In a recent paper, Island and colleagues1 described a heterozygous hepcidin (HAMP) promoter mutation, nc.- 153C>T, which in association with HFE p.C282Y homozygosity appeared to lead to very severe iron overload (IO). They demonstrated in vitro that this HAMP mutation decreases transcriptional activity of the hepcidin promoter, alters IL6 total responsiveness and impairs binding of the SMAD protein complex to the BPM-RE.
HFE genotypes other than the homozygous p.C282Y (YY) or the compound heterozygous state for the p.C282Y and p.H63D mutations are not considered responsible for causing symptomatic forms of IO. This led us to re-evaluate 30 unrelated patients with non-YY HFE genotype associated with the diagnostic criteria of hemochromatosis who were referred to our laboratory. Among them, 12 were previously reported H63D homozygotes.2 The diagnosis of IO was based on either liver biopsy and/or therapeutic criteria (phlebotomies). Informed written consent was obtained from all patients according to the French regulation.
DNA sequencing of the HAMP gene found no mutation in the coding sequence or in the exon-intron junctions of the 30 patients. However, two gene substitutions, a C to T replacement at position -153 upstream of the ATG and a C to T substitution in intron 1 at position -66, 5’ to exon 2 (c.91-66C>T, rs#2293689) were identified in 3 patients. Both sequence alterations were found in all 3 patients but we could not confirm whether they were in linkage disequilibrium or whether they were inherited on different chromosomes, as family segregation could not be performed. The patients, 3 white men living in different cities of the Southern part of France, were referred separately by three different physicians. To the best of our knowledge the men are not related. They all had ferritin levels over 1000 μg/L and transferrin saturation over 50%. They had been regularly phlebotomized for more than one year. Two of them, a 75-year old and a 54-year old male respectively, were p.H63D homozygotes (Table 1). The third patient, diagnosed at 69 years of age, was simply heterozygote for the p.S65C substitution on the HFE gene. His medical record indicated that he had myocardiopathy, cirrhosis and a liver biopsy compatible with the diagnosis of hemochromatosis (Table 1). Two patients had a family history of iron overload. Both substitutions, nc.-153C>T and c.91-66T>C, were also found in one out of 224 unrelated control chromosomes from white individuals of the same region. The iron status of this anonymous control was not available. The HAMP nc.-153C>T mutation was thus present at a high allele frequency (0.05) in this series of non-YY iron loaded patients. It was also found in one control subject, with a low allele frequency of 0.004 in this group (P=0.008).3
Table 1.
Characteristics of 3 iron loaded male patients with the HAMP nc.- 153 C>T.
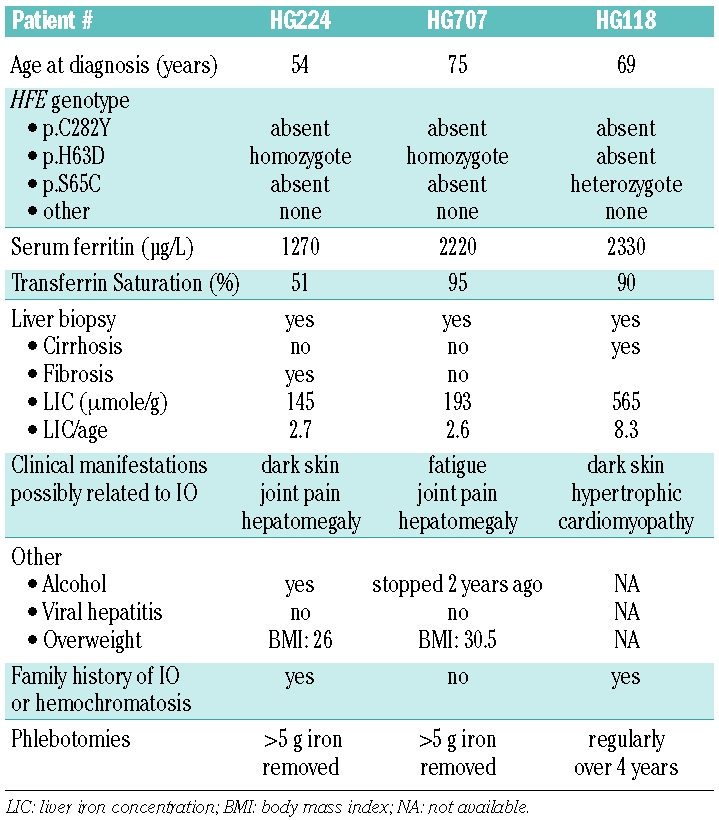
Barton and colleagues4 did not detect HAMP nc.- 153C>T in any of 191 HFE p.C282Y homozygotes from the HEIRS (Hemochromatosis and Iron Overload Screening) Study. They also screened a control group with various ethnic backgrounds, including non-Hispanic whites, Hispanics, blacks and Asian subjects, and found this mutation in only one Hispanic woman with apparently no IO at a control sample. They concluded that routine testing to detect HAMP nc.-153C>T is not indicated in population-based hemochromatosis and IO screening programs in North America. However, the data presented here indicate that in a selected sample of iron loaded individuals, especially those with HFE non-YY genotypes, the search for this mutation could be of interest as it can possibly explain IO in certain patients. Indeed, we found that 2 out of 12 iron loaded H63D homozygotes2 had the HAMP nc.-153 C>T as a potential genetic factor of IO. Screening for this mutant may be, therefore, indicated in this particular population, or for individuals with high transferrin saturation and unexplained IO, as suggested by Loréal.4
Acknowledgments
the authors are grateful to Dr Béatrice Bonafoux and Dr Nathalie Funakoshi for their helpful contribution.
Footnotes
Funding: supported by a grant from la Recherche Clinique, CHU de Montpellier, AOI 2004.
References
- 1.Island ML, Jouanolle AM, Mosser A, Deugnier Y, David V, Brissot P, et al. A new mutation in the hepcidin promoter impairs its BMP response and contributes to a severe phenotype in HFE related hemochromatosis. Haematologica. 2009;94(5):720–4. doi: 10.3324/haematol.2008.001784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguilar-Martinez P, Bismuth M, Picot MC, Thelcide C, Pageaux GP, Blanc F, et al. Variable phenotypic presentation of iron overload in H63D homozygotes: are genetic modifiers the cause? Gut. 2001;48(6):836–42. doi: 10.1136/gut.48.6.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguilar Martinez P, Giansily-Blaizot M, Jeanjean P, Igual H, Piganiol, Schved JF. BioIron 2005. Prague: Czech Republic; 2005. A rare haplotype of the HAMP gene with high allele frequency among patients with iron overload and an atypical HFE genotype; p. 73. [Google Scholar]
- 4.Barton JC, Leiendecker-Foster C, Li H, DelRio-LaFreniere S, Acton RT, Eckfeldt JH. HAMP promoter mutation nc.-153C>T in 785 HEIRS Study participants. Haematologica. 2009;94(10):1465. doi: 10.3324/haematol.2009.011486. author reply 1465–6. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tissue Antigens. 2001;58(5):281–92. doi: 10.1034/j.1399-0039.2001.580501.x. [DOI] [PubMed] [Google Scholar]


