4 (N=3). The pattern of CD34 expression in the different cell generations was studied. (C) Representative FACS analysis of CD34 and CFSE double staining at days 3 and 5. The dots in the gray region represent cells positive for CD34, the dots in the white region represent cells negative for CD34. Cell generations were identified according to the control cells which were treated with mitomycin at day 0. (D) CD34+ expression over eight cell generations within the three cell fractions (N = 8).
Immunophenotypes after each cell division
In order to determine the retention of the progenitor phenotype after each cell division, CFSE and CD34 double staining was performed in the three separated groups. After the first two cell divisions (in HSC, generations 0, 1 and 2), almost all cells from all three localizations were CD34+ (Figure 4C). However, in generations 3 and 4 only the phase-dim population retained a high percentage of CD34-expressing cells, which indicates their delayed differentiation compared to the other two cell fractions. As shown in Figure 4D, in the following cell divisions there were always more phase-dim cells that retained CD34 expression compared to the same HSC generation of the other two cell fractions. Taken together, the outcome of HSC division differed according to the localization of the cells, suggesting that the more slowly proliferating HSC grown beneath MSC retain their stem cell characteristics during cell division.
Immature hematopoietic stem cells migrate beneath the mesenchymal stromal cell layer
We then investigated whether more immature cells prefer the compartment beneath the MSC layer, migrating directly to this area in vitro. As already mentioned we detected phase-dim cells in newly established co-cultures only after day 2. However when purified HSC were cultured without MSC in a cytokine-supplemented medium for 4 days and then seeded on a MSC layer in a second culture, as described in the Design and Methods section, we saw up to 6% phase-dim cells already after 5 h of co-culture, indicating that primed HSC had an increased capacity for migration beneath the MSC layer. As shown in Figure 5A, HSC, of which 54.67±2.55% had a CD34+CD38− phenotype, were plated on the MSC layer. After 5 h the CD34+CD38− cells (78.23±7.65%) were enriched in the phase-dim fraction, suggesting that more immature HSC preferentially home beneath the MSC layer.
Figure 5.
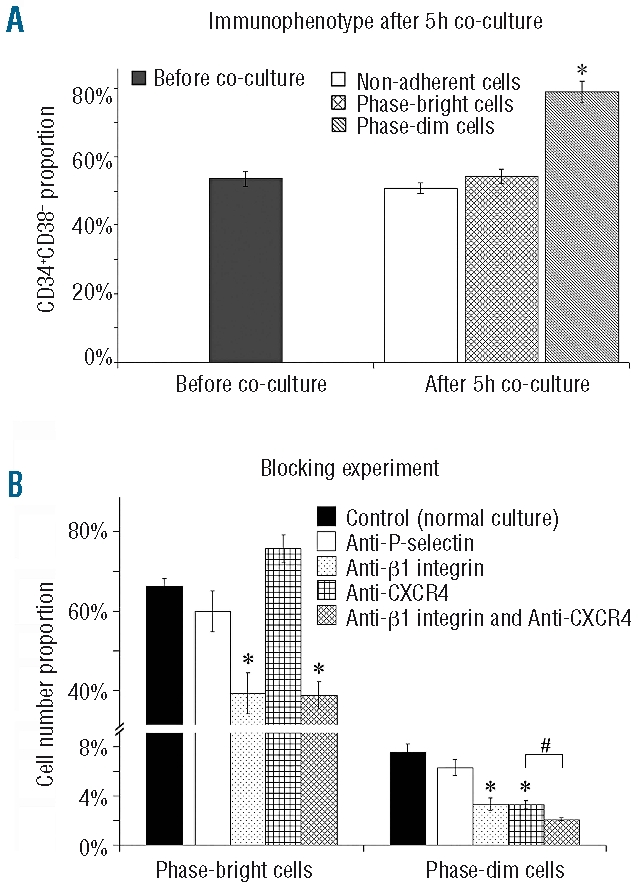
(A) Enrichment of CD34+CD38−cells in the phase-dim fraction. Cells containing around 50% CD34+CD38− cells were plated on the MSC layer. After 5 h the proportion of CD34+CD38− cells among the phase-dim fraction was enriched up to 80% (N=4, *P<0.01). (B) Blocking experiments for P-selectin, integrin β1 and CXCR4. By blocking integrin β1 or CXCR4 or both, percentages of phase-bright or phase-dim cells were down-regulated (N = 4, *P<0.01 (relative to control); #P<0.05 (relative to individual blocking for integrin β1 or CXCR4).
CXCR4 and integrins influence phase-dim cell formation
Recently we demonstrated that CXCR4 is up-regulated in the adherent-cell fraction in comparison to the non-adherent cell fraction.24 Other adhesion molecules, such as integrins, are also important for homing and migration. 28,32,33 The assay described above allowed us to investigate the impact of surface molecules on the primary attachment and migration of HSC underneath the MSC layer. First we blocked integrin β1. As shown in Figure 5B, this had the effect of reducing phase-bright cells from 69.89±4.08% to 40.56±12.45% (P<0.01) and phase-dim cells from 7.18±1.50% to 3.38%±1.19% (P<0.01), indicating that at least integrin β1 takes part in the process of HSC adhesion on the MSC surface and migration beneath the MSC layer. Next we blocked CXCR4 by adding AMD3100 (Figure 5B). Although the count of phase-bright cells was not reduced (69.89±4.08% versus 75.33±8.42%), phase-dim cells were diminished from 7.18±1.50% to 3.19±0.77% (P<0.01), indicating that in our assay CXCR4 plays a role in HSC migration beneath the MSC layer. Finally by blocking both CXCR4 and integrin β1, the count of phase-dim cells was reduced to 1.97±0.18%, which is a further reduction compared to that produced by individual blocking of integrin β1 or CXCR4 (3.38±1.19% and 3.19±0.77%, respectively), indicating that both integrins and CXCR4 are involved in the migration of HSC beneath the MSC feeder layer. Our data also confirm that CXCR4 has no impact on adhesiveness, as we have already recently described.24 Blocking P-selectin as a control did not cause significant decreases of phase-bright or phase-dim cells.
Discussion
Many studies have demonstrated that HSC can be expanded in cytokine-driven culture. However, this kind of expansion is accompanied by concomitant differentiation and gradual loss of ‘stemness’. In recent years, it has been shown that stromal feeder layers can be used to support HSC expansion. It has become evident that the interaction between HSC and MSC is an important issue for keeping HSC quiescent both in vivo and in vitro.11 We and others have shown that cell-to-cell contact in vitro has a significant impact on the functional, phenotypic, and clonogenic parameters of HSC. We have also demonstrated that direct contact with MSC affects the migratory behavior and gene expression profile of CD133+ HSC during ex vivo expansion.24
In the present study, we investigated the distribution of HSC in the co-culture assay in more detail. We observed that three different sites in the co-culture system represent distinct microenvironments for HSC, with a significant impact on their fate in vitro. By performing phase-contrast, confocal and electron microscopy, we separated HSC that were non-adherent, cells adherent to the surface of the MSC layer, and cells that had migrated beneath the feeder layer. A confluent MSC layer may serve as a boundary between two distinct compartments. These spatial constraints influence the proliferation and differentiation of HSC. One remarkable difference is the cell cycle status of cultured HSC. It is known that immediately after HSC have been isolated from peripheral blood, they usually are in G0/G1.34 Their status, however, changes if the cells are plated out in expansion assays with stimulating factors.13 In our study phase-bright cells significantly and consistently showed an increase of G2/M cells (over 10%) throughout the whole culture period. In contrast, among the non-adherent and phasedim fractions, there were almost no cells in the G2/M phase. Thus, G2/M phase cells were mainly on the surface and not in a non-adherent fraction. However, the number of non-adherent cells increased continuously, suggesting that the adherent fraction supplies the non-adherent fraction with detaching cells. We have evidence that the cell-to-cell contact on the surface of the MSC layer promotes the cell cycle, although the mechanism has not yet been clarified in detail.
Interestingly we found that in our co-culture system the translocation between HSC on the surface and those beneath the MSC layer was between two distinct compartments. Performing serial studies we found that phase-dim cells retained a more immature phenotype compared to that of the HSC on the surface of the MSC layer and had a significantly delayed rate of cell division in comparison to the other two cell fractions. We, therefore, speculate that the MSC boundary layer has a significant effect on HSC fate. Admittedly, HSC are highly motile and able to move between the compartments. Our daily measurements still represent snap-shots taken at defined time-points, which may not represent the whole complex dynamics of the process.
It is still not clear whether the environment beneath the MSC layer actively keeps HSC in an immature state or whether it creates a ‘niche’ atmosphere that specifically attracts HSC. We speculate that both mechanisms are involved. We were at least able to demonstrate that CD34+CD38− HSC preferentially migrate through the MSC layer.
The interaction between HSC and MSC, via soluble factors (SDF1/CXCR4) or adhesion molecules (integrins), also has an impact on HSC migration between the two microenvironments. Phase-dim cell formation was reduced by blocking integrins and CXCR4. This effect was further enhanced by combined blockade, indicating that β1-integrins and the SDF1/CXCR4 axis play synergistic roles in phase-dim cell formation. Interestingly the count of phase-bright cells was not reduced by AMD3100, indicating that CXCR4 is not relevant in adhesiveness, confirming our recently published data.24
In summary, we were able to distinguish three different compartments in our co-culture system: (i) the supernatant, i.e. the milieu in which HSC grow without direct contact with MSC; (ii) the surface of MSC; and (iii) the environment beneath the MSC layer. All three locations are dynamically linked with each other, and are characterized by special features. Specifically, the niche-like microenvironment (i.e. beneath MSC) probably recruits and retains HSC with more primitive properties whereas the MSC surface favors the active expansion of HSC.
Interestingly, in vivo there is a similar but certainly even more complex system of niches. It has been reported that quiescent HSC are preferentially located in osteoblastic niches, while more actively cycling and self-renewing HSC are kept in the perivascular niche.11,35 The three-compartment co-culture system probably mimics the cooperation of stem cell niches in vivo.
The model of the HSC in vitro niche is illustrated in Figure 6, showing the MSC surface as the major site of HSC proliferation and the microenvironment beneath the MSC layer as the storage site of more primitive cells, all affected by an orchestra of various soluble and non-soluble signals. Further investigations are needed to study the complex mechanism of HSC expansion in vitro, including investigation of the repopulating potential of the different cell fractions by performing in-vivo repopulation experiments.
Figure 6.
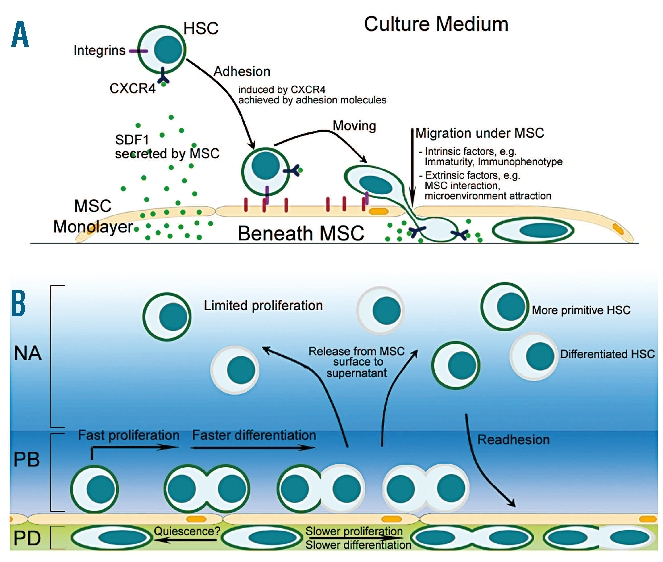
Graphic of the in vitro HSC/MSC co-culture system. (A) Migration towards and retention of HSC to MSC is mediated by SDF1/CXCR4 and adhesion molecules such as integrins. (B) The MSC layer serves in vitro as a boundary between two distinct compartments, i.e. the MSC surface mediating cell proliferation and a niche-like compartment beneath the layer. Here beneath the layer, phase-dim (PD) cells show a delayed cell-cycle activity and a more immature phenotype. In contrast, phase-bright (PB) HSC on the MSC surface revealed significantly more proliferation activity. We assume that cells from the MSC surface are released into the supernatant, the third microenvironment (non-adherent cells, NA) in the co-culture system. The graphic illustrates the dynamic interplay of HSC in the three compartments.
Acknowledgments
we thank Michelle Stewart for helpful discussions.
Footnotes
Funding: this work was supported by DFG grant “Collaborative Research Center 655”: Cells into tissues: stem cell and progenitor commitment and interactions during tissue formation (SFB 655).
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
JD performed the experiments, analyzed the data and wrote the paper; AVF performed electron microscopy and time-lapse microscopy studies; NA performed the co-culture studies; FAF analyzed the data; KM prepared primary human cells; MB and GE revised the paper; DC and RO designed the research, analyzed the data and revised the manuscript. All authors read the final version and agreed to the content of the manuscript.
The authors reported no potential conflicts of interest.
References
- 1.Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273(5272):242–5. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 2.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404(6774):193–7. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 3.Metcalf D. On hematopoietic stem cell fate. Immunity. 2007;26(6):669–73. doi: 10.1016/j.immuni.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Gordon MY. Stem cells for regenerative medicine – biological attributes and clinical application. Exp Hematol. 2008;36(6):726–32. doi: 10.1016/j.exphem.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Sorrentino BP. Clinical strategies for expansion of haematopoietic stem cells. Nat Rev Immunol. 2004;4(11):878–88. doi: 10.1038/nri1487. [DOI] [PubMed] [Google Scholar]
- 6.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425 (6960):778–9. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–41. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 8.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441(7097):1075–9. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 9.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–21. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Kiel MJ, Morrison SJ. Maintaining hematopoietic stem cells in the vascular niche. Immunity. 2006;25(6):977–88. doi: 10.1016/j.immuni.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6(2):93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 12.Blank U, Karlsson G, Karlsson S. Signaling pathways governing stem-cell fate. Blood. 2008;111(2):492–503. doi: 10.1182/blood-2007-07-075168. [DOI] [PubMed] [Google Scholar]
- 13.Heike T, Nakahata T. Ex vivo expansion of hematopoietic stem cells by cytokines. Biochim Biophys Acta. 2002;1592(3):313–21. doi: 10.1016/s0167-4889(02)00324-5. [DOI] [PubMed] [Google Scholar]
- 14.Ueda T, Tsuji K, Yoshino H, Ebihara Y, Yagasaki H, Hisakawa H, et al. Expansion of human NOD/SCID-repopulating cells by stem cell factor, Flk2/Flt3 ligand, thrombopoietin, IL-6, and soluble IL-6 receptor. J Clin Invest. 2000;105(7):1013–21. doi: 10.1172/JCI8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorrentino A, Ferracin M, Castelli G, Biffoni M, Tomaselli G, Baiocchi M, et al. Isolation and characterization of CD146+ multipotent mesenchymal stromal cells. Exp Hematol. 2008;36(8):1035–46. doi: 10.1016/j.exphem.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–13. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–36. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 18.Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP, Gerson SL. Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res. 2000;9(6):841–8. doi: 10.1089/152581600750062264. [DOI] [PubMed] [Google Scholar]
- 19.Noort WA, Kruisselbrink AB, in't Anker PS, Kruger M, van Bezooijen RL, de Paus RA, et al. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol. 2002;30(8):870–8. doi: 10.1016/s0301-472x(02)00820-2. [DOI] [PubMed] [Google Scholar]
- 20.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 21.Breems DA, Blokland EA, Siebel KE, Mayen AE, Engels LJ, Ploemacher RE. Stroma-contact prevents loss of hematopoietic stem cell quality during ex vivo expansion of CD34+ mobilized peripheral blood stem cells. Blood. 1998;91(1):111–7. [PubMed] [Google Scholar]
- 22.Gottschling S, Saffrich R, Seckinger A, Krause U, Horsch K, Miesala K, Ho AD. Human mesenchymal stromal cells regulate initial self-renewing divisions of hematopoietic progenitor cells by a beta1-integrin-dependent mechanism. Stem Cells. 2007;25(3):798–806. doi: 10.1634/stemcells.2006-0513. [DOI] [PubMed] [Google Scholar]
- 23.Wagner W, Roderburg C, Wein F, Diehlmann A, Frankhauser M, Schubert R, et al. Molecular and secretory profiles of human mesenchymal stromal cells and their abilities to maintain primitive hematopoietic progenitors. Stem Cells. 2007;25(10):2638–47. doi: 10.1634/stemcells.2007-0280. [DOI] [PubMed] [Google Scholar]
- 24.Alakel N, Jing D, Muller K, Bornhauser M, Ehninger G, Ordemann R. Direct contact with mesenchymal stromal cells affects migratory behavior and gene expression profile of CD133+ hematopoietic stem cells during ex vivo expansion. Exp Hematol. 2009;37(4):504–13. doi: 10.1016/j.exphem.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116(6):769–78. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 26.Oswald J, Boxberger S, Jørgensen B, Feldmann S, Ehninger G, Bornhäuser M, Werner C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22(3):377–84. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 27.Freund D, Bauer N, Boxberger S, Feldmann S, Streller U, Ehninger G, et al. Polarization of human hematopoietic progenitors during contact with multipotent mesenchymal stromal cells: effects on proliferation and clonogenicity. Stem Cells Dev. 2006;15(6):815–29. doi: 10.1089/scd.2006.15.815. [DOI] [PubMed] [Google Scholar]
- 28.Fierro FA, Taubenberger A, Puech PH, Ehninger G, Bornhauser M, Muller DJ, Illmer T. BCR/ABL expression of myeloid progenitors increases beta1-integrin mediated adhesion to stromal cells. J Mol Biol. 2008;377(4):1082–93. doi: 10.1016/j.jmb.2008.01.085. [DOI] [PubMed] [Google Scholar]
- 29.Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287(5459):1804–8. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 30.Krause DS, Fackler MJ, Civin CI, May WS. CD34: structure, biology, and clinical utility. Blood. 1996;87(1):1–13. [PubMed] [Google Scholar]
- 31.Petzer AL, Hogge DE, Landsdorp PM, Reid DS, Eaves CJ. Self-renewal of primitive human hematopoietic cells (long-term-culture- initiating cells) in vitro and their expansion in defined medium. Proc Natl Acad Sci USA. 1996;93(4):1470–4. doi: 10.1073/pnas.93.4.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106(6):1901–10. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 33.Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95(11):3289–96. [PubMed] [Google Scholar]
- 34.Uchida N, He D, Friera AM, Reitsma M, Sasaki D, Chen B, et al. The unexpected G0/G1 cell cycle status of mobilized hematopoietic stem cells from peripheral blood. Blood. 1997;89(2):465–72. [PubMed] [Google Scholar]
- 35.Xie Y, Yin T, Wiegraebe W, He XC, Miller D, Stark D, et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2009;457(7225):97–101. doi: 10.1038/nature07639. [DOI] [PubMed] [Google Scholar]