Abstract
Cross-species transplantation (xenotransplantation) has immense potential to solve the critical need for organs, tissues and cells for clinical transplantation. The increasing availability of genetically engineered pigs is enabling progress to be made in pig-to-nonhuman primate experimental models. Potent pharmacologic immunosuppressive regimens have largely prevented T-cell rejection and a T-cell-dependent elicited antibody response. However, coagulation dysfunction between the pig and primate is proving to be a major problem, and this can result in life-threatening consumptive coagulopathy. This complication is unlikely to be overcome until pigs expressing a human ‘antithrombotic’ or ‘anticoagulant’ gene, such as thrombomodulin, tissue factor pathway inhibitor or CD39, become available. Progress in islet xenotransplantation has been more encouraging, and diabetes has been controlled in nonhuman primates for periods in excess of 6 months, although this has usually been achieved using immunosuppressive protocols that might not be clinically applicable. Further advances are required to overcome the remaining barriers.
Keywords: antibodies, antipig, coagulation, consumptive coagulopathy, genetically engineered, nonhuman primate, pig, xenotransplantation
There is a well-known shortage of organs and tissues from deceased human donors for the purposes of clinical organ and cell transplantation. Although there are over 100,000 patients waiting for a donor organ in the USA today, the number of donor organs that will become available during the current year will be less than 30,000. The discrepancy between available transplantable organs and patients on the waiting list grows each year. Despite successful introduction of living related donors for kidney and liver transplantation, marginal (extended criteria) deceased donors and donation after cardiac death, it remains exceedingly unlikely that human organs will fulfill the needs of those who require organ transplantation.
The situation is even worse for patients in need of cell transplantation, such as islet transplantation for those affected by diabetes mellitus. Many of the 2–3 million patients with Type 1 diabetes in the USA would benefit from pancreatic islet transplantation, but clearly the number of deceased human donors available each year (<7000) will not resolve this problem. Indeed, the potential supply of islets from human donors will never be sufficient to treat the millions of patients with diabetes.
What are the alternatives to human organs and cells? Despite recent advances in stem cell biology and tissue engineering, the clinical application of these techniques realistically remains in the distant future. A readily available animal source of organs, tissues and cells for clinical transplantation (cross-species transplantation or ‘xenotransplantation’) would resolve this problem. There have been a small number of clinical attempts to use animal organs for transplantation during the past century [1] and, in the majority of cases, nonhuman primates were the sources of organs. The results were generally poor, although a baboon liver functioned in a human recipient for 70 days and a chimpanzee kidney supported a good quality of life for almost 9 months. Clinical experience with pigs as the source of organs or cells has been very limited, and the results have been extremely poor.
Although nonhuman primates are phylogenetically closer than other species to humans, for a number of reasons they are not considered to be a suitable source of organs for clinical xenotransplantation [2]. The potentially high risk of crossspecies transmission of infection to humans, difficulties in breeding, organ size disparities and other impracticalities, as well as ethical issues have largely excluded them from further consideration [3]. The pig is now the preferred source animal and the advantages and disadvantages of this animal have been discussed previously [2-4]. The advantages are many, but the primate immune response to transplanted pig organs and cells has proven to be a significant barrier that as yet may not have been fully overcome [5].
In this review, we shall briefly summarize the immunobiology of xenotransplantation based on current data, and discuss the major remaining obstacles. We shall concentrate our attention on the most relevant experimental model, namely pig-to-nonhuman primate xenotransplantation.
Immunobiology of pig-to-nonhuman primate transplantation
Hyperacute rejection
In initial experiments, when wild-type pig organs were transplanted into nonhuman primates, the binding of natural (preformed) antibodies to the pig vascular endothelium initiated activation of the complement cascade (Figure 1) [6-10]. The endothelial cells of the graft responded to the immune activation by converting from an anticoagulant to a procoagulant phenotype [11,12]. The result of activation of the complement and coagulation systems was hyperacute rejection.
Figure 1. Summary of the major known immunologic barriers to pig-to-primate organ transplantation, as exemplified in the transplanted pig heart.
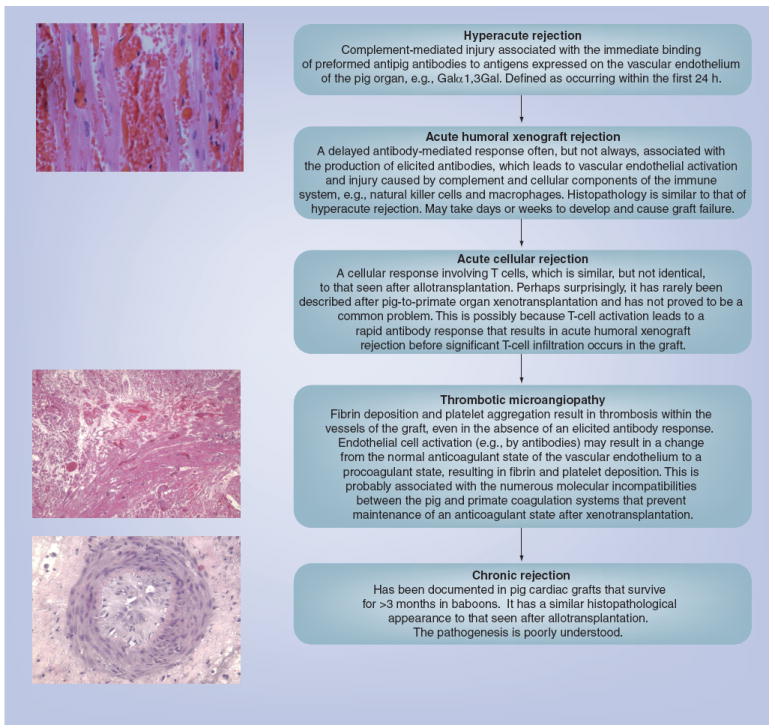
In hyperacute rejection, the graft develops microvascular thrombi, beginning in the venules. Occlusion of the vessels leads to rupture with interstitial hemorrhage and edema. Some cells of the innate immune system, such as neutrophils and macrophages, may be present. The appearance in acute humoral xenograft rejection is similar, although infiltration by cells of the innate immune system is more pronounced. Isolated acute cellular rejection is very rare, but T cells can be seen when thrombotic microangiopathy develops. The role of T cells in the development of thrombotic microangiopathy is uncertain and controversial. Reproduced with permission from [72].
Acute humoral xenograft rejection
When steps were taken to prevent hyperacute rejection (e.g., the depletion of antipig antibodies or complement from the nonhuman primate serum [7,13-22]) a delayed form of antibody-mediated rejection occurred, known variously as acute humoral xenograft rejection (AHXR), acute vascular rejection or delayed xenograft rejection (Figure 1) [23]. Natural antibody binding and complement activation resulted in vascular endothelial cell activation and injury caused by the complement and cellular components of the innate immune system. There is increasing evidence that primate neutrophils may be involved in pig endothelial cell activation [24-26], a topic that has been discussed previously by our group [27]. Natural killer (NK) cells play a role in AHXR [28-30], as do macrophages [31], but their exact importance remains unclear. AHXR may occur despite the administration of pharmacologic immunosuppressive agents, and is particularly seen following the development of a T-cell-dependent elicited antibody response.
Acute cellular rejection
Potent pharmacologic agents can largely prevent acute cellular rejection (i.e., T- and B-cell infiltration of the graft and T-cell activation) and a T-cell-dependent elicited antibody response, even though the T-cell response is believed to be stronger than the alloresponse [32-36]. This is possibly because T-cell activation leads to a rapid antibody response that results in AHXR before significant T-cell infiltration occurs in the graft. Acute cellular rejection is therefore typically not seen with intense immunosuppressive drug regimens [37-42]. Costimulation blockade agents, such as an antihuman CD154 monoclonal antibody, have been found to be particularly effective in preventing T-cell activation in the xenotransplantation setting [43].
Chronic rejection
In grafts that survive for more than a few weeks, features of chronic vasculopathy develop, similar to the chronic rejection seen in long-surviving allografts (Figure 1). The causative factors remain poorly understood.
Genetically engineered pigs
Most of the advances that have been made in this field have resulted from the introduction of genetically engineered pigs. The most significant advances to date have been the production of pigs expressing a human complement-regulatory protein (e.g., human decay-accelerating factor [CD55], membrane cofactor protein [CD46] or CD59 [44-48]) and pigs in which the gene for α1,3-galactosyltransferase has been knocked out (α1,3-galactosyltransferase gene-knockout [GTKO] pigs) [49-52].
The presence of a complement-regulatory protein on the surface of vascular endothelial cells in the pig largely protects the graft from hyperacute rejection. Of interest is the suggestion that overexpression of a pig complement-regulatory protein might be as effective as the expression of a human complement-regulatory protein in protecting the pig cell from lysis [53], although this theory has never been tested in an in vivo model.
The gene for α1,3-galactosyltransferase enables this enzyme to add Galα1,3Gal (Gal) oligosaccharides to various underlying glycoproteins and glycolipids in the pig [54,55]. Gal is the major target for human and nonhuman primate antipig antibodies [49,56,57] (reviewed in [58]), and its deletion from pigs has greatly reduced the incidence of hyperacute rejection of pig grafts in nonhuman primates [59-61]. GTKO pigs additionally transgenic for a human complement-regulatory protein provide increased benefit over each modification alone [62]. IgM, IgG and complement deposition in grafts is absent or less marked, and innate cellular infiltration has also been minimized.
Genetically engineered pig hearts placed heterotopically in baboons have functioned for periods of 3–6 months [59,60,63-65], life-supporting kidneys for periods approaching 3 months [38,39,61,66], livers for a matter of days [67,68] (reviewed in [69]), and lungs for a matter of hours [70,71]. Progress during the past 20 years or so can be illustrated by the prolongation of survival of pig hearts transplanted heterotopically into baboons using wild-type or genetically modified pigs and various immunosuppressive protocols (Figure 2) [72].
Figure 2. Progress in the results of pig heterotopic heart transplantation in baboons (1986–2005).
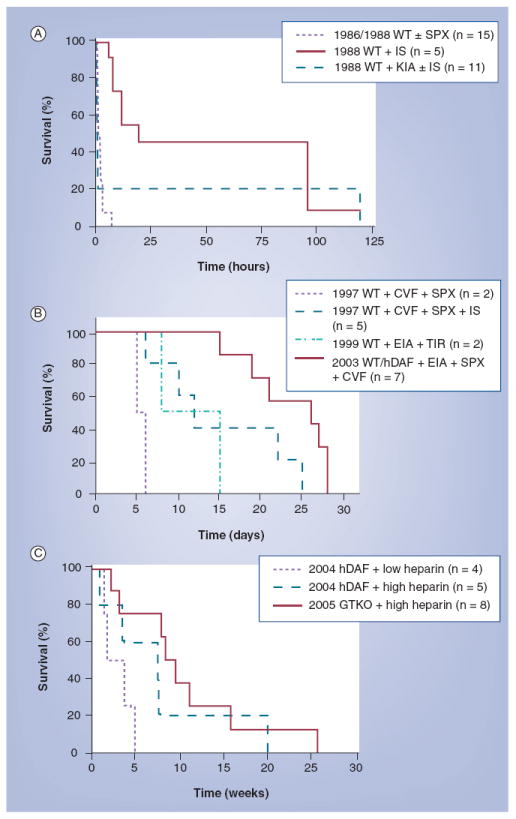
(A) Survival (in hours) of selected pig heterotopic heart grafts in baboons (1986–1996). (B) Survival (in days) of selected pig heterotopic heart grafts in baboons (1997–1999). (C) Survival (in weeks) of selected pig heterotopic heart grafts in baboons (2000–2005).
CVF: Cobra venom factor; EIA: Extracorporeal immunoadsorption; GTKO: α1,3-galactosyltransferase gene-knockout; hDAF: Pig transgenic for human decay-accelerating factor; IS: Pharmacologic immunosuppressive therapy; KIA: Prior pig kidney perfusion to deplete antipig antibodies; SPX: Splenectomy; TIR: Tolerance-inducing regimen; WT: Wild-type.
Reproduced with permission from [72].
Although the availability of GTKO pigs has been a major step forward, there are well-documented natural antibodies to nonGal antigens in humans and nonhuman primates [29,73-77], the nature of which remains unknown. Although there were reasons to believe that these may be directed to non-Gal oligosaccharides [78,79], recent data from our own center suggest that, with one exception, these antibodies are not directed to carbohydrate structures [Yeh Pet al., Manuscript In Preparation]. The exception are antibodies directed against N-glycolylneuraminic acid (NeuGc).
It has been known for some time that antibodies in humans directed to NeuGc may play a role in pig-graft destruction [80-84]. NeuGc is expressed on the vascular endothelium of all mammals with the exception of humans; chimpanzees also express this oligosaccharide. Although it may play a role when pig organs or cells are exposed to human serum, it cannot be a factor in the destruction of pig organs or cells after exposure to nonhuman primate serum. In pig-to-nonhuman primate transplantation models, therefore, other antigens must be the targets for anti-nonGal antibodies. These anti-non-Gal antibodies, whether directed to NeuGc or other antigens, are weaker and less destructive than anti-Gal antibodies, but nevertheless can be associated with hyperacute rejection or AHXR [41,85].
Genetic modifications to inhibit NK cell [85-88] and macrophage activity [89-91] are possible, but have not yet been tested in a pig-to-nonhuman primate model. Transgenic expression of HLA-E or HLA-G on porcine endothelial cells is known to inhibit NK cell cytotoxicity and adhesion [92,93], and HLA-E transgenic pigs have recently been produced [94]. The intraspecies incompatibility of the inhibitory interaction between CD47 and SIRP-α contributes to the phagocytosis of pig cells by primate macrophages [95,96].
Despite the absence of hyperacute rejection and classic AHXR, survival of pigorgan grafts in nonhuman primates is currently limited by either the development of a thrombotic microangiopathy [40,97-99] or a consumptive coagulopathy, or both [100-103]. These are clearly features of coagulation dysregulation between pig and primate, and this barrier has not yet been overcome. Following GTKO pig heart transplantation in baboons, thrombotic microangiopathy is the predominant feature, with subsequent consumptive coagulopathy in some cases [59,60]. However, after GTKO/CD46 pig kidney xenotransplantation, consumptive coagulopathy occurs relatively early in the absence of obvious features of thrombotic microangiopathy [Lin CC, Ekser B, Long Cet al., Manuscript In Preparation]. Following GTKO/CD46 pig liver xenotransplantation, thrombocytopenia develops within minutes and, although most coagulation parameters appear to remain within the normal ranges, the lack of platelets leads to spontaneous internal hemorrhage within days [68]. Pig lung xenotransplantation or ex vivo lung perfusion with human blood provides an accelerated sequence of events, as coagulation dysregulation occurs within minutes or hours [70,71].
Increasing experimental evidence suggests that the classic immune response is no longer the major problem, but physiologic incompatibilities between the coagulation systems of pig and primate are more problematic [102-112]. However, the immune response, particularly that of the innate immune system [27], may be playing a role in initiating this process.
Coagulation dysregulation
Despite considerable attention in recent years, the exact mechanisms by which coagulation disorders develop after xenotransplantation remain elusive. Previous reports suggested that consumptive coagulopathy is initiated by the expression of tissue factor (TF) in the porcine graft [113,114]. In response to the binding of xenoreactive antibody and/or activation by complement, endothelial cells in the graft are activated to increase TF activity and initiate intragraft thrombosis and consumptive coagulopathy [11,23].
During inflammation, type I activation of endothelial cells induces P-selectin and vascular leakiness of plasma proteins; this process takes 10–20 min. Type II activation of endothelial cells is triggered by the stimulation of TNF-α and IL-1, induces more effective leukocyte recruitment by synthesis of adhesion proteins, such as E-selectin and CD106 (VCAM-1), and is sustained for 6–24 h after cytokine-mediated activation. Type I and type II activations are believed to be associated with hyperacute rejection and AHXR, respectively [11]. The activated endothelial cells and the generated thrombin subsequently activate platelets, leukocytes and other inflammatory cells in the recipient, initiating a vicious cycle.
Recent in vitro studies at our center by Lin et al. have indicated that porcine aortic endothelial cells (PAECs) are able to induce human TF exposure on human platelets and monocytes through an immune response-independent pathway [115]. We have investigated this problem in vivo in pig-to-baboon kidney [Lin CC, Ezzelarab M, Shapiro Ret al., Manuscript In Preparation] and liver [Lin CC, Ekser B, Long Cet al., Manuscript In Preparation] transplantation models. For example, the rapid development of consumptive coagulopathy in a pig-to-baboon liver xenotransplantation model has been studied [Lin CC, Ekser B, Long Cet al., Manuscript In Preparation]. Using genetically modified pig liver transplantation into baboons, we observed that there is a massive loss of platelets from the circulation within minutes after reperfusion [68]. The development of trombocytopenia was accompanied by thrombin formation. Circulating platelets and peripheral blood mononuclear cells expressed functional TF and aggregated in the graft without the documented activation of donor endothelial cells (confirmed by negativity for P- and E-selectin, CD106 and TF expression on the porcine endothelial cells by immunofluorescence staining) [Lin CC, Ekser B, Long Cet al., Manuscript In Preparation]. Although there was a minimal measurable immune response (indicated by a lack of antibody and complement activity), consumptive coagulopathy still occurred. The severity and rapidity of thrombocytopenia were not alleviated by manipulation of the immune response (e.g., by prior depletion of complement by the administration of cobra venom factor). We therefore tentatively concluded that recipient TF initiated consumptive coagulopathy by a mechanism that is independent of the immune response.
These observations suggest that further manipulation of the immune response (with the increased risks of infection and other complications) will not completely overcome consumptive coagulopathy after xenotransplantation. Determination of the exact mechanism by which thrombotic microangiopathy and consumptive coagulopathy are initiated after xenotransplantation is important because it may enable further genetic modification in the pig or suggest therapy that might prevent them. The introduction of genes for human thrombomodulin, TF pathway inhibitor [116] or CD39 [117] have been suggested to overcome the coagulation incompatibilities between pig and primate.
Pig pancreatic islet xenotransplantation
In the field of porcine islet transplantation in diabetic nonhuman primates, the challenges are slightly different, and greater progress has been made.
Adult porcine islets do not express Gal [118,119], thus reducing the antibody-mediated response to them after transplantation into a primate. Fetal and neonatal islets do express Gal [120], and so GTKO pigs are likely to be advantageous. However, within purified adult pig islets remain fragments of vascular endothelial cells that express Gal, and it is possible that the immune response to these may be detrimental to survival of the surrounding islets. Therefore, it would seem advantageous to use GTKO pigs as the sources of all islets (fetal, neonatal and adult). Even though anti-Gal antibodies may not play a major role in islet graft rejection, it has been demonstrated that antibodies directed to unknown non-Gal antigens may be important [121,122].
When the islets are transplanted into the portal vein so that they reside within the liver (the current approach in clinical islet allotransplantation), there is a major loss of islets from what is known as the ‘immediate blood-mediated inflammatory reaction’ (IBMIR) (reviewed in [123]). Although IBMIR occurs following islet allotransplantation, it would appear to be of greater magnitude after pig islet xenotransplantation. It appears to be a nonspecific response to the presence of islets in the blood where, of course, they are normally not present. It involves both complement activation and activation of the coagulation system, and rapidly leads to destruction of a large number of islets either through complement activity or through ischemia following thrombus formation around the islets. The loss of islets is estimated to be in the region of 60–80%. However, there is also evidence that antibody-mediated complement activation may be playing a role [124].
If enough islets survive this attack – possibly a relatively small number – then normoglycemia may result. Pig islets that express the human complement-regulatory protein, CD46, appear to provide some protection from this response or from the antibody-mediated complement activation that occurs, but it is not yet certain how important CD46 expression is in contributing to the prolonged survival of CD46 pig islets reported in monkeys [125].
Even if sufficient islets remain viable after IBMIR to maintain a state of normoglycemia for a period of time, if the number of islets surviving is borderline, then islet function may fail and hyperglycemia will gradually return. It is currently unclear whether this slow loss of control of glycemia is related to immune system activity or just to physiologic ‘exhaustion’ of the islets.
T cells would appear to play a greater role in the rejection of pig islets than of pig organs. It would therefore appear to be even more important to suppress the T-cell response after pig islet xenotransplantation than after pig organ xenotransplantation. Fortunately, there are regimens that can do this. For example, at our own center, we have had encouraging results using a regimen consisting of induction therapy with antithymocyte globulin, and maintenance with an anti-CD154 monoclonal antibody and mycophenolate mofetil [125,126]. Others have had equally good results, but with more intensive immunosuppressive regimens [127,128].
An attempt is currently being made by several centers to reduce the intensity of the immunosuppressive therapy required and, in particular, to use agents that are clinically available at the present time or certainly will be in the near future. For example, at our own center, we are trying to replace anti-CD154 monoclonal antibody with another costimulation blockade agent, CTLA4-Ig. A regimen of antithymocyte globulin, CTLA4-Ig and mycophenolate mofetil would be clinically acceptable.
Immunological tolerance
The induction of immunological tolerance to the graft is the ultimate and ideal goal for xenotransplantation (and allotransplantation). Considerable efforts have been made to achieve this goal either by pig bone marrow transplantation (to induce mixed chimerism) (reviewed in [129]) or by pig thymus transplantation in the host [61,130]. After kidney allotransplantation, the induction of mixed chimerism, even if only transient, has been associated with the induction of tolerance to the graft in both nonhuman primate [131] and clinical models [132]. To date, however, neither of these approaches has been convincingly successful in models of xenotransplantation.
There is increasing interest in the potential role of T-regulatory cells (reviewed in [133]) and/or mesenchymal stem cells to induce a state of tolerance to a xenograft, but to date there has been very little exploratory work reported. The possibility of inducing B-cell tolerance in neonates, as has been achieved in ABO blood group-incompatible allografts [134,135], is also intriguing [136]. However, there are obviously a number of other barriers, such as thrombotic microangiopathy and consumptive coagulopathy, which need to be overcome before tolerance is likely to be induced.
In this review, we have not considered other areas of importance to clinical xenotransplantation. These include, first, the physiology of pig organ and cell grafts in primates and, second, the potential risk of infection with a pig microorganism that might be transferred to the recipient. These two topics will be very briefly discussed.
Pig organ function in primates
Even if the immunologic and coagulation barriers can be overcome, the question has been asked as to whether a pig organ will function satisfactorily in the primate bodily environment. Will the organ carry out all of the functions required of it (i.e., all of the functions of a native primate organ?). The physiological aspects of xenotransplantation have been reviewed relatively recently [137].
In summary, current evidence is that pig hearts function well in primates. Successful orthotopic life-supporting pig heart transplantation in baboons has been followed by satisfactory function for periods of up to 53 days [138,139]. The pig heart has been demonstrated to recover from an initial ischemic injury occurring during the transplant operative procedure [139].
Pig kidneys function adequately with one or two possible exceptions (e.g., handling of phosphate [140]). However, one major problem following pig kidney transplantation in nonhuman primates is the development of proteinuria, which can be considerable. This results in albuminemia with its accompanying complications, such as peripheral edema. Although this can be prevented or corrected by the continuous intravenous infusion of human albumin, this would clearly not be a realistic long-term therapeutic option in a patient with a pig kidney graft. Whether the proteinuria is related to the immune response or is simply a physiologic incompatibility remains uncertain. Our own observation that it develops rapidly (within hours) in the absence of significant antibody or complement deposition in the graft suggests that it may not be immune related.
Evidence for satisfactory function of the pig liver in a primate is limited and inconclusive. In addition to its detoxification functions, the liver synthesizes approximately 2000 different proteins, and it is unlikely that all the products of a pig liver will function adequately in a primate. However, we have evidence from our own studies that detoxification by a pig liver after orthotopic transplantation into a baboon is adequate, proteins are synthesized and that pig coagulation factors are produced that appear to function adequately in the primate [68]. If it is determined that one or two key or essential pig proteins do not function in the primate host, then it may be possible to genetically engineer the pig to produce the desired human protein.
The level of serum albumin in pigs is significantly lower than in primates. After pig liver transplantation, we observed that the albumin level falls from that seen in the baboon to that seen in the pig. The level can be maintained by the intravenous infusion of human albumin, but this again would be problematic if long-term albuminemia persisted.
Potential risk of transfer of a porcine microorganism to the human recipient (xenozoonosis)
The potential for the development of a xenozoonosis in the recipient of a pig graft (i.e., the potential for a porcine microorganism to cause infection in the recipient) has been of concern for a number of years [141-143]. These potential risks, particularly with regard to porcine endogenous retroviruses (PERV), are now considered to be much less significant than they were a few years ago [142-145], and a clinical trial would be deemed justified if there were a realistic possibility that the graft would be life-saving for the patient. Furthermore, activation of PERV can now be prevented by siRNA technology [146,147], although this is unlikely to be necessary. Nevertheless, largely because of the possibility of the transfer of a porcine-infectious microorganism, xenotransplantation will be highly regulated by national regulatory authorities, such as the US FDA. The likely regulatory requirements have recently been reviewed by Schuurman [148].
Clinical perspective
There are clearly problems that remain to be overcome before pig organs can be used in clinical transplantation, although pig islet transplantation is much closer to being translated into the clinic. As truly long-term survival of pig organ grafts may be limited for some time by the early onset of graft atherosclerosis or other forms of chronic rejection (until this problem can be resolved), initial clinical trials may involve ‘bridging’ a patient in end-stage organ failure, particularly of the liver [149] or heart [150], until a suitable allograft becomes available. This would not only be lifesaving – and therefore ethically justified – but would also enable valuable experience of pig organ function in humans, as opposed to nonhuman primates, to be gained.
However, ‘bridging’ would not be a clinical option if sensitization to pig antigens (e.g., swine leukocyte antigens), resulted in an increase in panel-reactive antibodies (i.e., antibodies to HLA), which might either preclude subsequent allotransplantation or be detrimental to the outcome of such a procedure. Fortunately, although limited, current evidence is that antibodies that develop after exposure to a pig xenograft (if immunosuppressive therapy has been unsuccessful in preventing sensitization) are not cross-reactive against HLA, and so would not be detrimental to a subsequent allograft (reviewed in [151]). By contrast, patients with a high level of HLA-reactive antibodies may be at greater risk of rejecting a pig xenograft, although again the evidence for this remains limited (reviewed in [151]).
The potential therapeutic possibilities offered by xenotransplantation are so considerable that it remains an area of research that should be pursued vigorously until the barriers have been overcome. Not only will pig organs and islets offer therapeutic options, but there are potential therapies related to pig corneal transplants, pig neural-cell transplants (in conditions such as Parkinson’s and Huntington’s disease), and even pig red blood cells for transfusion into humans [152]. The number of patients who might benefit from xenotransplantation may therefore run into the hundreds of thousands or even millions if it can achieve its potential.
Expert commentary & five-year view
The increasing availability of genetically modified pigs is steadily drawing clinical xenotransplantation closer. Treated ‘nonviable’ tissues from wild-type pigs, such as dermis scaffolds and small intestinal stroma, are already being used on a large scale in clinical surgery, and steps are underway to improve outcomes by using GTKO pigs for these purposes. There is evidence to indicate that tissues from GTKO pigs will generate a weaker inflammatory response in the recipient.
Work at our own and other centers is exploring the potential of pig corneas for corneal transplantation, and we have also investigated the possibility of using GTKO pig red blood cells for clinical transfusion [152]. The encouraging results of pig islet transplantation in diabetic monkeys [127,128], particularly when islets from genetically engineered pigs are transplanted [125], suggest that clinical islet xenotransplantation is almost certain to be instituted within a few years. Pig organ transplantation in patients with end-stage organ failure is likely to follow, initially as a bridge to allotransplantation.
In summary, therefore, further genetic engineering of pigs is required to protect the organs and islets from the primate immune response, particularly from the innate immune system. Most importantly, genetically engineered pigs are required whose organs and cells are protected from the coagulation dysregulation that occurs. In particular, modifications are required to prevent, first, TF activity on the graft [Lin CC, Ezzelarab M, Shapiro Ret al., Manuscript In Preparation] and, second, activation of recipient platelets to express TF and initiate consumptive coagulopathy [112,115].
An immunosuppressive regimen is required to prevent cellular rejection and a T-cell-dependent elicited antibody response, and this regimen must be one that is clinically applicable and not associated with a high incidence of complications, such as infection or malignant disease. In this respect, an alternative is to express the immunosuppressive agent in the graft. For example, CTLA4-Ig has been very successfully expressed ubiquitously in pigs – so successfully, in fact, that this resulted in complications of immunosuppression in the pig [153].
In 5 years time, therefore, we anticipate that clinical trials of islet xenotransplantation will have been initiated. The availability of GTKO/CD46 pigs transgenic for a human antithrombotic or anticoagulant gene will have resulted in improved organ graft survival in nonhuman primates, and may allow consideration of clinical trials of bridging to allotransplantation.
Key issues.
Genetic engineering of pigs to prevent the coagulation dysfunction that occurs between a pig organ graft and recipient primate may be achieved by the expression of thrombomodulin, tissue factor pathway inhibitor, CD39 or other mechanisms in the pig vascular endothelium.
Determination of an effective immunosuppressive regimen that is not so intensive that it results in complications, such as infection or malignancy, can be achieved by T-cell costimulation blockade, which offers great potential towards this goal.
Protection of pig islets from the instant blood-mediated inflammatory reaction following transplantation into the portal vein may be achieved by expression of anticomplement and anticoagulant genes on the islets. Alternatively, a different site for islet transplantation, such as the gastric submucosal space, should be explored.
Acknowledgments
The authors thank the many colleagues who have contributed to their own studies.
Footnotes
Financial & competing interests disclosure
Burcin Ekser was a recipient of an American Society of Transplantation/European Society for Organ Transplantation Exchange Grant. Work on xenotransplantation in the Thomas E Starzl Transplantation Institute of the University of Pittsburgh has been supported in part by NIH grants U01 AI068642 and R21 A1074844, and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Burcin Ekser, Thomas E Starzl Transplantation Institute, University of Pittsburgh Medical Center, Pittsburgh, PA, USA, and Department of Surgery and Organ Transplantation, University of Padua, Padua, Italy.
David KC Cooper, Thomas E Starzl Transplantation Institute, University of Pittsburgh Medical Center, Starzl Biomedical Science Tower, W1543, 200 Lothrop Street, Pittsburgh, PA 15261, USA, Tel.: +1 412 383 6961, Fax: +1 412 624 1172, cooperdk@upmc.edu.
References
Papers of special note have been highlighted as:
-
•
of interest
-
••
of considerable interest
- 1.Taniguchi S, Cooper DKC. Clinical xenotransplantation – past, present and future. Ann R Coll Surg Engl. 1994;79:13–19. [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper DKC, Lanza RP. Xeno – The Promise of Transplanting Animal Organs into Humans. Oxford University Press; NY, USA: 2000. [Google Scholar]
- 3.Cooper DKC, Gollackner B, Sachs DH. Will the pig solve the transplantation backlog? Annu Rev Med. 2002;53:133–147. doi: 10.1146/annurev.med.53.082901.103900. [DOI] [PubMed] [Google Scholar]
- 4.Cooper DKC, Ye Y, Rolf LL, Zuhdi N. The pig as potential organ donor for man. In: Cooper DKC, Kemp E, Reemtsma K, White DJG, editors. Xenotransplantation. Springer; Heidelberg, Germany: 1991. pp. 481–500. [Google Scholar]
- 5.Cooper DKC, Dorling A, Pierson RN, III, et al. α1,3-galactosyltransferase gene-knockout pigs for xenotransplantation: where do we go from here? Transplantation. 2007;84:1–7. doi: 10.1097/01.tp.0000260427.75804.f2. • Useful review of the recent progress in xenotransplantation and directions for the future.
- 6.Lexer G, Cooper DKC, Rose AG, et al. Hyperacute rejection in a discordant (pig to baboon) cardiac xenograft model. J Heart Transplant. 1986;5:411–418. • First description of hyperacute rejection of an organ graft in the pig-to-nonhuman primate model.
- 7.Cooper DKC, Human PA, Lexer G, et al. Effects of cyclosporine and antibody adsorption on pig cardiac xenograft survival in the baboon. J Heart Transplant. 1988;7:238–246. [PubMed] [Google Scholar]
- 8.Rose AG, Cooper DKC, Human PA, Reichenspurner H, Reichart B. Histopathology of hyperacute rejection of the heart – experimental and clinical observations in allografts and xenografts. J Heart Lung Transplant. 1991;10:223–234. [PubMed] [Google Scholar]
- 9.Rose AG, Cooper DKC. A histopathologic grading system of hyperacute (humoral, antibody-mediated) cardiac xenograft and allograft rejection. J Heart Lung Transplant. 1996;15:804–817. [PubMed] [Google Scholar]
- 10.Rose AG, Cooper DKC. Venular thrombosis is the key event in the pathogenesis of antibody-mediated cardiac rejection. Xenotransplantation. 2000;7:31–41. doi: 10.1034/j.1399-3089.2000.00042.x. [DOI] [PubMed] [Google Scholar]
- 11.Bach FH, Robson SC, Ferran C, et al. Endothelial cell activation and thromboregulation during xenograft rejection. Immunol Rev. 1994;141:5–30. doi: 10.1111/j.1600-065x.1994.tb00870.x. [DOI] [PubMed] [Google Scholar]
- 12.Saadi S, Platt JL. Role of complement in xenotransplantation. Clin Exp Pharmacol Physiol. 1999;26:1016–1019. doi: 10.1046/j.1440-1681.1999.03184.x. [DOI] [PubMed] [Google Scholar]
- 13.Alexandre GPJ, Gianello P, Latinne D, et al. Plasmapheresis and splenectomy in experimental renal xenotransplantation. In: Hardy MA, editor. Xenograft. Elsevier; NY, USA: 1989. pp. 259–266. [Google Scholar]
- 14.Ye Y, Neethling FA, Niekrasz M, et al. Evidence that intravenously administered a-galactosyl carbohydrates reduce baboon serum cytotoxicity to pig kidney cells (PK15) and transplanted pig hearts. Transplantation. 1994;58:330–337. [PubMed] [Google Scholar]
- 15.Simon P, Neethling FA, Taniguchi S, et al. Intravenous infusion of Galα1–3Gal oligosaccharides in baboons delays hyperacute rejection of porcine heart xenografts. Transplantation. 1998;65:346–353. doi: 10.1097/00007890-199802150-00009. [DOI] [PubMed] [Google Scholar]
- 16.Taniguchi S, Neethling FA, Korchagina EY, et al. In vivo immunoadsorption of antipig antibodies in baboons using a specific Galα1–3Gal column. Transplantation. 1996;62:1379–1384. doi: 10.1097/00007890-199611270-00001. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi T, Taniguchi S, Neethling FA, et al. Delayed xenograft rejection of pig-to-baboon cardiac transplants after cobra venom factor therapy. Transplantation. 1997;64:1255–1261. doi: 10.1097/00007890-199711150-00005. [DOI] [PubMed] [Google Scholar]
- 18.Xu Y, Lorf T, Sablinski T, et al. Removal of anti-porcine natural antibodies from human and nonhuman primate plasma in vitro and in vivo by a Galα1–3Galβ1–4βGlc-X immunoaffinity column. Transplantation. 1998;65:172–179. doi: 10.1097/00007890-199801270-00005. [DOI] [PubMed] [Google Scholar]
- 19.Kozlowski T, Shimizu A, Lambrigts D, et al. Porcine kidney and heart transplantation in baboons undergoing a tolerance induction regimen and antibody adsorption. Transplantation. 1999;67:18–30. doi: 10.1097/00007890-199901150-00004. [DOI] [PubMed] [Google Scholar]
- 20.Watts A, Foley A, Awwad M, et al. Plasma perfusion by apheresis through a Gal immunoaffinity column successfully depletes anti-Gal antibody: experience with 320 aphereses in baboons. Xenotransplantation. 2000;7:181–185. doi: 10.1034/j.1399-3089.2000.00068.x. [DOI] [PubMed] [Google Scholar]
- 21.Buhler L, Yamada K, Kitamura H, et al. Pig kidney transplantation in baboons: anti-Galα1–3Gal IgM alone is associated with acute humoral xenograft rejection and disseminated intravascular coagulation. Transplantation. 2001;72:1743–1752. doi: 10.1097/00007890-200112150-00007. [DOI] [PubMed] [Google Scholar]
- 22.Chen G, Sun H, Yang H, et al. The role of anti-non-Gal antibodies in the development of acute humoral xenograft rejection of hDAF transgenic porcine kidneys in baboons receiving anti-Gal antibody neutralization therapy. Transplantation. 2006;81:273–283. doi: 10.1097/01.tp.0000188138.53502.de. [DOI] [PubMed] [Google Scholar]
- 23.Gollackner B, Goh S-K, Qawi I, et al. Acute vascular rejection of xenografts: roles of natural and elicited xenoreactive antibodies in activation of vascular endothelial cells and induction of procoagulant activity. Transplantation. 2004;77:1735–1741. doi: 10.1097/01.tp.0000131167.21930.b8. [DOI] [PubMed] [Google Scholar]
- 24.Cardozo LAM, Rouw DB, Ambrose LR, et al. The neutrophil: unnoticed threat in xenotransplantation. Transplantation. 2004;78:1721–1728. doi: 10.1097/01.tp.0000147341.40485.b4. [DOI] [PubMed] [Google Scholar]
- 25.Gilli UO, Schneider MKJ, Loetscher P, et al. Human polymorphonuclear neutrophils are recruited by porcine chemokines acting on CXC chemokine receptor 2, and platelet-activating factor. Transplantation. 2005;79:1344–1331. doi: 10.1097/01.tp.0000155429.44902.44. [DOI] [PubMed] [Google Scholar]
- 26.Al-Mohanna F, Saleh S, Parhar RS, et al. Human neutrophil gene expression profiling following xenogeneic encounter with porcine aortic endothelial cells: the occult role of neutrophils in xenograft rejection revealed. J Leukoc Biol. 2005;78:51–61. doi: 10.1189/jlb.0904494. [DOI] [PubMed] [Google Scholar]
- 27.Ezzelarab M, Garcia B, Azimzadeh A, et al. The innate immune response and activation of coagulation in α1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009;87:805–812. doi: 10.1097/TP.0b013e318199c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inverardi L, Clissi B, Stolzer AL, et al. Human natural killer lymphocytes directly recognize evolutionarily conserved oligosaccharide ligands expressed by xenogeneic tissues. Transplantation. 1997;63:1318–1330. doi: 10.1097/00007890-199705150-00021. [DOI] [PubMed] [Google Scholar]
- 29.Baumann BC, Forte P, Hawley RJ, Rieben R, Schneider MK, Seebach JD. Lack of galactose-α1,3-galactose expression on porcine endothelial cells prevents complement-induced lysis but not direct xenogeneic NK cytotoxicity. J Immunol. 2004;172:6460–6467. doi: 10.4049/jimmunol.172.10.6460. [DOI] [PubMed] [Google Scholar]
- 30.Rieben R, Seebach JD. Xenograft rejection: IgG1, complement and NK cells team up to activate and destroy the endothelium. Trends Immunol. 2005;26:2–5. doi: 10.1016/j.it.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Fox A, Mountford J, Braakhuis A, et al. Innate and adaptive immune responses to nonvascular xenografts: evidence that macrophages are direct effectors of xenograft rejection. J Immunol. 2001;166:2133–2140. doi: 10.4049/jimmunol.166.3.2133. [DOI] [PubMed] [Google Scholar]
- 32.Yamada K, Sachs DH, DerSimonian H. Human anti-porcine xenogeneic T cell response. Evidence for allelic specificity of mixed leukocyte reaction and for both direct and indirect pathways of recognition. J Immunol. 1995;155:5249–5256. [PubMed] [Google Scholar]
- 33.Dorling A, Lechler RI. T cell-mediated xenograft rejection: specific tolerance is probably required for long term xenograft survival. Xenotransplantation. 1998;5:234–245. doi: 10.1111/j.1399-3089.1998.tb00034.x. [DOI] [PubMed] [Google Scholar]
- 34.Mirenda V, Golshayan D, Read J, et al. Achieving permanent survival of islet xenografts by independent manipulation of direct and indirect T-cell responses. Diabetes. 2005;54:1048–1055. doi: 10.2337/diabetes.54.4.1048. [DOI] [PubMed] [Google Scholar]
- 35.Buhler LH, Cooper DKC. How strong is the T cell response in the pig-to-primate model? Xenotransplantation. 2005;12:85–87. doi: 10.1111/j.1399-3089.2004.00185.x. [DOI] [PubMed] [Google Scholar]
- 36.Lin YJ, Hara H, Tai H-C, et al. Suppressive efficacy and proliferative capacity of human regulatory T cells in allogeneic and xenogeneic responses. Transplantation. 2008;86:1452–1462. doi: 10.1097/TP.0b013e318188acb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCurry KR, Parker W, Cotterell AH, et al. Humoral responses to pig-to-baboon cardiac transplantation: implications for the pathogenesis and treatment of acute vascular rejection and for accommodation. Hum Immunol. 1997;58:91–105. doi: 10.1016/s0198-8859(97)00229-2. [DOI] [PubMed] [Google Scholar]
- 38.Cozzi E, Bhatti F, Schmoeckel M, et al. Long-term survival of non-human primates receiving life-supporting transgenic porcine kidney xenografts. Transplantation. 2000;70:15–21. [PubMed] [Google Scholar]
- 39.Cozzi E, Vial C, Ostlie D, et al. Maintenance triple immunosuppression with cyclosporin A, mycophenolate sodium and steroids allows prolonged survival of primate recipients of hDAF porcine renal xenografts. Xenotransplantation. 2003;10:300–310. doi: 10.1034/j.1399-3089.2003.02014.x. [DOI] [PubMed] [Google Scholar]
- 40.Houser SL, Kuwaki K, Knosalla C, et al. Thrombotic microangiopathy and graft arteriopathy in pig hearts following transplantation into baboons. Xenotransplantation. 2004;11:416–425. doi: 10.1111/j.1399-3089.2004.00155.x. • Good description of thrombotic microangiopathy developing in pig hearts grafted into baboons.
- 41.Chen G, Qian H, Starzl T, et al. Induced anti-non-Gal antibodies lead to acute humoral xenograft rejection in baboons using α1,3-galactosyltransferase gene-knockout pigs as kidney donors. Nat Med. 2005;11:1295–1298. [Google Scholar]
- 42.Byrne GW, Davies WR, Oi K, et al. Increased immunosuppression, not anticoagulation, extends cardiac xenograft survival. Transplantation. 2006;82:1787–1791. doi: 10.1097/01.tp.0000251387.40499.0f. [DOI] [PubMed] [Google Scholar]
- 43.Buhler L, Awwad M, Basker M, et al. High-dose porcine hematopoietic cell transplantation combined with CD40 ligand blockade in baboons prevents an induced antipig humoral response. Transplantation. 2000;69:2296–2304. doi: 10.1097/00007890-200006150-00013. • The original report on the efficacy of costimulation blockade in preventing the primate T-cell-dependent elicited antibody response to a pig xenograft.
- 44.Dalmasso AP, Vercellotti GM, Platt JL, Bach FH. Inhibition of complement-mediated endothelial cell cytotoxicity by decay accelerating factor. Potential for prevention of xenograft hyperacute rejection. Transplantation. 1991;52:530–533. doi: 10.1097/00007890-199109000-00029. • Early report of the expression of a human complement-regulatory protein to protect pig vascular endothelial cells from the effects of antibody-mediated complement activation.
- 45.Oglesby TJ, White D, Tedja I, et al. Protection of mammalian cells from complement-mediated lysis by transfection of human membrane cofactor protein and decay-accelerating factor. Trans Assoc Am Phys. 1991;104:164–172. • Early report of the expression of a human complement-regulatory protein to protect pig vascular endothelial cells from the effects of antibody-mediated complement activation.
- 46.Cozzi E, White DJ. The generation of transgenic pigs as potential organ donors for humans. Nat Med. 1995;1:964–966. doi: 10.1038/nm0995-964. [DOI] [PubMed] [Google Scholar]
- 47.Byrne GW, McCurry KR, Martin MJ, McClellan SM, Platt JL, Logan JS. Transgenic pigs expressing human CD59 and decay-accelerating factor produce an intrinsic barrier to complement-mediated damage. Transplantation. 1997;63:149–155. doi: 10.1097/00007890-199701150-00027. [DOI] [PubMed] [Google Scholar]
- 48.Loveland BE, Milland J, Kyriakou P, et al. Characterization of a CD46 transgenic pig and protection of transgenic kidneys against hyperacute rejection in non-immunosuppressed baboons. Xenotransplantation. 2004;11:171–183. doi: 10.1046/j.1399-3089.2003.00103.x. [DOI] [PubMed] [Google Scholar]
- 49.Cooper DKC, Good AH, Koren E, et al. Identification of α-galactosyl and other carbohydrate epitopes that are bound by human antipig antibodies: relevance to discordant xenografting in man. Transpl Immunol. 1993;1:198–205. doi: 10.1016/0966-3274(93)90047-c. [DOI] [PubMed] [Google Scholar]
- 50.Cooper DKC, Koren E, Oriol R. Genetically engineered pigs. Lancet. 1993;342:682–683. doi: 10.1016/0140-6736(93)91791-j. • The initial suggestion that deletion of the gene for α1,3-galactosyltransferase should be undertaken in pigs to prevent expression of Gal antigens.
- 51.Phelps CJ, Koike C, Vaught TD, et al. Production of α1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–414. doi: 10.1126/science.1078942. • First report of production of α1,3-galactosyltransferase gene-knockout GTKO pigs.
- 52.Kolber-Simonds D, Lai L, Watt SR, et al. α1,3-galactosyltransferase null pigs via nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci USA. 2004;19:7335–7340. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morgan BP, Berg CW, Harris CL. ‘Homologous restriction’ in complement lysis: roles of membrane complement regulators. Xenotransplantation. 2005;12:258–265. doi: 10.1111/j.1399-3089.2005.00237.x. [DOI] [PubMed] [Google Scholar]
- 54.Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of a-galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263:17755–17762. • Original report of the presence of Gal in pigs and other nonprimate mammals.
- 55.Oriol R, Ye Y, Koren E, Cooper DKC. Carbohydrate antigens of pig tissues reacting with human natural antibodies as potential targets for hyperacute vascular rejection in pig-to-man organ xenotransplantation. Transplantation. 1993;56:1433–1442. doi: 10.1097/00007890-199312000-00031. [DOI] [PubMed] [Google Scholar]
- 56.Cooper DKC. Depletion of natural antibodies in non-human primates – a step towards successful discordant xenografting in man. Clin Transplantation. 1992;6:178–183. [PubMed] [Google Scholar]
- 57.Good AH, Cooper DKC, Malcolm AJ, et al. Identification of carbohydrate structures that bind human antiporcine antibodies: implications for discordant xenografting in man. Transplant Proc. 1992;24:559–562. • Original report of the importance of Gal antigens as the major target for human antipig antibodies.
- 58.Kobayashi T, Cooper DKC. Anti-Gal, α-Gal epitopes and xenotransplantation. In: Galili U, Avila JL, editors. αGal and Anti-Gal: α-1,3-Galactosyltransferase, α-Gal Epitopes, and the Natural Anti-Gal Antibody Subcellular Biochemistry Series. Vol. 32. Kluwer Academic/Plenum; NY, USA, London, UK: 1999. pp. 229–257. [Google Scholar]
- 59.Kuwaki K, Tseng YL, Dor FJMF, et al. Heart transplantation in baboons using α1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. • First report of transplantation of hearts from GTKO pigs into nonhuman primates, with survival extending for almost 6 months.
- 60.Tseng Y-L, Kuwaki K, Dor FJMF, et al. α1,3-galactosyltransferase gene-knockout pig heart transplantation in baboons with survival approaching six months. Transplantation. 2005;80:1493–1500. doi: 10.1097/01.tp.0000181397.41143.fa. [DOI] [PubMed] [Google Scholar]
- 61.Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of α1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–34. doi: 10.1038/nm1172. • First report of transplantation of kidneys from GTKO pigs into nonhuman primates.
- 62.Hara H, Long C, Lin YJ, et al. In vitro investigation of pig cells for resistance to human antibody-mediated rejection. Transpl Int. 2008;21:1163–1174. doi: 10.1111/j.1432-2277.2008.00736.x. [DOI] [PubMed] [Google Scholar]
- 63.Bhatti FN, Schmoeckel M, Zaidi A, et al. Three-month survival of hDAF transgenic pig hearts transplanted into primates. Transplant Proc. 1999;31:958. doi: 10.1016/s0041-1345(98)01855-7. [DOI] [PubMed] [Google Scholar]
- 64.McGregor CG, Teotia SS, Byrne GW, et al. Cardiac xenotransplantation: progress toward the clinic. Transplantation. 2004;78:1569–1575. doi: 10.1097/01.tp.0000147302.64947.43. [DOI] [PubMed] [Google Scholar]
- 65.McGregor CG, Davies WR, Oi K, et al. Cardiac xenotransplantation: recent preclinical progress with 3-month median survival. J Thorac Cardiovasc Surg. 2005;130(3):844–851. doi: 10.1016/j.jtcvs.2005.04.017. • Report of 3 months median survival of CD46-transgenic pig heart grafts in baboons using a conventional immunosuppressive regimen.
- 66.Zaidi A, Schmoeckel M, Bhatti F, et al. Life-supporting pig-to-primate renal xenotransplantation using genetically modified donors. Transplantation. 1998;65:1584–1590. doi: 10.1097/00007890-199806270-00008. [DOI] [PubMed] [Google Scholar]
- 67.Ramirez P, Chavez R, Majado M, et al. Life-supporting human complement regulator decay accelerating factor transgenic pig liver xenograft maintains the metabolic function and coagulation in the nonhuman primate for up to 8 days. Transplantation. 2000;70:989–998. doi: 10.1097/00007890-200010150-00001. [DOI] [PubMed] [Google Scholar]
- 68.Ekser B, Long C, Echeverri GJ, et al. Impact of thrombocytopenia on survival of baboons with genetically-modified pig liver transplants: clinical relevance. Am J Transplant. 2010;10:273–285. doi: 10.1111/j.1600-6143.2009.02945.x. [DOI] [PubMed] [Google Scholar]
- 69.Hara H, Gridelli B, Lin YJ, Marcos A, Cooper DKC. Liver xenografts for the treatment of acute liver failure: clinical and experimental experience and remaining immunologic barriers. Liver Transplant. 2008;14:425–434. doi: 10.1002/lt.21476. [DOI] [PubMed] [Google Scholar]
- 70.Nguyen BH, Zwets E, Schroeder C, Pierson RN, 3rd, Azimzadeh AM. Beyond antibody-mediated rejection: hyperacute lung rejection as a paradigm for dysregulated inflammation. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:255–269. doi: 10.2174/1568006054064753. [DOI] [PubMed] [Google Scholar]
- 71.Nguyen BN, Azimzadeh AM, Zhang T, et al. Life-supporting function of genetically modified swine lungs in baboons. J Thorac Cardiovasc Surg. 2007;133:1354–1363. doi: 10.1016/j.jtcvs.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 72.Zhu X, Dor FJMF, Cooper DKC. Pig-to-non-human primate heart transplantation: immunologic progress over 20 years. J Heart Lung Transplant. 2007;26:210–218. doi: 10.1016/j.healun.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 73.Baumann BC, Stussi G, Huggel K, et al. Reactivity of human natural antibodies to endothelial cells from Galα(1,3)Gal-deficient pigs. Transplantation. 2007;83:193–201. doi: 10.1097/01.tp.0000250478.00567.e5. [DOI] [PubMed] [Google Scholar]
- 74.Hara H, Ezzelarab M, Rood PPM, et al. Allosensitized humans are at no greater risk of humoral rejection of GT-KO pig organs than other humans. Xenotransplantation. 2005;13:357–365. doi: 10.1111/j.1399-3089.2006.00319.x. [DOI] [PubMed] [Google Scholar]
- 75.Ezzelarab M, Hara H, Busch J, et al. Antibodies directed to pig nonGal antigens in naïve and sensitized baboons. Xenotransplantation. 2006;13:400–407. doi: 10.1111/j.1399-3089.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- 76.Rood PPM, Hara H, Busch JL, et al. Incidence and cytotoxicity of antibodies in cynomolgus monkeys directed to nonGal antigens, and their relevance for experimental models. Transplant Int. 2006;19:158–165. doi: 10.1111/j.1432-2277.2005.00252.x. [DOI] [PubMed] [Google Scholar]
- 77.Wong BS, Yamada K, Koumi M, et al. Allosensitization does not increase the risk of xenoreactivity to α1,3-galactosyltransferase gene-knockout (GalT-KO) miniature swine in patients on transplantation waiting lists. Transplantation. 2006;82:314–319. doi: 10.1097/01.tp.0000228907.12073.0b. [DOI] [PubMed] [Google Scholar]
- 78.Cooper DKC. Xenoantigens and xenoantibodies. Xenotransplantation. 1998;5:6–17. doi: 10.1111/j.1399-3089.1998.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 79.Ezzelarab M, Ayares D, Cooper DKC. Carbohydrates in xenotransplantation. Immunol Cell Biol. 2005;83:396–404. doi: 10.1111/j.1440-1711.2005.01344.x. [DOI] [PubMed] [Google Scholar]
- 80.Bouhours D, Pourcel C, Bouhours JE. Simultaneous expression by porcine aorta endothelial cells of glycosphinogolipids bearing the major epitope for human xenoreactive antibodies (Galα1–3Gal), blood group H determinant and N-glycolylneuraminic acid. Glycoconj J. 1996;13:947–953. doi: 10.1007/BF01053190. [DOI] [PubMed] [Google Scholar]
- 81.Varki A. Loss of N-glycolylneuraminic acid in humans: mechanisms, consequences, and implications for hominid evolution. Am J Phys Anthropol. 2001;(Suppl 33):54–69. doi: 10.1002/ajpa.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu A, Hurst R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation. 2002;9:376–381. doi: 10.1034/j.1399-3089.2002.02138.x. [DOI] [PubMed] [Google Scholar]
- 83.Miwa Y, Kobayashi T, Nagasaka T, et al. Are N-glycolylneuraminic acid (Hanganutziu-Deicher) antigens important in pig-to-human xenotransplantation. Xenotransplantation. 2004;11:247–253. doi: 10.1111/j.1399-3089.2004.00126.x. [DOI] [PubMed] [Google Scholar]
- 84.Tangvoranuntakul P, Gagneux P, Diaz S, et al. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci USA. 2003;100:12045–12050. doi: 10.1073/pnas.2131556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seebach JD, Comrack C, Germana S, LeGuern C, Sachs DH, DerSimonian H. HLA-Cw3 expression on porcine endothelial cells protects against xenogeneic cytotoxicity mediated by a subset of human NK cells. J Immunol. 1997;159:3655–3661. [PubMed] [Google Scholar]
- 86.Dorling A, Monk NJ, Lechler RI. HLA-G inhibits the transendothelial migration of human NK cells. Eur J Immunol. 2000;30:586–593. doi: 10.1002/1521-4141(200002)30:2<586::AID-IMMU586>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 87.Forte P, Baumann BC, Weiss EH, Seebach JD. HLA-E expression on porcine cells: protection from human NK cytotoxicity depends on peptide loading. Am J Transplant. 2005;5:2085–2093. doi: 10.1111/j.1600-6143.2005.00987.x. [DOI] [PubMed] [Google Scholar]
- 88.Crew MD, Cannon MJ, Phanavanh B, Garcia-Borges CN. An HLA-E single trimer inhibits human NK cell reactivity towards porcine cells. Mol Immunol. 2005;42:1205–1214. doi: 10.1016/j.molimm.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 89.Ide K, Ohdan H, Kobayashi T, Hara H, Ishiyama K, Asahara T. Antibody- and complement-independent phagocytotic and cytolytic activities of human macrophages toward porcine cells. Xenotransplantation. 2005;12:181–188. doi: 10.1111/j.1399-3089.2005.00222.x. [DOI] [PubMed] [Google Scholar]
- 90.Burlak C, Twining LM, Rees MA. Terminal sialic acid residues on human glycophorin A are recognized by porcine Kupffer cells. Transplantation. 2005;80:344–352. doi: 10.1097/01.tp.0000162974.94890.9f. [DOI] [PubMed] [Google Scholar]
- 91.Rees MA, Butler AJ, Brons IG, et al. Evidence of macrophage receptors capable of direct recognition of xenogeneic epitopes without opsonization. Xenotransplantation. 2005;12:13–19. doi: 10.1111/j.1399-3089.2004.00195.x. [DOI] [PubMed] [Google Scholar]
- 92.Forte P, Matter-Reissmann UB, Strasser M, et al. Porcine aortic endothelial cells transfected with HLA-G are partially protected from xenogeneic human NK cytotoxicity. Hum Immunol. 2000;61:1066–1073. doi: 10.1016/s0198-8859(00)00202-0. [DOI] [PubMed] [Google Scholar]
- 93.Forte P, Pazmany L, Matter-Reissmann UB, et al. HLA-G inhibits rolling adhesion of activated human NK cells on porcine endothelial cells. J Immunol. 2001;167:6002–6008. doi: 10.4049/jimmunol.167.10.6002. [DOI] [PubMed] [Google Scholar]
- 94.Weiss EH, Lilienfeld BG, Müller S, et al. HLA-E/human β2-microglobulin transgenic pigs: protection against xenogeneic human antipig natural killer cell cytotoxicity. Transplantation. 2009;87:35–43. doi: 10.1097/TP.0b013e318191c784. [DOI] [PubMed] [Google Scholar]
- 95.Ide K, Wang H, Tahara H, et al. Role for CD47-SIRPa signaling in xenograft rejection by macrophages. Proc Natl Acad Sci USA. 2007;104:5062–5066. doi: 10.1073/pnas.0609661104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang YG, Sykes M. Xenotransplantation: current status and a perspective on the future. Nat Rev Immunol. 2007;7:519–531. doi: 10.1038/nri2099. [DOI] [PubMed] [Google Scholar]
- 97.Kuwaki K, Knosalla C, Dor FJMF, et al. Suppression of natural and elicited antibodies in pig-to-baboon heart transplantation using a human anti-CD154 monoclonal antibody-based regimen. Am J Transplant. 2004;4:363–372. doi: 10.1111/j.1600-6143.2004.00353.x. [DOI] [PubMed] [Google Scholar]
- 98.Shimizu A, Yamada K, Yamamoto S, et al. Thrombotic microangiopathic glomerulopathy in human decay accelerating factor-transgenic swine-to-baboon kidney xenograts. J Am Soc Nephrol. 2005;16:2732–2745. doi: 10.1681/ASN.2004121148. [DOI] [PubMed] [Google Scholar]
- 99.Shimizu A, Hisashi Y, Kuwaki K, et al. Thrombotic microangiopathy associated with humoral rejection of cardiac xenografts from α1,3-galactosyltransferase gene-knockout pigs in baboons. Am J Pathol. 2008;172:1471–1481. doi: 10.2353/ajpath.2008.070672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gaca JG, Lesher A, Aksoy O, et al. Disseminated intravascular coagulation in association with pig-to-primate pulmonary xenotransplantation. Transplantation. 2002;73:1717–1723. doi: 10.1097/00007890-200206150-00005. [DOI] [PubMed] [Google Scholar]
- 101.Buhler LH, Basker M, Alwayn IPJ, et al. Coagulation and thrombotic disorders associated with pig organ and hematopoietic cell transplantation in nonhuman primates. Transplantation. 2000;70:1323–1331. doi: 10.1097/00007890-200011150-00010. [DOI] [PubMed] [Google Scholar]
- 102.Robson SC, Cooper DKC, d’Apice AJF. Disordered regulation of coagulation and platelet activation in xenotransplantation. Xenotransplantation. 2000;7:166–176. doi: 10.1034/j.1399-3089.2000.00067.x. [DOI] [PubMed] [Google Scholar]
- 103.Chen D, Dorling A. Microcoagulation processes after xenotransplantation. Curr Opin Organ Transplant. 2005;10:240–245. [Google Scholar]
- 104.Robson SC, Young VK, Cook NS, et al. Thrombin inhibition in an ex vivo model of porcine heart xenograft hyperacute rejection. Transplantation. 1996;61:862–868. doi: 10.1097/00007890-199603270-00003. [DOI] [PubMed] [Google Scholar]
- 105.Lawson JH, Daniels LJ, Platt JL. The evaluation of thrombomodulin activity in porcine to human xenotransplantation. Transplant Proc. 1997;29:884–885. doi: 10.1016/s0041-1345(96)00192-3. [DOI] [PubMed] [Google Scholar]
- 106.Schulte am Esch J, Cruz MA, Siegel JB, Anrather J, Robson SC. Activation of human platelets by the membrane-expressed A1 domain of von Willebrand factor. Blood. 1997;90:4425–4437. [PubMed] [Google Scholar]
- 107.Schulte am Esch J, Rogiers X, Robson SC. Molecular incompatibilities in hemostasis between swine and men – impact on xenografting. Ann Transplant. 2001;6:12–16. [PubMed] [Google Scholar]
- 108.Kopp CW, Siegel JB, Hancock WW, et al. Effect of porcine endothelial tissue factor pathway inhibitor on human coagulation factors. Transplantation. 1997;63:749–758. doi: 10.1097/00007890-199703150-00023. [DOI] [PubMed] [Google Scholar]
- 109.Dorling A, Lechler RI. Disordered thromboregulation after xenografting. Curr Opin Organ Transplant. 2001;6:36–41. [Google Scholar]
- 110.Cowan PJ, Aminian A, Barlow H, et al. Protective effects of recombinant human antithrombin III in pig-to-primate renal xenotransplantation. Am J Transplant. 2002;2:520–525. doi: 10.1034/j.1600-6143.2002.20605.x. [DOI] [PubMed] [Google Scholar]
- 111.Cowan PJ, d’Apice AJF. The coagulation barrier in xenotransplantation: incompatibilities and strategies to overcome them. Curr Opin Organ Transplant. 2008;13:178–183. doi: 10.1097/MOT.0b013e3282f63c74. [DOI] [PubMed] [Google Scholar]
- 112.Lin CC, Cooper DK, Dorling A. Coagulation dysregulation as a barrier to xenotransplantation in the primate. Transpl Immunol. 2009;21:75–80. doi: 10.1016/j.trim.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Blakely ML, Van der Werf WJ, Berndt MC, Dalmasso AP, Bach FH, Hancock WW. Activation of intragraft endothelial and mononuclear cells during discordant xenograft rejection. Transplantation. 1994;58:1059–1066. [PubMed] [Google Scholar]
- 114.Gollackner B, Mueller NJ, Houser S, et al. Porcine cytomegalovirus and coagulopathy in pig-to-primate xenotransplantation. Transplantation. 2003;75:1841–1847. doi: 10.1097/01.TP.0000065806.90840.C1. [DOI] [PubMed] [Google Scholar]
- 115.Lin CC, Chen D, McVey JH, Cooper DKC, Dorling A. Expression of tissue factor and initiation of clotting by human platelets and monocytes after incubation with porcine endothelial cells. Transplantation. 2008;86:702–709. doi: 10.1097/TP.0b013e31818410a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen D, Weber M, McVey JH, et al. Complete inhibition of acute humoral rejection using regulated expression of membrane-tethered anticoagulants on xenograft endothelium. Am J Transplant. 2004;4:1958–1963. doi: 10.1111/j.1600-6143.2004.00625.x. [DOI] [PubMed] [Google Scholar]
- 117.Dwyer KM, Robson SC, Nandurkar HH, et al. Thromboregulatory manifestations in human CD39 transgenic mice and the implications for thrombotic disease and transplantation. J Clin Invest. 2004;113:1440–1446. doi: 10.1172/JCI19560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McKenzie IF, Xing PX, Vaughan HA, Prenzoska J, Dabkowski PL, Sandrin MS. Distribution of the major xenoantigen (Gal[α1–3]Gal) for pig to human xenografts. Transpl Immunol. 1994;2:81–86. doi: 10.1016/0966-3274(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 119.Dor FJ, Cheng J, Alt A, et al. Galα1,3Gal expression on porcine pancreatic islets, testis, spleen, and thymus. Xenotransplantation. 2004;11:101–106. doi: 10.1111/j.1399-3089.2004.00078.x. [DOI] [PubMed] [Google Scholar]
- 120.Soderlund J, Wennberg L, Castanos-Velez E, et al. Fetal porcine islet-like cell clusters transplanted to cynomolgus monkeys: an immunohistochemical study. Transplantation. 1999;67:784–791. doi: 10.1097/00007890-199903270-00002. [DOI] [PubMed] [Google Scholar]
- 121.McKenzie IF, Koulmanda M, Mandel TE, Sandrin MS. Pig islet xenografts are susceptible to “antipig” but not Galα(1,3) Gal antibody plus complement in Galo/o mice. J Immunol. 1998;161:5116–5119. [PubMed] [Google Scholar]
- 122.Komoda H, Miyagawa S, Kubo T, et al. A study of the xenoantigenicity of adult pig islets cells. Xenotransplantation. 2004;11:237–246. doi: 10.1111/j.1399-3089.2004.00121.x. [DOI] [PubMed] [Google Scholar]
- 123.van der Windt DJ, Bottino R, Casu A, Campanile N, Cooper DKC. Rapid loss of intraportally-transplanted islets: an overview of pathophysiology and preventive strategies. Xenotransplantation. 2004;14:288–297. doi: 10.1111/j.1399-3089.2007.00419.x. [DOI] [PubMed] [Google Scholar]
- 124.Goto M, Tjernberg J, Dufrane D, et al. Dissecting the instant blood-mediated inflammatory reaction in islet xenotransplantation. Xenotransplantation. 2008;15:225–234. doi: 10.1111/j.1399-3089.2008.00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.van der Windt DJ, Bottino R, Casu A, et al. Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. Am J Transplant. 2009;9(12):2716–2726. doi: 10.1111/j.1600-6143.2009.02850.x. • Reports control of diabetes and survival of pig islets for 1 year in a nonhuman primate.
- 126.Rood PPM, Bottino R, Balamurugan AN, et al. Reduction of early graft loss after intraportal porcine islet transplantation in monkeys. Transplantation. 2007;83:202–210. doi: 10.1097/01.tp.0000250680.36942.c6. [DOI] [PubMed] [Google Scholar]
- 127.Hering BJ, Wijkstrom M, Graham ML, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12:301–303. doi: 10.1038/nm1369. • Encouraging report of adult wild-type pig islet survival in diabetic nonhuman primates for 6-month periods using an intensive immunosuppressive regimen.
- 128.Cardona K, Korbutt GS, Milas Z, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12:304–306. doi: 10.1038/nm1375. • Encouraging report of neonatal wild-type pig islet survival in diabetic nonhuman primates for periods extending for 6 months using an immunosuppressive regimen based on costimulation blockade.
- 129.Tseng Y-L, Sachs DH, Cooper DKC. Porcine hematopoietic progenitor cell transplantation in nonhuman primates: a review of progress. Transplantation. 2005;79:1–9. doi: 10.1097/01.tp.0000146504.73727.13. [DOI] [PubMed] [Google Scholar]
- 130.Barth RN, Yamamoto S, LaMattina JC, et al. Xenogeneic thymokidney and thymic tissue transplantation in a pig-to-baboon model: I. Evidence for pig-specific T-cell unresponsiveness. Transplantation. 2003;75:1615–1624. doi: 10.1097/01.TP.0000064335.50622.20. [DOI] [PubMed] [Google Scholar]
- 131.Kawai T, Cosmi B, Colvin RB, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59:256–262. [PubMed] [Google Scholar]
- 132.Spitzer TR, Delmonico F, Tolkoff-Rubin N, et al. Combined histocompatibility leukocyte antigen-matched donor bone marrow and renal transplantation for multiple myeloma with end stage renal disease: the induction of allograft tolerance through mixed lymphohematopoietic chimerism. Transplantation. 1999;68:480–484. doi: 10.1097/00007890-199908270-00006. [DOI] [PubMed] [Google Scholar]
- 133.Muller YD, Golshayan D, Ehirchiou D, Wekerle T, Seebach JD, Buhler LH. T regulatory cells in xenotransplantation. Xenotransplantation. 2009;16:121–128. doi: 10.1111/j.1399-3089.2009.00531.x. [DOI] [PubMed] [Google Scholar]
- 134.West LJ, Pollock-Barziv SM, Dipchand AI, et al. ABO-incompatible heart transplantation in infants. N Engl J Med. 2001;344:793–800. doi: 10.1056/NEJM200103153441102. [DOI] [PubMed] [Google Scholar]
- 135.Fan X, Ang A, Pollock-Barziv SM, et al. Donor-specific B-cell tolerance after ABO-incompatible infant heart transplantation. Nat Med. 2004;10:1227–1233. doi: 10.1038/nm1126. [DOI] [PubMed] [Google Scholar]
- 136.Rood PPM, Tai H-C, Hara H, et al. Late onset of development of natural anti-nonGal antibodies in infant humans and baboons: implications for xenotransplantation in infants. Transplant Int. 2007;20:1050–1058. doi: 10.1111/j.1432-2277.2007.00546.x. [DOI] [PubMed] [Google Scholar]
- 137.Ibrahim Z, Busch J, Awwad M, Wagner R, Wells K, Cooper DKC. Selected physiologic compatibilities and incompatibilities between human and porcine organ systems. Xenotransplantation. 2006;13:488–499. doi: 10.1111/j.1399-3089.2006.00346.x. [DOI] [PubMed] [Google Scholar]
- 138.Vial CM, Ostlie DJ, Bhatti FN, et al. Life supporting function for over one month of a transgenic porcine heart in a baboon. J Heart Lung Transplant. 2000;19:224–229. doi: 10.1016/s1053-2498(99)00099-6. [DOI] [PubMed] [Google Scholar]
- 139.McGregor CGA, Davies WR, Oi K, et al. Recovery of cardiac function after pig-to-primate orthotopic heart transplant (Abstract) Am J Transplant. 2008;8(Suppl. 2):205. [Google Scholar]
- 140.Soin B, Smith KG, Zaidi A, et al. Physiological aspects of pig-to-primate renal xenotransplantation. Kidney Int. 2001;60:1592–1597. doi: 10.1046/j.1523-1755.2001.00973.x. [DOI] [PubMed] [Google Scholar]
- 141.Onions D, Cooper DKC, Alexander TJL, et al. An assessment of the risk of xenozoonotic disease in pig-to-human xenotransplantation. Xenotransplantation. 2000;7:143–155. doi: 10.1034/j.1399-3089.2000.00047.x. • Useful review of microorganisms that should be excluded from pigs being bred as sources of organs and cells for clinical xenotransplantation.
- 142.Fishman JA, Patience C. Xenotransplantation: infectious risk revisited. Am J Transplant. 2004;4:1383–1390. doi: 10.1111/j.1600-6143.2004.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Patience C, Patton GS, Takeuchi Y, et al. No evidence of pig DNA or retroviral infection in patients with short-term extracorporeal connection to pig kidneys. Lancet. 1998;352:699–701. doi: 10.1016/S0140-6736(98)04369-4. [DOI] [PubMed] [Google Scholar]
- 144.Paradis K, Langford G, Long Z, et al. Search for cross-species transmission of porcine endogenous retrovirus in patients with living pig tissue. The XEN 111 Study Group. Science. 1999;285:1236–1241. doi: 10.1126/science.285.5431.1236. [DOI] [PubMed] [Google Scholar]
- 145.Fishman JA. Xenosis and xenotransplantation: current concepts and challenges (Abstract PL5:2) Xenotransplantation. 2005;12:370. [Google Scholar]
- 146.Dieckhoff B, Petersen B, Kues WA, Kurth R, Niemann H, Denner J. Knockdown of porcine endogenous retrovirus (PERV) expression by PERV-specific siRNA in transgenic pigs. Xenotransplantation. 2008;15:36–45. doi: 10.1111/j.1399-3089.2008.00442.x. [DOI] [PubMed] [Google Scholar]
- 147.Ramsoondar J, Vaught T, Ball S, et al. Production of transgenic pigs that express porcine endogenous retrovirus small interfering RNAs. Xenotransplantation. 2009;16:164–180. doi: 10.1111/j.1399-3089.2009.00525.x. [DOI] [PubMed] [Google Scholar]
- 148.Schuurman HJ. Regulatory aspects of pig-to-human islet transplantation. Xenotransplantation. 2008;15:116–120. doi: 10.1111/j.1399-3089.2008.00467.x. [DOI] [PubMed] [Google Scholar]
- 149.Ekser B, Gridelli B, Tector AJ, Cooper DKC. Pig liver xenotransplantation as a bridge to allotransplantation: which patients might benefit? Transplantation. 2009;88(9):1041–1049. doi: 10.1097/TP.0b013e3181ba0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ibrahim Z, Ezzelarab M, Kormos R, Cooper DKC. Which patients first? Planning the first clinical trial of xenotransplantation: a case for cardiac bridging. Xenotransplantation. 2005;12:168–172. doi: 10.1111/j.1399-3089.2005.00218.x. [DOI] [PubMed] [Google Scholar]
- 151.Cooper DKC, Tseng Y-L, Saidman SL. Allo- and xeno-antibody cross-reactivity in transplantation. Transplantation. 2004;77:1–5. doi: 10.1097/01.TP.0000105116.74032.63. [DOI] [PubMed] [Google Scholar]
- 152.Long C, Hara H, Pawlikowski Z, et al. Genetically engineered pig red blood cells for clinical transfusion: initial in vitro studies. Transfusion. 2009;49(11):2418–2429. doi: 10.1111/j.1537-2995.2009.02306.x. [DOI] [PubMed] [Google Scholar]
- 153.Phelps C, Ball S, Vaught T, et al. Production and characterization of transgenic pigs expressing porcine CTLA4-Ig. Xenotransplantation. 2009;16(6):477–485. doi: 10.1111/j.1399-3089.2009.00533.x. [DOI] [PubMed] [Google Scholar]


