Abstract
TGF-β potently induces apoptosis in Burkitt’s Lymphoma (BL) cell lines and in explanted primary human B lymphocytes. The physiological relevance and mechanism of TGF-β-mediated apoptosis induction in these cells remains to be determined. Here we demonstrate the requirement for TGF-β-mediated regulation of BIK and BCL-XL to activate an intrinsic apoptotic pathway in centroblastic BL cells. TGF-β directly induced transcription of BIK and a consensus Smad binding element identified in the BIK promoter recruits TGF-β-activated Smad transcription factor complexes in vivo. TGF-β also transcriptionally repressed expression of the apoptosis inhibitor BCL-XL. Inhibition of BCL-XL sensitised BL cells to TGF-β-induced apoptosis while overexpression of BCL-XL or suppression of BIK by shRNA, diminished TGF-β-induced apoptosis. BIK and BCL-XL were also identified as TGF-β target genes in purified normal human centroblast B cells and immunohistochemical analyses of tonsil tissue revealed widespread TGF-β receptor-regulated Smad activation and a focal pattern of BIK expression. Furthermore, using a selective inhibitor of the TGF-β receptor we provide evidence that autocrine TGF-β signaling through ALK5 contributes to the default apoptotic program in normal human centroblasts undergoing spontaneous apoptosis. Our data suggests that TGF-β may act as a physiological mediator of human germinal centre homeostasis via regulation of BIK and BCL-XL.
Keywords: TGF-β, apoptosis, centroblast, BIK, BCL-XL
Introduction
Transforming growth factor-beta (TGF-β) is the prototypical member of a superfamily of pleiotropic cytokines which regulate a multitude of biological processes including tissue homeostasis, angiogenesis, migration and differentiation. TGF-β signals via activation of a heterooligomeric receptor complex consisting of the type II receptor, TGFβ-RII and the type I receptor ALK5. These receptors activate a diverse range of intracellular signal transduction pathways including the canonical Smad pathway. The receptor-associated Smads 2 and 3 (R-Smads), are directly phosphorylated on their carboxy termini by the serine/threonine kinase domain of ALK5. Following phosphorylation the R-Smads heterooligomerise with the co-Smad, Smad4, and accumulate in the nucleus where in conjunction with a number of cofactors they regulate target gene expression.1
TGF-β acts as a potent immunosuppressor by regulating the proliferation and survival of many cells of the immune system. Part of the homeostatic function of TGF-β is the cell-type specific induction of apoptosis which occurs in several cell types including B cells2. Gene ablation studies in mice have indicated that TGF-β signaling affects B cells at all stages of development and is critical for secreted IgA responses3. The observation that many lymphomas (including those derived from germinal centre B cells undergoing differentiation) have defective TGF-β signaling pathways4,5 suggests that disruption of TGF-β apoptotic signaling in human B cells may contribute to lymphomagenesis.
Germinal centres (GCs) are the main sites for generating high-affinity antibody responses through repeated rounds of selection by apoptotic elimination of unwanted B cells. Activated B cells entering GCs undergo a process where immunoglobulin genes are altered by somatic hypermutation. Over time, cells carrying mutated receptors with the highest affinity for foreign antigen out-compete others for limiting amounts of antigen and survival signals provided by T-cell help, so ensuring their continued survival and differentiation.6 At present, cell death at this site is generally thought to occur by FAS-dependent apoptosis (required for deletion of cells carrying low affinity mutated immunoglobulins7). In addition, B cell receptor (BCR) ligation in the absence of co-stimulatory receptor-ligand interactions is important for deletion of autoreactive B cells. In this context, FAS-mediated apoptosis is inhibited by cFLIPL induced by survival signals from CD40 and B cell receptor (BCR) ligation. In the absence of these signals (for example during in vitro culture) cFLIP is rapidly degraded leading to spontaneous apoptosis8, a process known as ‘death by neglect’. GC B cells are therefore primed to die and require continued environmental cues for survival.
Signaling via CD40 and the BCR may help to render B cells ‘Fas-resistant’ by induction of the pro-survival BCL-2 homologue, BCL-XL, although there are conflicting reports of its involvement in Fas-mediated cell death9. It is likely however, that BCL-XL or BCL-2 expression induced by survival signals is required to antagonise a mitochondrial ‘intrinsic’ apoptosis pathway activated by antigen-BCR interaction. BCR-mediated cell death correlates with induction of BIM10 which is a member of the pro-apoptotic BCL-2 family of proteins that also includes BIK, NOXA, BAD, BID, BMF and PUMA. These proteins antagonise the function of BCL-2 and its pro-survival homologues (BCL-XL, BCL-w, MCL-1, and A111) resulting in activation of BAX and BAK. Once activated, BAX and BAK permeabilise the mitochondrial outer membrane12 to release apoptogenic factors including cytochrome c which complexes with APAF1 and pro-caspase 9 to form the apoptosome. Knockout mouse studies have implicated several pro-apoptotic components of this pathway in B cell homeostasis.10
Previous findings suggest that TGF-β-mediated apoptosis may also be involved in normal human GC B cell homeostasis. For example, we and others have shown that some Burkitt’s Lymphoma (BL) cell lines (which derive from B cells differentiating within GCs) are highly sensitive to TGF-β-induced apoptosis.5,13,14 Exogenous TGF-β also enhances apoptosis of primary explanted human and murine B lymphocytes.3 However, the physiological role of TGF-β signaling in GC reactions, the cell types within this compartment which die in response to TGF-β, and the effector mechanisms TGF-β employs to induce apoptosis remain to be elucidated.
Here we demonstrate that TGF-β contributes to ‘death by neglect’ by regulating an intrinsic apoptotic pathway in human centroblastic B cells. TGF-β impacts on apoptosis regulators upstream of BAX and BAK, by inducing the pro-apoptotic BH3-only protein BIK and downregulating BCL-XL. These changes occurred both in BL cell lines and in their normal GC counterparts (centroblasts). Blocking the TGF-β-induced intrinsic pathway in primary human centroblasts provided cells with a survival advantage during spontaneous apoptosis. Our findings identify autocrine TGF-β signaling as a physiological regulator of a default mitochondrial apoptotic pathway in human B cells, and identify a novel function for BIK in B cell homeostasis.
Results
Engagement of the intrinsic mitochondrial apoptosis pathway by TGF-β
To determine how TGF-β regulates apoptosis in centroblastic Burkitt’s Lymphoma cells, we studied the response of a panel of BL cell lines to exogenous TGF-β. Ramos, BL2 and BL40 cells died following TGF-β addition shown by cleavage of the caspase substrate PARP, however, CA46 cells showed no PARP cleavage despite having an intact TGF-β signaling pathway (measured by phosphorylation of Smad2) (Figure 1a).
Figure 1.
TGF-β activates an intrinsic apoptotic program in centroblastic BL cells. (a) Western blot analysis of apoptosis (cleavage of the caspase substrate PARP) and TGF-β signaling (pSmad2) in a panel of BL cell lines treated with 1ng/ml TGF-β for the times indicated. (b) Loss of mitochondrial inner membrane potential (ΔΨm) during TGF-β-induced apoptosis of Ramos cells shown by reduced mean fluorescence of cells labelled with the mitochondrial stain tetramethlyrhodamine ethyl ester (TMRE). Cells were untreated (dash), labelled with TMRE alone (solid) or labelled with TMRE and treated with TGF-β (dotted). Cells were also treated with cyanide-m-chlorophenylhydrazone (CCCP) (thick solid) to cause complete membrane depolarisation and serve as a positive control for ΔΨm. Cells were treated for the times indicated on each histogram and were analysed by flow cytometry.
We next analysed mitochondrial membrane integrity in apoptotic BL cells to determine whether the intrinsic pathway was activated by TGF-β. The gradual loss of the mitochondrial stain TMRE during 48 hours treatment of BL cells is consistent with involvement of the intrinsic mitochondrial pathway in TGF-β-induced apoptosis of BL cells (Figure 1b).
TGF-β regulates multiple BCL-2 family members
Induction of the intrinsic apoptosis pathway requires activation of BAX/BAK which in turn is controlled by other members of the BCL-2 family. We therefore screened for transcriptional changes in BCL-2 family members during TGF-β-induced apoptosis. Multiprobe RNase protection assays (RPAs) demonstrated that TGF-β addition caused a rapid increase in transcripts of the pro-apoptotic BH3-only protein BIK in BL2 cells (Figure 2a) and in Ramos and CA46 BL cells (Supplementary Figure 1a). Elevated BIK expression in CA46 cells was initially surprising, since CA46 cells do not apoptose in response to TGF-β (Figure 1a and ref.5), however, further investigation confirmed previous reports15 that CA46 cells lack the mitochondrial membrane protein BAX (Supplementary Figure 1b) required for intrinsic apoptosis.
Figure 2.
TGF-β-induced apoptosis in BL cells is associated with induction of BIK and downregulation of BCL-XL. (a) Analysis of transcripts encoding BCL-2 family members by multiprobe RNAse protection assay (RPA) following TGF-β treatment of BL2 cells. Total RNA from untreated or TGF-β treated cells was analysed during a 6hr time course. Lanes corresponding to yeast negative control RNA (Y), undigested probe (P) and 32P-labelled pBR322 size markers (M) are shown. Sizes of the probe template set are also indicated. Confirmation of BIK induction (b) and BCL-XL downregulation (c) by Western blot analysis of RIPA lysates prepared from untreated and TGF-β (1ng/ml) treated BL cell lines. BIMEL expression was also measured and Smad2/3 levels are shown as a loading control. TGF-β signaling was analysed by Western blotting for phosphorylation of Smad3 (p-Smad3). Actin blots are shown as a loading control.
As well as inducing BIK, TGF-β treatment also rapidly decreased RNA expression of BCL-XL in BL2 (Figure 2a), Ramos and CA46 cells (Supplementary Figure 1c). These effects were mirrored at the level of protein expression (Figures 2b and 2c), although the kinetics of BIK induction were more rapid than loss of BCL-XL expression. No induction of BIM (a known TGF-β target gene16,17) was observed (Figure 2b and Supplementary Figure 2a, 2b). BL2 cells lacked BIM expression altogether, but died efficiently upon exposure to TGF-β, indicating that BIM is unlikely to mediate the apoptotic response in the BL cell lines studied. In addition, we observed no change in MCL-1 levels or in BID expression or cleavage to the pro-apoptotic 15kDa form, tBid (Supplementary Figure 2b). TGF-β-mediated apoptosis in BL cells therefore correlated primarily with effects on BIK and BCL-XL.
To address whether these events are critical for apoptosis, we obtained BL cells stably over-expressing BCL-XL18. Elevated levels of BCL-XL expression in the stably transfected cells (maintained throughout exposure to TGF-β) reduced the proportion of cells containing active caspase-3 (Figure 3a) and reduced cleavage of PARP induced by TGF-β (Figure 3b). Increased BCL-XL expression therefore largely protected BL cells from TGF-β-mediated apoptosis thus confirming the involvement of mitochondria in the response. To investigate further whether BCL-XL is critical for BL cell survival, we used an inhibitor to block the function of endogenous BCL-XL. BH3i-2′ inhibits BCL-2 and BCL-XL by binding to their BH3 binding pocket.19 Since BL2, Ramos and BL40 cell lines express BCL-XL but lack BCL-2 protein expression (Figure 3c) we used BH3i-2′ as a selective inhibitor of BCL-XL in BL cells. All three cell lines apoptosed upon treatment with 30μM BH3i-2′. BL2 cells, which express a lower amount of BCL-XL than either Ramos or BL40, were sensitive to 5μM of the inhibitor (Figure 3d). Significantly, we were able to sensitise BL cells to TGF-β-induced apoptosis using low dose BCL-XL inhibitor (5μM) (Figure 3e). Apoptosis induction was greater following combined treatment than was observed with either TGF-β or the BCL-XL inhibitor alone. The synergistic apoptotic response demonstrates that BCL-XL regulation is an important factor in TGF-β-induced apoptosis in BL cells.
Figure 3.
Regulation of BCL-XL and BIK are critical for optimal TGF-β-mediated apoptosis. (a) Flow cytometry analysis of active caspase-3 positive cells (mean ± s.d., n=3) induced by 24hr TGF-β treatment in L3055 BL cells and BCL-XL stable transfectants. (b) Western blot analysis of RIPA extracts from L3055 BL cells and BCL-XL stable transfectants after 48hrs TGF-β treatment for PARP cleavage, BCL-XL and phospho-Smad2 (p-Smad2). (c) Western blot analysis of 50μg RIPA extracts from untreated BL cells as indicated. Expression levels of BCL-XL and BCL-2 in each cell line are shown. A western blot for actin is included as a loading control. (d) BL2, Ramos and BL40 cells were seeded overnight at 5 × 105/ml and then treated for 48 hours with the BCL-XL inhibitor BH3i-2′ (Calbiochem) as indicated. Apoptosis induction was determined by PI staining and flow cytometry (mean ± s.d., sub-G1 DNA content (n=3)). (e) Ramos cells were left untreated or treated for 48 hours with TGF-β in the presence or absence of low dose BH3i-2′ (5μM). Apoptosis induction was determined by PI staining and flow cytometry (mean ± s.d., sub-G1 DNA content (n=3)). (f) Independent stable cell lines (labelled 1 and 2) expressing non-silencing (NS) and shRNAs targeted against Bik (Bik#1 and Bik#2) were generated by retroviral infection of Ramos BL cells. Quantitative real time PCR (qRT-PCR) was used to demonstrate knockdown of BIK in stable Ramos BL cells. Mean relative amounts of BIK RNA are expressed after normalisation to 18s rRNA levels. (g) Determination of apoptosis (mean ± s.d., sub-G1 DNA content (n=3)) by PI staining and flow cytometry following TGF-β treatment of cell lines described in (f).
To test whether BIK induction is also required for optimal induction of apoptosis, we used a stable shRNA knockdown approach. Non-silencing control and BIK shRNA retroviral vectors were generated to establish stable Ramos cell lines. qRT-PCR analysis revealed BIK shRNAs reduced expression to below basal levels in comparison with the non-silencing vector (Figure 3f). Importantly, BIK knockdown resulted in a substantial reduction in TGF-β-mediated apoptosis (Figure 3g) without affecting TGF-β signaling (data not shown). Taken together, these findings reveal that the levels of BIK and BCL-XL play a critical role in regulating TGF-β-dependent apoptosis in centroblastic BL cells.
BIK and BCL-XL are direct and indirect TGF-β target genes
We next investigated the mechanism of TGF-β regulation of BCL-2 family members firstly by testing whether BCL-XL and BIK are immediate early targets of TGF-β signaling. RPA analysis showed that repression of BCL-XL required protein synthesis since pre-treatment of cells with cycloheximide and anisomycin prevented the decrease in BCL-XL RNA levels (Figure 4a). Induction of BIK however, still occurred under these conditions but was completely blocked by inhibiting transcription with actinomycin D (Figure 4a and 4b) demonstrating that BIK is a direct, transcriptional target of TGF-β signaling.
Figure 4.
TGF-β directly regulates BIK transcription and induces recruitment of activated Smad complexes to the BIK promoter. (a) RPAs for BIK and BCL-XL, expression in cells treated with or without TGF-β and the protein synthesis inhibitors cycloheximide and anisomycin (C+A) as indicated. Time points -2 and 0 are samples harvested prior to and following inhibitor treatment respectively. TGF-β was added at 0 hours. (b) RPA for BIK and the loading control GAPDH following TGF-β treatment of BL cells pretreated for 1 hour with or without the transcription inhibitor actinomycin D (ActD). Time points -1 and 0 are samples harvested prior to and following inhibitor treatment respectively. TGF-β was added at 0 hours. M, P and Y are as described in Figure 2. (c) Real time qRT-PCR carried out on RNA isolated from BL cell lines treated with TGF-β in the presence or absence of the HDAC inhibitor TSA. Shown is the mean ± s.d. amount of RNA normalised to 18s levels and relative to TGF-β untreated samples. (d) Western blot analysis of 50μg RIPA extracts from BL cells treated with and without TGF-β in the presence or absence of TSA as indicated. TGF-β signaling was analysed by Western blotting for phosphorylation of Smads. A western blot for total Smad2/3 is included as a loading control. (e) Potential Smad binding regions (SBRs) within the human Bik promoter. Arrows indicate positions of PCR primers used in ChIP assays shown in (f). (f) ChIP assay for Smad recruitment to the endogenous Bik promoter in BL cells cultured with and without TGF-β for 1 hour. Input lanes are from 10% of samples used in the IPs performed with control IgG, Smad3 and Smad4 antibodies. (g) Sequence of ds oligos used in EMSAs shown in (h) containing the potential wildtype and mutant SBR2 (mSBR2). SBE sequences are shown in bold. (h) EMSA of CA46 nuclear extracts treated with and without TGF-β for 1 hour as indicated and incubated with wild type SBR1 and SBR2 and mSBR2. TGF-β inducible and antibody supershifted complexes are shown (arrowed).
The control of BCL-XL transcription is complex, involving multiple promoters whose activity appears to be cell type and context dependent.20 Since the histone deacetylases (HDACs)- 4 and 5 can be involved in repression of promoters by TGF-β21, we treated BL cells with an inhibitor of class I and class II HDACs, Trichostatin A (TSA), to gain insight into the possible mechanism of repression of BCL-XL.. As expected, TGF-β treatment for 8 hours decreased the amount of BCL-XL RNA expressed in both BL2 and BL40 cells. TSA pre-treatment of BL cells to inhibit HDAC function, completely blocked the ability of TGF-β to repress BCL-XL transcription (Figure 4c) without affecting TGF-β signaling (Figure 4d). In fact, loss of HDAC function switched the response to TGF-β from one of repression to one of activation (in agreement with previous studies of the osteocalcin promoter).21 TGF-β regulation of BCL-XL transcription may therefore involve chromatin remodelling through the recruitment of repressor complexes containing class I or II HDACs.
Our studies indicate that BIK is an immediate early target of TGF-β signaling in BL cells, and therefore is likely to be activated by Smad complexes. Smad binding regions (SBRs) often contain 2 copies of the Smad binding element (SBE) sequence 5′ GTCT 3′ or its reverse complement 5′AGAC 3′22. We identified two potential SBRs approximately 1.1Kb upstream of the BIK transcription start site (Figure 4e). Chromatin immunoprecipitation (ChIP) analysis using primers spanning this region demonstrated the TGF-β-dependent recruitment of Smads 3 and 4 to the endogenous BIK promoter in BL cells in vivo (Figure 4f). Radiolabelled dsDNA probes of SBR1 and SBR2 were prepared (wild type and mutant SBR2 sequences are shown in Figure 4g) and used in electrophoretic mobility-shift assays (EMSA) to determine which SBR in the region assayed by ChIP could bind Smad complexes in vitro. SBR2 bound a TGF-β inducible complex which could be supershifted with Smad3 and Smad4 antibodies (Figure 4h). SBR1 and a mutated form of SBR2 which abrogates the consensus SBEs, were unable to bind Smad3/4 complexes (Figure 4h). These data suggest that TGF-β activates BIK by direct binding of transcriptionally active Smad3/4 complexes to the SBR2 sequence within the promoter.
Autocrine TGF-β signaling in primary human centroblasts
Burkitt’s Lymphomas originate within GCs and phenotypically resemble centroblasts. Exogenously added TGF-β enhances apoptosis of GC B cells in vitro23 but the mechanism of induction and what contribution TGF-β makes to normal GC development is not fully understood. Having determined how TGF-β induces apoptosis in BL cell lines, we studied whether these responses occur in primary human tonsil cells. In addition to studying the effects of exogenously added TGF-β, we used SB-431542, a selective inhibitor of ALK524, to block endogenous TGF-β signaling. Total mononuclear cells (TMCs), purified CD77+ve centroblasts (isolated by positive selection), and TMCs depleted of CD77 positive cells responded to exogenous TGF-β as shown by increased Smad2 and Smad3 phosphorylation (Supplementary Figure 3a). Interestingly, active Smad2 was also detected in untreated cells cultured for 1 hour in serum free medium. This endogenous signaling was completely ablated by treatment with SB-431542 (Supplementary Figure 3a) which suggests that, in the absence of external sources of cytokine, autocrine TGF-β signaling via the ALK5 receptor occurs in GC B cells and isolated centroblasts. Consistent with this, we found that centroblasts express TGF-β1 mRNA (Supplementary Figure 3b).
ALK5 inhibition enhances centroblast survival
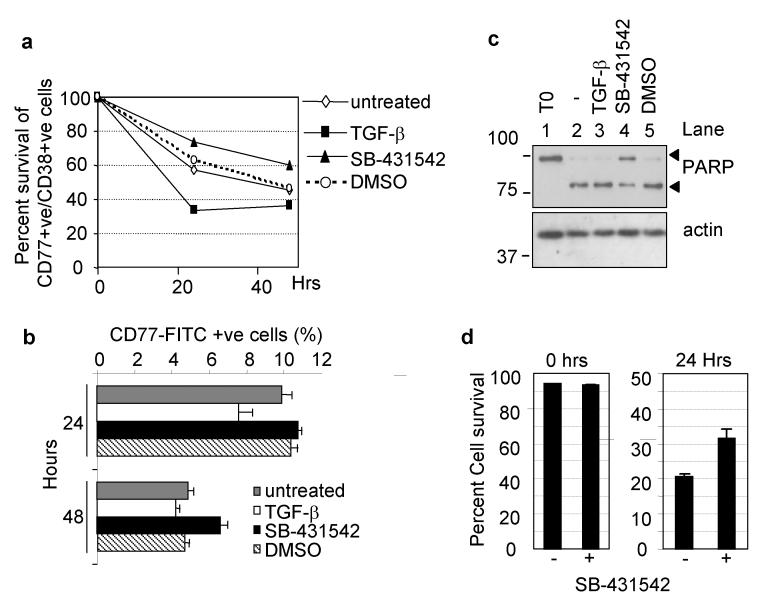
Since centroblasts exhibit endogenous TGF-β signaling and respond to increased doses of TGF-β, we examined the biological consequences of signaling. Firstly we analysed by flow cytometry (FCM) the survival of centroblasts (CD38/CD77 double positive cells) in populations of TMCs cultured with either TGF-β or SB-431542. During in vitro culture the percentage of double positive cells decreased. This process was enhanced by addition of TGF-β and partially suppressed by treatment with SB-431542 relative to controls (Figure 5a). Similar results were obtained with CD77 staining alone (Figure 5b), enabling the use of CD77 positive selection purification techniques to examine further the role of TGF-β signaling in these cells.
Figure 5.
Autocrine TGF-β signaling contributes to spontaneous centroblast apoptosis. Flow cytometry analysis of centroblasts in preparations of explanted normal human tonsil mononuclear cells (TMCs) showing the percent survival of CD77/CD38 +ve cells (a) and the percentage of anti-CD77-FITC stained cells remaining after 24 and 48 hours in vitro culture of TMCs (b) with either exogenous TGF-β or an inhibitor of TGF-β signaling (SB-431542, 10μM) or solvent control (DMSO). (c) Western blot analysis for PARP cleavage and actin of extracts from 1×105 purified centroblasts incubated for 36 hours with TGF-β, SB-431542 or DMSO solvent control. Actin levels are shown as a loading control. (d) Percent of active caspase-3 negative cells (mean ± s.d., n=3) determined by PhiPhiLux (G1D2) fluorescent caspase substrate and flow cytometry in purified centroblasts treated with DMSO (-) or SB-431542 for the indicated times.
Culture of purified centroblasts resulted in spontaneous apoptosis shown by PARP cleavage (Figure 5c, lane 2). Treatment with the mitogen, PMA, partially rescued centroblasts from spontaneous apoptosis but was unable to overcome cell death induced by addition of TGF-β (Supplementary Figures 4a and 4b). Interestingly, when centroblasts were cultured with SB-431542 we were able to consistently prolong cell survival shown by a reduced PARP cleavage (Figure 5c). In addition, we detected fewer centroblasts containing active caspase-3 following isolation and culture with the ALK5 inhibitor (Figure 5d). No PARP cleavage was evident in any CD77 depleted TMCs (Supplementary Figure 4a) despite apoptosis taking place in whole TMC cultures (data not shown). Thus we conclude that CD77+ve cells are the only cell type present in TMCs that apoptose in response to TGF-β and that blocking endogenous signaling with a selective inhibitor of ALK5 provides these cells with a survival advantage following withdrawal of environmental cues.
TGF-β regulates BIK and BCL-XL in primary human centroblasts
To assess whether apoptosis induction is mechanistically similar in both BL cell lines and their normal GC counterparts we analysed whether TGF-β signaling can be detected in intact germinal centres, and whether BIK and BCL-XL are also TGF-β target genes in primary human centroblasts. Formalin fixed, paraffin embedded tonsil tissues were stained for phosphorylated Smad2 and Ki67, a marker of proliferation (Figure 6a). Ki67 highlighted dark and light zones of the GC. Phosphorylated Smad2 was readily detected within both zones as well as in the surrounding mantle zone. A more restricted expression pattern of BIK was detected within the dark and light zones of GCs.
Figure 6.
TGF-β-mediated regulation of BIK and BCL-XL in primary human centroblasts. (a) Sections of formalin fixed paraffin-embedded human tonsil tissue were stained with antibodies as indicated and counterstained with haematoxylin. Scale bars represent 200μM. (b) Western blot analysis of BIK expression in TMCs, TMCs depleted of centroblasts (CD77 depleted) and isolated centroblasts (purified CD77). A western blot for actin is included as a loading control. (c) Real time qRT-PCR carried out on RNA isolated from purified centroblasts treated with zVAD-fmk and -/+ TGF-β for 2-4h (BIK and BIM) or 16-24h (BCL-XL). The mean fold changes in relative RNA expression compared to untreated controls are shown. Statistical analysis was carried out using paired t-tests (n=3). Statistically significant (p) values of <0.005 (**) are indicated.
RPA analysis demonstrated that purified CD77+ve cells express BCL-XL, and high levels of BIK RNA (Supplementary Figure 5). BCL-2 was poorly expressed confirming previous observations.25 Western blotting of lysates from tonsil mononuclear cells (TMC), purified CD77 positive cells and TMCs depleted of centroblasts revealed that while BIK protein could only be detected very weakly in TMCs, purified CD77 positive cells were strongly positive for BIK, demonstrating that BIK expression is restricted to centroblasts (Figure 6b). qRT-PCR analysis carried out on purified centroblasts treated with the pan-caspase inhibitor ZVAD to inhibit apoptosis showed that BIK and BCL-XL RNA levels were significantly increased and reduced respectively by TGF-β addition while the levels of BIM were unchanged (Figure 6c). Taken together, the findings reveal that TGF-β signaling is widespread during germinal centre reactions and that the effectors of apoptotic responses induced by TGF-β in BL cell lines and primary centroblasts are identical.
Discussion
This study reveals that TGF-β mediates apoptosis in germinal centre derived B cells via a mitochondrial intrinsic pathway (Figure 1b). The mechanism of apoptosis induction involved transcriptional regulation of the BCL-2 family members, BIK and BCL-XL (Figures 2 and 4). In addition, we provide the first evidence of a causal relationship between BIK and TGF-β-induced apoptosis (Figure 3g) and show that BCL-XL is important for BL cell survival and the outcome of their response to TGF-β signaling. Overall our data indicates that the relative levels of BIK and BCL-XL dictates the apoptotic response of centroblastic cells to TGF-β. We might therefore also predict that cross-talk between the TGF-β and BCR receptor signaling pathways is likely, since BIK (as well as BIM) may be regulated by BCR ligation.26
Upon induction, BH3-only proteins like BIK induce apoptosis by binding directly to pro-survival BCL-2 family members which constrain BAX and BAK activation. BIK has been described as promoting cell death by sequestering BCL-2 and BCL-XL27 and antagonising MCL-1 and BCL-XL during apoptosis caused by protein synthesis inhibition.28 Since centroblastic cells express very little BCL-2 (Supplementary Figure 5 and ref 25) induction of BIK in this cell type is more likely to antagonise the function of BCL-XL and/or MCL-1. Indeed, maintaining BCL-XL protein levels in BL cell lines partially overcame the apoptotic effects of TGF-β (Figures 3a and 3b), while inhibiting BCL-XL function sensitised BL cells to the effects of TGF-β (Figure 3e). Given the important role of BCL-XL in the TGF-β-mediated apoptotic response, it is possible that the defect in GC apoptosis seen in Bcl-XL transgenic mice, which results in “relaxed” negative selection and impaired affinity maturation29, could be a consequence of a defective default TGF-β apoptosis program.
BIK is transcriptionally regulated by p5330 and E2F-131,32, and we now identify BIK as a novel TGF-β target gene in human B cells via Smad recruitment to the promoter. Our conclusion that BIK is involved in B cell homeostasis is supported by the observations that mice with a heritable defect resulting in elevated levels of BIK RNA, have higher levels of apoptosis in splenic B cells, and normal B cell development can be restored by BCL-XL overexpression33. On the other hand, Bik-/- mice appear quite normal and their B cells are still sensitive to spontaneous apoptosis.34 To reconcile these observations, it is possible that murine and human B cells respond differently in some respects to TGF-β signaling. Significantly, the SBR recruiting Smads to the endogenous human BIK promoter (shown in Figure 4e and 4f) is not conserved in the mouse or rat (data not shown), indicating that BIK is unlikely to be involved in TGF-β-regulated germinal centre homeostasis in mice.
Using a selective inhibitor of TGF-β receptor function, we also show that TGF-β signaling through ALK5 is an important physiological modulator of apoptosis of human centroblasts in the absence of survival stimuli (Figure 5). It is possible that exogenous sources of TGF-β may contribute to the regulation of centroblasts in vivo, nevertheless, purified centroblasts exhibited phosphorylation of Smads in the absence of serum and express TGF-β1 RNA (Supplementary Figure 3b), suggesting that treatment of the cells with SB-431542 inhibits a functional autocrine TGF-β signaling pathway.
Thus far, we have not yet fully elucidated the mechanism of BCL-XL repression. Transcriptional regulation of BCL-XL is complex, involving numerous different transcription factors and three distinct promoters regulating initiation at the 5′end of two non-coding exons, IA and IB, and at the beginning of exon II.20 One possible mechanism of BCL-XL regulation by TGF-β is through increased expression of the TGF-β-induced early genes 1 and 2 (TIEG1 and TIEG2) which regulate BCL-XL levels in oligodendroglial precursor cells.35 TIEG-1 was undetectable in BL cells by Western blotting, and while TIEG2 was expressed its levels were unaltered by TGF-β (LS, unpublished observations). It therefore seems unlikely that induction of either TIEG1 or 2 is involved in BCL-XL regulation in BL cells. However, our data suggests that (as in the case of TGF-β-mediated repression of osteocalcin) chromatin remodelling in the form of recruitment of HDAC-containing repressor complexes may be involved. Studies are currently underway to characterise the mechanism of repression further.
A Model of Centroblast death
Overall our data provides evidence for a revised model of centroblast apoptosis (Figure 7) to include the novel involvement of TGF-β in regulating an intrinsic apoptosis pathway. We propose that autocrine TGF-β signaling through ALK5 contributes to the default apoptotic state apparent in centroblasts lacking survival stimuli. Parallel Fas (extrinsic) and TGF-β (intrinsic) pathways converge to regulate death by neglect, via caspase-8 and caspase-9 respectively. In line with this, chemical inhibition of caspase-9 activity in explanted GC B cells enhances their survival.36 Our data implies that survival signals provided during affinity maturation must overcome both pathways.
Figure 7. Model of centroblastic cell apoptosis pathways.
The contribution of TGF-β signaling to regulation of BCL-2 family members is included alongside known apoptosis pathways functional in germinal centres. The three separate apoptosis pathways converge to regulate death of human centroblasts. FAS signaling activates the extrinsic caspase-8/caspase-3 cascade while TGF-β signaling via its receptor complex represses the prosurvival factor BCL-XL and induces BIK to activate the intrinsic pathway. Fas and TGF-β signaling contribute to the default apoptotic state of ‘death by neglect’ while B cell receptor signaling also activates the mitochondrial apoptosis pathway in cells lacking environmental survival cues. Loss of TGF-β signaling through inhibition of TGF-β receptor signaling by SB-431542 would reduce the amount of caspase-3 activation and raise the threshold for apoptosis induction. The model highlights the requirement for maintenance of cFLIP and BCL-XL levels for cells to survive during germinal centre reactions.
Our data also suggests that defective TGF-β signaling would provide GC B cells with a survival advantage during selection. Indeed many lymphomas of B cell origin have aberrant TGF-β signaling or defects in downstream components of the signaling pathway4 while mutations in BIK have been reported in B cell lymphomas of GC origin.37 We hypothesise therefore that disruption of the TGF-β regulated apoptotic program in human B cells may contribute to lymphomagenesis and/or autoimmune pathology.
Materials and Methods
Cell Lines and reagents
BL cell lines were maintained in RPMI-1640 (Gibco-BRL) supplemented with 5-10% (v/v) heat-inactivated FCS, 2mM/ml glutamine and antibiotics. Cells were treated as required with 1ng/ml TGF-β1 (Peprotech), 50μM zVAD-fmk (Calbiochem) and 10μM SB-431542 (Tocris). Protein synthesis inhibition was carried out by pre-incubation for 2 hours with 50μg/ml cycloheximide and 100μM anisomycin (Sigma). Inhibition of transcription was carried out by pre-treatment of cells for 1 hour with 2.5μg/ml actinomycin D (Sigma). The inhibitor of BCL-XL function (BH3i-2′) was purchased from Calbiochem and reconstituted in DMSO at a concentration of 15mM. For inhibition of HDAC function cells were pretreated for 15 minutes with 330nM Trichostatin A (Sigma).
Isolation of centroblasts
Ethical permission was obtained for collection and use of normal tonsil tissue (confirmed on histology) from the Southern General Hospital Ethics Committee (Reference EC/04/S/06) and registered with the Research and Design Office (reference R040016) following routine tonsillectomy. Tonsil mononuclear cells (TMC) were isolated by Ficoll density gradient. CD77+ve cells were purified using rat anti-human CD77 (Immunotech) followed by mouse anti-rat IgM (BD biosciences) and rat anti-mouse IgG1 microbeads (Miltenyi Biotech). Cells were purified using an AutoMacs (Miltenyi Biotech) and were cultured at 37°C in RPMI plus 15% FCS at 1×107/ml.
Immunoblotting and antibodies
RIPA lysates were analysed by SDS-PAGE. Antibodies used in Western blotting were mouse monoclonals against PARP (BD Biosciences), BIK (FL-160, Santa Cruz), Smad 2/3 (BD Biosciences), and actin (Sigma) and rabbit polyclonal antibodies against phospho-Smad 2 (ser465/476), phospho-Smad 3 (ser 433/435), BID and BCL-XL (Cell Signaling), MCL-1 (Santa Cruz) and BIM (Chemicon). Secondary HRP-conjugated antibodies were obtained from Dako. Bound immunocomplexes were detected by enhanced chemiluminescence (ECL; Amersham). Prestained protein marker sizes are shown on each gel.
Analysis of cell surface markers by flow cytometry
1×106 cells in PBS/0.5% BSA were labelled on ice for 1 hour with the appropriate concentration of antibody before being washed and analysed by flow cytometry (BD FACScan or FacsCalibur). Antibodies used were PE-conjugated anti-CD38 and FITC-conjugated anti-CD77 (BD Biosciences). At least 104 events were acquired and analysed using CellQuest software. Analysis gates were set to include 1% of cells of an unstained or isotype matched control stained population.
Analysis of apoptosis by flow cytometry
Cells were fixed, labelled with propidium iodide (PI) and analysed by flow cytometry for sub-2N DNA content, or analysed by measuring intracellular caspase-3 activity using fluorogenic peptide substrates of caspase-3 (PhiPhiLux, G1D2, Calbiochem) as recommended by the manufacturer. To monitor mitochondrial membrane potential, cells were incubated for 20mins in medium containing 40nM of the mitochondrial stain TMRE (Molecular Probes). Treatment with CCCP induced complete mitochondrial membrane depolarisation as a positive control.
RNase protection assay (RPA)
Total cellular RNA was extracted using Trizol. RPAs were carried out as described previously38 using the hApo2b multiprobe template set (BD Biosciences Pharmingen).
Retroviral infection of BL cell lines
shRNA-mirs targeting BIK were generated using oligonucleotides described in supplementary text cloned into MSCV/LTRmiR30-PIGΔRI (LMP) [a kind gift of Ross Dickins and Scott Lowe]39 Amphotropic retroviruses were generated as described in supplementary materials and methods. BL cells, seeded at 5000 cells/well in 96-well plates, were infected with viral supernatants by centrifugation at 37°C, 4000×g for 1.5 hours. Cells were incubated overnight prior to addition of growth medium containing 0.6μg/ml puromycin. Stable cell pools were analysed directly after outgrowth in selection medium.
qRT-PCR
Total cell RNA was isolated using Trizol as recommended by the manufacturer. cDNA and qRT-PCR reactions were prepared using SYBR green 2-step qRT-PCR kits (Finnzymes) and specific primers for each gene (Qiagen). Amplified products were analysed by Chromo4 continuous fluorescence detector and Opticon Monitor3 software. The relative amount of RNA for each gene expressed after normalisation to the amount of 18s rRNA in each sample.
ChIP assay
2×107 CA46 cells were treated with 1ng/ml TGF-β for 1 hour and fixed in 1% formaldehyde for 15 minutes. Samples were prepared as described in supplementary text prior to analysis by PCR.
Immunohistochemistry
Sections (2μm) of formalin fixed- paraffin embedded human tonsil tissue were stained with antibodies raised against Ki67, BIK or phospho-Smad 2 as described in supplementary materials and methods.
EMSA
Band shifts were performed as described40 using the ds oligos described in supplementary materials and methods. A total of 5μg of nuclear extract derived from the appropriate cell type and condition was incubated with radiolabelled oligonucleotide probes. For supershifts, anti-Smad3 (Zymed) and anti-Smad4 (Santa Cruz) were also included.
Supplementary Material
Acknowledgements
We thank Colin Nixon for immunohistochemical staining; Brad Ozanne, John Wyke and Kevin Ryan for reviewing the manuscript, Ulf Klein and Ricardo Dalla-Favera for protocols on centroblast isolation and Ross Dickins and Scott Lowe for retroviral shRNAmir plasmids. L.C.S, D.O’B, D.D. and G.J.I. are supported by CRUK and an AICR fellowship to G.J.I. D.I. O’B and D.D. performed research, D.S. and L.J.C. collected patient material, C.D.G. supplied reagents, M.J.A designed initial research. L.C.S. and G.J.I. designed and performed research, analysed and interpreted data and drafted the manuscript. The authors have no conflicting interests.
Abbreviations
- BL
Burkitt’s Lymphoma
- GC
germinal centre
- RPA
RNase protection assay
- SBR
Smad binding region
- TMCs
total mononuclear cells
- FCM
flow cytometry
Footnotes
Supplementary information is available at Cell Death and Differentiation’s website
References
- 1.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 2.Schuster N, Krieglstein K. Mechanisms of TGF-beta-mediated apoptosis. Cell Tissue Res. 2002;307:1–14. doi: 10.1007/s00441-001-0479-6. [DOI] [PubMed] [Google Scholar]
- 3.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 4.Dong M, Blobe GC. Role of transforming growth factor-beta in hematologic malignancies. Blood. 2006;107:4589–96. doi: 10.1182/blood-2005-10-4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inman GJ, Allday MJ. Resistance to TGF-beta1 correlates with a reduction of TGF-beta type II receptor expression in Burkitt’s lymphoma and Epstein-Barr virus-transformed B lymphoblastoid cell lines. J Gen Virol. 2000;81:1567–78. doi: 10.1099/0022-1317-81-6-1567. [DOI] [PubMed] [Google Scholar]
- 6.Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi Y, Ohta H, Takemori T. Fas is required for clonal selection in germinal centers and the subsequent establishment of the memory B cell repertoire. Immunity. 2001;14:181–92. doi: 10.1016/s1074-7613(01)00100-5. [DOI] [PubMed] [Google Scholar]
- 8.van Eijk M, Defrance T, Hennino A, de Groot C. Death-receptor contribution to the germinal-center reaction. Trends Immunol. 2001;22:677–82. doi: 10.1016/s1471-4906(01)02086-5. [DOI] [PubMed] [Google Scholar]
- 9.Mizuno T, Zhong X, Rothstein TL. Fas-induced apoptosis in B cells. Apoptosis. 2003;8:451–60. doi: 10.1023/a:1025534223168. [DOI] [PubMed] [Google Scholar]
- 10.Opferman JT. Apoptosis in the development of the immune system. Cell Death Differ. 2008;15:234–42. doi: 10.1038/sj.cdd.4402182. [DOI] [PubMed] [Google Scholar]
- 11.Adams JM, Cory S. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr Opin Immunol. 2007;19:488–96. doi: 10.1016/j.coi.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George NM, Evans JJ, Luo X. A three-helix homo-oligomerization domain containing BH3 and BH1 is responsible for the apoptotic activity of Bax. Genes Dev. 2007;21:1937–48. doi: 10.1101/gad.1553607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inman GJ, Allday MJ. Apoptosis induced by TGF-beta 1 in Burkitt’s lymphoma cells is caspase 8 dependent but is death receptor independent. J Immunol. 2000;165:2500–10. doi: 10.4049/jimmunol.165.5.2500. [DOI] [PubMed] [Google Scholar]
- 14.Schrantz N, Bourgeade MF, Mouhamad S, Leca G, Sharma S, Vazquez A. p38-mediated regulation of an Fas-associated death domain protein-independent pathway leading to caspase-8 activation during TGFbeta-induced apoptosis in human Burkitt lymphoma B cells BL41. Mol Biol Cell. 2001;12:3139–51. doi: 10.1091/mbc.12.10.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doucet JP, Hussain A, Al-Rasheed M, Gaidano G, Gutierrez MI, Magrath I, et al. Differences in the expression of apoptotic proteins in Burkitt’s lymphoma cell lines: potential models for screening apoptosis-inducing agents. Leuk Lymphoma. 2004;45:357–62. doi: 10.1080/10428190310001595713. [DOI] [PubMed] [Google Scholar]
- 16.Wildey GM, Patil S, Howe PH. Smad3 potentiates transforming growth factor beta (TGFbeta )-induced apoptosis and expression of the BH3-only protein Bim in WEHI 231 B lymphocytes. J Biol Chem. 2003;278:18069–77. doi: 10.1074/jbc.M211958200. [DOI] [PubMed] [Google Scholar]
- 17.Ramjaun AR, Tomlinson S, Eddaoudi A, Downward J. Upregulation of two BH3-only proteins, Bmf and Bim, during TGF beta-induced apoptosis. Oncogene. 2007;26:970–81. doi: 10.1038/sj.onc.1209852. [DOI] [PubMed] [Google Scholar]
- 18.Gordon J, Challa A, Levens JM, Gregory CD, Williams JM, Armitage RJ, et al. CD40 ligand, Bcl-2, and Bcl-xL spare group I Burkitt lymphoma cells from CD77-directed killing via Verotoxin-1 B chain but fail to protect against the holotoxin. Cell Death Differ. 2000;7:785–94. doi: 10.1038/sj.cdd.4400710. [DOI] [PubMed] [Google Scholar]
- 19.Degterev A, Lugovskoy A, Cardone M, Mulley B, Wagner G, Mitchison T, et al. Identification of small-molecule inhibitors of interaction between the BH3 domain and Bcl-xL. Nat Cell Biol. 2001;3:173–82. doi: 10.1038/35055085. [DOI] [PubMed] [Google Scholar]
- 20.Habens F, Lapham AS, Dallman CL, Pickering BM, Michels J, Marcusson EG, et al. Distinct promoters mediate constitutive and inducible Bcl-XL expression in malignant lymphocytes. Oncogene. 2007;26:1910–9. doi: 10.1038/sj.onc.1209979. [DOI] [PubMed] [Google Scholar]
- 21.Kang JS, Alliston T, Delston R, Derynck R. Repression of Runx2 function by TGF-beta through recruitment of class II histone deacetylases by Smad3. Embo J. 2005;24:2543–55. doi: 10.1038/sj.emboj.7600729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang YC, Piek E, Zavadil J, Liang D, Xie D, Heyer J, et al. Hierarchical model of gene regulation by transforming growth factor beta. Proc Natl Acad Sci U S A. 2003;100:10269–74. doi: 10.1073/pnas.1834070100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holder MJ, Knox K, Gordon J. Factors modifying survival pathways of germinal center B cells. Glucocorticoids and transforming growth factor-beta, but not cyclosporin A or anti-CD19, block surface immunoglobulin-mediated rescue from apoptosis. Eur J Immunol. 1992;22:2725–8. doi: 10.1002/eji.1830221037. [DOI] [PubMed] [Google Scholar]
- 24.Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, et al. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 25.Klein U, Tu Y, Stolovitzky GA, Keller JL, Haddad J, Jr., Miljkovic V, et al. Transcriptional analysis of the B cell germinal center reaction. Proc Natl Acad Sci U S A. 2003;100:2639–44. doi: 10.1073/pnas.0437996100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang A, Clark EA. Involvement of Bik, a proapoptotic member of the Bcl-2 family, in surface IgM-mediated B cell apoptosis. J Immunol. 2001;166:6025–33. doi: 10.4049/jimmunol.166.10.6025. [DOI] [PubMed] [Google Scholar]
- 27.Gillissen B, Essmann F, Graupner V, Starck L, Radetzki S, Dorken B, et al. Induction of cell death by the BH3-only Bcl-2 homolog Nbk/Bik is mediated by an entirely Bax-dependent mitochondrial pathway. Embo J. 2003;22:3580–90. doi: 10.1093/emboj/cdg343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimazu T, Degenhardt K, Nur EKA, Zhang J, Yoshida T, Zhang Y, et al. NBK/BIK antagonizes MCL-1 and BCL-XL and activates BAK-mediated apoptosis in response to protein synthesis inhibition. Genes Dev. 2007;21:929–41. doi: 10.1101/gad.1522007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi Y, Cerasoli DM, Dal Porto JM, Shimoda M, Freund R, Fang W, et al. Relaxed negative selection in germinal centers and impaired affinity maturation in bcl-xL transgenic mice. J Exp Med. 1999;190:399–410. doi: 10.1084/jem.190.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathai JP, Germain M, Marcellus RC, Shore GC. Induction and endoplasmic reticulum location of BIK/NBK in response to apoptotic signaling by E1A and p53. Oncogene. 2002;21:2534–44. doi: 10.1038/sj.onc.1205340. [DOI] [PubMed] [Google Scholar]
- 31.Subramanian T, Vijayalingam S, Lomonosova E, Zhao LJ, Chinnadurai G. Evidence for involvement of BH3-only proapoptotic members in adenovirus-induced apoptosis. J Virol. 2007;81:10486–95. doi: 10.1128/JVI.00808-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Real PJ, Sanz C, Gutierrez O, Pipaon C, Zubiaga AM, Fernandez-Luna JL. Transcriptional activation of the proapoptotic bik gene by E2F proteins in cancer cells. FEBS Lett. 2006;580:5905–9. doi: 10.1016/j.febslet.2006.08.088. [DOI] [PubMed] [Google Scholar]
- 33.Amanna IJ, Dingwall JP, Hayes CE. Enforced bcl-xL gene expression restored splenic B lymphocyte development in BAFF-R mutant mice. J Immunol. 2003;170:4593–600. doi: 10.4049/jimmunol.170.9.4593. [DOI] [PubMed] [Google Scholar]
- 34.Coultas L, Bouillet P, Stanley EG, Brodnicki TC, Adams JM, Strasser A. Proapoptotic BH3-only Bcl-2 family member Bik/Blk/Nbk is expressed in hemopoietic and endothelial cells but is redundant for their programmed death. Mol Cell Biol. 2004;24:1570–81. doi: 10.1128/MCB.24.4.1570-1581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z, Spittau B, Behrendt M, Peters B, Krieglstein K. Human TIEG2/KLF11 induces oligodendroglial cell death by downregulation of Bcl-X(L) expression. J Neural Transm. 2007;114:867–75. doi: 10.1007/s00702-007-0635-6. [DOI] [PubMed] [Google Scholar]
- 36.Hennino A, Berard M, Krammer PH, Defrance T. FLICE-inhibitory protein is a key regulator of germinal center B cell apoptosis. J Exp Med. 2001;193:447–58. doi: 10.1084/jem.193.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arena V, Martini M, Luongo M, Capelli A, Larocca LM. Mutations of the BIK gene in human peripheral B-cell lymphomas. Genes Chromosomes Cancer. 2003;38:91–6. doi: 10.1002/gcc.10245. [DOI] [PubMed] [Google Scholar]
- 38.Spender LC, Whiteman HJ, Karstegl CE, Farrell PJ. Transcriptional cross-regulation of RUNX1 by RUNX3 in human B cells. Oncogene. 2005;24:1873–81. doi: 10.1038/sj.onc.1208404. [DOI] [PubMed] [Google Scholar]
- 39.Dickins RA, Hemann MT, Zilfou JT, Simpson DR, Ibarra I, Hannon GJ, et al. Probing tumor phenotypes using stable and regulated synthetic microRNA precursors. Nat Genet. 2005;37:1289–95. doi: 10.1038/ng1651. [DOI] [PubMed] [Google Scholar]
- 40.Inman GJ, Nicolás FJ, Hill CS. Nucleocytoplasmic shuttling of Smads 2, 3 and 4 permits sensing of TGF-β receptor activity. Mol. Cell. 2002;10:283–294. doi: 10.1016/s1097-2765(02)00585-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.