Abstract
Background
Muscarinic acetylcholine receptors (mAChRs) are well positioned to mediate ethanol’s stimulant effects. To investigate this possibility, we examined the effects of scopolamine, a receptor subtype nonselective mAChR antagonist, on ethanol-induced stimulation in genotypes highly sensitive to this effect of ethanol. We also investigated whether the dopamine D1-like receptor antagonist, SCH-23390 or the dopamine D2-like receptor antagonist, haloperidol, could block the extreme stimulant response found following co-administration of scopolamine and ethanol.
Methods
Scopolamine (0, 0.0625, 0.125, 0.25, or 0.5 mg/kg) was given 10 minutes prior to saline or ethanol (0.75 to 2 g/kg) to female FAST (Experiment I) or DBA/2J (Experiment II) mice that were then tested for locomotion for 30 minutes. In Experiments III and IV, respectively, SCH-23390 (0, 0.015, or 0.03 mg/kg) was given 10 minutes prior, and haloperidol (0, 0.08, or 0.16 mg/kg) was given 2 minutes prior, to scopolamine (0 or 0.5 mg/kg), followed 10 minutes later by saline or ethanol (1.5 g/kg) and female DBA/2J mice were tested for locomotion for 30 minutes.
Results
FAST and DBA/2J mice displayed a robust enhancement of the locomotor effects of ethanol following pretreatment with scopolamine that was suggestive of synergism. SCH-23390 had no effect on the response to the scopolamine + ethanol drug combination, nor did it attenuate ethanol- or scopolamine-induced locomotor activity. Haloperidol, while attenuating the effects of ethanol, was not able to block the effects of scopolamine or the robust response to the scopolamine-ethanol drug combination.
Conclusions
These results suggest that while muscarinic receptor antagonism robustly enhances acute locomotor stimulation to ethanol, dopamine receptors are not involved in the super-additive interaction of scopolamine and ethanol treatment. They also suggest that in addition to cautions regarding the use of alcohol when scopolamine is clinically prescribed due to enhanced sedative effects, enhanced stimulation may also be a concern.
Keywords: Dopamine, Ethanol, Locomotor Activity, Muscarinic Receptor, Selected Line
The FAST and SLOW lines of mice were created in replicate by selective breeding over multiple generations for extreme sensitivity (FAST-1 and -2) or insensitivity (SLOW-1 and -2) to the locomotor stimulant effects of ethanol (Crabbe et al., 1987; Phillips et al., 1991; Shen et al., 1995b). Several lines of rats and mice have been created for high and low ethanol drinking or high and low ethanol sedative sensitivity, but the FAST and SLOW lines are the only selected lines ever created to model extreme differences in behavioral stimulation to ethanol. The more stimulated FAST lines also exhibit heightened ethanol consumption, as well as reduced sensitivity to the sedative and ataxic effects of ethanol, compared to the SLOW lines (Phillips et al., 2002; Risinger et al., 1994; Shen et al., 1996), a suite of traits that has been sometimes argued to correspond with greater risk for alcohol addiction (Duranceaux et al., 2008; Holdstock et al., 2000; King et al., 2002; Newlin and Thomson, 1990; Schuckit, 1994; Schuckit et al., 2000, 2005). Studies designed to identify differences that are genetically related to the differential sensitivity of FAST and SLOW mice to ethanol have strongly implicated roles for dopaminergic and GABAergic processes (Boehm et al., 2002; Shen et al., 1995a, 1998a), as well as glutamatergic differences (Daniell and Phillips, 1994; Meyer and Phillips, 2003; Shen and Phillips, 1998b). However, several other possible neural substrates remain to be studied. The work described here focused on muscarinic acetylcholine receptor (mAChR)-mediated systems.
mAChRs are perfectly positioned in the brain to influence ethanol-related behaviors. All known subtypes of mAChRs (m1-m5) are G-protein coupled, with the m1, m3, and m5 subtypes positively coupled to phospholipase C (Gq), and the m2 and m4 subtypes negatively coupled to adenylyl cyclase (Gi) (Bymaster et al., 2003). Although there is some overlap, the mAChR subtypes are also differentially expressed and distributed throughout the brain, suggesting that they may serve different functions. The m1 receptor subtype is widely expressed in all major forebrain areas, such as the cortex, hippocampus, and striatum, and has been shown to play a role in learning and memory (Eglen, 2005; Smythies, 2005). The m2 receptor subtype is also widely expressed in the fore-brain, most significantly as a presynaptic autoreceptor in the striatum (Eglen, 2005; Smythies, 2005), where it functions as an inhibitor of neurotransmitter release. The m3 receptor is the subtype least expressed in the central nervous system, and is known to govern smooth muscle contraction (Eglen, 2005). The m4 receptor subtype is expressed in the striatum and colocalized with dopamine D1 receptors (Hersch et al., 1994; Ince et al., 1997); immunoreactivity for the m4 receptor subtype has also been detected in the nucleus accumbens, olfactory tubercle, islands of Calleja, substantia nigra, and cortex (Hersch et al., 1994; Levey et al., 1991). Finally, the m5 receptor subtype is expressed in the substantia nigra and ventral tegmental area (Weiner et al., 1990). Little is known about the roles of the m4 and m5 receptor subtypes in behavior or physiology; however, they are appropriately positioned to play a role in drug-induced changes in locomotor behavior, general locomotor coordination, and reward and reinforcement (Kalivas and Nakamura, 1999; Kalivas and Volkow, 2005; Phillips and Shen, 1996; Wise and Bozarth, 1987).
While it has been previously shown that scopolamine is able to counteract the sedative effects of ethanol in rats (Pohorecky et al., 1979), to the best of our knowledge no one has studied the effects of muscarinic drugs or receptors on acute locomotor stimulation to ethanol. When FAST and SLOW mice were tested for their locomotor response to scopolamine, 1 set of the replicated lines showed a difference in stimulant response (FAST-2 vs. SLOW-2), where as the other replicate set (FAST-1 vs. SLOW-1) did not (Bergstrom et al., 2003). These data provide moderate evidence (Crabbe et al., 1990) for common genetic influence on the locomotor response to scopolamine and ethanol, and thus suggest a possible role for mAChRs in sensitivity to ethanol-induced stimulation. The possible role of these receptors was examined here using the extremely sensitive FAST lines and the DBA/2J inbred mouse strain.
Whereas the FAST lines exhibit a large locomotor stimulant response to ethanol by design, the inbred DBA/2J mouse strain displays a robust locomotor response to an acute injection of ethanol by chance (Dudek et al., 1991). Utilization of these independent mouse populations increases the power for detecting important mechanisms underlying sensitivity to ethanol-induced stimulation. Consistent results across genotypes would provide more compelling evidence for the primary involvement of a neurochemical system or pathway. Three independently derived populations of mice (FAST-1, FAST-2, and DBA/2J) were utilized in these studies. Initial results showed that pretreatment with scopolamine robustly enhanced the locomotor stimulant effects of ethanol. We hypothesized that this was due to an increase in dopaminergic transmission (Di Chiara and Imperato, 1988; Yim and Gonzales, 2000), and examined the ability of SCH-23390, a D1-like dopaminergic receptor antagonist, and haloperidol, a mixed dopaminergic receptor antagonist with greater affinity for the D2 dopamine receptor, to block this response.
MATERIALS AND METHODS
Subjects
These experiments were performed in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals and with approval from the Portland Veterans Affairs Medical Center Institutional Animal Care and Use Committee. The animals were maintained on a 12 h/12 h light/dark cycle, with lights on at 6 am. Ambient temperature was kept at 21 ± 2°C. Food (Purina 5001; Animal Specialties Inc., Hubbard, OR) and tap water were available ad libitum, except during behavioral testing, which occurred during the light phase between 8:00 am and 4:00 pm. Animals were group-housed in polycarbonate/polysulfone cages (28 × 18 × 13 cm; length × width × height) lined with Bed-o’cobs bedding. Female mice were used in all experiments for reasons of availability and because they have a more robust stimulant response to ethanol compared to males; however, genetically determined differences in ethanol response have not been found to be dependent upon sex in the FAST and SLOW lines (Shen et al., 1995b) or standard inbred mouse strains (e.g., Dudek et al., 1991). Animals were distributed into experimental groups such that no cage contained more than 2 animals receiving the same treatment. All mice were experimentally naïve at the time of testing.
FAST-1 and -2 Selected Lines
FAST-1 and -2 offspring resided with dam and sire until 20 to 22 days old, at which time they were housed with same-sex littermates in groups of 2 to 5. Nonlittermates of the same line and similar age (±4 days) were occasionally housed together to avoid isolate housing. The FAST-1 and -2 replicate lines were selectively bred from independent breeding populations of a heterogeneous stock of mice, derived from the intercrossing of 8 genetically diverse inbred mouse strains (McClearn et al., 1970). Selection for extreme sensitivity to the locomotor stimulation induced by injection of 2.0 g/kg ethanol was practiced over multiple generations; 1.5 g/kg ethanol was used in the initial 6 generations of selection, but the dose was increased to maximize selection response (Crabbe et al., 1988). The details of the selection process have been described elsewhere (Crabbe et al., 1987; Phillips et al., 2002, 1991). The selection phenotype was magnitude of stimulation in a 4 minute locomotor test, measured beginning 2 minutes after acute ethanol injection, and corrected using activity results collected for the same time period after saline injection. The saline activity data were collected 24 hours after the ethanol activity data for most selection generations (see Crabbe et al., 1988, for details justifying alterations to the selection phenotype). Following generation 37, selection was relaxed and mice were bred randomly within replicate (Shen et al., 1995b); the stimulant response difference between FAST and SLOW mice has not regressed (Palmer et al., 2002). In the current experiments, FAST-1 mice were from S37G85–88 and FAST-2 mice were from S37G84–86 (Sxx denotes the last generation of selection and Gxx denotes the total number of generations). FAST-1 mice were 49 to 96 days old at the beginning of testing, and FAST-2 mice were 55 to 89 days old.
DBA/2J Inbred Strain
DBA/2J mice were purchased from The Jackson Laboratory West (PaloAlto, CA), and remained housed with their shipping mates in groups of 4. They were allowed at least 2 weeks to acclimate to the colony room before being used in the experiments, and were 52 to 75 days old at the beginning of testing.
Drug Sources and Preparation
Ethanol (200 proof) was obtained from Pharmco (Brookfield, CT). Haloperidol solution USP (2 mg/ml concentrate; Pharmaceutical Associates, Inc., Greenville, SC) was purchased from the Portland VA Pharmacy. R(+)-SCH-23390 hydrochloride and (−)scopolamine hydrobromide were obtained from Sigma (St. Louis, MO). All drugs were diluted in 0.9% saline (Baxter Healthcare Corporation, Deerfield, IL) to the appropriate concentrations on the day of the experiment. Scopolamine was prepared in dark bottles to avoid light-induced degradation. All drugs were given by intraperitoneal injection using Monoject 1cc tuberculin syringes (27 gauge, ½ needles and length; Sherwood Medical, St. Louis, MO) in a volume of 10 ml/kg of body weight (SCH-23390, scopolamine, haloperidol) or, in the case of ethanol, as a 20% v/v solution adjusted for body weight.
Apparatus
Locomotor activity was measured in automated Accuscan activity monitors (Accuscan Instruments Inc., Columbus, OH). Mice were housed in each of 8 monitors in a 40 × 40 × 30 cm (length × width × height) clear acrylic box during testing. Encompassing chambers provided external sound-attenuation due to foam lining, and a fan further masked outside noise and provided ventilation. Chambers were illuminated with an 8 W fluorescent light bulb during testing. The activity monitors had 2 sets of 8, evenly spaced photocell beams and detectors located 2 cm above the chamber floor. Beam breaks from the movement of the mice were automatically recorded and translated by Accuscan software into total distance traveled (cm).
Procedures
On each day of testing, mice were allowed to acclimate in their home cages to the testing room for 45 to 60 minutes. Mice were returned to group housing in their colony room following testing on Days 1 and 2. Injection sites were alternated between the 2 sides of the abdomen to minimize discomfort to the animals. On Day 3, immediately following removal from the activity chambers, periorbital blood samples were collected from each ethanol-treated mouse with 20 μl microcapillary pipets (Kimble Glass Inc., Vineland, NJ) to determine blood ethanol concentration (BEC). Details specific to each experiment are given below. Following each experiment, animals were humanely killed via CO2 inhalation.
Experiment I: Effect of Scopolamine on Acute Locomotor Response to Ethanol in FAST Mice
On Days 1 and 2, all mice were weighed and placed in individual holding cages, with less than 10 minutes of elapsed time between recording of weight and first injection. Mice were then injected with saline, and placed back in the holding cages for 10 minutes. Mice were injected again with saline and placed in the activity monitors where their total distance traveled was measured for 30 minutes in 5-minute time bins. Day 1 provided a measurement of locomotor activity in a novel environment, while Day 2 provided a baseline measurement of locomotor activity in the now familiar environment. On Day 3, mice were weighed, injected with either saline or 1 of 4 doses of scopolamine (0.0625, 0.125, 0.25, or 0.5 mg/kg), and placed in holding cages for 10 minutes. They were then injected with either saline or 1 of 2 doses of ethanol (1 or 2 g/kg) and placed in the activity monitors for 30 minutes. Eleven to 12 mice of each replicate line (FAST-1 and FAST-2) were included in each drug dose × ethanol dose group (total n = 180 per replicate line). The FAST-1 and -2 mice were run in 12 passes, with 27 to 48 animals per pass distributed across all treatment groups. The ethanol doses were chosen based on our previous experiments with the selected lines to produce little and maximal stimulation (Palmer et al., 2002; Phillips et al., 1991; Shen et al., 1995b). The doses of scopolamine were chosen from those that had minimal locomotor effects in a previously published study (Bergstrom et al., 2003). The decision to wait 10 minutes between scopolamine and ethanol injection was based on a previously published study (Drouin et al., 2002), showing peak scopolamine effects on locomotor behavior 10 to 15 minutes posttreatment.
Experiment II: Effect of Scopolamine on Acute Locomotor Response to Ethanol in DBA/2J Mice
All experimental details are identical to those for Experiment I except that we used doses of 0, 0.75, and 1.5 g/kg ethanol, chosen to produce little and maximal stimulation in this mouse strain (Dudek et al., 1991). Eleven to 12 mice were included in each drug dose × ethanol dose group (n = 179). This experiment was performed in 4 passes with 32 to 50 animals per pass distributed across all treatment groups.
Experiment III: Effect of SCH-23390 on the Acute Locomotor Response to Scopolamine and Ethanol in DBA/2J Mice
Experiment III was conducted to determine whether pretreatment with SCH-23390, a dopamine D1-like receptor antagonist, would block the robust stimulant response seen with the combination of scopolamine and ethanol. For reasons of availability and because similar results were obtained in FAST and DBA/2J mice in our initial studies, this experiment was performed only in DBA/2J mice. The experimental details are like those described for Experiments I and II, except that mice received 3 injections per day, with the second injection given 15 minutes after the first, and the third injection given 10 minutes after the second. Fourteen to 15 mice were injected per SCH-23390 dose (0, 0.015, 0.03 mg/kg) × scopolamine dose (0, 0.5 mg/kg) × ethanol dose (0, 1.5 g/kg) group; n = 178. This experiment was performed in 5 passes with 24 to 55 animals per pass distributed across all treatment groups. The SCH-23390 doses chosen were ones previously shown to attenuate ethanol-induced locomotor activation without affecting basal activity levels. Time between SCH-23390 and scopolamine injection was chosen based on previously published papers (Dickinson et al., 2003; Pastor et al., 2005; Shen et al., 1995a).
Experiment IV: Effect of Haloperidol on the Acute Locomotor Response to Scopolamine and Ethanol in DBA/2J Mice
Experiment IV was conducted to determine whether pretreatment with haloperidol, a dopamine D2-like receptor antagonist, would block the robust stimulant response seen with the combination of scopolamine and ethanol in DBA/2J mice. The experimental details are like those described for Experiment III, except that the second injection was given 2 minutes after the first. Eleven to 12 mice were injected per haloperidol dose (0, 0.08, 0.16 mg/kg) × scopolamine dose (0, 0.5 mg/kg) × ethanol dose (0, 1.5 g/kg) group; n = 143. This experiment was performed in 5 passes with 23 to 32 animals per pass distributed across all treatment groups. The haloperidol doses chosen were ones that attenuated ethanol-induced stimulation alone but did not retard basal locomotor activity (Shen et al., 1995a). The time between haloperidol and scopolamine injection was chosen based on a previously published paper (Shen et al., 1995a).
BEC Determination
The 20 μl blood samples were pipetted into 1.5 ml centrifuge tubes containing 50μl of ice-cold 5% ZnSO4, then 50μl 0.3 NBa(OH)2, and 300 μl ddH2O was added to the tubes. The samples were then centrifuged for 5 minutes at 12,000 rpm (11693 × g) in a Beckman centrifuge contained within a cold room. Following centrifugation, the supernatant was pipetted off into 2 ml glass chromatography vials, which were crimped with caps and analyzed for BEC via gas chromatography (Agilent 6890N; Agilent Technologies, Palo Alto, CA) using standard published methods (Boehm et al., 2000). Ethanol concentrations were interpolated from a standard curve generated from duplicate samples in the concentration range of 0.24 to 4.9 mg/ml.
Data Analysis
The critical dependent variable for locomotor drug response was total distance (cm) traveled on Day 3 corrected for saline baseline on Day 2 (Day 3 minus Day 2). Day 1 and Day 2 data were also analyzed to determine if there were group differences in locomotor behavior not related to drug treatments. Time-dependent effects were examined, and for the purpose of describing the patterns of effects, time course data are shown. Statistica software (StatSoft, Tulsa, OK) was used for all statistical analyses. Significance for all tests was set at α ≤ 0.05. Repeated measures analysis of variance (ANOVA) was first performed on Day 3 minus Day 2 scores to determine potential interactions of time (5-minute bins) with the possible factors SCH-23390 pretreatment dose, haloperidol pretreatment dose, scopolamine pretreatment dose, ethanol treatment dose, and replicate line. Significant interactions were considered further using 2-way ANOVA followed by simple main effects analyses and Newman-Keuls post hoc mean comparisons when appropriate. BEC data were similarly analyzed without the repeated measures factor. Results are expressed as mean ± SEM.
RESULTS
Initial analyses involving time indicated significant interaction effects involving this factor. We found key effects to be well-represented by data accumulated for the entire 30-minute test period in Experiments I and II. However, ethanol has pure and robust stimulant effects during the 5-minute period immediately after injection (Phillips et al., 1995; Shen et al., 1995a), and this time period was the most relevant for examination of the blockade of ethanol-induced stimulation addressed in Experiments III and IV. For consistency across studies, data from both the initial 5-minute and total 30-minute time periods are shown for all experiments.
Experiment I: Scopolamine Robustly Enhances Acute Locomotor Stimulation to Ethanol in FAST Mice
After saline injection on Day 1, FAST-1 mice had higher levels of locomotor activity than FAST-2 mice for the 30-minute time period. There was also a significant effect of ethanol dose group. However, although the replicate line difference was retained on basal activity Day 2 [F(1, 355) = 355, p < 0.01], locomotor differences among groups of animals assigned to specific doses for Day 3 treatment were absent. Both replicate lines displayed significant habituation from Day 1 to Day 2 [7608 ± 221 cm vs. 5990 ± 182 cm in FAST-1, F(1,163) = 51.3, p < 0.01; 4310 ± 114 cm vs. 2149 ± 68 cm in FAST-2, F(1,164) = 394, p < 0.01].
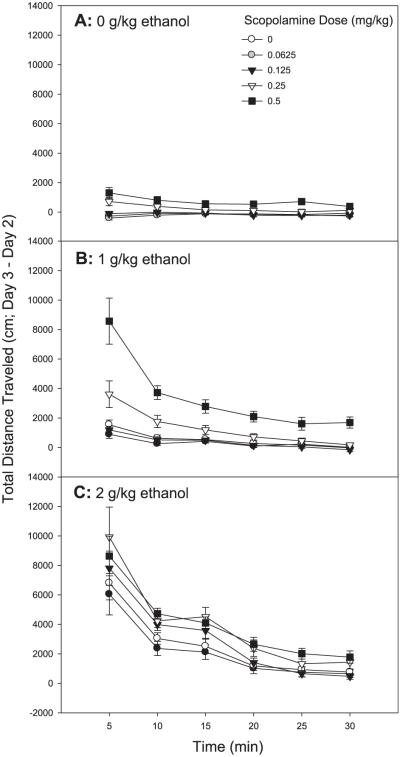
A repeated measures ANOVA of drug response data revealed a significant 3-way interaction of 5-minute time bin, scopolamine dose, and ethanol dose [F(40,1515) = 2.14, p < 0.01]. Overall, as can be seen in the pattern of time course results shown in Fig. 1, scopolamine generally enhanced the magnitude of stimulation to ethanol, while having little effect on the shape of the time–response curve. This pattern of results suggests that scopolamine had little effect on the duration of ethanol-induced stimulation in FAST mice.
Fig. 1.
Scopolamine pretreatment enhances the magnitude of stimulation to ethanol in FAST mice. Shown are Day 3 minus Day 2 drug stimulation scores for each 5-minute time bin of the 30-minute test. Mice were treated with saline or scopolamine followed 10 minutes later by saline or ethanol; locomotor activity was assessed for 30 minutes following the second injection; n = 23 to 24 female FAST mice/group. (A) Shown are data for mice treated with scopolamine then saline(0g/kg ethanol). (B) Shown are data for mice treated with scopolamine then 1 g/kg ethanol. (C) Shown are data for mice treated with scopolamine then 2 g/kg ethanol.
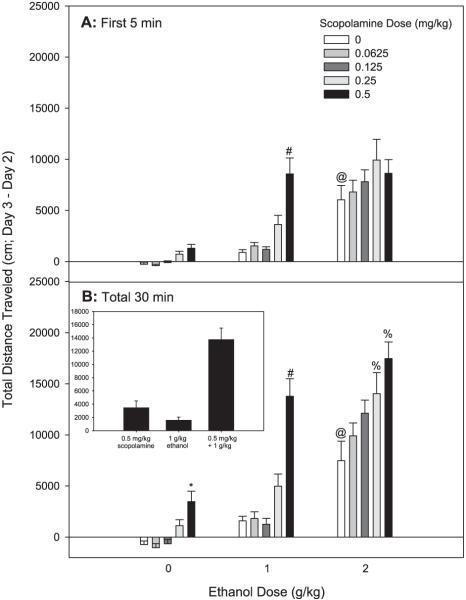
A factorial ANOVA (replicate line × scopolamine dose × ethanol dose) revealed a significant 2-way interaction of scopolamine pretreatment and ethanol treatment [F(8,288) = 2.99, p < 0.01] that did not significantly interact with replicate line. Data were thus reanalyzed without replicate as a factor, and the significant scopolamine dose × ethanol dose interaction was supported [F(8,303) = 2.55, p < 0.05]. Data are shown in Fig. 2 for the replicates combined. At the early 5-minute time point, FAST mice were not significantly stimulated by any dose of scopolamine alone. They did, however, exhibit acute stimulation to the 2, but not 1 g/kg dose of ethanol. Doses of scopolamine and ethanol that alone did not have significant effects produced a robust stimulant response when co-administered that was much greater than the additive effects of the drugs alone (Fig. 2A). In particular, FAST mice displayed extreme stimulation to the combination of 0.5 mg/kg scopolamine and 1 g/kg ethanol, a level of response not predicted from the value of the combined means.
Fig. 2.
Scopolamine and ethanol synergistically enhance locomotor activity in FAST mice. Shown are Day 3 minus Day 2 drug stimulation scores. Mice were treated with saline or scopolamine followed 10 minutes later by saline or ethanol treatment. Locomotor activity was assessed for 30 minutes following the second injection; n = 23 to 24 female FAST mice/group. (A) Shown are data from the first 5 minutes of the total 30-minute test. #Significant difference between activity levels of 0 mg/kg scopolamine + 1g/kg ethanol and those receiving 0.5 mg/kg scopolamine + 1 g/kg ethanol (p < 0.05). @Significant difference between activity levels of 0 mg/kg scopolamine + 0 g/kg ethanol and those receiving 0 mg/kg scopolamine + 2 g/kg ethanol (p < 0.05). (B) Shown are cumulative data for the 30-minute test. The inset shows the response to the combined 0.5 mg/kg dose of scopolamine and the 1 g/kg dose of ethanol, compared to each dose alone, to illustrate the super-additive effect. *Significant difference between activity levels of 0 mg/kg scopolamine + 0 g/kg ethanol and those receiving 0.5 mg/kg scopolamine + 0 g/kg ethanol (p < 0.05). #Significant difference between activity levels of 0 mg/kg scopolamine + 1 g/kg ethanol and those receiving 0.5 mg/kg scopolamine + 1 g/kg ethanol (p < 0.05). %Significant difference between activity levels of 0 mg/kg scopolamine + 2g/kg ethanol and those receiving 0.25–0.5 mg/kg scopolamine + 2 g/kg ethanol (p < 0.05). @Significant difference between activity levels of 0mg/kg scopolamine + 0 g/kg ethanol and those receiving 0 mg/kg scopolamine + 2 g/kg ethanol (p < 0.05).
When data were accumulated for the entire 30-minute test period, the stimulant effects of scopolamine alone were more apparent. A 2-way ANOVA revealed a significant interaction of scopolamine pretreatment and ethanol treatment [F(8,342) = 2.97, p < 0.01]. FAST mice were sensitive to the locomotor stimulant effects of 0.5 mg/kg scopolamine alone, and displayed acute stimulation to the 2, but not 1 g/kg dose of ethanol. Doses of scopolamine and ethanol that each alone did not have significant effects produced a robust stimulant response when co-administered that was much greater than the additive effects of the drugs alone (Fig. 2B). An example of this can be seen in the inset of Fig.2B by comparing the mean Day 3 minus Day 2 drug stimulation score for the 0.5 mg/kg scopolamine + 1 g/kg ethanol dose group to the combined means of the 0.5 mg/kg scopolamine group alone and the 1 g/kg ethanol group alone. This super-additive effect of the drug combination was also seen in groups treated with scopolamine and the higher 2 g/kg dose of ethanol. Scopolamine had no significant effect on BEC (Table 1), but there was a significant replicate line × ethanol dose interaction [F(2,327) = 5.174, p < 0.01]. This was due to a significant difference between FAST-1 and FAST-2 mice for mean BEC after treatment with the 2 g/kg, but not with the 1 g/kg ethanol dose. The mean BEC of FAST-2 mice (2.04 ± 0.07 mg/ml) was 13% higher than that of FAST-1 mice (1.80 ± 0.06 mg/ml).
Table 1.
Experiment I and II Mean (±SEM) BEC Values (mg/ml) 30 Minutes After Ethanol Injection
| Scopolamine dose (mg/kg) |
|||||
|---|---|---|---|---|---|
| Ethanol dose (g/kg) | 0 | 0.0625 | 0.125 | 0.25 | 0.5 |
| Experiment I | |||||
| FAST-1 | |||||
| 1 | 0.72 ± 0.04 | 0.79 ± 0.09 | 0.67 ± 0.08 | 0.72 ± 0.06 | 0.74 ± 0.05 |
| 2 | 1.94 ± 0.03 | 1.67 ± 0.18 | 1.72 ± 0.17 | 1.82 ± 0.11 | 1.82 ± 0.1 |
| FAST-2 | |||||
| 1 | 0.67 ± 0.06 | 0.76 ± 0.02 | 0.80 ± 0.02 | 0.76 ± 0.02 | 0.77 ± 0.02 |
| 2 | 2.07 ± 0.14 | 2.03 ± 0.2 | 2.15 ± 0.07 | 1.91 ± 0.18 | 2.05 ± 0.2 |
| Experiment II | |||||
| DBA/2J | |||||
| 0.75 | 0.38 ± 0.04 | 0.45 ± 0.05 | 0.47 ± 0.03 | 0.46 ± 0.07 | 0.44 ± 0.04 |
| 1.5 | 1.34 ± 0.06 | 1.47 ± 0.04 | 1.37 ± 0.05 | 1.44 ± 0.07 | 1.41 ± 0.08 |
Experiment II: Scopolamine Robustly Enhances Acute Locomotor Stimulation to Ethanol in DBA/2J Mice
On Days 1 and 2 after saline injection, there were no statistically significant differences in locomotor activity level for the total 30-minute test period among groups designated for specific drug treatments (data not shown). Day 1 levels of locomotion were higher than were Day 2 levels [3803 ± 98 cm vs. 3011 ± 97 cm, F(1, 160) = 58.8, p < 0.01], indicating significant habituation to the test environment.
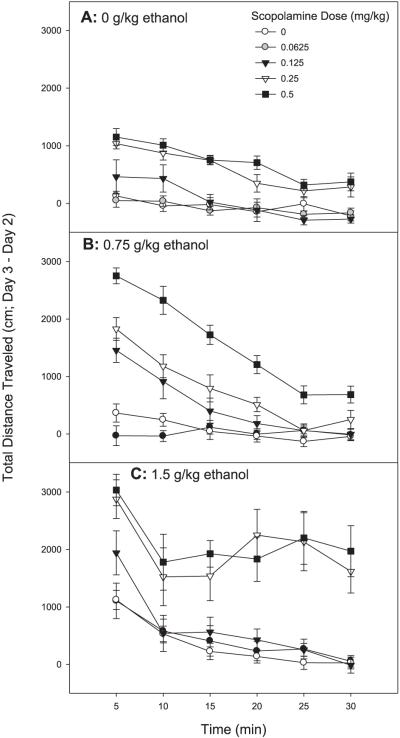
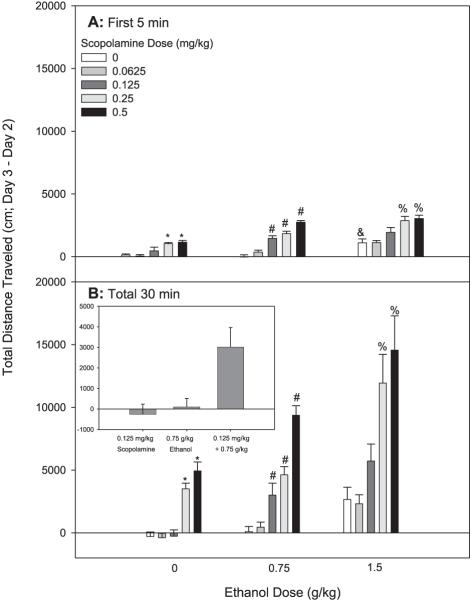
A repeated measures ANOVA revealed a significant 3-way interaction of 5-minute time bin, scopolamine dose, and ethanol dose [F(40,800) = 2.84, p < 0.01]. The pattern of results over time (Fig. 3) suggests that in DBA/2J mice, unlike FAST mice, some combined doses of scopolamine and ethanol increased both the magnitude and duration of stimulation. However, the overall results for the effects of combined scopolamine and ethanol treatment on locomotor activity in DBA/2J mice were similar to those in FAST mice, and again suggested synergistic responses to the drug combination. A factorial ANOVA indicated a significant interaction of scopolamine dose and ethanol dose [F(8, 160) = 2.49, p < 0.05] for the first 5-minute time period. DBA/2J mice were significantly stimulated by the 0.25 and 0.5 mg/kg doses of scopolamine alone during the first 5 minutes of the test (Fig. 4A). They were also significantly stimulated by the 1.5 g/kg dose of ethanol during this time period. Doses of scopolamine and ethanol that alone did not have significant stimulant effects produced a robust stimulant response when co-administered that was much greater than the additive effects of the drugs alone (Fig. 4A). DBA/2J mice displayed extreme stimulation to the combination of the 3 highest doses of scopolamine and 0.75 g/kg ethanol, a level of response not predicted from the value of the combined means. They also showed robust stimulation to the combination of the 2 highest doses of scopolamine and the 1.5 g/kg dose of ethanol.
Fig. 3.
Scopolamine pretreatment enhances the magnitude and duration of stimulation to ethanol in DBA/2J mice. Shown are Day 3 minus Day 2 drug stimulation scores for each 5-minute time bin of the 30-minute test. Mice were treated with saline or scopolamine followed 10 minutes later by saline or ethanol treatment; locomotor activity was assessed for 30 minutes following the second injection; n = 11 to 12 female DBA/2J mice/group. (A) Shown are data for mice treated with scopolamine then saline (0 g/kg ethanol). (B) Shown are data for mice treated with scopolamine then 0.75 g/kg ethanol. (C) Shown are data for mice treated with scopolamine then 1.5 g/kg ethanol.
Fig. 4.
Scopolamine and ethanol synergistically enhance locomotor activity in DBA/2J mice. Shown are Day 3 minus Day 2 drug stimulation scores. Mice were treated with saline or scopolamine followed 10 minutes later by saline or ethanol treatment. Locomotor activity was assessed for 30 minutes following the second injection; n = 11 to 12 female DBA/2J mice/group. (A) Shown are data from the first 5 minutes of the total 30-minute test. *Significant difference between activity levels of 0 mg/kg scopolamine + 0 g/kg ethanol and those receiving 0.25–0.5 mg/kg scopolamine + 0 g/kg ethanol (p < 0.05). #Significant difference between activity levels of 0 mg/kg scopolamine + 0.75 g/kg ethanol and those receiving 0.125–0.5 mg/kg scopolamine + 0.75 g/kg ethanol (p < 0.05). &Significant difference between activity levels of 0 mg/kg scopolamine + 0 g/kg ethanol and those receiving 0 mg/kg scopolamine + 2 g/kg ethanol (p < 0.05). %Significant difference between activity levels of 0 mg/kg scopolamine + 1.5 g/kg ethanol and those receiving 0.25–0.5 mg/kg scopolamine + 1.5 g/kg ethanol (p < 0.05). (B) Shown are cumulative data for the 30-minute test. The inset illustrates the response to the combined 0.125 mg/kg dose of scopolamine and the 0.75 g/kg dose of ethanol, compared to each dose alone, to illustrate the super-additive effect. *Significant difference between activity levels of 0 mg/kg scopolamine + 0 g/kg ethanol and those receiving 0.25–0.5 mg/kg scopolamine + 0 g/kg ethanol (p < 0.05). #Significant difference between activity levels of 0 mg/kg scopolamine + 0.75 g/kg ethanol and those receiving 0.125–0.5 mg/kg scopolamine + 0.75 g/kg ethanol (p < 0.05). %Significant difference between activity levels of 0 mg/kg scopolamine + 1.5 g/kg ethanol and those receiving 0.25–0.5 mg/kg scopolamine + 1.5 g/kg ethanol (p < 0.05).
Results for the total 30-minute time period were similar to those for the first 5 minutes (Fig. 4B). A 2-way ANOVA indicated a significant interaction of scopolamine pretreatment and ethanol treatment [F(8, 160) = 3.43, p < 0.01] for the total 30-minute time period. Subsequent analyses indicated that DBA/2J mice were significantly stimulated by the 2 highest doses of scopolamine alone (0.25 and 0.5 mg/kg). The stimulant effect of ethanol alone was not significant compared to saline alone when the data were analyzed accumulated over the total 30-minute. While the lower doses of scopolamine and doses of ethanol alone did not have significant effects on locomotor behavior, when combined there was a robust stimulant response greater than the sum of the effects of the 2 drugs alone (Fig. 4B inset). Ethanol dose-dependently increased BEC, but scopolamine had no effect on BEC (Table 1).
Experiment III: Effect of SCH-23390 on the Acute Locomotor Response to Scopolamine and Ethanol in DBA/2J Mice
There were no significant differences in Day 1 or Day 2 locomotor activity after saline injection for the 30-minute test period, among groups of animals assigned to receive different doses of SCH-23390, scopolamine, or ethanol. While animals displayed some habituation reflected as lower locomotor activity on Day 2 (3028.9 ± 121.9 cm) than on Day 1 (3956.1 ± 106 cm), the reduction in locomotor behavior was not statistically significant.
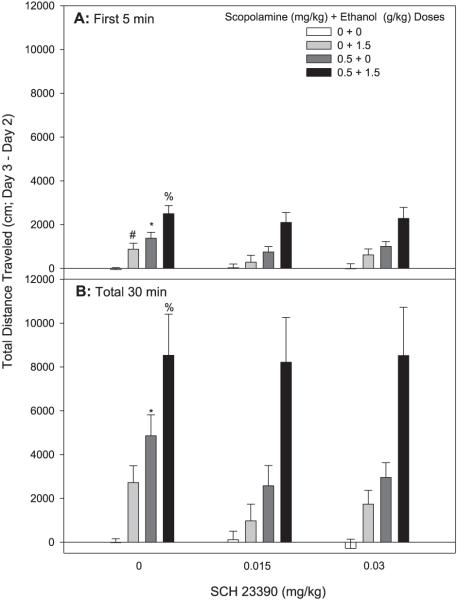
In a repeated measures ANOVA (SCH-23390 × scopolamine × ethanol × time), there was a significant interaction of scopolamine dose, ethanol dose, and time [F(5,835) = 5.75, p < 0.01]. While there was no significant 3-way interaction of SCH-23390, scopolamine, and ethanol dose when analyzed during the first 5 minutes, there was an interaction of scopolamine and ethanol dose [F(1,167) = 5.83, p < 0.02]. Consistent with the results of Experiment II, the 0.5 mg/kg scopolamine + 1.5 g/kg ethanol dose combination enhanced locomotor activity to a greater extent than predicted by the additive effects of each drug alone (Fig. 5A). As expected for this time period, there was also acute stimulation to ethanol. However, there was no time point at which SCH-23390, the dopamine D1-like receptor antagonist, was found to significantly attenuate ethanol- or scopolamine-induced stimulation, or their combined effects.
Fig. 5.
SCH-23390 has no effect on scopolamine-, ethanol-, or scopolamine + ethanol-induced locomotor activity. Shown are Day 3 minus Day 2 drug stimulation scores. Mice were treated with saline or SCH-23390 followed 15 minutes later by saline or scopolamine followed 10 minutes later by saline or ethanol treatment. Locomotor activity was assessed for 30 minutes following the third injection; n = 14 to 15 female DBA/2J mice/group. (A) Shown are cumulative data for the first 5 minutes of the test. *Significant difference in activity levels between 0 mg/kg SCH-23390 + 0 mg/kg scopolamine + 0 g/kg ethanol and 0 mg/kg SCH-23390 + 0.5 mg/kg scopolamine + 0 g/kg ethanol (p < 0.05). #Significant difference in activity levels between 0 mg/kg SCH-23390 + 0 mg/kg scopolamine + 0 g/kg ethanol and 0 mg/kg SCH-23390 + 0 mg/kg scopolamine + 1.5 g/kg ethanol (p < 0.05). %Significant difference in activity levels between 0 mg/kg SCH-23390 + 0 mg/kg scopolamine + 1.5 g/kg ethanol and 0 mg/kg SCH-23390 + 0.5 mg/kg scopolamine + 1.5 g/kg ethanol (p < 0.05). (B) Shown are cumulative data for the total 30-minute test period. *Significant difference in activity levels between 0 mg/kg SCH-23390 + 0 mg/kg scopolamine + 0 g/kg ethanol and 0 mg/kg SCH-23390 + 0.5 mg/kg scopolamine + 0 g/kg ethanol (p < 0.05). %Significant difference in activity levels between 0 mg/kg SCH-23390 + 0mg/kg scopolamine + 1.5 g/kg ethanol and 0 mg/kg SCH-23390 + 0.5 mg/kg scopolamine + 1.5 g/kg ethanol (p < 0.05).
A 3-way ANOVA performed on data for the entire 30-minute test period also revealed an interaction of scopolamine dose and ethanol dose [F(1,167) = 5.08, p < 0.05]. DBA/2J mice were sensitive to the locomotor stimulant effects of 0.5 mg/kg scopolamine alone (Fig. 5B), and while not statistically significant, there was a trend (p = 0.055) towards acute locomotor stimulation to the 1.5 g/kg dose of ethanol. As previously seen, mice showed a robust stimulant response to the combination of 0.5 mg/kg scopolamine and 1.5 g/kg ethanol. However, SCH-23390 had no effect on saline- or ethanol-induced locomotor activity, nor did it attenuate the effect of the scopolamine + ethanol drug combination. There were no differences in BEC among the groups (Table 2).
Table 2.
Experiment III and IV Mean (±SEM) BEC Values (mg/ml) 30 Minutes After Ethanol Injection
| Scopolamine dose (mg/kg) |
|||
|---|---|---|---|
| Ethanol dose (g/kg) | 0 | 0.5 | |
| Experiment III | |||
| DBA/2J | |||
| SCH-23390 dose (mg/kg) |
|||
| 1.5 | 0 | 1.36 ± 0.08 | 1.41 ± 0.10 |
| 1.5 | 0.015 | 1.40 ± 0.09 | 1.48 ± 0.04 |
| 1.5 | 0.03 | 1.40 ± 0.06 | 1.50 ± 0.03 |
| Experiment IV | |||
| DBA/2J | |||
| Haloperidol dose (mg/kg) |
|||
| 1.5 | 0 | 1.12 ± 0.07 | 1.18 ± 0.04 |
| 1.5 | 0.08 | 1.07 ± 0.08 | 1.03 ± 0.08 |
| 1.5 | 0.16 | 1.07 ± 0.13 | 1.16 ± 0.06 |
Experiment IV: Effect of Haloperidol on the Acute Locomotor Response to Scopolamine and Ethanol in DBA/2J Mice
There were no differences among the groups in basal locomotor activity for the 30-minute test period after saline treatment on Day 1 or Day 2, and the animals displayed a nonsignificant reduction in locomotor activity from Day 1 to Day 2 (3267 ± 174 cm vs. 2960 ± 112 cm).
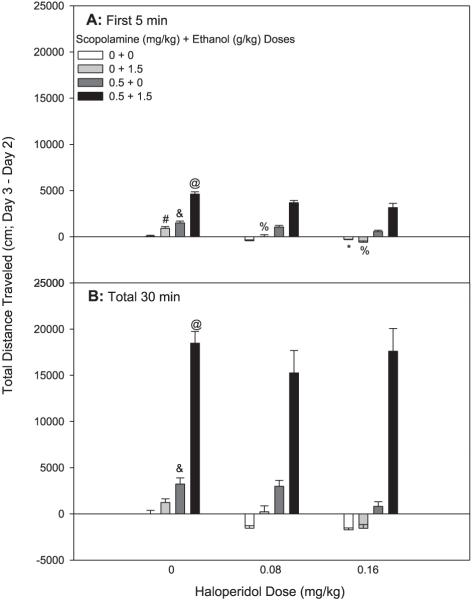
In a repeated measures ANOVA (haloperidol dose × scopolamine dose × ethanol dose × time), there was a significant interaction of haloperidol dose, ethanol dose, and time [F(6,390) = 3.96, p < 0.01]. While there was no significant 3-way interaction of the drug treatments within the first 5 minutes of the test, there was a significant interaction of scopolamine and ethanol dose [F(1,130) = 87.76, p < 0.001] and of haloperidol and ethanol dose [F(2,130) = 3.4, p < 0.05] for this early time period. The 0.5 mg/kg scopolamine + 1.5 g/kg ethanol dose combination enhanced locomotor activity to a greater extent than predicted by the additive effects of each drug alone (Fig. 6A). There was acute stimulation to ethanol, and haloperidol, the dopamine D2-like receptor antagonist, dose-dependently decreased ethanol-induced locomotor activation. The highest dose of haloperidol also significantly attenuated saline activity. Although the data visually suggest that haloperidol slightly decreased both locomotor stimulation to scopolamine alone and to the scopolamine + ethanol drug combination, these decreases were not statistically significant.
Fig. 6.
Haloperidol attenuates ethanol’s stimulant effects alone but not the locomotor activation following scopolamine or the scopolamine + ethanol combination. Shown are Day 3 minus Day 2 drug stimulation scores. Mice were treated with saline or haloperidol followed 2 minutes later by saline or scopolamine followed 10 minutes later by saline or ethanol treatment. Locomotor activity was assessed for 30 minutes following the third injection; n = 11 to 12 female DBA/2J mice/group. (A) Shown are cumulative data for the first 5 minutes of the test. *Significant difference in activity levels between 0 mg/kg haloperidol + 0 mg/kg scopolamine + 0 g/kg ethanol and 0.16 mg/kg haloperidol + 0 mg/kg scopolamine + 0 g/kg ethanol (p < 0.05). #Significant difference in activity levels between 0 mg/kg haloperidol + 0 mg/kg scopolamine + 0 g/kg ethanol and 0 mg/kg haloperidol + 0 mg/kg scopolamine + 1.5 g/kg ethanol (p < 0.05). &Significant difference in activity levels between 0 mg/kg haloperidol + 0 mg/kg scopolamine + 0 g/kg ethanol and 0 mg/kg haloperidol + 0.5 mg/kg scopolamine + 0 g/kg ethanol (p < 0.05). @Significant difference in activity levels between 0 mg/kg haloperidol + 0 mg/kg scopolamine + 0 g/kg ethanol and 0 mg/kg haloperidol + 0.5 mg/kg scopolamine + 1.5 g/kg ethanol (p < 0.05). %Significant difference in activity levels between 0 mg/kg haloperidol + 0 mg/kg scopolamine + 1.5 g/kg ethanol and 0.08–0.16 mg/kg haloperidol + 0 mg/kg scopolamine + 1.5 g/kg ethanol (p < 0.05). (B) Shown are cumulative data for the total 30-minute test. &Significant difference in activity levels between 0 mg/kg haloperidol + 0 mg/kg scopolamine + 0 g/kg ethanol and 0 mg/kg haloperidol + 0.5 mg/kg scopolamine + 0 g/kg ethanol (p < 0.05). @Significant difference in activity levels between 0 mg/kg haloperidol + 0 mg/kg scopolamine + 0 g/kg ethanol and 0 mg/kg haloperidol + 0.5 mg/kg scopolamine + 1.5 g/kg ethanol (p < 0.05).
Data analyzed over the total 30-minute test period revealed that DBA/2J mice were significantly stimulated by the 0.5 mg/kg dose of scopolamine (Fig. 6B); however, there was no acute locomotor stimulation to ethanol when the data were accumulated for 30 minutes. Again, the combination of 0.5 mg/kg scopolamine and 1.5 g/kg ethanol resulted in an extreme stimulant response much greater than predicted from the value of the combined means. For the 30-minute time period, however, haloperidol had no effect on saline-, ethanol-, or scopolamine-induced locomotion. There were no differences in BEC among the groups (Table 2).
DISCUSSION
The current experiments showed that combined treatment with scopolamine and ethanol induced a locomotor stimulant response in both FAST and DBA/2J mice that was greater than the additive effects of each drug alone. Although haloperidol, but not SCH-23390, attenuated ethanol-induced locomotor stimulation, neither the D1-like nor the D2-like dopamine receptor antagonist drug was able to block the robust locomotor response seen following the scopolamine and ethanol drug combination. SCH-23390 pretreatment also had no significant effect on the locomotor response to scopolamine alone. These data suggest that muscarinic receptor antagonism robustly enhances ethanol-induced locomotor activity, and that the dopamine system is not responsible for this super-additive response.
A Role for Muscarinic Receptors in the Behavioral Response to Stimulants
As seen here and in published work (Bergstrom et al., 2003; Pohorecky et al., 1979), scopolamine enhances locomotor activity on its own. Some data suggest that scopolamine may have reinforcing properties (Ranaldi and Woolverton, 2002; Rasmussen and Fink-Jensen, 2000). The interaction effect of scopolamine and ethanol was a robust phenomenon, as we replicated this effect in 2 mouse models and again in the studies with dopaminergic antagonists, and the effect was apparent at an early time period after drug administration. The published literature on muscarinic involvement in locomotor stimulation following drugs of abuse is scarce. Pretreatment with scopolamine has been shown to enhance amphetamine-induced hyperactivity, while pretreatment with the muscarinic agonist oxotremorine reduced the stimulant response to amphetamine (Ichikawa et al., 2002; Wang and McGinty, 1996). Microinfusion of scopolamine into the nucleus accumbens was able to reverse an oxotremorine-induced reduction in number of cocaine infusions in a rat self-administration paradigm (Mark et al., 2006). While not a measure of locomotor activity, these data suggest that the muscarinic system is able to interact with other brain systems, modulating psychostimulant reward-related behaviors. Data have not been published examining the effect of scopolamine or other muscarinic drugs on ethanol-induced stimulation. However, scopolamine has been shown to reverse ethanol-induced sedation in rats (Pohorecky et al., 1979).
Combined treatment with scopolamine and ethanol had a similar overall effect on the locomotor behavior of FAST and DBA/2J mice. In FAST mice, largely the magnitude, and not the duration of stimulation was increased in mice treated with scopolamine plus ethanol, compared to those treated with ethanol or scopolamine alone. In DBA/2J mice, both the magnitude and duration of stimulation appeared to be increased by combined treatment with some doses of scopolamine and ethanol. The reason for this difference between genotypes appears likely to be due to the greater sensitivity of the DBA/2J mice to scopolamine alone, compared to the sensitivity of FAST mice, which were generally less sensitive to scopolamine. Additionally, genetic differences associated with selective versus nonselective breeding of the FAST versus DBA/2J mice, and the difference in ethanol dose used in the 2 studies could have played a role. It is striking that similar super-additive stimulant responses were seen in both FAST and DBA/2J mice in response to the drug combination, regardless of their respective sensitivities to scopolamine alone.
The m4 and m5 mAChR subtype genes reside in the region of a quantitative trait locus (QTL) in 2 gene mapping studies for acute locomotor stimulation to ethanol (Demarest et al., 1999; Palmer et al., 2006). Pharmacological agents selective for these receptor subtypes do not exist. However, site-specific infusion of scopolamine into regions where these receptor subtypes are expressed (namely the VTA and substantia nigra), or the even more selective approach of RNA interference of gene expression are possible methods for testing their involvement. Studies with knockout mice suggest that mice lacking the m5 receptor have reduced sensitivity to reinforcement from stimulant drugs of abuse (Basile et al., 2002; Fink-Jensen et al., 2003; Thomsen et al., 2005; but see Yamada et al., 2001). The m5 knockout mice have not been tested for sensitivity to ethanol-induced stimulation, and mice lacking the m4 mAChR subtype have not been tested for their locomotor response to any drug with stimulant effects. Although there are other possible candidates in the QTL region, that the mAChR subtype 2 gene resides in the region of a QTL for ethanol consumption in both mice and humans is a finding that might further encourage continued research into the involvement of muscarinic systems in ethanol-related behaviors (Dick et al., 2006; Edenberg and Foroud, 2006; Phillips et al., 1998).
Cholinergic and Dopaminergic Neurocircuitry and Locomotor Stimulation
Acute ethanol injection causes an increase in extracellular levels of dopamine in the nucleus accumbens (Di Chiara and Imperato, 1988; Yim and Gonzales, 2000). Dopaminergic antagonists block this neurochemical response (Imperato and Di Chiara, 1988) as well as behavioral stimulation to ethanol (Enggasser and De Wit, 2001; Phillips and Shen, 1996; Risinger et al., 1992; Shen et al., 1995a). In vitro evidence suggests that the mAChR agonist oxotremorine facilitates striatal dopamine release (Zhang et al., 2002). However, at low doses, the mAChR antagonist scopolamine also enhances dopamine efflux in the nucleus accumbens in vivo (Ichikawa et al., 2002), which is consistent with the locomotor activating effect of this drug. Predicting the potential role of the muscarinic system in ethanol-related neural and behavioral changes is not straightforward. Muscarinic antagonists at high doses did not increase dopamine output from the nucleus accumbens in 1 study in Sprague-Dawley rats (Di Chiara and Imperato, 1988); however, low doses of scopolamine enhanced dopamine release in the nucleus accumbens in another study in Sprague-Dawley rats (Ichikawa et al., 2002). A low dose of scopolamine potentiated amphetamine-induced dopamine release in the rat nucleus accumbens (Ichikawa et al., 2002). Most cholinergic neurons in the striatum express m1, m2, and m4 receptor mRNA, indicating that acetylcholine is able to inhibit its own release via presynaptic autoreceptors (Bernard et al., 1992). Furthermore, dopamine is able to modulate acetylcholine release in the striatum (Di Chiara et al., 1994). The lack of selective ligands for the mAChR subtypes has made investigation more difficult.
In the current studies, neither SCH-23390 nor haloperidol significantly attenuated the robust stimulant response seen after co-administration of scopolamine and ethanol, or the activation induced by scopolamine alone. In the striatum, the m4 receptor subtype has been specifically implicated as exerting an inhibitory effect on D1 dopamine neurons. Mice lacking the m4 receptor subtype were less sensitive to the locomotor depressant effects of haloperidol at a dose comparable to the 1 used in this study, which was interpreted as a heightened stimulatory dopamine D1 receptor presence. However, there were no differences in sensitivity to the effects of SCH-23390 on locomotor activity between the knockout and wild-type mice (Gomeza et al., 1999). Another group showed that while scopolamine was able to rescue haloperidol-induced catalepsy in wild-type mice, it could not in m4 knockout mice; however, the doses used were much higher (10 mg/kg scopolamine, 0.5 mg/kg haloperidol) than used here and were given in an escalating fashion over a week-long period of time (Karasawa et al., 2003). Furthermore, in that study the inactivation of muscarinic receptors occurred prior to haloperidol administration, unlike ours.
While haloperidol’s interaction with the muscarinic system remains unclear from these studies, our results that haloperidol dose-dependently attenuated acute stimulation to ethanol indicates the effectiveness of the doses we used. The pattern of results shown in Fig. 6A suggests that a higher dose of haloperidol might have significantly attenuated the effects of scopolamine alone and of the scopolamine + ethanol drug combination. However, due to the depressant effects of higher doses of haloperidol on locomotor activity, interpretation would be difficult. In a previous study in DBA/2J mice, both the 0.015 and 0.03 mg/kg doses of SCH-23390 reduced baseline locomotor activity (Dickinson et al., 2003); however, these doses of SCH-23390 did not attenuate ethanol-induced conditioned place preference in DBA/2J mice. In our previous work, although SCH-23390 decreased ethanol-induced locomotion in FAST-1 mice, doses even as high as 0.045 mg/kg were not able to diminish ethanol stimulation in FAST-2 mice (Shen et al., 1995a). The doses of SCH-23390 in the current experiment were chosen to maximize effects on ethanol-induced stimulation while minimizing effects on basal locomotor activity. The results suggestthatthe 0.015 mg/kg dose of SCH-23390 had some effect on the ethanol response, but the retained stimulation in the mice tested with 0.03 mg/kg SCH-23390 is not consistent with the linear effect that would be predicted. Therefore, we must conclude that the smaller ethanol response in the mice treated with 0.015 mg/kg SCH-23390 was due to sampling error and that D1 receptors have little role in ethanol-induced locomotor stimulation in DBA/2J mice. We cannot rule out the possibility that combined antagonism of D1 and D2 dopamine receptors would block the extreme stimulant effect of scopolamine + ethanol or the stimulant response to scopolamine alone (Shen et al., 1995a). In our previous work, haloperidol pretreatment attenuated ethanol-stimulated activity in both FAST-1 and -2 mice, as did SCH-23390 in FAST-1, but not FAST-2, mice (Shen et al., 1995a); thus, the D2 receptor may play a more consistent role in ethanol-stimulated activity, as we found a significant effect of haloperidol, but not of SCH-23390.
Another possible explanation for our results is that scopolamine is enhancing locomotor activity by blocking inhibitory cholinergic autoreceptors in the nucleus accumbens. In this way, it could disinhibit GABAergic projection neurons to the ventral pallidum, serving to increase locomotor activity via a dopaminergic-independent mechanism. This may explain why the dopamine antagonists SCH-23390 or haloperidol did not block our robust stimulant response to the scopolamine-ethanol drug combination. The m1, m2, and m4 receptor subtypes have all been suggested to be presynaptic autoreceptors in the striatum and hippocampus (Bernard et al., 1992; Smythies, 2005; Zhou et al., 2003). Furthermore, baseline acetylcholine levels are higher in mice lacking the m4 receptor subtype than in their wild-type counterparts, and when infused through a probe located in the nucleus accumbens, scopolamine markedly increased acetylcholine efflux in the wild-type but not in knockout mice (Tzavara et al., 2004). In addition, we cannot rule out the possibility that the response, suggestive of synergism, is mediated by other neurotransmitter systems, for example, glutamate (Di Chiara et al., 1994; Gras et al., 2008; Zhou et al., 2003).
CONCLUSIONS
The similar results seen here in 2 genetic mouse models of high sensitivity to the stimulant effects of ethanol, suggest that mAChRs can have an impact on ethanol-induced stimulation. The interactive response to combined scopolamine and ethanol indicates an effect that cannot be explained by simple additive effects on a common neurochemical pathway, nor does dopamine appear to be involved in the robust response. Scopolamine has clinical usefulness as an aid in the treatment of motion sickness (Renner et al., 2005). Although prescribed doses generally cause sedation, and for this reason the combination of scopolamine and alcohol is contraindicated (Pappano, 2007; Renner et al., 2005), it is possible that people could take lower scopolamine doses within the stimulant range. Our data suggest that lower doses of scopolamine may actually potentiate the stimulant effects of low ethanol doses. Scopolamine is also used to treat morphine addiction in humans. Studies with rats have shown that repeated scopolamine pretreatment or co-administration with morphine attenuated morphine tolerance and withdrawal symptoms (Xiang et al., 2006; Zhou et al., 1999). While none of these studies addressed the acute effects of scopolamine on morphine responses, our results suggest that scopolamine could potentiate low dose stimulant effects that have been seen after morphine treatment (Cunningham et al., 1992; Marquez et al., 2008; Phillips et al., 1994; Puglisi-Allegra et al., 1986) or in combination with other drugs that have stimulant effects. Overall, muscarinic receptor-mediated mechanisms remain attractive targets in the study of ethanol-induced locomotor stimulation.
ACKNOWLEDGMENTS
This work was supported by a National Institute on Alcohol Abuse and Alcoholism grant P60AA010760, a grant from the National Institute on Drug Abuse T32DA07262, and a grant from the Department of Veterans Affairs.
Footnotes
No claim to original U.S. government works
REFERENCES
- Basile AS, Fedorova I, Zapata A, Liu X, Shippenberg T, Duttaroy A, Yamada M, Wess J. Deletion of the M5 muscarinic acetylcholine receptor attenuates morphine reinforcement and withdrawal but not morphine analgesia. Proc Natl Acad Sci USA. 2002;99:11452–11457. doi: 10.1073/pnas.162371899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom HC, Palmer AA, Wood RD, Burkhart-Kasch S, McKinnon CS, Phillips TJ. Reverse selection for differential response to the locomotor stimulant effects of ethanol provides evidence for pleiotropic genetic influence on locomotor response to other drugs of abuse. Alcohol Clin Exp Res. 2003;27:1535–1547. doi: 10.1097/01.ALC.0000091226.18969.B9. [DOI] [PubMed] [Google Scholar]
- Bernard V, Normand E, Bloch B. Phenotypical characterization of the rat striatal neurons expressing muscarinic receptor genes. J Neurosci. 1992;12:3591–3600. doi: 10.1523/JNEUROSCI.12-09-03591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm SL, II, Crabbe JC, Phillips TJ. Sensitivity to ethanol-induced motor incoordination in FAST and SLOW selectively bred mice. Pharmacol Biochem Behav. 2000;66:241–247. doi: 10.1016/s0091-3057(00)00264-1. [DOI] [PubMed] [Google Scholar]
- Boehm SL, II, Piercy MM, Bergstrom HC, Phillips TJ. Ventral tegmental area region governs GABAB receptor modulation of ethanol-stimulated activity in mice. Neuroscience. 2002;115:185–200. doi: 10.1016/s0306-4522(02)00378-0. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Carter PA, Yamada M, Gomeza J, Wess J, Hamilton SE, Nathanson NM, McKinzie DL, Felder CC. Role of specific muscarinic receptor subtypes in cholinergic parasympathomimetic responses, in vivo phosphoinositide hydrolysis, and pilocarpine-induced seizure activity. Eur J Neurosci. 2003;17:1403–1410. doi: 10.1046/j.1460-9568.2003.02588.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Deutsch CM, Tam BR, Young ER. Environmental variables differentially affect ethanol-stimulated activity in selectively bred mouse lines. Psychopharmacology (Berl) 1988;95:103–108. doi: 10.1007/BF00212776. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Kosobud A, Belknap JK. Estimation of genetic correlation: interpretation of experiments using selectively bred and inbred animals. Alcohol Clin Exp Res. 1990;14:141–151. doi: 10.1111/j.1530-0277.1990.tb00461.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Young ER, Deutsch CM, Tam BR, Kosobud A. Mice genetically selected for differences in open-field activity after ethanol. Pharmacol Biochem Behav. 1987;27:577–581. doi: 10.1016/0091-3057(87)90371-6. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Niehus DR, Malott DH, Prather LK. Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharmacology (Berl) 1992;107:385–393. doi: 10.1007/BF02245166. [DOI] [PubMed] [Google Scholar]
- Daniell LC, Phillips TJ. Ethanol sensitivity of brain NMDA receptors in mice selectively bred for differences in response to the low-dose locomotor stimulant effects of ethanol. Alcohol Clin Exp Res. 1994;18:1474–1481. doi: 10.1111/j.1530-0277.1994.tb01453.x. [DOI] [PubMed] [Google Scholar]
- Demarest K, McCaughran J, Jr, Mahjubi E, Cipp L, Hitzemann R. Identification of an acute ethanol response quantitative trait locus on mouse chromosome 2. J Neurosci. 1999;19:549–561. doi: 10.1523/JNEUROSCI.19-02-00549.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Morelli M, Consolo S. Modulatory functions of neuro-transmitters in the striatum: ACh/dopamine/NMDA interactions. Trends Neurosci. 1994;17:228–233. doi: 10.1016/0166-2236(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Dick DM, Jones K, Saccone N, Hinrichs A, Wang JC, Goate A, Bierut L, Almasy L, Schuckit M, Hesselbrock V, Tischfield J, Foroud T, Edenberg H, Porjesz B, Begleiter H. Endophenotypes successfully lead to gene identification: results from the collaborative study on the genetics of alcoholism. Behav Genet. 2006;36:112–126. doi: 10.1007/s10519-005-9001-3. [DOI] [PubMed] [Google Scholar]
- Dickinson SD, Lee EL, Rindal K, Cunningham CL. Lack of effect of dopamine receptor blockade on expression of ethanol-induced conditioned place preference in mice. Psychopharmacology (Berl) 2003;165:238–244. doi: 10.1007/s00213-002-1270-4. [DOI] [PubMed] [Google Scholar]
- Drouin C, Darracq L, Trovero F, Blanc G, Glowinski J, Cotecchia S, Tassin JP. α1b-Adrenergic receptors control locomotor and rewarding effects of psychostimulants and opiates. J Neurosci. 2002;22:2873–2884. doi: 10.1523/JNEUROSCI.22-07-02873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek BC, Phillips TJ, Hahn ME. Genetic analyses of the biphasic nature of the alcohol dose-response curve. Alcohol Clin Exp Res. 1991;15:262–269. doi: 10.1111/j.1530-0277.1991.tb01867.x. [DOI] [PubMed] [Google Scholar]
- Duranceaux NC, Schuckit MA, Luczak SE, Eng MY, Carr LG, Wall TL. Ethnic differences in level of response to alcohol between Chinese Americans and Korean Americans. J Stud Alcohol Drugs. 2008;69:227–234. doi: 10.15288/jsad.2008.69.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Foroud T. The genetics of alcoholism: identifying specific genes through family studies. Addict Biol. 2006;11:386–396. doi: 10.1111/j.1369-1600.2006.00035.x. [DOI] [PubMed] [Google Scholar]
- Eglen RM. Muscarinic receptor subtype pharmacology and physiology. Prog Med Chem. 2005;43:105–136. doi: 10.1016/S0079-6468(05)43004-0. [DOI] [PubMed] [Google Scholar]
- Enggasser JL, De Wit H. Haloperidol reduces stimulant and reinforcing effects of ethanol in social drinkers. Alcohol Clin Exp Res. 2001;25:1448–1456. [PubMed] [Google Scholar]
- Fink-Jensen A, Fedorova I, Wortwein G, Woldbye DP, Rasmussen T, Thomsen M, Bolwig TG, Knitowski KM, McKinzie DL, Yamada M, Wess J, Basile A. Role for M5 muscarinic acetylcholine receptors in cocaine addiction. J Neurosci Res. 2003;74:91–96. doi: 10.1002/jnr.10728. [DOI] [PubMed] [Google Scholar]
- Gomeza J, Zhang L, Kostenis E, Felder C, Bymaster F, Brodkin J, Shannon H, Xia B, Deng C, Wess J. Enhancement of D1 dopamine receptor-mediated locomotor stimulation in M4 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:10483–10488. doi: 10.1073/pnas.96.18.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras C, Amilhon B, Lepicard EM, Poirel O, Vinatier J, Herbin M, Dumas S, Tzavara ET, Wade MR, Nomikos GG, Hanoun N, Saurini F, Kemel ML, Gasnier B, Giros B, El Mestikawy S. The vesicular glutamate transporter VGLUT3 synergizes striatal acetylcholine tone. Nat Neurosci. 2008;11:292–300. doi: 10.1038/nn2052. [DOI] [PubMed] [Google Scholar]
- Hersch SM, Gutekunst CA, Rees HD, Heilman CJ, Levey AI. Distribution of m1-m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. J Neurosci. 1994;14:3351–3363. doi: 10.1523/JNEUROSCI.14-05-03351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdstock L, King AC, de Wit H. Subjective and objective responses to ethanol in moderate/heavy and light social drinkers. Alcohol Clin Exp Res. 2000;24:789–794. [PubMed] [Google Scholar]
- Ichikawa J, Chung YC, Li Z, Dai J, Meltzer HY. Cholinergic modulation of basal and amphetamine-induced dopamine release in rat medial prefrontal cortex and nucleus accumbens. Brain Res. 2002;958:176–184. doi: 10.1016/s0006-8993(02)03692-2. [DOI] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Effects of locally applied D-1 and D-2 receptor agonists and antagonists studied with brain dialysis. Eur J Pharmacol. 1988;156:385–393. doi: 10.1016/0014-2999(88)90284-1. [DOI] [PubMed] [Google Scholar]
- Ince E, Ciliax BJ, Levey AI. Differential expression of D1 and D2 dopamine and m4 muscarinic acetylcholine receptor proteins in identified striatonigral neurons. Synapse. 1997;27:357–366. doi: 10.1002/(SICI)1098-2396(199712)27:4<357::AID-SYN9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Nakamura M. Neural systems for behavioral activation and reward. Curr Opin Neurobiol. 1999;9:223–227. doi: 10.1016/s0959-4388(99)80031-2. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Karasawa H, Taketo MM, Matsui M. Loss of anti-cataleptic effect of scopolamine in mice lacking muscarinic acetylcholine receptor subtype 4. Eur J Pharmacol. 2003;468:15–19. doi: 10.1016/s0014-2999(03)01642-x. [DOI] [PubMed] [Google Scholar]
- King AC, Houle T, de Wit H, Holdstock L, Schuster A. Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res. 2002;26:827–835. [PubMed] [Google Scholar]
- Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci. 1991;11:3218–3226. doi: 10.1523/JNEUROSCI.11-10-03218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark GP, Kinney AE, Grubb MC, Zhu X, Finn DA, Mader SL, Berger SP, Bechthold AJ. Injection of oxotremorine in nucleus accumbens shell reduces cocaine but not food self-administration in rats. Brain Res. 2006;1123:51–59. doi: 10.1016/j.brainres.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez P, Borse J, Nguyen AT, Hamid A, Lutfy K. The role of the opioid receptor-like receptor in motor stimulatory and rewarding actions of buprenorphine and morphine. Neuroscience. 2008;155:597–602. doi: 10.1016/j.neuroscience.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClearn GE, Wilson JR, Meredith W. The use of isogenic and heterogenic mouse stocks in behavioral research. In: Lindzey G, Thiessen DD, editors. Contributions to Behavior-Genetic Analysis: The Mouse as a Prototype. Appleton-Centry-Crofts; New York: 1970. pp. 3–22. [Google Scholar]
- Meyer PJ, Phillips TJ. Sensitivity to ketamine, alone or in combination with ethanol, is altered in mice selectively bred for sensitivity to ethanol’s locomotor effects. Alcohol Clin Exp Res. 2003;27:1701–1709. doi: 10.1097/01.ALC.0000093602.00193.39. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol Bull. 1990;108:383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- Palmer AA, Lessov-Schlaggar CN, Ponder CA, McKinnon CS, Phillips TJ. Sensitivity to the locomotor-stimulant effects of ethanol and allopregnanolone: a quantitative trait locus study of common genetic influence. Genes Brain Behav. 2006;5:506–517. doi: 10.1111/j.1601-183X.2005.00198.x. [DOI] [PubMed] [Google Scholar]
- Palmer AA, McKinnon CS, Bergstrom HC, Phillips TJ. Locomotor activity responses to ethanol, other alcohols, and GABA-A acting compounds in forward- and reverse-selected FAST and SLOW mouse lines. Behav Neurosci. 2002;116:958–967. doi: 10.1037//0735-7044.116.6.958. [DOI] [PubMed] [Google Scholar]
- Pappano AJ. Cholinoceptor-blocking drugs. In: Katzung BG, editor. Basic and Clinical Pharmacology. 10th ed. McGraw-Hill Companies, Inc.; Columbus, Ohio: [Accessed July 11, 2008]. 2007. Available at: http://online.statref.com/Document/Document.aspx?DocId=54&FxId=2&Scroll=1&Index=2&SessionId=DE2BD7RKDVEXEGUK. [Google Scholar]
- Pastor R, Miquel M, Aragon CM. Habituation to test procedure modulates the involvement of dopamine D2-but not D1-receptors in ethanol-induced locomotor stimulation in mice. Psychopharmacology (Berl) 2005;182:436–446. doi: 10.1007/s00213-005-0115-3. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Belknap JK, Buck KJ, Cunningham CL. Genes on mouse chromosomes 2 and 9 determine variation in ethanol consumption. Mamm Genome. 1998;9:936–941. doi: 10.1007/s003359900903. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Burkhart-Kasch S, Terdal ES, Crabbe JC. Response to selection for ethanol-induced locomotor activation: genetic analyses and selection response characterization. Psychopharmacology (Berl) 1991;103:557–566. doi: 10.1007/BF02244259. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Dickinson S, Burkhart-Kasch S. Behavioral sensitization to drug stimulant effects in C57BL/6J and DBA/2J inbred mice. Behav Neurosci. 1994;108:789–803. doi: 10.1037//0735-7044.108.4.789. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Huson M, Gwiazdon C, Burkhart-Kasch S, Shen EH. Effects of acute and repeated ethanol exposures on the locomotor activity of BXD recombinant inbred mice. Alcohol Clin Exp Res. 1995;19:269–278. doi: 10.1111/j.1530-0277.1995.tb01502.x. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Shen EH. Neurochemical bases of locomotion and ethanol stimulant effects. Int Rev Neurobiol. 1996;39:243–282. doi: 10.1016/s0074-7742(08)60669-8. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Shen EH, McKinnon CS, Burkhart-Kasch S, Lessov CN, Palmer AA. Forward, relaxed, and reverse selection for reduced and enhanced sensitivity to ethanol’s locomotor stimulant effects in mice. Alcohol Clin Exp Res. 2002;26:593–602. [PubMed] [Google Scholar]
- Pohorecky LA, Makowski E, Newman B, Rassi E. Cholinergic mediation of motor effects of ethanol in rats. Eur J Pharmacol. 1979;55:67–72. doi: 10.1016/0014-2999(79)90148-1. [DOI] [PubMed] [Google Scholar]
- Puglisi-Allegra S, Cabib S, Oliverio A. Chronic stress reduces the analgesic but not the stimulant effects of morphine in mice. Brain Res. 1986;380:357–358. doi: 10.1016/0006-8993(86)90234-9. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Woolverton WL. Self-administration of cocaine: scopolamine combinations by rhesus monkeys. Psychopharmacology (Berl) 2002;161:442–448. doi: 10.1007/s00213-002-1069-3. [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Fink-Jensen A. Intravenous scopolamine is potently self-administered in drug-naïve mice. Neuropsychopharmacol. 2000;22:97–99. doi: 10.1016/S0893-133X(99)00088-3. [DOI] [PubMed] [Google Scholar]
- Renner UD, Oertel R, Kirch W. Pharmacokinetics and pharmacodynamics in clinical use of scopolamine. Ther Drug Monit. 2005;27:655–665. doi: 10.1097/01.ftd.0000168293.48226.57. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Dickinson SD, Cunningham CL. Haloperidol reduces ethanol-induced motor activity stimulation but not conditioned place preference. Psychopharmacology (Berl) 1992;107:453–456. doi: 10.1007/BF02245175. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Malott DH, Prather LK, Niehus DR, Cunningham CL. Motivational properties of ethanol in mice selectively bred for ethanol-induced locomotor differences. Psychopharmacology (Berl) 1994;116:207–216. doi: 10.1007/BF02245064. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J, Danko GP. A cross-generational comparison of alcohol challenges at about age 20 in 40 father-offspring pairs. Alcohol Clin Exp Res. 2005;29:1921–1927. doi: 10.1097/01.alc.0000187154.94681.65. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J, Tsuang J, Hesselbrock V, Bucholz K. Response to alcohol in daughters of alcoholics: a pilot study and a comparison with sons of alcoholics. Alcohol Alcohol. 2000;35:242–248. doi: 10.1093/alcalc/35.3.242. [DOI] [PubMed] [Google Scholar]
- Shen EH, Crabbe JC, Phillips TJ. Dopamine antagonist effects on locomotor activity in naïve and ethanol-treated FAST and SLOW selected lines of mice. Psychopharmacology (Berl) 1995a;118:28–36. doi: 10.1007/BF02245246. [DOI] [PubMed] [Google Scholar]
- Shen EH, Dorow J, Harland R, Burkhart-Kasch S, Phillips TJ. Seizure sensitivity and GABAergic modulation of ethanol sensitivity in selectively bred FAST and SLOW mouse lines. J Pharmacol Exp Ther. 1998a;287:606–615. [PubMed] [Google Scholar]
- Shen EH, Dorow JD, Huson M, Phillips TJ. Correlated responses to selection in FAST and SLOW mice: effects of ethanol on ataxia, temperature, sedation, and withdrawal. Alcohol Clin Exp Res. 1996;20:688–696. doi: 10.1111/j.1530-0277.1996.tb01673.x. [DOI] [PubMed] [Google Scholar]
- Shen EH, Harland RD, Crabbe JC, Phillips TJ. Bidirectional selective breeding for ethanol effects on locomotor activity: characterization of FAST and SLOW mice through selection generation 35. Alcohol Clin Exp Res. 1995b;19:1234–1245. doi: 10.1111/j.1530-0277.1995.tb01606.x. [DOI] [PubMed] [Google Scholar]
- Shen EH, Phillips TJ. MK-801 potentiates ethanol’s effects on locomotor activity in mice. Pharmacol Biochem Behav. 1998b;59:135–143. doi: 10.1016/s0091-3057(97)00389-4. [DOI] [PubMed] [Google Scholar]
- Smythies J. Section I. The cholinergic system. Int Rev Neurobiol. 2005;64:1–122. doi: 10.1016/S0074-7742(05)64001-9. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Woldbye DP, Wortwein G, Fink-Jensen A, Wess J, Caine SB. Reduced cocaine self-administration in muscarinic M5 acetylcholine receptor-deficient mice. J Neurosci. 2005;25:8141–8149. doi: 10.1523/JNEUROSCI.2077-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzavara ET, Bymaster FP, Davis RJ, Wade MR, Perry KW, Wess J, McKinzie DL, Felder C, Nomikos GG. M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J. 2004;18:1410–1412. doi: 10.1096/fj.04-1575fje. [DOI] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Muscarinic receptors regulate striatal neuropeptide gene expression in normal and amphetamine-treated rats. Neuroscience. 1996;75:43–56. doi: 10.1016/0306-4522(96)00277-1. [DOI] [PubMed] [Google Scholar]
- Weiner DM, Levey AI, Brann MR. Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc Natl Acad Sci USA. 1990;87:7050–7054. doi: 10.1073/pnas.87.18.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Xiang XH, Wang HL, Wu WR, Guo Y, Cao DY, Wang HS, Zhao Y. Ethological analysis of scopolamine treatment or pretreatment in morphine dependent rats. Physiol Behav. 2006;88:183–190. doi: 10.1016/j.physbeh.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Yamada M, Lamping KG, Duttaroy A, Zhang W, Cui Y, Bymaster FP, McKinzie DL, Felder CC, Deng CX, Faraci FM, Wess J. Cholinergic dilation of cerebral blood vessels is abolished in M5 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA. 2001;98:14096–14101. doi: 10.1073/pnas.251542998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim HJ, Gonzales RA. Ethanol-induced increases in dopamine extracellular concentration in rat nucleus accumbens are accounted for by increased release and not uptake inhibition. Alcohol. 2000;22:107–115. doi: 10.1016/s0741-8329(00)00121-x. [DOI] [PubMed] [Google Scholar]
- Zhang W, Yamada M, Gomeza J, Basile AS, Wess J. Multiple muscarinic acetylcholine receptor subtypes modulate striatal dopamine release, as studied with M1-M5 muscarinic receptor knock-out mice. J Neurosci. 2002;22:6347–6352. doi: 10.1523/JNEUROSCI.22-15-06347.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Ge X, Wang LZ, Ma L, Pei G. Attenuation of morphine tolerance and dependence in scopolamine-treated rats. NeuroReport. 1999;10:2007–2010. doi: 10.1097/00001756-199907130-00003. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Wilson C, Dani JA. Muscarinic and nicotinic cholinergic mechanisms in the mesostriatal dopamine systems. Neuroscientist. 2003;9:23–36. doi: 10.1177/1073858402239588. [DOI] [PubMed] [Google Scholar]