Abstract
In order to investigate the properties of water motion within and around brain tumors as a function of tumor growth, longitudinal diffusion tensor imaging (DTI) was carried out in a rat brain glioma (C6) model. As tumors grow in size, significant anisotropy of water diffusion was seen both within and around the tumor. The tissue water surrounding the tumor exhibited high planar anisotropy, as opposed to linear anisotropy normally seen in white matter, indicating that cells were experiencing stress in a direction normal to the tumor border. When tumors were sufficiently large, significant anisotropy was also seen within the tumor due to longer range organization of cancer cells within the tumor borders. These findings have important implications for diffusion-weighted MRI experiments studying tumor growth and response to therapy.
Keywords: DTI, cancer, glioma, rat
INTRODUCTION
Diffusion-weighted MRI (DWMRI) allows non-invasive measurement of the translational motion of water in living tissue. Because water diffusion is sensitive to the tissues cellular and sub-cellular architecture and integrity, DWMRI is being used to investigate a variety of diseases. In the area of cancer, DWMRI has been applied to evaluate tumors response to therapy in a number of preclinical studies in animal models (1–10) and in clinical trials (11–13). The working hypothesis in these studies is that the microscopic diffusion of water will be sensitive to cellular changes brought about by effective therapy before any detectable change in the size of the tumor. DWMRI is also being carried out to attempt to stage tumors and more accurately define their border (14–20). While contrast enhanced (CE) and T2-weighted MRI remain the conventional ways to identify the presence and extent of brain tumors, they often do not show the full extent of tumors. Consequently, DWMRI methods are being developed to detect the presence of tumor boundaries that may be different from those seen by more conventional methods (21–24). Because of the high clinical importance of these efforts, a more thorough understanding of the diffusion of water within and around tumors is warranted. In this report, we present a longitudinal diffusion tensor imaging (DTI) study of a common rat glioma model. Animal models of brain cancer allow DWMRI studies to be carried out before and after the implantation of tumors, allowing the diffusion properties of brain water to be studied in the same animals before and after the cancer has affected brain tissue.
There were two aims to the present study. The first was to determine whether the diffusion properties of water in tissue surrounding brain tumors were affected by tumor growth. If the presence of cancer cells change the macroscopic organization of cells in the surrounding area, it is possible that the translational motional properties of water in this area will be altered. Changes in the mean diffusivity of the tissue water as well as the anisotropic character of water movement in the surrounding tissue could be expected.
A second goal of this work was to determine whether the diffusion of water within a rat glioma brain tumor is isotropic. It is sometimes assumed that diffusion of water within solid tumors is isotropic, i.e., there is no macroscopic cellular organization that would impart anisotropy to the movement of water. If this is the case, then diffusion measurements made in any one direction will reflect the average diffusivity within the tumor. Practically, this allows reduction in the number of measurements made when trying to measure the apparent diffusion coefficient (ADC) which can be helpful in clinical settings. However, if this is not the case, then diffusion measurements must take care to account for existing anisotropy.
METHODS
Rats and Tumor Implantation
All animal studies were performed under protocols approved by The University of Arizona Institutional Animal Care and Use Committee. Six female adult Wistar rats (200–250 g in body weight) were used in this study. Rat glioma cells (C6) were obtained from the American Type Culture Collection (ATCC, Rockville, MD) and routinely cultured in Dulbecco’s modified Eagles medium (DMEM, (Sigma, St. Louis, MO)) supplemented with 10% fetal bovine serum (FBS, (Omega Scientific, Inc., Tarzana, CA)). For implantation, rats were anesthetized with ketamine/xylazine (80/10 mg kg−1, respectively, intra peritoneal) and placed in a stereotaxic frame. After exposing the skull by a midline scalp incision, a 1 mm hole was drilled through the skull, 3.5 mm to the right of bregma. Ten microliters of DMEM containing 105 C6 cells were injected at a depth of 5.5 mm using a Hamilton syringe. The craniotomy was then sealed with dental cement and the scalp sutured.
MRI Protocol
The six tumor-bearing rats were imaged prior to surgical implantation of C6 cells and 3–5 times after inoculation, between days 3 and 12 of growth. Rats were anesthetized with a 1.5 % (v/v) isoflurane in O2 at 1L/min and placed into a homemade head holder with plexiglass bite-bar and ear-bars for optimal head immobilization. Body temperature was monitored with a fiber optic rectal temperature probe (Luxtron, Santa Clara, CA) and maintained at 37 °C using a circulating heated water bath.
All imaging was carried out at 4.7 T using a horizontal bore Bruker Biospec Avance® MRI instrument (Bruker, Karlsruhe, Germany) equipped with actively shielded gradients capable of 200 mT/m with rise times of 200 µs. A 20 mm inner diameter (ID) surface coil placed on top of the rat head was used for signal reception. The rat holder and surface coil were placed into a 72 mm ID volume resonator that was used for excitation. T2-weighted fast spin-echo images were obtained in axial and sagittal planes to be used as reference scout images in order to choose similar coronal slices in the same rat brain over time. Imaging parameters for these images were: TR = 4000 ms, echo spacing = 16 ms, echo train length = 8, effective echo time (TEeff) = 72 ms, number of averages= 1, acquiring 15 contiguous 1 mm slices with a 4 × 4 cm2 field of view (FOV), and acquisition matrix = 256 × 256. Afterward, DTI was carried out in the coronal plane using a diffusion-weighted radial spin-echo pulse sequence (25). Six contiguous 1 mm thick sections were imaged using the following parameters: TR = 2000 ms, TE = 56 ms, matrix size = 256 × 256 (data points along a radial line × number of radial lines), FOV = 4 × 4 cm2, and number of averages = 3. A total of seven image sets were collected, one without diffusion weighting (T2-weighted, b=0 s/mm2) and six with diffusion weighting (b = 1065 s/mm2, Δ = 25 ms, δ = 9 ms) along six non-colinear directions (26) using a maximum diffusion gradient strength of 120 mT/m. The total scan time for each animal at each time point was approximately 3 hours.
Diffusion-weighted radial MRI data were reconstructed using a magnitude filtered back projection reconstruction (27) onto a 256 × 256 image matrix. The three rank-ordered principal diffusivities of the diffusion tensor (λ1, λ2 and λ3) were calculated from the diffusion weighted images using standard algorithms (28, 29). From these principal diffusivities, the mean diffusivity 〈λ〉, or apparent diffusion coefficient (ADC), was calculated as their arithmetic average and the fractional anisotropy, FA, was calculated according to (30)
and has values between 0 (isotropic diffusion) and 1.0 (diffusion restricted to a single direction). Planar anisotropy (CP), and linear anisotropy (CL) coefficients were also calculated according to
which describe the shape of the ellipsoid describing diffusion (31, 32). Higher values of CP are obtained in voxels that possess two directions of diffusivity that are higher than the third direction (oblate spheroid). CL will be high in voxels exhibiting one principal diffusivity that is higher than the other two (prolate spheroid). Voxels with equal diffusivities (isotropic, spherical) will have low values of both CP and CL. Directional encoded color (DEC) maps use color to designate the direction of the eigenvectors (ε1, ε2, ε3) of the corresponding principal diffusivities. In the DEC maps, intensities were scaled by FA value and pixels with FA < 0.3 were set to zero. All image reconstruction and parameter calculations were carried out using programs written in Interactive Data Language (IDL, Research System, Boulder, CO).
With careful placement of the rats in the magnet and careful planning of sections of interest, it was possible to obtain similar image sections over the 12 days of the study. However, because of subtle variability, parameter maps obtained on different days were not compared on a pixel by pixel basis, but through region of interest (ROI) analysis. ROIs were manually drawn on individual slices that contained fully volumed tissue of interest. To assess anisotropy in the ring of tissue surrounding the tumor, ROIs were drawn directly on FA maps calculated from images through the center of the tumor. Statistical significance for differences in FA in the ring of tissue surrounding the tumor and tissue farther away, or pre tumor tissue, was determined using a two-tailed Student t-Test. For white matter analysis (Fig. 7), ROIs were manually drawn on T2-weighted images, because of the ease of selecting anatomical structures in these images, and the ROIs were directly applied to FA maps. Statistical analysis of these data was carried out using 2-way ANOVA where the first factor was days after implantation and the second factor was side of the brain (tumor side or contralateral side).
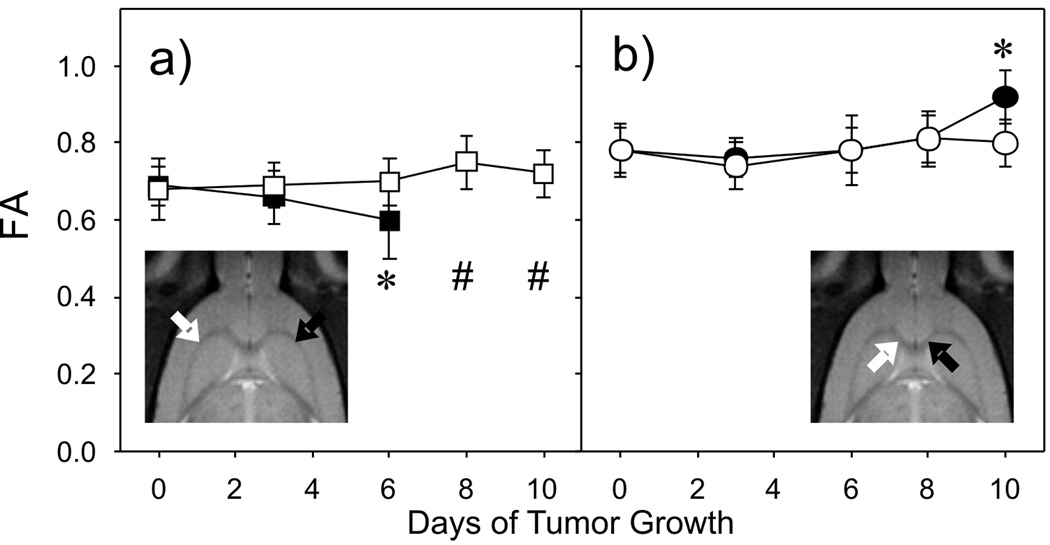
Fig. 7.
Fractional anisotropy measured in regions of white matter as a function of growth. FA values are from regions of interest in the external capsule (a) and the corpus callosum (b) from the tumor-bearing side of the brain (filled symbols) and the contralateral side (hollow symbols) as indicated in the inset T2-weighted images. Error bars correspond to the standard deviations of the FA values for each ROI. * Statistically different between sides and Day 0 values. # No values were available due to significant tumor infiltration.
Histology
Following the final imaging experiment, rats were euthanized by intravenous injection of sodium pentobarbital and their brains were removed and placed in formalin in preparation for histopathology. After fixation, brains were paraffin-embedded, sectioned (3 adjacent 5 µm slices every 500 µm through out the entire brain), and stained using both Hematoxylin and Eosin (H&E) and Luxol Fast Blue (LFB) with Hematoxylin as a counter stain. LFB stains myelinated fibers blue, neurophils pink, and nerve cells purple. Hematoxylin was added to the LFB to be able to localize tumor cells.
RESULTS
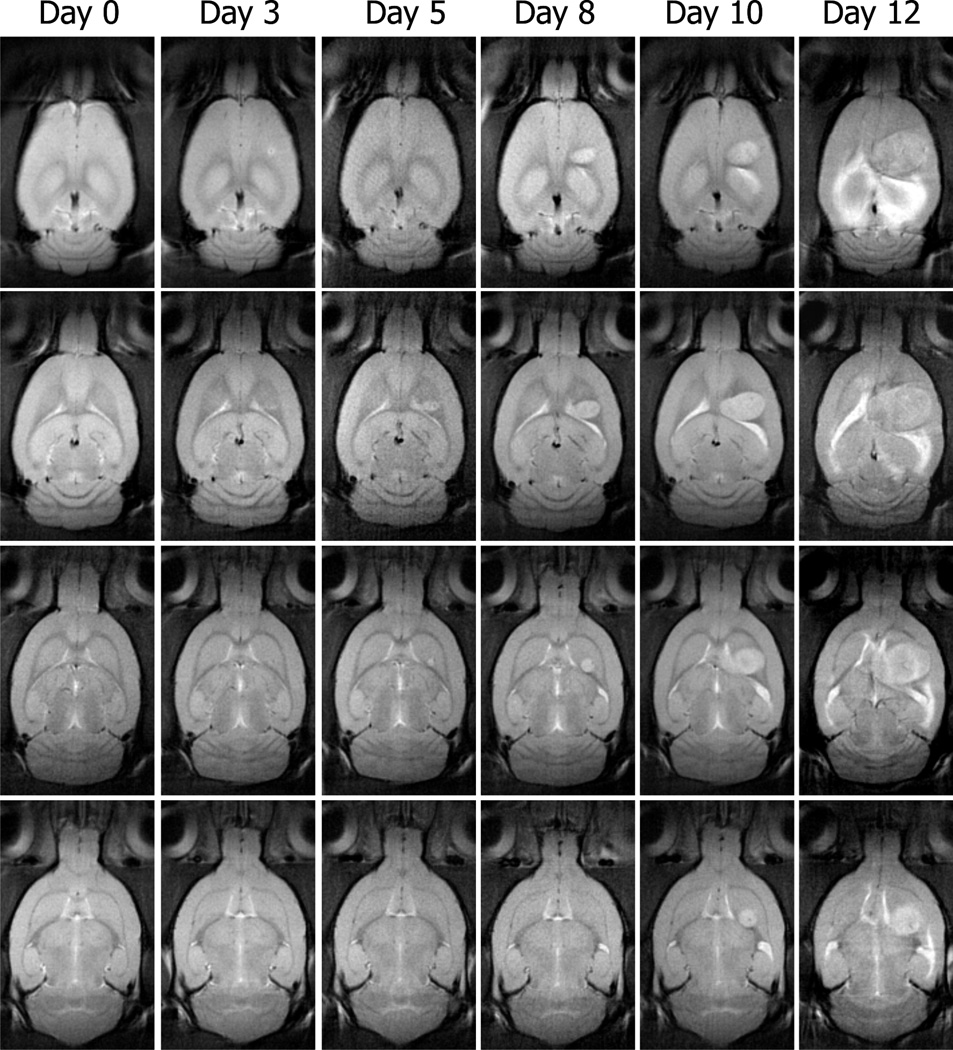
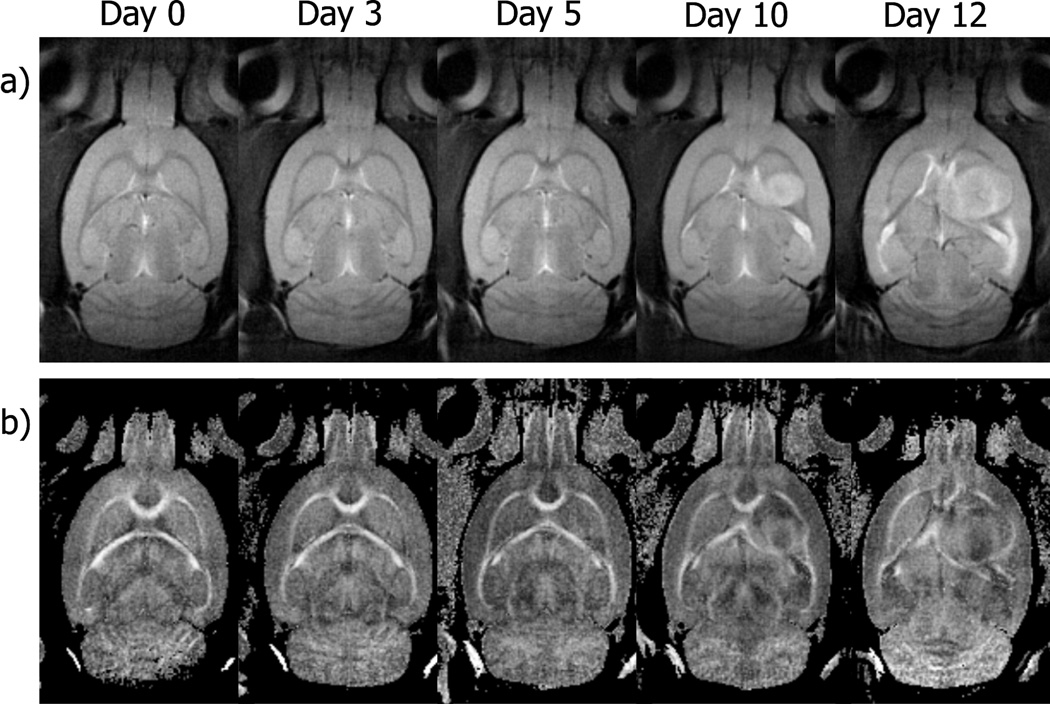
Representative T2-weighted axial images of rat brain, obtained before and after implantation of tumor cells, are shown in Fig. 1. The images shown are four contiguous 1 mm thick slices from the center of a six slice volume. The similarity of each image slice over time demonstrates the ability to image nearly identical sections of the brain over time. While our protocol did not allow a strict pixel to pixel correspondence from one imaging time to another it was adequate to monitor morphological changes in the same tissue regions over time. By day three after implantation, there is only a slight elevation of signal intensity observable at the injection sight and by day five, the tumor is readily visible in at least two of the sections (middle rows in Fig. 1). By day ten, the tumor is seen in all four sections shown in the figure and a mass effect is apparent as the ventricular space and white matter tracts are being displaced. These effects become exaggerated at day twelve where the tumor occupies greater that 50% of the right hemisphere of the brain. All tumors exhibited an exponential growth with a mean rate of 0.403 Day−1 (SD ±0.004).
Fig. 1.
T2-weighted (b = 0 s/mm2) images from four contiguous 1 mm coronal slices of a rat brain obtained before surgery (Day 0) and five times after implantation of C6 glioma cells (Day 3 to Day 12). At day three, only a slight hyperintensity is observed at the injection sight in the more dorsal sections. By day five the tumor is visible in two central sections and by day ten, the tumor is visible in all four slices. By day twelve after implantation the tumor occupied more than half of the right hemisphere.
T2-weighted and isotropically diffusion-weighted images, ADC and FA maps of a representative rat brain section obtained before and after implantation are shown in Fig. 2. As the tumor grows, there is a noticeable change in the diffusion properties of water within and surrounding the tumor. The most striking feature is the increase in the anisotropy (FA) of water diffusion in tissue surrounding the tumor compared to similar tissue farther away from the tumor (Fig. 2d). This increase in anisotropy was characteristic of all of the animals studied. There is also a decrease in the ADC values in these same areas (Fig. 2c), although it is less distinct than the changes in FA. There is also significant heterogeneity within the borders of the tumor at the later stages of growth. At days 8 and 10 following implantation, there are not only variations of signal intensity in the T2-weighted and diffusion-weighted images, but there are also substantial variations in ADC and FA within the tumor borders.
Fig. 2.
T2-weighted images (a), isotropically diffusion-weighted images (b), ADC maps (c) and FA maps (d) of a section of rat brain in the area of the tumor implantation site as a function of time. Images were obtained before (Day 0) and on days 3, 6, 8, and 10 after inoculation of tumor cells in the right caudate nucleus. On a pixel by pixel basis, the diffusion tensor was calculated using a reference image at b = 0 s/mm2 (a) and six images at b = 1065 s/mm2 along six hexahedral encoded diffusion directions. From these directional encoded diffusion-weighted images, the isotropically diffusion-weighted image was calculated as their geometric average. Changes in the translational motion of water within and around the tumor can be monitored via the ADC and FA maps as a function of tumor growth. Note the low values of ADC and high values of anisotropy surrounding the tumor at days 6–10.
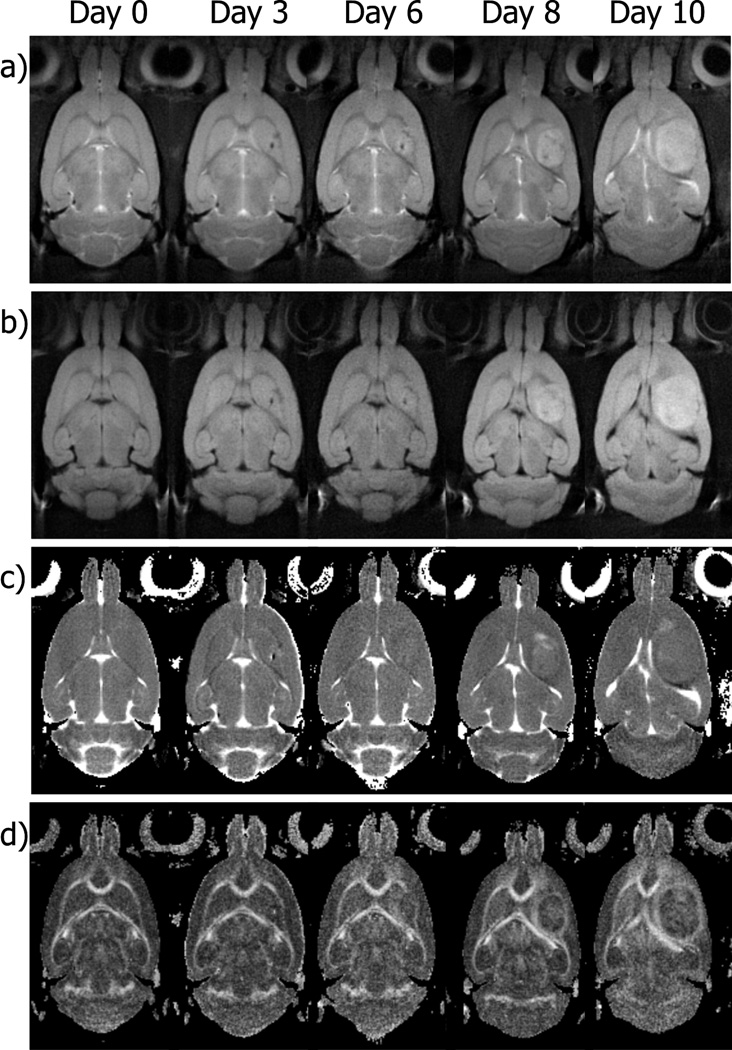
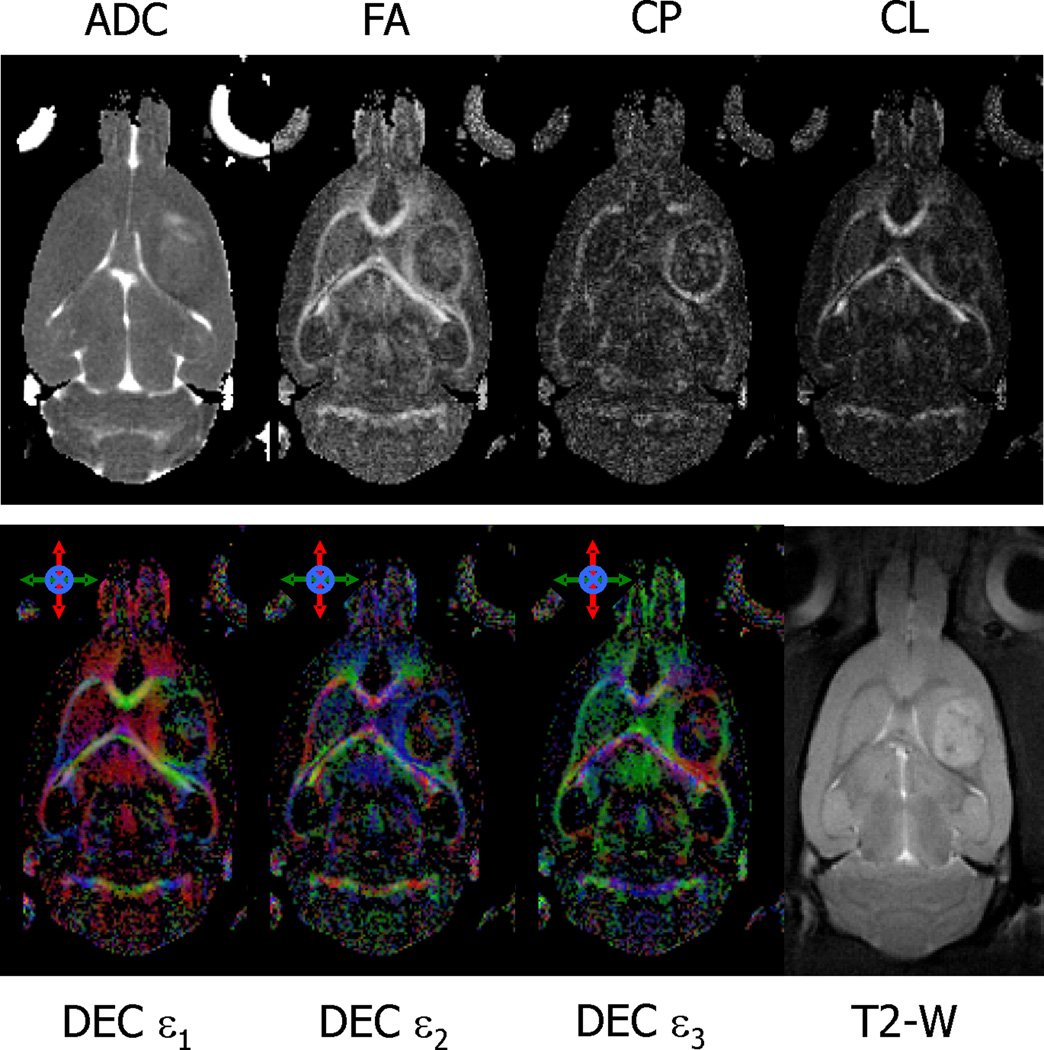
The change in diffusion properties of water in tissue surrounding the tumor is shown in more detail in Fig. 3. Maps of ADC, FA, CP and CL from a section of tissue through the center of a C6 tumor are shown in the top row of Fig. 3. Directional encoded color maps indicating the direction of the three principal eigenvectors (DEC ε1, ε2, ε3) are shown in the bottom row of Fig. 3, along with the corresponding T2-weighted image included for reference. All maps were generated from images obtained at day 8 after implantation. The FA map demonstrates high water diffusion anisotropy in tissue surrounding the tumor. The mean FA within an approximate 300 µm thick ring surrounding the tumor, corresponding to the visible hyperintensity on the FA maps, was significantly higher (0.47 ± 0.09, mean ± STD) compared with a mean FA of 0.27 (SD, 0.07) for a region in the cortex 600 µm away from the edge of the tumor (p<0.00001). The mean FA for that same area before the implantation of the glioma cells was 0.22 (SD, 0.06). The ADC map indicates a slight decrease of the mean diffusivity of water in the same area of tissue surrounding the tumor that exhibits high anisotropy (ADC = 0.692 ± 0.055 µm2/ms) compared with the value measured farther away from the tumor edge (ADC = 0.721 ± 0.047 µm2/ms) which could be due to an increased cellularity or changes in cell geometry (see discussion). Maps of CP and CL indicate that the region of tissue adjacent to the tumor border demonstrate high planar anisotropy compared to linear anisotropy. This was true for all animals studied and is in contrast to large white matter tracts, which typically exhibit higher values of CL compared to CP (see Fig. 3). The DEC maps use color to designate the direction of the eigenvectors of the diffusion tensor. In the tissue surrounding the tumor, the direction of the first (or major) eigenvector is consistently in a direction parallel to the surface of the tumor, i.e. moving from the top of the tumor in a clockwise direction around the tumor, the color changes from red to green to red to green and back to red. The direction of the second eigenvector is also parallel to the surface of the tumor, i.e. a constant blue color. The direction of the third eigenvector, on the other hand, is always in a direction perpendicular to the surface of the tumor. These results, common to all animals studied, indicate that water motion is greatest in a direction oriented tangentially to the edge of the tumor. That is, water can move more easily parallel to the surface of the tumor than it can perpendicular to it.
Fig. 3.
Representative images and parametric DTI maps of a rat brain acquired at day 8 of tumor growth. The CP and CL maps indicate relative amounts of planar and linear anisotropy, respectively. The colors in the directional encoded color, DEC, maps refer to the direction of the three principal diffusion directions (ε1, ε2, ε3) and are color coded as follows: red = rostral-caudal, green = right-left, and blue = dorsal-ventral. A T2-21 weighted image is included for anatomical reference. In the tissue surrounding the tumor, there is a slight decrease in the mean diffusivity and increase in anisotropy as seen in ADC and FA maps, respectively. This anisotropy surrounding the tumor also exhibits higher values of CP, and lower values of CL, indicating a planar geometry to water diffusion, i.e., water motion is restricted in one direction more than the other two orthogonal directions. This is also seen in the DEC maps where the two major diffusion directions (ε1, ε2) are parallel to the surface of the tumor and the direction of the lowest diffusion direction (ε3) is perpendicular to the surface.
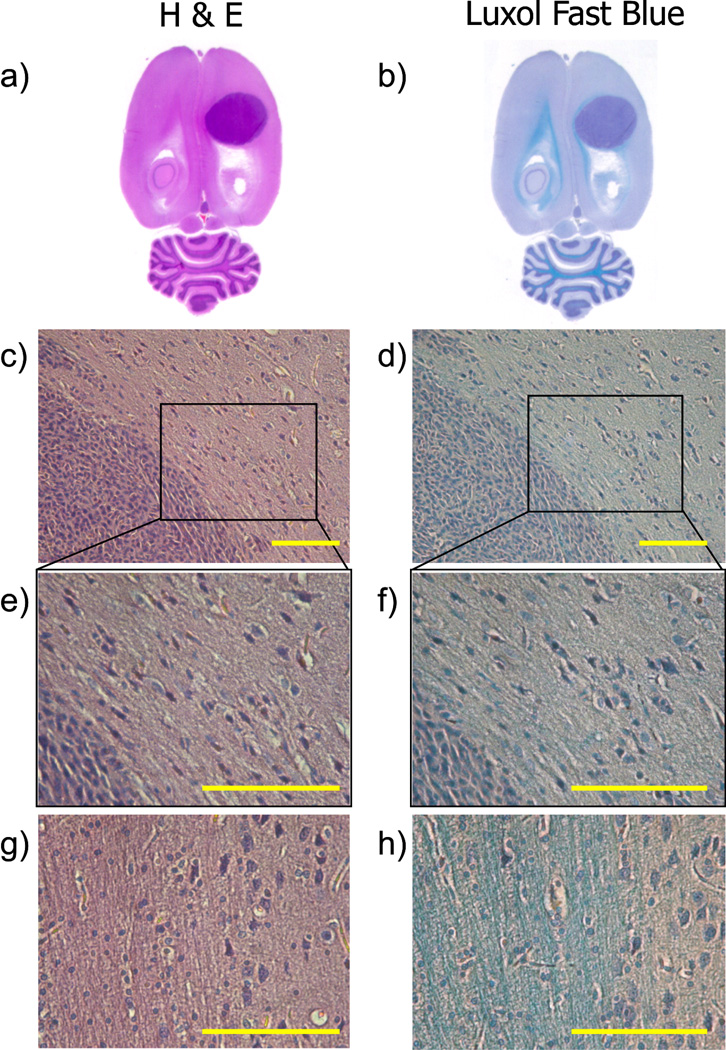
Histological sections of a rat brain stained with H&E and Luxol Fast Blue (LFB) are shown at different magnifications in Fig. 4. The presence of the tumor is easily seen in both H&E and LFB staining as shown in Figs. 4a and 4b, respectively. LFB staining is helpful for visualizing white matter and can be seen as the bright blue color in Fig. 4b. A magnified (20×) region of the edge of the tumor is shown in Figs. 4c and 4d. The high cellularity and dense packing of cell in the C6 tumor is readily apparent in both images. In the region of tissue immediately adjacent to the tumor border, cells are less dense than within the tumor, and appear somewhat elongated along the surface of the tumor. This elongation imparts some long range organization to the cells surrounding the tumor. This is more apparent in the higher magnification images (40×) shown in Figs. 4e and 4f where cells adjacent to the tumor have an asymmetric shape with a relatively long axis pointing along the tumor surface and a short axis in the direction normal to the surface. This asymmetry and organization is lost moving farther away from the surface of the tumor. Similar high magnification (40×) images of a region of white matter are shown in Fig. 4g and Fig. 4h for comparison. The bright blue color of myelin stain shown in Fig. 4h is not present in the tissue surrounding the tumor (Fig. 4f), indicating that the organized tissue immediately surrounding the tumor is not simply white matter adjacent to the tumor.
Fig. 4.
Histological slide of a rat brain at its endpoint stained with hematoxylin and eosin (a, c, e, and g), and luxol fast blue with hematoxylin (b, d, f, and h). Tumor cells are stained purple with the hematoxylin and white matter tracks are stained blue with LFB. The tumor is easily distinguished in the whole brain histology sections (a, b). A magnified (20×) region of the edge of the tumor (c, d) shows high cellularity in the tumor and also illustrates that cells near the outside of the tumor boundary are somewhat elongated along the surface of the tumor. This feature is more evident in the higher magnification (40×) images of the same region (e, f). Magnified images (40×) of a region of the brain containing white matter tracks are included in panels g and h for reference. Scale bars in panels c–h represent the in-plane pixel resolution used in DTI experiments (150 microns).
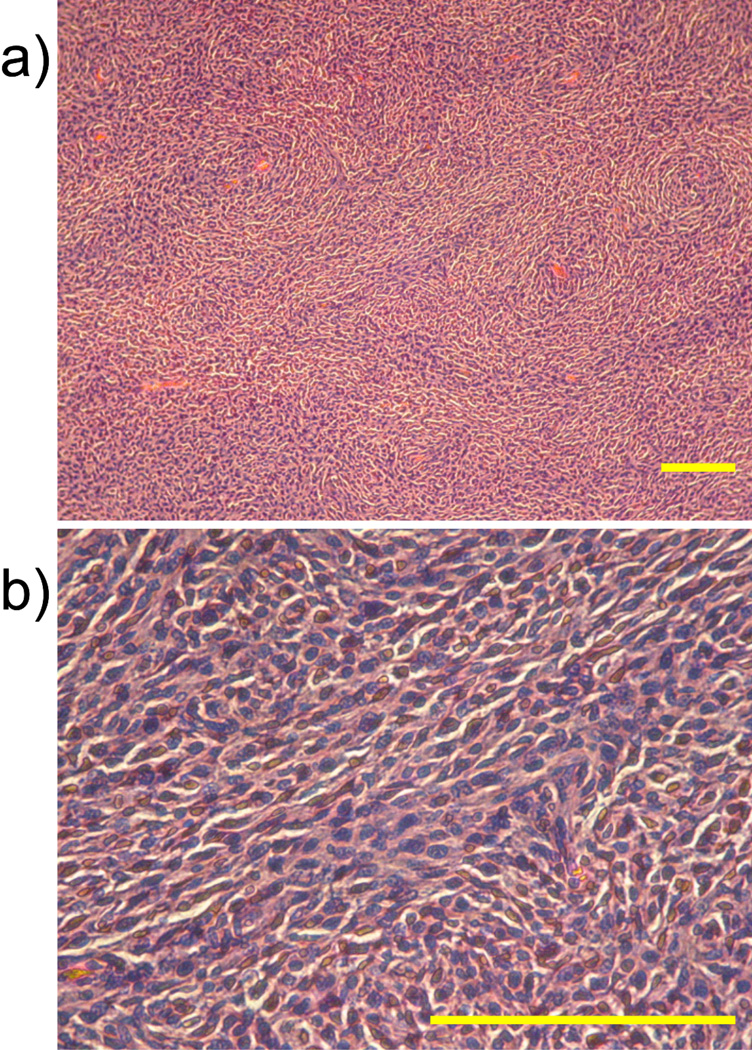
An example of the anisotropic nature of tissue within the tumor borders is shown in Fig. 5. T2-weighted images and FA maps of a section through the middle of a tumor are shown as a function of time following implantation. Not only are there high values of FA surrounding the tumor, but there is heterogeneity of anisotropy seen within the tumor. Similar results can also be seen in Fig. 2 and Fig. 3. Slides of H&E stained tissue obtained from within the tumor shown in Fig. 5 are shown in Fig. 6 at two different magnifications. While not all cells within the tumor are aligned in a particular direction, “rivers” of cells seem to exist that impart some macroscopic orientation to the tissue. The bars in the images represent the in-plane pixel dimension of the DTI studies.
Fig. 5.
Anisotropy within the tumor boundaries as a function of growth. T2-weighted images (a) and FA maps (b) of a rat brain obtained before (Day 0) and on days 3, 5, 10, and 12 after tumor cells inoculation. As tumor grows, heterogeneity is observed within the tumor borders as seen by the variation in intensity in the T2-weighted images and FA maps (days 10 and 12).
Fig. 6.
H&E stained section obtained within a tumor at two different magnifications: 10× (a) and 40× (b). Cells within the tumor appear to have some longer range organization which is consistent with the non-zero anisotropy observed in DTI maps. Scale bars represent in-plane pixel resolution used in DTI experiments (150 microns).
To evaluate the effect of tumor growth on white matter anisotropy, FA values were measured as a function of time in white matter tracts nearby and far away from the tumor location. Values of FA from regions of interest (ROIs) in the external capsule (EC) adjacent to the tumor and in the contralateral side of an individual rat are plotted in Fig. 7a. The values of FA in the EC contralateral to the tumor (left side) exhibit relatively high values that do not change significantly over time. Values of FA in the EC near the tumor (right side) start out with similar values as the contralateral side, but tend to decrease as tumor grows (F = 4.256, p = 0.0207). By day 6 of growth this reduction is statistically significant when compared to the contralateral side (F = 5.323, p = 0.0067) and also when compared to its baseline values on the tumor side (F = 4.256, p = 0.0207). Values in this region could only be measured through day 6 because after that time the tumor completely obscured the tract. FA values in the corpus callosum (CC) are plotted versus time in Fig. 7b. In this case the values of FA on the contralateral side remain statistically similar through day 10 of growth. The FA measured in the CC on the tumor side of the brain, however, is significantly higher than that of previous time points and higher than the FA measured in the CC on the contralateral side (F = 5.106, p = 0.0007). All rats showed results similar to those shown in Fig. 7, i.e. some areas exhibited 10 increases in anisotropy and others decreases. However, the exact location of decrease and increase depended on the location of the growth of the tumor.
DISCUSSION
A consistent and striking finding in this study was that the region of tissue immediately surrounding the tumor exhibited lower values of diffusivity (ADC) and higher values of diffusion anisotropy (FA). Histological results indicate that this region of tissue is comprised of geometrically asymmetric cells with longer dimensions parallel to the surface of the tumor and shorter axis normal to the border. We propose that the rapid growth in this tumor model exerts pressure on surrounding tissue in a direction normal to the surface of the tumor and that this pressure changes the average shape of cells in the immediate vicinity of the tumor from spherical to oblate spheroidal (i.e. compressed spheres). Farther away from the tumor this pressure decreases and this effect is relaxed. The decrease in diffusion (ADC), increase in anisotropy (FA), and higher values of planar anisotropy (CP) near the tumor border are all consistent with this interpretation. Average water diffusion would be reduced surrounding the tumor because tissue comprised of compressed cells would effectively have increased cellularity with more membranes per unit volume. Anisotropy would increase in this area because compression of cells in a direction normal to the surface of the tumor would reduce spacing of barriers perpendicular to the surface of the tumor, but not parallel to it. The observation of high values of planar anisotropy (as opposed to linear anisotropy) are consistent with spherical cells being compressed in a single direction, restricting diffusion parallel to the direction of compression, but not in two orthogonal directions perpendicular to it. The DEC maps representing principal directions of diffusion parallel to the surface of the tumor also indicate restricted diffusion perpendicular to the tumor border. In these studies, the imaging voxels were asymmetric with dimensions of 150 × 150 × 1000 µm3. This was to maintain adequate signal to noise, but also limit acquisition time. It is probable that there is averaging of anisotropy in the slice direction, and that within the tissues there is actually higher values of anisotropy than we are measuring. However, the fact that anisotropy is experimentally measured indicates a cellular organization that is maintained over these distances.
Another consistent feature of the results was that the ADC and FA values within the tumors were typically heterogeneous, indicating variability of long range cellular organization. That is, the tumor does not consist of a simple isotropic homogeneous cellular aggregate, but contains regions of more and less macroscopic cellular organization. This is evidenced by the heterogeneity in ADC and FA within the tumor borders as well as the histological results. For anisotropy to be measured from any given region of tissue, some amount of long range order of the barriers to water motion must exist over the dimensions of the voxel. To measure non-zero anisotropy (assuming adequate signal-to-noise ratio), macroscopic organization of cells would need to exist over these dimensions. Again, because of our relatively thick slices, there is likely some reduction of the true anisotropy that exists within the tumor borders. While the histological slides of the tumors do not yield information about out-of-plane tissue, they do show that there are regions of tissue that have multicellular organization in the order of several hundreds of microns.
The anisotropy measured in the corpus callosum and the external capsule of rats as a function of tumor growth exhibited interesting results. White matter regions on the opposite side of the brain from where the tumor was implanted showed high values of FA that remained relatively constant over the growth of the tumor. However, white matter regions on the tumor bearing side of the brain showed a variety of results. FA values in regions adjacent to the tumor showed a trend towards lower values of anisotropy with tumor growth, followed by a destruction of the white matter completely. If cancer cells infiltrate the surrounding tissue, it can be hypothesized that any anisotropy existing before the tumors invasion will be reduced by the infiltration of more randomly distributed cells. The reduction of FA values that was observed in the external capsule ROI of the tumor side of the brain would be consistent with such infiltration. Such a decrease in FA values in the corpus callosum of a patient with primary brain tumor was recently reported (33).
White matter regions farther away from the tumor, but on the same side of the brain, exhibited consistent values of anisotropy with tumor growth until the very last stages of growth where higher values of anisotropy were measured. By this time the tumor occupies more than half of the brain hemisphere and a lot of the structures surrounding the tumor have been altered or destroyed. The observed increase in anisotropy could be due to compression of that region of the corpus callosum via a mass effect of the tumor. This should increase the density of barriers to water motion across the fiber but not necessarily along it, making motion more anisotropic than it was without compression.
Although the methodology used in these studies of animal models are not directly translated to the clinic (e.g. the experimental time in our study was 3 hours), the findings could have significant implications for clinical and preclinical DWMRI experiments aimed at evaluating tumor growth and response to therapy. The observed heterogeneity of ADC and FA inside the tumor is not surprising and suggests that experiments aimed at measuring mean diffusivity inside tumors should be carried out in such a way to obtain isotropic diffusion weighting. This should not be a limitation in clinical DWMRI studies. Single-shot imaging techniques are inherently fast and there are also isotropic diffusion-weighting schemes that can be incorporated into virtually all DWMRI sequences (37–38). However, clinical DWMRI of the human brain is typically carried out at much lower resolution (several mm3) compared to the current study and effects of anisotropy will certainly be less noticeable. However, the presence of anisotropy, even if it exists for less than the full dimension of the imaging voxel, will affect DWMRI results. As clinical DWMRI pushes to higher resolution [25,38,39], these effects will be magnified.
The observed changes in ADC and FA outside the tumor also indicate that these features of water diffusion should be considered when using DWMRI to evaluate tumor borders and/or infiltration. As an alternative to conventional single exponential DWMRI analysis a diffusion heterogeneity index, α, obtained from a stretched exponential analysis of DWMRI data has been introduced (21). The parameter α was shown to be a marker of tumor invasion in rat brain (21) and to be relatively insensitive to the direction of the diffusion weighting in white matter of human brain (34). Because α was insensitive to diffusion direction in white matter of the human brain, it was concluded that measurements of α in a single direction could be used to investigate diffusion distributions in diseased brain. It should be noted that the anisotropy seen in large white matter tracts typically exhibit linear anisotropy while the anisotropy seen in the peritumoral gray matter, in the present study, demonstrates primarily planar anisotropy. It is not obvious how this difference would effect underlying diffusion distributions, but results from one type of anisotropic tissue may not be generally applicable to all types of anisotropic tissue.
There have also been recent clinical reports using DTI to evaluate the effects of tumor growth on white matter integrity (22, 33, 35–36). Our data (Fig 7) show that there could be a variety of results obtained in such cases. If the presence of tumor is compressing tissue, then higher values of anisotropy (compared to pre tumor values) may be expected. However, if the tumor is infiltrating or destroying white matter, then lower values of anisotropy could be seen. In an invasive tumor that invades tissue which was highly organized before invasion, e.g. white matter, a decrease in anisotropy would be expected. Alternatively, if the growth of a tumor compresses the tissue without direct invasion, then increases in anisotropy may be measured. It is possible that the presence of both infiltration and compression would have opposite effects on FA and cancel each other out. Results will certainly depend on the type of tumor, its invasiveness, its growth rate and its location. In the studies described here, the C6 tumors had a very rapid growth rate and a very strong and rapid mass effect was observed. It seems likely that a slower growing tumor, with less mass effect, would not tend to increase the anisotropy of local white matter as we have measured in the present study.
Finally, we note that the results we have presented here are for a specific model of rat brain glioma. The relationship to other brain tumor models has yet to be established. Depending on the degree of tumor encapsulation and/or tumor infiltration of surrounding tissue, the results could be significantly different. Additional longitudinal studies in a variety of implanted brain tumors in animal models are warranted and should aid the understanding and interpretation of the results of DWMRI in human brain cancer.
CONCLUSION
Longitudinal diffusion tensor imaging has been carried out in a rat brain glioma model to investigate the diffusion properties of water within and around the tumors. Significant diffusion anisotropy was observed within tumors that changes as a function of growth. Significant anisotropy was also seen surrounding the tumors, that was not present in the tissue before the presence of the tumor and which increased as the tumor grew. This anisotropy appears to come from pressure generated by the tumor forcing more spherical cells into more planar geometries. These results indicate that diffusion studies in brain tumors should account for anisotropy changes within and surrounding tumors. In white matter near the tumor, changes in diffusion anisotropy were variable with some white matter structures showing increased FA, presumably due to compression of the tissue by the tumor, and some showing decreased FA, presumably from infiltration of the tumor into the tissue.
ACKNOWLEGEMENTS
This study was supported by NIH grants EB000343 and CA88285, and the authors are grateful to Dr. Rexford Newbould for the development of the DTI analysis software.
REFERENCES
- 1.Chenevert TL, Mckeever PE, Ross BD. Monitoring early response of experimental brain tumors to therapy using diffusion magnetic resonance imaging. Clin Cancer Res. 1997;3(9):1457–1466. [PubMed] [Google Scholar]
- 2.Chenevert TL, Stegman LD, Taylor JMG, Robertson PL, Greenberg HS, Rehemtulla A, Ross BD. Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. J Natl Cancer Inst. 2000;92(24):2029–2036. doi: 10.1093/jnci/92.24.2029. [DOI] [PubMed] [Google Scholar]
- 3.Galons JP, Altbach MI, Gillian DP, Taylor CW, Gillies RJ. Early Increases in Breast Tumor Xenograft Water Mobility in Response to Paclitaxel Therapy Detected by Non-Invasive Diffusion Magnetic Resonance Imaging. Neoplasia. 1999;1(2):113–117. doi: 10.1038/sj.neo.7900009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jennings D, Hatton BN, Guo JY, Galons JP, Trouard TP, Raghunand N, Marshall J, Gillies RJ. Early response of prostate carcinoma xenografts to docetaxel chemotherapy monitored with diffusion MRI. Neoplasia. 2002;4(3):255–262. doi: 10.1038/sj.neo.7900225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemaire L, Franconi F, Saint-Andre JP, Roullin VG, Jallet P, Le Jeune JJ. High-field quantitative transverse relaxation time, magnetization transfer and apparent water diffusion in experimental rat brain tumour. NMR Biomed. 2000;13(3):116–123. doi: 10.1002/1099-1492(200005)13:3<116::aid-nbm616>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 6.Plaks V, Koudinova N, Nevo U, Pinthus JH, Kanety H, Eshhar Z, Ramon J, Scherz A, Neeman M, Salomon Y. Photodynamic therapy of established prostatic adenocarcinoma with TOOKAD: A biphasic apparent diffusion coefficient change as potential early MRI response marker. Neoplasia. 2004;6(3):224–233. doi: 10.1593/neo.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roth Y, Tichler T, Kostenich G, Ruiz-Cabello J, Maier SE, Cohen JS, Orenstein A, Mardor Y. High-b-value diffusion-weighted MR imaging for pretreatment prediction and early monitoring of tumor response to therapy in mice. Radiology. 2004;232(3):685–692. doi: 10.1148/radiol.2322030778. [DOI] [PubMed] [Google Scholar]
- 8.Poptani H, Puumalainen AM, Grohn OH, Loimas S, Kainulainen R, Yla-Herttuala S, Kauppinen RA. Monitoring thymidine kinase and ganciclovir-induced changes in rat malignant glioma in vivo by nuclear magnetic resonance imaging. Cancer Gene Ther. 1998;5(2):101–109. [PubMed] [Google Scholar]
- 9.Zhao M, Pipe JG, Bonnett J, Evelhoch JL. Early detection of treatment response by diffusion-weighted H-1-NMR spectroscopy in a murine tumour in vivo. Br J Cancer. 1996;73(1):61–64. doi: 10.1038/bjc.1996.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hakumaki JM, Poptani H, Puumalainen AM, Loimas S, Paljarvi LA, Yla-Herttuala S, Kauppinen RA. Quantitative H-1 nuclear magnetic resonance diffusion spectroscopy of BT4C rat glioma during thymidine kinase-mediated gene therapy in vivo: Identification of apoptotic response. Cancer Res. 1998;58(17):3791–3799. [PubMed] [Google Scholar]
- 11.Mardor Y, Roth Y, Ocherashvilli A, Spiegelmann R, Tichler T, Daniels D, Maier SE, Nissim O, Ram Z, Baram J, Orenstein A, Pfeffer R. Pretreatment prediction of brain tumors' response to radiation therapy using high b-value diffusion-weighted MRI. Neoplasia. 2004;6(2):136–142. doi: 10.1593/neo.03349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mardor Y, Roth Y, Lidar Z, Jonas T, Pfeffer R, Maier SE, Faibel M, Nass D, Hadani M, Orenstein A, Cohen JS, Ram Z. Monitoring response to convection-enhanced taxol delivery in brain tumor patients using diffusion-weighted magnetic resonance imaging. Cancer Res. 2001;61(13):4971–4973. [PubMed] [Google Scholar]
- 13.Theilmann RJ, Borders R, Trouard TP, Xia GW, Outwater E, Ranger-Moore J, Gillies RJ, Stopeck A. Changes in water mobility measured by diffusion MRI predict response of metastatic breast cancer to chemotherapy. Neoplasia. 2004;6(6):831–837. doi: 10.1593/neo.03343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunberg JA, Chenevert TL, Mckeever PE, Ross DA, Junck LR, Muraszko KM, Dauser R, Pipe JG, Betley AT. In-Vivo Mr Determination of Water Diffusion-Coefficients and Diffusion Anisotropy - Correlation with Structural Alteration in Gliomas of the Cerebral Hemispheres. Am J Neuroradiol. 1995;16(2):361–371. [PMC free article] [PubMed] [Google Scholar]
- 15.Tien RD, Felsberg GJ, Friedman H, Brown M, Macfall J. Mr-Imaging of High-Grade Cerebral Gliomas - Value of Diffusion-Weighted Echoplanar Pulse Sequences. Am J Roentgenol. 1994;162(3):671–677. doi: 10.2214/ajr.162.3.8109520. [DOI] [PubMed] [Google Scholar]
- 16.Krabbe K, Gideon P, Wagn P, Hansen U, Thomsen C, Madsen F. MR diffusion imaging of human intracranial tumours. Neuroradiology. 1997;39(7):483–489. doi: 10.1007/s002340050450. [DOI] [PubMed] [Google Scholar]
- 17.Maeda M, Sakuma H, Maier SE, Takeda K. Quantitative assessment of diffusion abnormalities in benign and malignant vertebral compression fractures by line scan diffusion-weighted imaging. Am J Roentgenol. 2003;181(5):1203–1209. doi: 10.2214/ajr.181.5.1811203. [DOI] [PubMed] [Google Scholar]
- 18.Desprechins B, Stadnik T, Koerts G, Shabana W, Breucq C, Osteaux M. Use of diffusion-weighted MR imaging in differential diagnosis between intracerebral necrotic tumors and cerebral abscesses. Am J Neuroradiol. 1999;20(7):1252–1257. [PMC free article] [PubMed] [Google Scholar]
- 19.Stadnik TW, Chaskis C, Michotte A, Shabana WM, van Rompaey K, Luypaert R, Budinsky L, Jellus V, Osteaux M. Diffusion-weighted MR imaging of intracerebral masses: Comparison with conventional MR imaging and histologic findings. Am J Neuroradiol. 2001;22(5):969–976. [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuruda JS, Chew WM, Moseley ME, Norman D. Diffusion-Weighted Mr Imaging of the Brain - Value of Differentiating Between Extraaxial Cysts and Epidermoid Tumors. Am J Neuroradiol. 1990;11(5):925–931. [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett KM, Hyde JS, Rand SD, Bennett R, Krouwer HGJ, Rebro KJ, Schmainda KM. Intravoxel distribution of DWI decay rates reveals C6 glioma invasion in rat brain. Magn Reson Med. 2004;52(5):994–1004. doi: 10.1002/mrm.20286. [DOI] [PubMed] [Google Scholar]
- 22.Lu S, Ahn D, Johnson G, Law M, Zagzag D, Grossman RI. Diffusion-tensor MR imaging of intracranial neoplasia and associated peritumoral edema: Introduction of the tumor infiltration index. Radiology. 2004;232(1):221–228. doi: 10.1148/radiol.2321030653. [DOI] [PubMed] [Google Scholar]
- 23.Provenzale JM, McGraw P, Mhatre P, Guo AC, Delong D. Peritumoral brain regions in gliomas and meningiomas: Investigation with isotropic diffusion-weighted MR imaging and diffusion-tensor MR imaging. Radiology. 2004;232(2):451–460. doi: 10.1148/radiol.2322030959. [DOI] [PubMed] [Google Scholar]
- 24.Tropine A, Vucurevic G, Delani P, Boor S, Hopf N, Bohl J, Stoeter P. Contribution of diffusion tensor imaging to delineation of gliomas and glioblastomas. J Magn Reson Imaging. 2004;20(6):905–912. doi: 10.1002/jmri.20217. [DOI] [PubMed] [Google Scholar]
- 25.Trouard TP, Theilmann RJ, Altbach MI, Gmitro AF. High-resolution diffusion imaging with DIFRAD-FSE (diffusion-weighted radial acquisition of data with fast spin-echo) MRI. Magn Reson Med. 1999;42(1):11–18. doi: 10.1002/(sici)1522-2594(199907)42:1<11::aid-mrm3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 26.Hasan KM, Parker DL, Alexander AL. Comparison of gradient encoding schemes for diffusion-tensor MRI. J Magn Reson Imaging. 2001;13(5):769–780. doi: 10.1002/jmri.1107. [DOI] [PubMed] [Google Scholar]
- 27.Trouard TP, Sabharwal Y, Altbach MI, Gmitro AF. Analysis and comparison of motion-correction techniques in diffusion-weighted imaging. Jmri-J Magn Reson Imaging. 1996;6(6):925–935. doi: 10.1002/jmri.1880060614. [DOI] [PubMed] [Google Scholar]
- 28.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 29.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36(6):893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- 30.Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. Diffusion tensor imaging: Concepts and applications. J Magn Reson Imaging. 2001;13(4):534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 31.Westin CF, Peled S, Gudbjartsson H, Kikinis R, Jolesz FA. Geometrical diffusion measures for MRI from tensor basis analysis; Abstract of the International Society of Magn Reson Med, 5th Annual Meeting; Vancouver, Canada; 1997. p. 1742. [Google Scholar]
- 32.Westin CF, Maier SE, Mamata H, Nabavi A, Jolesz FA, Kikinis R. Processing and visualization for diffusion tensor MRI. Med Image Anal. 2002;6(2):93–108. doi: 10.1016/s1361-8415(02)00053-1. [DOI] [PubMed] [Google Scholar]
- 33.Stieltjes B, Schlüter M, Didinger B, Weber MA, Hahn HK, Parzer P, Rexilius J, Konrad-Verse O, Peitgen HO, Essig M. Diffusion tensor imaging in primary brain tumors: reproducible quantitative analysis of corpus callosum infiltration and contralateral involvement using a probabilistic mixture model. Neuroimage. 2006;31(2):531–542. doi: 10.1016/j.neuroimage.2005.12.052. [DOI] [PubMed] [Google Scholar]
- 34.Bennett KM, Hyde JS, Schmainda KM. Water diffusion heterogeneity index in the human brain is insensitive to the orientation of applied magnetic field gradients. Magn Reson Med. 2006;56(2):235–239. doi: 10.1002/mrm.20960. [DOI] [PubMed] [Google Scholar]
- 35.Price SJ, Burnet NG, Donovan T, Green HAL, Pena A, Antoun NM, Pickard JD, Carpenter TA, Gillard JH. Diffusion tensor Imaging of brain tumours at 3 T: A potential tool for assessing white matter tract invasion? Clin Radiol. 2003;58(6):455–462. doi: 10.1016/s0009-9260(03)00115-6. [DOI] [PubMed] [Google Scholar]
- 36.Field AS, Alexander AL, Wu YC, Hasan KM, Witwer B, Badie B. Diffusion tensor eigenvector directional color imaging patterns in the evaluation of cerebral white matter tracts altered by tumor. J Magn Reson Imaging. 2004;20(4):555–562. doi: 10.1002/jmri.20169. [DOI] [PubMed] [Google Scholar]
- 37.Wong EC, Cox RW, Song AW. Optimized Isotropic Diffusion Weighting. Magn Reson Med. 1995;34(2):139–143. doi: 10.1002/mrm.1910340202. [DOI] [PubMed] [Google Scholar]
- 38.Sarlls J, Newbould R, Altbach MI, Gmitro A, Seeger J, Trouard TP. Isotropic Diffusion Weighting in Radial Fast Spin-Echo (Radial-FSE) MRI. Magn Reson Med. 2005;53:1347–1354. doi: 10.1002/mrm.20493. [DOI] [PubMed] [Google Scholar]
- 39.Pipe JG, Farthing VG, Forbes KP. Multishot Diffusion-Weighted FSE Using PROPELLER MRI. Magn Reson Med. 2002;47:42–52. doi: 10.1002/mrm.10014. [DOI] [PubMed] [Google Scholar]