Abstract
Previous studies have established cross-cultural methods to screen for ageing- related dementia and susceptibility genes, in particular Alzheimer’s disease (AD) among the Canadian Cree, African Americans and Yoruba in Nigeria. We determined whether the Community Screening Interview for Dementia (CSID), translated into Kikuyu, a major language of Kenya, could be used to evaluate dementia of the Alzheimer type. Using two sets of coefficients of cognitive and informant scores, two discriminant function (DF) scores were calculated for each of 100 elderly (>65 years) Nyeri Kenyans. When the cut-off points were selected for 100% sensitivities, the specificities of the DF scores were remarkably similar (93.75%) in the Kenyan sample. We propose the adapted CSID can be utilised to detect dementia among East Africans. We also show that apolipoprotein E ε4 allele frequencies were high (∼30%) and not different between normal subjects and those with probable AD. There was no evidence to suggest years of education or vascular factors were associated with dementia status.
Keywords: Africa, Alzheimer’s disease, Apolipoprotein E, Cognitive impairment, Dementia, Kenya, Neuropsychology
1. Introduction
The “greying” of the developing world is predicted to rise despite deaths due to malnutrition, poor economy, civil war and infectious diseases. The World Health Organization (WHO) predicts that by 2025 about three-quarters of the estimated 1200 million people aged 60 years and older will be living in developing countries (WHO, 2002) that bears a heavy cost in care (Wimo et al., 2006). Alzheimer’s disease (AD), as one of the main causes of dementia, is also expected to rise in the developing countries given the increasing demographic transition (Evans et al., 1989; Ferri et al., 2005) and prevalence of vascular disease (Kalaria, 2000, 2003). Recent studies have shown the importance of both genetic and environmental factors (Brayne, 1991; Hendrie et al., 2006). A valuable approach to the study of genes and environment and their interactions is to compare the disease in different environments using similar techniques. Cross-cultural studies pose major methodological and screening problems owing to differences in language, culture, and levels of education and literacy (Kalaria et al., 1997; Osuntokun et al., 1992). In certain communities more than 80% of elderly may not read or write (Hall et al., 1996). Various proposals have been made to standardize cognitive assessments and dementia estimates (Ferri et al., 2005), due to differences in culture and language (Buschke et al., 1999; Evans et al., 1989; Jorm and Jacomb, 1989; Maj et al., 1991; Prince et al., 2004).
Hendrie et al. (1993) have developed a community screening instrument for dementia (CSID) for use in populations with different cultural and linguistic identities including the Cree-speaking natives living on reserves in Manitoba and in Winnipeg, community-dwelling African Americans in Indianapolis and the Yoruba in Ibadan, Nigeria. The latter two communities with similar ancestries but widely differing environments (Grant, 2001; Hall et al., 1996; Hendrie et al., 1995, 2001; Osuntokun et al., 1992). The CSID has also been widely used, albeit effectively abridged, in the highly successful screening of dementia and AD in diverse populations of developing countries within the 10/66 studies conducted in more than 25 centres worldwide (Prince et al., 2007).
The aim of this study was to evaluate the utility of the CSID in diagnosing AD in a rural population sample in Nyeri, Kenya. The Kikuyu version of the CSID was administered to both the subjects and their informants, identified through non-random methods from registers at the local General hospital. Sensitivity and specificity of the CSID in the Nyeri sample of the elderly was compared to that obtained in Indianapolis and Ibadan (Hall et al., 1996). We also investigated vascular risk factors and the apolioprotein E (APOE) genotypes in the prospectively assessed Kikuyu sample to compare against others from developing countries.
2. Materials and methods
2.1. Subjects and location
The pilot sample comprised of 100 Kikuyu Kenyans aged 65 years and older from the Nyeri district. The assessments were performed on 84 controls and 16 demented subjects, and 100 informants, who were close relatives (or jamaa). Both subjects and their informants were identified from medical contacts through referrals at the Nyeri Provincial General Hospital (NPH) and a private clinic (MKK) in Nyeri town. Some cognitive impairment or reported memory problems was suspected in certain individuals invited to be screened. Table 1 shows the demographic statistics of the subjects by age, gender and education. None of the subjects assessed in the study were HIV positive or had AIDS. There was a rather negligible refusal rate (2%) to participate in the study. At beginning of the CSID interviews, a standard consent form was reviewed and signed by each subject. The project was also approved by the Office of the President, Republic of Kenya, the internal review board of NPH, the Provincial Medical Officer’s department and the registrar of births and deaths.
Table 1.
Demographic statistics of gender, age, and education of Nyeri sample compared to Indianapolis and Ibadan samples
| Clinical diagnosis | Men |
Women |
Total* |
|||
|---|---|---|---|---|---|---|
| Age (range) | Education (range) | Age (range) | Education (range) | Age (range) | Education (range) | |
| Control | 70.3 ± 6.5 (65–92) | 3.5 ± 3.0(0–14) | 69.1 ± 5.1 (65–84) | 1.4 ± 2.0 (0–8) | 69.7 ± 5.8 (65–92) | 2.4 ± 2.7 (0–14) |
| n=38 | n=46 | n=84 | ||||
| Demented | 79.4 ± 9.4 (70–96) | 1.7 ± 2.0 (0–5) | 73.4 ± 7.8 (67–85) | 2.3 ± 3.0 (0–8) | 76.4 ± 8.9 (67–96) | 2.0 ± 2.5 (0–8) |
| n=7 | n=9 | n=16 | ||||
| Total | 71.8 ± 7.7 (65–96) | 3.2 ± 3.0 (0–14) | 69.8 ± 5.7 (65–85) | 1.5 ± 2.2 (0–8) | 70.7 ± 6.8 (65–96) | 2.3 ± 2.7 (0–14) |
| n=45 | n=55 | n=100 | ||||
Numbers show mean±standard deviation (S.D.) with minimum–maximum range in parentheses in years.
Ten of the controls were showed some cognitive impairment but were not considered to have frank dementia. For comparison, in the Ibadan (Yoruba) a sample of n=50 the mean age was 73.5±8.1 years with 46% women and in the Indianapolis sample for n=50 the mean age was 72.2 ±5.7 years with 72% women. The % educated for 1–8 years were 20% in Ibadan and 40% in Indianapolis (Hall et al., 1996).
Nyeri is the main town situated 60 miles of north of Nairobi within a rural community in the Central Province of Kenya. At an elevation of 5800 ft, Nyeri and its residential environs are set in the Aberdares foothills with extensive small farms (vegetables and fruit), and modest manufacturing industry for soft drinks, ropes, clay and concrete products. Majority of the people are Kikuyu, whose main occupation is small hold farming. The main languages in Nyeri are Kikuyu, Swahili and English. The 1989 Central Bureau of Statistics survey indicates that the District comprised 130,541 households with a population of 607,292 and the age stratified data (65–80 plus years) revealed 28,803 above 65 years, comprising 4.7% of the population. Data (1999–2004) from the Registrar of births and deaths in Nyeri indicated that the three leading causes of death, which varied little, in the elderly >60 years with no apparent gender trends were heart disease (23%), pneumonia (20%) and cancer (14%).
2.2. Age determination
The age of each subject (Hall et al., 1996) in our study was confirmed by the Riika (or Mariika) system recognized to be the most efficient system known nationally for age determination (www.kenyaweb.com). Briefly, every Kikuyu person must know his or her Riika, i.e. their initiation into adulthood. For males this takes place at15 years and for females at 12 years. The initiation year is designated by an event known to the region and the specific Riika is established by the elders and informed to the community dwellers. For example, for the given event or introduction of an item, Njanjo (vaccination) = 1917 meaning that male subject was 98 years old in 2000. Similarly, Ndege (aeroplane) = 1927 equivalent to 88 years in 2000, Kababa Njabani (Japanese goods) = 1937 equivalent to 78 years in 2000 and so on. During the initiation ceremony, the eligible males and females in an area come together. Thus, members of a particular Riika class know one another and the social position and standing of each person is determined in the presence of others. In an urban setting, it would be possible for the older person (mzee) to lie about their Riika and not be discovered but in a rural setting it would be impossible to do so as the persons with the same Riika may live nearby and interact. The margin of error in the Riika system is calculated to be ±2 years (Muriuki, 1974). However, our experience showed that the respective spouse and relative independently knew the subject’s Riika, with complete agreement in all the subjects. The validity of the birth year was tested in 20% of the subjects who carried national identity cards lawfully required by every Kenyan.
2.3. Interviewer training and screening design
The CSID was administered to each subject and to one informant. Except for minor modifications related to cultural aspects, seasons (summer or winter only) and names of common fruits, for example, the Ibadan version of the CSID was used (Hall et al., 1993; WHO, 1984). The CSID was translated from English to Kikuyu and was then independently back translated to English for an accuracy check. A Swahili version of the CSID was also produced for occasional use as a reference. This was to obtain clarity of meaning of some Kikuyu terms derived from Swahili, which most informants also spoke as the second language.
We used a similar paradigm for first and second phase screening to that established at previous sites (Hall et al., 1996; Hall et al., 2000). The CSID interviews (with subject and jamaa) and more than 80% of neuropsychological testing took place at home. Inter-rater reliability between interviewers was calculated to be similar (>95%) to that experienced previously in Ibadan (Hall et al., 2000). Only in the case of controls, we experienced a refusal rate of 2% for participation in the study.
2.4. Clinical examination and dementia diagnosis
For the clinical phase, subjects were examined (MMK and PK) at a clinic in the NPH with the spouse or jamma. The protocol involved a general physical examination and brief neurological, and psychiatric evaluations. None of the subjects was taking medications that may have impaired his or her performance on the CSID. Five of the impaired subjects were also scanned (head CT) at the MRI Diagnostic Centre in Nairobi on separate occasions.
As previously established (Hendrie et al., 1995), the ICD-10 and DSM-IIIR diagnostic criteria for dementia and dementia subtypes and the clinical dementia rating (CDR) scale were used to confirm dementia in suspected subjects. Consensus meetings (MMK, PK, SG, and RNK) were held to review all the cases. The inter-rater reliability of 95% was apparent in the sample assessments given there was disagreement on five cases (whether cognitively impaired or demented) out of 100 evaluations (KH, FU, RFP and RNK).
2.5. Laboratory analysis
To obtain standard clinical laboratory values (Avenue Clinical Laboratories, Nairobi), 10–20 ml sample of blood was taken from each subject in EDTA tubes at the time of the clinical exam. B12, folic acid or thyroid function were not determined but each specimen was screened for HIV per WHO procedure and tested for syphilis to rule out CNS involvement using the VDRL (Venereal Disease Research Laboratory) and RPR (Rapid Plasma Reagin) methods.
2.6. APOE genotyping
For genetic analysis six drops (∼50 µl) of blood from each subject were spotted on filter (Gutherie) paper cards, which were air-dried for 15 min and individually collated in air-tight sealed bags for DNA analysis (Yang et al., 1996). As soon as the blood samples were cleared to be negative for HIV or VDRL (see above), filter cards were shipped to Newcastle for genotype analysis. DNA extracted from blood spots of 91 individuals was amplified by PCR to determine APOE genotypes and allele frequencies essentially as described previously (Premkumar et al., 1996).
2.7. Statistical design and analysis
The two resulting scores of the CSID from each subject and informant relative, i.e. cognitive and informant scores, were computed to derive the discriminant function (DF) score for each subject using the equation DF score = 0.4523 − 0.016699 × COGSCORE + 0.030338 × RELSCORE, to derive the combination of cognitive and informant scores that best discriminates between demented and non-demented subjects (Hall et al., 1996; Hendrie et al., 1993, 1995).
To accomplish multiple-site comparisons, we simply compared the two DF scores for the subjects in the Nyeri data: one DF score was calculated using the DF derived from the Indianapolis–Ibadan sample, and the other was calculated using the DF that we derived from the Nyeri data alone: DF score = 1.5960 − 0.2365 × COGSCORE + 0.2390 × RELSCORE.
Since the two DF scores have different scales and ranges, their ranks rather than the original scores were compared. We tested the difference between the distributions of the two ranks. If they were statistically different from each other, then we might choose the DF that had higher power, when appropriate cut-off points were chosen. To evaluate the agreement of the ranks of the two DF scores, Wilcoxon’s signed-rank test was used to test the difference between the ranks of two DF scores. In addition, we estimated the total percentage of disagreement between the two ranks, using percentiles as cut-off points.
In order to assess the ability of the DF scores to separate AD subjects from controls, sensitivity, specificity, and total errors in classification (1-sensitivity ± 1-specificity) were estimated at various cut-off points. An empirical receiver operating characteristic (ROC) curve was computed for the two logistic regression models that correlated with the clinical diagnosis of the two studies DF scores. The area under the curves was also computed for each logistic regression model (Hall et al., 1996). Since there was no significant difference between the distributions of the rank of the DF scores, we were justified in using cut-off scores derived from the previous study (Hall et al., 1993). The set of cut-off points of DF scores was chosen to maximize the specificity when sensitivity is set at 100%.
Standard statistical was performed using the SPSS v.10 software (Chicago, IL, USA). Comparisons between categories and numeric variables, e.g. ages, laboratory values were assessed by the two tailed t-test and Mann-Whitney U-test. Associations between numeric variables were determined using the Spearman’s rank correlation analysis (coefficient estimate r). Significance was considered at a probability (p) value equal or less than 0.05.
Comparisons of the distribution of APOE genotypes and allele frequencies in the two groups were performed using Pearson’s chi-square (χ2) and Fisher’s exact tests. Unless otherwise stated, the allele frequency comparisons between groups for each of the three alleles were determined in the absence and presence of at least one APOE allele (Premkumar et al., 1996).
3. Results
3.1. Clinical features, cognitive assessment and discriminant function scores
The mean age of the normal control and demented subjects in the Nyeri sample was 69.7 and 76.4 years (Table 1). The oldest intact and demented individuals, albeit men were 92 and 96 years old. The average period of dementia was 7 years. Of the 84 controls, eight subjects showed mild cognitive impairment or cognitive impairment with no dementia. There were no significant differences in age between the samples from the Nyeri and Indianapolis–Ibadan studies (two-sample t-tests, p > 0.05). In the Nyeri sample the mean age was 70.7 years whereas in the Ibadan and Indianapolis samples mean ages were 73.5 and 72.2 years.
In Nyeri, the average period of formal education was 2.4 years among the controls versus 2.0 years in the demented group. Generally, women were less educated by one year (Table 1). Despite a rural environment the overall level of education of subjects in Nyeri was slightly higher than in Ibadan. In Kenya, 45% were without formal education and 55% with an average of 4.0 years (±2.4) but in Ibadan 80% were without formal education. In contrast, in Indianapolis subjects had significantly higher levels of education vis a vis 40%: 1–8 years, 50%: 9–12 years, 10% > 12 years.
Among the non-demented group, we found following distribution of non-communicable conditions: 13% diabetes, 10.9% hypertensive, 7.6% angina, 1% heart attack, 2.2% at least one stroke and 1.1% depressive illness. Although these conditions occurred less frequently (<1%) in the demented group there were no apparent differences in frequencies. Whereas 39.1% of the non-demented group had ever used alcohol, only 6.6% of the demented group had used it. However, 23.9% of the controls regularly drank alcohol compared to 3.0% of the demented subjects. Similarly, 40.2% of the normal group smoked tobacco compared to 3% of demented subjects.
Fig. 1 shows the ranks of the two DF scores based on the Nyeri and Indianapolis–Ibadan models. The Wilcoxon’s signed-rank test indicated no significant differences between the ranks of the two DF scores (p = 0.42). Table 2 shows the estimated total percentage disagreement between the ranks of the two DF scores, using every 10 percentile cut-off points. The estimated 97.5% upper limit of disagreement is 0% at the 90th percentile and 5.45% at the 80th percentile, based on the binomial distribution. The percentage disagreement between the ranks of the two DF scores was at most 4% in upper 20%which corresponded to 5.45% for the 97.5%upper limit. The two DF scores gave roughly the same result when the cut-off point was chosen in the higher 20% (80th–100th percentiles) of ranks of the DF scores. By design, the higher DF score indicates a higher probability of being demented and with AD, indicated that the Indianapolis-Ibadan DF can be applied to the Nyeri study.
Fig. 1.
The ranks of the two DF scores based on the Nyeri, Kenya and Indianapolis, USA and Ibadan, Nigeria models. The numbers represent the status from the clinical diagnosis. For controls: 0 for cognitively normal (n = 74) and 1 for cognitively impaired but no dementia (n = 10). Cases are shown as 2 and met criteria for dementia (n = 16).
Table 2.
Estimated total percentages of disagreement between dementia and non-dementia
| Cut-off at percentile | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 |
|---|---|---|---|---|---|---|---|---|---|
| Total % of Disagreement | 0 | 2 | 2 | 6 | 6 | 8 | 6 | 2 | 0 |
| 97.5% Upper limit of disagreement (%) | 3.62 | 5.45 | 5.45 | 12.60 | 12.60 | 15.16 | 12.60 | 5.45 | 3.62 |
The numbers show the proportion of disagreement of subjects being categorized into different statuses (AD and non-AD) based on the ranks of the two DF scores when the cut-off point is chosen as the 10th to the 90th percentiles. The estimated 97.5% upper limit of disagreement was to estimate the percentage of subjects categorized into different status (AD and non-AD) based on the ranks of the two DF scores at each percentile. The categorized statuses of subjects based on the ranks of two DF scores are similar when the cut-off points at both ends the distribution. Thus, it was less than 4% if the cut-off point is at the 10th and 90th percentiles and less than 6% at the 20th and 80th percentiles.
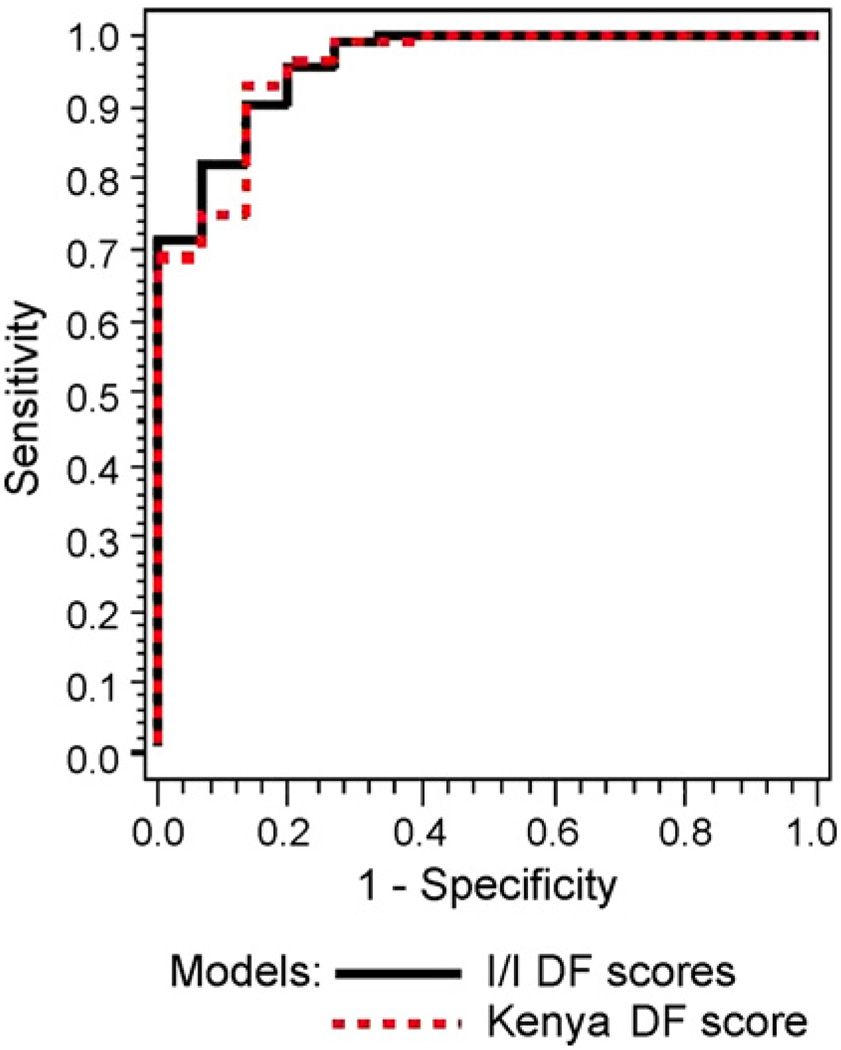
We determined the sensitivities and specificities for the two sites when the total error in classification was less than 40% (37.5% precisely). When the sensitivity is fixed at 81.3%, the specificity of the Nyeri DF score is 92.9%. However, when the sensitivity is fixed at 87.50%, the specificity of the Nyeri DF scores is 75%. Fig. 2 shows that the ROC curves of the two models were remarkably similar and the areas under the curves were not different (Nyeri = 0.911 and Indianapolis–Ibadan = 0.913).
Fig. 2.
ROC curves for discriminant function (DF) scores for Kenyan and Indianapolis–Ibadan (I–I) models.
3.2. Blood chemistry and APOE genotypes
Except for the MMSE scores, there were no significant differences in any of the mean values including haemoglobin (Hb) and lipids between the demented and control groups (Table 3). There was a tendency for increased triglyceride and cholesterol concentrations in the demented subjects but differences were not significant (p = 0.062). Five subjects (three controls and two demented) were severely anaemic indicated by <10 dg/L of Hb concentrations, which correlated (p < 0.01, Spearman’s rho) with their haematocrit values. None of the subjects were positive for HIV or syphilis.
Table 3.
Biochemical values and APOE ε4 allele frequencies in the Nyeri Ageing Study compared to Ibadan and non-African populations in developing countries
| Age, MMSE and biochemical indicesa | Controls (range) | Dementedb (range) |
|---|---|---|
| Age (years) | 70 ± 6.0 (n = 75) |
76.4 ± 8.9 (n = 16) |
| MMSE scoresc | 22 ± 1.0 | 14 ± 1.0 |
| Haematological values (normal range values) | ||
| Red blood cell count (male: 4.3–5.9 ×106; female: 3.5–5.5 ×106) | 4.8 ± 0.5 (3.3–5.8) | 4.7 ± 0.6 (3.7–5.5) |
| Haemoglobin (male: 13.5–17.5 g/dL; female: 12.0–16.0 g/dL | 14.2 ± 2.4 (4.8–18.5) | 13.7 ± 2.9 (4.4–16.6) |
| Haematocrit (packed cell volume) (male: 41–53%; female: 36–46%) | 43.8 ± 4.7 (30–55) | 43.1 ± 4.8 (37–55) |
| White Blood Cells (4.5–11.0 × 106 total) | 6.1 ± 1.9 (1.2–13.2) | 6.0 ± 1.4 (2.2–7.8) |
| Lipids | ||
| Triglycerides (0.4 – 1.8 mmol/L) | 1.8 ± 1.0 (0.7–5.7) | 2.4 ± 1.9 (0.8–7.7) |
| Cholesterol (3.6–6.5 mmol/L) | 4.7 ± 1.1 (1.2–6.8) | 5.3 ± 1.4 (3.3–8.7) |
| Other | ||
| Glucose (3.8–6.1 mmol/L; 2 h postprandial <6.6 mmol/L) | 6.4 ± 3.5 (3.7–16.9) | 6.2 ± 3.5 (4.3–18.3) |
| Total proteins (6.0–7.8 g/dL g/dl) | 7.8 ± 0.9 (6.2–10.4) | 7.9 ± 1.1 (5.3–9.2) |
| APOE allelesd | ||
| ε2% | 2.5 | 3.1 |
| ε3% | 65.3 | 65.6 |
| ε4% | 32.2 | 31.3 |
| APOEε4 allele frequencies in other studiese | ||
| Yoruba (Ibadan) n = 39 controls vs. 12 AD | 20.5 | 16.7 |
| Yoruba (Ibadan, Nigeria) n = 459 controls vs. 123 AD | 21.7 | 26.0 |
| Jamaicans (Jamaica) n = 59 controls vs. 14 AD | 22.0 | 39.2 |
| African Caribbean in UK (Jamaican, Barbadian and Guyanese) n = 36 controls vs. 50 CI | 31.0 | 42.0 |
| Wadi Ara Arabs (Israel) n = 25 AD | 3.5 | 4.5 |
| North Indians (Ballabgarh, India) n = 4450 controls vs. 28 AD | 7.0 | 16.7f |
Except for APOE allele frequencies (percent, %) values show mean ± S.D. derived from CSID and clinical data based on 91 subjects. Abbreviation: CI, cognitive impairment.
Biochemical values in Africans are within same range.
Demented group consists of cases with diagnoses of probable AD.
MMSE scores were significantly different (p = 0.000). Ten control subjects had a score below 15 but were considered unimpaired by CSID criteria.
There were no differences in the distribution of APOE alleles between AD and controls.
For comparison, reported data from our previous studies (Farrer et al., 2003; Gureje et al., 2006; Morgan et al., 1998; Osuntokun et al., 1995; Sayi et al., 1997; Stewart et al., 2001) compared to the Ballabgarh Indian study (Ganguli et al., 2000) shows APOEε4 frequencies were not significantly increased in AD group.
p < 0.05 by χ2 analysis (Ganguli et al., 2000)
More than 80% of the Nyeri Kikuyus carried the APOE ε3/ε3 and ε3/ε4 genotypes, with ε3/ε4 genotype as the most common genotype (48% versus 40% of ε3/ε3). There were no individuals with the rare ε2/ε2 genotype, only two with the ε2/ε3 and two with the ε2/ε4 signifying high frequency of ε4 allele in the sample. None of the demented subjects was homozygous for the ε4 allele. By contrast, the ε4/ε4 genotype was carried by five control subjects, who were between 65 and 75 years of age with no signs of cognitive impairment. The APOE ε4 allele frequencies were higher than 30% (50 alleles in each sample) in both controls and demented subjects (Table 3), with no significant differences in distribution between the groups. For the total sample, odds ratios for dementia were 0.93 (95% CI 0.60–1.44) if at least one copy of ε4 allele was present and 0.94 (95% CI 0.79–1.10) for the ε3 allele. In keeping with the Ibadan Nigerians, there were no differences in the ε4 allele frequencies between the demented (with probable AD) and normal Nyeri Kikuyus (Table 3). We also found that the APOE genotypes did not show any meaningful relationships with lipid levels or any of the vascular risk factors including hypertension, cardiovascular disease, diabetes and stroke.
4. Discussion
Our study carried out in the Nyeri district among life time residents demonstrates that the performance in the CSID is comparable to what has been published based on Indianapolis–Ibadan screening outcomes (Hendrie et al., 1995, 2001). We have extended the clinical utility of the CSID in a previously never studied elderly Kikuyu African cohort in Nyeri, Kenya. We also determined that the DF based on the previous studies could be applied to data using the CSID in Kikuyu. We showed if the sensitivity was assigned to be a 100% the specificity is nearly 90% for the detection of dementia, specifically probable AD. This analysis clearly allows us to compare the data from Kenya to the previous studies based on the similar measurement scales (Prince et al., 2007).
Our study also affords an opportunity to investigate risk factors for AD in the Kikuyu and by extension in other east African peoples such as the nomadic Maasai from different cultures and environments using similar techniques. These screening methods may also be used to examine familial AD in African populations (Heckmann et al., 2004). Besides genetics, other risk factors that would be particularly important to explore in the context of the developed communities include physical characteristics such as head circumference (Borenstein et al., 2005) and leg length (Mak et al., 2006), dietary trends and physical, and mental activities (Borenstein et al., 2005; Ogunniyi et al., 2006). Although vascular factors such as hypertension, history of altered cholesterol or triglyceride levels, hyperinsulinanaemia, diabetes and adiposity (body mass index) have been investigated in several developed nations, it is little known whether these factors (Hall et al., 2006) alter prevalence and incidence of dementia in middle to low income countries (Farrag et al., 1998; Hendrie et al., 1995, 2001). Indeed, a larger sample should also provide possibilities to explore the effects of increasing prevalence of stroke (Hachinski et al., 2006; Kalaria and Ballard, 2001; Richards et al., 2000) and depressive illness on cognitive function (Green et al., 2003) and depression frequency itself (Baiyewu et al., 2007; Prince et al., 2003) which comprises a large burden of mental health in Africa (WHO, 2002).
Our prospective data shows high APOE allele frequencies in cognitively intact elderly Nyeri Kenyans >65 years of age and a lack of correlation between ε4 allele frequency and probable AD. These new findings in controls accord with previous retrospective data in East Africans (Kalaria et al., 1997; Sayi et al., 1997). APOE ε4 allele occurrence, the so-called ancestral allele, has also been reported to be high in other African and non-African peoples. Frequencies were reported to be as high as 41% in Pygmies of Central African Republic, 37% in Khoi San of Southern Africa, 24–26% in the Aborigines of Malaysia and Australia, 37% in Papuans, 28% in some Native Americans and 31% in the Lapps (Corbo and Scacchi, 1999)and at the higher end of the spectrum at 14.3% and 15.5% in Africans from Ethiopia and Benin compared to the Caucasians (Corbo et al., 1999; Farrer et al., 1997). By contrast, certain people exhibit very low frequencies of the ε4 allele at 3.5% in the Wadi Ara Arabs in Israel (Farrer et al., 2003) and 7% in the North Indians of Ballabgarh (Ganguli et al., 2000).
In a meta-analysis encompassing data from several countries, Farrer et al. (1997) reported the APOE ε4 allele represented a major risk for AD in both genders in almost all ethnic groups across all ages between 40 and 90 years although the allele was not associated with AD in Italians >81 years (Scacchi et al., 1995). The relationship is weaker or inconsistent in African Americans and the Caribbean Hispanics (Farrer et al., 1997; Romas et al., 2002). Among the African-Caribbeans of largely Jamaican origin (78%), the APOE ε4 allele was weakly associated with cognitive impairment. Frequencies of ε4 were in the range 27–33% in the unimpaired group (Stewart et al., 2001). However, this is at variance with Nigerian Africans as we demonstrate in the Kikuyu Kenyans. Similar to autosomal dominant genes associated with AD (Heckmann et al., 2004), the APOE ε4 allele also does not appear to increase risk of AD in certain communities with high consangunity such as the Wadi Ara Arabs in North Israel (Farrer et al., 2003). The lack of effect of ε4 allele as a susceptibility factor for probable AD in Yoruba, Bantu and Nilotic Africans (Gureje et al., 2006; Heckmann et al., 2004; Osuntokun et al., 1995; Sayi et al., 1997) is unclear. The ε4 allele has been suggested to be the ‘thrifty’ allele in that exposure of APOE ε4 to the contemporary environmental conditions including dietary habits and sedentary life styles could have induced it a susceptibility factor for AD and coronary artery disease (Corbo et al., 2006).
Our sample was relatively limited in size but provides sufficient base data to explore potentially interesting risk factors for AD and other dementias in developing countries. Although not expected there were differences in levels of education between the Nyeri and Ibadan samples. In Nyeri, the elderly were marginally but significantly more literate, particularly the men. The level of basic education, literacy and mental activity as protective factors for cognitive impairment need to be investigated within peoples in developing countries. We could not adequately address the contribution of plasma factors such as homocysteine (Bowirrat et al., 2002) or Hb (Pandav et al., 2004) but assessing cognitive impairment and dementia in communities living in the developing world with reliable thoroughly tested methods opens up several future possibilities. In this context, the 10/66 dementia research group have published relevant protocols with similar goals (Prince et al., 2007). Our data, however, demonstrate that instruments such as the full CSID can be adapted for use in sub-Saharan Africa.
Acknowledgements
We are grateful for the co-operation of the subjects and families. We thank Mrs Anne M Ngari, (Kenyatta Hospital, Nairobi) for co-ordinating the study between the Nyeri and Nairobi sites, and keeping track of all the interviews. Mr Ngatia Hama, Agricultural Extension worker in Nyeri is gratefully acknowledged for contacting and locating the subjects and relatives. Members of the Nyeri Ageing study team of interviewers Humphrey Kariuki, Jennifer Kimunya, Pauline Muita, Irene Muruku, Alice Ngatia, Olive Wambugu and the Avenue Clinical laboratories staff are sincerely thanked for their invaluable contributions. Beverley Muscik (Indiana University) kindly helped in processing the data during the first phases of the analyses. Prof Carol Brayne (University of Cambridge) provided useful advice on early phases of the study. Dr Amrik Sahota (Rutgers University) helped to troubleshoot the extraction of DNA and genotyping from the blood spots on filter cards. Our work was supported in part by the Alzheimer’s Association, USA, the Nickman Family of Cleveland, Ohio, the National Institute on Aging (RO1AG09956, P30AG10133,1UO1AG17173-01A2, and the Alzheimer’s Disease Research Center Program, P50 AG 08012), the National Institute of General Medical Sciences (GM28356), the National Center for Research Resources (RR03655), the Joseph and Florence Mandel Foundation, The Institute for the Study of Aging, New York and the Medical Research Council, UK.
Footnotes
Disclosure statement
None of the authors withhold any actual or potential conflicts of interest connected with this study.
References
- Baiyewu O, Smith-Gamble V, Lane KA, Gureje O, Gao S, Ogunniyi A, Unverzagt FW, Hall KS, Hendrie HC. Prevalence estimates of depression in elderly community-dwelling African Americans in Indianapolis and Yoruba in Ibadan. Nigeria Int. Psychogeriatr. 2007;19:679–689. doi: 10.1017/S1041610207005480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein AR, Wu Y, Mortimer JA, Schellenberg GD, McCormick WC, Bowen JD, McCurry S, Larson EB. Developmental and vascular risk factors for Alzheimer’s disease. Neurobiol. Aging. 2005;26:325–334. doi: 10.1016/j.neurobiolaging.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Bowirrat A, Friedland RP, Farrer L, Baldwin C, Korczyn A. Genetic and environmental risk factors for Alzheimer’s disease in Israeli Arabs. J. Mol. Neurosci. 2002;19:239–245. doi: 10.1007/s12031-002-0040-4. [DOI] [PubMed] [Google Scholar]
- Brayne C. The EURODEM collaborative reanalysis of case-control studies of Alzheimer’s disease: implications for public health. Int. J. Epidemiol. 1991;20:568–571. doi: 10.1093/ije/20.supplement_2.s68. [DOI] [PubMed] [Google Scholar]
- Buschke H, Kuslansky G, Katz M, Stewart WF, Sliwinski MJ, Eck-holdt HM, Lipton RB. Screening for dementia with the memory impairment screen. Neurology. 1999;52:231–238. doi: 10.1212/wnl.52.2.231. [DOI] [PubMed] [Google Scholar]
- Corbo RM, Scacchi R. Apolipoprotein E (APOE) allele distribution in the world. Is APOE*4 a ‘thrifty’ allele? Ann. Hum. Genet. 1999;63:301–310. doi: 10.1046/j.1469-1809.1999.6340301.x. [DOI] [PubMed] [Google Scholar]
- Corbo RM, Scacchi R, Rickards O, Martinez-Labarga C, De Stefano GF. An investigation of human apolipoproteins B and E polymorphisms in two African populations from Ethiopia and Benin. Am. J. Hum. Biol. 1999;11:297–304. doi: 10.1002/(SICI)1520-6300(1999)11:3<297::AID-AJHB2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Corbo RM, Prevost M, Raussens V, Gambina G, Moretto G, Scacchi R. Structural and phylogenetic approaches to assess the significance of human Apolipoprotein E variation. Mol. Genet. Metab. 2006;89:261–269. doi: 10.1016/j.ymgme.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Evans DA, Funkenstein HH, Albert MS, Scherr PA, Cook NR, Chown MJ, Hebert LE, Hennekens CH, Taylor JO. Prevalence of Alzheimer’s diseasein acommunity population of older persons. Higher than previously reported. JAMA. 1989;262:2551–2556. [PubMed] [Google Scholar]
- Farrag A, Farwiz HM, Khedr EH, Mahfouz RM, Omran SM. Prevalence of Alzheimer’s disease and other dementing disorders: Assiut–Upper Egypt study. Dement. Geriatr. Cogn. Disord. 1998;9:323–328. doi: 10.1159/000017084. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- Farrer LA, Friedland RP, Bowirrat A, Waraska K, Korczyn A, Baldwin CT. Genetic and environmental epidemiology of Alzheimer’s disease in arabs residing in Israel. J. Mol. Neurosci. 2003;20:207–212. doi: 10.1385/JMN:20:3:207. [DOI] [PubMed] [Google Scholar]
- Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguli M, Chandra V, Kamboh MI, Johnston JM, Dodge HH, Thelma BK, Juyal RC, Pandav R, Belle SH, DeKosky ST. Apolipoprotein E polymorphism and Alzheimer disease: The Indo-US Cross-National Dementia Study. Arch. Neurol. 2000;57:824–830. doi: 10.1001/archneur.57.6.824. [DOI] [PubMed] [Google Scholar]
- Grant WB. Incidence of dementia and Alzheimer disease in Nigeria and the United States. JAMA. 2001;285:2448. (author reply 2448–2449) [PubMed] [Google Scholar]
- Green RC, Cupples LA, Kurz A, Auerbach S, Go R, Sadovnick D, Duara R, Kukull WA, Chui H, Edeki T, Griffith PA, Friedland RP, Bachman D, Farrer L. Depression as a risk factor for Alzheimer disease: the MIRAGE Study. Arch. Neurol. 2003;60:753–759. doi: 10.1001/archneur.60.5.753. [DOI] [PubMed] [Google Scholar]
- Gureje O, Ogunniyi A, Baiyewu O, Price B, Unverzagt FW, Evans RM, Smith-Gamble V, Lane KA, Gao S, Hall KS, Hendrie HC, Murrell JR. APOE epsilon4 is not associated with Alzheimer’s disease in elderly Nigerians. Ann. Neurol. 2006;59:182–185. doi: 10.1002/ana.20694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, Powers WJ, DeCarli C, Merino JG, Kalaria RN, Vinters HV, Holtzman DM, Rosenberg GA, Dichgans M, Marler JR, Leblanc GG. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- Hall K, Hendrie H, Brittain H, Norton JA. The development of a dementia screening interview in two distinct languages. Int. J. Methods Psychiatr. Res. 1993;3:1–28. [Google Scholar]
- Hall K, Ogunniyi A, Hendrie H, Osuntokun BO, Hui S, Musick B, Rodenberg CA, Unverzagt F, Gureje O, Baiyewu O. A cross-cultural community based study of dementias: methods and perfomance of the survey instrument Indianpolis, USA and Ibadan, Nigeria. Int. J. Methods Psychiatr. Res. 1996;6:129–142. [Google Scholar]
- Hall KS, Gao S, Emsley CL, Ogunniyi AO, Morgan O, Hendrie HC. Community screening interview for dementia (CSI ‘D’); performance in five disparate study sites. Int. J. Geriatr. Psychiatry. 2000;15:521–531. doi: 10.1002/1099-1166(200006)15:6<521::aid-gps182>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Hall K, Murrell J, Ogunniyi A, Deeg M, Baiyewu O, Gao S, Gureje O, Dickens J, Evans R, Smith-Gamble V, Unverzagt FW, Shen J, Hendrie H. Cholesterol, APOE genotype, and Alzheimer disease: an epidemiologic study of Nigerian Yoruba. Neurology. 2006;66:223–227. doi: 10.1212/01.wnl.0000194507.39504.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann JM, Low WC, de Villiers C, Rutherfoord S, Vorster A, Rao H, Morris CM, Ramesar RS, Kalaria RN. Novel presenilin1 mutation with profound neurofibrillary pathology in an indigenous Southern African family with early-onset Alzheimer’s disease. Brain. 2004;127:133–142. doi: 10.1093/brain/awh009. [DOI] [PubMed] [Google Scholar]
- Hendrie HC, Hall KS, Pillay N, Rodgers D, Prince C, Norton J, Brittain H, Nath A, Blue A, Kaufert J, et al. Alzheimer’s disease is rare in Cree. Int. Psychogeriatr. 1993;5:5–14. doi: 10.1017/s1041610293001358. [DOI] [PubMed] [Google Scholar]
- Hendrie HC, Osuntokun BO, Hall KS, Ogunniyi AO, Hui SL, Unverzagt FW, Gureje O, Rodenberg CA, Baiyewu O, Musick BS. Prevalence of Alzheimer’s disease and dementia in two communities: Nigerian Africans and African Americans. Am. J. Psychiatry. 1995;152:1485–1492. doi: 10.1176/ajp.152.10.1485. [DOI] [PubMed] [Google Scholar]
- Hendrie HC, Ogunniyi A, Hall KS, Baiyewu O, Unverzagt FW, Gureje O, Gao S, Evans RM, Ogunseyinde AO, Adeyinka AO, Musick B, Hui SL. Incidence of dementia and Alzheimer disease in 2 communities: Yoruba residing in Ibadan, Nigeria, and African Americans residing in Indianapolis, Indiana. JAMA. 2001;285:739–747. doi: 10.1001/jama.285.6.739. [DOI] [PubMed] [Google Scholar]
- Hendrie HC, Murrell J, Gao S, Unverzagt FW, Ogunniyi A, Hall KS. International studies in dementia with particular emphasis on populations of African origin. Alzheimer Dis. Assoc. Disord. 2006;20:S42–S46. doi: 10.1097/00002093-200607001-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol. Med. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- Kalaria RN. The role of cerebral ischemia in Alzheimer’s disease. Neurobiol. Aging. 2000;21:321–330. doi: 10.1016/s0197-4580(00)00125-1. [DOI] [PubMed] [Google Scholar]
- Kalaria RN. Dementia comes of age in the developing world. Lancet. 2003;361:888–889. doi: 10.1016/S0140-6736(03)12783-3. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Ballard C. Stroke and cognition. Curr. Atheroscler. Rep. 2001;3:334–339. doi: 10.1007/s11883-001-0028-5. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Ogeng’o JA, Patel NB, Sayi JG, Kitinya JN, Chande HM, Matuja WB, Mtui EP, Kimani JK, Premkumar DR, Koss E, Gatere S, Friedland RP. Evaluation of risk factors for Alzheimer’s disease in elderly east Africans. Brain Res. Bull. 1997;44:573–577. doi: 10.1016/s0361-9230(97)00310-9. [DOI] [PubMed] [Google Scholar]
- Maj M, Janssen R, Satz P, Zaudig M, Starace F, Boor D, Sughond-habirom B, Bing EG, Luabeya MK, Ndetei D, et al. The World Health Organization’s cross-cultural study on neuropsychiatric aspects of infection with the human immunodeficiency virus 1 (HIV-1). Preparation and pilot phase. Br. J. Psychiatry. 1991;159:351–356. doi: 10.1192/bjp.159.3.351. [DOI] [PubMed] [Google Scholar]
- Mak Z, Kim JM, Stewart R. Leg length, cognitive impairment and cognitive decline in an African-Caribbean population. Int. J. Geriatr. Psychiatry. 2006;21:266–272. doi: 10.1002/gps.1458. [DOI] [PubMed] [Google Scholar]
- Morgan OS, Eldemire DA, Thesiger CH, Luseko J, Sahota A, Gao S, Hall KS, Hendrie HC. APOE allele frequencies in demented and nondemented elderly Jamaicans. Ann. Neurol. 1998;43:545. doi: 10.1002/ana.410430423. [DOI] [PubMed] [Google Scholar]
- Muriuki G. A History of the Kikuyu 1500–1900. Nairobi, Kenya: Oxford University Press; 1974. [Google Scholar]
- Ogunniyi A, Hall KS, Gureje O, Baiyewu O, Gao S, Unverzagt FW, Smith-Gamble V, Evans RE, Dickens J, Musick BS, Hendrie HC. Risk factors for incident Alzheimer’s disease in African Americans and Yoruba. Metab. Brain Dis. 2006;21:235–240. doi: 10.1007/s11011-006-9017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuntokun BO, Hendrie HC, Ogunniyi AO, Hall KS, Lekwauwa UG, Brittain HM, Norton JA, Jr, Oyediran AB, Pillay N, Rodgers DD. Cross-cultural studies in Alzheimer’s disease. Ethn. Dis. 1992;2:352–357. [PubMed] [Google Scholar]
- Osuntokun BO, Sahota A, Ogunniyi AO, Gureje O, Baiyewu O, Adeyinka A, Oluwole SO, Komolafe O, Hall KS, Unverzagt FW, et al. Lack of an association between apolipoprotein E epsilon 4 and Alzheimer’s disease in elderly Nigerians. Ann. Neurol. 1995;38:463–465. doi: 10.1002/ana.410380319. [DOI] [PubMed] [Google Scholar]
- Pandav RS, Chandra V, Dodge HH, DeKosky ST, Ganguli M. Hemoglobin levels and Alzheimer disease: an epidemiologic study in India. Am. J. Geriatr. Psychiatry. 2004;12:523–526. doi: 10.1176/appi.ajgp.12.5.523. [DOI] [PubMed] [Google Scholar]
- Premkumar DR, Cohen DL, Hedera P, Friedland RP, Kalaria RN. Apolipoprotein E-epsilon4 alleles in cerebral amyloid angiopathy and cerebrovascular pathology associated with Alzheimer’s disease. Am. J. Pathol. 1996;148:2083–2095. [PMC free article] [PubMed] [Google Scholar]
- Prince M, Acosta D, Chiu H, Scazufca M, Varghese M. Dementia diagnosis in developing countries: a cross-cultural validation study. Lancet. 2003;361:909–917. doi: 10.1016/S0140-6736(03)12772-9. [DOI] [PubMed] [Google Scholar]
- Prince M, Graham N, Brodaty H, Rimmer E, Varghese M, Chiu H, Acosta D, Scazufca M. Alzheimer Disease International’s 10/66 Dementia Research Group – one model for action research in developing countries. Int. J. Geriatr. Psychiatry. 2004;19:178–181. doi: 10.1002/gps.1059. [DOI] [PubMed] [Google Scholar]
- Prince M, Ferri CP, Acosta D, Albanese E, Arizaga R, Dewey M, Gavrilova SI, Guerra M, Huang Y, Jacob KS, Krishnamoorthy ES, McKeigue P, Rodrigues JL, Salas A, Sosa AL, Sousa R, Stewart R, Uwakwe R. The protocols for the 10/66 Dementia Research Group population-based research programme. BMC Public Health. 2007;7:165. doi: 10.1186/1471-2458-7-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards SS, Emsley CL, Roberts J, Murray MD, Hall K, Gao S, Hendrie HC. The association between vascular risk factor-mediating medications and cognition and dementia diagnosis in a community-based sample of African-Americans. J. Am. Geriatr Soc. 2000;48:1035–1041. doi: 10.1111/j.1532-5415.2000.tb04777.x. [DOI] [PubMed] [Google Scholar]
- Romas SN, Santana V, Williamson J, Ciappa A, Lee JH, Rondon HZ, Estevez P, Lantigua R, Medrano M, Torres M, Stern Y, Tycko B, Mayeux R. Familial Alzheimer disease among Caribbean Hispanics: a reexamination of its association with APOE. Arch. Neurol. 2002;59:87–91. doi: 10.1001/archneur.59.1.87. [DOI] [PubMed] [Google Scholar]
- Sayi JG, Patel NB, Premkumar DR, Adem A, Winblad B, Matuja WB, Mtui EP, Gatere S, Friedland RP, Koss E, Kalaria RN. Apolipoprotein E polymorphism in elderly east Africans. East Afr. Med. J. 1997;74:668–670. [PubMed] [Google Scholar]
- Scacchi R, De Bernardini L, Mantuano E, Donini LM, Vilardo T, Corbo RM. Apolipoprotein E (APOE) allele frequencies in late-onset sporadic Alzheimer’s disease (AD), mixed dementia and vascular dementia: lack of association of epsilon 4 allele with AD in Italian octogenarian patients. Neurosci. Lett. 1995;201:231–234. doi: 10.1016/0304-3940(95)12190-0. [DOI] [PubMed] [Google Scholar]
- Stewart R, Russ C, Richards M, Brayne C, Lovestone S, Mann A. Apolipoprotein E genotype, vascular risk and early cognitive impairment in an African Caribbean population. Dement. Geriatr. Cogn.Disord. 2001;12:251–256. doi: 10.1159/000051267. [DOI] [PubMed] [Google Scholar]
- WHO. The use of epidemiology in the study of the elderly. Report of a WHO Scientific Group on the Epidemiology of Aging. WHO Techn. Rep. Ser. 1984;707:9. [PubMed]
- WHO. Geneva: World Health Organisation; Active ageing: a policy framework, 2002 health report. 2002
- Wimo A, Jonsson L, Winblad B. An estimate of the worldwide prevalence and direct costs of dementia in 2003. Dement. Geriatr. Cogn Disord. 2006;21:175–181. doi: 10.1159/000090733. [DOI] [PubMed] [Google Scholar]
- Yang M, Hendrie HC, Hall KS, Oluwole OS, Hodes ME, Sahota A. Improved procedure for eluting DNA from dried blood spots. Clin. Chem. 1996;42:1115–1116. [PubMed] [Google Scholar]