Abstract
Background
About one half of adults with acute lymphoblastic leukemia are not cured of the disease and ultimately die. The objective of this study was to explore the factors influencing the outcome of adult patients with relapsed acute lymphoblastic leukemia.
Design and Methods
We analyzed the characteristics, the outcome and the prognostic factors for survival after first relapse in a series of 263 adult patients with acute lymphoblastic leukemia (excluding those with mature B-cell acute lymphoblastic leukemia) prospectively enrolled in four consecutive risk-adapted PETHEMA trials.
Results
The median overall survival after relapse was 4.5 months (95% CI, 4–5 months) with a 5-year overall survival of 10% (95% CI, 8%–12%); 45% of patients receiving intensive second-line treatment achieved a second complete remission and 22% (95% CI, 14%–30%) of them remained disease free at 5 years. Factors predicting a good outcome after rescue therapy were age less than 30 years (2-year overall survival of 21% versus 10% for those over 30 years old; P<0.022) and a first remission lasting more than 2 years (2-year overall survival of 36% versus 17% among those with a shorter first remission; P<0.001). Patients under 30 years old whose first complete remission lasted longer than 2 years had a 5-year overall survival of 38% (95% CI, 23%–53%) and a 5-year disease-free survival of 53% (95% CI, 34%–72%).
Conclusions
The prognosis of adult patients with acute lymphoblastic leukemia who relapse is poor. Those aged less than 30 years with a first complete remission lasting longer than 2 years have reasonable possibilities of becoming long-term survivors while patients over this age or those who relapse early cannot be successfully rescued using the therapies currently available.
Keywords: acute lymphoblastic leukemia, relapse, rescue treatment, prognostic factors
Introduction
About one half of adults with acute lymphoblastic leukemia (ALL) are not cured of the disease and ultimately die. After front-line therapy, rates of complete remission have ranged between 78% and 93% in recent clinical trials. However, one third of patients with standard-risk ALL and two thirds of high-risk patients relapse.1–7 A second remission may be achieved, however post-relapse treatment rarely results in long-term survival.8–11 Some form of allogeneic stem cell transplantation (SCT) is considered as the best treatment option after achievement of a second remission if the patient’s age, performance status and donor availability allow.12 However, due to early relapse or poor performance status after re-induction therapy, allogeneic SCT is performed in only a subgroup of patients in second remission. The effect of prior submission to a SCT procedure during first complete remission on the feasibility of and outcome after a second procedure has seldom been explored.11 It is important to identify those patients who have reasonable survival options after salvage treatment including allogeneic SCT and, conversely, the patients with poor survival expectancy in order to include them in experimental therapies. Since 1989, the PETHEMA (Programa Español de Tratamiento en Hematologia) trials have used a common backbone of treatment for adult patients with newly diagnosed ALL, consisting of 4 weeks of induction therapy, early consolidation with short courses of combined active drugs and delayed consolidation, including further courses of combined cytotoxic drugs, followed by 2 years of maintenance treatment or, alternatively, an autologous or allogeneic SCT in some patients with high-risk ALL.13–16 Data from a very extended follow-up of this large cohort of prospectively enrolled patients after a common approach to initial therapy are currently available, thereby providing optimal information for identifying prognostic factors for the achievement of a second remission and subsequent survival in patients with relapsed ALL and for defining subsets of patients likely to benefit from rescue treatment. We analyzed the outcome of 263 adult patients who relapsed following treatment in the PETHEMA ALL89,13 ALL93HR,14 ALL96SR,16 and ALL03HR17 trials.
Design and Methods
Initial diagnostic procedures, classification and treatment
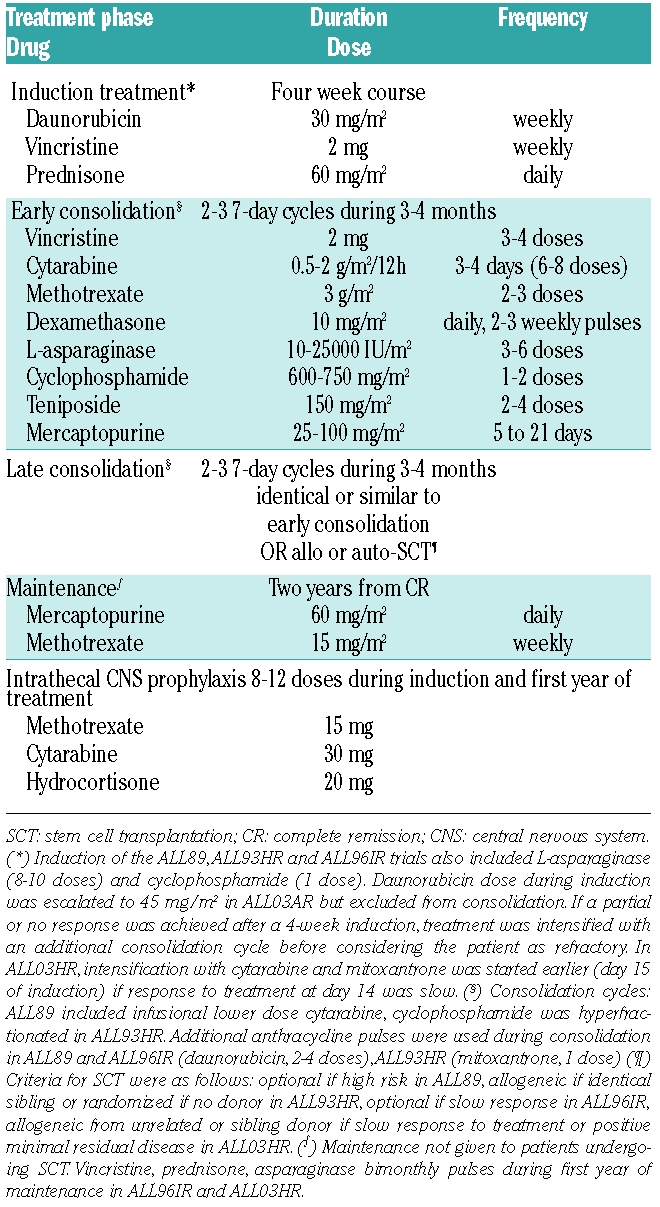
Patients aged 15 to 70 years old with newly diagnosed ALL (excluding mature B-ALL) and no prior condition preventing intensive treatment were included in the PETHEMA trials ALL89, ALL93HR, ALL96SR and ALL03HR. The institutional review boards of each participating center approved the studies. All patients gave informed consent to participation. The diagnosis of ALL was established according to standard criteria at each institution and central review of cytogenetics and immunophenotyping studies was performed. Philadelphia chromosome status was assessed by cytogenetic analysis and by molecular methods, when applicable. Patients were defined as having standard risk when their presenting white blood cell (WBC) count was below 30×109/L, they were under 30 years old and had no bad risk cytogenetic findings. Patients were considered as being at high-risk when their presenting WBC count was over 30×109/L, they were aged over 30 years old or had bad risk cytogenetic findings, defined as t(9;22), t(1;19) or t(4;11). The ALL89 trial included both standard- and high-risk patients (intensity of consolidation was greater in the high-risk patients), while the ALL93HR and ALL03HR trials enrolled only high-risk patients. The ALL96SR trial included standard-risk patients and high-risk patients of advanced age or with co-morbid conditions precluding the consolidation therapy planned for those with high-risk ALL. Standard-risk patients with a slow response or no response to the initial 4-week induction had the option to switch to the intensification or consolidation protocols planned for the high-risk patients. The results of each specific protocol have been fully published13–16 except those of the ALL03HR, for which only a preliminary report is available17 since the follow-up is still ongoing. A simplified scheme of the backbone characteristics of the four protocols is given in Table 1.
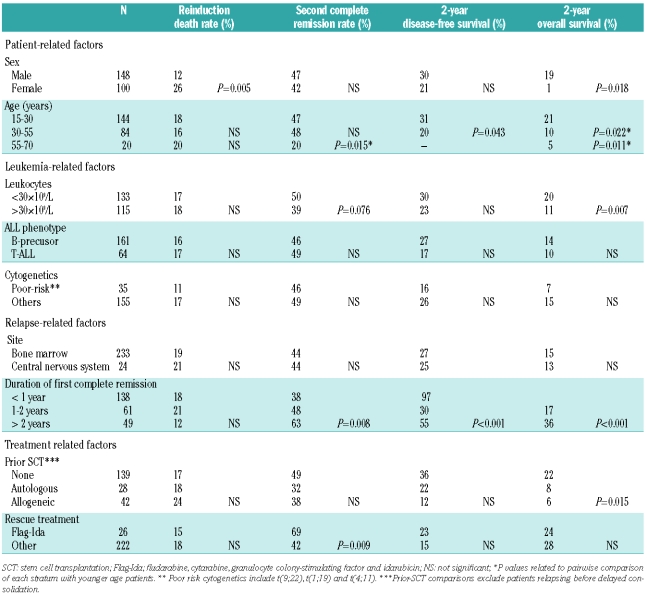
Table 1.
All patients included in the trials before January 2006 who achieved complete remission and for whom there was at least 3 years of follow-up after obtaining the complete remission were reviewed. Patients identified as having relapsed were selected and critical data (date of relapse, date of second remission if attained, date of subsequent relapse, survival status, date of last contact or death and cause of death) were obtained for all patients. Therapy after relapse, dates of second or subsequent complete remissions, autologous or allogeneic SCT procedures and subsequent relapses were also available for most patients. Data on all patients still alive were updated in January 2009.
Statistical analysis
Homogeneity of outcome across initial treatments was analyzed after stratifying the patients according to initial risk (standard versus high) and age (15–30, 30–55 and 55–70 years) using the χ2 and log-rank tests.18 Disease-free survival was defined as the time from second remission to last control, subsequent relapse or death; overall survival was defined as the time from first relapse to last control or death. The variables tested for prognostic significance after relapse included patient-related data (age, gender), leukemia-related factors (WBC count, phenotype and cytogenetics), relapse-related factors (duration of first complete remission and site of relapse) and treatment. Differences in relative risks of death during reinduction or achievement of second complete remission were assessed using the χ2 test. Kaplan-Meier curves19 and log-rank statistics were used for comparisons of disease-free survival and overall survival. Significant variables in univariate studies for second remission, disease-free survival and overall survival were included in multivariate logistic or Cox regression models. 20 Statistical analyses were performed using a SPSS package v15.0 (Chicago, IL, USA).
Results
Initial characteristics of patients and response to first-line treatment
Of the initial cohort of 589 patients, 427 (72%) had high-risk characteristics and 162 (28%) had standard-risk ALL. The complete remission rate was 69% in patients over 55 years old, 86% in patients 30–50 years old, 92% in high-risk patients under 30 years and 98% in standard-risk patients. Five-year overall survival rates for these four subgroups were 14% (95% CI, 6–23), 29% (95% CI, 23–35), 38% (95% CI, 32–45) and 69% (95% CI, 64–74), respectively. The outcome of patients was uniform across protocols when adjusted for age and risk. Of the 523 patients (89%) who achieved a first complete remission, 263 (50%) relapsed at a median of 11 months after attaining the remission (range, 1–10). Sixty (23%) had been initially classified as standard risk and 203 (77%) as high risk.
Patients’ characteristics at relapse
The median age of relapsed patients was 33 years (range, 15–69) and 150 (57%) were male. Relapses occurred during early consolidation in 46 patients (17%), after high-dose therapy and SCT in 71 (28 autologous - 10%, 43 allogeneic -16%) and during late consolidation, maintenance or beyond in 146 (55%). Most relapses occurred in the bone marrow either as the sole documented site (226 cases, 86%) or combined with central nervous system, testes or other locations (n=19, 7%). Exclusive extramedullary relapses occurred in the central nervous system (n=15); skin/soft tissue (n=2) and testes (n=1). Among patients with high-risk features at diagnosis, 41 (15%) carried high-risk translocations including 27 with t(9;22), 3 with t(1;19) and 11 with t(4;11).
Outcome after relapse
The median survival after relapse was 4.5 months (95% CI, 4–5 months). The 1-year overall survival was 24% (95% CI, 20–26%), and the 5-year overall survival was 10% (95% CI, 8–12%). In 15 patients (6%) no treatment was attempted to obtain a second remission. The median age of this subgroup of patients was 58 years (range, 43 to 69) and their median survival was 1 month (range, 0–2.3). SCT was attempted without re-induction therapy in 11 patients (4%) with a generally poor outcome. These cases included SCT without prior re-induction in two patients with incipient relapse (2 failures) and direct donor lymphocyte infusion in six patients submitted to allogeneic SCT (failure to achieve second remission, 1; non-relapse mortality, 3; subsequent relapse, 2). However, a durable second remission was obtained in two out of three patients with isolated central nervous system relapse conditioned with total body irradiation and receiving intrathecal therapy without systemic re-induction. An allogeneic SCT was attempted in two patients with active disease after re-induction failure and a durable second remission was obtained in one of them. Conventional re-induction treatment was given to 237 (90%) patients, and included regimens similar to first-line induction in 97 patients (37%), HyperCVAD6 or similar regimens in 83 (32%) and fludarabin-idarubicin (FlagIda)-based treatments in 26 (10%), whereas other regimens were administered to the remaining 31 patients. Death during induction occurred in 44 patients (17%) and second remission was achieved in 112 out of 248 patients (45%) receiving intensive second line treatment. The median disease-free survival after second remission was 6 months (95% CI, 5–7). The 1-year disease-free survival probability was 34% (95%CI, 26–42%) while the 5-year disease-free survival probability was 22% (95% CI, 14–30%). Thirty-three patients (30%) achieving second remission were not submitted to SCT (2 remain alive after standard consolidation, 30 relapsed before SCT and 1 died after further consolidation treatment in second remission), 14 (12%) received an autologous SCT (6 alive, 4 relapsed, 4 died of transplant-related events), 38 received a matched sibling allogeneic SCT (7 alive, 16 relapsed, 14 died of transplant-related events and 1 died of a second neoplasia) and 27 (24%) received an unrelated allogeneic SCT (14 alive, 8 relapsed, 5 died of transplant-related events). In all, 28 out of 113 relapsed patients who achieved a second remission (25%) remain alive without further relapse after a median follow-up of 6 years (range, 2–17).
Prognostic factors for achievement of second remission and survival after relapse
Table 2 summarizes the univariate analysis of factors predictive for re-induction-related death, second remission attainment, disease-free survival and overall survival. Baseline leukocytosis and gender were associated with a shorter overall survival. Females showed a higher rate of death during re-induction; however, the probability of achieving a second remission and disease-free survival were not significantly different between sexes. An analysis restricted to patients receiving active treatment showed that patients over 55 years had a lower second remission rate than those under this age (47% versus 20%, P=0.015). The median overall survival was 6.8 months (95% CI, 4.9–8.6) for patients up to 30 years compared to 4.2 months (95% CI, 3.7–4.8) for patients 30–55 years old (P=0.011) and 3.2 months (CI 95%, 2.4–4) for those over 55 years (P= 0.022) (Figure 1A). Disease-free survival was also significantly longer for younger patients (Figure 1B). Four patients over 55 years old achieved a second remission and none was subsequently submitted to any form of SCT. Only one of them remains alive and free of disease after achieving a second remission. Although phenotype did not have a significant influence on outcome after relapse, heterogeneity among small subsets of patients in each group could not be excluded (e.g. failure to respond in 4/4 mature thymic ALL). The complete remission rate of patients with normal cytogenetics (45%) was comparable to that of patients with t(9;22) (48%) or miscellaneous abnormalities (37%). However, only one out of three patients with t(1;19) and two out of 11 with t(4;11) achieved a second remission and none of these responses was durable. Similarly, the median disease-free survival of relapsed patients with t(9;22) was 4 months with only two out of 27 patients still alive and in second remission at the last control.
Table 2.
Univariate analysis of prognostic factors for reinduction death, second complete remission attainment, disease-free survival and overall survival after relapse in the 248 patients treated intensively after first relapse.
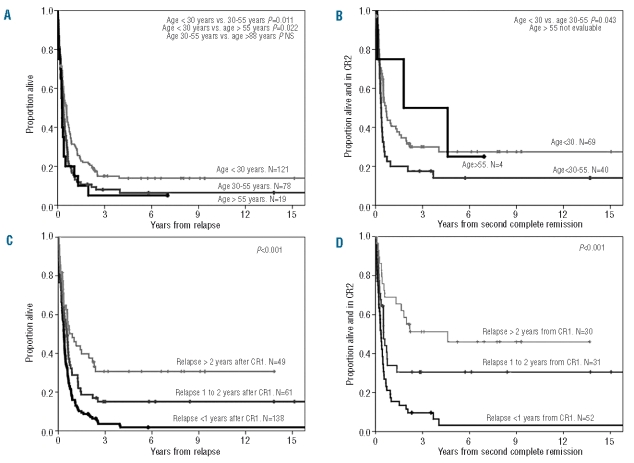
Figure 1.
Probabilities of overall survival and disease free survival from first relapse of 248 adult patients with ALL who received rescue treatment according to age at diagnosis (A and B) and time from diagnosis to relapse (C and D).
In patients relapsing within the first year of achieving first complete remission, the 5-year overall survival probability was 1.8% (95%CI, 0–4%), while it was 15% (95% CI, 7–23%) for patients relapsing between 1 and 2 years after achieving first remission and 31% (95% CI, 21–41%) for those relapsing later (Figure 1C). For patients achieving second remission, the 5-year disease-free survival probabilities were 3% (95% CI, 0–5%), 30% (95% CI, 16–44%) and 46% (95% CI, 29–63%) for patients relapsing during the first year, between 1 and 2 years or more than 2 years, respectively, after achieving first complete remission (Figure 1D).
The probabilities of successful treatment after relapse were not affected by SCT procedures during first complete remission among the 209 patients (84%) who relapsed late enough to receive late consolidation in first complete remission with conventional chemotherapy (67%), allogeneic SCT (20%) or autologous SCT (13%). Although those patients submitted to SCT in first complete remission tended to have a lower second remission rate (36% versus 49%, P=0.07) and a lower 5-year overall survival probability, 5% (95%CI, 0%–10%) versus 17% (95%CI, 11%–23%) (P=0.004), the differences lost statistical significance after adjustment for age and risk. Transplant-related mortality was higher for patients already submitted to a SCT in first complete remission (23% versus 45%, P=0.06). Patients treated upon relapse with FlagIda-like re-induction therapy had a higher rate of achievement of second remission although the difference was not statistically significant after adjustment for age.
The results of multivariate analysis are summarized in Table 3. Age and duration of first complete remission were the two independent prognostic factors for response and survival. Female gender retained prognostic significance for a higher probability of death during salvage induction therapy. The subgroup of patients under 30 years old and with a first complete remission lasting over 2 years (representing 13% of the whole cohort) had an 5-year overall survival of 38% (95% CI, 23–53%) and a 5-year disease-free survival of 53% (95% CI, 34–72%) (Figure 2).
Table 3.
Multivariate analysis for factors associated with death during rescue induction, second complete remission achievement, overall survival and disease-free survival in patients with relapsed ALL receiving intensive treatment.
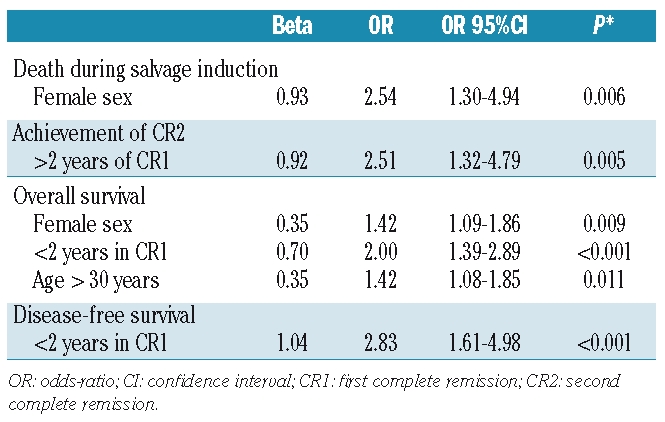
Figure 2.
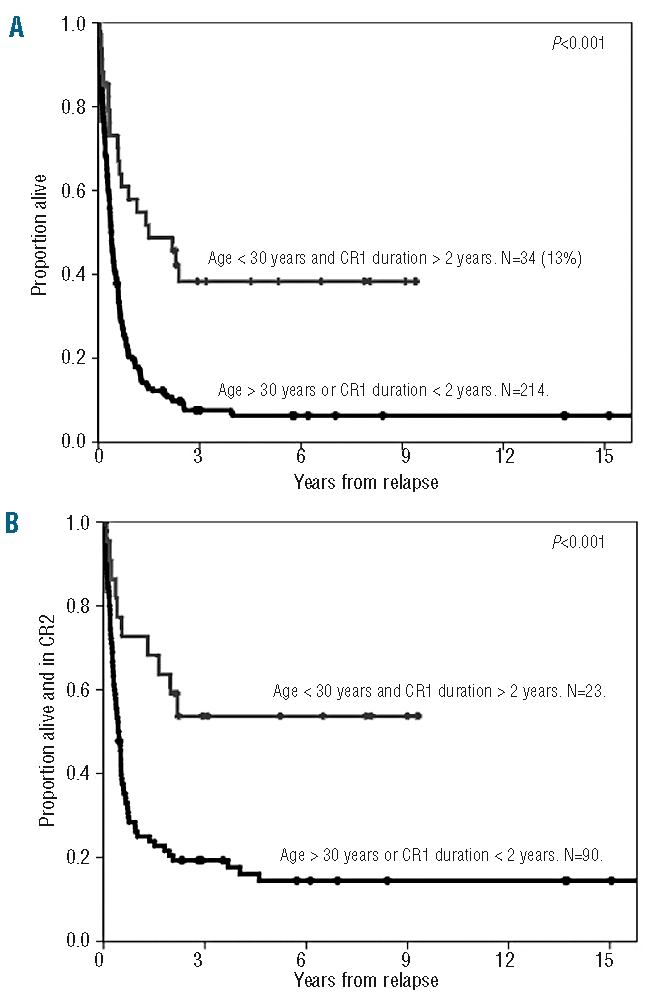
Comparison of the overall survival (A) and disease-free survival (B) from first relapse of patients with ALL up to 30 years of age and with a first remission lasting more than 2 years (N=34) versus all other patients who received intensive treatment (N=214).
Discussion
The aim of this study was to analyze the response to therapy and prognosis of adult ALL patients after first relapse in order to identify the subset of patients with chances for long-term survival. After receiving homogeneous risk-adapted first-line therapy, patients aged under 30 years old at diagnosis and with a first complete remission lasting more than 2 years emerged as those with the most favorable outcome. The subgroup of patients with these two characteristics represented only 13% of the whole cohort, but their prognosis, in terms of both overall survival and disease-free survival was similar to that of patients with newly diagnosed ALL. On the other hand, patients aged 55 years or older and those with a first remission lasting less than 1 year formed a subgroup with a dismal prognosis regardless of the rescue strategy pursued.
The PETHEMA trials have used a common backbone of treatment for adult patients with newly diagnosed ALL for more than a decade allowing the analysis of a cohort with mature follow-up. However, several drawbacks must be taken into account. First, as in other large cohorts,10,11,21 the use of risk-adapted initial therapy precluded the possibility of exploring the prognostic implications of initial treatment strategies. Secondly, as in the aforementioned studies, the rescue strategy was not uniform. Re-induction treatments have only been explored prospectively in short series.9 However, some type of allogeneic SCT after achievement of second remission is considered necessary. 21 Finally, analysis of specific cytogenetic subgroups was limited, given that some important cytogenetic abnormalities are infrequent. In the case of t(9;22), the emergence of novel specific therapies,22–26 which were available at relapse for some of the patients in the cohort, may have had an influence on rescue strategies.
The design of our study, based on a full-cohort followup, has provided other relevant data. Prior studies10,11 did not address the proportion of patients following alternative options of rescue treatment and their outcome or those receiving palliative treatment only. Donor lymphocyte infusion or other SCT strategies without prior chemotherapy had poor results, although the data were based on small numbers of patients. The proportion of untreated patients was relatively small (6%) and these patients were mostly elderly. In fact, the feasibility of standard rescue treatment was only anecdotal in patients over 55 years, with less than a quarter achieving second remission and with subsequent relapse occurring in most. Age has consistently been associated with a dismal prognosis in most studies.10,11.20 Although rescue treatment was attempted in most patients aged 30–55 years old, the study shows that these patients’ outcome was as poor as that of the older patients. Thus, patients over 30 years of age form a uniform group likely to benefit from being enrolled into clinical trials with novel drugs.
Another large cohort study described female sex as a predictor of poor outcome in adult ALL after relapse,11 a factor that had not been identified in previous studies.8–10 We found female sex to be specifically associated with a high rate of death during re-induction. Although the increased early death rate in females had an impact on their overall survival, female patients achieving second remission enjoyed the same disease-free survival as males. This finding might be partially explained by a high proportion of females in the older age groups but gender retained independent prognostic significance on multivariate analysis along with age. We also excluded the possibility of interactions with other variables such as less intensive treatment, different rates of failure to undergo allogeneic SCT or a higher incidence of poor-risk initial ALL characteristics among females. Although there is not a clear explanation for this finding, its appearance in the analysis of two large independent cohorts makes the possibility of a hazard effect unlikely.
As in prior studies,11 we failed to identify any statistically significant association between the initial characteristics of ALL and prognosis after relapse. High initial WBC counts lost statistical significance when included in the multivariate model. However, some associations cannot be completely excluded. No patient with mature thymic ALL could be rescued after relapse in our series. The prognostic significance of cytogenetics could also have been biased by the small numbers of patients analyzed as no patient with t(1;19) or t(4;11) obtained sustained second remission. Regarding t(9;22), despite having an identical second remission rate to patients with standard-risk cytogenetics (48%), second remissions lasted less than 1 year in more than half of these patients mainly due to relapse. Only two patients with t(9;22) ALL remained alive and in second remission at the last control. Our study failed to confirm the results of a previous report11 attributing a worse prognosis to patients relapsing in the central nervous system.
On the other hand, the dismal prognosis of early relapsers was clearly confirmed. An important proportion of the desperate attempts to achieve second remission in younger patients relapsing during consolidation or early afterwards may be related to the absence of available investigational alternatives until recent times. Currently, early relapsers of any age, along with patients over 30 years old, should be strongly encouraged to participate in clinical trials with newly available experimental agents.
The optimal first-line strategy in adult ALL is under debate, with evidence in favor of both pursuing an allogeneic SCT in first remission7 and of reserving SCT procedures for very high-risk patients.14,16,27,28 The role of initial therapy in determining outcome after relapse has been controversial.8,11 Patients from the ALL89, ALL96SR and ALL03HR trials received an allogeneic SCT only if slow response to treatment was observed, thus patients with adverse prognostic features were selected for SCT. The detrimental effect of a prior SCT in first complete remission in our series lost prognostic significance when age and risk factors were considered. When patients submitted to some type of SCT were matched with comparable patients and particularly when analysis was restricted to patients randomized between chemotherapy and maintenance, allogeneic SCT or autologous SCT (trial ALL93HR), no differences in outcome were observed, with roughly half of the patients achieving second remission and three quarters of them being able to undergo a SCT procedure. Within the select subset of patients undergoing a high-dose procedure, the proportions of patients who experienced transplant- related mortality, who relapsed or who maintained a durable second remission were about one third each, regardless of prior allogeneic or autologous SCT procedures in first complete remission. Thus, our study proves that first complete remission consolidation strategies are unlikely to hamper the possibility of a successful allogeneic SCT after relapse. Differences in efficacy among rescue treatments were largely affected by age, center effects and time to transplant. The best re-induction strategy remains to be determined. Our results confirm that any effective induction followed by prompt allogeneic SCT may be a judicious strategy. However, only younger patients with late relapses appear to benefit from such an approach. In the subgroup with the best prognosis, autologous SCT or even standard consolidation after second remission may be equally effective when a donor is not available.
In summary, we identified a small but relevant subgroup of adults with ALL in whom the chances of long-term survival may approach those of patients with newly diagnosed ALL. Late relapsers may retain chemosensitivity to previously used agents and have a relatively important second remission rate that allows subsequent high-dose therapy procedures with considerable chances of success. On the other hand, early relapsers and patients unfit to follow high-dose procedures should be included in trials involving novel therapeutic agents.29
Appendix
The following hospitals and doctors in Spain participated in the PETHEMA trials ALL89, ALL93HR, ALL96IR and ALL03HR: Institut Català d'Oncologia-Hospital Universitari Germans Trias i Pujol, Badalona: JM Ribera, A Oriol, S Vives, E Feliu; Hospital Clínico Universitario, Salamanca: JM Hernández-Rivas, JF San Miguel; Hospital Clínico Universitario Valencia: M Tormo, MJ Terol;Hospital Morales Meseguer, Murcia: MI Heras; Hospital Central de Asturias, Oviedo: T Bernal, E Martínez-Revuelta; Hospital Virgen de la Arrixaca Murcia: J Fuster; Hospital Clínic, Barcelona: J Esteve; Hospital Infantil Miguel Servet, Zaragoza: C Calvo, A Carboné; Hospital de Sant Pau, Barcelona: S Brunet, J Sierra; Hospital de San Pedro de Alcantara, Cáceres: JL Bergua; Hospital Nuestra Señora de Aranzazu San Sebastián: J Marín, I Egurbide; Hospital Virgen de la Concha, Zamora: J Sánchez; Hospital General, Castelló: R García-Boyero; Hospital Ramón y Cajal, Madrid: J Pérez de Oteyza; Hospital Duran y Reynals – Institut Català d’Oncologia, L’Hospitalet de Llobregat: J Sarrà; Hospital Vall d'Hebron, Barcelona: J Bueno, JJ Ortega, MP Bastida, T Olivé; Hospital Universitario Virgen del Rocío, Sevilla: JM Pérez-Hurtado, R Parody; Hospital Materno-Infantil Carlos Haya, Málaga: ME González-Valentín; Hospital General, Alicante: C Rivas, P Fernández-Abellán; Hospital Universitario La Fe, Valencia: MA Sanz, F Moscardó, P Montesinos; Hospital Clínico San Carlos, Madrid: E del Potro, J Díaz-Mediavilla; Hospital Txagorritxu, Vitoria-Gasteiz: JM Guinea; Hospital Josep Trueta, Girona: R Guardia. Hospital Mútua de Terrassa, Barcelona: JM Martí, F Vall-llobera; Hospital Xeral, Vigo: C Poderós;Hospital General, Segovia: JA Queizán, J Martínez; Hospital Carlos Haya, Málaga: C Bethencourt, J Maldonado; Hospital Puerta del Mar, Cádiz: V Martín-Reina, JL Gil;Hospital Universitario Virgen de la Victoria, Málaga: MJ Moreno, MP Queipo de Llano; Hospital Río Carrión, Palencia: F Ortega-Rivas; Hospital Virgen Blanca, León; JA Rodríguez, MJ Moro; Hospital Materno Infantil, Las Palmas: A Molinés, V Lodos; Hospital Arnau de Vilanova, Lleida: J Macià; Hospital Son Dureta, Palma de Mallorca: A Novo, J Besalduch; Hospital Rio Hortega, Valladolid: MD Peñarrubia;Hospital del Mar, Barcelona: C Pedro, E Abella; Hospital Juan Canalejo, A Coruña: G Deben; Hospital General Yagüe, Burgos: F Casanova; Hospital General de Especialidades, Jaén: F Gámez, A Alcalá;Hospital Xeral, Lugo: J Arias; Hospital Dr Peset, Valencia: P León; Hospital Mexoeiro, Vigo: A Ares; Hospital Joan XXIII, Tarragona: A Llorente; Hospital de Galdakao, Bilbao: K Atutxa; Hospital General Universitario, La Laguna: L Hernández-Nieto; Hospital General, Guadalajara: G Díaz-Morfa; Centro Médico Teknon, Barcelona: P Vivancos;Hospital Reina Sofía, Córdoba: A Rodríguez-Villa; Hospital Xeral, Santiago de Compostela: JL Bello; Hospital General, Valencia: F Carbonell, M Orts; Hospital Clínico, Valladolid: J Fernández-Calvo, D Borrego; Hospital Doce de Octubre, Madrid: C Grande.
Footnotes
Funding: supported in part by grants P-EF/08 from the José Carreras Leukemia Foundation and RD06/0020/1056 from RETICS.
Authorship and Disclosures
JMR designed and coordinated the original trials; SV collected and verified the data; AO analyzed the data and wrote the paper. All authors participated in data collection and contributed to writing the paper. JMR checked the final version of the manuscript; JMR was study chair of the original trials and the joint final analysis. All other authors participated in the original clinical trials, followed patients clinically, updated information on outcome and reviewed the manuscript critically. No potential conflicts of interests relevant to this article were reported.
References
- 1.Larson RA, Dodge RK, Burns CP, Lee EJ, Stone RM, Schulman P, et al. A five-drug remission induction regimen with intensive consolidation for adults with acute lymphoblastic leukemia: Cancer and Leukemia Group B study 8811. Blood. 1995;85(8):2025–37. [PubMed] [Google Scholar]
- 2.Gökbuget N, Hoelzer D, Arnold R, Böhme A, Bartram CR, Freund M, et al. Treatment of adult ALL according to protocols of the German Multicenter Study Group for Adult ALL (GMALL) Hematol Oncol Clin North Am. 2000;14(6):1307–25. doi: 10.1016/s0889-8588(05)70188-x. [DOI] [PubMed] [Google Scholar]
- 3.Annino L, Vegna ML, Camera A, Specchia G, Visani G, Fioritoni G, et al. Treatment of adult acute lymphoblastic leukemia (ALL): long-term follow-up of the GIMEMA ALL 0288 randomized study. Blood. 2002;99(3):863–71. doi: 10.1182/blood.v99.3.863. [DOI] [PubMed] [Google Scholar]
- 4.Takeuchi J, Kyo T, Naito K, Sao H, Takahashi M, Miyawaki S, et al. Induction therapy by frequent administration of doxorubicin with four other drugs, followed by intensive consolidation and maintenance therapy for adult acute lymphoblastic leukemia: the JALSG-ALL93 study. Leukemia. 2002;16(7):1259–66. doi: 10.1038/sj.leu.2402526. [DOI] [PubMed] [Google Scholar]
- 5.Thomas X, Boiron JM, Huguet F, Dombret H, Bradstock K, Vey N, et al. Outcome of treatment in adults with acute lymphoblastic leukemia: analysis of the LALA-94 trial. J Clin Oncol. 2004;22(20):4075–86. doi: 10.1200/JCO.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian H, Thomas D, O'Brien S, Cortes J, Giles F, Jeha S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101(12):2788–801. doi: 10.1002/cncr.20668. [DOI] [PubMed] [Google Scholar]
- 7.Rowe JM, Buck G, Burnett AK, Chopra R, Wiernik PH, Richards SM, et al. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood. 2005;106(12):3760–7. doi: 10.1182/blood-2005-04-1623. [DOI] [PubMed] [Google Scholar]
- 8.Giona F, Annino L, Rondelli R, Arcese W, Meloni G, Testi AM, et al. Treatment of adults with acute lymphoblastic leukaemia in first bone marrow relapse: results of the ALL R-87 protocol. Br J Haematol. 1997;97(4):896–903. doi: 10.1046/j.1365-2141.1997.1102926.x. [DOI] [PubMed] [Google Scholar]
- 9.Montillo M, Tedeschi A, Centurioni R, Leoni P. Treatment of relapsed adult acute lymphoblastic leukemia with fludarabine and cytosine arabinoside followed by granulocyte colony-stimulating factor (FLAGGCSF) Leuk Lymphoma. 1997;25(5–6):579–83. doi: 10.3109/10428199709039047. [DOI] [PubMed] [Google Scholar]
- 10.Thomas DA, Kantarjian H, Smith TL, Koller C, Cortes J, O'Brien S, et al. Primary refractory and relapsed adult acute lymphoblastic leukemia: characteristics, treatment results, and prognosis with salvage therapy. Cancer. 1999;86(7):1216–30. doi: 10.1002/(sici)1097-0142(19991001)86:7<1216::aid-cncr17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 11.Fielding AK, Richards SM, Chopra R, Lazarus HM, Litzow MR, Buck G, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109(3):944–50. doi: 10.1182/blood-2006-05-018192. [DOI] [PubMed] [Google Scholar]
- 12.Popat U, Carrum G, Heslop HE. Haemopoietic stem cell transplantation for acute lymphoblastic leukaemia. Cancer Treat Rev. 2003;29(1):3–10. doi: 10.1016/s0305-7372(02)00092-0. [DOI] [PubMed] [Google Scholar]
- 13.Ribera JM, Ortega JJ, Oriol A, Fontanillas M, Hernandez-Rivas JM, Brunet S, et al. Late intensification chemotherapy has not improved the results of intensive chemotherapy in adult acute lymphoblastic leukemia. Results of a prospective multicenter randomized trial (PETHEMA ALL-89) Haematologica. 1998;83(3):222–30. [PubMed] [Google Scholar]
- 14.Ribera JM, Oriol A, Bethencourt C, Parody R, Hernández-Rivas JM, Moreno MJ, et al. Comparison of intensive chemotherapy, allogeneic or autologous stem cell transplantation as post-remission treatment for adult patients with high-risk acute lymphoblastic leukemia. Results of the PETHEMA ALL-93 trial. Haematologica. 2005;90(10):1346–56. [PubMed] [Google Scholar]
- 15.Sancho JM, Ribera JM, Xicoy B, Morgades M, Oriol A, Tormo M, et al. Results of the PETHEMA ALL-96 trial in elderly patients with Philadelphia chromosome-negative acute lymphoblastic leukemia. Eur J Haematol. 2007;78(2):102–10. doi: 10.1111/j.1600-0609.2006.00778.x. [DOI] [PubMed] [Google Scholar]
- 16.Ribera JM, Oriol A, Sanz MA, Tormo M, Fernández-Abellán P, del Potro E, et al. Comparison of the results of the treatment of adolescents and young adults with standard- risk acute lymphoblastic leukemia with the Programa Español de Tratamiento en Hematología pediatric-based protocol ALL-96. J Clin Oncol. 2008;26(11):1843–9. doi: 10.1200/JCO.2007.13.7265. [DOI] [PubMed] [Google Scholar]
- 17.Ribera JM, Oriol A, Morgades M, Sarra J, Montesinos P, Brunet S, et al. Treatment of high-risk (HR) Philadelphia chromosome-negative (Ph-) adult acute lymphoblastic leukemia (ALL) according to baseline risk factors and minimal residual disease (MRD). Results of the PETHEMA ALL-AR- 03 trial. Blood. 2008;112(supp11) doi: 10.1200/JCO.2013.52.2425. abs3291. [DOI] [PubMed] [Google Scholar]
- 18.Peto R, Pike MC. Conservatism of the approximation sigma(O-E)2-E in the logrank test for survival data or tumour incidence data. Biometrics. 1973;29(3):579–84. [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assoc. 1958;53:457–81. [Google Scholar]
- 20.Cox DR. Regression models and life tables. J Roy Stat Soc Series B. 1972;34(2):187–220. [Google Scholar]
- 21.Garcia-Manero G, Thomas DA. Salvage therapy for refractory or relapsed acute lymphocytic leukemia. Hematol Oncol Clin North Am. 2001;15(1):163–205. doi: 10.1016/s0889-8588(05)70204-5. [DOI] [PubMed] [Google Scholar]
- 22.Wassmann B, Pfeifer H, Goekbuget N, Beelen DW, Beck J, Stelljes M, et al. Alternating versus concurrent schedules of imatinib and chemotherapy as front-line therapy for Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL) Blood. 2006;108(5):1469–77. doi: 10.1182/blood-2005-11-4386. [DOI] [PubMed] [Google Scholar]
- 23.Yanada M, Takeuchi J, Sugiura I, Akiyama H, Usui N, Yagasaki F, et al. High complete remission rate and promising outcome by combination of imatinib and chemotherapy for newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia: a phase II study by the Japan Adult Leukemia Study Group. J Clin Oncol. 2006;24(3):460–6. doi: 10.1200/JCO.2005.03.2177. [DOI] [PubMed] [Google Scholar]
- 24.Thomas DA, Faderl S, Cortes J, O'Brien S, Giles FJ, Kornblau SM, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103(12):4396–407. doi: 10.1182/blood-2003-08-2958. [DOI] [PubMed] [Google Scholar]
- 25.Lee KH, Lee JH, Choi SJ, Lee JH, Seol M, Lee YS, et al. Clinical effect of imatinib added to intensive combination chemotherapy for newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukaemia. Leukemia. 2005;19(9):1509–16. doi: 10.1038/sj.leu.2403886. [DOI] [PubMed] [Google Scholar]
- 26.de Labarthe A, Rousselot P, Huguet-Rigal F, Delabesse E, Witz F, Maury S, et al. Imatinib combined with induction or consolidation chemotherapy in patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: results of the GRAAPH-2003 study. Blood. 2007;109(4):1408–13. doi: 10.1182/blood-2006-03-011908. [DOI] [PubMed] [Google Scholar]
- 27.Ramanujachar R, Richards S, Hann I, Webb D. Adolescents with acute lymphoblastic leukaemia: emerging from the shadow of paediatric and adult treatment protocols. Pediatr Blood Cancer. 2006;47(6):748–56. doi: 10.1002/pbc.20776. [DOI] [PubMed] [Google Scholar]
- 28.Boissel N, Auclerc MF, Lheritier V, Perel Y, Thomas X, Leblanc T, et al. Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults? comparison of the French FRALLE-93 and LALA-94 trials. J Clin Oncol. 2003;21(5):774–80. doi: 10.1200/JCO.2003.02.053. [DOI] [PubMed] [Google Scholar]
- 29.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354(2):166–78. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]